SUMMARY
Objective
Focal cortical dysplasias (FCDs) constitute a prevalent cause of intractable epilepsy in children, and one of the leading conditions requiring epilepsy surgery. Despite the recent advances on the cellular and molecular biology of these conditions, the pathogenetic mechanisms of FCDs remain largely unknown. The purpose if this work is to review the molecular underpinnings of FCDs and to highlight potential therapeutic targets.
Methods
A systematic review of the literature regarding the histological, molecular, and electrophysiological aspects of FCDs was conducted.
Results
Disruption of the mTOR signaling comprises a common pathway underlying the structural and electrical disturbances of some FCDs. Other mechanisms such as viral infections, prematurity, head trauma, and brain tumors are also posited. mTOR inhibitors (i.e., rapamycin) have shown positive results on seizure management in animal models and in a small cohort of patients with FCD.
Significance
Encouraging progresses have been achieved on the molecular and electrophysiological basis of constitutive cells in the dysplastic tissue. Despite the promising results of mTOR inhibitors, large-scale randomized trials are in need to evaluate their efficacy and side effects, along with additional mechanistic studies for the development of novel, molecular-based diagnostic and therapeutic approaches.
INTRODUCTION
The development of the human cerebral cortex proceeds through stages including cell proliferation, differentiation, migration, synaptogenesis and re-organization to generate a functional laminated cortex. The disruption of the cortical assemblage can result in malformations of cortical development (MCDs). Cortical malformations constitute a heterogeneous group of diseases whose pathological patterns rely on the pathogenesis and timing of the insult(s) during brain development. These conditions are commonly associated with intractable epilepsy, cognitive impairment, motor and sensory deficits.
Focal cortical dysplasias (FCDs) comprise a subgroup of MCDs characterized by abnormal cortical lamination, defects of neuronal migration, growth and differentiation involving one discrete cortical region, several lobes or even the entire hemisphere. FCDs often result in medically intractable epilepsy constituting, in fact, the most common cortical malformation encountered in epilepsy surgery.24 The association between genetic mutations, the involvement of specific molecular pathways, their implications on cortex development and the subsequent mechanisms leading to epilepsy are still under intensive investigation. Recent work has linked the activation of the mammalian target of rapamycin (mTOR) pathway with changes in the structural and electrical properties of nerve cells in some FCDs, which could account for the epileptogenic and disorganized cortical lamination of these conditions. Here we review the molecular basis of FCDs and highlight potential targets for future diagnostic and therapeutic measures.
NEUROPATHOLOGY AND CLINICO-RADIOLOGICAL CORRELATIONS
Focal cortical dysplasias typically exhibit varying degrees of disorganized cortical lamination. Constituent cells, in turn, display morphological changes and/or abnormal organization throughout the cortex. These findings were originally described in resected dysplastic cortices from patients with intractable epilepsy.64 This initial report distinguished enlarged, round neurons (dysplastic cells) distributed throughout the affected cortex but sparing the first cortical layer; and balloon cells, described as malformed cells with, at times, multiple nuclei surrounded by excessive cytoplasm and located deeply in the cortex and subjacent white matter. Since this original description, several classifications have been proposed based on new histological findings.44; 51 However, the variable nomenclature led to the lack of agreement upon defining constituent cells, which impacted subsequent studies on their electrophysiological properties and protein expression.
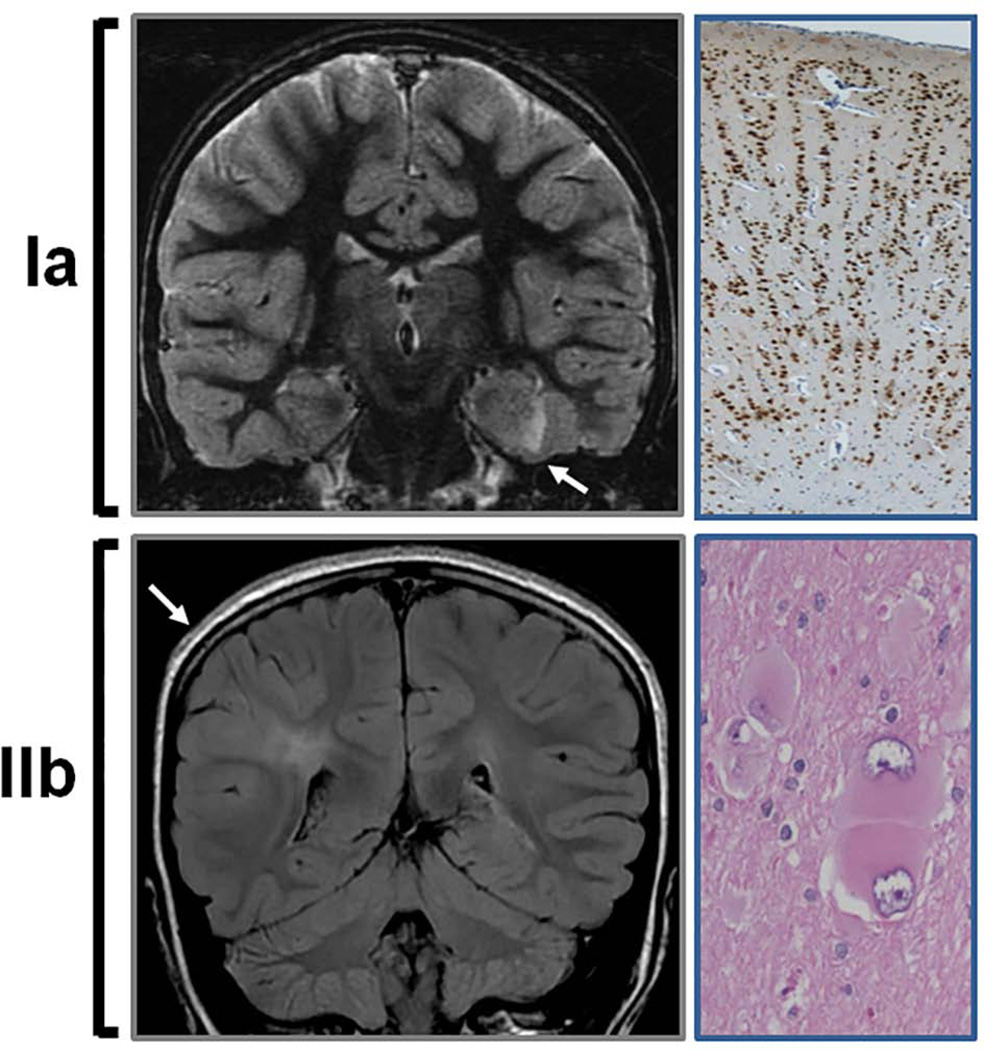
In order to establish a global consensus, the International League Against Epilepsy (ILAE) reported in 2011 a three-level classification system based not only on histological features, but also on clinical presentation and neuroimaging findings.8 This classification was further adapted to the ongoing progress of the molecular basis of FCD (Table 1).6 It is postulated that FCD type I and type III result from cortical defects/injury at postmigrational stages. In this sense, patients with history of severe prematurity, hypoxic-ischemic insults, head trauma from violent shaking, intracranial bleeding or stroke occurring during prenatal or perinatal stages may manifest features of FCD type I.32; 42 Patients commonly exhibit psychomotor retardation, focal deficits and drug-resistant seizures. FCD type I is considered an isolated malformation with abnormal cortical layering in a radial (Ia), tangential (Ib) or mixed (Ic) patterns (Figure 1). Neuronal density is commonly increased, along with lessened cortical thickness and abundance of neuronal microcolumns.46 MRI demonstrates focal cortical hypoplasia and occasionally moderate abnormal signal intensity in the subcortical white matter (Figure 1).18 FCD type III, on the other hand, encompasses a wide group of conditions included, for the first time, in the last classification of FCDs.8 FCD type III is associated with additional primary underlying pathology that occurred during early development, such as hippocampal sclerosis (IIIa), tumors causing epilepsy such as gangliogliomas (IIIb), adjacent vascular malformations (IIIc), and epileptogenic lesions including head trauma, hypoxic-ischemic injury, or encephalitis (IIId).46 These conditions usually manifest abnormal features on MRI, although it may not always be possible to assign a causal event.
TABLE 1. Classification of focal cortical dysplasia.
This table illustrates the last classification system reported by the International League Against Epilepsy in 2011, adapted to the current knowledge of the pathogenesis of FCD6; 8.
| Focal cortical dysplasias due to developmental disturbances |
| Type I (dyslamination) |
| Ia. Abnormal radial lamination |
| Ib. Abnormal tangential lamination |
| Ic. Abnormal radial and tangential lamination |
| Type III (associated pathology) |
| IIIa. Hippocampal sclerosis |
| IIIb. Tumors associated with epilepsy |
| IIIc. Vascular malformations |
| IIId. Other principal lesions during early life |
| Cortical dysgenesis with abnormal cell proliferation without neoplasia |
| Type II (dyslamination with dysmorphic neurons) |
| IIa. Large, dysmorphic neurons |
| IIb. Large, dysmorphic neurons and balloon cells. |
FIGURE 1. Neuroimaging and histological features in FCD type Ia and IIb.
(Upper panel) Coronal T2-weighted MRI illustrating a dysplastic left medial temporal cortex (arrow) corresponding to FCD type Ia. NeuN staining (neuronal marker) of the resected specimen reveals absence of normal lamination and the characteristic radial distribution of neurons (10×). (Lower panel) Coronal fluid-attenuated inversion recovery (FLAIR) MRI illustrates cortical thickening and hyperintense signal in cortex and subcortical regions of the right parietal lobe in FCD type IIb. Note that the subcortical hyperintensity extends to the margin of the right ventricle (transmantle sign). Hematoxylin and eosin staining of the corresponding resected specimen demonstrates the typical balloon cells (20×).
On the other hand, FCD type II results from abnormal cell development and maturation.6 It is characterized by the presence of dysmorphic neurons, dramatic cortical dyslamination, and some specimens display balloon cells. FCD type II is subdivided in type IIa, when balloon cells are not observed, and type IIb, when balloon cells are present (Figure 1).46 Balloon cells, therefore, constitute the defining cell type of FCD IIb and they can also be present in cortical tubers (known as giant cells) and hemimegalencephaly (HME).4 Patients typically exhibit drug-resistant epilepsy at earlier age, higher degree of cognitive decline, and manifest better surgical outcome compared to patients with FCD type I.74 The most common MRI findings include cortical thickening, abnormal gyral and sulcal patterns, and subcortical white matter hyperintensity extending to the margin of the lateral ventricle, the so-called transmantle sign (Figure 1).17 The transmantle sign, constituted by 1) grey-white junction blurring, 2) cortical thickening appearance, 3) bottom-of-the-sulcus dysplasia, is most commonly observed in FCD type IIb and it is associated with favorable seizure control after surgical resection.70 However, it is not known whether the better outcome is related to intrinsic lower epileptogenicity or the easier anatomic recognition of the epileptogenic tissue.
An additional characteristic of FCD type II is the presence of cells expressing immature markers located in deep cortical layers, in contrast to FCD type I, in which cells express few progenitor proteins in superficial layers.49 The markers expressed by balloon cells can be neuronal (i.e., NeuN MAP1B, vimentin, nestin and α-internexin), glial (GFAP) or both, indicating that these cells retain features of immaturity and/or a mixed lineage of origin.19 In addition, balloon cells also express stem cell markers such as Sox2, Oct4, c-Myc, and Klf4, a feature that has been linked to enhanced mTOR signaling.49 Altogether, the impaired cell differentiation in FCD type II could represent a histological hallmark of this cortical malformation.
A MECHANISTIC OVERVIEW
While the histopathological classification is an essential starting point for the definition of the different types of FCD, it will be important to update the current classification according to new findings on the cellular and molecular basis of these conditions. In this section, we review the molecular biology and electrophysiology of FCDs, placing a special emphasis on potential therapeutic targets.
mTOR and interrelated pathways
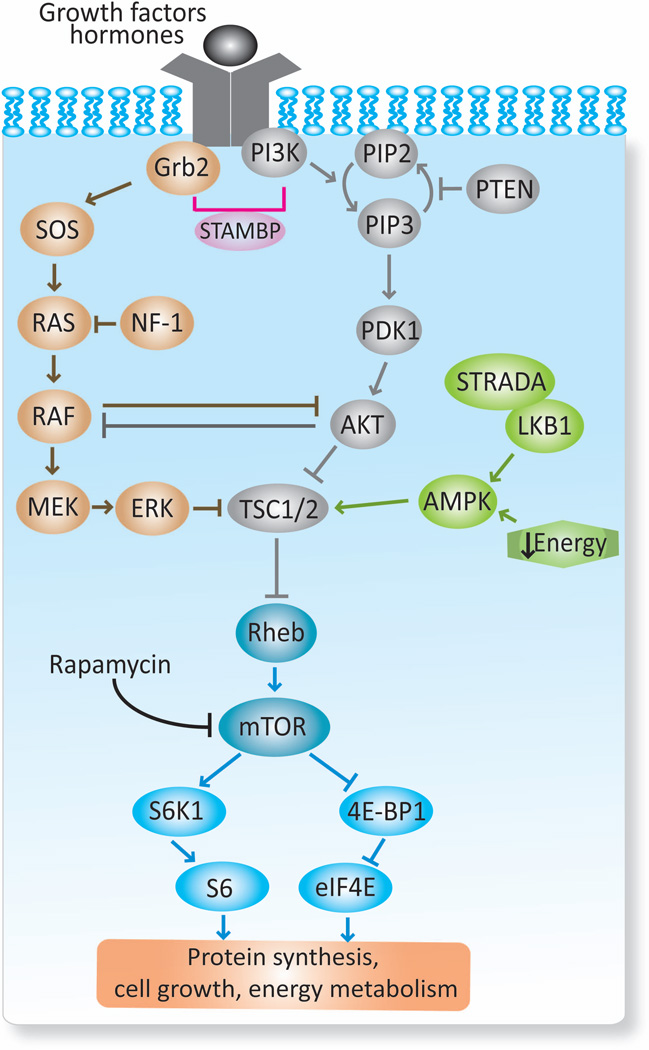
The mTOR pathway is a cellular signaling cascade implicated in the pathogenesis of numerous cortical malformations. mTOR is a conserved serine/threonine kinase protein that serves as central regulator of multiple functions during development and maturational stages, such as cell growth and proliferation, energy metabolism, inflammation and prevention of autophagy.34 mTOR constitutes a focal node integrating multiple extracellular and intracellular inputs, including nutrients, growth factors, cytokines, hormones, cellular stressors and oxygen sensors (Figure 2). mTOR forms two distinct protein complexes, mTORC1 and mTORC2. mTORC1 is rapamycin sensitive and constitutes the integrator of several inputs through the phosphoinositide 3-kinase (PI3K)-protein kinase B(AKT) pathway.25 Within other functions, mTORC1 promotes protein synthesis through the activation of several downstream signaling cascades, such as the ribosomal protein S6 (S6)-ribosomal protein S6 kinase beta-1 (S6K1) pathway and the eukaryotic initiation factor 4E binding protein-1 (4E-BP1)-eukaryotic translation initiation factor 4E (eIF4E) pathway. On the other hand, mTORC2 is relatively insensitive to rapamycin and moderates different kinases as well as regulators of cytoskeletal organization.25 mTORC1 and mTORC2 are inhibited upstream by regulatory genes, including the tumor suppressor genes Tuberous Sclerosis Complex 1 (TSC1) and TSC2, phosphatase and tensin homolog (PTEN) gene, neurofibromin 1 (NF1) gene and other gene regulators such as STE20-related kinase adaptor alpha (STRADA) (Figure 2 and 3).28; 30; 38; 54
FIGURE 2. Schematic representation of the mTOR pathway.
The mammalian target of rapamycin (mTOR) complex, particularly mTORC1, integrates multiple signals derived from growth factors, hormones and energy status. A first level of integration occurs at TSC1/TSC2 complex where several pathways converge, including RAS-MAKP, PI3K-AKT-mTOR and AMPK. Based on its degree of activation, the TSC1/TSC2 negatively modulates mTOR through the Ras homolog enriched in brain (Rheb). mTOR, in turn, regulates downstream substrates (S6K1/S6, 4E-BP1/eIF4E) that subsequently control protein synthesis, cell growth, and energy metabolism. Other important regulatory proteins of this pathway (PTEN, NF-1 and STRADA) and their sites of action are depicted.28; 30; 34; 38; 43; 54 Grb2: growth factor receptor-bound protein 2; SOS: Son of sevenless; RAS: Rat sarcoma; RAF: Rapidly Accelerated Fibrosarcoma; MEK: MAPK/ERK kinase; ERK: extracellular signal regulated kinase; PIK3: phosphoinositide 3-kinase; PIP2: phosphatidylinositol-4,5-biphosphate; PIP3: phosphatidylinositol-3,4,5-triphosphate; PDK1: phosphoinositide-3-dependent kinase 1; AKT: protein kinase B; AMPK: AMP activated protein kinase; LKB1: liver kinase B1; STAMBP: STAM-binding protein; NF-1: neurofibromin 1; PTEN: phosphatase and tensin homolog; STRADA: STE20-related kinase adapter protein alpha; TSC1/2: tuberous sclerosis complex 1 and 2; S6K1: ribosomal protein S6 kinase beta-1; S6: ribosomal protein S6; 4E-BP1: 4E binding protein-1; eIF4E: eukaryotic translation initiation factor 4E (abbreviations also apply to Figure 3).
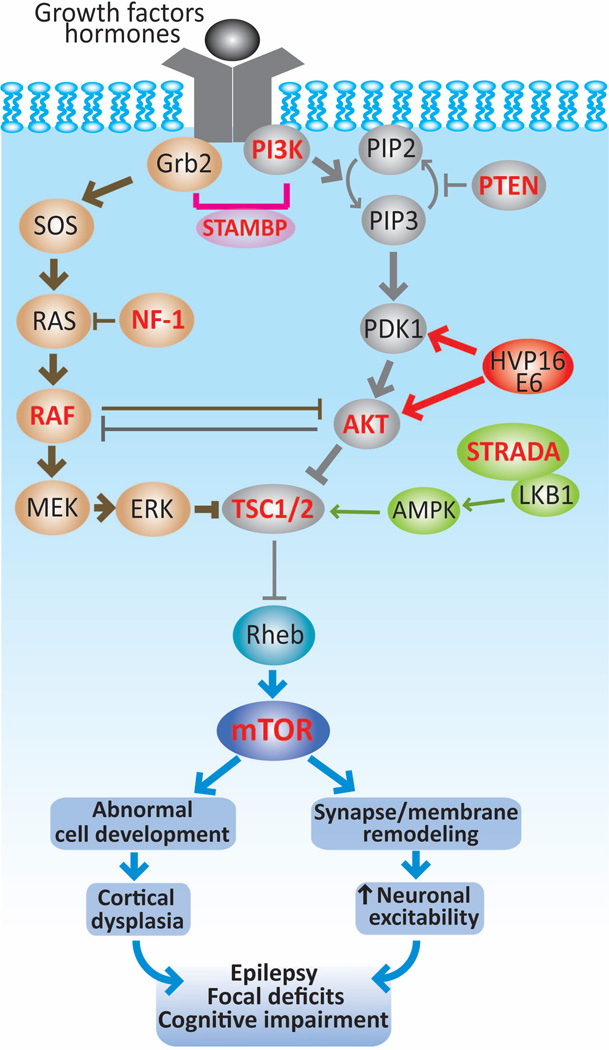
FIGURE 3. Role of mTOR pathway in cortical dysplasias and epileptogenesis.
The mTOR pathway can become hyperactive (thick lines) by mutations of genes encoding upstream regulators (TSC1, TSC2, STRADA, PTEN or NF-1), components of the pathways that converge on TCS1/2, such as RAF, AKT, PI3K and also of mTOR (highlighted in red). The recent discovery of HPV16 E6 in FCD type IIa and IIb along with other DNA viruses suggest that an infectious etiology can contribute to the pathogenesis of this condition. Together, activated mTOR pathway results in abnormally increased cell growth and proliferation that could account for the anatomical lesions encountered in cortical dysplasias. In addition, enhanced mTOR signaling can modify the expression of neurotransmitter receptors and ion channels, which can change membrane properties and synaptic organization leading to neuronal hyperexcitability. Both morphological and functional alterations at the cellular and circuit levels may lead to epilepsy, focal deficits and cognitive dysfunction in patients with cortical dysplasias.15; 30; 31; 35; 38; 43; 54; 59; 67
After the discovery of the inhibitory role of proteins TSC1 and TSC2 on mTOR, the structural disturbances encountered in TSC patients were attributed to the activated mTOR cascade.65 The histological resemblance between tubers and FCD type IIb led to pursue this pathway as a potential underlying cause of FCDs. In fact, an increased mTOR signaling was first identified in FCD type IIb, and then in HME and gangliogliomas based on the enhanced phosphorylated downstream molecules S6K1 and S6.5; 7; 58 This is particularly pertinent in FCD type IIb and tubers, in which more than 80% of balloon cells and giant cells, respectively, manifest increased phosphorylated S6K1 and S6.7; 49 In contrast, enhanced mTOR signaling has not been reported in FCD type I. Some cases of FCD type IIb exhibit also activation of the upstream molecules phosphoinositide-3-dependent kinase 1 (PDK1) and AKT, and of the downstream substrates vascular endothelial growth factor (VEGF) and signal transducer and activator of transcription 3 (STAT3).9; 41 The role of mTOR signaling in the pathophysiology of FCD was further corroborated in the PTEN knockout mouse model. These animals manifest similar histological features to FCD, many of which can be rescued with rapamycin.80
Recently, the human papilloma virus (HPV) 16 oncoprotein E6 has been identified in a group of patients with FCD type IIb15, suggesting the intriguing possibility of a viral source for FCD. In resected tissues, the expression of E6 colocalized with the phosphoactivated S6 protein, mainly in balloon cells, indicating that HPV16 may alter cortical development through mTOR (Figure 3). These findings were further supported in mice transfected with HPV16 at fetal stages, which showed focal cortical malformations with increased expression of phosphorylated S6.
Two mechanisms have linked HPV16 E6 oncoprotein to mTORC1 activation: 1) binding to TSC2 and promoting its ubiquitin-mediated degradation39, 2) activation of PDK1 and AKT, which subsequently inhibits TSC1/TSC2.61 It has been shown that PI3K-AKT-mTOR pathway favors the replication of cytomegalovirus (CMV), herpes simplex virus (HSV-1) and other double-stranded DNA viruses by promoting protein translation, which may contribute to spread the infection to other cells.11 A recent study supported this model by detecting HPV16 in resected specimens from 18 of 20 patients with FCD type IIb and in 6 of 27 individuals with FCD type IIa.36 Other pathogens such as CMV, HSV-1 and human herpes virus 6B (HHV-6B) were also identified in balloon cells and dysplastic neurons in some specimens. More studies are warranted to confirm the mechanistic role of viral infections on FCD, to elucidate the mode of transmission, timing of infection and spreading mechanisms, and to evaluate their epidemiological impact on cortical malformations.
Based on the wide range of mTOR functions, it is not surprising that some developmental brain tumors are associated with cortical malformations, epilepsy and activation of mTOR pathway. This is the case of gangliogliomas, the most common pediatric brain tumor associated with intractable epilepsy.60 This tumor can be associated with an adjacent or directly connected FCD, and based on its epileptogenic tendency it is classified as FCD type IIIb.8 Histologically, gangliogliomas are mixed neoplasms constituted by a heterogeneous population of dysplastic neurons and neoplastic glial cells.40 Atypical ganglion cells can also be observed, having a similar morphology and protein expression to balloon cells.58
The role of the mTOR pathway in the pathogenesis of gangliogliomas was first established by the demonstration of enhanced phosphorylated S6K1 and S6 in neurons and atypical ganglion cells.58 These findings were later supported by the increased labeling of phosphorylated upstream substrates (PDK1, AKT), mTOR, and downstream molecules (4E-BP1, eIF4G, S6K1 and S6).10 As occurs in melanoma, the potential mechanism for enhanced mTOR signaling in gangliogliomas may reside on the dual implication of RAF-MEK (Rapidly Accelerated Fibrosarcoma-MAPK-ERK kinase) and PI3K-AKT-mTOR pathways due to V600E mutation in v-raf murine sarcoma viral oncogene homolog B (BRAF) (Figure 3).14 It has been recently reported that approximately 20 to 60% of gangliogliomas carry the V600E mutation in BRAF, particularly in neurons and atypical ganglion cells.31; 59 This aspect has therapeutic implications since the inhibition of both signaling cascades in other tumor types showed a significant decrease of tumor cell survival.16
Additional regulators or components of the mTOR pathway play an important role in the pathogenesis of other malformations of cortical development. For example, de novo somatic mutations of PI3K, AKT3 or MTOR have been identified in resected specimens of patients with HME and megalencephaly-polymicrogyria-polydactyly-hydrocephalus (MPPH) syndrome (Figure 3).35; 57 Mutations of STRADA have been reported in patients with polyhydramnios, megalencephaly, and symptomatic epilepsy (PMSE) syndrome.54 On the other hand, a dual dysfunction of RAS-MAPK (Rat sarcoma-Mitogen protein kinase) and PI3K-AKT-mTOR seems to play a role in cortical dysplasia. Both pathways are interrelated at different levels, for example through the interaction of STAM-binding protein (STAMBP) with growth factor receptor-bound protein 2 (Grb2) (Figure 2).67 Mutations of STAMBP have been associated with enhanced RAS-MAPK and PI3K-AKT-mTOR pathways in patients with microcephaly-capillary malformation syndrome (MIC-CAP) (Figure 3)43, underscoring the importance of both signaling pathways in cell growth, proliferation, and angiogenesis.
Epileptogenesis in focal cortical dysplasias
Seizures represent one of the most common and challenging clinical manifestations of patients with FCD. In the last decade, encouraging advances have been made on the understanding of the electrophysiological properties of nerve cells within and surrounding the dysplastic cortex, and the potential link between aberrant molecular pathways and neuronal hyperexcitability. An enhanced mTOR signaling has been found in several animal models of acquired epilepsy, such as temporal lobe epilepsy78 and infantile spasms.56 The fact that their epileptic phenotype was rescued with rapamycin, highlighted the potential implication of mTOR on epileptogenesis.
A still controversial question is to what extent seizures originate within the dysplastic cortex, in the surrounding brain or in both regions. This is a relevant matter as a significant proportion of patients remain refractory after surgical resection of the abnormal tissue. Electrophysiological recordings on freshly resected dysplastic specimens from pediatric patients demonstrated that not all abnormal nerve cells manifest aberrant electrophysiological features.13 In these tissues, large pyramidal neurons showed signs of hyperexcitability based on their ample Ca2+ currents and influx upon depolarization. This contrasted with dysmorphic and immature pyramidal neurons that did not display considerable electrical abnormalities. Balloon cells were not excitable per se, but it has been hypothesized that the presence of these electrically “silent” cells could promote synaptic reorganization in the surrounding cortex resulting in net excitability of the tissue.13
A proposed underlying mechanism for neuronal hyperexcitability in FCD is the enhanced expression of glutamatergic receptors in dysplastic neurons, for example the NMDA receptor subunits NR1-1a, 1b, 2a, 2b, NR2A/B, NR2B, and the AMPA receptor subunits GluR1 and GluR2/3.45; 48; 76; 77 However, there is variability across studies in terms of the degree of expression of glutamate receptors, probably as a consequence of the variable histological nomenclature or the different experimental conditions.3 Studies on the ultrastructure of dysplastic cortices revealed significant differences in the synaptic density and proportion of excitatory and inhibitory synapses relative to the adjacent cortex, which might result in critical changes of neuronal circuits within and surrouding the dysplastic tissue that may favor epileptogenesis.2 Based on intracranial EEG recordings on FCD patients, the epileptogenic zone may extend beyond the imaging-identified lesion, and it may dysplay complex propagation patterns to neighboring or distant regions.20 Certain electrographic patterns are more characteristic of FCD than other conditions, specifically of the ictal zone. For example, ictal and interictal paroxysmal, commonly low-voltage, fast activity are typically seen in intracranial recordings of FCD, and the presence of paroxysmal fast and slow repetitive spikes are, in turn, predictive of the ictal zone.53; 73 Other patterns associated with seizure-onset in these patients include spike and wave activity, burst of polyspikes and delta brush.53
An additional mechanism that may explain drug-resistant epilepsy in FCD is the overexpression of multidrug resistance gene-1 p-glycoprotein (MDR1) and multidrug resistance-associated protein-1 (MRP1) in neurons, astrocytes, dysplastic neurons and balloon cells within the dysplastic cortex.1 Positing that multidrug resistant proteins (MDRP) can expunge antiepileptic drugs from the nervous system, reducing the effective concentration in the brain. However, it is still unclear whether the overexpression of these transporters in the epileptogenic tissue is primary or secondary to chronic seizure or antiepileptic use, and the functional relevance to treatment strategies.69
Epilepsy in FCD: lessons from Tuberous Sclerosis Complex
TSC is an autosomal dominant disorder resulting from mutations of the mTOR regulatory genes TSC1 or TSC2. This condition is typically associated with formation of cortical tubers, which resemble histologically to some FCDs and are invariably linked to intractable epilepsy. Based on this and the clearly established role of mTOR signaling in its pathogenesis, TSC constitutes a valuable model to study epileptogenesis in FCD. In this sense, mutations of TSC2 favor glutamate-mediated neuronal hyperexcitability as demonstrated in a TSC patient whose resected dysplastic brain exhibited increased excitation with preserved inhibition.72 These results were further corroborated in a conditional knockout mouse for TSC1, in which whole-cell patch clamp recordings revealed enhanced AMPA-mediated excitatory synaptic currents.72 On the other hand, the proportion of abnormal neuronal and glial cells may play a role in the epileptogenesis of tubers owing to their different expression of glutamate receptors.62 Thus, tubers with a high proportion of dysplastic neurons expressing abnormal AMPA and NMDA receptor subunits are more likely to be epileptogenic than those mainly composed of giant cells or dysplastic astroglia.
The dysfunction of glutamate homeostasis has also been associated with the mechanism of seizures in TSC. Mice with astrocyte-specific TSC1 inactivation manifest progressive epilepsy68, which could result from the reduced astrocytic expression of glutamate transporters (GLT-1 and GLAST), and the impaired extracellular potassium uptake by astrocytes through inward rectifier potassium (Kir) channels.29; 75 This correlates with the high epileptogenic tendency found in knockout mouse for GLT-163, supporting the conception that an impaired synaptic glutamate clearance in astrocytes could potentiate excitatory neurotransmission and thus epileptogenesis in TSC.
TARGETED TREATMENTS IN FCD
Currently, the available therapeutic options in FCD remain primarily symptomatic. Since seizures are frequently refractory to pharmacological agents, epilepsy surgery is often required. Surgical resection of the dysplastic lesion leads to seizure-freedom in approximately 50–80% of patients, depending on the type of FCD, age and type of resection.50 Yet, a significant proportion of patients continue having seizures after surgery, and a small percentage will manifest surgical complications. Hence, the development of novel, molecular-based treatments is in need.
There are significant ongoing efforts to target the mTOR pathway due to its involvement in multiple human diseases other than FCD. The most widely used pharmacological agents to inhibit mTOR are the rapalogs, which include rapamycin and its analogs temsirolimus and everolimus. They act specifically on mTORC1 reducing the phosphorylation of downstream effectors (Figure 2), which prevents abnormal cell growth, axonal sprouting and neuronal intrinsic hyperexcitability associated with enhanced mTOR signaling.26 In this regard, it has been shown that chronic administration of rapamycin can reduce cortical excitability by decreasing the expression of AMPA receptors after blockade of synaptic activity and by increasing GABAA receptor–mediated neurotransmission.23; 71 In addition, rapamycin also reduces neuronal intrinsic excitability by prolonging the opening time of Ca2+-dependent K+ channels and increasing the expression of the voltage-gated potassium channel Kv1.1 in cortical and hippocampal neurons.55; 66
The potential antiepileptic effects of rapamycin have been evaluated in animal models of cortical dysplasia. In mice with inactivated TSC1, rapamycin prevented epilepsy and premature death when administered at early age, and ameliorated seizure frequency and prolonged survival when given at later stages.79 Moreover, rapamycin recued the synaptic plasticity and behavioral deficits of mice with inactivating mutation of TSC2.21 In PTEN knock-out mice, rapamycin significantly suppressed the severity and duration of seizures, prevented neuronal hypertrophy and prolonged the survival rate of these animals.37 Rapamycin has been successfully used in other mTOR-associated conditions. For example, it was able to prevent the migrational defects of cell lines and mouse brain depleted of STRADA.52 An optimal response, however, is not the norm in other animal models of epilepsy. For example, rapamycin did not ameliorate the frequency or severity of epileptic events in animals with pilocarpine-induced seizures.12 This indicates that the activation of the mTOR pathway may not be the core mechanism of all forms of epilepsy, and therefore rapamycin may not provide universal antiepileptic effects.
As a corollary, mTOR inhibitors have recently been introduced in patients with cortical dysplasias. For example, the administration of rapamycin decreased drastically the duration and frequency of seizures in a child with TSC47, and reduced the size of subependymal giant astrocytomas (SEGA) in patients with TSC in an open label study.33 On the other hand, patients with PMSE syndrome manifested a significant amelioration of seizure frequency and an improvement of receptive language when treated with sirolimus (rapamycin).52 However, larger trials are necessary to assess the efficacy and side effects of rapamycin, particularly in patients with FCD.
Additional treatments for cortical dysplasias are still under investigation. For instance, multidrug resistance gene-1 p-glycoprotein (MDR1) inhibitors (i.e., verapamil) have been used in patients with refractory epilepsy providing some amelioration in seizure frequency and severity.27 Personal observations have been reported regarding the benefit of verapamil on seizure control in selected patients with FCD.22 On the other hand, the combination of rapamycin with a RAF-MEK inhibitor may be considered as a potential treatment for ganglioglioma, since targeting both pathways in colon cancer decreased notably the survival rate of tumor cells.16 The recent association of viral infections with FCD constitutes a potential avenue for the development of alternative therapeutic approaches. Overall, the ongoing progress on the molecular basis of FCD will support the design of personalized treatments by targeting specific pathways, ion channels, neurotransmitter receptors or transporters involved in each particular condition, minimizing, in turn, the collateral adverse effects.
FUTURE DIRECTIONS
Focal cortical dysplasia remains a veritable challenge for the clinician due its likely causal heterogeneity, impact on patients’ neurological status and the lack of refined treatments. The identification of the mTOR signaling as an underlying mechanism of FCD and other cortical malformations comprises a unique opportunity for the development of more effective treatments. Despite of the promising results of mTOR inhibitors on seizure management in animal models and patients, large, randomized controlled trials are necessary to evaluate their efficacy and long-term side effects, specifically in patients with FCD. Moreover, the recent detection of indicators of viral pathogens in FCD specimens opens a potential avenue in the understanding of these conditions and favors the design of preventive, diagnostic and therapeutic measures. For this purpose, upcoming efforts will need to verify the pathogenic role of viral agents on cortical dysplasia, and to answer relevant inquiries regarding the route of infection, prevalent timing during development and spreading mechanisms. On the other hand, the heterogeneous etiology of FCD type I and III portends that several mechanisms may underlay the pathogenesis of these conditions. The foundations of their common anatomical and clinical manifestations, however, remain elusive and constitute a major dare to solve in the near future.
Supplementary Material
ACKNOWLEDGEMENTS
The authors acknowledge the helpful advice from and discussions with Drs. Juan M Pascual, Tracy Dixon-Salazar and Gary Mathern. This work is supported by NIH grant P01HD070494, R01NS083823 and the Howard Hughes Medical Institute.
Footnotes
CONFLICT OF INTEREST
None to declare.
AUTHORS DECLARATION
We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
REFERENCES
- 1.Ak H, Ay B, Tanriverdi T, et al. Expression and cellular distribution of multidrug resistance-related proteins in patients with focal cortical dysplasia. Seizure. 2007;16:493–503. doi: 10.1016/j.seizure.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 2.Alonso-Nanclares L, Garbelli R, Sola RG, et al. Microanatomy of the dysplastic neocortex from epileptic patients. Brain. 2005;128:158–173. doi: 10.1093/brain/awh331. [DOI] [PubMed] [Google Scholar]
- 3.Andre VM, Flores-Hernandez J, Cepeda C, et al. NMDA receptor alterations in neurons from pediatric cortical dysplasia tissue. Cereb Cortex. 2004;14:634–646. doi: 10.1093/cercor/bhh024. [DOI] [PubMed] [Google Scholar]
- 4.Arai Y, Edwards V, Becker LE. A comparison of cell phenotypes in hemimegalencephaly and tuberous sclerosis. Acta Neuropathol. 1999;98:407–413. doi: 10.1007/s004010051101. [DOI] [PubMed] [Google Scholar]
- 5.Aronica E, Boer K, Baybis M, et al. Co-expression of cyclin D1 and phosphorylated ribosomal S6 proteins in hemimegalencephaly. Acta Neuropathol. 2007;114:287–293. doi: 10.1007/s00401-007-0225-6. [DOI] [PubMed] [Google Scholar]
- 6.Barkovich AJ, Guerrini R, Kuzniecky RI, et al. A developmental and genetic classification for malformations of cortical development: update 2012. Brain. 2012;135:1348–1369. doi: 10.1093/brain/aws019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baybis M, Yu J, Lee A, et al. mTOR cascade activation distinguishes tubers from focal cortical dysplasia. Ann Neurol. 2004;56:478–487. doi: 10.1002/ana.20211. [DOI] [PubMed] [Google Scholar]
- 8.Blumcke I, Thom M, Aronica E, et al. The clinicopathologic spectrum of focal cortical dysplasias: a consensus classification proposed by an ad hoc Task Force of the ILAE Diagnostic Methods Commission. Epilepsia. 2011;52:158–174. doi: 10.1111/j.1528-1167.2010.02777.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boer K, Troost D, Spliet WG, et al. Cellular distribution of vascular endothelial growth factor A (VEGFA) and B (VEGFB) and VEGF receptors 1 and 2 in focal cortical dysplasia type IIB. Acta Neuropathol. 2008;115:683–696. doi: 10.1007/s00401-008-0354-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boer K, Troost D, Timmermans W, et al. Pi3K-mTOR signaling and AMOG expression in epilepsy-associated glioneuronal tumors. Brain Pathol. 2010;20:234–244. doi: 10.1111/j.1750-3639.2009.00268.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Buchkovich NJ, Yu Y, Zampieri CA, et al. The TORrid affairs of viruses: effects of mammalian DNA viruses on the PI3K-Akt-mTOR signalling pathway. Nat Rev Microbiol. 2008;6:266–275. doi: 10.1038/nrmicro1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Buckmaster PS, Lew FH. Rapamycin suppresses mossy fiber sprouting but not seizure frequency in a mouse model of temporal lobe epilepsy. J Neurosci. 2011;31:2337–2347. doi: 10.1523/JNEUROSCI.4852-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cepeda C, Hurst RS, Flores-Hernandez J, et al. Morphological and electrophysiological characterization of abnormal cell types in pediatric cortical dysplasia. J Neurosci Res. 2003;72:472–486. doi: 10.1002/jnr.10604. [DOI] [PubMed] [Google Scholar]
- 14.Chen B, Tardell C, Higgins B, et al. BRAFV600E negatively regulates the AKT pathway in melanoma cell lines. PLoS One. 2012;7:e42598. doi: 10.1371/journal.pone.0042598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen J, Tsai V, Parker WE, et al. Detection of human papillomavirus in human focal cortical dysplasia type IIB. Ann Neurol. 2012;72:881–892. doi: 10.1002/ana.23795. [DOI] [PubMed] [Google Scholar]
- 16.Coffee EM, Faber AC, Roper J, et al. Concomitant BRAF and PI3K/mTOR blockade is required for effective treatment of BRAF(V600E) colorectal cancer. Clin Cancer Res. 2013;19:2688–2698. doi: 10.1158/1078-0432.CCR-12-2556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Colombo N, Tassi L, Deleo F, et al. Focal cortical dysplasia type IIa and IIb: MRI aspects in 118 cases proven by histopathology. Neuroradiology. 2012;54:1065–1077. doi: 10.1007/s00234-012-1049-1. [DOI] [PubMed] [Google Scholar]
- 18.Colombo N, Tassi L, Galli C, et al. Focal cortical dysplasias: MR imaging, histopathologic, and clinical correlations in surgically treated patients with epilepsy. AJNR Am J Neuroradiol. 2003;24:724–733. [PMC free article] [PubMed] [Google Scholar]
- 19.Crino PB, Trojanowski JQ, Eberwine J. Internexin, MAP1B, and nestin in cortical dysplasia as markers of developmental maturity. Acta Neuropathol. 1997;93:619–627. doi: 10.1007/s004010050660. [DOI] [PubMed] [Google Scholar]
- 20.Duchowny M, Jayakar P, Levin B. Aberrant neural circuits in malformations of cortical development and focal epilepsy. Neurology. 2000;55:423–428. doi: 10.1212/wnl.55.3.423. [DOI] [PubMed] [Google Scholar]
- 21.Ehninger D, Han S, Shilyansky C, et al. Reversal of learning deficits in a Tsc2+/− mouse model of tuberous sclerosis. Nat Med. 2008;14:843–848. doi: 10.1038/nm1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gaitanis JN, Donahue J. Focal cortical dysplasia. Pediatr Neurol. 2013;49:79–87. doi: 10.1016/j.pediatrneurol.2012.12.024. [DOI] [PubMed] [Google Scholar]
- 23.Galanopoulou AS, Gorter JA, Cepeda C. Finding a better drug for epilepsy: the mTOR pathway as an antiepileptogenic target. Epilepsia. 2012;53:1119–1130. doi: 10.1111/j.1528-1167.2012.03506.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harvey AS, Cross JH, Shinnar S, et al. Defining the spectrum of international practice in pediatric epilepsy surgery patients. Epilepsia. 2008;49:146–155. doi: 10.1111/j.1528-1167.2007.01421.x. [DOI] [PubMed] [Google Scholar]
- 25.Hsu PP, Kang SA, Rameseder J, et al. The mTOR-regulated phosphoproteome reveals a mechanism of mTORC1-mediated inhibition of growth factor signaling. Science. 2011;332:1317–1322. doi: 10.1126/science.1199498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang X, Zhang H, Yang J, et al. Pharmacological inhibition of the mammalian target of rapamycin pathway suppresses acquired epilepsy. Neurobiol Dis. 2010;40:193–199. doi: 10.1016/j.nbd.2010.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Iannetti P, Parisi P, Spalice A, et al. Addition of verapamil in the treatment of severe myoclonic epilepsy in infancy. Epilepsy Res. 2009;85:89–95. doi: 10.1016/j.eplepsyres.2009.02.014. [DOI] [PubMed] [Google Scholar]
- 28.Inoki K, Li Y, Zhu T, et al. TSC2 is phosphorylated and inhibited by Akt and suppresses mTOR signalling. Nat Cell Biol. 2002;4:648–657. doi: 10.1038/ncb839. [DOI] [PubMed] [Google Scholar]
- 29.Jansen LA, Uhlmann EJ, Crino PB, et al. Epileptogenesis and reduced inward rectifier potassium current in tuberous sclerosis complex-1-deficient astrocytes. Epilepsia. 2005;46:1871–1880. doi: 10.1111/j.1528-1167.2005.00289.x. [DOI] [PubMed] [Google Scholar]
- 30.Johannessen CM, Reczek EE, James MF, et al. The NF1 tumor suppressor critically regulates TSC2 and mTOR. Proc Natl Acad Sci U S A. 2005;102:8573–8578. doi: 10.1073/pnas.0503224102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Koelsche C, Sahm F, Paulus W, et al. BRAF V600E expression and distribution in desmoplastic infantile astrocytoma/ganglioglioma. Neuropathol Appl Neurobiol. 2013 doi: 10.1111/nan.12072. [DOI] [PubMed] [Google Scholar]
- 32.Krsek P, Jahodova A, Maton B, et al. Low-grade focal cortical dysplasia is associated with prenatal and perinatal brain injury. Epilepsia. 2010;51:2440–2448. doi: 10.1111/j.1528-1167.2010.02730.x. [DOI] [PubMed] [Google Scholar]
- 33.Krueger DA, Care MM, Holland K, et al. Everolimus for subependymal giant-cell astrocytomas in tuberous sclerosis. N Engl J Med. 2010;363:1801–1811. doi: 10.1056/NEJMoa1001671. [DOI] [PubMed] [Google Scholar]
- 34.Laplante M, Sabatini DM. mTOR signaling in growth control and disease. Cell. 2012;149:274–293. doi: 10.1016/j.cell.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee JH, Huynh M, Silhavy JL, et al. De novo somatic mutations in components of the PI3K-AKT3-mTOR pathway cause hemimegalencephaly. Nat Genet. 2012;44:941–945. doi: 10.1038/ng.2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu S, Lu L, Cheng X, et al. Viral infection and focal cortical dysplasia. Ann Neurol. 2013 doi: 10.1002/ana.24037. [DOI] [PubMed] [Google Scholar]
- 37.Ljungberg MC, Sunnen CN, Lugo JN, et al. Rapamycin suppresses seizures and neuronal hypertrophy in a mouse model of cortical dysplasia. Dis Model Mech. 2009;2:389–398. doi: 10.1242/dmm.002386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lu Y, Lin YZ, LaPushin R, et al. The PTEN/MMAC1/TEP tumor suppressor gene decreases cell growth and induces apoptosis and anoikis in breast cancer cells. Oncogene. 1999;18:7034–7045. doi: 10.1038/sj.onc.1203183. [DOI] [PubMed] [Google Scholar]
- 39.Lu Z, Hu X, Li Y, et al. Human papillomavirus 16 E6 oncoprotein interferences with insulin signaling pathway by binding to tuberin. J Biol Chem. 2004;279:35664–35670. doi: 10.1074/jbc.M403385200. [DOI] [PubMed] [Google Scholar]
- 40.Luyken C, Blumcke I, Fimmers R, et al. Supratentorial gangliogliomas: histopathologic grading and tumor recurrence in 184 patients with a median follow-up of 8 years. Cancer. 2004;101:146–155. doi: 10.1002/cncr.20332. [DOI] [PubMed] [Google Scholar]
- 41.Ma J, Meng Y, Kwiatkowski DJ, et al. Mammalian target of rapamycin regulates murine and human cell differentiation through STAT3/p63/Jagged/Notch cascade. J Clin Invest. 2010;120:103–114. doi: 10.1172/JCI37964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Marin-Padilla M, Parisi JE, Armstrong DL, et al. Shaken infant syndrome: developmental neuropathology, progressive cortical dysplasia, and epilepsy. Acta Neuropathol. 2002;103:321–332. doi: 10.1007/s00401-001-0470-z. [DOI] [PubMed] [Google Scholar]
- 43.McDonell LM, Mirzaa GM, Alcantara D, et al. Mutations in STAMBP, encoding a deubiquitinating enzyme, cause microcephaly-capillary malformation syndrome. Nat Genet. 2013;45:556–562. doi: 10.1038/ng.2602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mischel PS, Nguyen LP, Vinters HV. Cerebral cortical dysplasia associated with pediatric epilepsy. Review of neuropathologic features and proposal for a grading system. J Neuropathol Exp Neurol. 1995;54:137–153. doi: 10.1097/00005072-199503000-00001. [DOI] [PubMed] [Google Scholar]
- 45.Moddel G, Jacobson B, Ying Z, et al. The NMDA receptor NR2B subunit contributes to epileptogenesis in human cortical dysplasia. Brain Res. 2005;1046:10–23. doi: 10.1016/j.brainres.2005.03.042. [DOI] [PubMed] [Google Scholar]
- 46.Muhlebner A, Coras R, Kobow K, et al. Neuropathologic measurements in focal cortical dysplasias: validation of the ILAE 2011 classification system and diagnostic implications for MRI. Acta Neuropathol. 2012;123:259–272. doi: 10.1007/s00401-011-0920-1. [DOI] [PubMed] [Google Scholar]
- 47.Muncy J, Butler IJ, Koenig MK. Rapamycin reduces seizure frequency in tuberous sclerosis complex. J Child Neurol. 2009;24:477. doi: 10.1177/0883073808324535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Najm IM, Ying Z, Babb T, et al. Epileptogenicity correlated with increased N-methyl-D-aspartate receptor subunit NR2A/B in human focal cortical dysplasia. Epilepsia. 2000;41:971–976. doi: 10.1111/j.1528-1157.2000.tb00281.x. [DOI] [PubMed] [Google Scholar]
- 49.Orlova KA, Tsai V, Baybis M, et al. Early progenitor cell marker expression distinguishes type II from type I focal cortical dysplasias. J Neuropathol Exp Neurol. 2010;69:850–863. doi: 10.1097/NEN.0b013e3181eac1f5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Otsuki T, Honda R, Takahashi A, et al. Surgical management of cortical dysplasia in infancy and early childhood. Brain Dev. 2013;35:802–809. doi: 10.1016/j.braindev.2013.04.008. [DOI] [PubMed] [Google Scholar]
- 51.Palmini A, Najm I, Avanzini G, et al. Terminology and classification of the cortical dysplasias. Neurology. 2004;62:S2–S8. doi: 10.1212/01.wnl.0000114507.30388.7e. [DOI] [PubMed] [Google Scholar]
- 52.Parker WE, Orlova KA, Parker WH, et al. Rapamycin prevents seizures after depletion of STRADA in a rare neurodevelopmental disorder. Sci Transl Med. 2013;5:182ra153. doi: 10.1126/scitranslmed.3005271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Perucca P, Dubeau F, Gotman J. Intracranial electroencephalographic seizure-onset patterns: effect of underlying pathology. Brain. 2014;137:183–196. doi: 10.1093/brain/awt299. [DOI] [PubMed] [Google Scholar]
- 54.Puffenberger EG, Strauss KA, Ramsey KE, et al. Polyhydramnios, megalencephaly and symptomatic epilepsy caused by a homozygous 7-kilobase deletion in LYK5. Brain. 2007;130:1929–1941. doi: 10.1093/brain/awm100. [DOI] [PubMed] [Google Scholar]
- 55.Raab-Graham KF, Haddick PC, Jan YN, et al. Activity- and mTOR-dependent suppression of Kv1.1 channel mRNA translation in dendrites. Science. 2006;314:144–148. doi: 10.1126/science.1131693. [DOI] [PubMed] [Google Scholar]
- 56.Raffo E, Coppola A, Ono T, et al. A pulse rapamycin therapy for infantile spasms and associated cognitive decline. Neurobiol Dis. 2011;43:322–329. doi: 10.1016/j.nbd.2011.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Riviere JB, Mirzaa GM, O'Roak BJ, et al. De novo germline and postzygotic mutations in AKT3, PIK3R2 and PIK3CA cause a spectrum of related megalencephaly syndromes. Nat Genet. 2012;44:934–940. doi: 10.1038/ng.2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Samadani U, Judkins AR, Akpalu A, et al. Differential cellular gene expression in ganglioglioma. Epilepsia. 2007;48:646–653. doi: 10.1111/j.1528-1167.2007.00925.x. [DOI] [PubMed] [Google Scholar]
- 59.Schindler G, Capper D, Meyer J, et al. Analysis of BRAF V600E mutation in 1,320 nervous system tumors reveals high mutation frequencies in pleomorphic xanthoastrocytoma, ganglioglioma and extra-cerebellar pilocytic astrocytoma. Acta Neuropathol. 2011;121:397–405. doi: 10.1007/s00401-011-0802-6. [DOI] [PubMed] [Google Scholar]
- 60.Schramm J, Kral T, Grunwald T, et al. Surgical treatment for neocortical temporal lobe epilepsy: clinical and surgical aspects and seizure outcome. J Neurosurg. 2001;94:33–42. doi: 10.3171/jns.2001.94.1.0033. [DOI] [PubMed] [Google Scholar]
- 61.Spangle JM, Munger K. The human papillomavirus type 16 E6 oncoprotein activates mTORC1 signaling and increases protein synthesis. J Virol. 2010;84:9398–9407. doi: 10.1128/JVI.00974-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Talos DM, Kwiatkowski DJ, Cordero K, et al. Cell-specific alterations of glutamate receptor expression in tuberous sclerosis complex cortical tubers. Ann Neurol. 2008;63:454–465. doi: 10.1002/ana.21342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tanaka K, Watase K, Manabe T, et al. Epilepsy and exacerbation of brain injury in mice lacking the glutamate transporter GLT-1. Science. 1997;276:1699–1702. doi: 10.1126/science.276.5319.1699. [DOI] [PubMed] [Google Scholar]
- 64.Taylor DC, Falconer MA, Bruton CJ, et al. Focal dysplasia of the cerebral cortex in epilepsy. J Neurol Neurosurg Psychiatry. 1971;34:369–387. doi: 10.1136/jnnp.34.4.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tee AR, Fingar DC, Manning BD, et al. Tuberous sclerosis complex-1 and -2 gene products function together to inhibit mammalian target of rapamycin (mTOR)-mediated downstream signaling. Proc Natl Acad Sci U S A. 2002;99:13571–13576. doi: 10.1073/pnas.202476899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Terashima A, Nakai M, Hashimoto T, et al. Single-channel activity of the Ca2+-dependent K+ channel is modulated by FK506 and rapamycin. Brain Res. 1998;786:255–258. doi: 10.1016/s0006-8993(97)01435-2. [DOI] [PubMed] [Google Scholar]
- 67.Tsang HT, Connell JW, Brown SE, et al. A systematic analysis of human CHMP protein interactions: additional MIT domain-containing proteins bind to multiple components of the human ESCRT III complex. Genomics. 2006;88:333–346. doi: 10.1016/j.ygeno.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 68.Uhlmann EJ, Wong M, Baldwin RL, et al. Astrocyte-specific TSC1 conditional knockout mice exhibit abnormal neuronal organization and seizures. Ann Neurol. 2002;52:285–296. doi: 10.1002/ana.10283. [DOI] [PubMed] [Google Scholar]
- 69.Volk HA, Loscher W. Multidrug resistance in epilepsy: rats with drug-resistant seizures exhibit enhanced brain expression of P-glycoprotein compared with rats with drug-responsive seizures. Brain. 2005;128:1358–1368. doi: 10.1093/brain/awh437. [DOI] [PubMed] [Google Scholar]
- 70.Wang DD, Deans AE, Barkovich AJ, et al. Transmantle sign in focal cortical dysplasia: a unique radiological entity with excellent prognosis for seizure control. J Neurosurg. 2013;118:337–344. doi: 10.3171/2012.10.JNS12119. [DOI] [PubMed] [Google Scholar]
- 71.Wang Y, Barbaro MF, Baraban SC. A role for the mTOR pathway in surface expression of AMPA receptors. Neurosci Lett. 2006;401:35–39. doi: 10.1016/j.neulet.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 72.Wang Y, Greenwood JS, Calcagnotto ME, et al. Neocortical hyperexcitability in a human case of tuberous sclerosis complex and mice lacking neuronal expression of TSC1. Ann Neurol. 2007;61:139–152. doi: 10.1002/ana.21058. [DOI] [PubMed] [Google Scholar]
- 73.Widdess-Walsh P, Jeha L, Nair D, et al. Subdural electrode analysis in focal cortical dysplasia: predictors of surgical outcome. Neurology. 2007;69:660–667. doi: 10.1212/01.wnl.0000267427.91987.21. [DOI] [PubMed] [Google Scholar]
- 74.Widdess-Walsh P, Kellinghaus C, Jeha L, et al. Electro-clinical and imaging characteristics of focal cortical dysplasia: correlation with pathological subtypes. Epilepsy Res. 2005;67:25–33. doi: 10.1016/j.eplepsyres.2005.07.013. [DOI] [PubMed] [Google Scholar]
- 75.Wong M, Ess KC, Uhlmann EJ, et al. Impaired glial glutamate transport in a mouse tuberous sclerosis epilepsy model. Ann Neurol. 2003;54:251–256. doi: 10.1002/ana.10648. [DOI] [PubMed] [Google Scholar]
- 76.Yamanouchi H. Activated remodeling and N-methyl-D-aspartate (NMDA) receptors in cortical dysplasia. J Child Neurol. 2005;20:303–307. doi: 10.1177/08830738050200040601. [DOI] [PubMed] [Google Scholar]
- 77.Ying Z, Bingaman W, Najm IM. Increased numbers of coassembled PSD-95 to NMDA-receptor subunits NR2B and NR1 in human epileptic cortical dysplasia. Epilepsia. 2004;45:314–321. doi: 10.1111/j.0013-9580.2004.37703.x. [DOI] [PubMed] [Google Scholar]
- 78.Zeng LH, Rensing NR, Wong M. The mammalian target of rapamycin signaling pathway mediates epileptogenesis in a model of temporal lobe epilepsy. J Neurosci. 2009;29:6964–6972. doi: 10.1523/JNEUROSCI.0066-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zeng LH, Xu L, Gutmann DH, et al. Rapamycin prevents epilepsy in a mouse model of tuberous sclerosis complex. Ann Neurol. 2008;63:444–453. doi: 10.1002/ana.21331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhou J, Blundell J, Ogawa S, et al. Pharmacological inhibition of mTORC1 suppresses anatomical, cellular, and behavioral abnormalities in neural-specific Pten knock-out mice. J Neurosci. 2009;29:1773–1783. doi: 10.1523/JNEUROSCI.5685-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.