Abstract
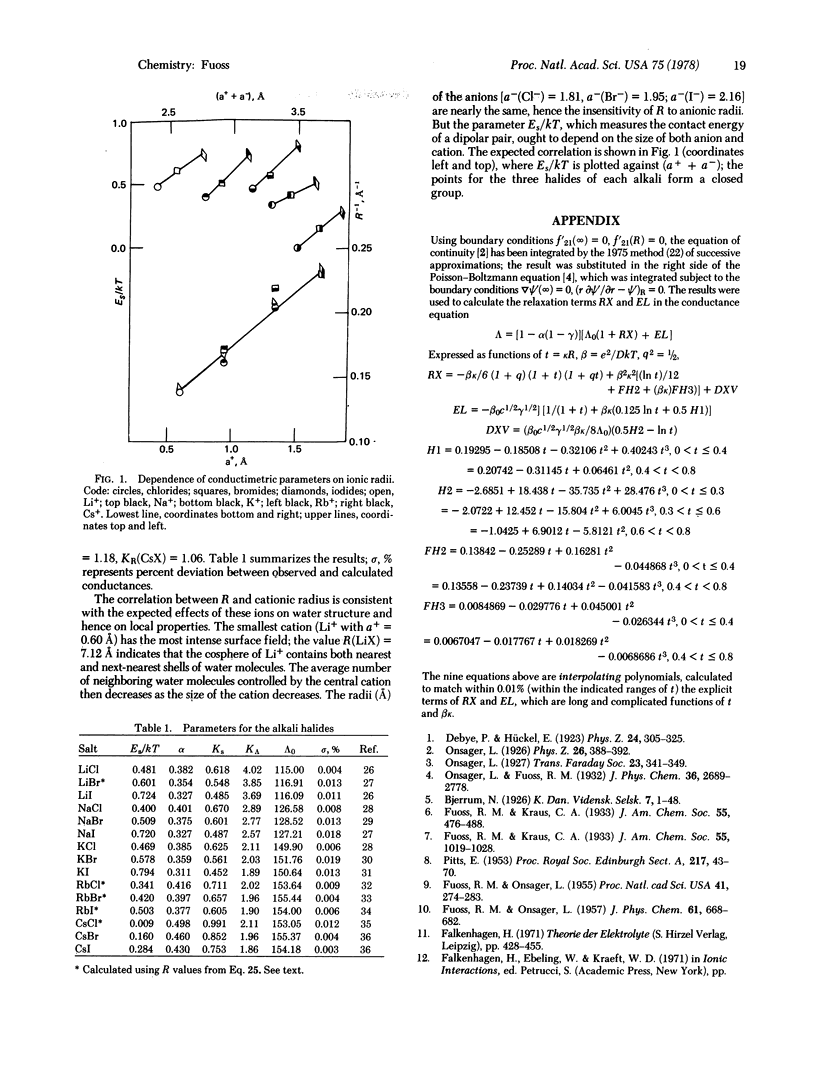
Previous models for which theories of electrolytic conductance have been developed are reviewed. Discrepancies between theoretically derived values of parameters and parameters characteristic of real physical systems suggested the following revised model. Ions are counted as diffusion pairs if their center-to-center distance r is in the range a ≤ r ≤ R, in which a is contact distance and R is the diameter of the Gurney cosphere. A fraction α of these pairs diffuse to contact to form nonconducting dipolar pairs; α/(1-α) = exp(-Es/kT), in which Es is the difference in energy between a diffusion pair at r = R and a contact pair, k is the Boltzmann constant, and T is the absolute temperature. This model permits separate treatment of long-range and short-range interionic effects. The former (relaxation field and electrophoresis) depend on R and the values of the dielectric constant and viscosity of the pure solvent. The latter (formation of dipolar pairs) is described by Es, or alternatively by Ks = exp(-Es/kT) in which Ks is the constant describing the steady state between solvent-separated diffusion pairs and dipolar (contact) pairs. For solutions of the alkali halides, a simple empirical correlation is found between R and the Pauling radii of the cations, and also between Es and the sum of the radii of cation and anion.
Keywords: ion pairs, association constants, electrolyte models, conductance theory
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Fuoss R. M., Hsia K. L. Association of 1-1 salts in water. Proc Natl Acad Sci U S A. 1967 Jun;57(6):1550–1557. doi: 10.1073/pnas.57.6.1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuoss R. M., Onsager L. CONDUCTANCE OF STRONG ELECTROLYTES AT FINITE DILUTIONS. Proc Natl Acad Sci U S A. 1955 May 15;41(5):274–283. doi: 10.1073/pnas.41.5.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuoss R. M. Parametric analysis of conductance data. Proc Natl Acad Sci U S A. 1974 Nov;71(11):4491–4495. doi: 10.1073/pnas.71.11.4491. [DOI] [PMC free article] [PubMed] [Google Scholar]


