Abstract
In previous papers of this series, emphasis has been placed on the steady-state phase transition and critical properties of large lattices of interacting, symmetrical, and identical enzyme molecules. The present paper is concerned with a number of examples of enzyme--enzyme interactions that do not belong to the class of models of the earlier papers. These are more biochemically oriented and include heterologous dimers, a linear chain with unsymmetrical interactions, and concerted isologous dimers (half-the-sites reactivity).
Full text
PDF
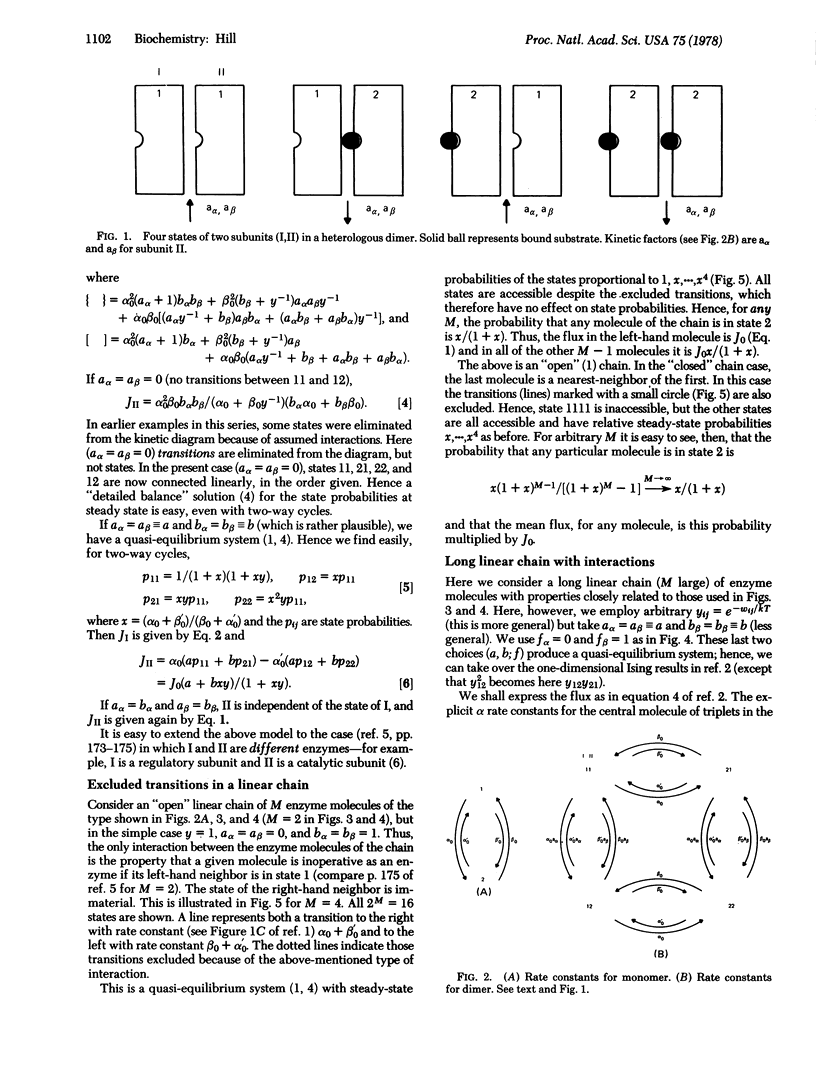
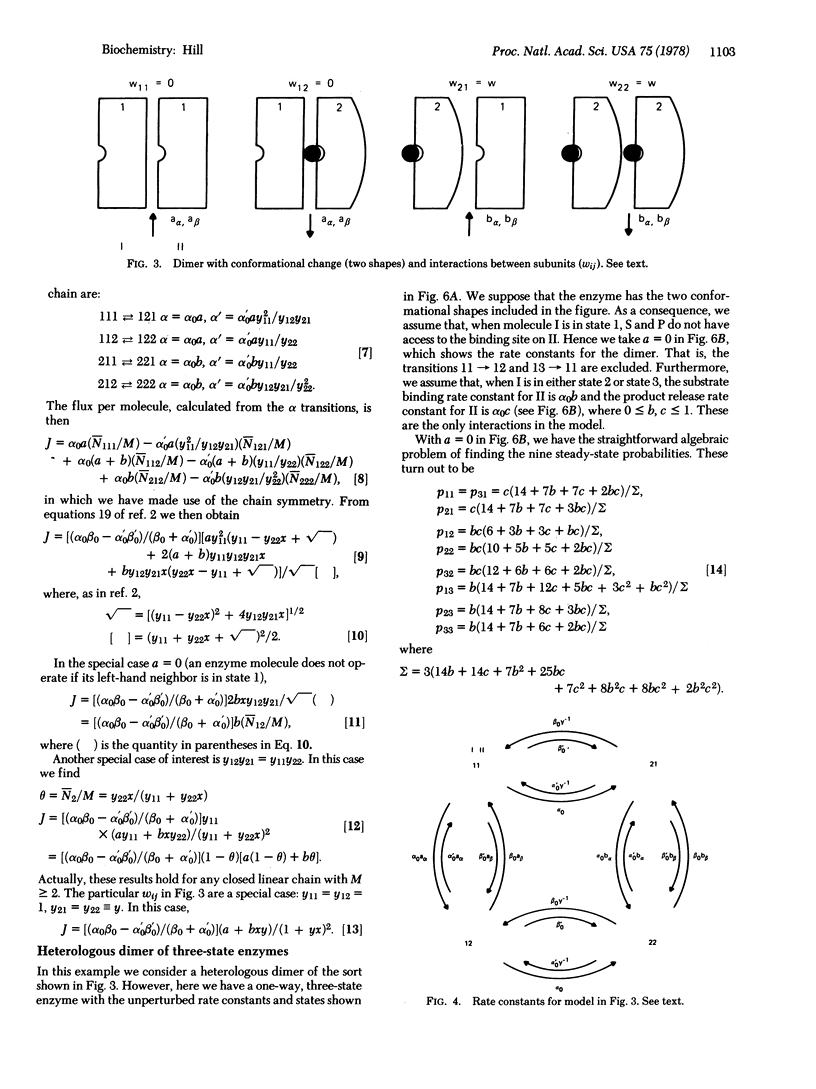
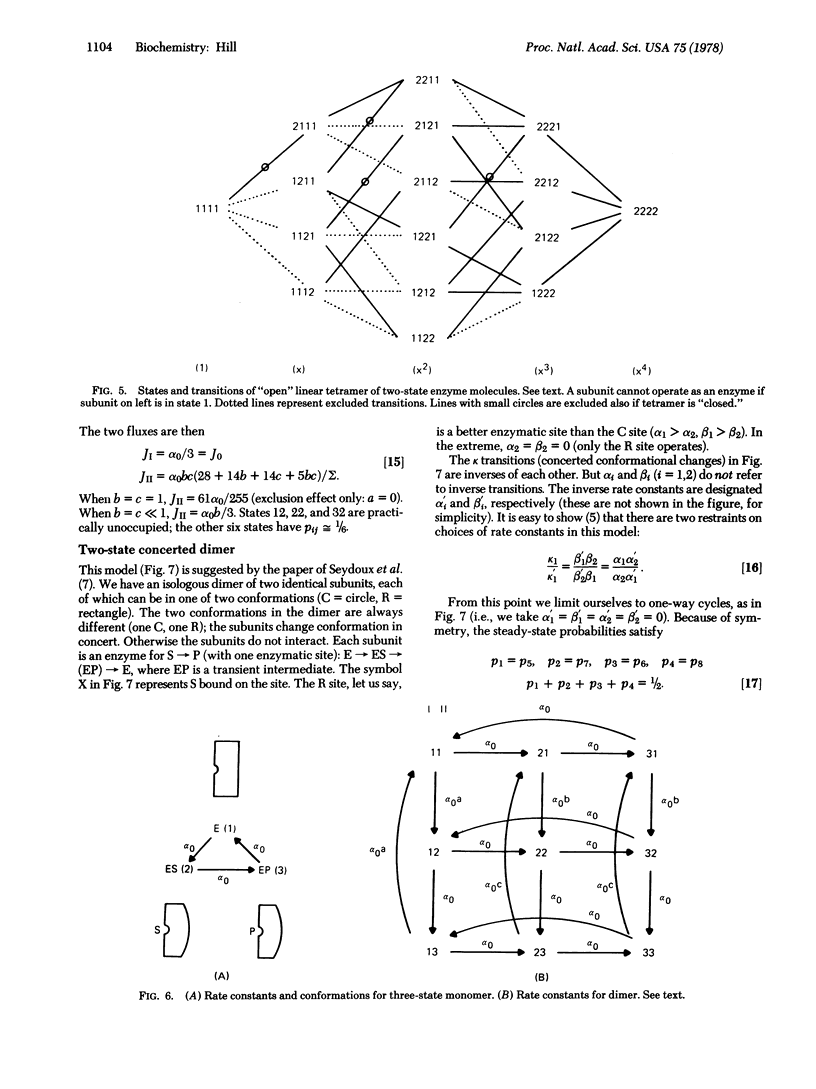
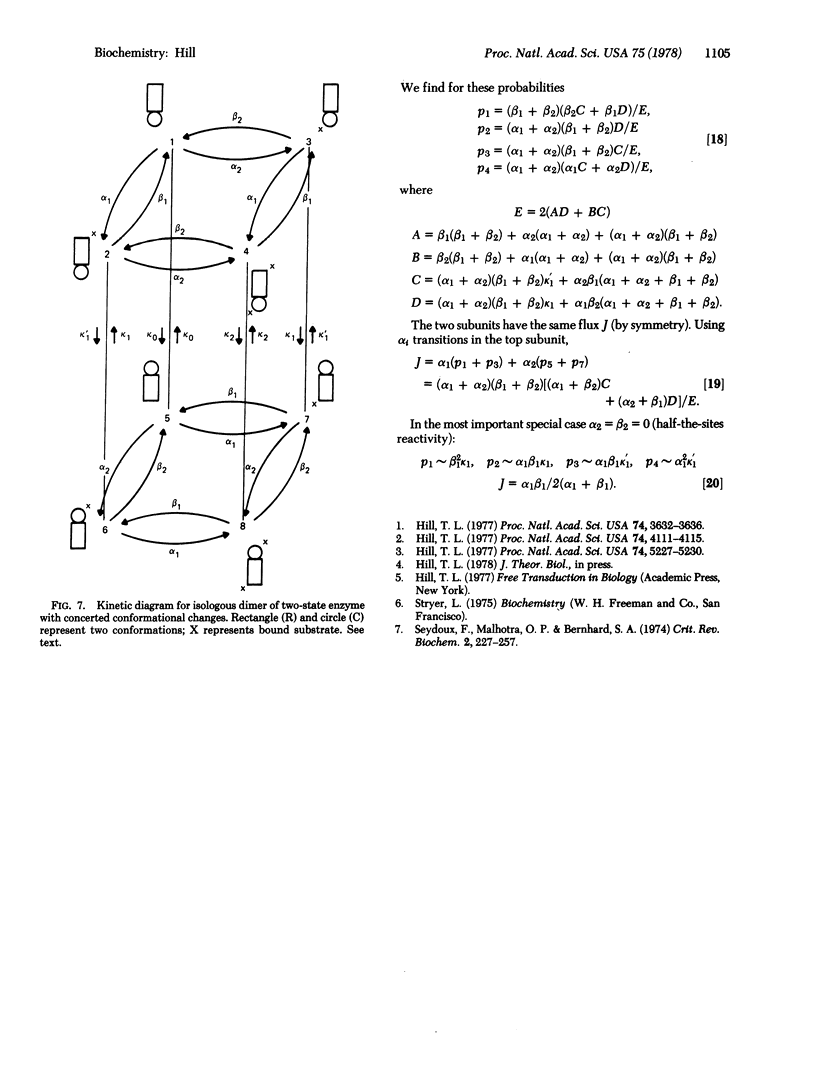
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Hill T. L. "Viral" expansion of enzyme flux and use of quasi-chemical approximation for two-state enzymes with enzyme-enzyme interactions. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5227–5230. doi: 10.1073/pnas.74.12.5227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill T. L. Further study of the effect of enzyme-enzyme interactions on steady-state enzyme kinetics. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4111–4115. doi: 10.1073/pnas.74.10.4111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill T. L. Theoretical study of the effect of enzyme-enzyme interactions on steady-state enzyme kinetics. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3632–3636. doi: 10.1073/pnas.74.9.3632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seydoux F., Malhotra O. P., Bernhard S. A. Half-site reactivity. CRC Crit Rev Biochem. 1974 Mar;2(2):227–257. doi: 10.3109/10409237409105448. [DOI] [PubMed] [Google Scholar]


