Abstract
Objectives
Children with an immunocompromised condition and requiring invasive mechanical ventilation have high risk of death. Such patients are commonly transitioned to rescue modes of non-conventional ventilation, including airway pressure release ventilation and high-frequency oscillatory ventilation, for acute respiratory distress syndrome refractory to conventional ventilation. Our aim was to describe our experience with airway pressure release ventilation and high-frequency oscillatory ventilation in children with an immunocompromised condition and acute respiratory distress syndrome refractory to conventional ventilation and to identify factors associated with survival.
Design
Retrospective cohort study.
Setting
Tertiary care, university-affiliated PICU.
Patients
Sixty pediatric patients with an immunocompromised condition and acute respiratory distress syndrome refractory to conventional ventilation transitioned to either airway pressure release ventilation or high-frequency oscillatory ventilation.
Interventions
None.
Measurements and Main Results
Demographic data, ventilator settings, arterial blood gases, oxygenation index, and Pao2/Fio2 were recorded before transition to either mode of nonconventional ventilation and at predetermined intervals after transition for up to 5 days. Mortality in the entire cohort was 63% and did not differ between patients transitioned to airway pressure release ventilation and high-frequency oscillatory ventilation. For both airway pressure release ventilation and high-frequency oscillatory ventilation, improvements in oxygenation index and Pao2/Fio2 at 24 hours expressed as a fraction of pretransition values (oxygenation index24/oxygenation indexpre and Pao2/Fio224/Pao2/FIO2pre) reliably discriminated nonsurvivors from survivors, with receiver operating characteristic areas under the curves between 0.89 and 0.95 (p for all curves < 0.001). Sensitivity-specificity analysis suggested that less than 15% reduction in oxygenation index (90% sensitive, 75% specific) or less than 90% increase in Pao2/Fio2 (80% sensitive, 94% specific) 24 hours after transition to airway pressure release ventilation were the optimal cutoffs to identify nonsurvivors. The comparable values 24 hours after transition to high-frequency oscillatory ventilation were less than 5% reduction in oxygenation index (100% sensitive, 83% specific) or less than 80% increase in Pao2/Fio2 (91% sensitive, 89% specific) to identify nonsurvivors.
Conclusions
In this single-center retrospective study of pediatric patients with an immunocompromised condition and acute respiratory distress syndrome failing conventional ventilation transitioned to either airway pressure release ventilation or high-frequency oscillatory ventilation, improved oxygenation at 24 hours expressed as Pao2/Fio224/Pao2/Fio2pre or oxygenation index24/oxygenation indexpre reliably discriminates nonsurvivors from survivors. These findings should be prospectively verified.
Keywords: acute lung injury, acute respiratory distress syndrome, airway pressure release ventilation, high-frequency oscillatory ventilation, mechanical ventilation, pediatric
Patients admitted to the PICU with an immunocompromised condition (ICC) have high risk of death (1, 2). Respiratory failure requiring invasive mechanical ventilation is a risk factor for mortality (1–3), and children with an ICC and acute respiratory distress syndrome (ARDS) have a higher mortality than non-ICC patients with ARDS (4, 5). Such patients are commonly transitioned from conventional ventilation to alternative modes of nonconventional ventilation (NCV) for refractory hypoxemia or to limit cyclic high peak distending pressures. Two such modes employed are high-frequency oscillatory ventilation (HFOV) and airway pressure release ventilation (APRV).
HFOV achieves gas exchange using very high respiratory rates oscillating around a fixed mean airway pressure (mPaw) delivering subdead space volumes. HFOV has demonstrated improved gas exchange in neonatal and pediatric respiratory failure without consistent demonstration of benefits on clinical outcomes (4, 6–9), including mortality or duration of mechanical ventilation. Recently, APRV has been described as an alternate mode for refractory hypoxemia (10). APRV applies a prolonged continuous positive airway pressure to recruit available lung units of varying time constants while using lower peak pressures and inspiratory flow rates than conventional, with periodic time-cycled releases to facilitate Co2 clearance. Experience with APRV is limited, but initial reports suggest potential advantages (11–13), including improved cardiac output, decreased vasopressor requirement, and cardiorespiratory benefits of spontaneous respiration throughout the ventilator cycle.
To further clarify the role of different types of NCV in pediatric patients with ICC with ARDS, we conducted a retrospective study on a cohort of patients in our PICU with ICC who failed conventional ventilation and transitioned to either APRV or HFOV. We sought to identify physiologic predictors of survival, especially early in the course of NCV, to assess the response to changing modes of ventilation in this cohort with high mortality risk. We hypothesized that improved oxygenation after transition to NCV would be associated with survival. Given the a priori small sample size, single center, and retrospective nature of our study, we did not aim to compare superiority of either APRV or HFOV with respect to clinical outcomes in this population.
Methods
Design
We conducted a retrospective cohort study in pediatric patients with an ICC receiving either APRV or HFOV at the Children's Hospital of Philadelphia, a tertiary care PICU. The study was approved by the hospital's institutional review board with waiver of the requirement for informed consent.
Patient Selection
Patients were identified by query of a prospectively collected PICU admission database. Patients were initially screened for the presence of an immunocompromised diagnosis (using the diagnostic category terms of “oncologic,” “immunologic,” “rheumatologic,” and “transplant”) and a requirement for mechanical ventilation. All patients were verified during chart review to be immunocompromised at the time of mechanical ventilation, most commonly by verifying active immunosuppressive chemotherapy as per prior studies on immunocompromised children (3, 14, 15). All consecutive ICC patients between 2 weeks old and 21 years old who transitioned from conventional ventilation to either APRV or HFOV between January 1, 2004, and June 30, 2012, were eligible. There were no exclusion criteria.
Conventional Ventilation Strategy
Determination of failure of conventional ventilation and decision to employ alternate modes was left to the discretion of the attending physician. Despite the lack of a formal protocol, our institutional practice for respiratory failure is to initiate conventional ventilation with a minimum of 5 cm H2O of positive end-expiratory pressure (PEEP), 6–8 mL/kg of tidal volume, and to attempt to wean Fio2 to less than or equal to 0.60. Inability to wean Fio2 prompts escalation of PEEP and subsequent repeat efforts to wean Fio2, with the goal to maintain peak inspiratory pressures less than or equal to 35 cm H2O. Persistently elevated peak pressures (≥ 35 cm H2O), ongoing hypercarbia (Paco2 ≥ 80 or pH < 7.30), or oxygenation difficulties (inability to wean Fio2 ≤ 0.60 despite increasing PEEP) prompted consideration for a change in the mode of ventilation.
APRV Strategy
APRV was initiated selecting peak pressure (Phigh) and inspiratory time (Thigh) to at least match the mPaw being delivered on conventional, with stepwise increases in mPaw by adjusting Phigh or Thigh if unable to wean Fio2 to less than or equal to 0.60. Our institutional practice is to set the low pressure on APRV (Plow) to 0 to facilitate rapid emptying and to adjust expiratory time (Tlow) to terminate at 50–75% of peak expiratory flow (10).
HFOV Strategy
HFOV was initiated by setting mPaw to at least match that being delivered on conventional ventilation, with escalation of mPaw until Fio2 could be weaned to less than or equal to 0.60. Amplitude was increased to achieve oscillations visible to the pelvis and initial frequency set based on patient weight. For both APRV and HFOV, the oxygenation goal was an oxygen saturation of greater than or equal to 88% on Fio2 less than or equal to 0.60, and the ventilation goal was Paco2 less than or equal to 60 with pH greater than or equal to 7.3. Use of inhaled nitric oxide, neuromuscular blockade, or conversion to a different mode of NCV or to extracorporeal support was left to the discretion of the attending physician.
Data Collection
Data abstracted from eligible patients' medical records included admission diagnoses, demographics, severity of illness scores at PICU admission (Pediatric Risk of Mortality III [PRISM III] at 24 hr), comorbidities, indication for mechanical ventilation, length of mechanical ventilation, and survival to hospital discharge. We recorded ventilatory settings, vasopressor use in the first 72 hours after transition, evidence of pneumomediastinum or pneumothorax at any time on NCV, and the use of adjunctive therapies for acute lung injury (inhaled nitric oxide, neuromuscular blockade in the first 72 hr after transition to NCV). Oxygenation index (OI) was calculated every 12 hours for the first 5 days of NCV if arterial blood gases from indwelling catheters were available and if the patient had not expired, escalated to extracorporeal support, or changed modes of ventilation including transition back to conventional.
Equations and Definitions
Measures of oxygenation calculated were the PF (Pao2/Fio2) ratio and the OI ([mPaw × Fio2 × 100]/Pao2). The PF ratio and OI before transition are designated “PFpre” and “OIpre,” respectively; the values at 24 hours after transition to NCV are PF24 and OI24.
Statistical Analysis
Continuous data were nonnormally distributed and reported as median [25th and 75th percentiles]. Categorical data were reported as frequencies and percentages. Continuous variables were compared using Wilcoxon rank-sum test and categorical variables compared using the Fisher exact test. Multilevel mixed effects linear regression was used to model the change in OI over time after transition to NCV. Receiver operating characteristic (ROC) curves were used to assess the performance of predictive variables in discriminating between survivors and nonsurvivors. Calculations were performed in Stata 10.0 (StataCorp, LP, College Station, TX).
Results
Patient Characteristics
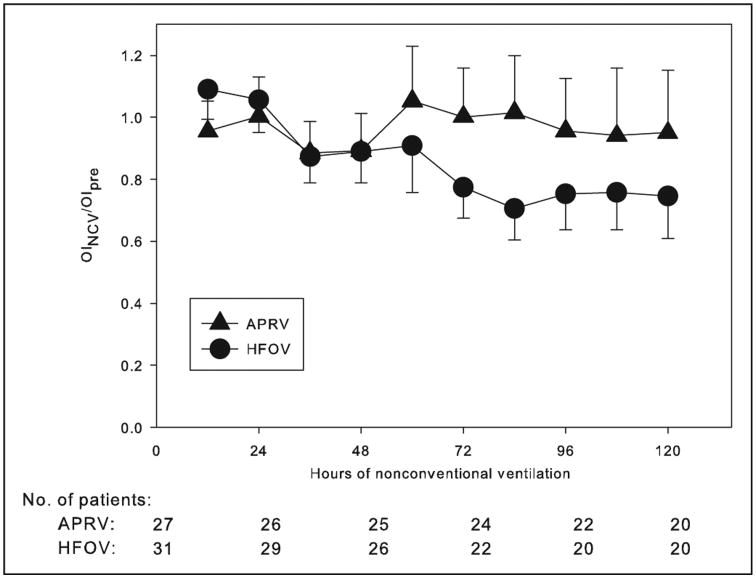
Sixty patients (28 male, 47%) with ICC transitioned from conventional ventilation to either APRV or HFOV during the study period (Table 1). The most common diagnoses were leukemia and stem cell transplant (SCT): 20 patients with leukemia, SCT, or both were transitioned to APRV, and 18 such patients to HFOV. All patients met radiographic and oxygenation criteria for moderate/severe ARDS (all PFpre < 150) at the time of transition. Patients transitioned to HFOV (Table 2) were younger, had worse oxygenation as measured by lower PF (p = 0.054), higher Paco2 (p < 0.001), and a higher rate of NCV failure requiring transition to extracorporeal support (p = 0.043). Patients with an ICC failed conventional ventilation and transitioned to APRV or HFOV at a median length of 1 day [interquartile range, IQR, 0, 4] of conventional ventilation, and at a median peak inspiratory pressure of 39.5 [34, 43], before transfer to NCV. The mPaw at 1 hour (30 cm H2O [27, 32]) and at 24 hours after transition (31 cm H2O [27, 34]) were substantially higher in both modes of NCV relative to pretransition (22 cm H2O [19, 24], p < 0.001 for comparisons with both 1-hr and 24-hr mPaw). PF at 1 hour (103 [81, 141]) and at 24 hours (138 [100, 218]) were significantly improved relative to pretransition (81 [60, 100], p < 0.001 for comparisons with both 1-hr and 24-hr PF). However, a similar improvement in OI in the entire cohort was not observed at either 1 or 24 hours relative to pretransition. Characteristics of patients with SCT (Supplementary Table 1, Supplemental Digital Content 1, http://links.lww.com/PCC/A84) and all malignancies (Supplementary Table 2, Supplemental Digital Content 1, http://links.lww.com/PCC/A84) are similar to those of the entire cohort. Patients transitioned to HFOV experienced a greater decrease in OI over the first 5 days after transition compared to those transitioned to APRV (Fig. 1), with a significant effect of mode of NCV on change in OI over time (p = 0.022). Nonsurvivors on APRV had worsening OI over the first 5 days after transition, whereas non-survivors transitioned to HFOV had an essentially unchanged OI, and survivors in both cohorts had improving OI over time (Supplementary Fig. 1, Supplemental Digital Content 2, http://links.lww.com/PCC/A85).
Table 1. Types of Immunocompromised Conditions (n = 60).
| Diagnosisa | Airway Pressure Release Ventilation (n = 29) | High-Frequency Oscillatory Ventilation (n = 31) |
|---|---|---|
| Stem cell transplant | ||
| Allogeneic | 11 | 9 |
| Autologous | 5 | 4 |
|
| ||
| Congenital or acquired anemia | 7 | 3 |
|
| ||
| Congenital immunodeficiency | 0 | 4 |
|
| ||
| Leukemia or lymphoma | 11 | 10 |
|
| ||
| Solid organ malignancy | 5 | 7 |
|
| ||
| Solid organ transplant | 3 | 2 |
|
| ||
| Autoimmune disease and hemophagocytic lymphohistiocytosis | 3 | 5 |
|
| ||
| HIV | 1 | 0 |
More than one category is possible.
Table 2. Characteristics of Immunocompromised Patients (n = 60).
| Variablea | All (n = 60) | Airway Pressure Release Ventilation (n = 29) | High-Frequency Oscillatory Ventilation (n = 31) | pb |
|---|---|---|---|---|
| Age (yr) | 5.9 [2.0, 12.2] | 10.8 [4.2, 17.8] | 3.3 [1.3, 6.6] | 0.001 |
|
| ||||
| Pediatric Risk of Mortality version III at 24 hr | 15 [7, 20] | 14 [9, 20] | 15.5 [6, 22] | 0.761 |
|
| ||||
| Length of conventional ventilation before switch to NCV (d) | 1 [0, 4] | 1 [0, 4] | 1 [0, 4] | 0.760 |
|
| ||||
| Before switch to NCV | ||||
| mPaw (cm H2O) | 22 [19, 24] | 23 [17, 26] | 22 [19, 23] | 0.641 |
| Pao2/Fio2 | 81 [60, 100] | 93 [66, 115] | 75 [59, 93] | 0.054 |
| OIpre | 27.8 [20.5, 32.2] | 24.0 [17.5, 31.4] | 28.9 [24.5, 33.6] | 0.130 |
| Peak inspiratory pressure | 39.5 [34, 43] | 40 [33.5, 42.5] | 39 [35, 43.5] | 0.683 |
| Paco2 | 72.5 [57.5, 86.5] | 59 [53, 68] | 85 [73, 107] | < 0.001 |
|
| ||||
| Lung disease (%) | ||||
| Viral pneumonia | 25 (42) | 12 (41) | 13 (42%) | |
| Bacterial pneumonia | 6 (10) | 3 (10) | 3 (9.5%) | |
| Fungal pneumonia | 8 (13) | 4 (14) | 4 (13%) | 0.912 |
| Alveolar hemorrhage | 5 (8) | 2 (7) | 3 (9.5%) | |
| Sepsis | 12 (20) | 5 (17) | 7 (23%) | |
| Other | 4 (7) | 3 (10) | 1 (3%) | |
|
| ||||
| Vasopressor infusions (%) | ||||
| <2 | 16 (26) | 9 (31) | 7 (22%) | 0.563 |
| ≥ 2 | 44 (73) | 20 (69) | 24 (77%) | |
|
| ||||
| Stem cell transplant (%) | ||||
| Allogeneic | 21 (35) | 12 (41) | 9 (29%) | 0.561 |
| Autologous | 8 (13) | 4 (14) | 4 (13%) | |
| None | 31 (52) | 13 (45) | 18 (58%) | |
|
| ||||
| Continuous neuromuscular blockade (%) on NCV | 38 (63) | 9 (31) | 29 (94%) | < 0.001 |
|
| ||||
| Inhaled nitric oxide on NCV (%) | 44 (73) | 19 (66) | 25 (81%) | 0.247 |
|
| ||||
| After 1 hr of NCV | ||||
| mPaw (cm H2O) | 30 [27, 32] | 28 [27, 31] | 31 [28.5, 34] | 0.013 |
| Pao2/Fio2 | 103 [81, 141] | 120 [84, 142] | 96 [76, 131] | 0.249 |
| OI1 | 28.5 [20.5, 37.2] | 24.0 [19.3, 32.6] | 29.9 [23.3, 42.3] | 0.072 |
| Paco2 | 56 [44, 73] | 49 [44, 58] | 69 [50, 99.5] | < 0.001 |
|
| ||||
| After 24 hr of NCV | ||||
| mPaw (cm H2O) | 31 [27, 34] | 29 [27, 35] | 32 [29, 34] | 0.191 |
| Pao2/Fio2 | 138 [100, 218] | 146 [105, 225] | 137 [88, 184] | 0.308 |
| OI24 | 22.7 [13.4, 33.2] | 19.1 [12.1, 32.0] | 25.0 [18.2, 38.3] | 0.157 |
| Paco2 | 50.5 [44, 59] | 48 [43, 54] | 54 [46.5, 62] | 0.014 |
|
| ||||
| Length of NCV (d) | 6.5 [3, 13] | 7 [3, 10] | 6 [2, 19] | 0.917 |
|
| ||||
| New air leak (%) | 7 (12) | 3 (10) | 4 (13%) | 1 |
|
| ||||
| Failure of NCV (%) | ||||
| Change NCV modes | 5 (8) | 5 (7) | 0 | 0.043 |
| Extracorporeal membrane oxygenation | 6 (10) | 2 (17) | 4 (13%) | |
|
| ||||
| Mortality (nonsurvivor) (%) | 38 (63) | 18 (62) | 20 (65%) | 1 |
NCV = nonconventional ventilation, mPaw = mean airway pressure, OI = oxygenation index.
Continuous data are in the form of median [25th and 75th percentile], and categorical data are in the form of n (%)
Medians are compared using a Wilcoxon rank-sum test for unpaired data. Categorical variables are compared using a Fisher exact test.
Figure 1.
Change in fractional oxygenation index (OI) (OINCV/OIpre) over hours of nonconventional ventilation (NCV) between patients transitioned to airway pressure release ventilation (APRV) or high-frequency oscillatory ventilation (HFOV). The OI before transition to NCV is designated OIpre. OINCV was calculated every 12 hr for the first 5 d after transition and is reported as a fraction of OIpre. Values of OINCV/OIpre are expressed as mean (± sem). Sample sizes for each grouping, representing patients with arterial access still alive on NCV, are given at hour 0, 24, 48, 72, 96, and 120. Patients were dropped from data collection if they lost arterial access, changed modes of ventilation, transitioned to extracorporeal support, or died. Multilevel mixed effects linear regression was used to test the relationship between the mode of NCV and the change in OI over time, with a significant effect (p = 0.022) of ventilator mode on OINCV/OIpre over time.
Overall mortality was 63%, with no difference between modes of NCV. Patients with an SCT had a notably higher mortality (76%) than the subgroup with malignancies (64%) and the whole cohort (63%). Six patients (two APRV and four HFOV) transitioned to extracorporeal membrane oxygenation (ECMO) at a median of 2.5 days (range, 2–9 d) after transition to NCV and at a median of 4 days (range, 2–9 d) after starting any mechanical ventilation. The median OI at the time of ECMO cannulation was 42 (range, 40–82). One of the patients in the APRV cohort briefly was placed on HFOV prior to ECMO initiation, and the other was cannulated while on APRV. All four HFOV patients were cannulated while on HFOV. Five of these six patients died; the one survivor with common variable immunodeficiency was cannulated to venovenous ECMO after 4 days of HFOV with a pre-ECMO OI of 44.
Eighteen of 29 patients (62%) with ICC who transitioned to APRV died, and 20 of 31 patients (65%) transitioned to HFOV died. In both cohorts, there were no demographic or physiologic variables associated with mortality before transition to either APRV or HFOV. The presence and type of SCT was not significantly associated with mortality in either cohort, but for both APRV and HFOV, the highest mortality occurred in patients who had undergone allogeneic SCT (83% of allogeneic SCT died in the APRV cohort, and 89% in the HFOV). Twenty-four hours after transition to both APRV and HFOV, survivors had lower mPaw, higher PF, and lower OI at 24 hours (Table 3) than nonsurvivors. Similar to the larger cohort, in the subgroup who had undergone SCT (n = 29), survivors (n = 7) demonstrated lower OI (11.3 [9.1, 18.8] vs 30.3 [19.1, 42.3]; p = 0.003) and higher PF (240 [143, 309] vs 103 [86, 157]; p = 0.006) 24 hours after transition to NCV than did nonsurvivors (n = 22).
Table 3. Characteristics of Patients Transitioned to Airway Pressure Release Ventilation (n = 29) and High-Frequency Oscillatory Ventilation (n = 31).
| Airway Pressure Release Ventilation | High-Frequency Oscillatory Ventilation | |||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Variablea | Survivors (n = 11) | Nonsurvivors (n = 18) | pb | Survivors (n = 11) | Nonsurvivors (n = 20) | pb |
| Age (yr) | 10.8 [4.3, 17.2] | 10.4 [4.0, 18.7] | 0.946 | 3.0 [1.7, 6.8] | 3.4 [1.1, 6.5] | 0.951 |
|
| ||||||
| Pediatric Risk of Mortality version III at 24 hr | 17 [12, 19] | 12 [8, 20] | 0.174 | 17 [3, 22] | 15 [6, 21] | 0.860 |
|
| ||||||
| Before switch to NCV | ||||||
| mPaw (cm H2O) | 19 [16, 23] | 24 [21, 26] | 0.096 | 21 [18, 23] | 22 [20, 23] | 0.207 |
| Pao2/Fio2 | 87 [63, 117] | 98 [69, 111] | 0.980 | 78 [60, 96] | 73 [58, 84] | 0.409 |
| OIpre | 20.4 [17.1, 25.4] | 28.5 [20.0, 31.5] | 0.327 | 25.0 [22.3, 29.4] | 30.0 [24.8, 38.0] | 0.193 |
| Paco2 | 58 [53, 66] | 61 [49, 70] | 1 | 81 [74.5, 100.5] | 85 [73, 116.5] | 0.788 |
|
| ||||||
| Lung disease (%) | ||||||
| Viral pneumonia | 1 (9) | 11 (61) | 4 (36%) | 9 (45%) | ||
| Bacterial pneumonia | 2 (18) | 1 (5.5) | 2 (18%) | 1 (5%) | ||
| Fungal pneumonia | 2 (18) | 2 (11) | 0.158 | 1 (9%) | 3 (15%) | 0.389 |
| Alveolar hemorrhage | 1 (9) | 1 (5.5) | 0 | 3 (15%) | ||
| Sepsis | 3 (27) | 2 (11) | 3 (27%) | 4 (20%) | ||
| Other | 2 (18) | 1 (5.5) | 1 (9%) | 0 | ||
|
| ||||||
| Vasopressor infusions (%) | ||||||
| <2 | 5 (45) | 4 (22) | 0.237 | 3 (27%) | 4 (20%) | 0.676 |
| ≥ 2 | 6 (55) | 14 (78) | 8 (73%) | 16 (80%) | ||
|
| ||||||
| Stem cell transplant (%) | ||||||
| Allogeneic | 2 (18) | 10 (56) | 0.110 | 1 (73%) | 8 (40%) | 0.199 |
| Autologous | 2 (18) | 2 (11) | 2 (9%) | 2 (10%) | ||
| None | 7 (64) | 6 (33) | 8 (18%) | 10 (50%) | ||
|
| ||||||
| After 24 hr of NCV | ||||||
| mPaw (cm H2O) | 26 [24, 28] | 31 [28, 37] | 0.012 | 31 [27, 32] | 33 [31, 35] | 0.076 |
| PF | 248 [198, 309] | 115 [88, 159] | < 0.001 | 224 [145, 309] | 101 [69, 138] | < 0.001 |
| PF24/PFpre | 2.8 [1.9, 3.8] | 1.3 [0.9, 1.6] | 0.001 | 2.7 [2.2, 3.2] | 1.2 [0.9, 1.5] | < 0.001 |
| OI24 | 11.8 [8.3, 13.0] | 29.7 [18.3, 35.2] | < 0.001 | 14.3 [9.2, 22.0] | 33.7 [25.0, 52.2] | < 0.001 |
| OI24/OIpre | 0.5 [0.4, 0.7] | 1 [0.8, 1.7] | < 0.001 | 0.5 [0.4, 0.7] | 1.3 [1.0, 1.6] | < 0.001 |
| Paco2 | 43 [40, 56] | 49 [43, 54] | 0.300 | 48 [45, 56.5] | 59 [51, 64.5] | 0.102 |
NCV = nonconventional ventilation, mPaw = mean airway pressure, OI = oxygenation index, PF = Pao2/Fio2
Continuous data are in the form of median [25th and 75th percentile], and categorical data are in the form of n (%)
Medians are compared using a Wilcoxon rank-sum test for unpaired data. Categorical variables are compared using a Fisher exact test.
Variables Associated With Mortality
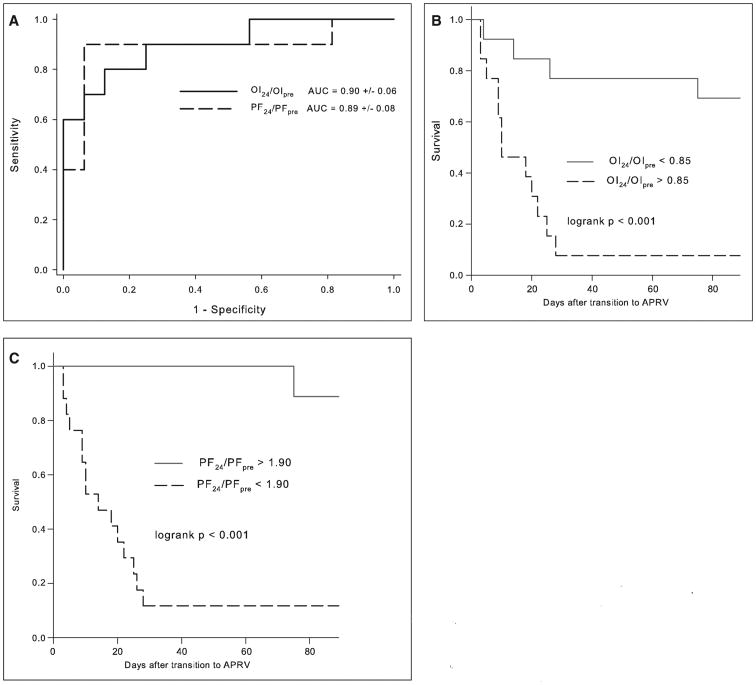
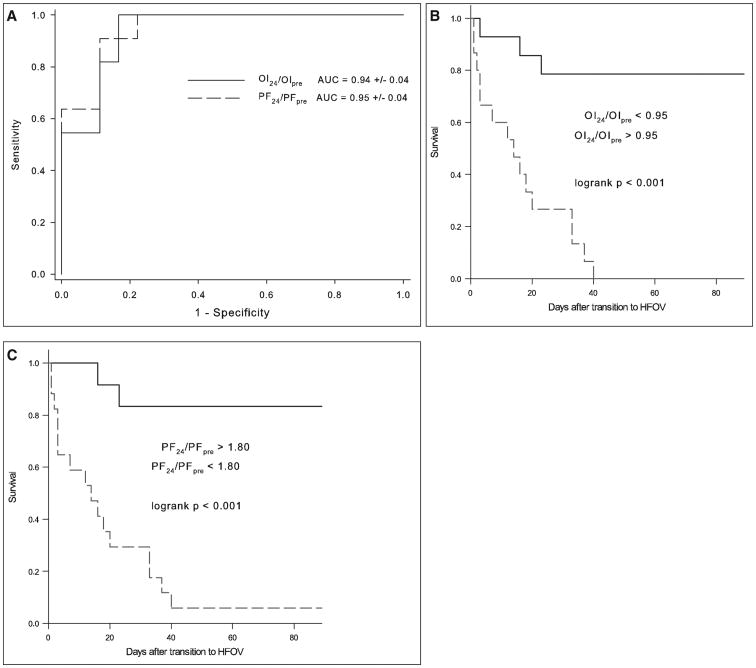
To assess the utility of physiologic variables to discriminate nonsurvivors from survivors, we computed ROC curves and calculated areas under the curve (AUC) for all variables associated with mortality p less than 0.01 separately for the APRV and HFOV cohorts. Specifically, ROC curves were constructed for PF at 24 hours, OI at 24 hours, PF at 24 hours as a fraction of the PF pretransition to NCV (PF24/PFpre), and the OI at 24 hours as a fraction of the OI pretransition to NCV (OI24/OIpre). For both the APRV and HFOV cohorts, the best predictors of mortality were PF24/PFpre and OI24/OIpre. In the APRV cohort, PF24/PFpre has an AUC of 0.89 ± 0.08 (sem), and OI24/OIpre has an AUC of 0.90 ± 0.08 (both p < 0.001) (Fig. 2A). Optimal cutoffs for PF24/PFpre and OI24/OIpre and the associated Kaplan-Meier survival curves are shown in Table 4 and Figure 2, B and C. In the HFOV cohort, the PF24/PFpre and OI24/OIpre AUCs are 0.95 ± 0.08 and 0.94 ± 0.08, respectively (both p < 0.0001) (Fig. 3A). Optimal cutoffs and survival curves are shown in Table 4 and Figure 3, B and C.
Figure 2.
A, Receiver operating characteristic (ROC) curves and associated calculated areas under the curve (AUC) for oxygenation index (OI) and Pao2/Fio2 (PF) 24 hr after transition to airway pressure release ventilation (APRV) as a fraction of pretransition values (OI24/OIpre and PF24/PFpre). AUCs are expressed as fractions (± sem). Kaplan-Meier survival curves for OI24/OIpre (B) and PF24/PFpre (C) after determination of optimal cutoffs from sensitivity-specificity analysis.
Table 4. Threshold Values of Oxygenation Response to Discriminate Survivors and Nonsurvivors.
| Cutoffs for Nonsurvival | Sensitivity (%) | Specifcity (%) | Positive Predictive Value (%) | Negative Predictive Value (%) |
|---|---|---|---|---|
| Airway pressure release ventilation | ||||
| PF24/PFpre < 1.90 | 80 | 94 | 89 | 88 |
| OI24/OIpre > 0.85 | 90 | 75 | 69 | 92 |
|
| ||||
| High-frequency oscillatory ventilation | ||||
| PF24/PFpre < 1.80 | 91 | 89 | 83 | 94 |
| OI24/OIpre > 0.95 | 100 | 83 | 79 | 100 |
PF = Pao2/Fio2, OI = oxygenation index.
Figure 3.
A, Receiver operating characteristic (ROC) curves and associated calculated areas under the curve (AUC) for oxygenation index (OI) and Pao2/Fio2 (PF) 24 hr after transition to high-frequency oscillatory ventilation (HFOV) as a fraction of pretransition values (OI24/OIpre and PF24/PFpre). AUCs are expressed as fractions (± sem). Kaplan-Meier survival curves for OI24/OIpre (b) and PF24/PFpre (C) after determination of optimal cutoffs from sensitivity-specificity analysis.
Discussion
In this single-center cohort, pediatric patients with an ICC and ARDS transitioned to either APRV or HFOV have a high mortality rate. Survival was significantly associated with improvement in oxygenation variables 24 hours after transition to NCV. Select cut-points of PF24/PFpre or OI24/OIpre for these distinct ventilator modes are highly sensitive and specific for hospital mortality and accurately discriminate between nonsurvivors and survivors. OI improved more on HFOV than on APRV over the first 5 days after transition, but this did not translate into a mortality benefit.
Patients with an ICC failing conventional ventilation transitioned to APRV or HFOV relatively early in the course of respiratory failure, with substantial oxygenation defects. Median mPaw before transition to NCV was 22 cm H2O [IQR, 19, 24], somewhat lower than the pressures described for determination of failure of conventional ventilation in a previous pediatric series (7), where children with respiratory failure transitioned to HFOV at a median mPaw of 26 cm H2O [IQR, 19, 24], but is similar to the mPaw of 23 (± 5) cm H2O at which a large Canadian adult cohort transitioned to HFOV (16). The relatively early transition may have been due to greater comfort with NCV in our institution and an unwillingness to exceed peak inflating pressures of 35 cm H2O. At 1 hour and at 24 hours, there was substantial increase in mPaw with improvement in PF but not in OI, suggesting increased alveolar recruitment at the cost of higher mPaw. Our study suggests that younger and sicker ICC patients were preferentially transitioned to HFOV relative to APRV. This may reflect greater familiarity with HFOV, especially in infants, and reluctance to transition patients with worse oxygenation to APRV at our institution, and may have biased our HFOV cohort toward worse outcomes. Despite this, mortality was similar between the two modes of NCV (62% in APRV and 65% in HFOV cohort) and slightly higher than the 56% mortality rate of ARDS patients with an ICC reported in a prior pediatric study (5). Patients in our study had higher OI, which may explain the higher mortality in our study. A study composed entirely of mechanically ventilated pediatric SCT recipients (17) demonstrated a mortality rate of 70%, slightly higher than our total population, in which only 29 of 60 patients (48%) had undergone SCT, but consistent with the mortality of 76% in our subgroup who had undergone SCT (Supplementary Table 1, Supplemental Digital Content 1, http://links.lww.com/PCC/A84). Although demographic variables were generally not predictive of mortality in either mode, with similar PRISM III scores, admission diagnoses, and types of lung disease, the highest mortality occurred in allogeneic SCT patients transitioned to both APRV (10 nonsurvivors among 12 patients) and HFOV (eight non-survivors among nine allogeneic SCT patients). This finding is consistent with the increased mortality risk in allogeneic SCT compared with autologous (1, 2).
For both patients transitioned to APRV and HFOV, the best predictors of mortality were measures of oxygenation 24 hours after transition as a fraction of the pretransition values reported as the PF24/PFpre and OI24/OIpre. In both APRV and HFOV cohorts, the median OI24 for survivors was nearly 40% lower than the median OIpre, whereas for nonsurvivors, the OI24 and OIpre were nearly identical (Table 3). The discriminating value of PF24/PFpre and OI24/OIpre may be a reflection of more highly recruitable lung tissue in survivors, such that these children experience a much larger increase in PF at 24 hours (> 90% increase in PF on APRV, > 80% increase on HFOV) with the increased mPaw than do nonsurvivors. This is corroborated by the relative improvements in OI (> 15% reduction in OI on APRV, > 5% reduction on HFOV), suggesting that survivors with more recruitability are able to improve their PF at relatively lower mPaw, whereas nonsurvivors cannot. Interestingly, a study of adult ARDS patients assessing lung recruitability by CT demonstrated that patients with more potentially recruitable lung had worse oxygenation and higher mortality (18), possibly reflecting greater disease severity as the recruitability of normal healthy lungs is close to zero. Furthermore, increased recruitable lung tissue by CT was positively correlated with mortality. The authors of this study themselves noted a dissociation between “anatomic” and “functional” lung recruitment as assessed by imaging versus gas exchange, respectively. Our finding of improved mortality with improved gas exchange after transition to NCV may be more accurately characterized as improved “functional” recruitability, suggesting that improvements in PF at relatively lower mPaw at the least do not adversely affect the ventilation-perfusion ratio. One of the limitations of using OI is the potential differences in the reported (and delivered) mPaw between conventional and nonconventional modes of ventilation, despite the convention of using the reported mPaw in calculating OI irrespective of mode of ventilation. The calculation of PF is insensitive to mPaw and may be a more appropriate metric for comparing oxygenation across modes of ventilation.
Prior reports on HFOV have made the association between improved OI at 24 hours and mortality (4, 6, 19, 20), although the overlap between the OI of survivors and nonsurvivors has generally precluded the utility of this measure as a prognostic tool. In this high-risk population of pediatric ICC, however, there is minimal overlap, possibly because of the sicker baseline and higher consequent mortality. Arnold et al (4) in 2000 reported an association between OI 24 hours after transition to HFOV, with an OI24 of 28 predicting 70% mortality, and an OI24 of 58 predicting 90% mortality. In our cohort, the isolated OI24 also had acceptable accuracy, with an OI24 greater than 19 in the APRV cohort 24 hours after transition (90% sensitivity, 75% specificity), and an OI24 greater than 24 in the HFOV cohort (90% sensitivity, 78% specificity), predicting mortality. However, the relative ratios PF24/PFpre and OI24/OIpre are useful as they allow comparison of the degree of improvement, if any, after transition to NCV, and may serve as a more useful marker of success than an isolated OI value. PF24/PFpre and OI24/OIpre are also relatively early markers of success or failure of NCV, thereby allowing more invasive therapies to be instituted earlier in ICC patients with ARDS. Early identification of ICC patients transitioned to either APRV or HFOV who remain at high risk of mortality will allow more directed use of potentially beneficial treatments, including exogenous surfactant, prone positioning, or extracorporeal support. The potential for earlier transition to ECMO may be especially beneficial as prolonged time on any mechanical ventilation pre-ECMO has been associated with higher mortality (15, 21).
Interestingly, OI improvement was greater for patients on HFOV over the first 5 days after transition than on APRV (Fig. 1), although this was not reflected in an improved survival for HFOV. Nonsurvivors on APRV actually had worsening OI over the first 5 days after transition, whereas nonsurvivors transitioned to HFOV had an essentially unchanged OI (Supplementary Fig. 1, Supplemental Digital Content 2, http://links.lww.com/PCC/A85), possibly reflecting ongoing derecruitment with the periodic releases seen on APRV, a phenomenon not observed with the sustained mPaw of HFOV. Periodic derecruitment has been shown to be detrimental to gas exchange in animal models (22). Alternatively, the improved OI on HFOV may be a reflection of improved continued recruitment with HFOV relative to APRV, or an effect of the higher use of neuromuscular blockade with HFOV, which has also been shown to improve oxygenation in severe ARDS (23–25).
The association between mortality and improvement in OI and PF within 24 hours after transition to NCV should be validated in a prospective population of immunocompromised pediatric patients. If the observation holds, lack of early improvements in oxygenation might be used as physiologic indicators for determining “failure of NCV” and for switching modes in future trials of these modes of ventilation. Recent adult ARDS trials suggested increased mortality (26) or no effect (27) of HFOV compared to conventional ventilation, calling into question the use of early oscillatory ventilation. The applicability of these trials to our study is limited as the protocols in these trials do not reflect typical HFOV utilization at our institution. In our study, children were transitioned to both HFOV and APRV with higher OI and lower PF ratios than in either adult HFOV trial, and both modes of NCV were used to “rescue” hypoxemia refractory to conventional ventilation. However, given these adult data, the current use of HFOV as rescue ventilation, as opposed to early initiation, seems justified.
Our study has several limitations. The retrospective observational design, small sample size, and lack of a specific mechanical ventilation protocol limit firm conclusions regarding the significance of associations between improvements in oxygenation and mortality. Evolution of care over the 8-year study period may preclude the grouping of all patients with an ICC as a common cohort. Although the association between improved oxygenation and survival is robust, these findings need to be replicated in a comparable population, ideally at different PICUs. The single-center nature may limit its generalizability, although this study represents a relatively large cohort of immunocompromised patients with ARDS transitioned to APRV and HFOV over several years, and the case mix is likely representative of many tertiary care PICUs. The mixed population encompasses many diagnoses of ICC, although a majority (63%) of this cohort has leukemia or has undergone SCT, representing a very high-risk population which is already the target of a focused prospective investigation on the use of exogenous surfactant for ARDS (14). These limitations are best addressed by a prospective trial between APRV and HFOV in pediatric ICC, with clearly defined indications for transitioning onto and off of either mode of NCV.
Our study has the advantage of describing the characteristics of relatively uncommon modes of ventilation for ARDS in an ICC population with an extremely high mortality risk. In addition to describing the value of PF24/PFpre and OI24/OIpre in discriminating between survivors and nonsurvivors, we recorded OI over 5 days, longer than most other studies of NCV, which generally assess gas exchange for the first 24 to 48 hours after transition.
Conclusions
Pediatric patients with an ICC failing conventional ventilation and transitioning to either APRV or HFOV have a high mortality rate. Improved oxygenation at 24 hours expressed as PF24/PFpre or OI24/OIpre reliably discriminates survivors from nonsurvivors. A prospective trial between these two modes of NCV for pediatric ARDS in an ICC population is warranted.
Supplementary Material
Acknowledgments
Supported, in part, by Russell C. Raphaely Endowed Chair in Critical Care Medicine, Department of Anesthesiology and Critical Care, Children's Hospital of Philadelphia.
Dr. Yehya is employed by the Children's Hospital of Philadelphia. Dr. Topjian is employed by UPENN. Her institution received grant support from the National Institutes of Health (NIH). Dr. Thomas consulted for Discovery Laboratories (advisory board). His institution received grant support from the Food and Drug Administration (research grant). Dr. Friess' institution received grant support from NIH.
Footnotes
See also p. 379.
This work was performed at Children's Hospital of Philadelphia.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's website (http://journals.lww.com/pccmjournal).
References
- 1.Bratton SL, Van Duker H, Statler KD, et al. Lower hospital mortality and complications after pediatric hematopoietic stem cell transplantation. Crit Care Med. 2008;36:923–927. doi: 10.1097/01.CCM.0B013E318161FAC1. [DOI] [PubMed] [Google Scholar]
- 2.Duncan CN, Lehmann LE, Cheifetz IM, et al. Pediatric Acute Lung Injury and Sepsis (PALISI) Network. Clinical outcomes of children receiving intensive cardiopulmonary support during hematopoietic stem cell transplant. Pediatr Crit Care Med. 2013;14:261–267. doi: 10.1097/PCC.0b013e3182720601. [DOI] [PubMed] [Google Scholar]
- 3.Piastra M, De Luca D, Pietrini D, et al. Noninvasive pressure-support ventilation in immunocompromised children with ARDS: A feasibility study. Intensive Care Med. 2009;35:1420–1427. doi: 10.1007/s00134-009-1558-5. [DOI] [PubMed] [Google Scholar]
- 4.Arnold JH, Anas NG, Luckett P, et al. High-frequency oscillatory ventilation in pediatric respiratory failure: A multicenter experience. Crit Care Med. 2000;28:3913–3919. doi: 10.1097/00003246-200012000-00031. [DOI] [PubMed] [Google Scholar]
- 5.Willson DF, Thomas NJ, Markovitz BP, et al. Pediatric Acute Lung Injury and Sepsis Investigators. Effect of exogenous surfactant (calfactant) in pediatric acute lung injury: A randomized controlled trial. JAMA. 2005;293:470–476. doi: 10.1001/jama.293.4.470. [DOI] [PubMed] [Google Scholar]
- 6.Arnold JH, Hanson JH, Toro-Figuero LO, et al. Prospective, randomized comparison of high-frequency oscillatory ventilation and conventional mechanical ventilation in pediatric respiratory failure. Crit Care Med. 1994;22:1530–1539. [PubMed] [Google Scholar]
- 7.Ben Jaballah N, Khaldi A, Mnif K, et al. High-frequency oscillatory ventilation in pediatric patients with acute respiratory failure. Pediatr Crit Care Med. 2006;7:362–367. doi: 10.1097/01.PCC.0000227108.38119.2E. [DOI] [PubMed] [Google Scholar]
- 8.Bojan M, Gioanni S, Mauriat P, et al. High-frequency oscillatory ventilation and short-term outcome in neonates and infants undergoing cardiac surgery: A propensity score analysis. Crit Care. 2011;15:R259. doi: 10.1186/cc10521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Faqih NA, Qabba'h SH, Rihani RS, et al. The use of high frequency oscillatory ventilation in a pediatric oncology intensive care unit. Pediatr Blood Cancer. 2012;58:384–389. doi: 10.1002/pbc.23294. [DOI] [PubMed] [Google Scholar]
- 10.Habashi NM. Other approaches to open-lung ventilation: Airway pressure release ventilation. Crit Care Med. 2005;33:S228–S240. doi: 10.1097/01.ccm.0000155920.11893.37. [DOI] [PubMed] [Google Scholar]
- 11.Schultz TR, Costarino AJA, Durning SM, et al. Airway pressure release ventilation in pediatrics. Pediatr Crit Care Med. 2001;2:243–246. doi: 10.1097/00130478-200107000-00010. [DOI] [PubMed] [Google Scholar]
- 12.Walsh MA, Merat M, La Rotta G, et al. Airway pressure release ventilation improves pulmonary blood flow in infants after cardiac surgery. Crit Care Med. 2011;39:2599–2604. doi: 10.1097/CCM.0b013e318228297a. [DOI] [PubMed] [Google Scholar]
- 13.Kaplan LJ, Bailey H, Formosa V. Airway pressure release ventilation increases cardiac performance in patients with acute lung injury/adult respiratory distress syndrome. Crit Care. 2001;5:221–226. doi: 10.1186/cc1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tamburro RF, Thomas NJ, Pon S, et al. Pediatric Acute Lung Injury and Sepsis Investigators (PALISI) Network. Post hoc analysis of calfactant use in immunocompromised children with acute lung injury: Impact and feasibility of further clinical trials. Pediatr Crit Care Med. 2008;9:459–464. doi: 10.1097/PCC.0b013e3181849bec. [DOI] [PubMed] [Google Scholar]
- 15.Zabrocki LA, Brogan TV, Statler KD, et al. Extracorporeal membrane oxygenation for pediatric respiratory failure: Survival and predictors of mortality. Crit Care Med. 2011;39:364–370. doi: 10.1097/CCM.0b013e3181fb7b35. [DOI] [PubMed] [Google Scholar]
- 16.Adhikari NK, Bashir A, Lamontagne F, et al. High-frequency oscillation in adults: A utilization review. Crit Care Med. 2011;39:2631–2644. doi: 10.1097/CCM.0b013e318226675e. [DOI] [PubMed] [Google Scholar]
- 17.Rowan CM, Hege KM, Speicher RH, et al. Oxygenation index predicts mortality in pediatric stem cell transplant recipients requiring mechanical ventilation. Pediatr Transplant. 2012;16:645–650. doi: 10.1111/j.1399-3046.2012.01745.x. [DOI] [PubMed] [Google Scholar]
- 18.Gattinoni L, Caironi P, Cressoni M, et al. Lung recruitment in patients with the acute respiratory distress syndrome. N Engl J Med. 2006;354:1775–1786. doi: 10.1056/NEJMoa052052. [DOI] [PubMed] [Google Scholar]
- 19.Sarnaik AP, Meert KL, Pappas MD, et al. Predicting outcome in children with severe acute respiratory failure treated with high-frequency ventilation. Crit Care Med. 1996;24:1396–1402. doi: 10.1097/00003246-199608000-00020. [DOI] [PubMed] [Google Scholar]
- 20.Camporota L, Sherry T, Smith J, et al. Physiological predictors of survival during high-frequency oscillatory ventilation in adults with acute respiratory distress syndrome. Crit Care. 2013;17:R40. doi: 10.1186/cc12550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Minneci PC, Kilbaugh TJ, Chandler HK, et al. Factors associated with mortality in pediatric patients requiring extracorporeal life support for severe pneumonia. Pediatr Crit Care Med. 2013;14:e26–e33. doi: 10.1097/PCC.0b013e31826e7254. [DOI] [PubMed] [Google Scholar]
- 22.Suh GY, Koh Y, Chung MP, et al. Repeated derecruitments accentuate lung injury during mechanical ventilation. Crit Care Med. 2002;30:1848–1853. doi: 10.1097/00003246-200208000-00029. [DOI] [PubMed] [Google Scholar]
- 23.Gainnier M, Roch A, Forel JM, et al. Effect of neuromuscular blocking agents on gas exchange in patients presenting with acute respiratory distress syndrome. Crit Care Med. 2004;32:113–119. doi: 10.1097/01.CCM.0000104114.72614.BC. [DOI] [PubMed] [Google Scholar]
- 24.Forel JM, Roch A, Marin V, et al. Neuromuscular blocking agents decrease inflammatory response in patients presenting with acute respiratory distress syndrome. Crit Care Med. 2006;34:2749–2757. doi: 10.1097/01.CCM.0000239435.87433.0D. [DOI] [PubMed] [Google Scholar]
- 25.Papazian L, Forel JM, Gacouin A, et al. ACURASYS Study Investigators. Neuromuscular blockers in early acute respiratory distress syndrome. N Engl J Med. 2010;363:1107–1116. doi: 10.1056/NEJMoa1005372. [DOI] [PubMed] [Google Scholar]
- 26.Ferguson ND, Cook DJ, Guyatt GH, et al. OSCILLATE Trial Investigators; Canadian Critical Care Trials Group. High-frequency oscillation in early acute respiratory distress syndrome. N Engl J Med. 2013;368:795–805. doi: 10.1056/NEJMoa1215554. [DOI] [PubMed] [Google Scholar]
- 27.Young D, Lamb SE, Shah S, et al. OSCAR Study Group. High-frequency oscillation for acute respiratory distress syndrome. N Engl J Med. 2013;368:806–813. doi: 10.1056/NEJMoa1215716. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.