Abstract
Cocaine abuse disrupts reward and homeostatic processes through diverse processes, including those involved in circadian clock regulation. Recently we showed that cocaine administration to mice disrupts nocturnal photic phase resetting of the suprachiasmatic (SCN) circadian clock, whereas administration during the day induces non-photic phase shifts. Importantly, the same effects are seen when cocaine is applied to the SCN in vitro, where it blocks photic-like (glutamate-induced) phase shifts at night and induces phase advances during the day. Furthermore, our previous data suggest that cocaine acts in the SCN by enhancing serotonin (5-HT) signaling. For example, the in vitro actions of cocaine mimic those of 5-HT and are blocked by the 5-HT antagonist, metergoline, but not the dopamine receptor antagonist, fluphenazine. Although our data are consistent with cocaine acting through enhance 5-HT signaling, the nonselective actions of cocaine as an antagonist of monoamine transporters raises the question of whether inhibition of the 5-HT transporter (SERT) is key to its circadian effects. Here we investigate this issue using transgenic mice expressing a SERT that exhibits normal 5-HT recognition and transport but significantly reduced cocaine potency (SERT Met172). Circadian patterns of SCN behavioral and neuronal activity did not differ between WT and SERT Met172 mice, nor did they differ in the ability of the 5-HT1A,2,7 receptor agonist, 8-OH-DPAT to reset SCN clock phase, consistent with the normal SERT expression and activity in the transgenic mice. However, 1) cocaine administration does not induce phase advances when administered in vivo or in vitro in SERT Met172 mice; 2) cocaine does not block photic or glutamate-induced (phase shifts in SERT Met172 mice; and 3) cocaine does not induce long-term changes in free-running period in SERT Met172 mice. We conclude that SERT antagonism is required for the phase shifting of the SCN circadian clock induced by cocaine.
Keywords: cocaine, suprachiasmatic nucleus, circadian rhythms, serotonin, glutamate, phase-shift
Cocaine abuse disrupts homeostatic, immune, and reward processes by affecting diverse neuronal processes, including those involved in circadian clock regulation. Recent work focusing on interactions between cocaine and the circadian system has revealed a reciprocal relationship whereby cocaine affects circadian timekeeping, and conversely, the circadian system modulates cocaine intake, sensitization and reward (Baird and Gauvin, 2000;Roberts et al., 2002;Abarca et al., 2002). The rhythm in cocaine self-administration is regulated by the circadian clock, since it free-runs when animals are housed in constant conditions (Falk et al., 1996). Conversely, cocaine administration disrupts multiple circadian behavioral and physiological rhythms, including sleep, feeding, and endocrine fluctuations (McClung, 2008;Morgan et al., 2006). Additionally, daily cocaine injections can lead to anticipatory activity suggestive of circadian entrainment (Tornatzky and Miczek, 1999). Recently we showed that cocaine administration to mice at night disrupts photic phase resetting of the suprachiasmatic (SCN) circadian clock, whereas administration during the day induces non-photic phase shifts (Glass et al., 2012). Notably, the same effects are seen when cocaine is applied directly to the SCN in vitro, where it blocks photic-like (glutamate-induced) phase shifts at night and induces phase advances during the day, demonstrating that cocaine is likely acting within the SCN to modulate circadian clock phase (Glass et al., 2012).
At a molecular level, the interplay between cocaine and the circadian system includes clear bi-directional interactions between cocaine and circadian clock genes. For example, cocaine disrupts circadian rhythms in Clock, Per1 and Per2 expression in a variety of brain areas (Ahmed et al., 2005;Manev and Uz, 2006;Uz et al., 2005;Yuferov et al., 2003). Conversely, decreased expression in each of these genes can alter cocaine preference and use (Abarca et al., 2002;McClung et al., 2005;Gabriele et al., 2009). Moreover, decreased Per2 expression leads to enhanced cocaine-induced phase shifts during the day and decreased cocaine-induced inhibition of photic phase resetting at night (Brager et al., 2013).
Although the findings outlined above demonstrate important connections between cocaine and the SCN circadian clock, they provide little information regarding the neurochemical basis of these interactions. With respect to its rewarding properties, cocaine is thought to act primarily by inhibiting the dopamine (DA) transporter, which regulates DA signaling in the mesolimbic reward system (Ron and Jurd, 2005). However, cocaine also inhibits the transporters for norepinephrine and serotonin (5-HT, SERT), and these actions could contribute to the addictive effects of the psychostimulant (Uchimura and North, 1990;Sofuoglu and Sewell, 2008;Nonkes et al., 2011). With respect to cocaine's actions on the circadian system, it seems less likely that they involve changes in DA signaling, since DA receptors present in the fetal SCN are no longer expressed in the adult (Weaver et al., 1992). Moreover, our previous findings suggest that cocaine acts in the SCN by enhancing 5-HT signaling (Glass et al., 2012). Thus, the in vitro actions of cocaine on SCN clock phase mimic those of 5-HT and are blocked by the 5-HT receptor antagonist, metergoline but not by the DA receptor antagonist, fluphenazine. These phase shifts are also prevented by pre-treatments with a low concentration of the 5-HT1A,2,7 receptor agonist, 8-OH-DPAT, which has previously been shown to inhibit serotonergic phase resetting in vitro, likely by down-regulating 5-HT receptors (Prosser et al., 2006).
Although our behavioral and pharmacological data are consistent with cocaine acting within the SCN through enhanced 5-HT rather than DA signaling, a contributory role of 5-HT reuptake inhibition has not been demonstrated. Here we explored this hypothesis using recently developed transgenic mice (SERT Met172 ) that express a SERT protein bearing the amino acid substitution, Ile172Met (Thompson et al., 2011;Ye and Blakely, 2011). The substitution yields normal SERT protein expression and paroxetine-sensitive, 5-HT reuptake in vitro and in vivo. However, the alteration leads to a more than 50 fold reduction in cocaine sensitivity for 5-HT reuptake blockade (Thompson et al., 2011;Ye and Blakely, 2011). Here, we use the SERT Met172 mice to demonstrate a critical role of SERT reuptake blockade in the phase shifting properties of cocaine, findings that underscore a key contribution of 5-HT signaling to the plasticities that arise in the context of drug addiction. .
METHODS
2.1 Animals
All experiments used adult male mice ~8-20 weeks of age. For the in vitro experiments, SERT Met172 homozygous mice maintained on either a 129S6/S4 background or C57BL/6J background were obtained from colonies maintained at Vanderbilt University. Wild-type mice for the in vitro experiments were C57BL/6 mice from Harlan Laboratories (Indianapolis, IN, USA). Wild-type mice for the in vivo experiments were C57BL/6J mice from Jackson Laboratories (Bar Harbor, Maine). All animals were housed in polycarbonate cages under a 12:12 light-dark (LD) photoperiod in a temperature-controlled vivarium (23°C) with food and water provided ad libitum. The experiments followed the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals and were approved by the Kent State University and University of Tennessee Institutional Animal Care and Use Committees.
2.2 Brain slice preparation and single unit recording
Coronal brain slices (500 μm) containing the SCN were prepared during the daytime from adult wild-type or SERT Met172 mice (2-5 months old), housed in LD conditions, as reported previously (Prosser and Gillette, 1989;Prosser et al., 1993;Prosser, 2003). Slices were prepared between Zeitgeber time (ZT) 0–4, where ZT 0 = lights-on and ZT 12 = lights-off in the animal colony. Slices were maintained at the interface of a Hatton-style brain slice chamber (Hatton et al., 1980), where they were perfused continuously with warm (37°C), oxygenated (95% O2/5%CO2), glucose/bicarbonate-supplemented Earle's Balanced Salt Solution (EBSS; MP Biomedicals, Solon, OH, USA), pH 7.4–7.5. Gentamicin (Sigma-Aldrich; St. Louis, MO; 0.05%) was also added to the perfusion medium. All drugs were prepared in warm, oxygenated EBSS and were bath-applied to the brain slices. At the onset of the drug treatments, perfusion of the standard medium was stopped, the medium was completely removed from the chamber, and fresh medium containing the drugs was applied. Previous experiments have demonstrated that changing the perfusion medium by itself does not affect the phase of the circadian clock.
The procedure for neuronal recordings has been described previously (Prosser et al., 1993;Prosser, 2003). Briefly, the spontaneous activity of single SCN neurons was recorded extracellularly using glass capillary microelectrodes filled with 3M NaCl. Each neuron was recorded for 5 min, and the data stored for later determination of firing rate using a DataWave system (Berthoud, CO). Typically, 4–7 cells were recorded during each hour. These individual firing rates were then used to calculate 2 h running averages, lagged by 1 h (± SEM), to obtain a measure of population neuronal activity. As in previous studies (Prosser et al., 1993;Prosser, 2003), the time of peak neuronal activity was assessed visually by estimating, to the nearest quarter hour, the time of symmetrically highest activity. For example, if the two highest 2 h means are equal, then the time of peak is estimated to be halfway between them. Phase shifts were calculated as the difference in time-of-peak of untreated slices vs. drug treated slices. Using these methods, the consistency of the results obtained for each experimental manipulation is such that differences in phase of as little as one hour are often statistically significant with few (n=2 to 3) replicates (Prosser, 2003;Prosser et al., 2006).
2.3 Circadian activity measurements
General circadian locomotor activity was measured using overhead passive infrared motion detectors interfaced with a computerized data acquisition system (Clocklab: Coulbourn Instruments, Whitehall, PA). The data were collected in 1 min bins, and activity onset associated with lights-off (ZT 12) was defined by the initial 6 min period that 1) coincided with an intensity of activity that exceeded 10% of the maximum rate for the day, 2) was preceded by at least 4 hr of activity quiescence, and 3) was followed by at least 60 min of sustained activity. Under constant darkness (DD),activity onset is designated as circadian time (CT) 12 and is the phase reference point for the onset of the subjective night. Phase shifts were determined using a modified Aschoff II protocol, where mice were released to DD immediately after drug or control treatments (Aschoff, 1965). Release of animals into DD is used to reveal the extent to which a phase-resetting treatment shifts clock time in the absence of an entraining photocycle that would otherwise mask a phase-resetting effect. Shifts were calculated as the difference between the projected times of activity onset of baseline entrainment under LD and days following the cocaine/photic treatment under DD as determined by 1) back extrapolation of the least-squares line through activity onsets on days 3-7 after cocaine-photic treatment and 2) extrapolation of the least-squares line calculated from activity onset data collected the last 5 days of baseline entrainment. In the chronic cocaine drinking experiment, free-running circadian period was measured during the last week of DD exposure using the Clocklab function for computing rhythm period from activity onsets. Activity bout analyses were conducted using the total number of individual activity episodes registered by the motion detectors across the entire 24-hour day averaged over the last week immediately prior to release into DD.
2.4 Drugs
Cocaine hydrochloride, glutamate and 8-OH-DPAT were obtained from Sigma-Aldrich Corp St. Louis, MO, USA. For oral cocaine experiments, aqueous stock cocaine solutions were adjusted to pH 3 and stored for 1 week or less at 4°C to prevent hydrolysis (Murray and Al-Shora, 1978). In this regard, when cocaine is stored at room temperature in a solution of pH 5.5 or higher it decomposes (mainly via hydrolysis to benzoylecgonine) within approximately 13 days (Murray and Al-Shora, 1978). However, at low pH (4 or below) cocaine can be stored at room temperature for up to 45 days without significant degradation. To ensure the accurate dosing of cocaine, fresh solutions were made and replaced approximately every 5 days. The water-only controls received water that was acidified to pH 3.
2.5 Experimental Protocols
2.5.1 Forced oral cocaine administration
After 2 weeks of baseline activity measurements in LD, SERT Met172 and wild-type mice were given access to a single bottle containing either cocaine solution (0.5%) or water alone for 3 weeks. Actogram analyses of circadian locomotor behavior were continued throughout. For all experiments, fluid consumption was measured daily and estimated to the nearest 0.25 ml using 50 ml graduated drinking tubes. Thereafter, the cocaine solution was replaced with water and mice were released into DD for 40 days to assess cocaine effects on free-running circadian period.
2.5.2 In vitro phase-resetting experiments using the SCN brain slice preparation
Drugs were bath-applied for 10 min at either ZT 6 or ZT 16 during the initial day in vitro. In the glutamate inhibition experiments, cocaine or 8-OH DPAT was applied to the brain slices 5 min prior to and extended 5 min after its 10 min co-application with glutamate (1 μM) at ZT 16.
2.5.3. In vivo non-photic phase-resetting experiments
SERT Met172 and control mice under LD were caged individually, and their circadian locomotor activity rhythms measured over a two-week period prior to experimentation. On the day of treatment, animals received an injection of cocaine (20.0 mg/kg i.p) dissolved in physiological saline or saline alone at ZT 6, the time of maximal phase-advances of the non-photic phase-response curve. Immediately following drug injection, the animals were released into DD for 2 wks to assess phase-advancing responses according to the Aschoff Type II procedure.
2.5.4. In vivo photic phase-resetting experiments
SERT Met172 and control mice under LD were individually caged and their daily activity rhythms measured for a 2 wk period prior to experimentation. On the day of experimentation, mice received an i.p. injection of cocaine (20 mg/kg) dissolved in physiological saline or saline alone 15 min preceding a 30 min light pulse (25 lux) delivered from ZT 16-16.5, the time of maximal phase-delays of the photic phase-response curve. Immediately following the light pulse, the animals were released into DD for 2 wks to assess the extent of phase-delaying according to the Aschoff Type II procedure.
2.6 Statistics
The effects of cocaine on non-photic and photic shifting and circadian rhythm period were assessed by one- or two-way ANOVA. A Student Neuman-Keuls post-hoc comparison test was utilized when the analysis of variance revealed significant treatment effects. In all cases, the level of significance was set at p<0.05.
RESULTS
3.1 Baseline circadian rhythms in SERT Met172 transgenic mice
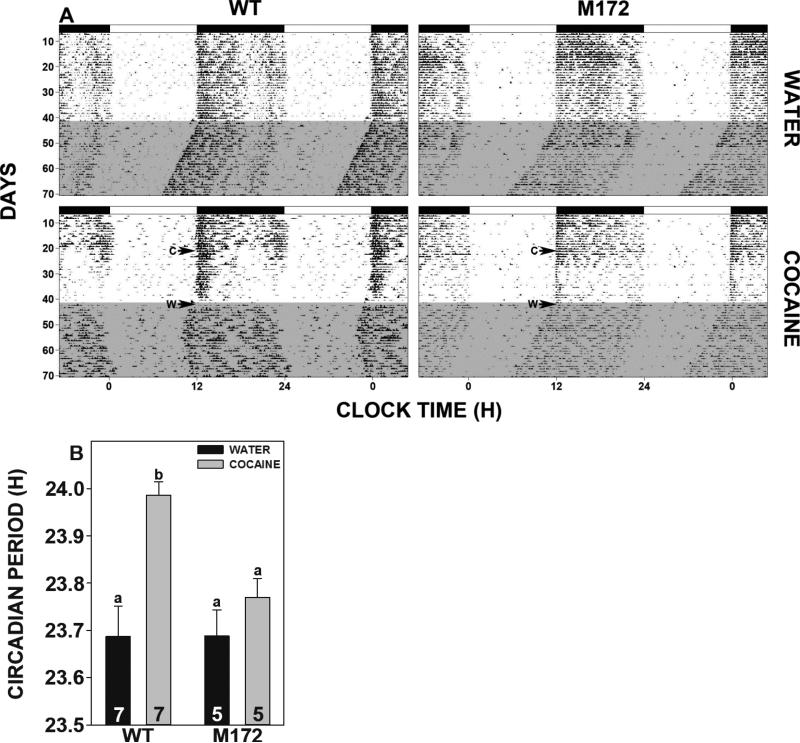
Before investigating potential changes in circadian clock phase resetting in the SERT Met172 transgenic mice, we first assessed their baseline circadian rhythms under LD in vivo (general locomotor activity) and in vitro (SCN neuronal activity). As seen in Fig 1, the circadian rhythm of behavioral activity exhibited by SERT Met172 mice is the same as wild-type controls, with respect to the phase angle of entrainment to LD and length of the nocturnal activity period.
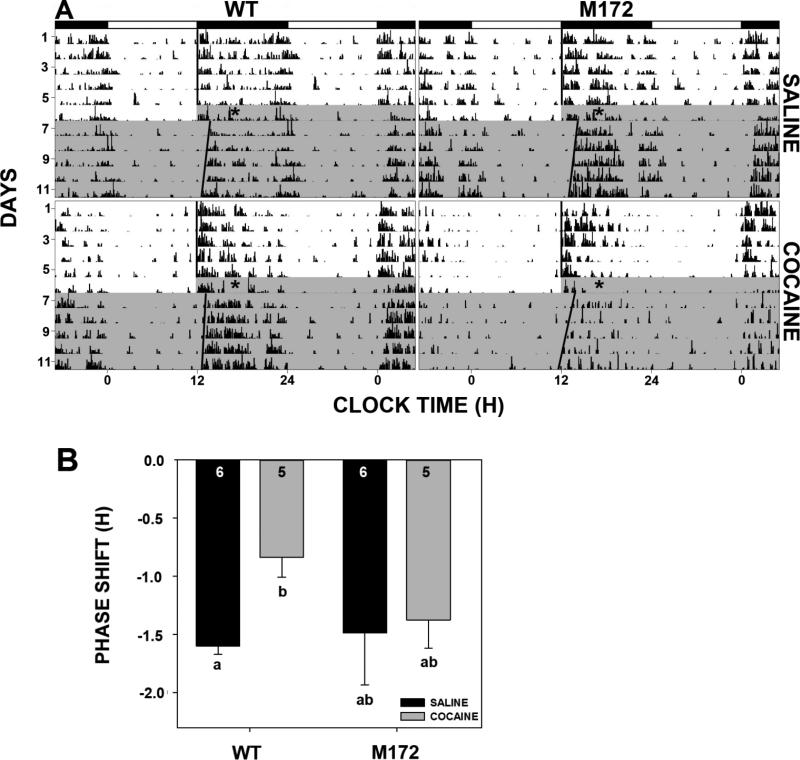
Figure 1.
Effects of SERT Met172 mutation and chronic cocaine on circadian rhythms. A. Representative double-plotted actograms of general locomotor activity of wild-type (WT; left panels) and SERT Met172 mutant mice (right panels) receiving water alone (top panels) or chronic forced oral cocaine (0.5 mg/ml) for 3 wk (bottom panels). Onset and withdrawal from cocaine are designated by “C” and “W”, respectively. Shading represents release to DD. Overhead filled horizontal bars represent the dark phase of the LD cycle. B. Histogram representation (means ± SEM) of behavioral rhythm data showing the cocaine-induced shortening of the free-running circadian period in wild-type mice that is not exhibited by the SERT Met172 mutants. Bars with different letters are significantly different (p<0.05). Numbers in bars are N's per group.
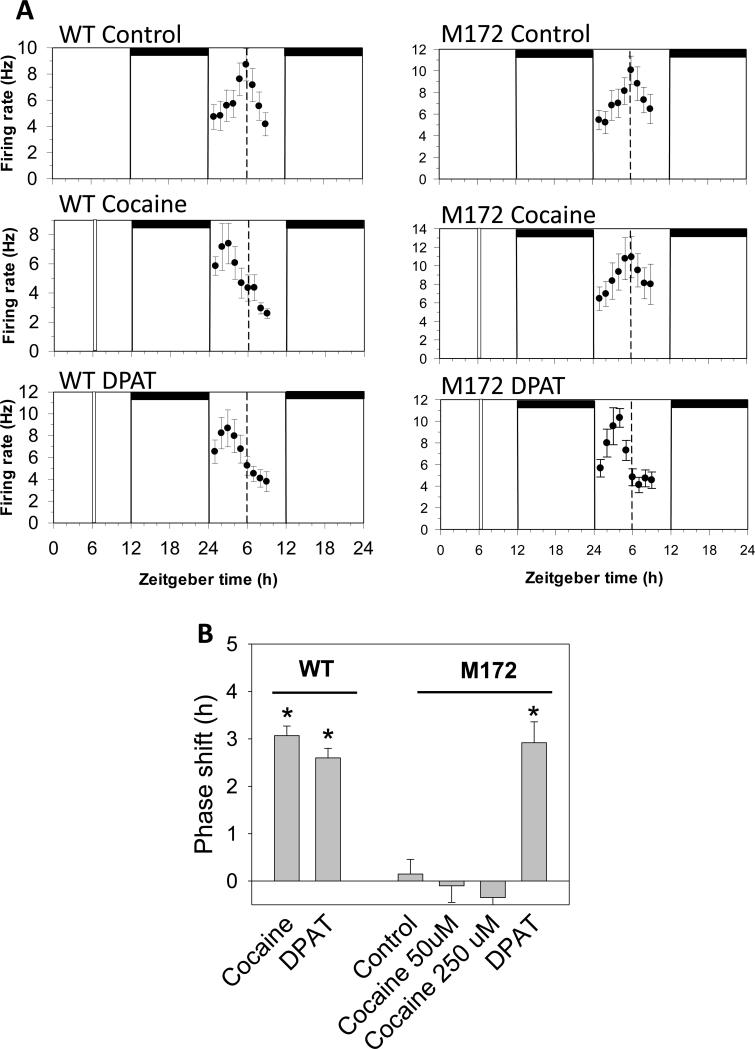
With respect to the in vitro neuronal activity rhythms, SCN-containing brain slices were prepared from wild-type C57 mice and from SERT Met172 mice with both the C57 and 129 background. Slices were maintained for 2 days in vitro, and neuronal activity was monitored on the second day. As shown in Fig 2, the time of peak activity in these brain slices was ~ZT 6, consistent with previous studies (Prosser et al., 1993; Prosser, 2003). Because in these and subsequent experiments there were no differences between the SERT Met172 mice from the two backgrounds the data from the two groups were combined.
Figure 2.
In vitro non-photic phase resetting of the SCN circadian clock. A. Shown are the 2 h means ± SEM of SCN neuronal activity in representative experiments. Neuronal activity peaks near ZT 6 on the second day in vitro in a control (no drug) experiment. Neuronal activity peaks approximately 3 h earlier after treatment with cocaine (50 μM) applied alone at ZT 6 to wild-type (WT) SCN brain slices, indicating the SCN clock had been phase-advanced by 3 h. In contrast, cocaine application to SCN brain slices from SERT Met172 mice induces no phase shift. Conversely, DPAT application to both WT and SERT Met172 brain slices induces similar phase advances. Horizontal bars: time of lights-off in the animal colony; vertical bars: time of drug treatment; dotted line: mean time-of-peak in control (untreated) brain slices. B. Histogram plot of mean ± SEM phase shifts induced by the different in vitro treatments at ZT 6. *p<0.01 vs. mean time-of-peak in untreated WT brain slices.
3.2 Effects of oral cocaine on free-running rhythms
Exposure of mice to a single bottle regimen of forced cocaine drinking (0.5 mg/ml) resulted in an average daily intake of 64.6±5.0 mg/kg for SERT Met172 and 153.6±27.1 mg/kg for wild-type mice (p<0.03). As previously shown (Stowie et al., 2012), when wild-type mice previously given oral cocaine for 3 wks were placed in DD, they exhibited a persistent lengthening of free-running period, vs. drug naïve mice, after cocaine withdrawal (23.9 ± 0.03 h vs. 23.69 ± 0.06 h, respectively; p<0.01; Fig. 1), as measured during the last week of DD exposure. In contrast, SERT Met172 mice demonstrated no such cocaine-induced alteration in free-running periods when compared to cocaine-naïve SERT Met172 mice (23.77 ± 0.04 h vs. 23.69 ± 0.046 h, respectively; p>0.12). Activity bout analysis averaged over the last week of cocaine treatment revealed no effect of drug treatment vs. water on daily activity in wild-types (10.7×103±1.7×103 bouts/day vs. 7.2×103±3.0×103 bouts/day, respectively; p>0.4) or SERT Met172 mice (8.4×103±1.9×103_bouts/day vs 5.7×103±2.3×103 bouts/day, respectively; p>0.4).
3.3 In vitro non-photic phase resetting in SCN brain slices
To begin assessing the effects of acute cocaine, we investigated non-photic phase resetting in brain slices prepared from SERT Met172 mice. Bath application of the 5-HT1A,2,7 receptor agonist, 8-OH-DPAT (10 μM) to SERT Met172 brain slices for 10 min at ZT 6 induced 3 h phase advances (Fig 2), similar to that seen in wild-type SCN brain slices (Glass et al., 2012). In contrast, cocaine (50 μM) treatment failed to induce changes in the phase of the SCN neuronal activity in the SERT Met172 brain slices. To confirm the insensitivity of the SERT Met172 SCN to cocaine, we tested a higher concentration (250 μM), which also did not induce a phase shift (Fig. 2). These data indicate that the phase-resetting actions of cocaine within the SCN require a cocaine-sensitive 5-HT transporter.
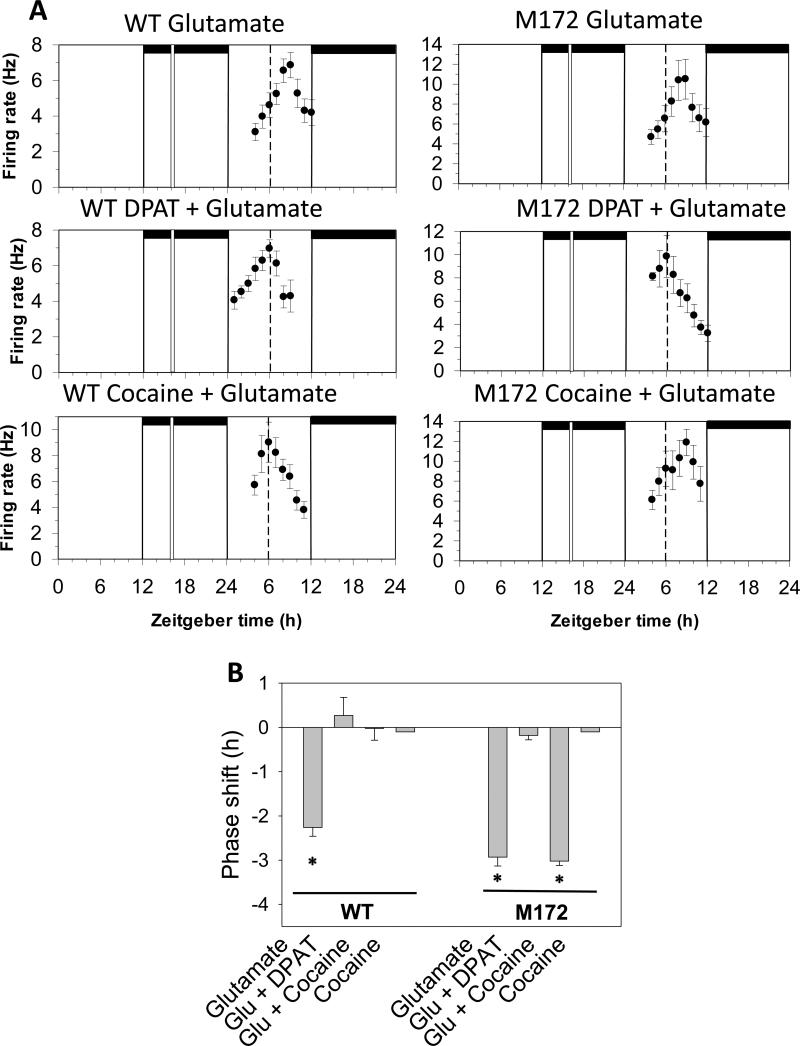
3.4 In vitro glutamate-induced phase resetting in SCN brain slices
Previously we demonstrated that cocaine applied directly to SCN tissue isolated from wild-type mice blocks glutamate-induced phase shifts at night (Glass et al., 2012). Here we investigated whether cocaine induces similar effects in SCN brain slices prepared from SERT Met172 mice. Bath application of glutamate (1 mM) to SERT Met172 brain slices for 10 min at ZT 16 induced a 3 h phase delay, similar to that seen in wild-type SCN brain slices (Fig 3). The glutamate-induced phase delays were inhibited by co-application of 8-OH-DPAT in both wild-type and SERT Met172 brain slices. However, cocaine co-application was unable to block the glutamate-induced phase delays in the SERT Met172 brain slices. In control experiments, cocaine and DPAT applied alone at ZT 16 had no effect. Thus, the ability of cocaine to inhibit photic-like (glutamate-induced) phase shifts in vitro also requires a cocaine-sensitive 5-HT transporter.
Figure 3.
In vitro photic phase resetting of the SCN circadian clock. A. Shown are the 2 h means ± SEM of SCN neuronal activity in individual experiments. Glutamate (1mM) treatment at ZT 16 induces similar 2-3 h phase delays in SCN brain slices from WT and SERT Met172 mice. Further, cocaine treatment alone induces no phase shifts in WT and SERT Met172 brain slices. However, cocaine co-application blocks glutamate-induced phase shifts in WT SCN brain slices, but does not block glutamate-induced phase shifts in brain slices from SERT Met172 mice. B. Histogram plot of mean ± SEM phase shifts induced by the different in vitro treatments at ZT 16. *p<0.01 vs. mean time-of-peak in untreated WT brain slices. See Fig 2 legend for details.
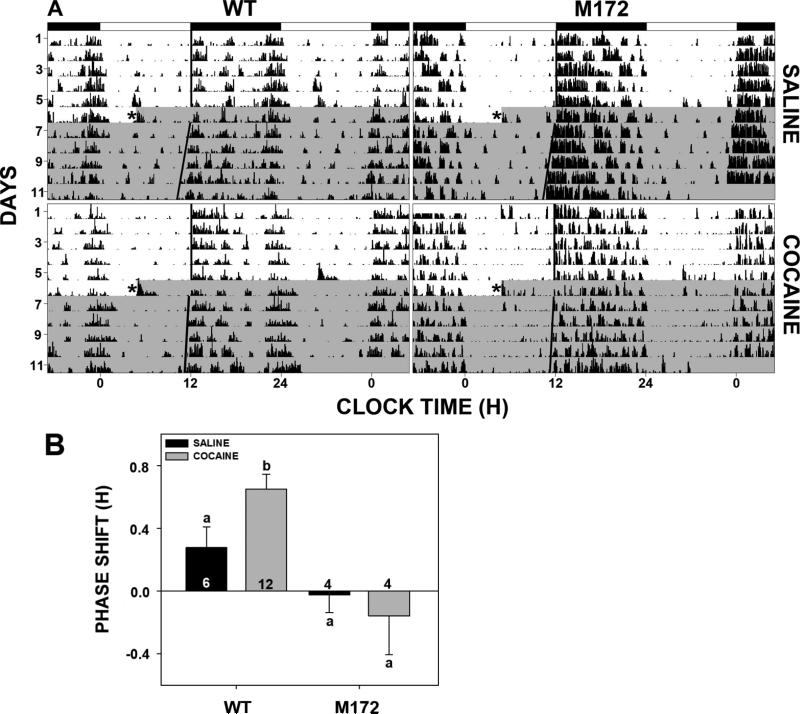
3.5 In vivo non-photic phase resetting
Similar to our previous findings (Glass et al., 2012), acute i.p. injection of cocaine (20 mg/kg) in wild-type mice at midday elicited a phase advance response that was significantly greater than in saline injected controls (0.65±0.10 h vs. 0.28±0.13 h, respectively; p<0.04; Fig. 4). In contrast, similar cocaine treatment in SERT Met172 mice did not induce a significant phase advance relative to saline controls (−0.16±0.25 h vs.−0.02±0.01 h, respectively; p>0.4), indicating that the mutation renders the animals insensitive to the in vivo non-photic clock-resetting action of cocaine.
Figure 4.
In vivo non-photic phase resetting of the SCN circadian clock. A. Representative double-plotted actograms of general locomotor activity showing phase-advance shifting in response to acute cocaine (20 mg/kg i.p. ) in wild-type mice (bottom left panel) that was not expressed in SERT Met172 mutant mice (bottom right panel) or in saline injected controls (top panels). B. Histogram representation (means ± SEM) of behavioral rhythm data showing the phase-resetting action of acute cocaine (20 mg/kg i.p.) in wild-type mice (WT) but not in SERT Met172 mutant mice. Saline injections had little phase-resetting effect. Bars with different letters are significantly different (p<0.05). See Fig 1 legend for details.
3.6 In vivo photic phase resetting
Also consistent with our previous report (Glass et al., 2012), acute i.p. injection of cocaine (20 mg/kg) in wild-type mice markedly suppressed the phase-delaying effect of light during the early night compared to saline controls (0.84±0.17 h vs. 1.60±0.07 h, respectively; p<0.003; Fig. 5). In SERT Met172 controls receiving saline injection, exposure to the same photic stimulus elicited a similar phase-resetting response as in the saline treated wild-type controls; however, pretreatment with cocaine had little suppressive effect on photic phase resetting compared to SERT Met172 saline controls (1.38±0.24 h vs. 1.49±0.45 h, respectively; p>0.84). Thus, the SERT I172M mutation also renders the animals insensitive to the inhibitory action of cocaine on photic phase-resetting responses.
Figure 5.
In vivo photic phase resetting of the SCN circadian clock. A. Representative double-plotted actograms of general locomotor activity showing acute cocaine (20 mg/kg i.p.) attenuation of photic phase-delay responses to light pulses delivered at ZT 16.5 in wild-type mice (lower left panel) but not in SERT Met172 mutant mice (lower right panel) relative to saline controls (top panels). B. Histogram representation (means ± SEM) of behavioral rhythm data showing inhibition of the phase-resetting action of acute cocaine (20 mg/kg i.p.) in wild-type mice (WT) but not in SERT Me172 mutant mice relative to saline controls. Bars with different letters are significantly different (p<0.05). Numbers in bars are N's per group. See Fig 1 legend for details.
DISCUSSION
Research on substance abuse is complicated by the reality that most drugs of abuse, including cocaine, affect multiple neurochemical signaling systems throughout the brain. Therefore, it is important to assess the neurochemistry of drug actions at both the systemic and local levels. Such was the scope of the present study, where complementary behavioral (in vivo) and electrophysiological (in vitro) approaches were used to explore the role of 5-HT in mediating cocaine's actions in the mammalian circadian timing system.
Cocaine abuse disrupts circadian rhythms in sleep, endocrine, and immune processes (McClung, 2008;Morgan et al., 2006). Our research has shown that cocaine functions, at least in part, through direct actions within the SCN, where it can inhibit photic signaling processes in vivo and in vitro and can induce its own phase shifts in circadian clock-regulated behavioral and SCN neuronal activity rhythms when applied during the day (Glass et al., 2012;Brager et al., 2013). Although cocaine can enhance DA, norepinephrine, and 5-HT signaling by inhibiting their respective transporters throughout the brain, our previous pharmacological data suggest that cocaine primarily acts by enhancing 5-HT signaling in the SCN (Glass et al., 2012). Here we extended our investigation of the neurochemical mechanisms through which cocaine affects the circadian system through the use of genetically modified mice that express a SERT modified to limit cocaine potency (~60 fold). These mice, originally generated to evaluate the SERT specificity of antidepressant actions, have normal 5-HT- and paroxetine-inhibited 5-HT uptake, but significantly decreased responsiveness to many SERT inhibitors, including cocaine (Thompson et al., 2011;Ye and Blakely, 2011). Theoretically, because cocaine increases DA signaling but not 5-HT signaling in these mice, their circadian clock should not respond to cocaine's non-photic and photic actions. Consistent with this idea, both of these effects of cocaine were absent in the SERT Met172 mice in vivo and in vitro. Moreover, the long-lasting effect of forced oral cocaine administration on free-running period was also absent in the SERT Met172 mice. An important caveat to these results stems from the 50% lower cocaine self-administration in the SERT Met172 mice. Therefore, it will be critical to further evaluate this issue in subsequent experiments where cocaine administration is experimentally regulated.
The in vitro effects of cocaine in the SCN are consistent with the psychostimulant acting to increase 5-HT signaling in this brain region. The SCN receives direct serotonergic input from the midbrain median raphe nuclei (Meyer-Bernstein et al., 1997), and there is a pronounced circadian rhythm in 5-HT output in the SCN that is driven by rhythmic behavioral activity/arousal (Dudley et al., 1998;Grossman et al., 2000). Similar to cocaine, 5-HT receptor agonists induce phase advances when administered during the day (Prosser, 2003), whereas at night these drugs inhibit photic (glutamate-mediated) phase shifts (present data) (Rea et al., 1994;Rea and Pickard, 2000). How these circadian/SCN effects of cocaine relate to its effects on the reward system are not clear. Circadian clock regulation of mesolimbic neuronal activity (McClung, 2008;Hampp et al., 2008;Baltazar et al., 2013) may contribute to circadian rhythms in cocaine use and reward properties, but this hypothesis has not yet been tested directly, and the neurochemistry of these effects is not clear. The prevailing view is that the rewarding properties of cocaine occur through increased DA signaling (e.g., (Ron and Jurd, 2005)). However, recent studies suggest that increases in 5-HT (Nonkes et al., 2011) and norepinephrine (Sofuoglu and Sewell, 2008) signaling may also participate, particularly in cue saliency (Liu et al., 2013;Cunningham and Anastasio, 2014). The fact that the SERT Met172 mice drank less cocaine in the forced consumption paradigm used here is consistent with either decreased reward or decreased palatability, but this would need more extensive investigation.
A second question is whether cocaine can also modulate circadian rhythms through non-serotonergic processes. Our previous data suggest that, in addition to its actions in the SCN, cocaine is also able to alter circadian rhythms through extra-SCN actions. We have shown that both the photic and non-photic effects of systemically administered cocaine on circadian rhythms are potentiated in Per2 mutant mice (Brager et al., 2013). However, the enhanced non-photic responsiveness in these mice is not seen when cocaine is administered directly into the SCN via reverse microdialysis (Brager et al., 2013). Thus, at least with respect to its interactions with the Per2 gene, cocaine can influence circadian behavioral rhythms through distinct intra- and extra-SCN mechanisms. Whether this regional difference in action also holds for other circadian clock genes that have been linked to cocaine actions, including Clock and Per1 (Abarca et al., 2002;McClung et al., 2005;Spencer et al., 2012;Falcon et al., 2013), remains to be determined.
A third important issue is how the effects of cocaine shown here and in previous studies (Glass et al., 2012;Brager et al., 2013) relate to long-term disruptions in circadian rhythms that occur during chronic cocaine use. The cocaine-induced phase advances, inhibition of photic-like phase delays, and altered free-running period are all consistent with cocaine-induced disruptions of circadian clock genes, as shown in other studies (Yuferov et al., 2005;Falcon et al., 2013). Notably, chronic intake of cocaine is associated with long-term alterations in circadian-related processes, including free-running period (present results), clock gene expression discussed above (Ahmed et al., 2005;Manev and Uz, 2006;Uz et al., 2005;Yuferov et al., 2003), central neurotransmitter release and binding (Zeigler et al., 1991), hormone secretion (Vescovi et al., 1992) and wheel-running behavior (Asami et al., 1996), as well as deficits in learning, cognition (Calu et al., 2007;Zeigler et al., 1991) and sleep architecture (Zeigler et al., 1991). In particular, the neurochemical basis for changes in these functions is unknown, but could involve disruptions in circadian clock genes caused by perturbed serotonin signaling in the SCN. The serotoninergic and circadian systems are reciprocally linked anatomically and genetically: key clock genes are expressed in 5-HT neurons, and the SCN clock receives substantial serotonergic input (Ciarleglio et al., 2011). Thus, long-term cocaine-induced changes in 5-HT signaling theoretically could promote prolonged changes in circadian processes. It will be important to determine the extent to which SERT Met172 mice exhibit any of the drug-induced disruptions listed above, and thus support a role for 5-HT (if any) in their etiology. These issues are of fundamental importance when considering the degree to which behavioral, physiological, and clock gene disruptions like those presented and discussed here contribute to the processes underlying cocaine addiction (Abarca et al., 2002;Hasler et al., 2012;McClung et al., 2005;Perreau-Lenz et al., 2007).
Highlights.
Cocaine in the day and night alters circadian clock phase regulation in vivo and in vitro.
Pharmacological experiments suggest this involves increased serotonergic signaling.
Here we used transgenic mice expressing a serotonin (5-HT) transporter (SERT) with reduced cocaine potency (SERT Met172).
The mice have normal circadian rhythms and phase shifting to light and glutamate but the clock does not respond to cocaine.
Thus, cocaine modulates the circadian clock by SERT-associated increases in 5-HT signaling in the suprachiasmatic nucleus.
Abbreviations
- 5-HT
Serotonin
- CT
Circadian time
- DA
Dopamine
- DD
Constant dark
- LD
Light-dark
- SCN
Suprachiasmatic nucleus
- SERT
Serotonin transporter
- ZT
Zeitgeber time
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Abarca C, Albrecht U, Spanagel R. Cocaine sensitization and reward are under the influence of circadian genes and rhythm. PNAS. 2002;99:9026–9030. doi: 10.1073/pnas.142039099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed SH, Lutiens R, van der Stap LD, Ledic D, Romano-Spica V, Morales M, Koob GF, Repunte-Canonigo V, Sanna PP. Gene expression evidence for remodeling of lateral hypothalamic pituitary circuitry in cocaine addiction. PNAS. 2005;102:11533–11538. doi: 10.1073/pnas.0504438102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asami T, Shinoda M, Kuribara H, Ida I, Mikuni M, Machiyama Y, Yukio I. Circadian variation in the ambulatory and drinking activities after repeated administration of cocaine in rats. Eur Neuropsychopharm. 1996;6:103. [Google Scholar]
- Aschoff J. Response curves in circadian periodicity. In: Aschoff J, editor. Circadian clocks. North-Holland; Amsterdam: 1965. pp. 95–111. [Google Scholar]
- Baird TJ, Gauvin DV. Characterization of cocaine self-administration and pharmacokinetics as a function of time of day in the rat. Pharmacol Biochem Beh. 2000;65:289–299. doi: 10.1016/s0091-3057(99)00207-5. [DOI] [PubMed] [Google Scholar]
- Baltazar RM, Coolen LM, Webb IC. Diurnal rhythms in neural activation in he mesolimbic award system: critical role of the medial prefrontal cortex. Eur J Neurosci. 2013;38:2319–2327. doi: 10.1111/ejn.12224. [DOI] [PubMed] [Google Scholar]
- Brager AJ, Stowie AC, Prosser RA, Glass JD. The mPer2 clock gene modulates cocaine actions in the mouse circadian system. Beh Brain Res. 2013;243:255–260. doi: 10.1016/j.bbr.2013.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calu DJ, Stalnaker TA, Franz TM, Singh T, Shaham Y, Schoenbaum G. Withdrawal from cocaine self-administration produces long-lasting deficits in orbitofrontal-dependent reversal learning in rats. Learn Mem. 2007;14:325–328. doi: 10.1101/lm.534807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciarleglio CM, Resuehr HES, McMahon DG. Interactions of the serotonin an circadian systems: nature and nurture in rhythms and blues. Neuroscience. 2011;197:8–16. doi: 10.1016/j.neuroscience.2011.09.036. [DOI] [PubMed] [Google Scholar]
- Cunningham KA, Anastasio NC. Serotonin at the nexus of impulsivity and cue reactivity in cocaine addiction. Neuropharmacology. 2014;76:460–478. doi: 10.1016/j.neuropharm.2013.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudley TE, DiNardo LA, Glass JD. Endogenous regulation of serotonin release in the hamster suprachiasmatic nucleus. J Neurosci. 1998;18:5045–5052. doi: 10.1523/JNEUROSCI.18-13-05045.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falcon E, Ozburn A, Mukherjee S, Roybal K, McClung CA. Differential regulation of the Period gene in striatal regions following cocaine exposure. PLOS One. 2013;8:e66438. doi: 10.1371/journal.pone.0066438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falk JL, Siris A, Lau CE. Conditions sufficient for the production of oral cocaine or lidocaine self-administration in preference to water. Drug Alcohol Dep. 1996;40:241–247. doi: 10.1016/0376-8716(96)01220-3. [DOI] [PubMed] [Google Scholar]
- Gabriele A, Setlow B, Packard MG. Cocaine self-administration alters the relative effectiveness of multiple memory systems during extinction. Learn Mem. 2009;16:296–299. doi: 10.1101/lm.1253409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass JD, Brager AJ, Stowie AC, Prosser RA. Cocaine modulates pathways for photic and non-photic entrainment of the mammalian SCN circadian clock. Am J Physiol. 2012;302:R740–R750. doi: 10.1152/ajpregu.00602.2011. [DOI] [PubMed] [Google Scholar]
- Grossman GH, Mistlberger RE, Antle MC, Ehlen JC, Glass JD. Sleep deprivation stimulates serotonin release in the suprachiasmatic nucleus. NeuroReport. 2000;11:1929–1932. doi: 10.1097/00001756-200006260-00024. [DOI] [PubMed] [Google Scholar]
- Hampp G, Ripperger JA, Houben T, Schmutz I, Blex C, Perreau-Lenz S, Brunk I, Spanagel R, Ahnert-Hilger G, Meijer JH, Albrecht U. Regulation of monoamine oxidase A by circadian-clock components implies clock influence on mood. Curr Biol. 2008;18:678–683. doi: 10.1016/j.cub.2008.04.012. [DOI] [PubMed] [Google Scholar]
- Hasler BP, Smith LJ, Cousins JC, Bootzin RR. Circadian rhythms, sleep, and substance abuse. Sleep Med Rev. 2012;16:67–81. doi: 10.1016/j.smrv.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatton GI, Doran AD, Salm AK, Tweedle CD. Brain slice preparation: Hypothalamus. Brain Res Bull. 1980;5:405–414. doi: 10.1016/s0361-9230(80)80010-4. [DOI] [PubMed] [Google Scholar]
- Liu S, Lane SD, Schmitz JM, Cunningham KA, John VP, Moeller FG. Effects of escitalopram on attentional bias to cocaine-related stimuli and inhibitory control in cocaine-dependent subjects. J Psychopharmacol. 2013;27:801–807. doi: 10.1177/0269881113492898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manev H, Uz T. Clock genes: influencing and being influenced by psychoactive drugs. TIPS. 2006;27:186–189. doi: 10.1016/j.tips.2006.02.003. [DOI] [PubMed] [Google Scholar]
- McClung CA. Circadian rhythms, the mesolimbic dopamine circuit, and drug addiction. The Scient World Jour. 2008;7:202. doi: 10.1100/tsw.2007.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClung CA, Sidiropoulou K, Vitaterna M, Takahashi JS, White FJ, Cooper DC, Nestler EJ. Regulation of dopaminergic transmission and cocaine reward by the Clock gene. PNAS. 2005;102:9377–9381. doi: 10.1073/pnas.0503584102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer-Bernstein EL, Blanchard JH, Morin LP. The serotonergic projection from the median raphe nucleus to the suprachiasmatic nucleus modulates activity phase onset, but not other circadian rhythm parameters. Brain Res. 1997;755:112–120. doi: 10.1016/s0006-8993(97)00111-x. [DOI] [PubMed] [Google Scholar]
- Morgan PT, Pace-Schott EF, Sahul ZH, Coric V, Stickgold R, Malison RT. Sleep, sleep-dependent procedural learning and vigilance in chronic cocaine users: evidence for occult insomnia. Drug Alcohol Dep. 2006;82:238–249. doi: 10.1016/j.drugalcdep.2005.09.014. [DOI] [PubMed] [Google Scholar]
- Murray JB, Al-Shora HI. Stability of cocaine in aqueous solution. J Clin Pharm Therap. 1978;3:1–6. [Google Scholar]
- Nonkes LJP, van Bussel IPG, Verheij MMM, Homberg JR. The interplay between brain 5-hydroxytryptamine levels and cocaine addiction. Beh Pharmacol. 2011;22:723–738. doi: 10.1097/FBP.0b013e32834d6260. [DOI] [PubMed] [Google Scholar]
- Perreau-Lenz S, Zhgoul T, Spanagel R. Clock genes running amok. EMBO reports. 2007;8:S20–S23. doi: 10.1038/sj.embor.7401016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prosser RA. Serotonin phase-shifts the mouse suprachiasmatic circadian clock in vitro. Brain Res. 2003;966:110–115. doi: 10.1016/s0006-8993(02)04206-3. [DOI] [PubMed] [Google Scholar]
- Prosser RA, Gillette MU. The mammalian circadian clock in the suprachiasmatic nuclei is reset in vitro by cAMP. J Neurosci. 1989;9:1073–1081. doi: 10.1523/JNEUROSCI.09-03-01073.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prosser RA, Lee H-M, Wehner A. Serotonergic pre-treatments block in vitro serotonergic phase shifts of the mouse suprachiasmatic nucleus circadian clock. Neuroscience. 2006;142:547–555. doi: 10.1016/j.neuroscience.2006.06.014. [DOI] [PubMed] [Google Scholar]
- Prosser RA, Miller JD, Heller HC. A K+ channel mediates serotonergic- and cAMP-induced phase advances of the suprachiasmatic circadian clock. Soc Neurosci Abst. 1993;19:1817. [Google Scholar]
- Rea MA, Glass JD, Colwell CS. Serotonin modulates photic responses in the hamster suprachiasmatic nuclei. J Neurosci. 1994;14:3635–3642. doi: 10.1523/JNEUROSCI.14-06-03635.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rea MA, Pickard GE. Serotonergic modulation of photic entrainment in the Syrian hamster. Biol Rhythms Res. 2000;31:284–314. [Google Scholar]
- Roberts D, Brebner K, Vincler M, Lynch WJ. Patterns of cocaine self-administration in rats produced by various access conditions under a discrete trials procedure. Drug Alcohol Dep. 2002;67:291–299. doi: 10.1016/s0376-8716(02)00083-2. [DOI] [PubMed] [Google Scholar]
- Ron D, Jurd R. The “ups and downs” of signaling cascades in addiction. Science's stke 309:re14. 2005 doi: 10.1126/stke.3092005re14. [DOI] [PubMed] [Google Scholar]
- Sofuoglu M, Sewell RA. Norepinephrine and stimulant addiction. Addict Biol. 2008;14:119–129. doi: 10.1111/j.1369-1600.2008.00138.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer S, Torres-Altoro MI, Falcon E, Arey R, Marvin M, Goldberg M, Bibb JA, McClung CA. A mutation in CLOCK leads to altered dopamine receptor function. J Neurochem. 2012;123:124–134. doi: 10.1111/j.1471-4159.2012.07857.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stowie AC, Amicarelli MJ, Prosser RA, Glass JD. Effects of chronic oral cocaine self-administration and withdrawal on circadian regulation. Soc Neurosci Abst. 2012;94 [Google Scholar]
- Thompson BJ, Jessen T, Henry LK, Field JR, Gamble KL, Gresch PJ, Carneiro AM, Horton RE, Chisnell PJ, Belova Y, McMahon DG, Daws LC, Blakely RD. Transgenic elimination of high-affinity antidepressant and cocaine sensitivity in the presynaptic serotonin transporter. PNAS. 2011;108:3785–3790. doi: 10.1073/pnas.1011920108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tornatzky W, Miczek KA. Repeated limited access to IV cocaine self-administration: conditioned autonomic rhythmicity illustrating “predictive homeostasis”. Psychopharmacology. 1999;145:144–152. doi: 10.1007/s002130051043. [DOI] [PubMed] [Google Scholar]
- Uchimura N, North RA. Actions of cocaine on rat nucleus accumbens neurones in vitro. Br J Pharmacol. 1990;99:736–740. doi: 10.1111/j.1476-5381.1990.tb12999.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uz T, Ahmed R, Akhisaroglu M, Kurtuncu M, Imbesi M, Arslan A, Manev H. Effect of fluoxetine and cocaine on the expression of clock genes in the mouse hippocampus and striatum. Neuroscience. 2005;134:1309–1316. doi: 10.1016/j.neuroscience.2005.05.003. [DOI] [PubMed] [Google Scholar]
- Vescovi PP, Coiro V, Volpi R, Passeri M. Diurnal variations in plasma ACTH, cortisol and beta-endorphin levels in cocaine addicts. Horm Res. 1992;37:221–224. doi: 10.1159/000182316. [DOI] [PubMed] [Google Scholar]
- Weaver DR, Rivkees SA, Reppert SM. D1-dopamine receptors activate c-fos expression in the fetal suprachiasmatic nuclei. Proc Natl Acad Sci. 1992;89:9201–9204. doi: 10.1073/pnas.89.19.9201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye R, Blakely RD. Natural and engineered coding variation in antidepressant-sensitive serotonin transporters. Neuroscience. 2011;197:28–36. doi: 10.1016/j.neuroscience.2011.08.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuferov V, Butelman ER, Kreek MJ. Biological clocks may modulate drug addiction. Eur J Human Gen. 2005;13:1101–1103. doi: 10.1038/sj.ejhg.5201483. [DOI] [PubMed] [Google Scholar]
- Yuferov V, Kroslak T, Laforge KS, Zhou Y, Ho A, Kreek MJ. Differential gene expression in the rat caudate putamen after “binge” cocaine administration: advantages of triplicate microarray analysis. Synapse. 2003;48:157–169. doi: 10.1002/syn.10198. [DOI] [PubMed] [Google Scholar]
- Zeigler S, Lipton J, Toga A, Ellison G. Continuous cocaine administration produces persisting changes in brain neurochemistry and behavior. Brain Res. 1991;552:27–35. doi: 10.1016/0006-8993(91)90655-f. [DOI] [PubMed] [Google Scholar]