Abstract
Upper airway viral infection in patients with airway allergy often exacerbates olfactory dysfunction, but the mechanism for this exacerbation remains unclear. Here, we examined the effects of respiratory syncytial virus (RSV) infection, in the presence or absence of airway allergy, on olfactory receptor neurons (ORNs) and their progenitors in mice. Immunohistological analyses revealed that cockroach allergen (CRA)-induced airway allergy alone did not affect the number of OMP+ mature ORNs and SOX2+ ORN progenitors. Intranasal RSV line 19 infection in allergy-free mice resulted in a transient decrease in SOX2+ ORN progenitors without affecting OMP+ ORNs. In contrast, the RSV-induced decrease in SOX2+ ORN progenitors was exacerbated and prolonged in allergic mice, which resulted in eventual loss of OMP+ ORNs. In the allergic mice, reduction of RSV in the olfactory epithelium was delayed as compared with allergy-free mice. These results suggest that ORN progenitors were impaired by RSV infection and that airway allergy exacerbated damage to ORN progenitors by reducing viral clearance.
Keywords: respiratory syncytial virus, airway allergy, olfactory dysfunction, olfactory receptor neuron, SOX2
1. Introduction
Olfaction is a sensor for environmental chemicals that facilitates the identification of foods as well as environmental danger signals. Olfaction is mediated by the olfactory system, which is composed of the olfactory receptor neurons (ORNs) in the nasal cavity and the olfactory bulb (OB) in the forebrain. ORNs sense odor molecules and transmit the odor information to olfactory bulbs passing through the cribriform plate [1].
ORNs are unique among adult mammalian peripheral nervous system in that they possess regenerative potential through the olfactory epithelial stem cell system [2]. Two cell types in the basal layer of olfactory epithelia (OE), globose basal cells (GBCs) and horizontal basal cells (HBCs), act as ORN stem cells/progenitors and give rise to mature ORNs expressing olfactory marker protein (OMP), which is exclusively expressed in mature ORNs and modulates olfactory signal transduction [3]. Accumulating evidences suggest that GBCs are actively proliferating stem cells/multi-potent progenitors and increase their proliferation potential upon OE damage [4], whereas HBCs are quiescent stem cells and contribute to OE regeneration only after severe OE damage [5, 6]. SOX2 is a transcription factor that is widely expressed in stem cell populations including neural stem cells and plays a role in maintaining their undifferentiated state [7]. In the OE, SOX2 is expressed by proliferating stem cells or progenitor cells and regulates the homeostasis of OE [2, 8–10].
Olfactory dysfunction affects roughly 1–2 percent of individuals in North America, especially in elderly populations, and impairs their mental health and quality of life [11–13]. Olfactory dysfunction is associated with the damage to ORNs and/or OB from a variety of causes, such as airway allergy, upper-airway virus infections such as rhinovirus, influenza viruses and respiratory syncytial viruses (RSVs), head trauma, and neurodegenerative diseases. Among these, severe upper-airway virus infections are the most common cause of prolonged olfactory dysfunction, which appears to occur from the continuous or irreversible damage to ORNs and possibly to ORN stem cells/ progenitors or microenvironment required to support ORN regeneration[11, 14]. Interestingly, airway allergy and upper airway infections are closely associated with each other; acute RSV infection during infancy is a risk factor of allergic asthma, which is strongly associated with airway allergy and exacerbates the severity of RSV infection in the respiratory mucosa[15–17]. Also, patients with asthma and allergic rhinitis are often troubled by severe exacerbation of their smell disorder and more prolonged nasal symptoms after upper airway infection, compared with patients without allergic condition. Despite extensive research efforts in understanding the immunological mechanisms of RSV exacerbation of allergic responses, the impact of RSV infection in the presence or absence of airway allergy on ORNs and their progenitors remains elusive.
In this study, we explored the effects of RSV infection with or without airway allergy on ORNs and ORN progenitors using a mouse model of allergic airway disease and RSV infection. We found that RSV infection impairs the ORN progenitor SOX2+ basal cells and the association of RSV with airway allergy exacerbates the pathology possibly due to the delay in RSV clearance in allergic mice.
2. Methods
2.1. Mice
Female BALB/c mice, 6 to 8 weeks old, were purchased from the Jackson Laboratory (Bar Harbor, ME). All mice were maintained in specific-pathogen-free facilities in the Unit for Laboratory Animal Medicine at the University of Michigan. The University Committee of Use and Care of Animals (UCUCA), University of Michigan, Ann Arbor, approved all animal experimental protocols, and experiments were conducted according to the guidelines provided by the UCUCA review committee.
2.2. Airway allergy model and respiratory syncytial virus infection
Airway allergy and RSV infection were performed as described previously, with some modifications[18]. In brief, mice were simultaneously immunized both subcutaneously and intraperitoneally with 200 ul of a 1:1 mixture of clinical-grade CRA (Hollister-Stier Laboratories, Spokane, WA) in incomplete Freund’s adjuvant (Sigma-Aldrich) on day −22. The mice next received CRA intranasally three times on day −8, −4 and 0 to induce asthma and allergic inflammation (allergic mice). Both naïve and allergic mice were infected intranasally with 1×105 plaque-forming units (pfu) of RSV strain line 19 [19, 20] on day 0 and sacrificed on days 2, 4, 7 and 14 post-infection.
2.3. Tissue preparation
Septal nasal mucosa and olfactory bulbs were harvested on days 2, 4, 7 and 14 for histological and RT-PCR analyses. In brief, immediately after euthanasia, nasal cavities were gently irrigated with 4% paraformaldehyde in order to minimize any mechanical damage to the olfactory neuroepithelium. Mandibles were discarded, trimmed heads were skinned and further fixed in 4% paraformaldehyde for 24 hrs followed by decalcification using Decalcifying Solution B (Wako Pure Chemical Industries, Ltd, Japan) for 7 days. After decalcification, specimens were embedded in paraffin or in Optimal Cutting Temperature compound for frozen sectioning.
2.4. Histological analyses
All samples were cut at the level of the anterior end of the olfactory bulb. Four μm-thick paraffin sections were deparaffinized in xylene and rehydrated in alcohol before immunostaining. Deparaffinized sections or six μm-thick frozen sections were treated with 3% H202 to block endogenous peroxidase and then incubated in Blocking One (Nacalai Tesque) to block non-specific immunoglobulin binding. Primary antibodies against mouse SOX2 (rabbit monoclonal, EPR3131, Abcam), Cytokeratin 5 (CK5, rabbit monoclonal EP1601Y, Abcam), and OMP (Goat polyclonal, Wako) were detected with peroxidase conjugated appropriate secondary antibodies and a diaminobenzidine (DAB) substrate. The numbers of OMP+ ORNs was analyzed quantitatively on these histologic sections. To control for variation between specimens, analyses were restricted to the olfactory neuroepithelium. Three different microscopic fields (dorsal, middle, and ventral) of the bilateral septal olfactory neuroepithelium were randomly captured by a digital microscope camera (AxioCam, Carl Zeiss) with a 40x objective lens. The width of each field was 350 um. The number of counted cells in each of the three microscopic fields was averaged across the sections. The number of SOX2+ ORN progenitors and CK5+ quiescent stem cells per mm basal layer length were counted manually on three different fields (dorsal, middle and ventral) per mouse using digital imaging software (CellSens Dimension, Olympus). Results are presented as mean ± SD for each time point.
2.5. Enzyme-linked immunosorbent assay (ELISA)
Sera from allergic and allergy-free mice were prepared and 500-fold serum dilutions were tested for IgE to confirm the presence of allergic responses before RSV infection using a mouse IgE ELISA MAX Deluxe kit (Biolegend), according to the manufacturer’s instructions.
2.6. RT-PCR
Total RNA was isolated from septal nasal mucosa and olfactory bulbs using TRIzol reagents (Life Technologies) at days 0, 2, 4, 7 and 14 after RSV infection and reverse-transcribed with iScript Reverse Transcription Supermix for RT-qPCR (Bio-Rad). Real-time PCR was performed on 7500 Real-Time PCR System (Applied Biosystems) using a TaqMan probes or SYBR green (both from Life Technologies). We employed two RSV-derived genes for quantification of RSV burden, RSV-G that encodes glycoprotein mediating attachment to target cells and RSV-F that encodes protein essential for fusion of virus particle and target cells. Gene specific primes and probes used were: 18sRNA as endogenous control (Life technologies); RSV-G (forward 5′-CCAAGCAAACCCAATAATGATTT-3′, reverse 5′ GCCCAGCAGGTTGGATTGT-3′ and probe 5′-CTTTGAAGTGTTCAACTTTGTACCCTGCAT-3′); RSV-F (forward 5′-AATGATATGCCTATAACAAATGATCAGAA-3′ and reverse 5′-TGGACATGATAGAGTAACTTTGCTGTCT-3′). The quantity of target mRNA in each sample was normalized relative to the respective 18sRNA measurement. Results are presented as mean ± SD for each time point, and are representative of two independent experiments.
2.7. Statistical Analysis
Statistical differences among groups or time-points were determined with one-way ANOVA for kinetic studies, or Mann-Whitney u-test for ELISA, using GraphPad Prism software (version 6.0; GraphPad Software, Inc.). Logarithmic transformations were performed to obtain normality of the RT-PCR data. p < 0.05 was considered to be statistically significant.
3. Results
3.1. RSV infection impairs the olfactory receptor system
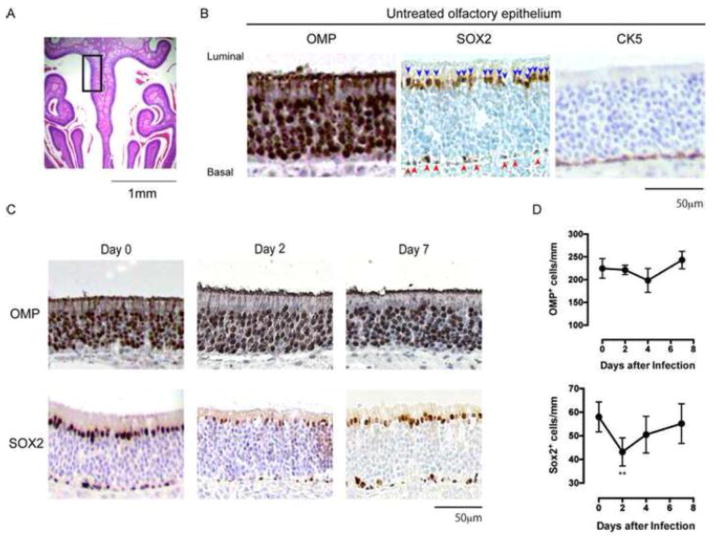
Olfactory receptor neurons (ORNs) are continuously replenished from progenitors/stem cells in the basal layer[2]. To explore the effects of RSV-infection on the ORNs and their progenitors/stem cells, BALB/c mice were infected intranasally with RSV line 19 and the nose tissues were collected for immunohistological analyses at days 2, 4 and 7. Mature ORNs were identified as olfactory marker protein-positive (OMP+) cells. ORN progenitors and quiescent stem cells were identified as SOX2+ cells and cytokeratin 5-positive (CK5+) cells, respectively, in the basal layer of the OE (Figure 1A and B). Time course analyses revealed that the number of OMP+ ORNs were unchanged by day 7 after RSV infection, whereas those of SOX2+ ORN progenitors in the basal layer significantly decreased to about 75% of those in untreated mice on day 2 (Figure 1C and D). SOX2 expression was also found in sustentacular cells in the luminal layer, and its expression level was decreased on day 2 after RSV infection, although the biological significance of this observation remains unclear. The mean number of SOX2+ ORN progenitors in RSV infected mice returned to steady-state levels by day 7 (Figure 1C and D). There were no obvious changes in the number of CK5+ quiescent stem cells over the duration of the experiment (Figure 1B and data not shown). Given that OMP+ ORNs are replenished by SOX2+ ORN progenitors, these data suggest that RSV disrupts OE by targeting and impairing SOX2+ ORN progenitors.
Figure 1.
Histogical changes in the olfactory epithelium following RSV infection. A: Olfactory epithelium in the indicated gated area was analyzed for the immunohistological identification of OMP+ ORNs, SOX2+ ORN progenitors and CK5+ quiescent stem cells. B: Representative images of the olfactory epithelium in untreated mice. Brown shows OMP or SOX2 or CK5 positive cells in the indicated panels. Blue shows nuclear counterstain with hematoxylin. Arrow head: ORN progenitors in basal layer. C: Mice were infected with 1×105 pfu of RSV line 19 and the olfactory epithelium was analyzed for OMP+ ORNs and SOX2+ ORN progenitors on the days indicated. Representative images from two independent experiments. D: Kinetics of the numbers of OMP+ ORNs and SOX2+ ORN progenitors/mm basal layer after RSV infection. Data represent means ± SEM (n = 6) and are pooled from two independent experiments. **, P = 0.0052 (vs day 0, one-way ANOVA)
3.2. Airway allergy exacerbates the RSV-induced damage to ORN progenitors
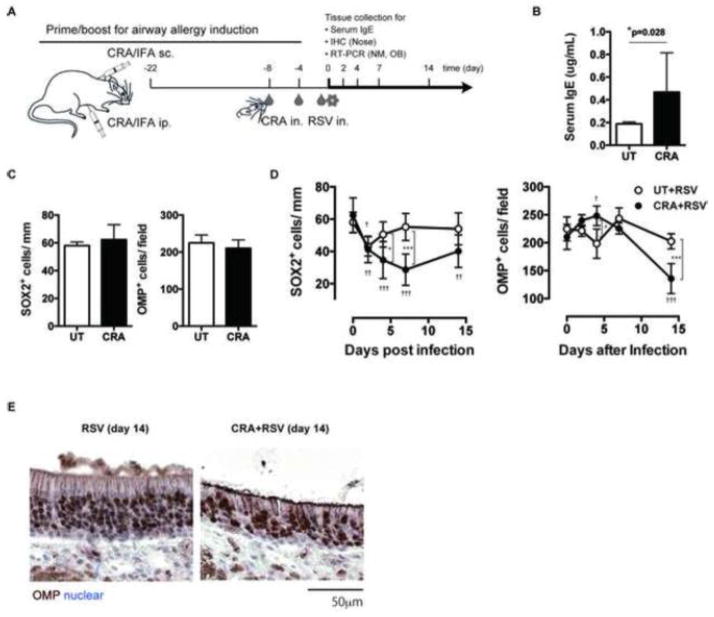
Since RSV infection often becomes severe in patients and mice with airway allergy, we next examined RSV-induced damage to ORN progenitors in mice with or without airway allergy. A systemic priming with subsequent nasal boosting protocol was used to induce airway allergy to CRA (Figure 2A), and the magnitude of the allergic response was evaluated by measuring IgE serum levels. The CRA-immunized group showed significantly higher IgE serum levels as compared to those in the unimmunized group, suggesting the successful induction of an allergic response (Figure 2B). The CRA-immunized mice and unimmunized control mice were then infected intranasally with RSV and the nasal tissue was analyzed for SOX2+ ORN progenitors and OMP+ ORNs at days 0, 2, 4, 7 and 14 post-infection. The results showed that before RSV infection, the numbers of SOX2+ ORN progenitors and OMP+ ORNs in allergic mice were equivalent to those found in the allergy-free group (Figure 2C). When RSV-infected allergic (CRA+RSV) and allergy-free (UT+RSV) groups were compared, the results demonstrated that although the numbers of SOX2+ ORN progenitors in both groups similarly decreased at day 2 after RSV-infection, the reduction became more severe at day 4 and was prolonged to day 14 in allergic mice (Figure 2D). The numbers of OMP+ ORNs were stable by day 7 in both groups, however, in RSV-infected allergic mice OMP+ ORN numbers were significantly decreased at day 14 after infection (Figure 2D and E). These data suggest that RSV-induced damage to ORN progenitors is exacerbated and prolonged in the presence of airway allergy that eventually results in the loss of ORNs.
Figure 2.
Airway allergy exacerbates RSV-induced damage to ORN progenitors. A: Mice were immunized subcutaneously (s.c.) and intraperitoneally (i.p.) with cockroach antigen (CRA) emulsified in incomplete Freund’s adjuvant (IFA) on day −22, then administrated intranasally (i.n.) with CRA every 4 days from day −8 to day 0 to induce airway allergy. Allergic- (CRA) and allergy-free mice were infected intranasally with 1×105 pfu of RSV line 19 on day 0 and the tissue samples were collected for time-course analyses on the days indicated. B: Serum IgE levels of untreated (UT) and allergic mice were determined by ELISA. Data represents means ± SEM (n = 9 and 34) and are pooled from seven independent experiments. *, p=0.028 (Mann-Whitney u-test). C: Nose sections from untreated and allergic mice were analyzed for the OMP+ ORNs and SOX2+ ORN progenitors in olfactory epithelium. Data represent means ± SEM (n = 4–6) from two independent experiments. D: Allergy free- (RSV) and allergic-mice (CRA+RSV) were infected with RSV on day 0 and the numbers of OMP+ ORNs and SOX2+ ORN progenitors in the olfactory epithelium were quantified in nose sections on the days indicated. Data represent means ± SEM (n = 4–6) and are pooled from two independent experiments. *, p < 0.05; **, p<0.01; ***, p<0.001(RSV vs CRA+RSV, by one-way ANOVA); †, p<0.05; ††, p ≤ 0.01; †††, p<0.001 (vs day 0, by one-way ANOVA). E: Representative images of day 14 sections. Brown; OMP+ORNs, Blue; nuclear counterstain.
3.3. Airway allergy delays RSV clearance in the nasal mucosa and olfactory bulbs
Finally, we examined the mechanisms of how airway allergy exacerbates the ORN progenitor damage induced by RSV-infection. Since the Th2 dominant allergic microenvironment often inhibits the induction of Th1- and Tc1- T cells required for optimal virus clearance, we hypothesized that RSV clearance is impaired in the nasal tissue of these allergic mice. To address our hypothesis, nasal mucosa and OB were collected from RSV-infected mice with or without airway allergy, and RT-PCR was performed with gene specific primers to quantify RSV related structural proteins RSV-G and RSV-F. Relative expression of RSV-G and RSV-F mRNA rapidly decreased and became almost undetectable by day 14 in the nasal mucosa of allergy-free mice following intranasal RSV-infection, whereas RSV-infected allergic mice had high levels of RSV-G at day 14 compared to RSV-infected allergy-free mice (Figure 3). Expression levels of RSV-G and -F in OB also appeared higher (RSV-G but not RSV-F reached statistical significance) in RSV-infected allergic mice when compared to allergy-free mice at day 14 post-infection, and the relative expression of RSV-G and RSV-F in the OB were 10–1000 times lower than those found in nasal mucosa of the same group (Figure 3). Taken together, these results suggest that airway allergy inhibits RSV clearance in nasal mucosa and OB.
Figure 3.
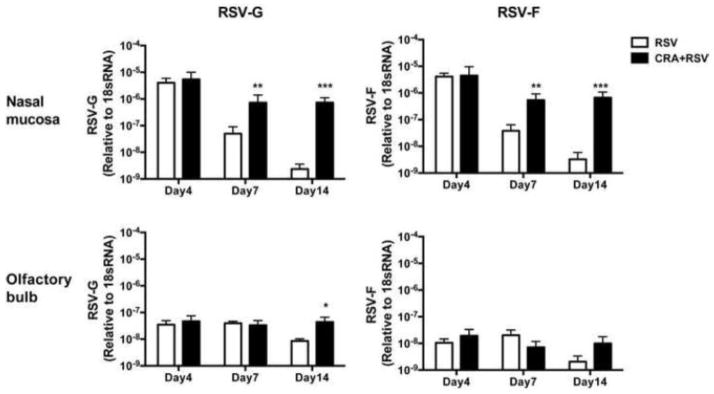
Airway allergy delays RSV clearance in the nasal mucosa and olfactory bulb.
Allergy free- (RSV) and allergic- (CRA+RSV) mice were infected with RSV on day 0 and the RSV proteins in the nasal mucosa (A) and olfactory bulbs (B) were quantified by RT-PCR with gene specific primers for RSV-G and RSV-F on the days indicated. Data represent means ± SEM (n = 3–4), and are representative of two independent experiments. *, p < 0.05; **, p<0.01; ***, p<0.001 (by one-way ANOVA).
4. Discussion
In the present study, we demonstrate for the first time that SOX2+ ORN progenitors are damaged a few days after RSV infection, before the manifestation of ORN impairment. The damage to ORN progenitor cells became severe and was prolonged in the presence of airway allergy, and was associated with defective virus clearance from the nasal mucosa and OB. Our results suggest that RSV impairs olfaction by upstream disruption of ORN development and that airway allergy exacerbates RSV-induced olfactory dysfunction by inhibiting virus clearance.
OE contains mature ORNs and their stem cells/progenitors, which constitute the regenerative peripheral nerve system [2]. Due to the technical difficulties in accessing OE within hard nasal cavities, the effects of RSV infection and airway allergy on this unique nerve system have remained elusive. However, our mouse models of RSV infection and airway allergy enabled us to dissect the kinetics of ORN and ORN progenitors numbers after RSV infection, and revealed that ORN progenitors are the primary targets of RSV. Nonetheless, it remains to be determined whether damage to ORN progenitors is responsible for the virus-induced olfactory dysfunction found in patients, since we could not quantitatively evaluate the olfaction of the mice with or without ORN progenitor impairments. Considering that some viruses directly invade the central nervous system and that cytopathic damage or virus-induced inflammation could also affect olfaction [21], damage to ORN progenitors alone might not be responsible for olfactory dysfunction. In fact, we detected a low level of RSV-derived mRNA in OB of RSV-infected mice, which is consistent with a recent report by Espinoza et al. [22]. Future neurophysiological studies that quantify the signal transduction from ORN to OB, as well as behavior tests to evaluate olfaction, will reveal the pathophysiological significance of RSV-induced ORN progenitor impairments. In addition, virus strains, host species, pre-existing inflammation and immunological memory to the virus could also affect virus tropism and the nature of the inflammatory response. Thus, establishment of a clinically available detection method to evaluate damage to ORNs and ORN progenitors is required in order to investigate the impact of virus infection on these cells in patients with olfactory dysfunction.
In our model, reduction of SOX2+ ORN progenitors manifested as early as day 2 after RSV infection independently of airway allergy. A possible mechanism of this reduction is the direct effects of the RSV on ORN progenitors or on undefined olfactory niche cells involved in the maintenance of ORN progenitors/stem cells. Despite its tropism for epithelial cells, Li et al. have demonstrated that RSV could directly infect nerve cells in the lung [23]. Thus, RSV might directly infect and impair ORNs or ORN progenitors in OE. Since RSV is primarily a low cytopathic virus [17], growth inhibition or functional modification rather than cytopathic damage to infected cells are likely to effect the reduction of SOX2+ ORN progenitors.
Alternatively, it is also possible that RSV impairs SOX2+ ORN progenitors through RSV-induced inflammation and immune responses, since the pathogenesis of RSV is largely mediated by inflammation and immunotoxicity in lungs [17]. We have previously demonstrated that the line 19 stain of RSV used in the current study induces expression of IL-6 [18], an inflammatory cytokine that suppress OE regeneration following injury [24]. Since the loss of SOX2+ ORN progenitors by RSV infection occurs as early as day 2, and virus specific immune responses require several days before effector T cells are seen at the site of infection, it is unlikely that cytokines produced by effector T cells are responsible for the damage to SOX2+ ORN progenitors. However, the possibility remains that effector T cell-derived cytokines synergistically disrupt ORN progenitors homeostasis.
RSV-induced reduction of SOX2+ ORN progenitors became severe and was prolonged in the presence of airway allergy. Since airway allergy itself did not affect the number of SOX2+ ORN progenitors, airway allergy likely amplified the effects of RSV infection in our model. In fact, RSV gene transcripts remained significantly higher in allergic mice when compared to non-allergic mice, indicating that the pathological action of RSV persisted longer in the allergic mice. The delay in RSV clearance in allergic mice might be due to suppression of anti-viral pro-inflammatory Th1 response under the Th2 dominant microenvironment in allergic mice. However, there have been contradictory reports on the effects of allergy on the interferon gamma response (IFNγ). Although IFNγ responses were suppressed in a rat allergy model induced by Aspergillus fumigatus [25, 26], no changes in IFNγ levels were observed in a mouse allergy model induced by CRA [25, 26]. This discrepancy might be due to a difference in the antigens used to induce allergy or viruses and host species. Further studies are required to identify the molecules and cells involved in defective virus clearance from nasal mucosa in an allergic microenvironment.
In conclusion, we show for the first time that SOX2+ ORN progenitors are damaged by RSV infection. The damage to ORN progenitor cells became severe and was prolonged in the presence of airway allergy, with defects in virus clearance from the nasal mucosa and OB. The pathophysiological and clinical significance of these findings in virus-induced olfactory dysfunctions, and the mechanisms underlying the damage to ORN progenitors remain to be addressed. Our findings provide a basis for these future studies and contribute to the development of preventive and therapeutic approaches to virus-induced olfactory dysfunction.
Highlights.
RSV infection transiently impairs SOX2+ ORN progenitors in nasal mucosa.
Airway allergy exacerbates RSV-induced impairments in ORN progenitors.
Airway allergy delays RSV clearance from nasal mucosa.
Acknowledgments
This work was supported in part by NIH grant HL089216. We are deeply grateful to Lisa Riggs and Dr. Keigo Suzukawa for special assistance with histological studies, Drs. Matthew Schaller, Takehiko Shibata, William F Carson, Ron Allen, Sihyug Jang, Syu Kikuta and Koichi Tsunoda for technical assistance, and Drs. Francis H.W. Shand and Judith Connett for editorial assistance in preparing this article.
Abbreviations
- RSV
respiratory syncytial virus
- ORNs
olfactory receptor neurons
- CRA
cockroach allergen
- OB
olfactory bulb
- OE
olfactory epithelia
- GBC
globose basal cell
- HBC
horizontal basal cell
- OMP
olfactory marker protein
- pfu
plaque-forming units
- CK5
Cytokeratin 5
- DAB
diaminobenzidine
Footnotes
Competing interests
The authors declare that they have no competing interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Su CY, Menuz K, Carlson JR. Olfactory perception: receptors, cells, and circuits. Cell. 2009;139:45–59. doi: 10.1016/j.cell.2009.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bermingham-McDonogh O, Reh TA. Regulated reprogramming in the regeneration of sensory receptor cells. Neuron. 2011;71:389–405. doi: 10.1016/j.neuron.2011.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buiakova OI, Baker H, Scott JW, Farbman A, Kream R, Grillo M, et al. Olfactory marker protein (OMP) gene deletion causes altered physiological activity of olfactory sensory neurons. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:9858–63. doi: 10.1073/pnas.93.18.9858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen X, Fang H, Schwob JE. Multipotency of purified, transplanted globose basal cells in olfactory epithelium. The Journal of comparative neurology. 2004;469:457–74. doi: 10.1002/cne.11031. [DOI] [PubMed] [Google Scholar]
- 5.Iwai N, Zhou Z, Roop DR, Behringer RR. Horizontal basal cells are multipotent progenitors in normal and injured adult olfactory epithelium. Stem cells. 2008;26:1298–306. doi: 10.1634/stemcells.2007-0891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leung CT, Coulombe PA, Reed RR. Contribution of olfactory neural stem cells to tissue maintenance and regeneration. Nature neuroscience. 2007;10:720–6. doi: 10.1038/nn1882. [DOI] [PubMed] [Google Scholar]
- 7.Pevny L, Placzek M. SOX genes and neural progenitor identity. Current opinion in neurobiology. 2005;15:7–13. doi: 10.1016/j.conb.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 8.Guo Z, Packard A, Krolewski RC, Harris MT, Manglapus GL, Schwob JE. Expression of pax6 and sox2 in adult olfactory epithelium. The Journal of comparative neurology. 2010;518:4395–418. doi: 10.1002/cne.22463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kawauchi S, Kim J, Santos R, Wu HH, Lander AD, Calof AL. Foxg1 promotes olfactory neurogenesis by antagonizing Gdf11. Development. 2009;136:1453–64. doi: 10.1242/dev.034967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kawauchi S, Shou J, Santos R, Hebert JM, McConnell SK, Mason I, et al. Fgf8 expression defines a morphogenetic center required for olfactory neurogenesis and nasal cavity development in the mouse. Development. 2005;132:5211–23. doi: 10.1242/dev.02143. [DOI] [PubMed] [Google Scholar]
- 11.Doty RL. The olfactory system and its disorders. Seminars in neurology. 2009;29:74–81. doi: 10.1055/s-0028-1124025. [DOI] [PubMed] [Google Scholar]
- 12.Strous RD, Shoenfeld Y. To smell the immune system: olfaction, autoimmunity and brain involvement. Autoimmunity reviews. 2006;6:54–60. doi: 10.1016/j.autrev.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 13.NIDCD, Health information
- 14.Xie F, Fang C, Schnittke N, Schwob JE, Ding X. Mechanisms of permanent loss of olfactory receptor neurons induced by the herbicide 2,6-dichlorobenzonitrile: Effects on stem cells and noninvolvement of acute induction of the inflammatory cytokine IL-6. Toxicology and applied pharmacology. 2013 doi: 10.1016/j.taap.2013.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Black CP. Systematic review of the biology and medical management of respiratory syncytial virus infection. Respiratory care. 2003;48:209–31. discussion 31–3. [PubMed] [Google Scholar]
- 16.Stensballe LG, Devasundaram JK, Simoes EA. Respiratory syncytial virus epidemics: the ups and downs of a seasonal virus. The Pediatric infectious disease journal. 2003;22:S21–32. doi: 10.1097/01.inf.0000053882.70365.c9. [DOI] [PubMed] [Google Scholar]
- 17.van Drunen Littel-van den Hurk S, Watkiss ER. Pathogenesis of respiratory syncytial virus. Current opinion in virology. 2012;2:300–5. doi: 10.1016/j.coviro.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 18.Mukherjee S, Lindell DM, Berlin AA, Morris SB, Shanley TP, Hershenson MB, et al. IL-17-induced pulmonary pathogenesis during respiratory viral infection and exacerbation of allergic disease. The American journal of pathology. 2011;179:248–58. doi: 10.1016/j.ajpath.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Herlocher ML, Ewasyshyn M, Sambhara S, Gharaee-Kermani M, Cho D, Lai J, et al. Immunological properties of plaque purified strains of live attenuated respiratory syncytial virus (RSV) for human vaccine. Vaccine. 1999;17:172–81. doi: 10.1016/s0264-410x(98)00155-8. [DOI] [PubMed] [Google Scholar]
- 20.Moore ML, Chi MH, Luongo C, Lukacs NW, Polosukhin VV, Huckabee MM, et al. A chimeric A2 strain of respiratory syncytial virus (RSV) with the fusion protein of RSV strain line 19 exhibits enhanced viral load, mucus, and airway dysfunction. Journal of virology. 2009;83:4185–94. doi: 10.1128/JVI.01853-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Doty RL. The olfactory vector hypothesis of neurodegenerative disease: is it viable? Annals of neurology. 2008;63:7–15. doi: 10.1002/ana.21327. [DOI] [PubMed] [Google Scholar]
- 22.Espinoza JA, Bohmwald K, Cespedes PF, Gomez RS, Riquelme SA, Cortes CM, et al. Impaired learning resulting from respiratory syncytial virus infection. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:9112–7. doi: 10.1073/pnas.1217508110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li XQ, Fu ZF, Alvarez R, Henderson C, Tripp RA. Respiratory syncytial virus (RSV) infects neuronal cells and processes that innervate the lung by a process involving RSV G protein. Journal of virology. 2006;80:537–40. doi: 10.1128/JVI.80.1.537-540.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kobayashi M, Tamari K, Miyamura T, Takeuchi K. Blockade of interleukin-6 receptor suppresses inflammatory reaction and facilitates functional recovery following olfactory system injury. Neuroscience research. 2013;76:125–32. doi: 10.1016/j.neures.2013.03.015. [DOI] [PubMed] [Google Scholar]
- 25.Anderson VE, Nguyen Y, Weinberg JB. Effects of allergic airway disease on mouse adenovirus type 1 respiratory infection. Virology. 2009;391:25–32. doi: 10.1016/j.virol.2009.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hassantoufighi A, Oglesbee M, Richter BW, Prince GA, Hemming V, Niewiesk S, et al. Respiratory syncytial virus replication is prolonged by a concomitant allergic response. Clinical and experimental immunology. 2007;148:218–29. doi: 10.1111/j.1365-2249.2007.03341.x. [DOI] [PMC free article] [PubMed] [Google Scholar]