Abstract
Changes in brain pathology as schizophrenia progresses have been repeatedly suggested by previous studies. Meta-analyses of previous proton magnetic resonance spectroscopy (1H MRS) studies at each clinical stage of schizophrenia indicate that the abnormalities of N-acetylaspartate (NAA) and glutamatergic metabolites change progressively. However, to our knowledge, no single study has addressed the possible differences in 1H MRS abnormalities in subjects at 3 different stages of disease, including those at ultrahigh risk for psychosis (UHR), with first-episode schizophrenia (FES), and with chronic schizophrenia (ChSz). In the current study, 24 patients with UHR, 19 FES, 25 ChSz, and their demographically matched 3 independent control groups (n = 26/19/28 for the UHR, FES, and ChSz control groups, respectively) underwent 1H MRS in a 3-Tesla scanner to examine metabolites in medial prefrontal cortex. The analysis revealed significant decreases in the medial prefrontal NAA and glutamate + glutamine (Glx) levels, specifically in the ChSz group as indexed by a significant interaction between stage (UHR/FES/ChSz) and clinical status (patients/controls) (P = .008). Furthermore, the specificity of NAA and Glx reductions compared with the other metabolites in the patients with ChSz was also supported by a significant interaction between the clinical status and types of metabolites that only occurred at the ChSz stage (P = .001 for NAA, P = .004 for Glx). The present study demonstrates significant differences in 1H MRS abnormalities at different stages of schizophrenia, which potentially correspond to changes in glutamatergic neurotransmission, plasticity, and/or excitotoxicity and regional neuronal integrity with relevance for the progression of schizophrenia.
Key words: anterior cingulate cortex, at-risk mental state, biomarkers, frontal lobe, magnetic resonance imaging, neurochemical abnormality
Introduction
Identifying reliable biomarkers for the emergence and progression of schizophrenia is a fundamental priority for the development of efficient ways of detecting and protecting against the transition to psychosis and further progression.1–4 Although prefrontal or temporal gray matter (GM) volume reductions are promising candidate for the biomarker,5–10 neurochemical biomarkers have not been well established.
Proton magnetic resonance spectroscopy (1H MRS) allows for in vivo measurements of certain brain chemicals. These include N-acetylaspartate (NAA), an amino acid hypothesized to be a marker of neuronal integrity,11–14 and glutamate + glutamine (Glx), metabolites presumed to be involved in excitatory neurotransmission, plasticity, and excitotoxicity.14–19 Although 1H MRS does not selectively measure synaptic glutamate, brain glutamate abnormalities may be a major neurochemical contributor to schizophrenia.20–22 Previous studies have consistently reported lower-than-normal NAA and significant deviations in Glx levels in various brain regions, including the medial prefrontal cortex (mPFC), in patients with schizophrenia.15,23–26
A limited number of previous studies have compared 1H MRS metabolites across different stages of schizophrenia (ie, at ultrahigh risk for psychosis [UHR],27 first-episode schizophrenia [FES], and chronic schizophrenia [ChSz]). One cross-sectional study examined 2 clinical groups of antipsychotic naive UHR and first-episode psychosis patients and reported increased glutamate levels in the dorsal caudate in both groups compared with controls.28 Bustillo and coworkers29 longitudinally studied NAA, including in frontal areas for up to 2 years, in patients with schizophrenia with less than 3 weeks of lifetime antipsychotic exposure. They reported lower-than-normal global NAA levels in the patients before treatment and no changes during follow-ups. Using a similar longitudinal design, a previous study reported higher-than-normal glutamine levels in the anterior cingulate and thalamus of never-treated patients with FES and later reductions of thalamic glutamine levels at a 30-month reexamination.30 Although these well-designed previous studies suggest how and when 1H MRS metabolite abnormalities emerge or progress, it would be difficult to implement long-term follow-up examinations across 3 different stages.
A recent meta-analysis25 reported NAA reductions in the frontotemporal cortices and thalamus of patients with schizophrenia and suggested that critical changes in the frontotemporal NAA abnormalities may occur in the transition from the premorbid stage to FES. A meta-analysis of Glx showed significantly decreased medial frontal glutamate and increased glutamine with not significantly lower Glx in patients with schizophrenia as compared with healthy individuals. It also reported that both Glx levels decreased faster-than-normal as the patients aged.15 These meta-analyses suggested that abnormalities in NAA, glutamate, and glutamine levels depend on the stage of the disease. However, to our knowledge, no previous study using a homogeneous study protocol has compared these metabolites in subjects with UHR, FES, and ChSz.
The current study was designed to examine whether the 1H MRS abnormalities in patients with schizophrenia depend on the stage of the disease. Based on the previous literature, it was reasonably expected that reductions in medial prefrontal NAA and Glx levels would be gradually prominent with the illness progression and would be most marked in patients at the chronic phase and relatively less evident in those at the FES and UHR. To test this hypothesis, our study examined clinical populations at the UHR, FES, and ChSz stages and 3 independent healthy control groups demographically matched to each of the 3 different stages. Because recent studies have pointed out methodological difficulties in independently quantifying Glx with a short echo time (TE) at 3-Tesla scanners,31–33 we utilized the composite Glx as a relatively reliable measure of the Glx.
Methods
Subjects
One hundred and forty-one Japanese participated in this study. Of these, 68 subjects were in the clinical groups, consisting of 24 UHR, 19 FES, and 25 ChSz. These subjects were recruited from the Department of Neuropsychiatry, The University of Tokyo Hospital, Japan. The diagnoses of schizophrenia for the ChSz patients were determined (paranoid [n = 17], disorganized [n = 3], catatonic [n = 1], undifferentiated [n = 2], and residual [n = 2]) according to the Structured Clinical Interview for Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) Axis I Disorder (SCID-I) Clinical Version.34 The inclusion criteria for FES and UHR described in our previous studies4,35 were used. Briefly, the FES subjects were 15–40 years of age, had received antipsychotic medications for less than 16 cumulative weeks, and had exhibited their first experience of psychosis according to the Structured Interview for Prodromal Symptoms (SIPS).36 Their diagnoses were confirmed as schizophrenia (paranoid [n = 18] and undifferentiated [n = 1]) over more than 6 months of examinations. The inclusion criteria for UHR were subjects between the ages of 15–30 years who had received a diagnosis of UHR according to the SIPS (attenuated positive symptom syndrome [APS] [n = 20], brief intermittent psychotic symptom syndrome [BIPS] [n = 1], genetic risk and deterioration syndrome without schizotypal personality disorder [n = 2], and comorbidity of APS and BIPS [n = 1]). Psychiatric symptoms for each patient were evaluated using the Positive and Negative Syndrome Scale (PANSS)37 within 7 days before and after their magnetic resonance imaging (MRI) scan. Fourteen UHR and one FES subjects had not previously received antipsychotic medications, whereas the other patients were currently medicated with antipsychotics at the time of MR scans (only atypical antipsychotics [n = 38], only typical antipsychotics [n = 3], and both types of antipsychotics [n = 12]). The subjects with UHR included those with comorbid major depressive disorder (n = 2), anxiety disorder not otherwise specified (n = 3), and adjustment disorder (n = 1). Similarities in clinical manifestations and cognitive deficits have been pointed out between schizophrenia, UHR, and autism spectrum disorders (ASD).38,39 Additionally, because ASD subjects show aberrant prefrontal metabolite levels,40 all the participants were confirmed not to have diagnosis of ASD according to the DSM-IV based on clinical histories from all subjects and their family members. All clinical evaluations were performed by a psychiatrist (T.N., N.I., H.I., or Y.T.) fully trained to maintain reliability and consistency.
Seventy-three healthy control subjects participated, and they were assigned to 3 groups based on their demographic information with fully blind to the 1H MRS data as in the similar process to the previous studies.35,41 The first normal control (NC) group was matched to the UHR (NCUHR, n = 26), the second was to the FES (NCFES, n = 19), and the third to the ChSz (NCChSz, n = 28). The controls were screened for neuropsychiatric disorders through the SCID-I Non-patient Edition.34 Assessments of the subjects’ and their parents’ socioeconomic status (SES) were conducted using the Hollingshead scale,42 handedness using the Edinburgh Inventory,43 and premorbid intelligence quotients (IQ) using the Japanese version of the National Adult Reading Test.44,45
The exclusion criteria for all groups were a current or past neurological illness, a traumatic brain injury with any known cognitive consequences or loss of consciousness for more than 5 min, a history of electroconvulsive therapy, and previous substance abuse or dependence based on clinical histories. Additional exclusion criteria for the controls were a history of psychiatric disease in the subjects or of axis I disorders in their first-degree relatives. The ethical committee of The University of Tokyo Hospital approved this study (no. 397 and 2226). After a complete explanation of the study to the participants, written informed consent was obtained from every individual (table 1).
Table 1.
Clinical and Demographic Characteristics of the Subjects
| NCUHR (n = 26) | UHR (n = 24) | NCFES (n = 19) | FES (n = 19) | NCChSz (n = 28) | ChSz (n = 25) | Clinical Status | Clinical Status × Stage | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | F(1,135) | P Value | F(2,135) | P Value | |
| Sex | 13/13 | 12/12 | 14/5 | 14/5 | 17/11 | 15/10 | 0.003a | .96a | ||||||||
| Age (range) | 22.3 (16–26) | 3.2 | 21.7 (16–29) | 3.8 | 26.3 (21–29) | 1.5 | 25.4 (17–37) | 6.3 | 32.8 (27–41) | 4.3 | 32.7 (17–50) | 8.6 | 0.3 | .56 | 0.06 | .94 |
| SESb | 2.0 | 1.0 | 2.9c | 1.3 | 1.4 | 0.5 | 3.2c | 1.5 | 1.7 | 0.6 | 3.8c | 1.3 | 72.0 | <.001 | 3.7 | .03 |
| Parental SES | 1.9 | 0.5 | 2.2 | 0.8 | 1.8 | 0.5 | 2.4c | 0.6 | 2.0 | 0.6 | 2.6c | 0.6 | 19.4 | <.001 | 0.9 | .42 |
| Height | 166.0 | 8.9 | 165.5 | 7.7 | 173.1 | 9.8 | 165.7c | 8.6 | 167.5 | 7.9 | 164.9 | 8.7 | 5.7 | .02 | 1.8 | .18 |
| Weight | 58.3 | 11.2 | 57.4 | 8.7 | 65.4 | 13.3 | 60.7 | 9.9 | 60.7 | 11.5 | 66.6 | 14.5 | 0.003 | .95 | 2.4 | .09 |
| Handednessd | 85.0 | 40.4 | 87.0 | 18.8 | 93.9 | 17.5 | 94.6 | 11.5 | 95.2 | 8.6 | 93.7 | 13.8 | 0.01 | .91 | 0.09 | .92 |
| IQ (JART25)e | 106.8 | 10.3 | 108.4 | 9.1 | 109.0 | 7.0 | 103.2 | 13.3 | 108.5 | 9.3 | 102.7 | 11.3 | 3.6 | .06 | 2.1 | .13 |
| PANSS | ||||||||||||||||
| Positive symptoms | 13.6 | 2.9 | 16.0 | 4.5 | 16.2 | 6.0 | ||||||||||
| Negative symptoms | 18.1 | 6.3 | 18.8 | 4.8 | 21.6 | 7.1 | ||||||||||
| General psychopathology | 33.1 | 7.0 | 36.4 | 7.4 | 38.4 | 11.3 | ||||||||||
| GAF | 48.0 | 11.8 | 38.4 | 8.4 | 38.3 | 14.3 | ||||||||||
| Onset of illness (y) | 25.1 | 6.0 | 25.4 | 7.7 | ||||||||||||
| Duration of untreated psychosis (wk) | 15.7 | 20.9 | 45.4 | 77.2 | ||||||||||||
| Duration of illness (mo) | 8.4 | 10.0 | 92.8 | 59.2 | ||||||||||||
| Antipsychotic dosef (mg/d) | 117.6 | 194.6 | 558.1 | 512.9 | 839.8 | 845.0 | ||||||||||
| Antipsychotic type (atypical/typical/both/ none) | 9/0/1/14 | 16/1/1/1 | 13/2/10/0 | |||||||||||||
Note: NC, normal control; UHR, ultrahigh risk for psychosis; FES, first-episode schizophrenia; ChSz, chronic schizophrenia; SES, socioeconomic status; IQ, intelligence quotients; PANSS, Positive and Negative Syndrome Scale; GAF, Global Assessment of Functioning.
aChi-square test for ChSz stage.
bAssessed with the Hollingshead scale. Higher scores indicate lower educational and/or occupational status.
cPost hoc test indicated that the patient group was significantly different from each control group (P < .05, independent 2-tailed t tests).
dDetermined with Edinburgh Inventory: scores more than 0 indicate right handedness. A score of 100 indicates strong right handedness.
eEstimated from scores on the Japanese Adult Reading Test.
fBased on chlorpromazine equivalents.
MRI Acquisition
The methods for MRI and MRS acquisition, processing, and quantification were the same as in our recent study.46 Briefly, MRI data were obtained using a 3-Tesla scanner (GE Signa HDxt). Axial T2-weighted images—TE = 82.32 ms, repetition time (TR) = 4400 ms, field of view (FOV) = 240 × 240mm2, matrix = 256 × 256, slice thickness = 2.5 mm, number of axial slices = 62—were acquired for positioning of the volume-of-interest (VOI). Three-dimensional fast spoiled gradient recalled acquisition with steady state (3D-FSPGR) images—TE = 1.94 ms, TR = 6.80 ms, FOV = 240 × 240 mm, matrix = 256 × 256, flip angle = 20°, slice thickness = 1.0 mm, number of axial slices = 176—were acquired for tissue segmentation of the VOI. A trained neuroradiologist (W.G., H.S., M.K., or H.T.) evaluated the MRI scans and found no gross abnormalities in any of the subjects.
1H MRS Acquisition
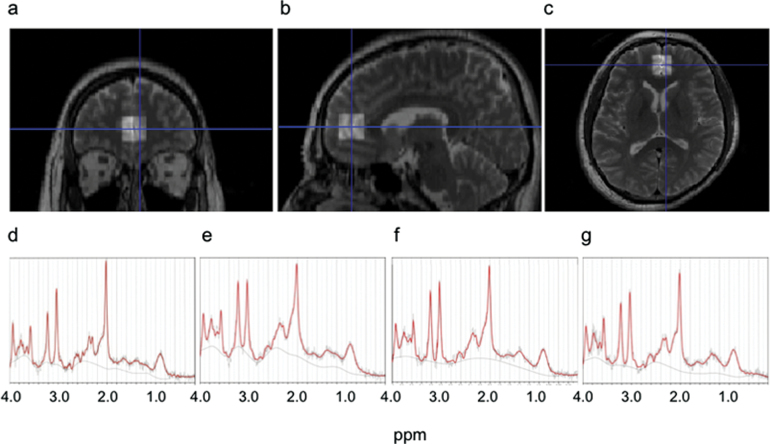
A stimulated echo acquisition mode (STEAM) imaging sequence—TR = 3000 ms, TE = 15 ms, mixing time = 13.7 ms, 128 water-suppressed, and 8 water-unsuppressed averages—was used to obtain the proton MR spectra. The location of the VOIs was based on our previous studies.46,47 Briefly, in the mid-sagittal T2 slice, a VOI (20 × 20 × 20 mm3) was placed closest to the most anterior part of genu of the corpus callosum with the center of the posterior plane of the VOI. This VOI contained predominantly the GM of mPFC, including primarily the anterior cingulate and paracingulate gyri bilaterally (figure 1).
Fig. 1.
Location of the volume-of-interest (VOI) and representative proton magnetic resonance spectroscopy (1H MRS) spectra. (a–c) Representative T2-weighted brain images in the orthogonal slices in a control subject. The square indicates the 20×20×20mm3 VOI in the medial prefrontal cortex. (d–g) Representative 1H MRS spectra from subjects in the groups of (d) controls, (e) ultrahigh risk for psychosis, (f) first-episode schizophrenia, and (g) chronic schizophrenia as fitted by LCModel.
Spectrum Quantification
All spectra were quantified with LCModel (ver.6.2-3A). The raw spectral data were read into LCMgui in which spectrum processing was performed automatically. Based on the comparison of the in vitro spectra from measurements analyzed with the LCModel basis set, the absolute levels for 17 metabolites were estimated from the in vivo spectra. Among these, the current study focused on NAA, Glx, creatine and phosphocreatine (Cre), myoinositol (mI), and glycerophosphocholine and phosphocholine (Cho). Representative spectra from patients and controls are shown in figure 1.
Spectrum Quality
Only metabolite spectra that showed Cramer-Rao lower bounds (CRLB) <20% were included in the analysis. Additionally, full width at half maximum (FWHM) less than 0.16 ppm and signal-to-noise ratio (S/N) ≥5 were required for inclusion. Based on these criteria, all metabolites in all the 141 subjects were included in the analysis as shown in table 2. The spectrum quality of the included subjects was good (see online supplementary tables 1 and 2).
Table 2.
Descriptive and Inferential Statistical Results of the Metabolite Concentrations in the Participants
| Metabolites (mmol/ml) | Clinical Status | Stage | Repeated-Measures ANCOVA: Main Analysis | MANCOVA in Stage of ChSza | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| UHR | FES | ChSz | Clinical Status | Stage | Clinical Status × Stage | Clinical Status | ||||||||||||||||||
| N | Mean | SD | N | Mean | SD | N | Mean | SD | F(1,132) | P | F(2,132) | P | F(2,132) | P | F(1,48) | P | ||||||||
| NAA | Controls | 26 | 8.00 | 1.22 | 19 | 8.45 | 1.11 | 28 | 8.00 | 1.20 | 2.42 | .12 | 0.44 | .64 | 4.98 | .008 | 13.80 | .001 | ||||||
| Patients | 24 | 8.12 | 1.69 | 19 | 8.20 | 1.28 | 25 | 6.83 | 1.41 | |||||||||||||||
| Glx | Controls | 26 | 12.34 | 2.06 | 19 | 12.39 | 2.29 | 28 | 12.62 | 2.50 | Follow-up Analysesb
|
9.05 | .004 | |||||||||||
| Patients | 24 | 13.04 | 2.58 | 19 | 12.39 | 2.35 | 25 | 10.96 | 2.85 | |||||||||||||||
| Cre | Controls | 26 | 7.88 | 1.36 | 19 | 8.26 | 1.02 | 28 | 8.60 | 0.92 | UHR | FES | ChSz | 9.54 | .04 | |||||||||
| Patients | 24 | 8.09 | 1.63 | 19 | 8.18 | 1.37 | 25 | 7.87 | 1.89 | Clinical Status | Clinical Status × Metabolites | Clinical Status | Clinical Status × Metabolites | Clinical Status | Clinical Status × Metabolites | |||||||||
| Cho | Controls | 26 | 2.44 | 0.49 | 19 | 2.55 | 0.39 | 28 | 2.64 | 0.35 | 0.38 | .16 | ||||||||||||
| Patients | 24 | 2.44 | 0.58 | 19 | 2.62 | 0.43 | 25 | 2.57 | 0.58 | F(1,45) | P | F(4,180) | P | F(1,33) | P | F(4,132) | P | F(1,48) | P | F(4,192) | P | |||
| mI | Controls | 26 | 5.91 | 1.27 | 19 | 6.20 | 0.92 | 28 | 6.81 | 1.30 | 7.98 | .10 | ||||||||||||
| Patients | 24 | 6.21 | 1.09 | 19 | 6.79 | 1.01 | 25 | 6.22 | 2.04 | 0.51 | .48 | 0.35 | .84 | 0.001 | .97 | 1.06 | .38 | 12.66 | .001 | 3.71 | .006 | |||
Note: Abbreviations are explained in the first footnote to table 1. NAA, N-acetylaspartate; Glx, glutamate + glutamine; Cre, creatine and phosphocreatine; mI, myoinositol; Cho, glycerophosphocholine and phosphocholine. Bold values indicate statistical significance.
aPost hoc MANCOVA for the stage of ChSz after the significant interaction of clinical status × metabolites in follow-up analyses in the stage of ChSz. Threshold for statistical significance was set at P < .01 with Bonferroni correction.
bPost hoc repeated-measures ANCOVA for each stage after the significant interaction of clinical status × stage in main analysis. Threshold for statistical significance was set at P < .017 with Bonferroni correction.
Tissue Segmentation
As in our recent study,46 3D-FSPGR images were used to calculate GM, white matter (WM), and cerebrospinal fluid (CSF) volumes. To obtain the tissue-composition-corrected metabolite intensities, each metabolite value was corrected for the CSF content of the VOI using the following formula as in a previous study48: corrected metabolite level = uncorrected metabolite level/(1−C), where C is the fractional CSF content of the VOI.
Statistical Method
All statistical analyses were conducted using PASW Statistics 18 (SPSS Inc). GM, WM, and CSF volumes within the VOI and demographic variables, including age, height, body weight, subjects’ SES, parental SES, handedness, and IQ, were compared using MANOVA with these indices as the dependent variables and with main factors of clinical status (patients/controls) and stage (UHR and NCUHR/FES and NCFES/ChSz and NCChSz). The sex ratios were compared using chi-square tests between the patient and the matched controls. The significance level was set at a P < .05 without correction for multiple comparisons to strictly assess the effects of potential confounds.
For the group comparisons of metabolite levels, we employed a repeated-measures ANCOVA using metabolite concentrations as the dependent variables with 2 between-subject factors (clinical status: patients/controls; stage: UHR and NCUHR/FES and NCFES/ChSz and NCChSz), 1 within-subject factor (metabolites: NAA, Glx, Cre, Cho, and mI). The current analysis treated metabolites levels as a within-subject factor because metabolite levels were suggested to be interrelated in previous studies49–53 as well as in the present study (ie, most metabolites pairs of all of the 10 possible combinations of 5 metabolites showed significant correlations: 9 of the 10 pairs at P < .05 and 7 of 10 at P < .005 Bonferroni corrected for 10 pairs). Three covariates (age, parental SES, and GM volume) were also included in the analysis because significant differences between different clinical status or stages were found for age and parental SES. Because the CSF components had already been accounted for by calculating the corrected metabolite level (see “Tissue Segmentation” section), GM components were further treated as covariates to account for possible difference in metabolite levels between GM and WM.29,48,54 If a significant interaction between clinical status and any other factor was found, post hoc analyses were performed using repeated-measures ANCOVA (see “Results” section). If a significant interaction between metabolites and any other factor was found, further post hoc analyses using multivariate ANCOVA (MANCOVA) with the level of metabolites as a dependent variable and age, parental SES, and GM volume as covariates were performed (see “Results” section).
The associations between NAA and Glx levels, which showed significant deviations from the normal in the ChSz (see “Results” section), and clinical indices including PANSS (Positive symptoms, Negative symptoms, and General psychopathology) scores, age at illness onset, duration of illness (DOI), and duration of untreated psychosis (DUP) were tested by Spearman’s correlation coefficients in the patients with ChSz as well as UHR and FES. Considering the exploratory nature of the correlational analyses, the threshold for statistical significance was set at P < .0017 = .05/30 correlations using Bonferroni correction. Additionally, the correlations between NAA and Glx levels and potential confounding factors, including age, SES, parental SES, IQ, and daily antipsychotic dose based on chlorpromazine equivalents, were also tested separately in each diagnostic group using Spearman’s correlation coefficients. The threshold for statistical significance was set at P < .001 = .05/54 correlations using Bonferroni correction.
Furthermore, to test the potential interrelationship between the metabolites abnormalities,49 post hoc correlational analyses between NAA and Glx levels, which showed significant deviations from the normal in the ChSz (see “Results” section), were conducted in the patients with ChSz as well as UHR, FES and their controls. Significance level was set at P < .0083 = .05/6 correlations.
Results
Demographic and Clinical Measures
The MANOVA showed significant interaction of clinical status and stage on SES (F[2,135] = 3.7, P = .03), a main effect of clinical status in SES (F[1,135] = 72.0, P < .001), parental SES (F[1,135] = 19.4, P < .001), and height (F[1,135] = 5.7, P = 0.02). The interaction between clinical status and stage and the main effect of clinical status were not significant for the other dependent variables (P > .06). Post hoc independent 2-tailed t tests revealed significant differences as follows: compared with their matched controls, the subjects with ChSz (t[51] = −7.49, P < .001), FES (t[36] = −4.94, P < .001), and UHR (t[48] = −2.72, P = .009) had significantly lower SES. The subjects with ChSz and FES had significantly lower parental SES (t[51] = −3.48, P = 0.001 and t[36] = −2.94, P = .006, respectively) than their matched controls. The subjects with FES had significantly lower height (t[36] = 2.45, P = 0.019) than their matched controls (table 1).
1H MRS Measures
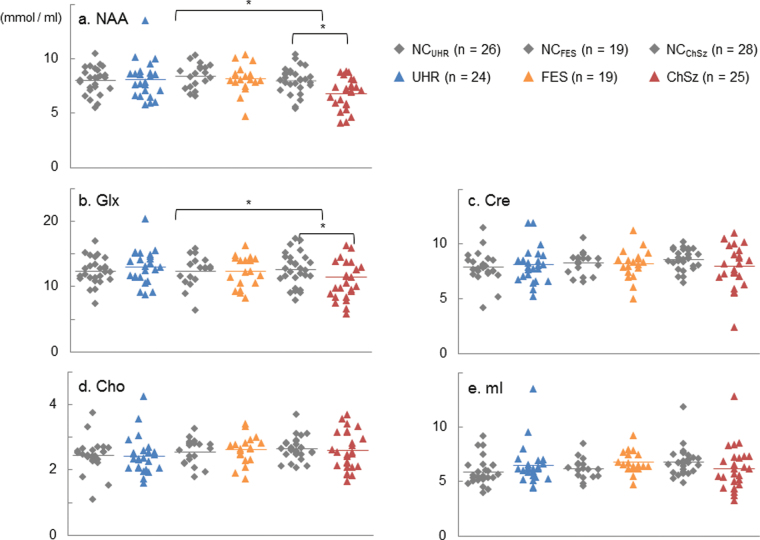
Repeated-measures ANCOVA showed a significant interaction between stage and clinical status (F[2,132] = 4.98, P = 0.008) with no significant main effect of stage or clinical status (P > .12) and no interaction between metabolite concentration and stage or clinical status. Post hoc repeated-measures ANCOVA for each stage with “clinical status” as a between-subject factor, “metabolites” as a within-subject factor, and the same covariates as the main analysis were conducted with the threshold for statistical significance set at P < .017 = .05/3 stages using Bonferroni correction. These post hoc analyses revealed a significant main effect of clinical status (F[1,48] = 12.66, P = 0.001) and a significant interaction between the metabolites and clinical status (F[4,192] = 3.71, P = .006) in the ChSz subjects, whereas there were no significant main effects of clinical status or significant interactions in the FES or UHR subjects. Post hoc MANCOVA for ChSz with clinical status as a main factor, metabolite concentrations as the dependent variables, and the same covariates as the main analysis revealed a significant effect of clinical status for NAA (F[1,48] = 13.80, P = .001) and Glx (F[1,48] = 9.05, P = .004) with the threshold for statistical significance set at P < .01 = 0.05/5 metabolites using Bonferroni correction. In contrast, no significant effects for the other metabolites were found. These results demonstrate significant reductions of both the NAA and Glx levels in the mPFC in subjects with ChSz but not in those with FES or UHR (table 2; figure 2a–c).
Fig. 2.
Plots of the metabolite levels. Scatter plots showing the concentrations of (a) N-acetylaspartate (NAA), (b) glutamate + glutamine (Glx), (c) creatine and phosphocreatine (Cre), (d) glycerophosphocholine and phosphocholine (Cho), and (e) myoinositol (mI) in the subjects with the ultrahigh risk for psychosis (UHR), first-episode schizophrenia (FES), and chronic schizophrenia (ChSz) and matched controls for each group. *Statistically significant at P < .05.
When 6 UHR subjects with nonpsychotic axis I DSM-IV disorders (see “Methods” section) were excluded from the analyses, the statistical conclusions were similar to the above. If we used a criteria of FWHM <0.13 or group comparisons of metabolite levels with FWHM as an additional covariate to account for the relatively high threshold of FWHM < 0.16,22,28,48,55,56 we found equivalent statistical results. When we analyzed every metabolite individually, the results were substantially similar to those determined by the analysis using metabolites as a within-subject factor. And statistical analyses using GM/(GM + WM) or WM/(GM + WM) as one of the covariates instead of using GM in the group comparisons of metabolite levels to account for CSF variations within the VOI provided equivalent results whose statistical conclusions were totally preserved from those analyses using GM volume as the covariate.
If we replaced NAA to NAA + N-acetylasparty lglutamate (NAAG), the statistical conclusions were totally preserved. The means (SD) of NAA + NAAG levels were 8.2 (1.2) and 8.3 (1.6) for NCUHR and UHR, 8.6 (1.0) and 8.4 (1.3) for NCFES and FES, and 8.3 (1.2) and 7.0 (1.5) for NCChSz and ChSz, respectively. Repeated-measures ANCOVA with NAA + NAAG instead of NAA showed a significant interaction between stage and clinical status (F[2,132] = 5.16, P = .007) with no significant main effect of stage or clinical status (P > .11) and no interaction between metabolite concentration and stage or clinical status. Post hoc repeated-measures ANCOVA for each stage with “clinical status” as a between-subject factor, “metabolites” as a within-subject factor, and the same covariates as the main analysis were conducted. These post hoc analyses revealed a significant main effect of clinical status (F[1,48] = 13.43, P = .001) and a significant interaction between the metabolites and clinical status (F[4,192] = 3.88, P = .005) in the ChSz subjects, whereas there were no significant main effects of clinical status or significant interactions in the FES or UHR subjects. Post hoc MANCOVA for ChSz with clinical status as a main factor, metabolite concentrations as the dependent variables, and the same covariates as the main analysis revealed significant effects of clinical status for NAA + NAAG (F[1,48] = 17.13, P < .001) as well as Glx.
Correlations With Metabolite Concentrations
Correlational analyses showed relationships between the Glx level and DOI (ρ = 0.524, P = .021) and DUP (ρ = 0.638, P = .008) in the FES, although not statistically significant after correction for multiple comparisons.
Several correlations were found between the Glx level and parental SES in the NCChSz (ρ = 0.422, P = .025), the NAA level and SES (ρ = 0.477, P = .039), daily antipsychotic medication dose (ρ = −0.491, P = .033) in the patients with FES, and Glx level and age in the individuals at UHR (ρ = −0.513, P = .010). However, these correlations were not significant after correction for multiple comparisons.
In the patients with ChSz, the reduced NAA level was significantly correlated with the reduced Glx level (ρ = 0.720, P < .001) while not in the subjects with UHR or FES. The correlation was similar in pattern to those observed in the NCChSz (ρ = 0.571, P = .001) or for NCUHR (ρ = 0.553, P = 0.003).
Discussion
The current study revealed significantly decreased medial prefrontal NAA and Glx levels specifically in the subjects at chronic stage of schizophrenia. The specificity of these reductions to the patients at chronic stage compared with those at the UHR and FES stages was shown by a significant interaction between diagnosis and illness stage. The specificity of reduced NAA and Glx levels compared with Cre, Cho, and mI levels was shown by a significant interaction between diagnosis and metabolite types in the patients with ChSz and their matched controls. Thus, the present study demonstrates significant differences in 1H MRS abnormalities between patients at different stages of schizophrenia, which differentially occur with different metabolites.
The chronic patients-specific decrements of medial frontal NAA are generally in line with previous studies, indicating robust reductions in medial frontal NAA in chronic but not in early phase schizophrenia. Although a meta-analysis of 97 1H MRS studies25 found a reduced NAA in the frontal lobe, both in FES and ChSz, they suggested that the criteria for FES were not explicitly reported in any of the 19 studies included in their meta-analysis. Furthermore, among the 19 studies, 7 of the 9 studies employed a medial frontal VOI reported no change in NAA consistent with our study,55,57–62 whereas the other 2 reported reduced NAA. One of these latter studies recruited 13 children and adolescents with schizophrenia, of whom only 3 subjects were first-episode patients.63 The other recruited 30 patients with and without Gilbert’s syndrome, whose mean durations of illnesses were relatively long with 1.3/1.7 years, respectively.61 Thus, the current and the latter 2 studies are different in the age, illness stage, and mean durations of illness in the participants. The current study suggests that there is no decrease in the medial frontal NAA of FES, and this result does not conflict with those of most of the previous studies.
We also observed significantly decreased medial prefrontal Glx specifically in the patients with ChSz. A meta-analysis of 28 1H MRS studies in schizophrenia15 found significantly decreased glutamate and increased glutamine in medial frontal regions of patients with schizophrenia compared with healthy controls. Our finding of chronic patients-specific decreases in the medial frontal Glx levels is in keeping with the result of this meta-analysis that both Glx levels decreased at a faster-than-normal rate with age in patients. Because the current participants with FES were sufficiently treated with antipsychotics, the preserved Glx level in the current patients with FES is in line with a recent study which demonstrated significantly higher anterior cingulate glutamate/Cr or Glx/Cr in patients who were still symptomatic than in those in remission.64
Decreased NAA and Glx levels in ChSz may be associated with the progressive brain volume reductions in the mPFC that are associated with longer durations of schizophrenia.65–67 However, it is not possible to completely explain these metabolite decreases by volume reductions because of the use of tissue composition correction. Correlation between Glx and NAA in schizophrenia was previously reported in line with the N-methyl-d-aspartate receptor hypofunction model of schizophrenia14,56,68,69 and contributions from glutamate related dendritic toxicity. In accordance with the notion, the decreased NAA and Glx levels were significantly correlated in the patients with ChSz but not in the UHR or FES. Because Glx and NAA are thought to be inherently linked through a series of biochemical reactions, mainly the tricarboxylic acid and glutamate-glutamine cycles in neurons and glia,14,49,70 both of the altered Glx and NAA measures may commonly reflect dysfunction and/or loss of neuronal tissues. The specific loss of NAA and Glx, as compared with no change in Cho or mI, in the patients with ChSz is also consistent with previous reports, suggesting brain tissue loss occurs as a result of reductions of neuropil and potential rearrangements of cortical architecture, rather than by neuronal loss or degeneration.15,71–76
Antipsychotic medications may have some effect on MRS measures.30,49,56,77–79 Most previous studies of unmedicated patients with schizophrenia have reported abnormally elevated glutamine or Glx levels in the mPFC30,51,57,58 with one study showing effects in the striatum.28 In addition, one previous study compared medial prefrontal Glx levels in medicated patients with those in unmedicated patients, and found elevated Glx levels only in the unmedicated patients.51 Even though no significant correlation between the daily antipsychotic dose and metabolite levels was found in the current patients with ChSz and no significant difference in any metabolites levels was found between the medicated (n = 10) and nonmedicated (n = 14) individuals at UHR (P > .2), the fact that all 3 patient groups were not all similarly medicated is a potential confound of the present study. Future study should adequately address the effects of antipsychotic medications on the Glx and NAA levels.
The current study extends the findings of previous meta-analyses by making direct comparisons in a single study. For the integration of findings across different studies, heterogeneity in magnetic field strength, acquisition mode, quantification method, and scanner type between studies should also be taken into account because these could have a significant impact on the variability of the metabolites quantified.7,57 Although meta-analyses cannot totally rule out these effects,15,25 our study confirmed ChSz-specific NAA and Glx abnormalities under uniform conditions in a single study.
There are several methodological considerations and limitations of our study. First, our MRS measurement was limited to the mPFC, even though other regions are likely involved in the pathology of schizophrenia. Although the single VOI model yields high S/N,80 future studies should examine the regional specificity of these findings. Second, although the present study examined patients at 3 different stages of schizophrenia, which would be difficult in a longitudinal study, the cross-sectional design supports descriptive rather than causal interpretations. Although 2 of the 24 individuals at UHR developed psychosis later in the mean of 12.4-month follow-up, in which the transition rate does not conflict with those in the previous studies,9,81 the small number of subjects with transition make any statistical comparison difficult. Third, because MRS does not selectively measure synaptic glutamatergic metabolites, the results should be carefully interpreted regarding this matter. Fourth, the STEAM sequence with a TE = 15 ms left room for improvement, although the currently described low CRLB values indicate good Glx data quality. Future study should expand on the current findings using an optimized TE82,83 or improved handling of macromolecule signals.84,85 Fifth, although the main findings were based on the analysis controlling potential confounding effect of parental SES, a potential cohort effect (as distinct from a stage of illness effect) cannot be totally ruled out because of the lack of matching the subjects in the ChSz group in terms of their parental SES.
Overall, the present results indicate ChSz-specific NAA and Glx reductions in the mPFC in a single study that included subjects at 3 different stages of schizophrenia. These reductions are potentially related to changes in glutamatergic transmissions and regional neuronal integrity and may be related to the pathophysiology and progressive brain morphological changes seen in schizophrenia.
Supplementary Material
Supplementary material is available at http://schizophre niabulletin.oxfordjournals.org.
Funding
“Development of biomarker candidates for social behavior” project carried out under the Strategic Research Program for Brain Sciences by the Ministry of Education, Culture, Sports, Science and Technology (MEXT); KAKENHI (22689034 to H.Y., 20591378 to N.Y., 21249064 to K.K.); Global Center of Excellence (COE) Program “Comprehensive Center of Education and Research for Chemical Biology of the Diseases” (to N.Y.); Health and Labour Sciences Research Grants for Comprehensive Research on Disability, Health and Welfare (H22-seishin-ippan-015 to K.K.).
Supplementary Material
Acknowledgments
The authors have declared that there are no conflicts of interest in relation to the subject of this study.
References
- 1. Stone JM, Day F, Tsagaraki H, et al. Glutamate dysfunction in people with prodromal symptoms of psychosis: relationship to gray matter volume. Biol Psychiatry. 2009;66:533–539 [DOI] [PubMed] [Google Scholar]
- 2. Jessen F, Scherk H, Träber F, et al. Proton magnetic resonance spectroscopy in subjects at risk for schizophrenia. Schizophr Res. 2006;87:81–88 [DOI] [PubMed] [Google Scholar]
- 3. Fusar-Poli P, Bonoldi I, Yung AR, et al. Predicting psychosis: meta-analysis of transition outcomes in individuals at high clinical risk. Arch Gen Psychiatry. 2012;69:220–229 [DOI] [PubMed] [Google Scholar]
- 4. Koike S, Takano Y, Iwashiro N, et al. A multimodal approach to investigate biomarkers for psychosis in a clinical setting: the integrative neuroimaging studies in schizophrenia targeting for early intervention and prevention (IN-STEP) project. Schizophr Res. 2013;143:116–124 [DOI] [PubMed] [Google Scholar]
- 5. Smieskova R, Fusar-Poli P, Allen P, et al. Neuroimaging predictors of transition to psychosis–a systematic review and meta-analysis. Neurosci Biobehav Rev. 2010;34:1207–1222 [DOI] [PubMed] [Google Scholar]
- 6. Olabi B, Ellison-Wright I, McIntosh AM, Wood SJ, Bullmore E, Lawrie SM. Are there progressive brain changes in schizophrenia? A meta-analysis of structural magnetic resonance imaging studies. Biol Psychiatry. 2011;70:88–96 [DOI] [PubMed] [Google Scholar]
- 7. Hirayasu Y, Shenton ME, Salisbury DF, et al. Lower left temporal lobe MRI volumes in patients with first-episode schizophrenia compared with psychotic patients with first-episode affective disorder and normal subjects. Am J Psychiatry. 1998;155:1384–1391 [DOI] [PubMed] [Google Scholar]
- 8. Kasai K, Shenton ME, Salisbury DF, et al. Progressive decrease of left Heschl gyrus and planum temporale gray matter volume in first-episode schizophrenia: a longitudinal magnetic resonance imaging study. Arch Gen Psychiatry. 2003;60:766–775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pantelis C, Velakoulis D, McGorry PD, et al. Neuroanatomical abnormalities before and after onset of psychosis: a cross-sectional and longitudinal MRI comparison. Lancet. 2003;361:281–288 [DOI] [PubMed] [Google Scholar]
- 10. Suzuki M, Zhou SY, Takahashi T, et al. Differential contributions of prefrontal and temporolimbic pathology to mechanisms of psychosis. Brain. 2005;128:2109–2122 [DOI] [PubMed] [Google Scholar]
- 11. Bates TE, Strangward M, Keelan J, Davey GP, Munro PM, Clark JB. Inhibition of N-acetylaspartate production: implications for 1H MRS studies in vivo. Neuroreport. 1996;7:1397–1400 [PubMed] [Google Scholar]
- 12. Lentz MR, Kim JP, Westmoreland SV, et al. Quantitative neuropathologic correlates of changes in ratio of N-acetylaspartate to creatine in macaque brain. Radiology. 2005;235:461–468 [DOI] [PubMed] [Google Scholar]
- 13. Stork C, Renshaw PF. Mitochondrial dysfunction in bipolar disorder: evidence from magnetic resonance spectroscopy research. Mol Psychiatry. 2005;10:900–919 [DOI] [PubMed] [Google Scholar]
- 14. Moffett JR, Ross B, Arun P, Madhavarao CN, Namboodiri AM. N-Acetylaspartate in the CNS: from neurodiagnostics to neurobiology. Prog Neurobiol. 2007;81:89–131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Marsman A, van den Heuvel MP, Klomp DW, Kahn RS, Luijten PR, Hulshoff Pol HE. Glutamate in schizophrenia: a focused review and meta-analysis of ¹H-MRS studies. Schizophr Bull. 2013;39:120–129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cull-Candy S, Brickley S, Farrant M. NMDA receptor subunits: diversity, development and disease. Curr Opin Neurobiol. 2001;11:327–335 [DOI] [PubMed] [Google Scholar]
- 17. Paoletti P, Neyton J. NMDA receptor subunits: function and pharmacology. Curr Opin Pharmacol. 2007;7:39–47 [DOI] [PubMed] [Google Scholar]
- 18. Magistretti PJ, Pellerin L. Cellular mechanisms of brain energy metabolism. Relevance to functional brain imaging and to neurodegenerative disorders. Ann N Y Acad Sci. 1996;777:380–387 [DOI] [PubMed] [Google Scholar]
- 19. Petroff OA. GABA and glutamate in the human brain. Neuroscientist. 2002;8:562–573 [DOI] [PubMed] [Google Scholar]
- 20. Krystal JH. Capitalizing on extrasynaptic glutamate neurotransmission to treat antipsychotic-resistant symptoms in schizophrenia. Biol Psychiatry. 2008;64:358–360 [DOI] [PubMed] [Google Scholar]
- 21. Stone JM, Howes OD, Egerton A, et al. Altered relationship between hippocampal glutamate levels and striatal dopamine function in subjects at ultra high risk of psychosis. Biol Psychiatry. 2010;68:599–602 [DOI] [PubMed] [Google Scholar]
- 22. Fusar-Poli P, Stone JM, Broome MR, et al. Thalamic glutamate levels as a predictor of cortical response during executive functioning in subjects at high risk for psychosis. Arch Gen Psychiatry. 2011;68:881–890 [DOI] [PubMed] [Google Scholar]
- 23. Wood SJ, Yücel M, Wellard RM, et al. Evidence for neuronal dysfunction in the anterior cingulate of patients with schizophrenia: a proton magnetic resonance spectroscopy study at 3 T. Schizophr Res. 2007;94:328–331 [DOI] [PubMed] [Google Scholar]
- 24. Steen RG, Hamer RM, Lieberman JA. Measurement of brain metabolites by 1H magnetic resonance spectroscopy in patients with schizophrenia: a systematic review and meta-analysis. Neuropsychopharmacology. 2005;30:1949–1962 [DOI] [PubMed] [Google Scholar]
- 25. Brugger S, Davis JM, Leucht S, Stone JM. Proton magnetic resonance spectroscopy and illness stage in schizophrenia–a systematic review and meta-analysis. Biol Psychiatry. 2011;69:495–503 [DOI] [PubMed] [Google Scholar]
- 26. Kraguljac NV, Reid M, White D, et al. Neurometabolites in schizophrenia and bipolar disorder - a systematic review and meta-analysis. Psychiatry Res. 2012;203:111–125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yung AR, Phillips LJ, Yuen HP, et al. Psychosis prediction: 12-month follow up of a high-risk (“prodromal”) group. Schizophr Res. 2003;60:21–32 [DOI] [PubMed] [Google Scholar]
- 28. De la Fuente-Sandoval C, Leon-Ortiz P, Favila R, et al. Higher levels of glutamate in the associative-striatum of subjects with prodromal symptoms of schizophrenia and patients with first-episode psychosis. Neuropsychopharmacology. 2011;36:1781–1791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bustillo JR, Rowland LM, Jung R, et al. Proton magnetic resonance spectroscopy during initial treatment with antipsychotic medication in schizophrenia. Neuropsychopharmacology. 2008;33:2456–2466 [DOI] [PubMed] [Google Scholar]
- 30. Théberge J, Williamson KE, Aoyama N, et al. Longitudinal grey-matter and glutamatergic losses in first-episode schizophrenia. Br J Psychiatry. 2007;191:325–334 [DOI] [PubMed] [Google Scholar]
- 31. Yang S, Hu J, Kou Z, Yang Y. Spectral simplification for resolved glutamate and glutamine measurement using a standard STEAM sequence with optimized timing parameters at 3, 4, 4.7, 7, and 9.4T. Magn Reson Med. 2008;59:236–244 [DOI] [PubMed] [Google Scholar]
- 32. Jensen JE, Licata SC, Ongür D, et al. Quantification of J-resolved proton spectra in two-dimensions with LCModel using GAMMA-simulated basis sets at 4 Tesla. NMR Biomed. 2009;22:762–769 [DOI] [PubMed] [Google Scholar]
- 33. Henry ME, Lauriat TL, Shanahan M, Renshaw PF, Jensen JE. Accuracy and stability of measuring GABA, glutamate, and glutamine by proton magnetic resonance spectroscopy: a phantom study at 4 Tesla. J Magn Reson. 2011;208:210–218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders, Non-patient Edition. New York, NY: Biometrics Research Department, New York State Psychiatric Institute; 1997. (Japanese translation: Kitamura T, Okano T. Tokyo, Japan: Nihon Hyoron-sha Publishers; 2003). [Google Scholar]
- 35. Iwashiro N, Suga M, Takano Y, et al. Localized gray matter volume reductions in the pars triangularis of the inferior frontal gyrus in individuals at clinical high-risk for psychosis and first episode for schizophrenia. Schizophr Res. 2012;137:124–131 [DOI] [PubMed] [Google Scholar]
- 36. McGlashan TH, Miller TJ, Woods SW. Structured Interview for Prodromal Syndromes (Version 3.0). New Haven, CT: PRIME Research Clinic, Yale School of Medicine; 2001 [Google Scholar]
- 37. Kay SR, Fiszbein A, Opler LA. The Positive and Negative Syndrome Scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–276 [DOI] [PubMed] [Google Scholar]
- 38. Barneveld PS, Pieterse J, de Sonneville L, et al. Overlap of autistic and schizotypal traits in adolescents with autism spectrum disorders. Schizophr Res. 2011;126:231–236 [DOI] [PubMed] [Google Scholar]
- 39. Solomon M, Olsen E, Niendam T, et al. From lumping to splitting and back again: atypical social and language development in individuals with clinical-high-risk for psychosis, first episode schizophrenia, and autism spectrum disorders. Schizophr Res. 2011;131:146–151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Aoki Y, Kasai K, Yamasue H. Age-related change in brain metabolite abnormalities in autism: a meta-analysis of proton magnetic resonance spectroscopy studies. Transl Psychiatry. 2012;2:e69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Brans RG, van Haren NE, van Baal GC, Schnack HG, Kahn RS, Hulshoff Pol HE. Heritability of changes in brain volume over time in twin pairs discordant for schizophrenia. Arch Gen Psychiatry. 2008;65:1259–1268 [DOI] [PubMed] [Google Scholar]
- 42. Hollingshead AB. Two Factor Index of Social Position. New Haven, CT: Yale Univ Press; 1965 [Google Scholar]
- 43. Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113 [DOI] [PubMed] [Google Scholar]
- 44. Matsuoka K, Uno M, Kasai K, Koyama K, Kim Y. Estimation of premorbid IQ in individuals with Alzheimer’s disease using Japanese ideographic script (Kanji) compound words: Japanese version of National Adult Reading Test. Psychiatry Clin Neurosci. 2006;60:332–339 [DOI] [PubMed] [Google Scholar]
- 45. Uetsuki M, Matsuoka K, Kim Y, et al. Estimation of premorbid IQ by JART in schizophrenia. Seishin Igaku. 2006;48:15–22 [Google Scholar]
- 46. Aoki Y, Abe O, Yahata N, et al. Absence of age-related prefrontal NAA change in adults with autism spectrum disorders. Transl Psychiatry. 2012;2:e178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Yamasue H, Fukui T, Fukuda R, et al. 1H-MR spectroscopy and gray matter volume of the anterior cingulate cortex in schizophrenia. Neuroreport. 2002;13:2133–2137 [DOI] [PubMed] [Google Scholar]
- 48. Lutkenhoff ES, van Erp TG, Thomas MA, et al. Proton MRS in twin pairs discordant for schizophrenia. Mol Psychiatry. 2010;15:308–318 [DOI] [PubMed] [Google Scholar]
- 49. Kraguljac NV, Reid MA, White DM, den Hollander J, Lahti AC. Regional decoupling of N-acetyl-aspartate and glutamate in schizophrenia. Neuropsychopharmacology. 2012;37:2635–2642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Reid MA, Stoeckel LE, White DM, et al. Assessments of function and biochemistry of the anterior cingulate cortex in schizophrenia. Biol Psychiatry. 2010;68:625–633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Kegeles LS, Mao X, Stanford AD, et al. Elevated prefrontal cortex γ-aminobutyric acid and glutamate-glutamine levels in schizophrenia measured in vivo with proton magnetic resonance spectroscopy. Arch Gen Psychiatry. 2012;69:449–459 [DOI] [PubMed] [Google Scholar]
- 52. Walter M, Henning A, Grimm S, et al. The relationship between aberrant neuronal activation in the pregenual anterior cingulate, altered glutamatergic metabolism, and anhedonia in major depression. Arch Gen Psychiatry. 2009;66:478–486 [DOI] [PubMed] [Google Scholar]
- 53. Waddell KW, Zanjanipour P, Pradhan S, et al. Anterior cingulate and cerebellar GABA and Glu correlations measured by ¹H J-difference spectroscopy. Magn Reson Imaging. 2011;29:19–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Tal A, Kirov II, Grossman RI, Gonen O. The role of gray and white matter segmentation in quantitative proton MR spectroscopic imaging. NMR Biomed. 2012;25:1392–1400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. O’Neill J, Levitt J, Caplan R, et al. 1H MRSI evidence of metabolic abnormalities in childhood-onset schizophrenia. Neuroimage. 2004;21:1781–1789 [DOI] [PubMed] [Google Scholar]
- 56. Bustillo JR, Rowland LM, Mullins P, et al. 1H-MRS at 4 Tesla in minimally treated early schizophrenia. Mol Psychiatry. 2010;15:629–636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Bartha R, Williamson PC, Drost DJ, et al. Measurement of glutamate and glutamine in the medial prefrontal cortex of never-treated schizophrenic patients and healthy controls by proton magnetic resonance spectroscopy. Arch Gen Psychiatry. 1997;54:959–965 [DOI] [PubMed] [Google Scholar]
- 58. Théberge J, Bartha R, Drost DJ, et al. Glutamate and glutamine measured with 4.0 T proton MRS in never-treated patients with schizophrenia and healthy volunteers. Am J Psychiatry. 2002;159:1944–1946 [DOI] [PubMed] [Google Scholar]
- 59. Bertolino A, Sciota D, Brudaglio F, et al. Working memory deficits and levels of N-acetylaspartate in patients with schizophreniform disorder. Am J Psychiatry. 2003;160:483–489 [DOI] [PubMed] [Google Scholar]
- 60. Fannon D, Simmons A, Tennakoon L, et al. Selective deficit of hippocampal N-acetylaspartate in antipsychotic-naive patients with schizophrenia. Biol Psychiatry. 2003;54:587–598 [DOI] [PubMed] [Google Scholar]
- 61. Yasukawa R, Miyaoka T, Mizuno S, et al. Proton magnetic resonance spectroscopy of the anterior cingulate gyrus, insular cortex and thalamus in schizophrenia associated with idiopathic unconjugated hyperbilirubinemia (Gilbert’s syndrome). J Psychiatry Neurosci. 2005;30:416–422 [PMC free article] [PubMed] [Google Scholar]
- 62. Ohrmann P, Kugel H, Bauer J, et al. Learning potential on the WCST in schizophrenia is related to the neuronal integrity of the anterior cingulate cortex as measured by proton magnetic resonance spectroscopy. Schizophr Res. 2008;106:156–163 [DOI] [PubMed] [Google Scholar]
- 63. Thomas MA, Ke Y, Levitt J, et al. Preliminary study of frontal lobe 1H MR spectroscopy in childhood-onset schizophrenia. J Magn Reson Imaging. 1998;8:841–846 [DOI] [PubMed] [Google Scholar]
- 64. Egerton A, Brugger S, Raffin M, et al. Anterior cingulate glutamate levels related to clinical status following treatment in first-episode schizophrenia. Neuropsychopharmacology. 2012;37:2515–2521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Velakoulis D, Wood SJ, Smith DJ, et al. Increased duration of illness is associated with reduced volume in right medial temporal/anterior cingulate grey matter in patients with chronic schizophrenia. Schizophr Res. 2002;57:43–49 [DOI] [PubMed] [Google Scholar]
- 66. Koo MS, Levitt JJ, Salisbury DF, Nakamura M, Shenton ME, McCarley RW. A cross-sectional and longitudinal magnetic resonance imaging study of cingulate gyrus gray matter volume abnormalities in first-episode schizophrenia and first-episode affective psychosis. Arch Gen Psychiatry. 2008;65:746–760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Premkumar P, Fannon D, Kuipers E, Cooke MA, Simmons A, Kumari V. Association between a longer duration of illness, age and lower frontal lobe grey matter volume in schizophrenia. Behav Brain Res. 2008;193:132–139 [DOI] [PubMed] [Google Scholar]
- 68. Olney JW, Farber NB. Glutamate receptor dysfunction and schizophrenia. Arch Gen Psychiatry. 1995;52:998–1007 [DOI] [PubMed] [Google Scholar]
- 69. McCarley RW, Hsiao JK, Freedman R, Pfefferbaum A, Donchin E. Neuroimaging and the cognitive neuroscience of schizophrenia. Schizophr Bull. 1996;22:703–725 [DOI] [PubMed] [Google Scholar]
- 70. Clark JF, Doepke A, Filosa JA, et al. N-acetylaspartate as a reservoir for glutamate. Med Hypotheses. 2006;67:506–512 [DOI] [PubMed] [Google Scholar]
- 71. Harrison PJ. The neuropathology of schizophrenia. A critical review of the data and their interpretation. Brain. 1999;122 (Pt 4):593–624 [DOI] [PubMed] [Google Scholar]
- 72. Meyer-Lindenberg A. Neuroimaging and the question of neurodegeneration in schizophrenia. Prog Neurobiol. 2011;95:514–516 [DOI] [PubMed] [Google Scholar]
- 73. Kim H, McGrath BM, Silverstone PH. A review of the possible relevance of inositol and the phosphatidylinositol second messenger system (PI-cycle) to psychiatric disorders–focus on magnetic resonance spectroscopy (MRS) studies. Hum Psychopharmacol. 2005;20:309–326 [DOI] [PubMed] [Google Scholar]
- 74. Yoo SY, Yeon S, Choi CH, et al. Proton magnetic resonance spectroscopy in subjects with high genetic risk of schizophrenia: investigation of anterior cingulate, dorsolateral prefrontal cortex and thalamus. Schizophr Res. 2009;111:86–93 [DOI] [PubMed] [Google Scholar]
- 75. Sijens PE, Knopp MV, Brunetti A, et al. 1H MR spectroscopy in patients with metastatic brain tumors: a multicenter study. Magn Reson Med. 1995;33:818–826 [DOI] [PubMed] [Google Scholar]
- 76. Moore CM, Bonello CM, Sherwood AR, Cohen BM, Renshaw PF, Yurgulen-Todd DA. Mesial temporal lobe Cho to Cr(PCr) ratio asymmetry in chronic schizophrenics. Schizophr Res. 2002;57:35–42 [DOI] [PubMed] [Google Scholar]
- 77. Szulc A, Galinska B, Tarasow E, et al. The effect of risperidone on metabolite measures in the frontal lobe, temporal lobe, and thalamus in schizophrenic patients. A proton magnetic resonance spectroscopy (1H MRS). Pharmacopsychiatry. 2005; 38:214–219 [DOI] [PubMed] [Google Scholar]
- 78. Szulc A, Galinska B, Tarasow E, et al. Proton magnetic resonance spectroscopy study of brain metabolite changes after antipsychotic treatment. Pharmacopsychiatry. 2011;44:148–157 [DOI] [PubMed] [Google Scholar]
- 79. Goto N, Yoshimura R, Kakeda S, et al. Six-month treatment with atypical antipsychotic drugs decreased frontal-lobe levels of glutamate plus glutamine in early-stage first-episode schizophrenia. Neuropsychiatr Dis Treat. 2012;8:119–122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Qayyum A. MR spectroscopy of the liver: principles and clinical applications. Radiographics 2009;29:1653–1664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Fusar-Poli P, Deste G, Smieskova R, et al. Cognitive functioning in prodromal psychosis: a meta-analysis. Arch Gen Psychiatry. 2012;69:562–571 [DOI] [PubMed] [Google Scholar]
- 82. Wijtenburg SA, Knight-Scott J. Very short echo time improves the precision of glutamate detection at 3T in 1H magnetic resonance spectroscopy. J Magn Reson Imaging. 2011;34:645–652 [DOI] [PubMed] [Google Scholar]
- 83. Mullins PG, Chen H, Xu J, Caprihan A, Gasparovic C. Comparative reliability of proton spectroscopy techniques designed to improve detection of J-coupled metabolites. Magn Reson Med. 2008;60:964–969 [DOI] [PubMed] [Google Scholar]
- 84. Seeger U, Klose U, Mader I, et al. Parameterized evaluation of macromolecules and lipids in proton MR spectroscopy of brain diseases. Magn Reson Med. 2003;49:19–28 [DOI] [PubMed] [Google Scholar]
- 85. Auer DP, Gossl C, Schirmer T, et al. Improved analysis of 1H-MR spectra in the presence of mobile lipids. Magn Reson Med. 2001;46:615–618 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.