Abstract
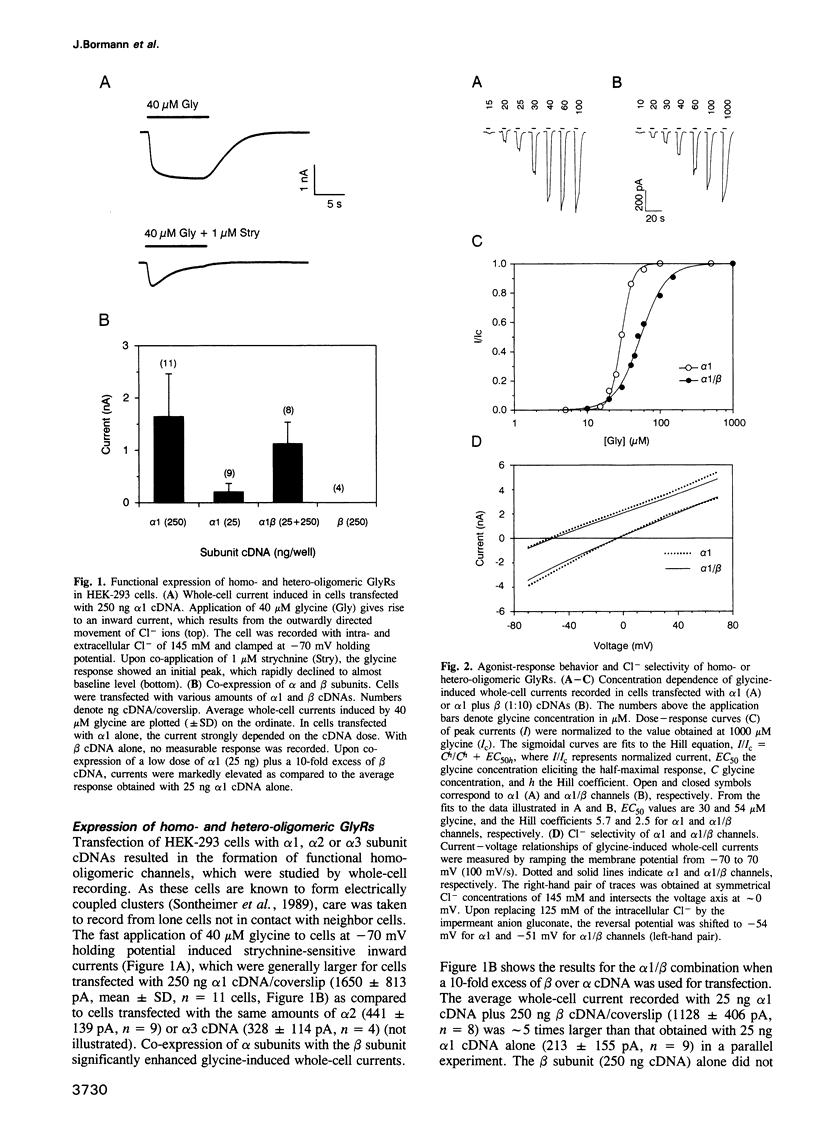
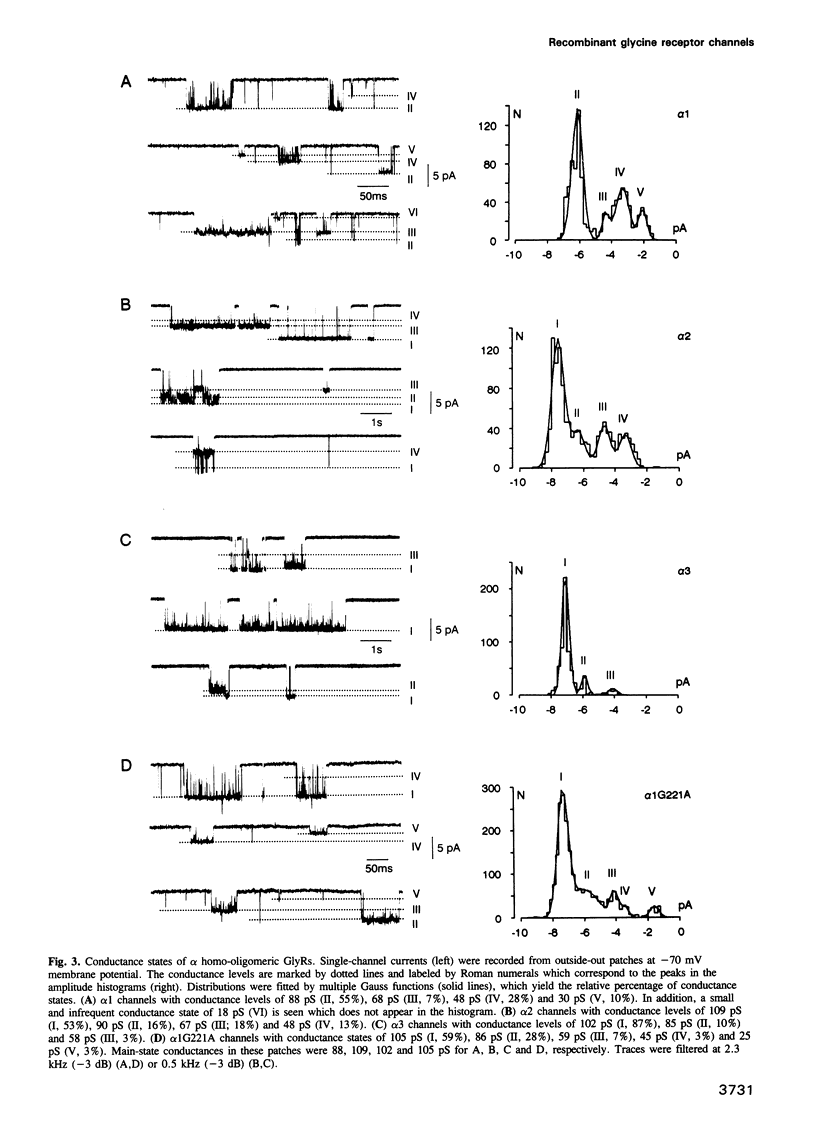
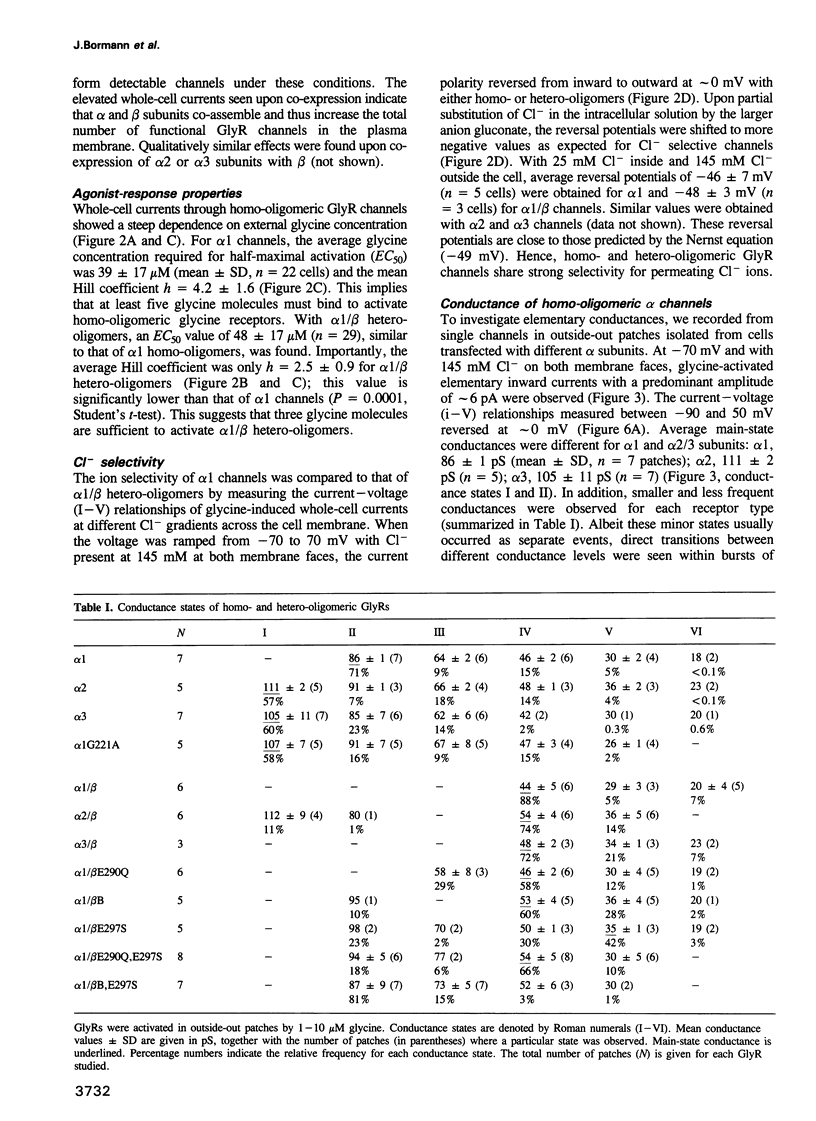
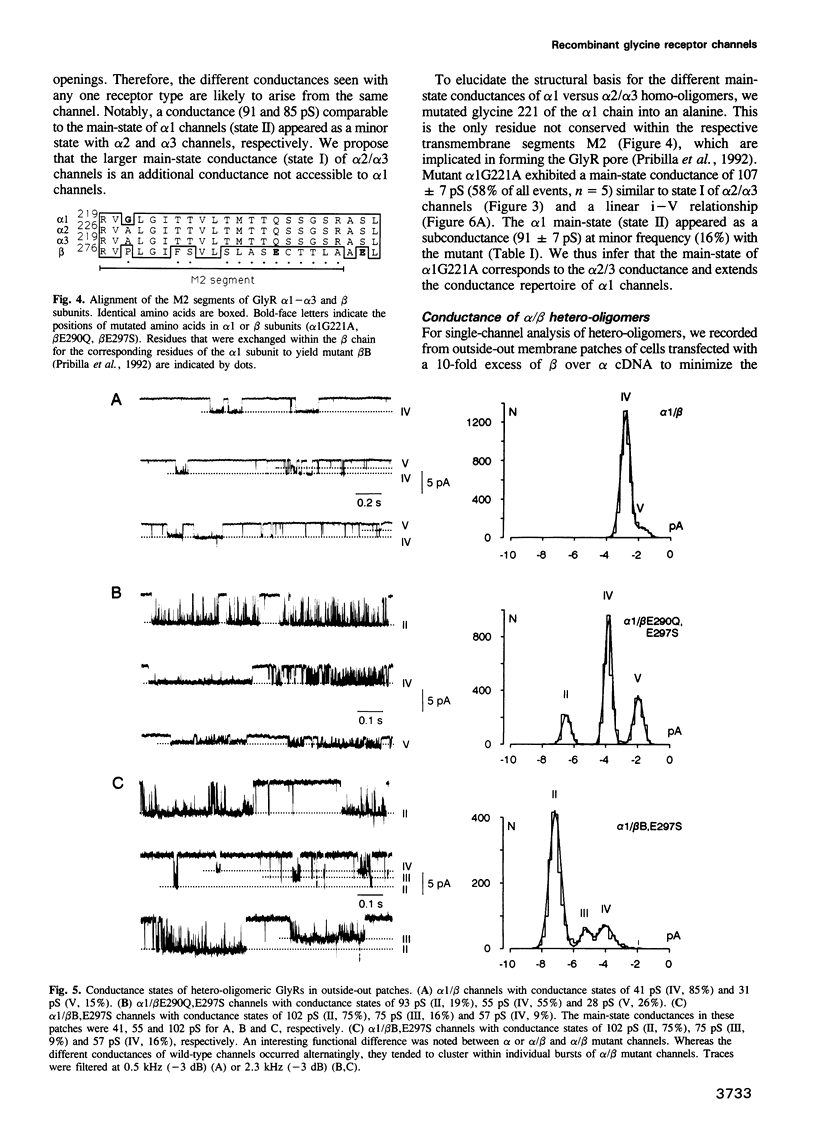
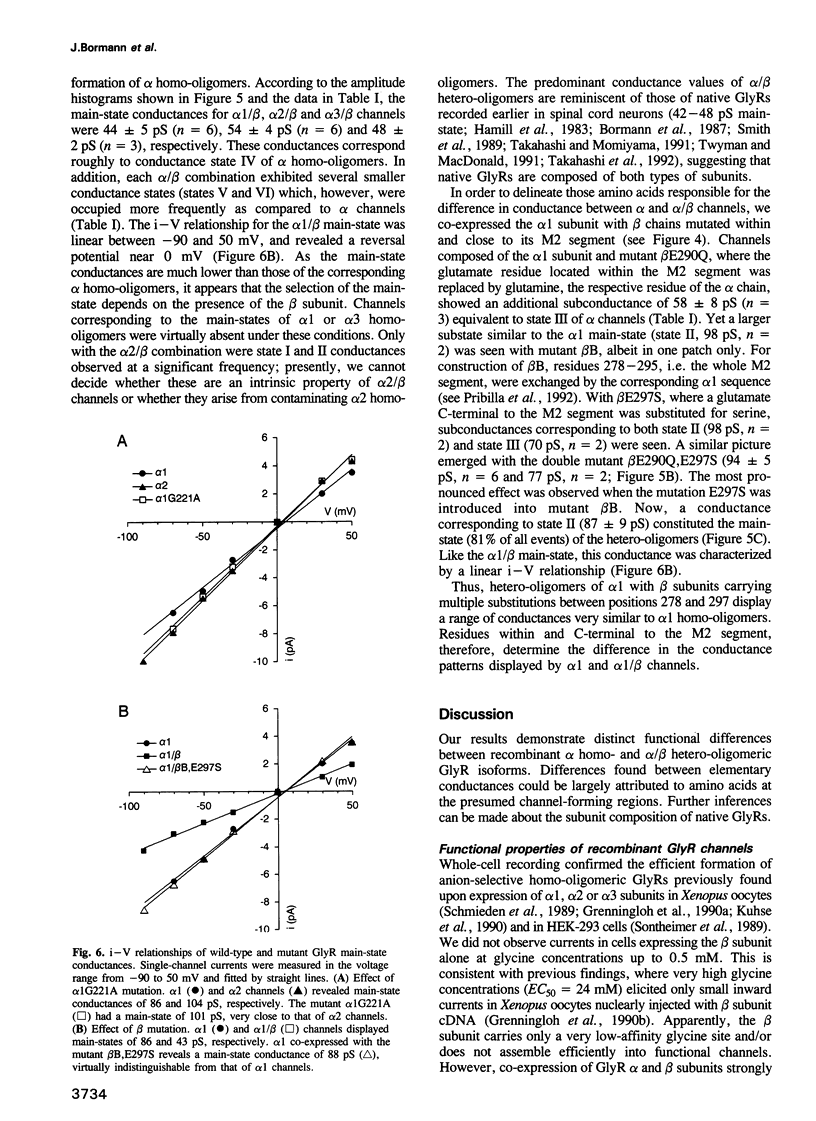
We have expressed glycine receptor (GlyR) alpha and beta subunit cDNAs in HEK-293 cells to study the functional properties of homo- versus hetero-oligomeric GlyR channels. Dose-response curves of whole-cell currents in cells expressing alpha 1 subunits revealed an average Hill coefficient of h = 4.2. Co-expression with the beta subunit markedly increased glycine-gated whole-cell currents, which now exhibited a mean Hill coefficient of only h = 2.5. For alpha 1, alpha 2 and alpha 3 homo-oligomers, the main-state single-channel conductances were 86, 111 and 105 pS, respectively, recorded at symmetrical Cl- concentrations of 145 mM. The mutant alpha 1 G221A gave rise to a main-state of 107 pS. This indicates that the main-state of alpha homo-oligomers depends on residue 221 which is located within transmembrane segment M2. Importantly, the main-state conductances of alpha 1/beta, alpha 2/beta and alpha 3/beta hetero-oligomers were only 44, 54 and 48 pS, respectively. The latter values are similar to those found in spinal neurons, suggesting that native GlyRs are predominantly alpha/beta hetero-oligomers. Co-expression of alpha 1 with mutant beta subunits revealed that residues within and close to segment M2 of the beta subunit determine the conductance differences between homo- and hetero-oligomers.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akaike N., Kaneda M. Glycine-gated chloride current in acutely isolated rat hypothalamic neurons. J Neurophysiol. 1989 Dec;62(6):1400–1409. doi: 10.1152/jn.1989.62.6.1400. [DOI] [PubMed] [Google Scholar]
- Becker C. M., Hoch W., Betz H. Glycine receptor heterogeneity in rat spinal cord during postnatal development. EMBO J. 1988 Dec 1;7(12):3717–3726. doi: 10.1002/j.1460-2075.1988.tb03255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker C. M., Schmieden V., Tarroni P., Strasser U., Betz H. Isoform-selective deficit of glycine receptors in the mouse mutant spastic. Neuron. 1992 Feb;8(2):283–289. doi: 10.1016/0896-6273(92)90295-o. [DOI] [PubMed] [Google Scholar]
- Betz H. Structure and function of inhibitory glycine receptors. Q Rev Biophys. 1992 Nov;25(4):381–394. doi: 10.1017/s0033583500004340. [DOI] [PubMed] [Google Scholar]
- Bormann J., Hamill O. P., Sakmann B. Mechanism of anion permeation through channels gated by glycine and gamma-aminobutyric acid in mouse cultured spinal neurones. J Physiol. 1987 Apr;385:243–286. doi: 10.1113/jphysiol.1987.sp016493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C., Okayama H. High-efficiency transformation of mammalian cells by plasmid DNA. Mol Cell Biol. 1987 Aug;7(8):2745–2752. doi: 10.1128/mcb.7.8.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claudio T., Paulson H. L., Green W. N., Ross A. F., Hartman D. S., Hayden D. Fibroblasts transfected with Torpedo acetylcholine receptor beta-, gamma-, and delta-subunit cDNAs express functional receptors when infected with a retroviral alpha recombinant. J Cell Biol. 1989 Jun;108(6):2277–2290. doi: 10.1083/jcb.108.6.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feigenspan A., Wässle H., Bormann J. Pharmacology of GABA receptor Cl- channels in rat retinal bipolar cells. Nature. 1993 Jan 14;361(6408):159–162. doi: 10.1038/361159a0. [DOI] [PubMed] [Google Scholar]
- Galzi J. L., Devillers-Thiéry A., Hussy N., Bertrand S., Changeux J. P., Bertrand D. Mutations in the channel domain of a neuronal nicotinic receptor convert ion selectivity from cationic to anionic. Nature. 1992 Oct 8;359(6395):500–505. doi: 10.1038/359500a0. [DOI] [PubMed] [Google Scholar]
- Gorman C. M., Gies D., McCray G., Huang M. The human cytomegalovirus major immediate early promoter can be trans-activated by adenovirus early proteins. Virology. 1989 Aug;171(2):377–385. doi: 10.1016/0042-6822(89)90605-3. [DOI] [PubMed] [Google Scholar]
- Grenningloh G., Pribilla I., Prior P., Multhaup G., Beyreuther K., Taleb O., Betz H. Cloning and expression of the 58 kd beta subunit of the inhibitory glycine receptor. Neuron. 1990 Jun;4(6):963–970. doi: 10.1016/0896-6273(90)90149-a. [DOI] [PubMed] [Google Scholar]
- Grenningloh G., Rienitz A., Schmitt B., Methfessel C., Zensen M., Beyreuther K., Gundelfinger E. D., Betz H. The strychnine-binding subunit of the glycine receptor shows homology with nicotinic acetylcholine receptors. Nature. 1987 Jul 16;328(6127):215–220. doi: 10.1038/328215a0. [DOI] [PubMed] [Google Scholar]
- Grenningloh G., Schmieden V., Schofield P. R., Seeburg P. H., Siddique T., Mohandas T. K., Becker C. M., Betz H. Alpha subunit variants of the human glycine receptor: primary structures, functional expression and chromosomal localization of the corresponding genes. EMBO J. 1990 Mar;9(3):771–776. doi: 10.1002/j.1460-2075.1990.tb08172.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Bormann J., Sakmann B. Activation of multiple-conductance state chloride channels in spinal neurones by glycine and GABA. 1983 Oct 27-Nov 2Nature. 305(5937):805–808. doi: 10.1038/305805a0. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hoch W., Betz H., Becker C. M. Primary cultures of mouse spinal cord express the neonatal isoform of the inhibitory glycine receptor. Neuron. 1989 Sep;3(3):339–348. doi: 10.1016/0896-6273(89)90258-4. [DOI] [PubMed] [Google Scholar]
- Hurtley S. M., Helenius A. Protein oligomerization in the endoplasmic reticulum. Annu Rev Cell Biol. 1989;5:277–307. doi: 10.1146/annurev.cb.05.110189.001425. [DOI] [PubMed] [Google Scholar]
- Imoto K., Busch C., Sakmann B., Mishina M., Konno T., Nakai J., Bujo H., Mori Y., Fukuda K., Numa S. Rings of negatively charged amino acids determine the acetylcholine receptor channel conductance. Nature. 1988 Oct 13;335(6191):645–648. doi: 10.1038/335645a0. [DOI] [PubMed] [Google Scholar]
- Imoto K., Konno T., Nakai J., Wang F., Mishina M., Numa S. A ring of uncharged polar amino acids as a component of channel constriction in the nicotinic acetylcholine receptor. FEBS Lett. 1991 Sep 9;289(2):193–200. doi: 10.1016/0014-5793(91)81068-j. [DOI] [PubMed] [Google Scholar]
- Kuhse J., Schmieden V., Betz H. Identification and functional expression of a novel ligand binding subunit of the inhibitory glycine receptor. J Biol Chem. 1990 Dec 25;265(36):22317–22320. [PubMed] [Google Scholar]
- Langosch D., Hartung K., Grell E., Bamberg E., Betz H. Ion channel formation by synthetic transmembrane segments of the inhibitory glycine receptor--a model study. Biochim Biophys Acta. 1991 Mar 18;1063(1):36–44. doi: 10.1016/0005-2736(91)90350-h. [DOI] [PubMed] [Google Scholar]
- Langosch D., Thomas L., Betz H. Conserved quaternary structure of ligand-gated ion channels: the postsynaptic glycine receptor is a pentamer. Proc Natl Acad Sci U S A. 1988 Oct;85(19):7394–7398. doi: 10.1073/pnas.85.19.7394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis C. A., Ahmed Z., Faber D. S. A characterization of glycinergic receptors present in cultured rat medullary neurons. J Neurophysiol. 1991 Oct;66(4):1291–1303. doi: 10.1152/jn.1991.66.4.1291. [DOI] [PubMed] [Google Scholar]
- Lewis C. A., Ahmed Z., Faber D. S. Characteristics of glycine-activated conductances in cultured medullary neurons from embryonic rat. Neurosci Lett. 1989 Jan 16;96(2):185–190. doi: 10.1016/0304-3940(89)90055-4. [DOI] [PubMed] [Google Scholar]
- Malosio M. L., Marquèze-Pouey B., Kuhse J., Betz H. Widespread expression of glycine receptor subunit mRNAs in the adult and developing rat brain. EMBO J. 1991 Sep;10(9):2401–2409. doi: 10.1002/j.1460-2075.1991.tb07779.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pribilla I., Takagi T., Langosch D., Bormann J., Betz H. The atypical M2 segment of the beta subunit confers picrotoxinin resistance to inhibitory glycine receptor channels. EMBO J. 1992 Dec;11(12):4305–4311. doi: 10.1002/j.1460-2075.1992.tb05529.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmieden V., Grenningloh G., Schofield P. R., Betz H. Functional expression in Xenopus oocytes of the strychnine binding 48 kd subunit of the glycine receptor. EMBO J. 1989 Mar;8(3):695–700. doi: 10.1002/j.1460-2075.1989.tb03428.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S. M., Zorec R., McBurney R. N. Conductance states activated by glycine and GABA in rat cultured spinal neurones. J Membr Biol. 1989 Apr;108(1):45–52. doi: 10.1007/BF01870424. [DOI] [PubMed] [Google Scholar]
- Sontheimer H., Becker C. M., Pritchett D. B., Schofield P. R., Grenningloh G., Kettenmann H., Betz H., Seeburg P. H. Functional chloride channels by mammalian cell expression of rat glycine receptor subunit. Neuron. 1989 May;2(5):1491–1497. doi: 10.1016/0896-6273(89)90195-5. [DOI] [PubMed] [Google Scholar]
- Takahashi T., Momiyama A., Hirai K., Hishinuma F., Akagi H. Functional correlation of fetal and adult forms of glycine receptors with developmental changes in inhibitory synaptic receptor channels. Neuron. 1992 Dec;9(6):1155–1161. doi: 10.1016/0896-6273(92)90073-m. [DOI] [PubMed] [Google Scholar]
- Takahashi T., Momiyama A. Single-channel currents underlying glycinergic inhibitory postsynaptic responses in spinal neurons. Neuron. 1991 Dec;7(6):965–969. doi: 10.1016/0896-6273(91)90341-v. [DOI] [PubMed] [Google Scholar]
- Tokutomi N., Kaneda M., Akaike N. What confers specificity on glycine for its receptor site? Br J Pharmacol. 1989 Jun;97(2):353–360. doi: 10.1111/j.1476-5381.1989.tb11961.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twyman R. E., Macdonald R. L. Kinetic properties of the glycine receptor main- and sub-conductance states of mouse spinal cord neurones in culture. J Physiol. 1991 Apr;435:303–331. doi: 10.1113/jphysiol.1991.sp018512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdoorn T. A., Burnashev N., Monyer H., Seeburg P. H., Sakmann B. Structural determinants of ion flow through recombinant glutamate receptor channels. Science. 1991 Jun 21;252(5013):1715–1718. doi: 10.1126/science.1710829. [DOI] [PubMed] [Google Scholar]
- Villarroel A., Herlitze S., Koenen M., Sakmann B. Location of a threonine residue in the alpha-subunit M2 transmembrane segment that determines the ion flow through the acetylcholine receptor channel. Proc Biol Sci. 1991 Jan 22;243(1306):69–74. doi: 10.1098/rspb.1991.0012. [DOI] [PubMed] [Google Scholar]
- Werman R., Davidoff R. A., Aprison M. H. Inhibition of motoneurones by iontophoresis of glycine. Nature. 1967 May 13;214(5089):681–683. doi: 10.1038/214681a0. [DOI] [PubMed] [Google Scholar]


