Abstract
Multiple sclerosis (MS) is an inflammatory central nervous system (CNS) disorder, which typically occurs in early adulthood while it is rare in children. We tested in the MS-model, experimental autoimmune encephalomyelitis (EAE), whether functional maturation of innate immune cells may determine susceptibility to CNS autoimmune disease. 2 week-old mice were resistant to active EAE causing fulminant paralysis in adult mice, which was associated with an impaired development of Th1- and Th17 cells. Resistant, young mice contained a higher frequency of myeloid derived suppressor cells and plasmacytoid dendritic cells. Further, myeloid antigen-presenting cells (APC) as well as B-cells expressed lower levels of MHC class II and CD40, produced decreased amounts of pro-inflammatory cytokines, while release of anti-inflammatory IL-10 was enhanced. When used as APC, splenocytes from 2 week-old mice failed to differentiate naïve T-cells into Th1- and Th17 cells irrespective of the T-cell donor's age, and promoted development of regulatory T-cells and Th2 cells instead. Adoptive transfer of adult APC restored the ability of 2 week-old mice to generate encephalitogenic T-cells and to develop EAE. Collectively, these findings indicate that the innate immune cell compartment functionally matures during development which may be a prerequisite for development of T-cell-mediated CNS autoimmune disease.
Keywords: multiple sclerosis, experimental autoimmune encephalomyelitis, age, susceptibility, antigen-presenting cells, MHC class II, co-stimulatory molecules, myeloid-derived suppressor cells, plasmacytoid dendritic cells, development, maturation
Introduction
Multiple sclerosis (MS) is the most common inflammatory demyelinating disorder of the central nervous system (CNS) in humans [1]. Its prevalence peaks in early adulthood, while a first-time diagnosis of MS before puberty is remarkably rare [2]. Furthermore, the estimated 5% of MS cases with an onset before the age of 16 have a better clinical prognosis [3]. Pathophysiological mechanisms by which the risk to develop MS may increase after childhood are largely unknown.
Much of our current knowledge regarding the assumed autoimmune pathogenesis of MS derived from experimental autoimmune encephalomyelitis (EAE), the animal model of MS. Activated, myelin-reactive CD4+ Th1 cells are thought to have a central role in the pathogenesis of both MS and EAE [4]. Initial activation of CD4+ T cells occurs through recognition of antigen presented in the context of MHC class II. Processing of antigen and presentation of linearized peptides is provided by MHC II-expressing antigen presenting cells (APC) [5], such as myeloid monocytes and macrophages, dendritic cells as well as B cells. Following antigen recognition, efficient activation of CD4+ T cells requires further ligation with co-stimulatory molecules expressed on the APC surface. Besides density of MHC II expression [6,7] and composition of co-stimulatory molecules [8,9], the fate of the corresponding T cell to either differentiate into a pro-inflammatory Th1 or Th17 phenotype or to alternatively develop into an anti-inflammatory Th2 cell or regulatory T cell is determined by the cytokine milieu present at the site of APC-T cell interaction [10,11]. Thus, a variety of signals provided by the APC is required for efficient development of pro-inflammatory T cells in vivo. Based on this conception, we tested in the EAE model, whether an age-associated alteration of innate immune cell function may determine susceptibility to CNS autoimmune disease.
Results
Susceptibility to experimental autoimmune encephalitis is age-dependent
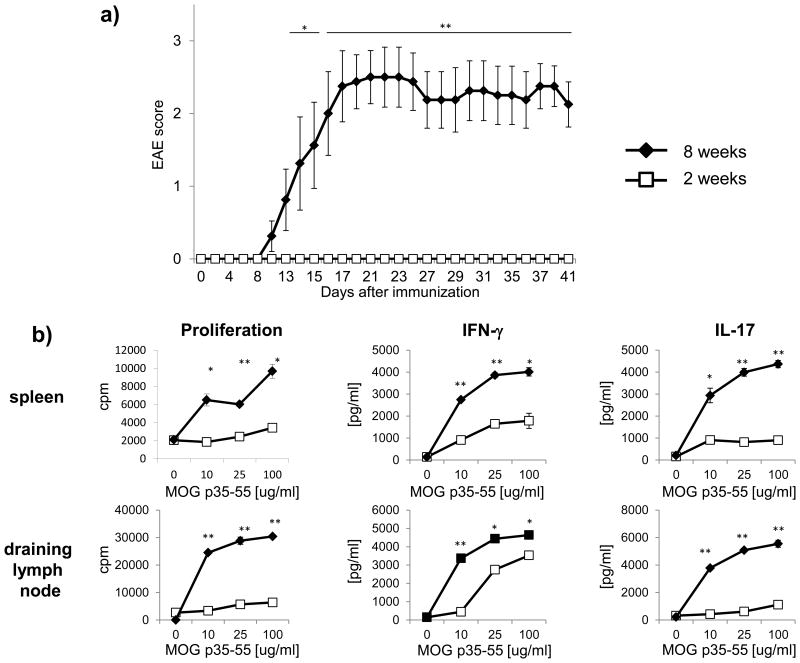
EAE is traditionally induced by active immunization with CNS auto-antigen in 8 to 20 week old mice, as EAE susceptibility is maximal at this age [12]. To establish that susceptibility may be lower at an earlier age, EAE was induced in C57Bl/6 mice at the age of 2 weeks using an active immunization protocol with MOG p35-55 in CFA and PTx. As indicated in figure 1a, none of the 2 week old mice showed any clinical signs of EAE (0/13), whereas 8/8 mice at the age of 8 weeks developed ascending paralysis around day 10 after immunization. 12 days after immunization, a subgroup of mice was analyzed for development of myelin-reactive T cells. As shown in figure 1b, splenocytes from 2 week old mice revealed a strongly reduced proliferation of T cells in response to MOG p35-55. Furthermore, secretion of IFN-γ and IL-17 was decreased suggesting that EAE resistance of 2 week old mice relates to an inability of younger mice to generate encephalitogenic T cells.
Figure 1.
a) C57Bl/6 mice at the age of 2 weeks (12 mice) or 8 weeks (8 mice) were immunized with 100 μg MOG p35-55 followed by PTx injections (200 ng) on day 0 and 2. EAE severity was scored on a scale from 0-5 and presented as mean group score +/-SEM. Significance of differences between the groups was calculated using the Mann-Whitney-U Test. * p ≤ 0.05; ** p ≤ 0.001. Shown is one representative out of three independent experiments. b) 12 days after immunization, 3 additional mice per group were sacrificed and T cell responses to MOG p35-55 in spleen and draining lymph nodes were evaluated by [3H]-thymidine incorporation (proliferation, in counts per minute; cpm) and ELISA (production of IFN-γ or IL-17). Data was obtained in triplicates and is presented as mean +/- SEM. Significance of differences of mean values was calculated using unpaired two-sided student's t-Test. * p ≤ 0.05; ** p ≤ 0.001. Shown is one representative out of three independent experiments.
Frequency and ability of T cells to differentiate into Th1 and Th17 cells is not impaired in 2 week old mice
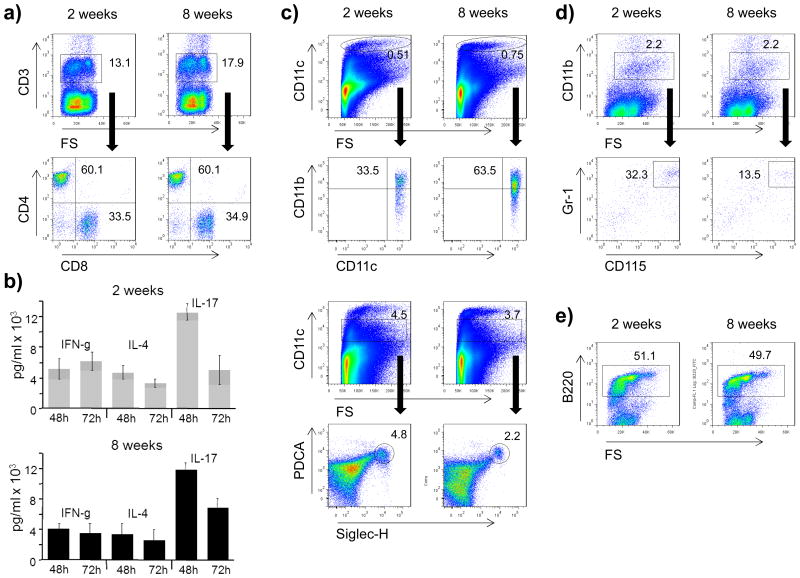
In order to elucidate mechanistically why young mice are unable to generate EAE-inducing, pro-inflammatory T cells, we first confirmed that the frequency of peripheral T cells was unchanged. As indicated in figure 2a, there was no difference in 2 or 8 week old mice in the frequency of total CD3+ T cells as well as the ratio of CD4+ to CD8+ T cells. To investigate whether T cells of 2 week old mice are generally capable of differentiating into encephalitogenic IFN-γ-producing Th1 cells and IL-17-producing Th17 cells, purified splenic T cells were stimulated directly by anti-CD3 and anti-CD28. In the presence of polarizing cytokines, this APC-independent activation regimen generated effector T cells producing equivalent amounts of IFN-γ and IL-17, irrespective of the naïve T cell donor age (figure 2b). When T cell activation was titrated to include lower doses of anti-CD3 in the absence of polarizing cytokines, 2 week old T cells produced even higher amounts of IFN-γ and slightly elevated levels of IL-17 (supplementary figure 1). These findings highlight that T cells are generally capable of differentiating into encephalitogenic Th1- and Th17 cells at the age of 2 weeks, suggesting that an immaturity of peripheral T cells is unlikely to explain EAE resistance in 2 week old mice.
Figure 2.
Splenocytes from 2 or 8 week old mice were evaluated for the frequency of leucocyte subsets by FACS staining identifying a) T cells (CD3; mother gate on lymphocytes) further divided into CD4+ and CD8+ T cells, c) dendritic cells (CD11c; mother gate on viable leucocytes), further divided into myeloid (CD11chiCD11b+) and plasmacytoid (CD11cintPDCA+Siglec-H+) subsets, d) monocytes/macrophages (CD11b; mother gate on viable leucocytes) further analysed by co-expression of CD115/Gr-1, and e) B cells (B220; mother gate on lymphocytes). Black arrows indicate mother gate → daughter population. For a detailed description of the gating strategy see also suppl. Fig. 3. Shown is one representative out of three independent experiments, mean frequencies from all experiments are provided in table 1. b) For APC-independent T cell activation MACS-separated T cells from 2 or 8 week old C57Bl/6 mice were activated by plate-bound anti-CD3 (1 μg/ml) and anti-CD28 (2 μg/ml) in the presence of polarizing cytokines: 5 ng/ml IL-12 for Th1 (evaluated by IFN-γ production), 10 ng/ml IL-4, and 5 μg/ml anti-IFNγ for Th2 (evaluated by IL-4 production), and 25 ng/ml IL-6, 0.5 ng/ml TGF-β, 10 ng/ml IL-1β and 10 ng/ml TNF for Th17 differentiation (evaluated by IL-17 production). Cytokine secretion was measured in triplicates after 48 and 72h by ELISA. Values are presented as mean +/- SEM. Individual values obtained from T cells from 2 week versus 8 week old mice were compared using an unpaired two-sided student's t-Test. None of the values reached significance defined as p ≤ 0.05. Shown is one representative out of two independent experiments.
Antigen-presenting cells of 2 week old mice contain a higher frequency of anti-inflammatory and suppressive phenotypes
Activation and pro-inflammatory differentiation of CD4+ T cells depends on recognition of antigen provided by antigen-presenting cells, such as dendritic cells, monocytes and B cells [13]. Accordingly, we investigated next, whether the insufficiency of young mice to generate encephalitogenic T cells may relate to an age-dependent alteration within the APC compartment. Similar to the investigations on T cells, we first determined that the overall frequency of dendritic cells, monocytes and B cells in 2 week old mice was comparable to adult mice (figure 2c-e, table 1). Recent findings suggest that subclasses of dendritic cells and myeloid cells may differ in their capacity to activate T cells, with subtypes rather suppressing than promoting pro-inflammatory T cell differentiation. In this regard, further phenotyping of dendritic cells revealed that at an age of 2 weeks, mice contained a higher frequency of CD11cintPDCA+Siglec-H+ plasmacytoid dendritic cells which can promote development of regulatory T cells and inhibit CNS autoimmune disease [14]. In contrast, the frequency of CD11b+ myeloid dendritic cells with a strong capacity to generate Th1 and Th17 cell responses, but also to reactivate encephalitogenic T cells in the inflamed CNS [15] was reduced (Figure 2c, table 1). Along the same lines, the frequency of CD115+Gr-1+ myeloid-derived suppressor cells, which can impair expansion and homeostasis of pro-inflammatory T cells [16] and development of EAE [17] was elevated in 2 week old mice (Figure 2d, table 1). Taken together, within the compartment of APC of myeloid origin young mice contained a markedly higher percentage of phenotypes with the potential to suppress autoimmune T cell responses.
Table 1. Frequencies of leucocytes in 2 or 8 week old naïve mice.
Splenocytes were isolated from 3 mice per group and evaluated for frequencies of leukocyte populations by FACS. Subpopulations are presented as frequency within the mother population.
| 2 weeks | 8 weeks | |
|---|---|---|
| CD3+ T cells | 14.2 +/- 3.3 | 17.2 +/- 4.5 |
| CD4+ T cells | 60.0 +/- 1.1 | 60.1 +/- 0.8 |
| CD8+ T cells | 33.8 +/- 0.9 | 34.8 +/- 1.1 |
| CD11chi | 0.51 +/- 0.04 | 0.72 +/- 0.01 * |
| CD11chiCD11b+ mDC | 34.6 +/- 0.5 | 60.5 +/- 1.3 * |
| CD11cint | 5.7 +/- 0.8 | 3.9 +/- 0.1 * |
| CD11cintPDCA+Siglec-H+ pDC | 4.8 +/- 0.3 | 2.2 +/- 0.1 * |
| CD11b+ | 2.2 +/- 0.1 | 2.1 +/- 0.3 |
| CD11b+CD115+Gr-1+ MDSC | 33.1 +/- 0.7 | 13.5 +/- 0.1 * |
| B220+ B cells | 52.0 +/- 2.3 | 49.4 +/- 3.2 |
Shown is the mean +/- SEM;
= p < 0.05;
hi = high,
int = intermediate,
m = myeloid,
p = plasmacytoid,
MDSC = myeloid-derived suppressor cells
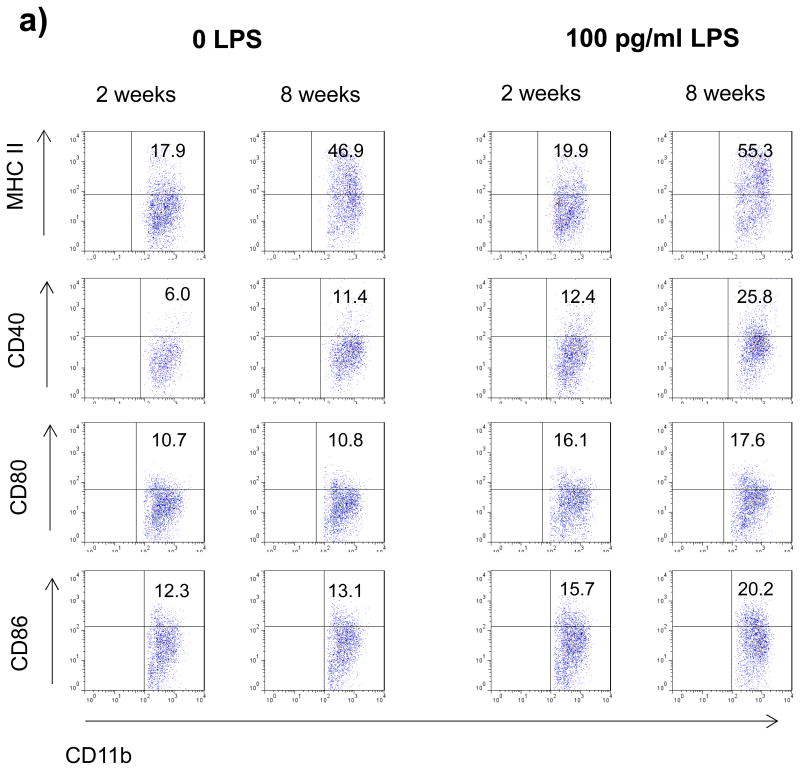
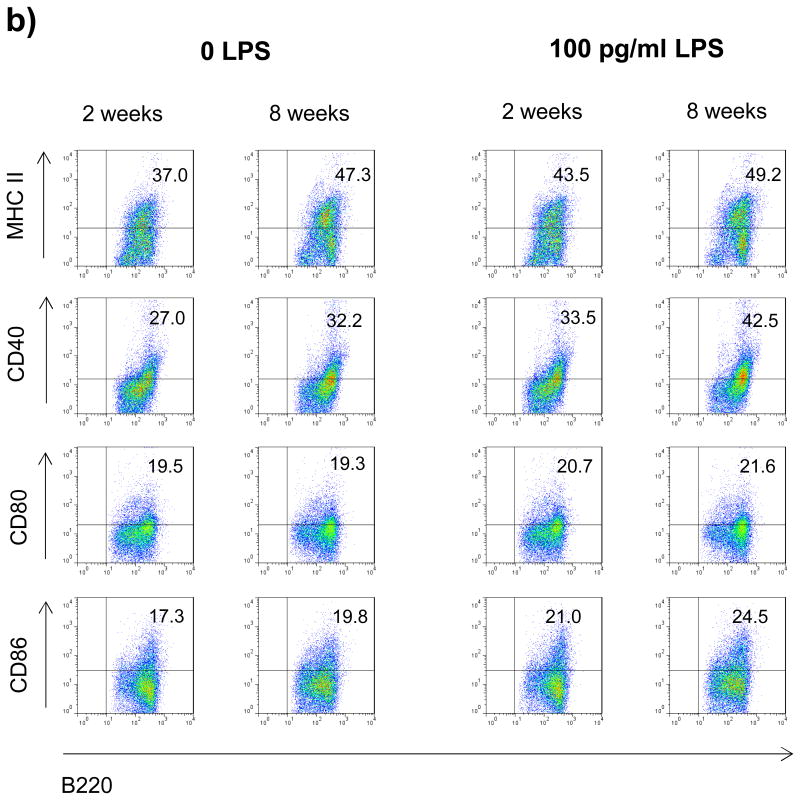
Myeloid APC and B cells of 2 week old mice display a reduced constitutive and inducible expression of MHC class II and co-stimulatory CD40
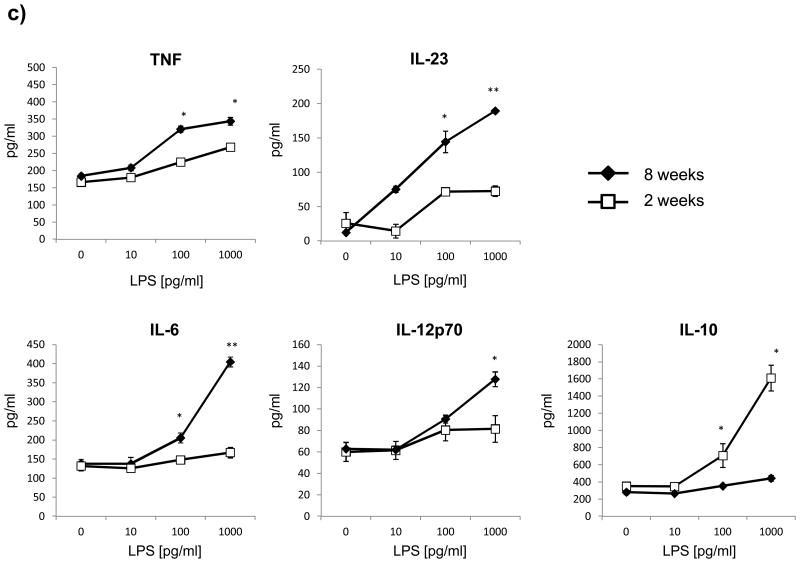
Pro-inflammatory differentiation of CD4+ T cell requires two signals [18]. The first signal is antigen recognition in the context of MHC II via their T cell receptor, the second mandatory interaction consists of ligation of co-stimulatory molecules. In order to investigate whether APC from 2 week old mice may differ in quantity or quality of these signals, myeloid CD11b+ APC as well as B cells from 2 or 8 week old mice were evaluated for surface expression of MHC II and the costimulatory molecules CD40, CD80 and CD86. As shown in figure 3a+b, both myeloid CD11b+ cells as well as B cells from 2 week old mice showed a decreased constitutive and inducible surface expression of MHC class II and CD40, whereas levels of CD80 and CD86 were essentially unchanged. The reduction of MHC II and CD40 was particularly evident on myeloid APC (figure 3a). Besides the composition of co-stimulatory molecules, T cell differentiation is primarily determined by the cytokine milieu present at the time of initial activation [10]. Therefore, 2 or 8 week old splenocytes were evaluated for cytokine production upon stimulation with increasing concentrations of LPS. As shown in figure 3c, 2 week old splenocytes produced significantly lower amounts of the pro-inflammatory cytokines TNF, IL-23, IL-6 and IL-12, while the release of anti-inflammatory IL-10 was enhanced.
Figure 3.
Splenocytes from 2 or 8 week old mice were cultured with 0 or 100 pg/ml LPS for 48h. Surface expression of MHC II and co-stimulatory molecules (CD40, CD80, CD86) by a) myeloid APC (CD11b+; mother gate viable leucocytes) and b) B cells (B220+; mother gate viable leucocytes) was evaluated by FACS staining. For a detailed description of the gating strategy see also suppl. Fig. 3. c) For evaluation of cytokine production splenocytes were stimulated with increasing concentrations of LPS and secretion of TNF, IL-6, IL-23, IL-10 and IL-12 was determined by ELISA after 72h. Data was obtained in triplicates and is presented as mean +/- SEM. Significance of differences of mean values was calculated using unpaired two-sided student's t-Test. * p ≤ 0.05; ** p ≤ 0.001. Shown is one representative out of two independent experiments.
APC from older mice drive efficient T cell activation and pro-inflammatory differentiation while APC from younger mice promote development of regulatory T cells
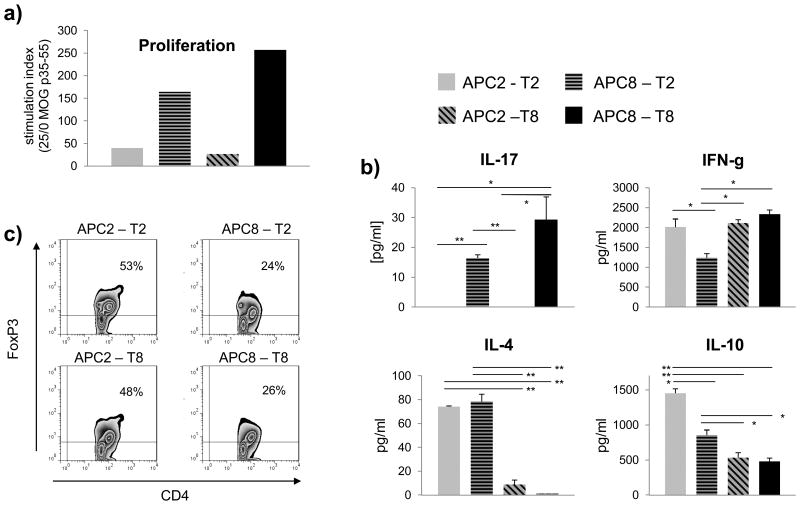
The data acquired to this point suggested that the inability to generate an encephalitogenic T cell response and to induce CNS autoimmune disease could refer to the immature phenotype of APC in younger mice with an insufficient expression of MHC II as well as to a higher frequency of phenotypes with regulatory and/or suppressive properties. To elucidate this possibility functionally, we co-cultured APC and purified T cells obtained from 2 week or 8 week old mice in the presence of antigen in a cross-over design [19]. Splenic APC were obtained from wild-type C57Bl/6 mice, whereas T cells were isolated from MOG p35-55 T cell receptor transgenic mice. As indicated in figure 4a, myelin-reactive T cells proliferated irrespective of their own age when activated by APC obtained from 8 week old mice. 2 week old APC failed to induce proliferation of both 2 week and 8 week old myelin-reactive T cells. Along the same lines, only 8 week old, but not two week old APC promoted development ofTh17 cells, while release of IFN-γ was only reduced when APC were 8 week and T cells 2 week old (figure 4b). Based on the observation that certain phenotypes of APC, such as plasmacytoid DC are capable of promoting development of anti-inflammatory T cell phenotypes instead [20], we expanded our investigations to generation of Th2 cells and CD4+CD25+FoxP3+ regulatory T cells. As indicated in figure 4b+c, two week old APC in contact with two week old T cells promoted development of Th2 cells and Treg as evaluated by release of IL-4, IL-10 or expression of FoxP3, respectively. In conjunction with the observation that T cell differentiation upon direct, APC-independent activation of T cells did not markedly differ between 2 or 8 week old mice (Figure 2b and supplementary figure 1), these data corroborate that the age of the APC rather than the age of the corresponding T cell determines development of encephalitogenic T cells.
Figure 4.
For evaluation of APC-dependent T cell activation, 5 × 105 splenocytes (MACS-depleted of T cells) from naïve 2 or 8 week old wild-type C57Bl/6 mice (APC2/8) were co-cultured with 1 × 104 naïve T cells isolated from 2 or 8 week old MOG T cell receptor transgenic mice (T2/8) in the presence of 0, 1, 10 and 25 μg/ml MOG p35-55. T cell activation and differentiation was evaluated by a) proliferation ([3H]-thymidine incorporation; simulation index as ratio 25/0 μg/ml MOG p35-55), b) production of IL-17, IFN-γ, IL-4, IL-10 (ELISA; cytokine production at 25 μg/ml MOG p35-55) and c) development of regulatory T cells (CD4/CD25/FoxP3 FACS staining at 5 μg/ml MOG p35-55). Shown is one of two independent experiments. Cytokine secretion is presented as mean of ELISA triplicates +/- SEM. Significance of differences of mean values was calculated using unpaired two-sided student's t-Test. * p ≤ 0.05; ** p ≤ 0.001.
Peripheral and CNS MHC class II expression increases with age
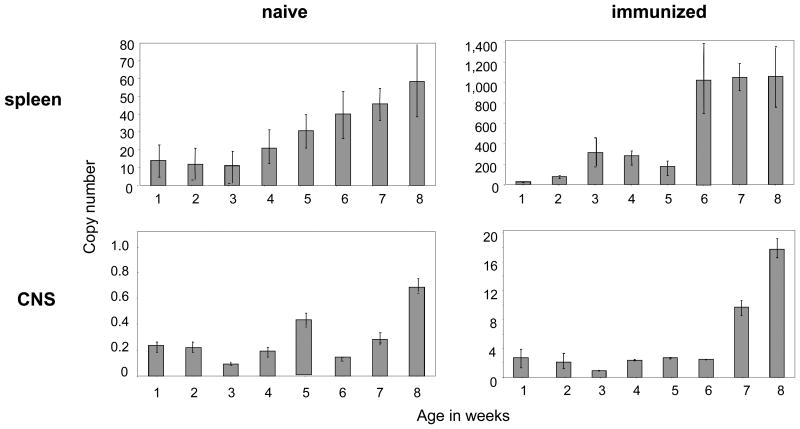
In order to further elaborate the association between MHC class II upregulation, APC maturation and age, we investigated the expression of MHC II mRNA starting in newborn mice over the period of 8 weeks. Importantly, since most APC stabilize MHC class II surface molecules, the amount of MHC class II mRNA does not necessarily reflect its surface expression, but rather the relative upregulation of MHC class II. As indicated in figure 5, splenocytes from naïve mice contained a consistently low overall copy number of MHC class II RNA up to the age of 3 weeks. From week 4 on, MHC class II copy numbers continuously increased through week 8. A similar scenario occurred in mice immunized with MOG p35-55, although the upregulation of MHC II appeared to be more abrupt between week 5 and 6. We applied the same technique to evaluate upregulation of MHC II within the CNS. Here, the copy numbers also increased in an age-dependent manner in immunized mice, although upregulation of MHC class II appeared to occur at a later age, suggesting that this overall increase in copy numbers within the CNS may primarily relate to infiltration of peripheral immune cells starting to express MHC class II.
Figure 5.
Naïve (left panels) or MOG p35-55 immunized mice (right panels) were evaluated for expression of MHC class II (I-A) in spleen (upper panels) and CNS (lower panels) by RT-PCR at the age indicated (weekly, week 1 through 8). Mice were immunized with 100 μg MOG p35-55 7 days prior to analysis and received 200 ng PTx on the day of immunization and 2 days thereafter. Three separate experiments were performed. Values were generated as RNA fraction of β-actin. Data were checked for normality by using the Kolmogorov-Smirnov test; the mean of values +/- SEM is presented. Mean values from naive versus immunized mice were compared individually using an unpaired two-sided student's t-Test and found to be significantly different for each time point in spleen and CNS (p ≤ 0.001).
Adoptive transfer of adult encephalitogenic T cells fails to induce experimental autoimmune encephalomyelitis in young mice
In order to induce EAE, T cells require MHC class II-restricted activation twice, first in the periphery followed by their reactivation within the CNS [5]. The data presented in the paragraph above indicated that besides peripheral APC function, MHC class II restricted reactivation of T cells within the CNS may be similarly impaired in young mice. To elucidate this possibility we transferred readily primed encephalitogenic T cells from adult mice into 2 week old recipients, an induction regimen which bypasses peripheral APC function. As demonstrated in table 2, encephalitogenic T cells induced EAE in 8 week old recipients, but failed to do so in 2 week old mice. In conjunction with the lower CNS MHC II mRNA expression presented in figure 5, this finding suggests that in young mice both peripheral as well as CNS APC are incapable of sufficiently activating or reactivating auto-reactive T cells, respectively.
Table 2. Adoptive transfer of encephalitogenic T cells fails to induce EAE in 2 week old recipients.
For generation of donor cells, splenocytes from 8 week old MBP Ac 1-11 transgenic mice were stimulated with 6 ug/ml MBP Ac1-11 and 0.5 ng/ml IL 12 for 72hrs. Following purification, 5×106 T cells were injected i.p. into naïve 8 week or 2 week old B10PL mice. Two independent experiments were conducted with a minimum of 10 mice per group. EAE severity is provided as the group mean of the maximal EAE severity of an individual mouse +/- SEM.
| age recipients | age T cell donors | EAE incidence of recipients | Mean day of onset | Mean max. EAE severity |
|---|---|---|---|---|
| 2 weeks | 8 weeks | 0/10 | n/a | n/a |
| 8 weeks | 8 weeks | 9/10 | 8 +/- 0.4 | 4.25+/- 0.16 |
Adoptive transfer of 8 week old APC restores EAE susceptibility in 2 week old mice
In an approach to formally proof that protection of young mice from EAE refers to the observed alterations and immaturity within the innate immune cell compartment, we adoptively transferred splenic myeloid APC and B cells from 8 week old mice into 2 week old recipients at the time-point of immunization and two days thereafter. Prior to transfer, CD3+ T cells were removed by MACS separation. As indicated in table 3, adoptive transfer of adult APC into 2 week old mice restored susceptibility to actively induced EAE in three out of three independent experiments. When recipient mice were evaluated for splenic T cell responses to the immunogen, recipients of adult APC showed an increased proliferation of myelin-reactive T cells (supplementary figure 2), indicating that donor adult APC restored the ability of young mice to generate an encephalitogenic T cell response.
Table 3. Adoptive transfer of adult APC confers EAE susceptibility to 2 weeks old mice.
APC were obtained from spleens of 2 weeks or 8 weeks old mice by MACS-mediated removal of CD3+ T cells. 2 × 107 cells were injected i.p. immediately before immunization and 2 days thereafter. Shown are 3 independent experiments. EAE severity is provided as the group mean of the maximal EAE severity of an individual mouse +/- SEM.
| age (recipients) at immunization | age (donors) | EAE incidence of recipients | Mean day of onset | Mean max. EAE severity of recipients | |
|---|---|---|---|---|---|
| Experiment 1 | 2 weeks | 2 weeks | 0/3 | n/a | n/a |
| 2 weeks | 8 weeks | 1/3 | 13 | 2.5 | |
| 8 weeks | n/a | 5/5 | 13+/- 0.5 | 2.9 +/- 0.3 | |
| Experiment 2 | 2 weeks | 2 weeks | 0/3 | n/a | n/a |
| 2 weeks | 8 weeks | 2/3 | 14+/- 1 | 3.3+/- 0.3 | |
| 8 weeks | n/a | 4/5 | 13.3+/- 0.6 | 2.6 +/- 0.4 | |
| Experiment 3 | 2 weeks | 2 weeks | 0/3 | n/a | n/a |
| 2 weeks | 8 weeks | 2/3 | 14 +/- 1 | 2.3 +/- 0.2 | |
| 8 weeks | n/a | 4/5 | 13.8+/- 0.5 | 3.1 +/-0.5 |
Collectively, these data highlight the conclusion that the age-related increase in susceptibility to CNS autoimmune disease may be determined by a paralleling maturation of the predominant APC phenotype. With aging, expression of molecules required for sufficient antigen presentation increases, which determines the ability to trigger an auto-reactive T cell response and to develop CNS autoimmune disease. In contrast the overall immature phenotype of APC containing higher frequencies of subpopulations with regulatory or suppressive properties may render younger mice largely incapable of generating encephalitogenic T cells and may further protect them by promoting development of Th2 cells and Treg.
Discussion
In this study, we demonstrate that the animal model of MS, EAE cannot be induced with a standard protocol in otherwise susceptible mice that are below a certain age. Disease resistance in younger mice was associated with a higher frequency of plasmacytoid dendritic cells and myeloid-derived suppressor cells, two APC subtypes with immunosuppressive properties [14,17]. Furthermore, APC from younger mice displayed a functionally immature phenotype characterized by a decreased expression of MHC II and co-stimulatory CD40, a reduced production of pro-inflammatory TNF, IL-6, IL-23 and IL-12 and an enhanced release of anti-inflammatory IL-10. These APC were incapable of generating encephalitogenic T cells and promoted development of regulatory T cell populations instead. As adoptive transfer of adult APC restored inducibility of EAE in young mice, we propose that during development the innate immune cell compartment may gradually shift from regulatory/suppressive properties to pro-inflammatory function, which may represent one immunological factor that facilitates susceptibility to CNS autoimmune disease.
Our results hence favor an age-related decline of regulatory APC phenotypes and myeloid derived suppressor cells and an increase in the expression of constitutive and inducible MHC II and co-stimulatory molecules on myeloid APC and B cells as explanation why young mice are protected from T cell-mediated CNS autoimmune disease. It is clear that overall MHC class II expression is required for initiation of EAE, as mice genetically engineered to lack MHC II molecules are resistant to development of CNS autoimmune disease [21]. Further, it has been demonstrated that the density of MHC II-antigen complexes and thereby the strength of TCR signaling can determine the fate of the corresponding T cell [22]. While a strong interaction between APC and T cells was required to generate pro-inflammatory T cells, a weaker molecular contact triggered development of an anti-inflammatory T cell response [23]. Besides sufficient stimulation via MHC II, CD40-CD40-L ligation is critical to further stabilize the APC-T cell interaction after antigen recognition [24]. In vivo disruption of CD40-CD40-L interaction via a monoclonal anti-CD40L antibody completely prevented the development of EAE [25], suggesting that cross-ligation via CD40 is a requirement for effector T cell development. In context with our new findings, these data further consolidate the conclusion that younger mice are protected from CNS autoimmune disease as lower expression levels of MHC class II and CD40 on APC may not suffice to generate encephalitogenic Th1- and Th17 effector T cells.
Although recent years have brought several new MS susceptibility genes [26], allelic variation in the MHC class II region remains the single strongest effect on genetic risk to develop this disease. Various MHC class II haplotypes clearly differ in their ability to mount an encephalitogenic T cell response [27,28], which may relate to the signal strength they can possibly provide to the corresponding T cell. In context with the findings described in the paragraph above, it appears likely, that besides molecular differences in the composition of MHC II, an enhanced expression level of the individual MHC II may independently increase the risk to trigger a pro-inflammatory autoimmune response. In light of our novel preclinical finding, that an age-related upregulation of MHC II permits EAE development in adult mice, it will thus be instrumental to investigate whether expression levels of MHC class II on blood-borne and CNS resident APC may similarly vary throughout human development.
Besides the presented developmental alterations in the innate immune cell compartment, several other age-associated mechanisms could contribute to the lower prevalence of CNS autoimmune disease at younger age. Mechanistically, completed myelination which occurs during early childhood could be a prerequisite for development of MS, as immune responses against myelin autoantigens [29,30] may be required for its initiation. Studies in EAE indeed suggest that a relative lack of CNS myelination in immature brain and spinal cord may contribute to relative EAE resistance in immature rodents [31,32]. However, incomplete CNS myelination is unlikely to explain the results of our study; first, CNS myelination in mice is completed at the age of 3 weeks [33], when in our hands mice were still entirely resistant to EAE. Second, and probably most important, protection from EAE development was associated with the inability of younger mice to generate a pro-inflammatory auto-reactive T cell response following an active EAE induction protocol. This insufficiency cannot be explained by any effect within the CNS including lack of myelination and instead points towards an immaturity of the immunological synapse as plausible explanation.
While we present one immunological mechanism by which the low incidence and prevalence of MS in infancy could be determined, it is evident that other factors have to be considered as well. Besides MHC class II-dependent development of CD4+ T cells, MHC class I-restricted immune responses mediated by CD8+ T cells may play a similarly critical role in pathogenesis of CNS autoimmune diseases. Several studies indicate that CD8+ T cells may also participate as effector cells in EAE induction [34,35]. In MS, clonally expanded CD8+ T cells accumulate within the CNS [36,37]; in vitro, CD8+ T cells can kill oligodendrocytes [38] and neurons [39]. These findings are clearly suggestive of a pathogenic role of CD8+ T cells in CNS autoimmune disease. It will be thus instrumental to investigate in a complementary manner whether expression level of MHC I may also alter and possibly increase during development.
In summary, our data demonstrate an important role of antigen presentation in age-related susceptibility to CNS autoimmune disease. They suggest a scenario in which the phenotype of APC matures during development; while younger individuals may be widely protected from CNS autoimmune disease through an elevated frequency of myeloid derived suppressor cells and plasmacytoid dendritic cells preferentially promoting development of regulatory T cells, upregulation of MHC class II, co-stimulatory molecules and pro-inflammatory cytokines may enable APC to generate CNS autoimmune disease-initiating T cells at a later maturation stage. Hereby, our data provide one immunological mechanism which may explain the increased susceptibility to CNS autoimmune disease after childhood and concomitantly highlight modulation of APC function as an attractive therapeutic goal in Th1/Th17-mediated autoimmunity.
Methods
Mice
C57Bl/6 female mice were purchased from Charles River (Sulzfeld, Germany) and bred in our facilities. Vα2.3/Vβ8.2 (MBP Ac1-11) transgenic B10.PL mice were also bred in our facilities. MOG TCR transgenic (2D2) mice were kindly provided by Thomas Korn (Technische Universität München, Munich, Germany).
Active EAE induction
The animal protocol was approved by the ethics committee at the Technische Universität München, Munich, Germany (protocol approval number 55.2-1-54-2531-67-09). Female C57Bl/6 mice were injected subcutaneously with 100 μg MOG p35-55 (Auspep, Parkville, Australia) in complete Freund's adjuvant (CFA, Sigma-Aldrich, Taufkirchen, Germany). After immunization and 48 hours thereafter, mice received an intravenous injection of 200 ng pertussis toxin (PTx, Sigma-Aldrich, Taufkirchen, Germany). Mice immunized to analyse MHC class II mRNA at various ages received this immunization regimen 7 days prior to analysis. Individual animals were observed daily and clinical scores were assessed as follows: 0 = no clinical disease, 1 = loss of tail tone only, 2 = mild monoparesis or paraparesis, 3 = severe paraparesis, 4 = paraplegia and/or quadraparesis, and 5 = moribund or death.
FACS stainings
Maturation, differentiation and activation of leucocyte subsets was evaluated by surface staining for CD11b, CD11c, B220, CD3, CD4, CD8, CD115, Gr-1, PDCA, Siglec-H, AF6.1, CD40, CD80 and CD86 (all BD Pharmingen, Heidelberg, Germany). Frequency of regulatory T cells was evaluated by staining for CD4//FoxP3 (all BD Pharmingen). Samples were acquired on a Beckman Coulter Cyan ADP FACS.
APC-dependent and –independent T cell activation
For APC-independent T cell activation in vitro, MACS separated (negative selection for CD3) T cells from 2 or 8 week old C57Bl/6 mice were activated by plate-bound anti-CD3 and anti-CD28 at the indicated concentrations. For T cell polarization, medium was supplemented as follows: 5 ng/ml IL-12 for Th1, 10 ng/ml IL-4, and 5 μg/ml anti-IFNγ for Th2 and 25 ng/ml IL-6, 0.5 ng/ml TGF-β, 10 ng/ml IL-1β and 10 ng/ml TNF for Th17 differentiation. On day 3 cells were split into fresh antibody coated plates and 1 ml of fresh medium supplemented with cytokines was added to the appropriate wells: 10 U/ml IL-2 and 5 ng/ml IL-12 for Th1, 10 ng/ml IL-4 for Th2 and 25 ng/ml IL-6, 0.5 ng/ml TGF-β, 10 ng/ml IL-1β and 10 ng/ml TNF for Th17. At 48 and 72h of the second stimulation culture supernatants were collected. In an alternative approach aiming to titrate the T cell activating stimulus, MACS separated (negative selection for CD3) T cells from 2 or 8 week old C57Bl/6 mice were activated by various concentrations of plate-bound anti-CD3 and anti-CD28 in the absence of polarizing cytokines and supernatants were collected after 72h.
For APC-dependent T cell activation 5 × 105 splenocytes from naïve 2 or 8 week old wild-type C57Bl/6 mice were cocultured with 1 × 104 naïve T cells isolated from 2 or 8 week old MOG T cell receptor transgenic mice (negative selection for CD3) in the presence of MOG p35-55. T cell activation and differentiation was evaluated by proliferation or ELISA and FACS staining for CD4+CD25+FoxP3+ T cells, respectively.
Proliferation Assays
Cellular proliferation was measured by pulsing cultures with 1 μCi [3H]-thymidine. 16 hours thereafter, cells were harvested. Mean counts per minute (cpm) of [3H]-thymidine incorporation was calculated for triplicate cultures (Perkin-Elmar 1450 MicroBeta Trilux beta scintillitation counter). Data is presented as absolute counts per minute (cpm) or as stimulation index (cpm of stimulated cells/unstimulated cells).
Cytokine analysis
ELISA for analysis of IFN-γ, IL-17, IL-4, IL-10, IL-6, IL-23, IL-12, TNF were performed using paired monoclonal antibodies specific for corresponding cytokines per manufacturer's recommendations (BD Pharmingen, San Diego, CA). Plates were read on a Tecan GENios (Crailsheim, Germany). The results for ELISA assays are expressed as an average of triplicate wells +/- SEM.
Kinetic polymerase chain reaction
RNA from spleen and brain tissue was prepared from approximately 108 cells using the Rneasy Mini Kit (Qiagen, Valencia, CA). One step kinetic RT-PCR for I-A expression was performed using the following primers: 5′-CTTGAACAGCCCAATGTCTG forward, and 5′-CATGACCAGGACC TGGAAGG reverse. Following an initial incubation for 10 min at 450C with activating uracyl N-glycosylase followed by RT 30 min; 50 cycles at 950C for 15 sec and 570C for 30 sec. β-actin was amplified from all samples as a housekeeping gene to normalize expression. A control (no template) was included for each primer set. To validate the primers, a template titration assay was performed, followed by plotting or a standard curve and a dissociation curve for each target gene with the Applied Biosystems 7900HT instrument software. Each sample was run in triplicate with an ABI 7900HT thermocycler. The quantity of transcript in each unknown sample was calculated by the instrument software based on the linear regression formula of the standard curve. Samples were normalized to β-actin mRNA, to account for the variability in the initial concentration of the total RNA and the conversion efficiency of the PCR reaction. Normalization of samples was performed by dividing the copies of the gene of interest by copies of the reference gene β-actin mRNA.
Adoptive transfer of APC or T cells
APC to be transferred were obtained from spleens of 2 weeks or 8 weeks old mice by MACS-separation (removal) of CD3+ T cells. 2 × 107 cells were injected i.p. immediately before immunization and 2 days thereafter. For the induction of EAE by adoptive transfer of encephalitogenic T cells, spleens from 8 week old MBP Ac1-11 TCR-transgenic mice were removed and splenocytes were stimulated with 6 ug/ml MBP Ac1-11 and 0.5 ng/ml IL 12 for 72hrs. Following purification, 5×106 T cells were injected i.p. into naïve 8 week or 2 week old B10PL mice. Two independent experiments were conducted with a minimum of 10 mice per group.
Statistical analysis
Groups were compared using the Mann-Whitney-U Test. For parametric tests, data were checked for normality by using the Kolmogorov-Smirnov test. Normally distributed values were compared using the unpaired two-sided Student t-test. All values are presented as mean +/- standard error of the mean (SEM). If not indicated differently, three independent experiments were performed for all data presented.
Supplementary Material
Acknowledgments
M.S.W. is supported by the Else Kröner Fresenius Stiftung (A69/2010), TEVA, the Deutsche Forschungsgemeinschaft (DFG; WE 3547/4-1), the US National Multiple Sclerosis Society (NMSS; PP 1660) and the ProFutura program of the University of Göttingen. This study was further supported by a Start-up Grant from the Dallas VA Research Corporation, a New Investigator Award from VISN 17, Veterans Administration, Research Grants from National Multiple Sclerosis Society (NMSS; RG3427A8/T, and RG2969B7/T), and a grant from the Viragh Foundation (O.S.). Support for this study was provided to S.S.Z. by the NIH (RO1 AI073737 and RO1 NS063008), the NMSS (RG 4124), The Guthy Jackson Charitable Foundation and The Maisin Foundation.
Footnotes
Conflict of interest: The authors declare they have no conflict of interest.
References
- 1.Frohman EM, Racke MK, Raine CS. Multiple sclerosis--the plaque and its pathogenesis. N Engl J Med. 2006;354:942–955. doi: 10.1056/NEJMra052130. [DOI] [PubMed] [Google Scholar]
- 2.Kurtzke JF, Page WF, Murphy FM, Norman JE., Jr Epidemiology of multiple sclerosis in US veterans. 4. Age at onset. Neuroepidemiology. 1992;11:226–235. doi: 10.1159/000110935. [DOI] [PubMed] [Google Scholar]
- 3.Renoux C, Vukusic S, Confavreux C. The natural history of multiple sclerosis with childhood onset. Clin Neurol Neurosurg. 2008;110:897–904. doi: 10.1016/j.clineuro.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 4.Zamvil SS, Steinman L. The T lymphocyte in experimental allergic encephalomyelitis. Ann Rev Immunol. 1990;8:579–621. doi: 10.1146/annurev.iy.08.040190.003051. [DOI] [PubMed] [Google Scholar]
- 5.Slavin AJ, Soos JM, Stuve O, Patarroyo JC, Weiner HL, et al. Requirement for endocytic antigen processing and influence of invariant chain and H-2M deficiencies in CNS autoimmunity. J Clin Invest. 2001;108:1133–1139. doi: 10.1172/JCI13360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Murray JS, Madri J, Pasqualini T, Bottomly K. Functional CD4 T cell subset interplay in an intact immune system. J Immunol. 1993;150:4270–4276. [PubMed] [Google Scholar]
- 7.Constant S, Pfeiffer C, Woodard A, Pasqualini T, Bottomly K. Extent of T cell receptor ligation can determine the functional differentiation of naive CD4+ T cells. J Exp Med. 1995;182:1591–1596. doi: 10.1084/jem.182.5.1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Racke MK, Scott DE, Quigley L, Gray GS, Abe R, et al. Distinct roles for B7-1 (CD-80) and B7-2 (CD-86) in the initiation of experimental allergic encephalomyelitis. J Clin Invest. 1995;96:2195–2203. doi: 10.1172/JCI118274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kuchroo VK, Prabhu Das M, Brown JA, Ranger AM, Zamvil SS, et al. B7-1 and B7-2 costimulatory molecules activate differentially the Th1/Th2 developmental pathways: application to autoimmune disease therapy. Cell. 1995;80:707–718. doi: 10.1016/0092-8674(95)90349-6. [DOI] [PubMed] [Google Scholar]
- 10.Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 11.Korn T, Mitsdoerffer M, Croxford AL, Awasthi A, Dardalhon VA, et al. IL-6 controls Th17 immunity in vivo by inhibiting the conversion of conventional T cells into Foxp3+ regulatory T cells. Proc Natl Acad Sci U S A. 2008;105:18460–18465. doi: 10.1073/pnas.0809850105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Steinman L, Zamvil SS. How to successfully apply animal studies in experimental allergic encephalomyelitis to research on multiple sclerosis. Ann Neurol. 2006;60:12–21. doi: 10.1002/ana.20913. [DOI] [PubMed] [Google Scholar]
- 13.Weber MS, Hemmer B. Cooperation of B cells and T cells in the pathogenesis of multiple sclerosis. Results Probl Cell Differ. 2010;51:115–126. doi: 10.1007/400_2009_21. [DOI] [PubMed] [Google Scholar]
- 14.Irla M, Kupfer N, Suter T, Lissilaa R, Benkhoucha M, et al. MHC class II-restricted antigen presentation by plasmacytoid dendritic cells inhibits T cell-mediated autoimmunity. J Exp Med. 2010;207:1891–1905. doi: 10.1084/jem.20092627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bailey SL, Schreiner B, McMahon EJ, Miller SD. CNS myeloid DCs presenting endogenous myelin peptides ‘preferentially’ polarize CD4+ T(H)-17 cells in relapsing EAE. Nat Immunol. 2007;8:172–180. doi: 10.1038/ni1430. [DOI] [PubMed] [Google Scholar]
- 16.Apolloni E, Bronte V, Mazzoni A, Serafini P, Cabrelle A, et al. Immortalized myeloid suppressor cells trigger apoptosis in antigen-activated T lymphocytes. J Immunol. 2000;165:6723–6730. doi: 10.4049/jimmunol.165.12.6723. [DOI] [PubMed] [Google Scholar]
- 17.Zhu B, Bando Y, Xiao S, Yang K, Anderson AC, et al. CD11b+Ly-6C(hi) suppressive monocytes in experimental autoimmune encephalomyelitis. J Immunol. 2007;179:5228–5237. doi: 10.4049/jimmunol.179.8.5228. [DOI] [PubMed] [Google Scholar]
- 18.Lafferty KJ, Cunningham AJ. A new analysis of allogeneic interactions. Aust J Exp Biol Med Sci. 1975;53:27–42. doi: 10.1038/icb.1975.3. [DOI] [PubMed] [Google Scholar]
- 19.Weber MS, Prod'homme T, Patarroyo JC, Molnarfi N, Karnezis T, et al. B-cell activation influences T-cell polarization and outcome of anti-CD20 B-cell depletion in central nervous system autoimmunity. Ann Neurol. 2010;68:369–383. doi: 10.1002/ana.22081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weber MS, Prod'homme T, Youssef S, Dunn SE, Rundle CD, et al. Type II monocytes modulate T cell-mediated central nervous system autoimmune disease. Nat Med. 2007;13:935–943. doi: 10.1038/nm1620. [DOI] [PubMed] [Google Scholar]
- 21.Stuve O, Youssef S, Slavin AJ, King CL, Patarroyo JC, et al. The Role of the MHC Class II Transactivator in Class II Expression and Antigen Presentation by Astrocytes and in Susceptibility to Central Nervous System Autoimmune Disease. J Immunol. 2002;169:6720–6732. doi: 10.4049/jimmunol.169.12.6720. [DOI] [PubMed] [Google Scholar]
- 22.Constant SL, Bottomly K. Induction of Th1 and Th2 CD4+ T cell responses: the alternative approaches. Annu Rev Immunol. 1997;15:297–322. doi: 10.1146/annurev.immunol.15.1.297. [DOI] [PubMed] [Google Scholar]
- 23.Tao X, Constant S, Jorritsma P, Bottomly K. Strength of TCR signal determines the costimulatory requirements for Th1 and Th2 CD4+ T cell differentiation. J Immunol. 1997;159:5956–5963. [PubMed] [Google Scholar]
- 24.Grewal IS, Flavell RA. The role of CD40 ligand in costimulation and T-cell activation. Immunol Rev. 1996;153:85–106. doi: 10.1111/j.1600-065x.1996.tb00921.x. [DOI] [PubMed] [Google Scholar]
- 25.Gerritse K, Laman JD, Noelle RJ, Aruffo A, Ledbetter JA, et al. CD40-CD40 ligand interactions in experimental allergic encephalomyelitis and multiple sclerosis. Proc Natl Acad Sci U S A. 1996;93:2499–2504. doi: 10.1073/pnas.93.6.2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Patsopoulos NA, de Bakker PI, Esposito F, Reischl J, Lehr S, et al. Genome-wide meta-analysis identifies novel multiple sclerosis susceptibility loci. Ann Neurol. 2011;70:897–912. doi: 10.1002/ana.22609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mangalam A, Luckey D, Basal E, Jackson M, Smart M, et al. HLA-DQ8 (DQB1*0302)-restricted Th17 cells exacerbate experimental autoimmune encephalomyelitis in HLA-DR3-transgenic mice. J Immunol. 2009;182:5131–5139. doi: 10.4049/jimmunol.0803918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mangalam A, Luckey D, Basal E, Behrens M, Rodriguez M, et al. HLA-DQ6 (DQB1*0601)-restricted T cells protect against experimental autoimmune encephalomyelitis in HLA-DR3.DQ6 double-transgenic mice by generating anti-inflammatory IFN-gamma. J Immunol. 2008;180:7747–7756. doi: 10.4049/jimmunol.180.11.7747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zang YC, Li S, Rivera VM, Hong J, Robinson RR, et al. Increased CD8+ cytotoxic T cell responses to myelin basic protein in multiple sclerosis. J Immunol. 2004;172:5120–5127. doi: 10.4049/jimmunol.172.8.5120. [DOI] [PubMed] [Google Scholar]
- 30.Genain CP, Cannella B, Hauser SL, Raine CS. Identification of autoantibodies associated with myelin damage in multiple sclerosis. Nat Med. 1999;5:170–175. doi: 10.1038/5532. [DOI] [PubMed] [Google Scholar]
- 31.Umehara F, Qin YF, Goto M, Wekerle H, Meyermann R. Experimental autoimmune encephalomyelitis in the maturing central nervous system. Transfer of myelin basic protein-specific T line lymphocytes to neonatal Lewis rats. Lab Invest. 1990;62:147–155. [PubMed] [Google Scholar]
- 32.Smith ME, Eller NL, McFarland HF, Racke MK, Raine CS. Age dependence of clinical and pathological manifestations of autoimmune demyelination. Implications for multiple sclerosis. Am J Pathol. 1999;155:1147–1161. doi: 10.1016/S0002-9440(10)65218-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Baumann N, Pham-Dinh D. Biology of oligodendrocyte and myelin in the mammalian central nervous system. Physiol Rev. 2001;81:871–927. doi: 10.1152/physrev.2001.81.2.871. [DOI] [PubMed] [Google Scholar]
- 34.Huseby ES, Liggitt D, Brabb T, Schnabel B, Ohlen C, et al. A pathogenic role for myelin-specific CD8(+) T cells in a model for multiple sclerosis. J Exp Med. 2001;194:669–676. doi: 10.1084/jem.194.5.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sun D, Whitaker JN, Huang Z, Liu D, Coleclough C, et al. Myelin antigen-specific CD8+ T cells are encephalitogenic and produce severe disease in C57BL/6 mice. J Immunol. 2001;166:7579–7587. doi: 10.4049/jimmunol.166.12.7579. [DOI] [PubMed] [Google Scholar]
- 36.Babbe H, Roers A, Waisman A, Lassmann H, Goebels N, et al. Clonal expansions of CD8(+) T cells dominate the T cell infiltrate in active multiple sclerosis lesions as shown by micromanipulation and single cell polymerase chain reaction. J Exp Med. 2000;192:393–404. doi: 10.1084/jem.192.3.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jacobsen M, Cepok S, Quak E, Happel M, Gaber R, et al. Oligoclonal expansion of memory CD8+ T cells in cerebrospinal fluid from multiple sclerosis patients. Brain. 2002;125:538–550. doi: 10.1093/brain/awf059. [DOI] [PubMed] [Google Scholar]
- 38.Jurewicz A, Biddison WE, Antel JP. MHC class I-restricted lysis of human oligodendrocytes by myelin basic protein peptide-specific CD8 T lymphocytes. J Immunol. 1998;160:3056–3059. [PubMed] [Google Scholar]
- 39.Medana IM, Gallimore A, Oxenius A, Martinic MM, Wekerle H, et al. MHC class I-restricted killing of neurons by virus-specific CD8+ T lymphocytes is effected through the Fas/FasL, but not the perforin pathway. Eur J Immunol. 2000;30:3623–3633. doi: 10.1002/1521-4141(200012)30:12<3623::AID-IMMU3623>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.