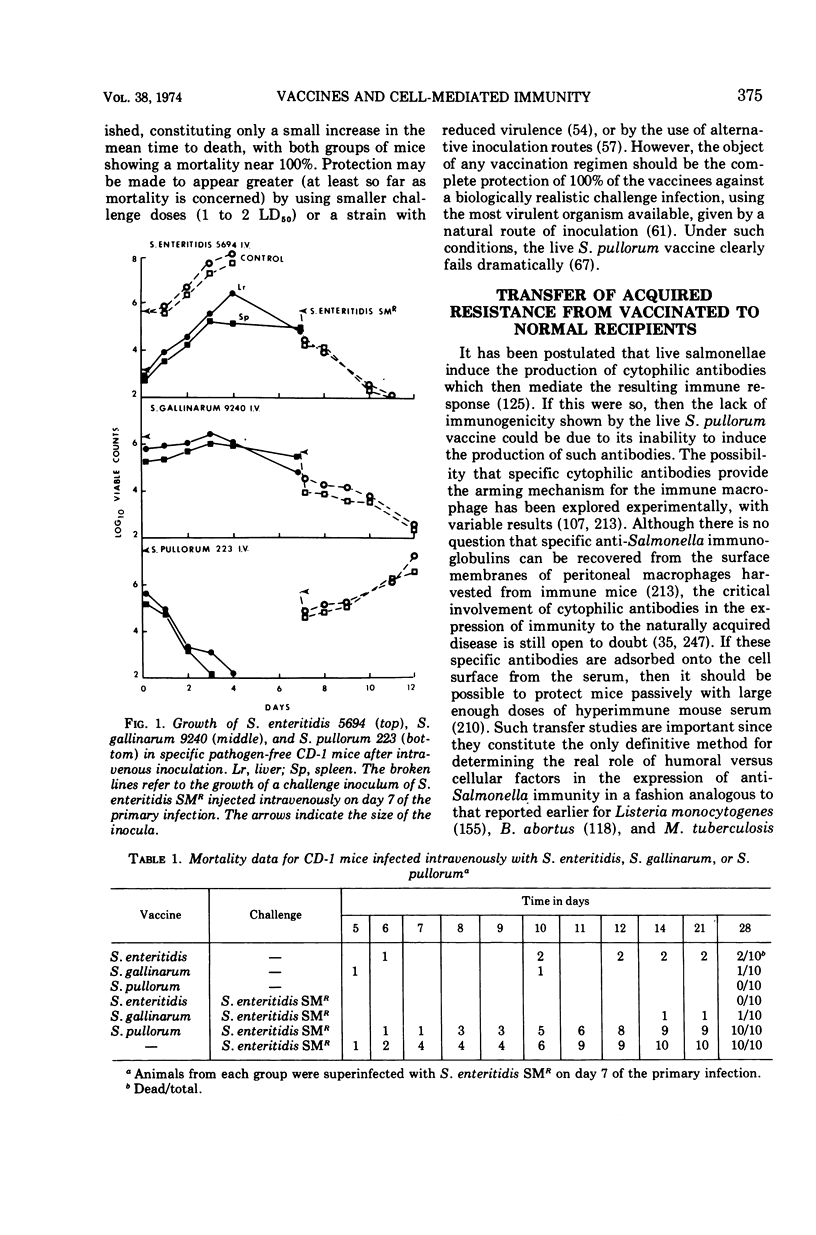
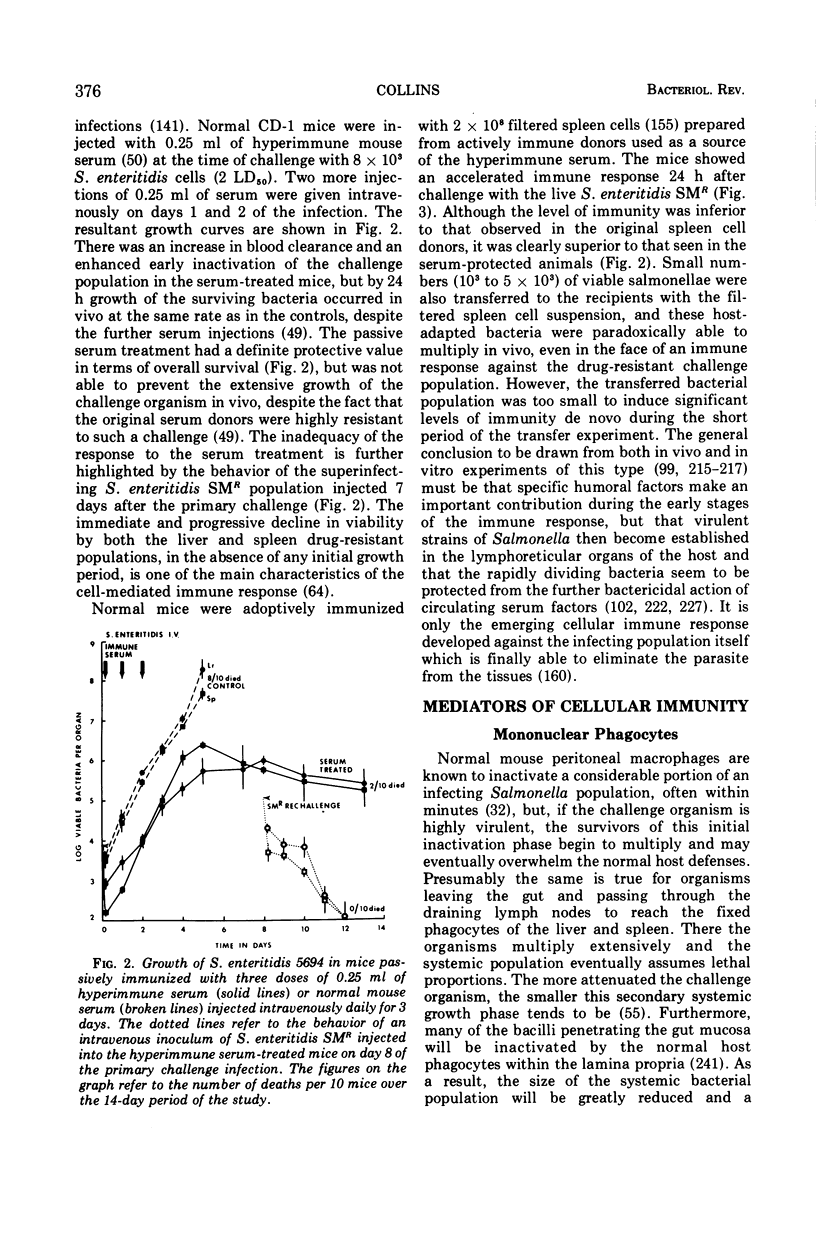
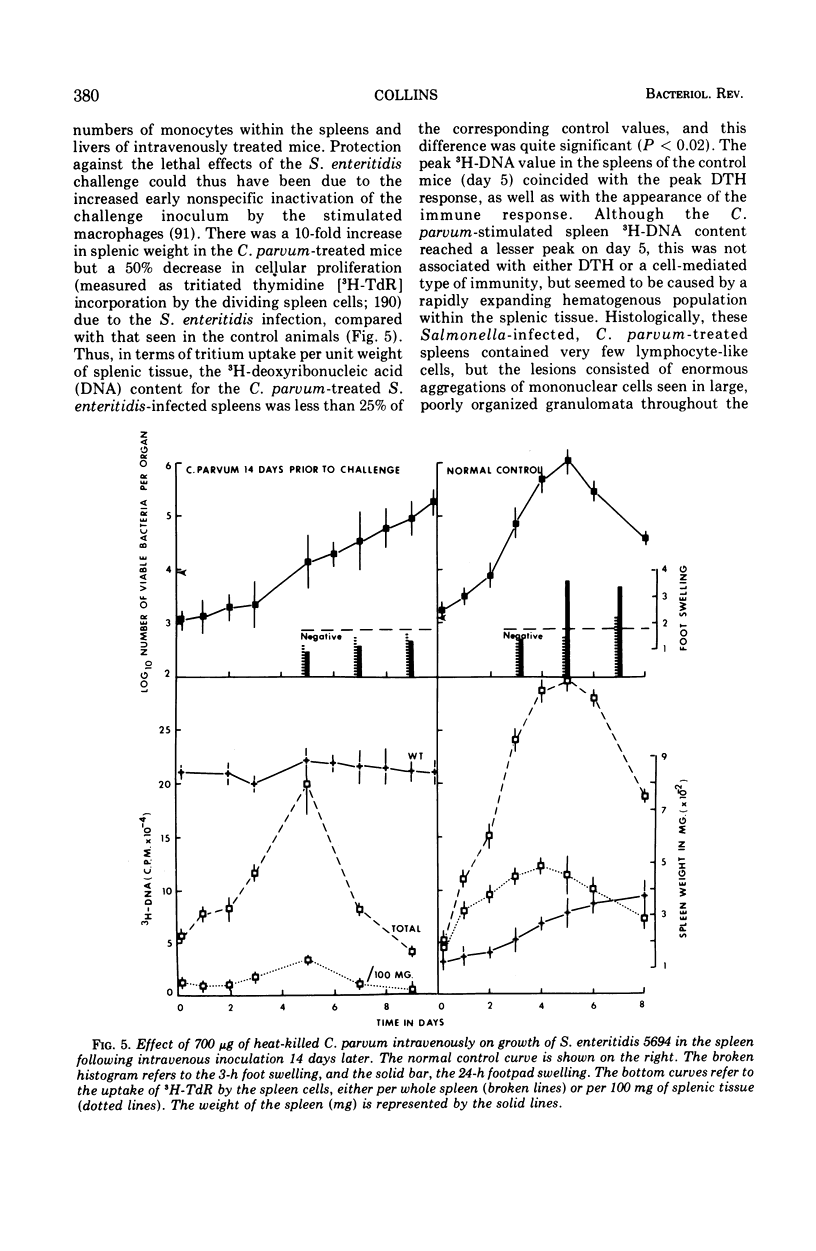
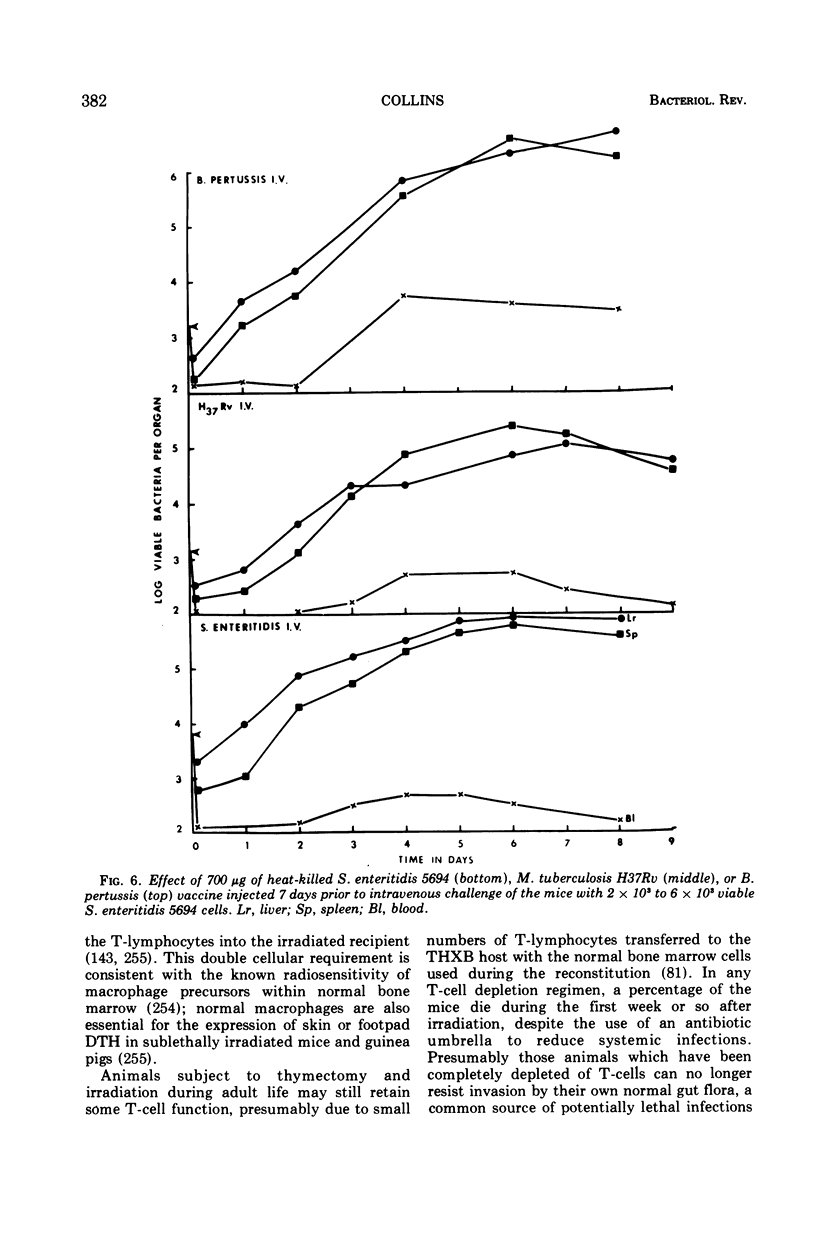
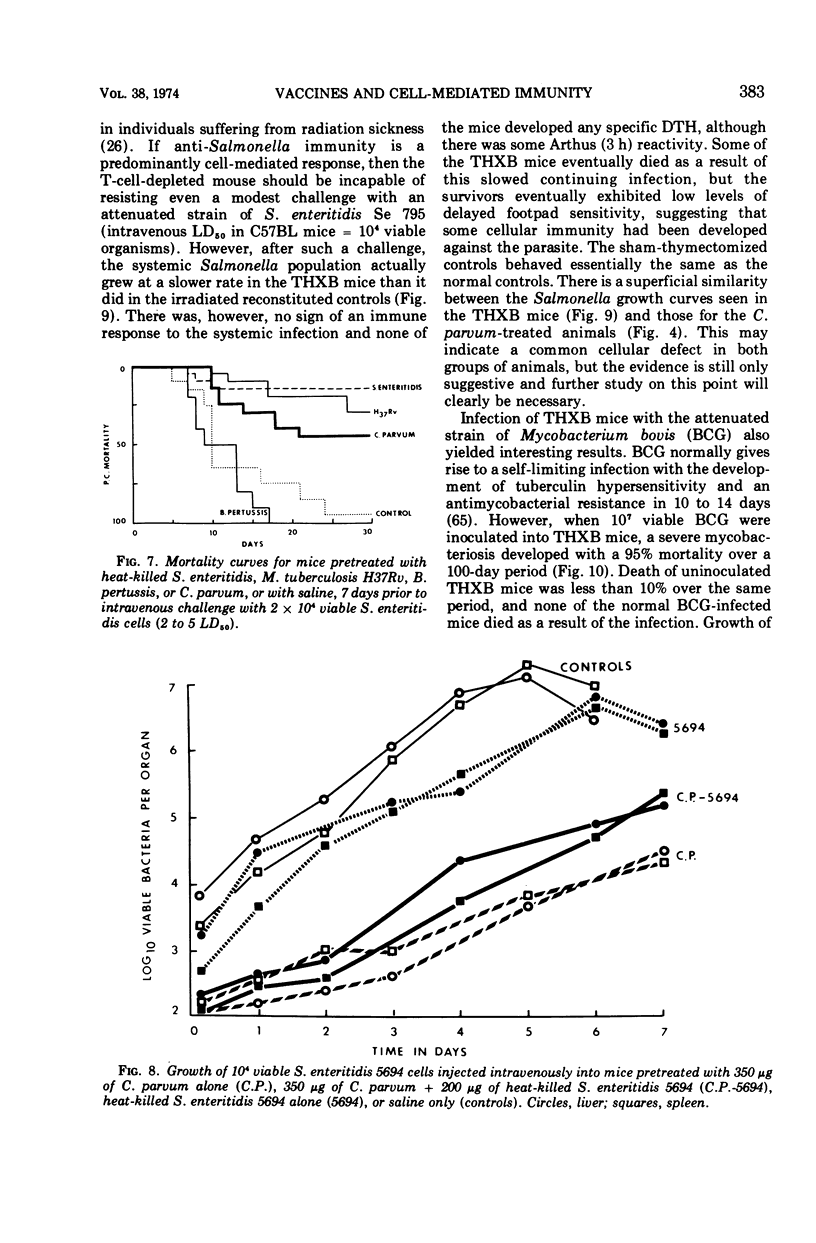
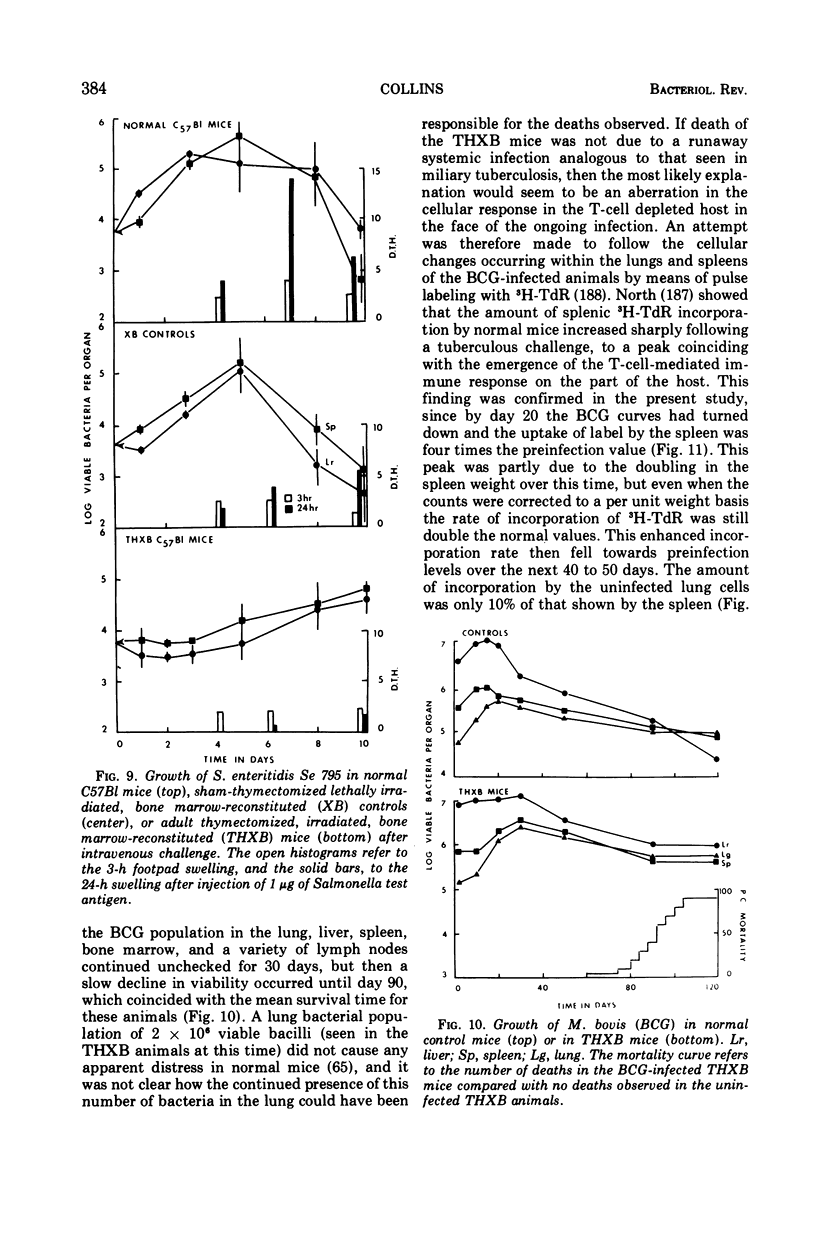
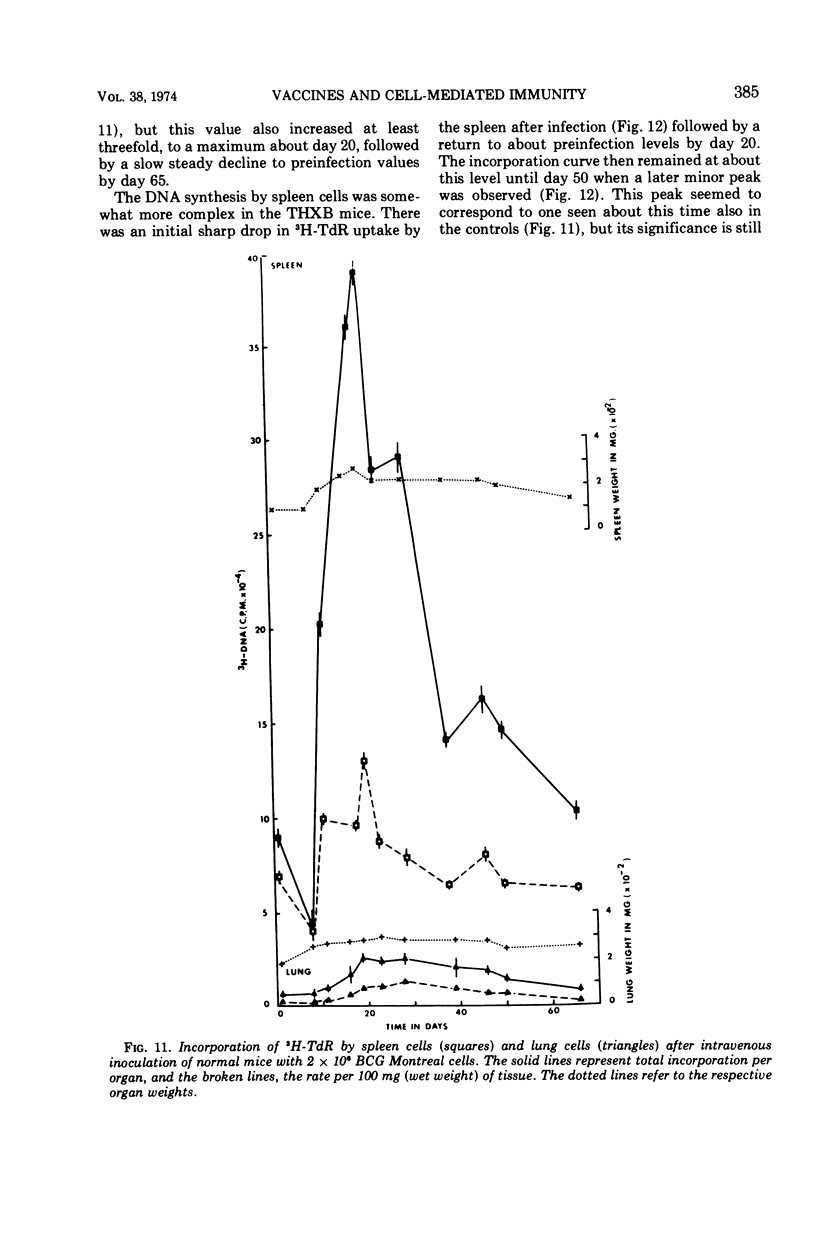
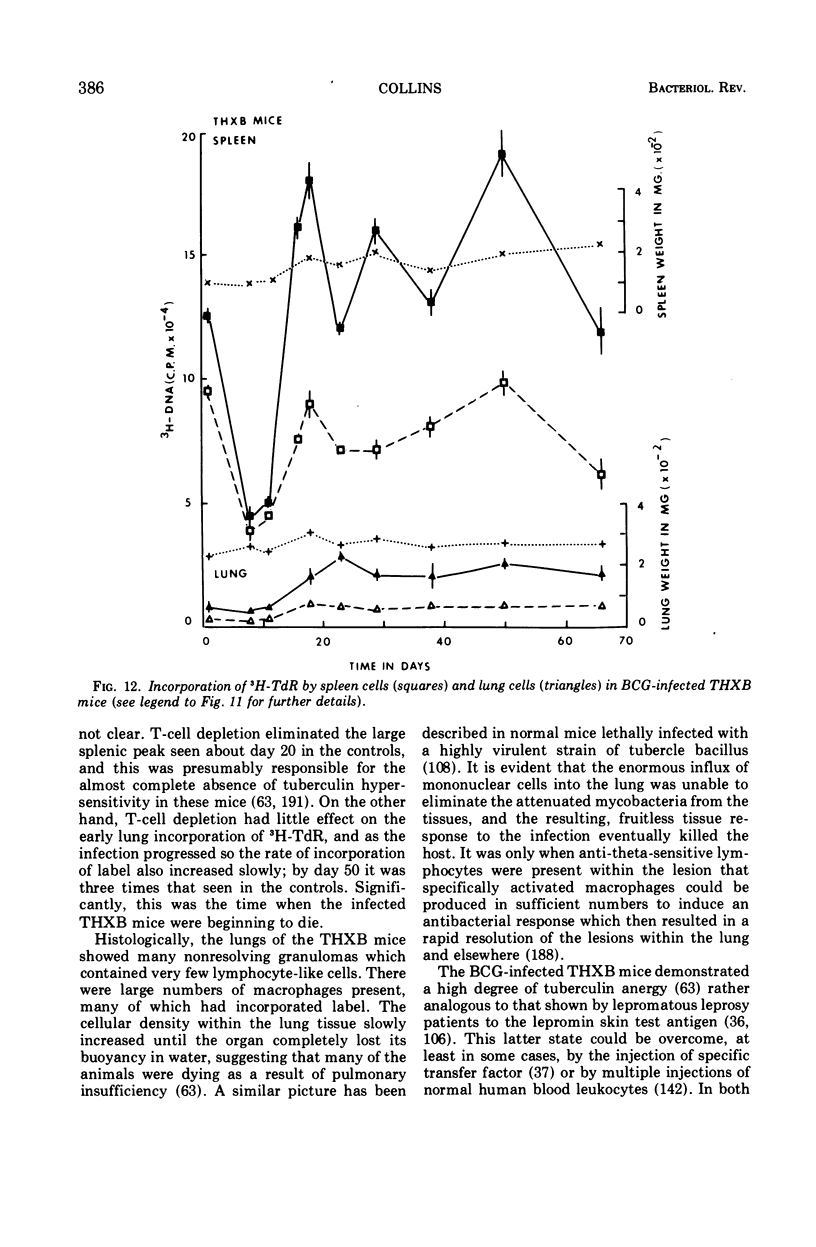
Full text
PDF





















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AKIYAMA T., MAEDA K., ASABA G., NAGATOMI H., WATABIKI S., USHIBA D. THE USE OF DIFFUSION CHAMBERS IN INVESTIGATING THE CELLULAR NATURE OF IMMUNITY IN EXPERIMENTAL TYPHOID AND TUBERCULOSIS. Jpn J Microbiol. 1964 Jun;8:37–48. doi: 10.1111/j.1348-0421.1964.tb00257.x. [DOI] [PubMed] [Google Scholar]
- AKIYAMA T., MAEDA K., USHIBA D. [Studies on immunity in experimental typhoid. Challenge of mice passively immunized with antiserum through various routes (mechanism of immunization with killed vaccines)]. Nihon Saikingaku Zasshi. 1962 Oct;17:829–834. [PubMed] [Google Scholar]
- ARNASON B. G., JANKOVIC B. D., WAKSMAN B. H., WENNERSTEN C. Role of the thymus in immune reactions in rats. II. Suppressive effect of thymectomy at birth on reactions of delayed (cellular) hypersensitivity and the circulating small lymphocyte. J Exp Med. 1962 Aug 1;116:177–186. doi: 10.1084/jem.116.2.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ASHCROFT M. T., RITCHIE J. M., NICHOLSON C. C. CONTROLLED FIELD TRIAL IN BRITISH GUIANA SCHOOL CHILDREN OF HEAT-KILLED-PHENOLIZED AND ACETONE-KILLED LYOPHILIZED TYPHOID VACCINES. Am J Hyg. 1964 Mar;79:196–206. doi: 10.1093/oxfordjournals.aje.a120376. [DOI] [PubMed] [Google Scholar]
- AUZINS I., ROWLEY D. FACTORS INVOLVED IN THE ADHERENCE OF S. TYPHIMURIUM C5 AND MOUSE PERITONEAL MACROPHAGES. Aust J Exp Biol Med Sci. 1963 Oct;41:539–546. doi: 10.1038/icb.1963.44. [DOI] [PubMed] [Google Scholar]
- AUZINS I., ROWLEY D. On the question of the specificity of cellular immunity. Aust J Exp Biol Med Sci. 1962 Aug;40:283–291. doi: 10.1038/icb.1962.32. [DOI] [PubMed] [Google Scholar]
- Adam A., Ciorbaru R., Petit J. F., Lederer E. Isolation and properties of a macromolecular, water-soluble, immuno-adjuvant fraction from the cell wall of Mycobacterium smegmatis. Proc Natl Acad Sci U S A. 1972 Apr;69(4):851–854. doi: 10.1073/pnas.69.4.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adlam C., Broughton E. S., Scott M. T. Enhanced resistance of mice to infection with bacteria following pre-treatment with Corynebacterium parvum. Nat New Biol. 1972 Feb 16;235(59):219–220. doi: 10.1038/newbio235219a0. [DOI] [PubMed] [Google Scholar]
- Adlam C., Scott M. T. Lympho-reticular stimulatory properties of Corynebacterium parvum and related bacteria. J Med Microbiol. 1973 Aug;6(3):261–274. doi: 10.1099/00222615-6-3-261. [DOI] [PubMed] [Google Scholar]
- Agarwal S. C., Ganguly N. K. Oral immunization with L-forms of Vibrio cholerae in human volunteers. Infect Immun. 1972 Jul;6(1):17–20. doi: 10.1128/iai.6.1.17-20.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anacker R. L., Barclay W. R., Brehmer W., Goode G., List R. H., Ribi E., Tarmina D. F. Effectiveness of cell walls of Mycobacterium bovis strain BCG administered by various routes and in different adjuvants in protecting mice against airborne infection with Mycobacterium tuberculosis strain H37Rv. Am Rev Respir Dis. 1969 Feb;99(2):242–248. doi: 10.1164/arrd.1969.99.2.242. [DOI] [PubMed] [Google Scholar]
- Anacker R. L., Barclay W. R., Brehmer W., Larson C. L., Ribi E. Duration of immunity to tuberculosis in mice vaccinated intravenously with oil-treated cell walls of Mycobacterium bovis strain BCG. J Immunol. 1967 Jun;98(6):1265–1273. [PubMed] [Google Scholar]
- Archer J. R., Rowley D. A quantitative comparison of the antigenic structure of a virulent and an avirulent strain of Salmonella typhimurium. Immunology. 1969 Oct;17(4):551–558. [PMC free article] [PubMed] [Google Scholar]
- Azurin J. C., Cruz A., Pesigan T. P., Alvero M., Camena T., Suplido R., Ledesma L., Gomez C. Z. A controlled field trial of the effectiveness of cholera and cholera El Tor vaccines in the Philippines. Bull World Health Organ. 1967;37(5):703–727. [PMC free article] [PubMed] [Google Scholar]
- BAKER E. E., WHITESIDE R. E., BASCH R., DEROW M. A. The VI antigens of the Enterobacteriaceae. I. Purification and chemical properties. J Immunol. 1959 Dec;83:680–696. [PubMed] [Google Scholar]
- BARON L. S., FORMAL S. B. Immunization studies with living vaccine of Salmonella typhimurium. Proc Soc Exp Biol Med. 1960 Aug-Sep;104:565–567. doi: 10.3181/00379727-104-25909. [DOI] [PubMed] [Google Scholar]
- BENACERRAF B. Influence of irradiation on resistance to infection. Bacteriol Rev. 1960 Mar;24(1):35–40. doi: 10.1128/br.24.1.35-40.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BENENSON A. S. SEROLOGICAL RESPONSES OF MAN TO TYPHOID VACCINES. Bull World Health Organ. 1964;30:653–662. [PMC free article] [PubMed] [Google Scholar]
- BENOIT J. C., PANISSET M. SURVIE ET MULTIPLICATION DU BCG ET DU BACILLE TUBERCULEUX CHEZ LA SOURIS. IV. SURVIE ET MULTIPLICATION DE SOUCHES-FILLES DE BCG EN FONCTION DU TEMPS ET DE LA DOSE. Acta Tuberc Pneumol Scand. 1963;43:125–136. [PubMed] [Google Scholar]
- BOYDEN S. V., SUTER W. E. Stimulating effect of tuberculin upon production of circulating antibodies in guinea pigs infected with tubercle bacilli. J Immunol. 1952 May;68(5):577–589. [PubMed] [Google Scholar]
- Baker R. E., Hill W. E., Larson C. L. Delayed hypersensitivity reactions provoked by ribosomes from acid-fast bacilli. I. Ribosomal isolation, characterization, delayed hypersensitivity, and specificity. Infect Immun. 1972 Sep;6(3):258–265. doi: 10.1128/iai.6.3.258-265.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber C., Eylan E. Immunochimie de Salmonella typhimurium. Variations de la fraction protéique de l'antigène somatique. Rev Immunol (Paris) 1972 Jan-Mar;36(1):1–13. [PubMed] [Google Scholar]
- Barber C., Eylan E., Keydar Y. Contributions à l'étude des salmonelles. Protéines communes des salmonelles appartenant aux groupes B, C, D et E, et leur relation avec Citrobacter ballerup. Pathol Microbiol (Basel) 1968;31(3):165–174. [PubMed] [Google Scholar]
- Barber C., Vlădoianu I. R., Dimache G. Contributions to the study of Salmonella. II. Protein determinants shared by S. typhi, S. enteritidis and S. typhimurium. Immunology. 1967 Apr;12(4):411–416. [PMC free article] [PubMed] [Google Scholar]
- Barclay W. R., Anacker R., Brehmer W., Ribi E. Effects of oil-treated mycobacterial cell walls on the organs of mice. J Bacteriol. 1967 Nov;94(5):1736–1745. doi: 10.1128/jb.94.5.1736-1745.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekierkunst A., Levij I. S., Yarkoni E., Vilkas E., Adam A., Lederer E. Granuloma formation induced in mice by chemically defined mycobacterial fractions. J Bacteriol. 1969 Oct;100(1):95–102. doi: 10.1128/jb.100.1.95-102.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanden R. V., Lefford M. J., Mackaness G. B. The host response to Calmette-Guérin bacillus infection in mice. J Exp Med. 1969 May 1;129(5):1079–1107. doi: 10.1084/jem.129.5.1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanden R. V., Mackaness G. B., Collins F. M. Mechanisms of acquired resistance in mouse typhoid. J Exp Med. 1966 Oct 1;124(4):585–600. doi: 10.1084/jem.124.4.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanden R. V., Mims C. A. Macrophage activation in mice infected with ectromelia or lymphocytic choriomeningitis viruses. Aust J Exp Biol Med Sci. 1973 Jun;51(3):393–398. doi: 10.1038/icb.1973.35. [DOI] [PubMed] [Google Scholar]
- Blanden R. V. Modification of macrophage function. J Reticuloendothel Soc. 1968 Jun;5(3):179–202. [PubMed] [Google Scholar]
- Blanden R. V. T cell response to viral and bacterial infection. Transplant Rev. 1974;19(0):56–88. doi: 10.1111/j.1600-065x.1974.tb00128.x. [DOI] [PubMed] [Google Scholar]
- Boyle W. An antigen common to mouse cells and Salmonella typhimurium. J Immunol. 1967 Feb;98(2):256–259. [PubMed] [Google Scholar]
- Bullock W. E., Fields J. P., Brandriss M. W. An evaluation of transfer factor as immunotherapy for patients with lepromatous leprosy. N Engl J Med. 1972 Nov 23;287(21):1053–1059. doi: 10.1056/NEJM197211232872101. [DOI] [PubMed] [Google Scholar]
- CAMERON J. A., HOLTMAN D. F., JEFFRIES C. D. The association of virulence with endotoxin in Salmonella pullorum. J Infect Dis. 1960 Mar-Apr;106:159–161. doi: 10.1093/infdis/106.2.159. [DOI] [PubMed] [Google Scholar]
- CAREY W. F., BARON L. S. Comparative immunologic studies of cell structures isolated from Salmonella typhosa. J Immunol. 1959 Nov;83:517–520. [PubMed] [Google Scholar]
- CVJETANOVIC B., UEMURA K. THE PRESENT STATUS OF FIELD AND LABORATORY STUDIES OF TYPHOID AND PARATYPHOID VACCINES WITH SPECIAL REFERENCE TO STUDIES SPONSORED BY WORLD HEALTH ORGANIZATION. Bull World Health Organ. 1965;32:29–36. [PMC free article] [PubMed] [Google Scholar]
- Carter P. B., Collins F. M. The route of enteric infection in normal mice. J Exp Med. 1974 May 1;139(5):1189–1203. doi: 10.1084/jem.139.5.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chedid L., Parant M., Parant F., Gustafson R. H., Berger F. M. Biological study of a nontoxic, water-soluble immunoadjuvant from mycobacterial cell walls. Proc Natl Acad Sci U S A. 1972 Apr;69(4):855–858. doi: 10.1073/pnas.69.4.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chernokhvostova E., Luxemburg K. I., Starshinova V., Andreeva N., German G. Study on the production of IgG, IgA- and IgM-antibodies to somatic antigens of Salmonella typhi in humans. Clin Exp Immunol. 1969 Apr;4(4):407–421. [PMC free article] [PubMed] [Google Scholar]
- Ciznár I., Shands J. W. Effect of Alkali on the Immunological Reactivity of Lipopolysaccharide from Salmonella typhimurium. Infect Immun. 1970 Nov;2(5):549–555. doi: 10.1128/iai.2.5.549-555.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark C., Munoz J. Delayed hypersensitivity to insulin and its component polypeptide chains. J Immunol. 1970 Sep;105(3):574–583. [PubMed] [Google Scholar]
- Cluff L. E. Effects of endotoxins on susceptibility to infections. J Infect Dis. 1970 Sep;122(3):205–215. doi: 10.1093/infdis/122.3.205. [DOI] [PubMed] [Google Scholar]
- Collins F. M., Boros D. L., Warren K. S. The effect of Schistosoma mansoni infection on the response of mice to Salmonella enteritidis and Listeria monocytogenes. J Infect Dis. 1972 Mar;125(3):249–256. doi: 10.1093/infdis/125.3.249. [DOI] [PubMed] [Google Scholar]
- Collins F. M., Carter P. B. Comparative immunogenicity of heat-killed and living oral Salmonella vaccines. Infect Immun. 1972 Oct;6(4):451–458. doi: 10.1128/iai.6.4.451-458.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins F. M. Effect of adjuvant on immunogenicity of a heat-killed salmonella vaccine. J Infect Dis. 1972 Jul;126(1):69–76. doi: 10.1093/infdis/126.1.69. [DOI] [PubMed] [Google Scholar]
- Collins F. M. Effect of specific immune mouse serum on the growth of Salmonella enteritidis in mice preimmunized with living or ethyl alcohol-killed vaccines. J Bacteriol. 1969 Feb;97(2):676–683. doi: 10.1128/jb.97.2.676-683.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins F. M. Effect of specific immune mouse serum on the growth of Salmonella enteritidis in nonvaccinated mice challenged by various routes. J Bacteriol. 1969 Feb;97(2):667–675. doi: 10.1128/jb.97.2.667-675.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins F. M. Growth of Pasteurella multocida in vaccinated and normal mice. Infect Immun. 1973 Dec;8(6):868–875. doi: 10.1128/iai.8.6.868-875.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins F. M. Immunity to enteric infection in mice. Infect Immun. 1970 Mar;1(3):243–250. doi: 10.1128/iai.1.3.243-250.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins F. M. Immunogenicity of various mycobacteria and the corresponding levels of cross-protection developed between species. Infect Immun. 1971 Dec;4(6):688–696. doi: 10.1128/iai.4.6.688-696.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins F. M., Mackaness G. B., Blanden R. V. Infection-immunity in experimental salmonellosis. J Exp Med. 1966 Oct 1;124(4):601–619. doi: 10.1084/jem.124.4.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins F. M., Mackaness G. B. Delayed hypersensitivity and arthus reactivity in relation to host resistance in salmonella-infected mice. J Immunol. 1968 Nov;101(5):830–845. [PubMed] [Google Scholar]
- Collins F. M., Mackaness G. B. The relationship of delayed hypersensitivity to acquired antituberculous immunity. I. Tuberculin sensitivity and resistance to reinfection in BCG-vaccinated mice. Cell Immunol. 1970 Sep;1(3):253–265. doi: 10.1016/0008-8749(70)90047-x. [DOI] [PubMed] [Google Scholar]
- Collins F. M., Mackaness G. B. The relationship of delayed hypersensitivity to acquired antituberculous immunity. II. Effect of adjuvant on the allergenicity and immunogenicity of heat-killed tubercle bacilli. Cell Immunol. 1970 Sep;1(3):266–275. doi: 10.1016/0008-8749(70)90048-1. [DOI] [PubMed] [Google Scholar]
- Collins F. M. Mechanisms in antimicrobial immunity. J Reticuloendothel Soc. 1971 Jul;10(1):58–99. [PubMed] [Google Scholar]
- Collins F. M., Miller T. E. Growth of a drug-resistant strain of Mycobacterium bovis (BCG) in normal and immunized mice. J Infect Dis. 1969 Nov;120(5):517–533. doi: 10.1093/infdis/120.5.517. [DOI] [PubMed] [Google Scholar]
- Collins F. M., Milne M. Heat-labile antigens of Salmonella enteritidis. II. Mouse-protection studies. J Bacteriol. 1966 Sep;92(3):549–557. doi: 10.1128/jb.92.3.549-557.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins F. M., Montalbine V. Relative immunogenicity of streptomycin-sensitive and -resistant strains of BCG. Infect Immun. 1973 Sep;8(3):381–387. doi: 10.1128/iai.8.3.381-387.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins F. M. Recall of immunity in mice vaccinated with Salmonella enteritidis or Salmonella typhimurium. J Bacteriol. 1968 Jun;95(6):2014–2021. doi: 10.1128/jb.95.6.2014-2021.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins F. M. Salmonellosis in orally infected specific pathogen-free C57B1 mice. Infect Immun. 1972 Feb;5(2):191–198. doi: 10.1128/iai.5.2.191-198.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins F. M., Scott M. T. Effect of Corynebacterium parvum treatment on the growth of Salmonella enteritidis in mice. Infect Immun. 1974 May;9(5):863–869. doi: 10.1128/iai.9.5.863-869.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins F. M. Serum mediated killing of three group D salmonellas. J Gen Microbiol. 1967 Feb;46(2):247–253. doi: 10.1099/00221287-46-2-247. [DOI] [PubMed] [Google Scholar]
- Collins F. M. The relative immunogenicity of virulent and attenuated strains of tubercle bacilli. Am Rev Respir Dis. 1973 Jun;107(6):1030–1040. doi: 10.1164/arrd.1973.107.6.1030. [DOI] [PubMed] [Google Scholar]
- Coppel S., Youmans G. P. Specificity of the anamnestic response produced by Listeria monocytogenes or Mycobacterium tuberculosis to challenge with Listeria monocytogenes. J Bacteriol. 1969 Jan;97(1):127–133. doi: 10.1128/jb.97.1.127-133.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronly-Dillon S. Comparison of the virulence for mice of Salmonella typhimurium given by the intraperitoneal and subcutaneous routes. J Hyg (Lond) 1973 Jun;71(2):223–225. doi: 10.1017/s0022172400022695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowle A. J., Hu C. C. Delayed hypersensitivity in mice to dextran. Int Arch Allergy Appl Immunol. 1967;31(2):123–144. doi: 10.1159/000229861. [DOI] [PubMed] [Google Scholar]
- Cvjetanović B., Mel D. M., Felsenfeld O. Study of live typhoid vaccine in chimpanzees. Bull World Health Organ. 1970;42(4):499–507. [PMC free article] [PubMed] [Google Scholar]
- DUBOS R. J., PIERCE C. H. Differential characteristics in vitro and in vivo of several substrains of BCG. I. Multiplication and survival in vitro. Am Rev Tuberc. 1956 Nov;74(5):655–666. doi: 10.1164/artpd.1956.74.5.655. [DOI] [PubMed] [Google Scholar]
- DUBOS R. J., PIERCE C. H. Differential characteristics in vitro and in vivo of several substrains of BCG. IV. Immunizing effectiveness. Am Rev Tuberc. 1956 Nov;74(5):699–717. doi: 10.1164/artpd.1956.74.5.699. [DOI] [PubMed] [Google Scholar]
- DUBOS R. J., PIERCE C. H., SCHAEFER W. B. Differential characteristics in vitro and in vivo of several substrains of BCG. III. Multiplication and survival in vivo. Am Rev Tuberc. 1956 Nov;74(5):683–698. doi: 10.1164/artpd.1956.74.5.683. [DOI] [PubMed] [Google Scholar]
- Dannenberg A. M., Jr Cellular hypersensitivity and cellular immunity in the pathogensis of tuberculosis: specificity, systemic and local nature, and associated macrophage enzymes. Bacteriol Rev. 1968 Jun;32(2):85–102. doi: 10.1128/br.32.2.85-102.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies A. J. The thymus and the cellular basis of immunity. Transplant Rev. 1969;1:43–91. doi: 10.1111/j.1600-065x.1969.tb00136.x. [DOI] [PubMed] [Google Scholar]
- Diena B. B., Johnson E. M., Baron L. S., Wallace R., Greenberg L. Assay of typhoid vaccines with Salmonella typhosa-Salmonella typhimurium hybrids. Infect Immun. 1973 Jan;7(1):5–8. doi: 10.1128/iai.7.1.5-8.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doenhoff M. J., Davies A. J., Leuchars E., Wallis V. The thymus and circulating lymphocytes of mice. Proc R Soc Lond B Biol Sci. 1970 Oct 13;176(1042):69–85. doi: 10.1098/rspb.1970.0035. [DOI] [PubMed] [Google Scholar]
- DuPont H. L., Hornick R. B., Snyder M. J., Libonati J. P., Formal S. B., Gangarosa E. J. Immunity in shigellosis. II. Protection induced by oral live vaccine or primary infection. J Infect Dis. 1972 Jan;125(1):12–16. doi: 10.1093/infdis/125.1.12. [DOI] [PubMed] [Google Scholar]
- DuPont H. L., Hornick R. B., Snyder M. J., Libonati J. P., Woodward T. E. Immunity in typhoid fever: evaluation of live streptomycin-dependent vaccine. Antimicrob Agents Chemother (Bethesda) 1970;10:236–239. [PubMed] [Google Scholar]
- Eddie D. S., Schulkind M. L., Robbins J. B. The isolation and biologic activities of purified secretory IgA and IgG anti-Salmonella typhimurium "O" antibodies from rabbit intestinal fluid and colostrum. J Immunol. 1971 Jan;106(1):181–190. [PubMed] [Google Scholar]
- Esposito V. M., Feeley J. C., Leeder W. D., Pittman M. Immunological response of three mouse strains to typhoid vaccine and Vi antigen. J Bacteriol. 1969 Jul;99(1):8–12. doi: 10.1128/jb.99.1.8-12.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FAUVE R. M., PIERCE-CHASE C. H., DUBOS R. CORYNEBACTERIAL PSEUDOTUBERCULOSIS IN MICE. II. ACTIVATION OF NATURAL AND EXPERIMENTAL LATENT INFECTIONS. J Exp Med. 1964 Aug 1;120:283–304. doi: 10.1084/jem.120.2.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FELIX A. The preparation, testing and standardization of typhoid vaccine. J Hyg (Lond) 1951 Jun-Sep;49(2-3):268–297. doi: 10.1017/s0022172400044156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FREUND J. The mode of action of immunologic adjuvants. Bibl Tuberc. 1956;(10):130–148. [PubMed] [Google Scholar]
- FURNESS G., FERREIRA I. The role of macrophages in natural immunity to Salmonellae. J Infect Dis. 1959 Mar-Apr;104(2):205–206. [PubMed] [Google Scholar]
- FURNESS G., ROWLEY D. Transduction of virulence within the species Salmonella typhimurium. J Gen Microbiol. 1956 Aug;15(1):140–145. doi: 10.1099/00221287-15-1-140. [DOI] [PubMed] [Google Scholar]
- Fahey K. J., Cooper G. N. Oral Immunization in Experimental Salmonellosis II. Characteristics of the Immune Response to Temperature-Sensitive Mutants Given by Oral and Parenteral Routes. Infect Immun. 1970 Aug;2(2):183–191. doi: 10.1128/iai.2.2.183-191.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahey K. J., Cooper G. N. Oral immunization against experimental salmonellosis I. Development of temperature-sensitive mutant vaccines. Infect Immun. 1970 Mar;1(3):263–270. doi: 10.1128/iai.1.3.263-270.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fauve R. M., Hevin M. B. Pouvoir bactéricide des macrophages spléniques et hépatiques de souris envers Listeria monocytogenes. Influence du traitement préalable des animaux par des glucocorticoides, une endotoxine, Corynebacterium parvum et l'acide polyinosinique, polycytidylique (Poly: I.C.) Ann Inst Pasteur (Paris) 1971 Mar;120(3):399–411. [PubMed] [Google Scholar]
- Fubara E. S., Freter R. Protection against enteric bacterial infection by secretory IgA antibodies. J Immunol. 1973 Aug;111(2):395–403. [PubMed] [Google Scholar]
- GAINES S., TULLY J. G., TIGERTT W. D. Enhancement of the mouse virulence of a non-Vi variant of Salmonella typhosa by Vi antigen. J Immunol. 1961 May;86:543–551. [PubMed] [Google Scholar]
- GELZER J., SUTER E. The effect of antibody on intracellular parasitism of Salmonella typhimurium in mononuclear phagocytes in vitro: prolonged survival of infected monocytes in presence of antibody. J Exp Med. 1959 Nov 1;110:715–730. doi: 10.1084/jem.110.5.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRAY D. F. Immunity, natural anergy, and artificial desensitization in experimental tuberculosis. Am Rev Tuberc. 1958 Aug;78(2):235–250. doi: 10.1164/artpd.1958.78.2.235. [DOI] [PubMed] [Google Scholar]
- GREISMAN S. E., WOODWARD W. E. MECHANISMS OF ENDOTOXIN TOLERANCE. 3. THE REFRACTORY STATE DURING CONTINUOUS INTRAVENOUS INFUSIONS OF ENDOTOXIN. J Exp Med. 1965 Jun 1;121:911–933. doi: 10.1084/jem.121.6.911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerety R. J., Ferraresi R. W., Raffel S. Polysaccharide in delayed hypersensitivity. I. Pneumococcal polysaccharide as inducer and elicitor of delayed reactivity in guinea pigs. J Exp Med. 1970 Jan 1;131(1):189–206. doi: 10.1084/jem.131.1.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germanier R. Immunity in Experimental Salmonellosis I. Protection Induced by Rough Mutants of Salmonella typhimurium. Infect Immun. 1970 Sep;2(3):309–315. doi: 10.1128/iai.2.3.309-315.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germanier R. Immunity in experimental salmonellosis. 3. Comparative immunization with viable and heat-inactivated cells of Salmonella typhimurium. Infect Immun. 1972 May;5(5):792–797. doi: 10.1128/iai.5.5.792-797.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godal T., Myklestad B., Samuel D. R., Myrvang B. Characterization of the cellular immune defect in lepromatous leprosy: a specific lack of circulating Mycobacterium leprae-reactive lymphocytes. Clin Exp Immunol. 1971 Dec;9(6):821–831. [PMC free article] [PubMed] [Google Scholar]
- Gordon B. L., 2nd The case for cytophilic antibodies in cellular immunity. Ann Allergy. 1967 Jan;25(1):1–5. [PubMed] [Google Scholar]
- Gupta J. D., Reed C. E. Amount, class and specificity of antibody to the lipopolysaccharide of Salmonella enteritidis after immunization by various schedules. Int Arch Allergy Appl Immunol. 1971;40(2):256–263. doi: 10.1159/000230410. [DOI] [PubMed] [Google Scholar]
- HOBSON D. Resistance to reinfection in experimental mouse typhoid. J Hyg (Lond) 1957 Sep;55(3):334–343. doi: 10.1017/s0022172400037244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOLLAND J. J., PICKETT M. J. A cellular basis of immunity in experimental Brucella infection. J Exp Med. 1958 Sep 1;108(3):343–360. doi: 10.1084/jem.108.3.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOWARD J. G., BIOZZI G., HALPERN B. N., STIFFEL C., MOUTON D. The effect of Mycobacterium tuberculosis (BCG) infection on the resistance of mice to bacterial endotoxin and Salmonella enteritidis infection. Br J Exp Pathol. 1959 Jun;40(3):281–290. [PMC free article] [PubMed] [Google Scholar]
- Halliburton B. L., Hinsdill R. D. Recall of acquired cellular resistance in mice by antigens from killed Brucella. Infect Immun. 1972 Jan;5(1):42–47. doi: 10.1128/iai.5.1.42-47.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hejfec L. B., Levina L. A., Kuz'minova M. L., Slavina A. M., Drozd A. K., Tonojan I. A., Koenman L. I., Levites N. E., Abidov L. A., Abidov A. Z. A controlled field trial to evaluate the protective capacity of a single dose of acetone-killed agar-grown and heat-killed broth-grown typhoid vaccines. Bull World Health Organ. 1969;40(6):903–907. [PMC free article] [PubMed] [Google Scholar]
- Hejfec L. B. Size of the immunizing dose and postvaccination immunity to typhoid and paratyphoid B from the results of controlled field trials. J Hyg Epidemiol Microbiol Immunol. 1971;15(2):173–182. [PubMed] [Google Scholar]
- Herzberg M., Nash P., Hino S. Degree of immunity induced by killed vaccines to experimental salmonellosis in mice. Infect Immun. 1972 Jan;5(1):83–90. doi: 10.1128/iai.5.1.83-90.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornick R. B., Greisman S. E., Woodward T. E., DuPont H. L., Dawkins A. T., Snyder M. J. Typhoid fever: pathogenesis and immunologic control. N Engl J Med. 1970 Sep 24;283(13):686–691. doi: 10.1056/NEJM197009242831306. [DOI] [PubMed] [Google Scholar]
- Hornick R. B., Woodward T. E. Appraisal of typhoid vaccine in experimentally infected human subjects. Trans Am Clin Climatol Assoc. 1967;78:70–78. [PMC free article] [PubMed] [Google Scholar]
- Houchens D. P., Wright G. L., Jr Immunity to Salmonella typhimurium infection: characterization of antigens in active protection by polyacrylamide gel electrophoresis. Infect Immun. 1973 Mar;7(3):507–511. doi: 10.1128/iai.7.3.507-511.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard J. G., Christie G. H., Scott M. T. Biological effects of Corynebacterium parvum. IV. Adjuvant and inhibitory activities on B lymphocytes. Cell Immunol. 1973 May;7(2):290–301. doi: 10.1016/0008-8749(73)90251-7. [DOI] [PubMed] [Google Scholar]
- Hunt S. V., Ellis S. T., Gowans J. L. The role of lymphocytes in antibody formation. IV. Carriage of immunological memory by lymphocyte fractions separated by velocity sedimentation and on glass bead columns. Proc R Soc Lond B Biol Sci. 1972 Sep 19;182(1067):211–231. doi: 10.1098/rspb.1972.0076. [DOI] [PubMed] [Google Scholar]
- JENKIN C. R., ROWLEY D., AUZINS I. THE BASIS FOR IMMUNITY TO MOUSE TYPHOID. I. THE CARRIER STATE. Aust J Exp Biol Med Sci. 1964 Apr;42:215–228. doi: 10.1038/icb.1964.23. [DOI] [PubMed] [Google Scholar]
- JENKIN C. R., ROWLEY D. BASIS FOR IMMUNITY TO TYPHOID IN MICE AND THE QUESTION OF "CELLULAR IMMUNITY". Bacteriol Rev. 1963 Dec;27:391–404. doi: 10.1128/br.27.4.391-404.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JENKIN C. R., ROWLEY D. PARTIAL PURIFICATION OF THE "PROTECTIVE" ANTIGEN OF SALMONELLA TYPHIMURIUM AND ITS DISTRIBUTION AMONGST VARIOUS STRAINS OF BACTERIA. Aust J Exp Biol Med Sci. 1965 Feb;43:65–78. doi: 10.1038/icb.1965.5. [DOI] [PubMed] [Google Scholar]
- Joó I., Pusztai Z., Juhász V. P. Mouse-protective ability of the international reference preparations of typhoid vaccine. Z Immunitatsforsch Allerg Klin Immunol. 1968 Jun;135(4):365–387. [PubMed] [Google Scholar]
- KARTHIGASU K., JENKIN C. R., TURNER K. J. THE NATURE OF THE OPSONINS IN ADULT HEN SERUM AND DEVELOPING CHICK EMBRYOS TO CERTAIN GRAM-NEGATIVE BACTERIA. Aust J Exp Biol Med Sci. 1964 Aug;42:499–510. doi: 10.1038/icb.1964.47. [DOI] [PubMed] [Google Scholar]
- KHASANOV M. I., KHEIFETS L. B., SALMIN L. V. A controlled field trial of the typhoid component of polyvalent enteric vaccine (NIISI polyvaccine). Bull World Health Organ. 1962;26:371–379. [PMC free article] [PubMed] [Google Scholar]
- Kawakami M., Nakata H., Mitsuhashi S. Experimental salmonellosis. Immunizing effect of live vaccine prepared from various mutants of Salmonella having different cell wall polysaccharides. Jpn J Microbiol. 1969 Dec;13(4):315–324. doi: 10.1111/j.1348-0421.1969.tb00474.x. [DOI] [PubMed] [Google Scholar]
- Kawakami M., Osawa N., Mitsuhashi S. Experimental salmonellosis. VII. Comparison of the immunizing effect of live vaccine and materials extracted from Salmonella enteritidis. J Bacteriol. 1966 Dec;92(6):1585–1589. doi: 10.1128/jb.92.6.1585-1589.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenny K., Herzberg M. Antibody response and protection induced by immunization with smooth and rough strains in experimental salmonellosis. J Bacteriol. 1968 Feb;95(2):406–417. doi: 10.1128/jb.95.2.406-417.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenny K., Schlecht S., Westphal O. Antibody response to various single-factor o antigens of salmonella. Infect Immun. 1970 Jan;1(1):41–50. doi: 10.1128/iai.1.1.41-50.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klasky S., Pickett M. J. Immunogenicity and specificity of Listeria monocytogenes cell walls. J Infect Dis. 1968 Feb;118(1):65–75. doi: 10.1093/infdis/118.1.65. [DOI] [PubMed] [Google Scholar]
- Krishnapillai V., Karthigasu K. Salmonella abony-Salmonella typhimurium recombinant nonvirulent for the mouse. J Bacteriol. 1969 Mar;97(3):1343–1351. doi: 10.1128/jb.97.3.1343-1351.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane F. C., Unanue E. R. Requirement of thymus (T) lymphocytes for resistance to listeriosis. J Exp Med. 1972 May 1;135(5):1104–1112. doi: 10.1084/jem.135.5.1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Layton H. W., Youmans G. P. Effect of dietary factors upon the resistance of albino mice to experimental infection with Mycobacterium tuberculosis. J Bacteriol. 1965 Oct;90(4):958–964. doi: 10.1128/jb.90.4.958-964.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefford M. J., McGregor D. D., Mackaness G. B. Immune response to Mycobacterium tuberculosis in rats. Infect Immun. 1973 Aug;8(2):182–189. doi: 10.1128/iai.8.2.182-189.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim S. D., Kiszkiss D. F., Jacobson R. R., Choi Y. S., Good R. A. Thymus-dependent lymphocytes of peripheral blood in leprosy patients. Infect Immun. 1974 Feb;9(2):394–399. doi: 10.1128/iai.9.2.394-399.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubaroff D. M., Waksman B. H. Bone marrow as a source of cells in reactions of cellular hypersensitivity. I. Passive transfer of tuberculin sensitivity in syngeneic systems. J Exp Med. 1968 Dec 1;128(6):1425–1435. doi: 10.1084/jem.128.6.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lüderitz O., Staub A. M., Westphal O. Immunochemistry of O and R antigens of Salmonella and related Enterobacteriaceae. Bacteriol Rev. 1966 Mar;30(1):192–255. doi: 10.1128/br.30.1.192-255.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MACKANESS G. B. Cellular resistance to infection. J Exp Med. 1962 Sep 1;116:381–406. doi: 10.1084/jem.116.3.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MACKANESS G. B., SMITH N., WELLS A. Q. The growth of intracellular tubercle bacilli in relation to their virulence. Am Rev Tuberc. 1954 Apr;69(4):479–494. doi: 10.1164/art.1954.69.4.479. [DOI] [PubMed] [Google Scholar]
- MACKANESS G. B. THE IMMUNOLOGICAL BASIS OF ACQUIRED CELLULAR RESISTANCE. J Exp Med. 1964 Jul 1;120:105–120. doi: 10.1084/jem.120.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARCUS S., DONALDSON D. M., ESPLIN D. W. Role of host defenses in systemic Klebsiella pneumoniae infection: effect of antibiotics in normal and x-irradiated mice. J Immunol. 1955 Jun;74(6):494–497. [PubMed] [Google Scholar]
- MARMION D. E., NAYLOR G. R., STEWART I. O. Second attacks of typhoid fever. J Hyg (Lond) 1953 Jun;51(2):260–267. doi: 10.1017/s0022172400015680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MILLER C. P., BOHNHOFF M. CHANGES IN THE MOUSE'S ENTERIC MICROFLORA ASSOCIATED WITH ENHANCED SUSCEPTIBILITY TO SALMONELLA INFECTION FOLLOWING STREPTOMYCIN TREATMENT. J Infect Dis. 1963 Jul-Aug;113:59–66. doi: 10.1093/infdis/113.1.59. [DOI] [PubMed] [Google Scholar]
- MUNOZ J. J. SYMPOSIUM ON RELATIONSHIP OF STRUCTURE OF MICROORGANISMS TO THEIR IMMUNOLOGICAL PROPERTIES. I. IMMUNOLOGICAL AND OTHER BIOLOGICAL ACTIVITIES OF BORDETELLA PERTUSSIS ANTIGENS. Bacteriol Rev. 1963 Dec;27:325–340. doi: 10.1128/br.27.4.325-340.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackaness G. B., Auclair D. J., Lagrange P. H. Immunopotentiation with BCG. I. Immune response to different strains and preparations. J Natl Cancer Inst. 1973 Nov;51(5):1655–1667. doi: 10.1093/jnci/51.5.1655. [DOI] [PubMed] [Google Scholar]
- Mackaness G. B., Blanden R. V. Cellular immunity. Prog Allergy. 1967;11:89–140. [PubMed] [Google Scholar]
- Mackaness G. B., Blanden R. V., Collins F. M. Host-parasite relations in mouse typhoid. J Exp Med. 1966 Oct 1;124(4):573–583. doi: 10.1084/jem.124.4.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackaness G. B. Cellular immunity. Ann Inst Pasteur (Paris) 1971 Mar;120(3):428–437. [PubMed] [Google Scholar]
- Mackaness G. B., Hill W. C. The effect of anti-lymphocyte globulin on cell-mediated reistance to infection. J Exp Med. 1969 May 1;129(5):993–1012. doi: 10.1084/jem.129.5.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackaness G. B. Resistance to intracellular infection. J Infect Dis. 1971 Apr;123(4):439–445. doi: 10.1093/infdis/123.4.439. [DOI] [PubMed] [Google Scholar]
- Mackaness G. B. The J. Burns Amberson LECTURE The induction and expression of cell-mediated hypersensitivity in the lung. Am Rev Respir Dis. 1971 Dec;104(6):813–828. doi: 10.1164/arrd.1971.104.6.813. [DOI] [PubMed] [Google Scholar]
- Mackaness G. B. The immunology of antituberculous immunity. Am Rev Respir Dis. 1968 Mar;97(3):337–344. doi: 10.1164/arrd.1968.97.3.337. [DOI] [PubMed] [Google Scholar]
- Mackaness G. B. The influence of immunologically committed lymphoid cells on macrophage activity in vivo. J Exp Med. 1969 May 1;129(5):973–992. doi: 10.1084/jem.129.5.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackaness G. B. The monocyte in cellular immunity. Semin Hematol. 1970 Apr;7(2):172–184. [PubMed] [Google Scholar]
- Mackaness G. B. The relationship of delayed hypersensitivity to acquired cellular resistance. Br Med Bull. 1967 Jan;23(1):52–54. doi: 10.1093/oxfordjournals.bmb.a070516. [DOI] [PubMed] [Google Scholar]
- Mackenzie G. M., Pike R. M., Swinney R. E. Virulence of Salmonella typhimurium: II. Studies of the Polysaccharide Antigens of Virulent and Avirulent Strains. J Bacteriol. 1940 Aug;40(2):197–214. doi: 10.1128/jb.40.2.197-214.1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malley A., Baecher L. Hypersensitivity to bacterial enzymes. I. Atopic hypersensitivity induced in rhesus monkeys. J Allergy Clin Immunol. 1972 Jan;49(1):36–42. doi: 10.1016/0091-6749(72)90121-2. [DOI] [PubMed] [Google Scholar]
- McCune R. M., Feldmann F. M., McDermott W. Microbial persistence. II. Characteristics of the sterile state of tubercle bacilli. J Exp Med. 1966 Mar 1;123(3):469–486. doi: 10.1084/jem.123.3.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDERMOTT W. Microbial persistence. Yale J Biol Med. 1958 Feb;30(4):257–291. [PMC free article] [PubMed] [Google Scholar]
- McGregor D. D., Hahn H. H., Mackaness G. B. The mediator of cellular immunity. V. Development of cellular resistance to infection in thymectomized irradiated rats. Cell Immunol. 1973 Feb;6(2):186–199. doi: 10.1016/0008-8749(73)90021-x. [DOI] [PubMed] [Google Scholar]
- Miller J. F. The thymus and the immune system. Vox Sang. 1971 Jun;20(6):481–491. doi: 10.1111/j.1423-0410.1971.tb00459.x. [DOI] [PubMed] [Google Scholar]
- Mäkelä P. H., Valtonen V. V., Valtonen M. Role of O-antigen (lipopolysaccharide) factors in the virulence of Salmonella. J Infect Dis. 1973 Jul;128(Suppl):81–85. doi: 10.1093/infdis/128.supplement_1.s81. [DOI] [PubMed] [Google Scholar]
- NELSON D. S., BOYDEN S. V. THE CUTANEOUS REACTIVITY OF GUINEA PIGS TO PURE PROTEIN ANTIGENS. I. A CRITICAL EVALUATION OF METHODS FOR THE INDUCTION OF DELAYED-TYPE HYPERSENSITIVITY TO PURE PROTEINS. Int Arch Allergy Appl Immunol. 1964;25:279–303. doi: 10.1159/000229529. [DOI] [PubMed] [Google Scholar]
- NELSON E. L., BECKER J. R. The effect of whole-body x irradiation on the bactericidal activity of pagocytic cells. II. Survival of Pseudomonas aeruginosa within livers and spleens of mice. J Infect Dis. 1959 Jan-Feb;104(1):20–23. doi: 10.1093/infdis/104.1.20. [DOI] [PubMed] [Google Scholar]
- NEVA F. A., MORGAN H. R. Tolerance to the action of endotoxins of enteric bacilli in patients convalescent from typhoid and paratyphoid fevers. J Lab Clin Med. 1950 Jun;35(6):911–922. [PubMed] [Google Scholar]
- Nakano M., Saito K. Chemical components in the cell wall of Salmonella typhimurium affecting its virulence and immunogenicity in mice. Nature. 1969 Jun 14;222(5198):1085–1086. doi: 10.1038/2221085a0. [DOI] [PubMed] [Google Scholar]
- Nakano M., Saito K. The chemical compositions of the cell wall of Salmonella typhimurium affecting the clearance-rate in mouse; influence of specific antibody. Jpn J Microbiol. 1970 Nov;14(6):451–461. [PubMed] [Google Scholar]
- Neiburger R. G., Youmans G. P., Youmans A. S. Relationship between tuberculin hypersensitivity and cellular immunity to infection in mice vaccinated with viable attenuated Mycobacterial cells or with Mycobacterial ribonucleic acid preparations. Infect Immun. 1973 Jul;8(1):42–47. doi: 10.1128/iai.8.1.42-47.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North R. J. Cellular mediators of anti-Listeria immunity as an enlarged population of short lived, replicating T cells. Kinetics of their production. J Exp Med. 1973 Aug 1;138(2):342–355. doi: 10.1084/jem.138.2.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North R. J. Importance of thymus-derived lymphocytes in cell-mediated immunity to infection. Cell Immunol. 1973 Apr;7(1):166–176. doi: 10.1016/0008-8749(73)90193-7. [DOI] [PubMed] [Google Scholar]
- North R. J., Mackaness G. B., Elliott R. W. The histogenesis of immunologically committed lymphocytes. Cell Immunol. 1972 Apr;3(4):680–694. doi: 10.1016/0008-8749(72)90130-x. [DOI] [PubMed] [Google Scholar]
- North R. J., Mackaness G. B. Immunological control of macrophage proliferation in vivo. Infect Immun. 1973 Jul;8(1):68–73. doi: 10.1128/iai.8.1.68-73.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North R. J. The relative importance of blood monocytes and fixed macrophages to the expression of cell-mediated immunity to infection. J Exp Med. 1970 Sep 1;132(3):521–534. doi: 10.1084/jem.132.3.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North R. J. The uptake of particulate antigens. J Reticuloendothel Soc. 1968 Jun;5(3):203–229. [PubMed] [Google Scholar]
- OLITZKI A. L., GODINGER D. COMPARATIVE STUDIES ON SALMONELLA TYPHI GROWN IN VIVO AND IN VITRO. III. THE IMMUNIZING POTENCIES OF ACETONE-KILLED VACCINES PREPARED FROM IN VIVO- AND IN VITRO-GROWN BACTERIA AND THE IMMUNIZING POTENCY OF SUBSTANCES ISOLATED FROM INFECTED ORGANS. J Hyg (Lond) 1963 Sep;61:353–363. doi: 10.1017/s0022172400039644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parish C. R. The relationship between humoral and cell-mediated immunity. Transplant Rev. 1972;13:35–66. doi: 10.1111/j.1600-065x.1972.tb00059.x. [DOI] [PubMed] [Google Scholar]
- Pearson M. N., Raffel S. Macrophage-digested antigen as inducer of delayed hypersensitivity. J Exp Med. 1971 Mar 1;133(3):494–505. doi: 10.1084/jem.133.3.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plant J., Glynn A. A. Natural resistance to Salmonella infection, delayed hypersensitivity and Ir genes in different strains of mice. Nature. 1974 Mar 22;248(446):345–347. doi: 10.1038/248345a0. [DOI] [PubMed] [Google Scholar]
- ROBBINS J. B., KENNY K., SUTER E. THE ISOLATION AND BIOLOGICAL ACTIVITIES OF RABBIT GAMMA M- AND GAMMA G-ANTI-SALMONELLA TYPHIMURIUM ANTIBODIES. J Exp Med. 1965 Aug 1;122:385–402. doi: 10.1084/jem.122.2.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROWLEY D., JENKIN C. R. Partial purification of opsonins in pig serum to a strain of salmonella typhimurium. Immunology. 1962 Sep;5:557–565. [PMC free article] [PubMed] [Google Scholar]
- ROWLEY D. Rapidly induced changes in the level of non-specific immunity in laboratory animals. Br J Exp Pathol. 1956 Jun;37(3):223–234. [PMC free article] [PubMed] [Google Scholar]
- ROWLEY D., TURNER K. J., JENKIN C. R. THE BASIS FOR IMMUNITY TO MOUSE TYPHOID. 3. CELL-BOUND ANTIBODY. Aust J Exp Biol Med Sci. 1964 Apr;42:237–248. doi: 10.1038/icb.1964.25. [DOI] [PubMed] [Google Scholar]
- Rees R. J. New prospects for the study of leprosy in the laboratory. Bull World Health Organ. 1969;40(5):785–800. [PMC free article] [PubMed] [Google Scholar]
- Remington J. S., Merigan T. C. Synthetic polyanions protect mice against intracellular bacterial infection. Nature. 1970 Apr 25;226(5243):361–363. doi: 10.1038/226361a0. [DOI] [PubMed] [Google Scholar]
- Ribi E., Anacker R. L., Barclay W. R., Brehmer W., Harris S. C., Leif W. R., Simmons J. Efficacy of mycobacterial cell walls as a vaccine against airborne tuberculosis in the Rheusus monkey. J Infect Dis. 1971 May;123(5):527–538. doi: 10.1093/infdis/123.5.527. [DOI] [PubMed] [Google Scholar]
- Ribi E., Anacker R. L., Brehmer W., Goode G., Larson C. L., List R. H., Milner K. C., Wicht W. C. Factors influencing protection against experimental tuberculosis in mice by heat-stable cell wall vaccines. J Bacteriol. 1966 Oct;92(4):869–879. doi: 10.1128/jb.92.4.869-879.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribi E. Currents in tuberculosis research. J Infect Dis. 1971 May;123(5):562–565. doi: 10.1093/infdis/123.5.562. [DOI] [PubMed] [Google Scholar]
- Ribi E., Larson C., Wicht W., List R., Goode G. Effective nonliving vaccine against experimental tuberculosis in mice. J Bacteriol. 1966 Mar;91(3):975–983. doi: 10.1128/jb.91.3.975-983.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roantree R. J. Salmonella O antigens and virulence. Annu Rev Microbiol. 1967;21:443–466. doi: 10.1146/annurev.mi.21.100167.002303. [DOI] [PubMed] [Google Scholar]
- Robson H. G., Vas S. I. Resistance of inbred mice to Salmonella typhimurium. J Infect Dis. 1972 Oct;126(4):378–386. doi: 10.1093/infdis/126.4.378. [DOI] [PubMed] [Google Scholar]
- Rowley D., Auzins I., Jenkin C. R. Further studies regarding the question of cellular immunity in mouse typhoid. Aust J Exp Biol Med Sci. 1968 Aug;46(4):447–463. doi: 10.1038/icb.1968.38. [DOI] [PubMed] [Google Scholar]
- Rowley D. Endotoxins and bacterial virulence. J Infect Dis. 1971 Mar;123(3):317–327. doi: 10.1093/infdis/123.3.317. [DOI] [PubMed] [Google Scholar]
- Ruskin J., McIntosh J., Remington J. S. Studies on the mechanisms of resistance to phylogenetically diverse intracellular organisms. J Immunol. 1969 Aug;103(2):252–259. [PubMed] [Google Scholar]
- SAITO K., AKIYAMA T., NAKANO M., USHBA D. Interaction between Salmonella enteritidis and tissue cultured macrophages derived from immunized animals. Jpn J Microbiol. 1960 Oct;4:395–407. doi: 10.1111/j.1348-0421.1960.tb00188.x. [DOI] [PubMed] [Google Scholar]
- SAITO K., NAKANO M., AKIYAMA T., USHIBA D. Passive transfer of immunity to typhoid by macrophages. J Bacteriol. 1962 Sep;84:500–507. doi: 10.1128/jb.84.3.500-507.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SATO I., TANAKA T., SAITO K., MITSUHASHI S. Inhibition of Salmonella enteritidis ingested in mononuclear phagocytes from liver and subcutaneous tissue of mice immunized with live vaccine. J Bacteriol. 1962 Jun;83:1306–1312. doi: 10.1128/jb.83.6.1306-1312.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHAFFER J. M., KUCERA C. J., SPINK W. W. The protection of intracellular brucella against therapeutic agents and the bactericidal action of serum. J Exp Med. 1953 Jan;97(1):77–90. doi: 10.1084/jem.97.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHAW C. M., ALVORD E. C., Jr, FAHLBERG W. J., KIES M. W. SUBSTITUTES FOR THE MYCOBACTERIA IN FREUND'S ADJUVANTS IN THE PRODUCTION OF EXPERIMENTAL "ALLERGIC" ENCEPHALOMYELITIS IN THE GUINEA PIG. J Immunol. 1964 Jan;92:28–40. [PubMed] [Google Scholar]
- SMITH H. W. The use of live vaccines in experimental Salmonella gallinarum infection in chickens with observations on their interference effect. J Hyg (Lond) 1956 Sep;54(3):419–432. doi: 10.1017/s0022172400044685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SMITH H., FITZGEORGE R. B. THE EFFECT OF OPSONIZATION WITH ANTISERUM ON THE SURVIVAL OF BRUCELLA ABORTUS WITHIN BOVINE PHAGOCYTES. Br J Exp Pathol. 1964 Dec;45:672–674. [PMC free article] [PubMed] [Google Scholar]
- SPAUN J. STUDIES ON THE INFLUENCE OF THE ROUTE OF IMMUNIZATION IN THE ACTIVE MOUSE PROTECTION TEST WITH INTRAPERITONEAL CHALLENGE FOR POTENCY ASSAY OF TYPHOID VACCINES. Bull World Health Organ. 1964;31:793–798. [PMC free article] [PubMed] [Google Scholar]
- SPAUN J., UEMURA K. INTERNATIONAL REFERENCE PREPARATIONS OF TYPHOID VACCINE. A REPORT ON INTERNATIONAL COLLABORATIVE LABORATORY STUDIES. Bull World Health Organ. 1964;31:761–791. [PMC free article] [PubMed] [Google Scholar]
- STUART C. A. The unfortunate role of precedent in bacteriology. Bacteriol Rev. 1956 Dec;20(4):203–206. doi: 10.1128/br.20.4.203-206.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STUHL L., BENDA R., FREY N. PREPARATION OF DRIED ACETONE-INACTIVATED AND HEAT-PHENOL-INACTIVATED TYPHOID VACCINES. Bull World Health Organ. 1964;30:635–646. [PMC free article] [PubMed] [Google Scholar]
- SUTER E. Hyperreactivity to endotoxin in mice infected with BCG. Studies on the role of concomitant infection. J Immunol. 1962 Sep;89:377–381. [PubMed] [Google Scholar]
- SUTER E. Interaction between phagocytes and pathogenic microorganisms. Bacteriol Rev. 1956 Jun;20(2):94–132. doi: 10.1128/br.20.2.94-132.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito K., Mitsuhashi S. Experimental Salmonellosis VI. In Vitro Transfer of Cellular Immunity of Mouse Mononuclear Phagocytes. J Bacteriol. 1965 Sep;90(3):629–634. doi: 10.1128/jb.90.3.629-634.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlossman S. F., Levine H. Immunochemical studies on delayed and arthus-type hypersensitivity reactions. I. The relationship between antigenic determinant size and antibody combining site size. J Immunol. 1967 Feb;98(2):211–219. [PubMed] [Google Scholar]
- Scott M. T. Biological effects of the adjuvant Corynebacterium parvum. II. Evidence for macrophage-T-cell interaction. Cell Immunol. 1972 Nov;5(3):469–479. doi: 10.1016/0008-8749(72)90073-1. [DOI] [PubMed] [Google Scholar]
- Sher N. A., Chaparas S. D., Pearson J., Chirigos M. Virulence of six strains of Mycobacterium bovis (BCG) in mice. Infect Immun. 1973 Nov;8(5):736–742. doi: 10.1128/iai.8.5.736-742.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith H. W., Halls S. The immunity produced by a rough Salmonella dublin variant against Salmonella typhimurium and Salmonella choleraesuis infection guinea-pigs. J Hyg (Lond) 1966 Sep;64(3):357–359. doi: 10.1017/s0022172400040638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R. A., Bigley N. J. Detection of delayed hypersensitivity in mice injected with ribonucleic acid-protein fractions of Salmonella typhimurium. Infect Immun. 1972 Sep;6(3):384–389. doi: 10.1128/iai.6.3.384-389.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R. A., Bigley N. J. Ribonucleic acid-protein fractions of virulent Salmonella typhimurium as protective immunogens. Infect Immun. 1972 Sep;6(3):377–383. doi: 10.1128/iai.6.3.377-383.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S. E., Scott M. T. Biological effects of Corynebacterium parvum. 3. Amplification of resistance and impairment of active immunity to murine tumours. Br J Cancer. 1972 Oct;26(5):361–367. doi: 10.1038/bjc.1972.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon J. B. Immunity to Salmonella gallinarum during ontogeny of the chicken. I. Onset of resistance to infection; the minor role of opsonins. Immunology. 1968 Aug;15(2):197–206. [PMC free article] [PubMed] [Google Scholar]
- Stubbs M., Kühner A. V., Glass E. A., David J. R., Karnovsky M. L. Metabolic and functonal studies on activated mouse macrophages. J Exp Med. 1973 Feb 1;137(2):537–542. doi: 10.1084/jem.137.2.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeya K., Mori R., Nomoto K., Nakayama H. Experimental mycobacterial infections in neonatally thymectomized mice. Am Rev Respir Dis. 1967 Sep;96(3):469–477. doi: 10.1164/arrd.1967.96.3.469. [DOI] [PubMed] [Google Scholar]
- Tomasi T. B., Jr, Bienenstock J. Secretory immunoglobulins. Adv Immunol. 1968;9:1–96. doi: 10.1016/s0065-2776(08)60441-1. [DOI] [PubMed] [Google Scholar]
- Tripathy S. P., Mackaness G. B. The effect of cytotoxic agents on the primary immune response to Listeria monocytogenes. J Exp Med. 1969 Jul 1;130(1):1–16. doi: 10.1084/jem.130.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turcotte R. Yield of non-dialyzable mycobacterial constituents during the growth cycle. Can J Microbiol. 1969 Jan;15(1):35–41. doi: 10.1139/m69-006. [DOI] [PubMed] [Google Scholar]
- Uhr J. W. Delayed hypersensitivity. Physiol Rev. 1966 Jul;46(3):359–419. doi: 10.1152/physrev.1966.46.3.359. [DOI] [PubMed] [Google Scholar]
- Ushiba D. Two types of immunity in experimental typhoid; "cellular immunity" and "humoral immunity". Keio J Med. 1965 Jun;14(2):45–61. doi: 10.2302/kjm.14.45. [DOI] [PubMed] [Google Scholar]
- Valtonen V. V., Mäkelä P. H. The effect of lipopolysaccharide modification--antigenic factors 1,5,12 2 and 27--on the virulence of salmonella strains for mice. J Gen Microbiol. 1971 Nov;69(1):107–115. doi: 10.1099/00221287-69-1-107. [DOI] [PubMed] [Google Scholar]
- Venneman M. R., Berry L. J. Cell-mediated resistance induced with immunogenic preparations of Salmonella typhimurium. Infect Immun. 1971 Oct;4(4):381–387. doi: 10.1128/iai.4.4.381-387.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venneman M. R., Berry L. J. Experimental salmonellosis: differential passive transfer of immunity with serum and cells obtained from ribosomal and ribonucleic acid-immunized mice. J Reticuloendothel Soc. 1971 May;9(5):491–502. [PubMed] [Google Scholar]
- Venneman M. R., Berry L. J. Serum-mediated resistance induced with immunogenic preparations of Salmonella typhimurium. Infect Immun. 1971 Oct;4(4):374–380. doi: 10.1128/iai.4.4.374-380.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venneman M. R., Bigley N. J. Isolation and partial characterization of an immunogenic moiety obtained from Salmonella typhimurium. J Bacteriol. 1969 Oct;100(1):140–148. doi: 10.1128/jb.100.1.140-148.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkman A., Collins F. M. Polyarthritis associated with Salmonella infection in rats. Infect Immun. 1973 Nov;8(5):814–818. doi: 10.1128/iai.8.5.814-818.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkman A., Collins F. M. Recovery of delayed-type hypersensitivity in mice following suppressive doses of X-radiation. J Immunol. 1968 Nov;101(5):846–859. [PubMed] [Google Scholar]
- Volkman A., Collins F. M. The restorative effect of peritoneal macrophages on delayed hypersensitivity following ionizing radiation. Cell Immunol. 1971 Dec;2(6):552–566. doi: 10.1016/0008-8749(71)90004-9. [DOI] [PubMed] [Google Scholar]
- WHITE R. G., JOLLES P., SAMOUR D., LEDERER E. CORRELATION OF ADJUVANT ACTIVITY AND CHEMICAL STRUCTURE OF WAX D FRACTIONS OF MYCOBACTERIA. Immunology. 1964 Mar;7:158–171. [PMC free article] [PubMed] [Google Scholar]
- Weinstein M. J., Waitz J. A., Came P. E. Induction of resistance to bacterial infections of mice with poly I-poly C. Nature. 1970 Apr 11;226(5241):170–170. doi: 10.1038/226170a0. [DOI] [PubMed] [Google Scholar]
- Weissman J. B., Rice P. A., Krogstad D. J., Baine W. B., Gangarosa E. J. Risk of severe intestinal infection to the traveler in Mexico. J Infect Dis. 1973 Oct;128(4):574–577. doi: 10.1093/infdis/128.4.574. [DOI] [PubMed] [Google Scholar]
- Wiegeshaus E. H., Smith D. W. Biology of the mycobacterioses. Experimental models for study of immunity in tuberculosis. Ann N Y Acad Sci. 1968 Sep 5;154(1):194–199. doi: 10.1111/j.1749-6632.1968.tb16709.x. [DOI] [PubMed] [Google Scholar]
- Winston S., Berry L. J. Immunity induced by ribosomal extracts from Staphylococcus aureus. J Reticuloendothel Soc. 1970 Jul;8(1):66–73. [PubMed] [Google Scholar]
- Wong K. H., Feeley J. C. Isolation of Vi antigen and a simple method for its measurement. Appl Microbiol. 1972 Oct;24(4):628–633. doi: 10.1128/am.24.4.628-633.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong K. H., Feeley J. C., Pittman M. Effect of a Vi-degrading enzyme on potency of typhoid vaccines in mice. J Infect Dis. 1972 Apr;125(4):360–366. doi: 10.1093/infdis/125.4.360. [DOI] [PubMed] [Google Scholar]
- Woolcock J. B. Resistance to microbial infection--vaccines in theory and practice. Aust Vet J. 1973 Jun;49(6):307–317. doi: 10.1111/j.1751-0813.1973.tb06813.x. [DOI] [PubMed] [Google Scholar]
- YOUMANS A. S., YOUMANS G. P. IMMUNOGENIC ACTIVITY OF A RIBOSOMAL FRACTION OBTAINED FROM MYCOBACTERIUM TUBERCULOSIS. J Bacteriol. 1965 May;89:1291–1298. doi: 10.1128/jb.89.5.1291-1298.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YOUMANS G. P., YOUMANS A. S. The measurement of the response of immunized mice to infection with Mycobacterium tuberculosis va. hominis. J Immunol. 1957 May;78(5):318–329. [PubMed] [Google Scholar]
- Yamamoto K., Anacker R. L., Ribi E. Macrophage Migration Inhibition Studies with Cells from Mice Vaccinated with Cell Walls of Mycobacterium bovis BCG: Relationship Between Inhibitory Activity of Lung Cells and Resistance to Airborne Challenge with Mycobacterium tuberculosis H37Rv. Infect Immun. 1970 Jun;1(6):595–599. doi: 10.1128/iai.1.6.595-599.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youdim S., Stuntman O., Good R. A. Thymus dependency of cells involved in transfer of delayed hypersensitivity to Listeria monocytogenes in mice. Cell Immunol. 1973 Sep;8(3):395–402. doi: 10.1016/0008-8749(73)90129-9. [DOI] [PubMed] [Google Scholar]
- Youmans A. S., Youmans G. P. Effect of trypsin and ribonuclease on the immunogenic activity of ribosomes and ribonucleic acid isolated from Mycobacterium tuberculosis. J Bacteriol. 1966 Jun;91(6):2146–2154. doi: 10.1128/jb.91.6.2146-2154.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youmans A. S., Youmans G. P. Immunogenic mycobacterial ribosomal and ribonucleic Acid preparations: chemical and physical characteristics. Infect Immun. 1970 Nov;2(5):659–668. doi: 10.1128/iai.2.5.659-668.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youmans G. P., Youmans A. S. Allergenicity of mycobacterial ribosomal and ribonucleic acid preparations in mice and guinea pigs. J Bacteriol. 1969 Jan;97(1):134–139. doi: 10.1128/jb.97.1.134-139.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youmans G. P., Youmans A. S. Nonspecific factors in resistance of mice to experimental tuberculosis. J Bacteriol. 1965 Dec;90(6):1675–1681. doi: 10.1128/jb.90.6.1675-1681.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zbar B., Rapp H. J., Ribi E. E. Tumor suppression by cell walls of mycobacterium bovis attached to oil droplets. J Natl Cancer Inst. 1972 Mar;48(3):831–835. [PubMed] [Google Scholar]


