Abstract
Tandem mass spectrometry (MS/MS) plays an essential role in modern chemical analysis. It is used for differentiating isomers and isobars and suppressing chemical noises, which allows high precision quantitation. The MS/MS analysis has been typically applied by isolating the target precursor ions, while wasting other ions, followed by a fragmentation that produces the product ions. In this study, configurations of dual linear ion traps were explored to develop high efficiency MS/MS analysis. The ions trapped in the first linear ion trap were axially, mass-selectively transferred to the second linear ion trap for MS/MS analysis. Ions from multiple compounds simultaneously introduced into the mass spectrometer could be sequentially analyzed. This development enables a highly efficient use of the sample. For miniature ion trap mass spectrometers with discontinuous atmospheric pressure interfaces, the analysis speed and the quantitation precision can be significantly improved.
Tandem mass spectrometry (MS/MS)1 has been widely used for analysis of chemical and biological compounds in samples with complex matrices. The precursor ions are isolated and fragmented and the product ions are then mass analyzed. Ion trap is a popular mass analyzer that can perform multiple-stage MS/MS analysis. In the current commercial mass spectrometers, the MS/MS analysis can be performed fast with a scan time of about 100ms for each compound; however, the sample usage is of low efficiency since the ions other than the precursors are wasted during the isolation process. For miniature mass spectrometry (MS) systems,2 the MS/MS plays an even more essential role and the efficiency improvement for the MS/MS process may also have more significant impacts. The sample preparation and chromatographic separation for in-situ or in-field analysis need to be highly simplified or completely eliminated, as demonstrated with the recent development of miniature MS systems3, 4 with ambient ionization5, 6 sources. However, it is necessary to use MS/MS to differentiate isomers and isobars. The limit of detection (LOD) and limit of quantitation (LOQ) can also be significantly improved4 with the chemical noise removed through the MS/MS process.7 Using the characteristic fragmentation pattern for confirmation of the chemical identification, high specificity potentially could also be retained for miniature mass spectrometers without ultra-high mass accuracy or resolution.
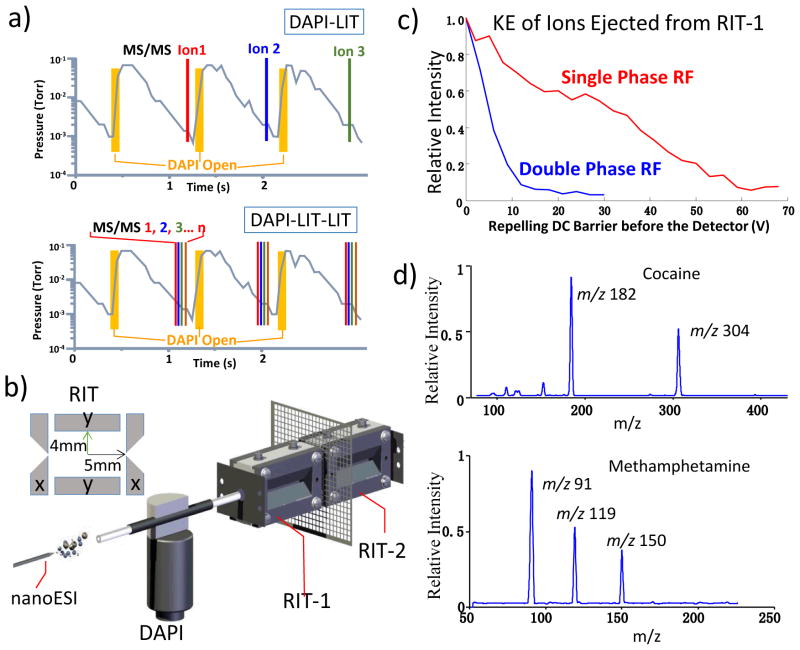
Currently, discontinuous atmospheric pressure interface (DAPI)8–10 has been used to enable the coupling of atmospheric pressure ionization and ambient ionization sources with miniature linear ion trap (LIT) mass spectrometers.3, 4, 9 The ions are introduced in a pulsed fashion with about 20 ms opening of DAPI in about every 1.5s. The ions injected are trapped in the LIT at an elevated pressure and then analyzed after the pressure drops back to millitorrs level in 500–800 ms (Figure 1a). The required pumping capacity is highly reduced for DAPI-LIT systems, while the efficiency and scan speed for MS and MS/MS analysis are also reduced. The waste of samples due to the low duty cycle, e.g. 20 ms ion introduction in every 1s or longer, could be minimized by using a pulsed ion source synchronized with the DAPI operation.10 However, for MS/MS analysis of multiple analytes in a sample, the entire process could take a significantly long time.
Figure 1.
a) Comparison of MS/MS analysis for DAPI MS instruments with one LIT and dual LITs. b) DAPI-RIT-RIT configuration for MS/MS analysis. c) Signal intensities measured for amitriptyline ions m/z 278 reaching the ion detectors as a function of the repelling voltage (see Figure S2c for experimental setup). d) MS spectra recorded by RIT-2 for protonated cocaine m/z 304 and methamphetamine m/z 150, each mass-selectively transferred from RIT-1 to RIT-2, ions generated by ESI of methanol solutions containing only 1μg/mL cocaine or only 500 ng/mL methamphetamine, AC excitation of 80 mV and 168 kHz, q = 0.45 in RIT-1, low mass cutoff = 67 for trapping the ions in RIT-2
In this study, we explore a solution for this problem using a dual LIT configuration to execute multiple MS/MS analyses with a single ion introduction (Figure 1a). The ions in a broad mass-to-charge ratio (m/z) range were trapped in the first LIT and the precursor ions within different m/z ranges could be sequentially, mass selectively transferred to the second LIT for MS/MS analysis. Dual LIT configurations11, 12 have been previously used in commercial instruments such as the LTQ Velos (Thermo Scientific, San Jose, CA),13 but the precursor ions were typically isolated and fragmented in same (first) LIT without saving the ions from other analytes; the fragmented ions were transferred with no mass selection to the second trap at a lower pressure for mass analysis at a better resolution. In a recent study,14 ions trapped in a first LIT were scanned to a second trap, trapped for a short period and then scanned out for spectrum recording, which minimized the space charge effect and hence helped to retain a high resolution with high populations of ions. In the current study, the precursor ions of a m/z value was axially, mass selectively transferred to the second LIT for MS/MS analysis, while other ions were still trapped in the first LIT for subsequent mass-selective transfers and MS/MS analyses. For implementation of the concept, we also combined the axial15 and radial16 mass-selective ion ejection methods, previously developed for two popular commercial ion trap instruments, to demonstrate the high efficiency MS/MS analysis.
Instrumentation, Results and Discussions
Rectilinear ion traps (RIT),17 previously used in development of a series of miniature mass spectrometers,3, 4, 18, 19 were used for an initial test of this dual-LIT concept (Figure 1b and S1a). Each of the two RITs has a stretched geometry with an inter-electrode distance of 5.0 mm in the x direction and 4.0 mm in the y direction. A stainless steel mesh was used as the common end electrode between these RITs. A home built testing system previously reported10 was modified for the experimental characterization. Single phase RFs of 1015 kHz and 995 kHz were applied on the y electrodes of the RIT-1 and RIT-2, respectively. The ions were trapped in RIT-1 during the DAPI opening period and then an axial mass selective ejection toward RIT-2 was attempted using two methods, an RF scan with a resonance ejection by a dipolar AC,15 or an AC excitation with a steady RF. Adjustments in wide ranges were performed for the RF voltages, AC excitation frequency (q value) and amplitude, as well as the DC voltage on the common mesh end electrode. However, no ions were found to be trapped in the RIT-2.
The axial mass-selective ion ejection from RIT-1 was then characterized using the setup shown in Figure S2a. Efficient axial ion ejection with AC excitation was observed and MS spectra were recorded with one example shown in Figure S2b for amitriptyline (3μg/mL) and amitriptyline-d6 (2μg/mL) in methanol ionized by nanoESI (electrospray ionization).20 This led to a hypothesis that the kinetic energy (KE) of the ions ejected from RIT-1 might have been too high to be trapped in RIT-2. A mesh electrode was then added between the mesh end electrode of the RIT and the ion detector assembly to apply a repelling voltage, as shown in Figure S2c). The signal intensity of amitriptyline m/z 277 was measured as a function of the repelling voltage, reflecting a wide KE distribution up to 70 eV (Figure 1c).
It is known that the field at the center axis of the LIT oscillates with unbalanced RF voltages applied on the LIT, which was responsible for the wide distribution of the KE for the axially ejected ions. A double phase RF was then applied on the RIT-1. The characterization of KE showed a much narrower distribution below 10 eV for the axially ejected ions (Figure 1c). However, when the double phase RFs were applied on both RITs for testing, the ions ejected from RIT-1 still could not be trapped in the RIT-2. As an attempt of increasing the collisional cooling, a second DAPI was then used to introduce a gas flow for 10 ms immediately before the ion transfer to increase the manifold pressure from 3 mtorr to 10 mTorr. An AC excitation of 80 mV at 148 kHz (q = 0.4) was used to mass selectively eject the ions from the RIT-1, followed by an MS analysis in RIT-2 after the pressure decrease to 3 mtorr in about 100 ms. The trapping of transferred ions in RIT-2 was observed; however, intense fragmentations was also observed, as shown by the MS spectra recorded for the protonated cocaine m/z 304 and methamphetamine m/z 150 (Figure 1d). The collisions helped to reduce the kinetic energies of the ions but also facilitated the collisional induced dissociation (CID) that is efficient at elevated pressures. A complete transfer of the ions could take as long as 80 ms. For practical implementation, the fragmentation of the precursor ion during the mass selective transfer represents an advantage since operation for CID is not required for RIT-2, which saves about 20 ms or more and simplifies the control procedure; however, an additional DAPI would be required and an additional 100 ms delay for pressure drop was needed for each MS/MS scan.
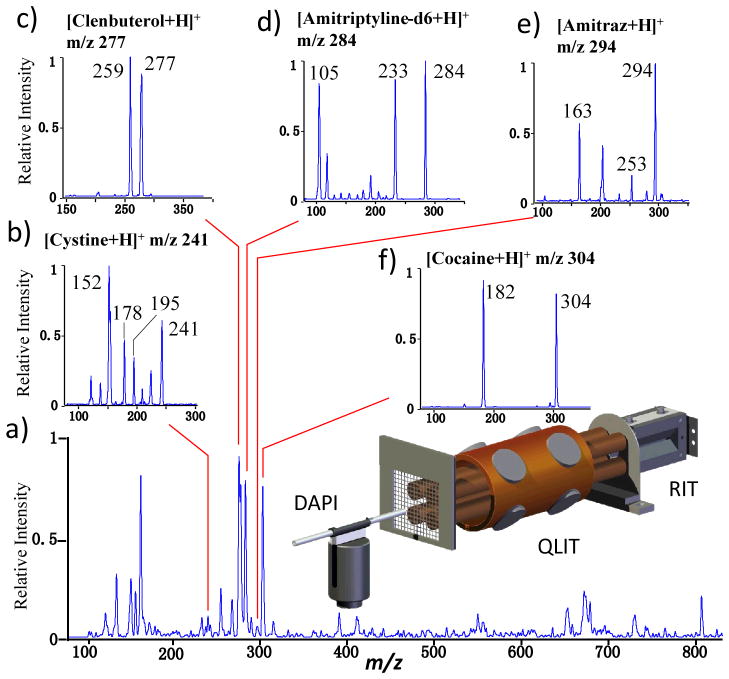
To further reduce the KE of the axially ejected ions, an LIT of a symmetric configuration (QLIT) was used to replace the RIT-1 (Figure 2 and S1c). The QLIT was previously used in development of the QTrap instruments15 (AB Sciex, Ltd, Toronto, CA), constructed with round electrodes (r = 4.17) and has a symmetric configuration with r0 = 4.0 mm. Simulations were carried out for the comparison of the electric fields for the RIT-RIT and the QLIT-RIT configurations (Figure S3). The narrowest KE distribution was expected for the QLIT operated with a balanced, double phase RF. The mass selective transfer of the intact precursor ions was indeed achieved for the QLIT-RIT configuration without elevated pressure. For analysis of a mixture of clenbuterol (1 μg/mL), amitriptyline-d6 (1 μg/mL), amitraz (200 ng/mL), cysteine (500 ng/mL) and cocaine (500 ng/mL) in a methanol solution (Figure 2 and S4), each of the precursor ions from these compounds could be mass-selectively transferred with minimal fragmentation during the transfer (Figure S4) and then MS/MS analyzed in RIT (Figure 2b–e). The mass selective ion transfers could all be completed within 30 ms. In comparison with the non-selective transfer using DC potential (Figure 2a and S4a), the efficiency for mass-selective transfer of the ions is about 55%.
Figure 2.
a) MS spectrum recorded by RIT for ions transferred from QLIT without mass selection. MS/MS spectra of protonated b) cysteine m/z 241, c) clenbuterol m/z 277, d) amitriptyline-d6 m/z 284, e) amitraz m/z 294 and f) cocaine m/z 304, precursor ions of each compounds mass selectively transferred from QLIT to RIT, fragmented in RIT, and MS analyzed. AC excitation of 80 mV and 169 kHz, q = 0.45 in QLIT; q = ~0.2 for the trapping the ions in RIT, q = ~0.2 for CID in RIT.
The ions were introduced through DAPI for 20 ms, trapped and cooled in LIT for 500 ms. The MS/MS analysis of the precursor ions from each compound took about 100 ms, including 30 ms for the mass-selective transfer from the QLIT to RIT, 20 ms for the fragmentation by CID, and about 50 ms for MS analysis of the fragment ions. With five MS/MS analysis executed for each scan cycle, the average analysis speed for each MS/MS analysis is 0.2 s, which is comparable with commercial instruments operated with continuous atmospheric pressure interface. The testing system used in this study had a large manifold and also a pumping system for lab scale mass spectrometer, viz. a 30 m3/h rotary vane pump (UNO-030M, Pfeiffer Vacuum Inc., New Hampshire) and a 345 L/s turbo pump (TurboVac 361, Leybold Vacuum, Germany).10 The scan with DAPI opening could be repeatedly run at a speed of 0.6s per scan. For miniature DAPI-RIT systems with small pumping systems,4, 19 the scan period is typically 1.5s for one MS/MS analysis. With a DAPI-LIT-LIT configuration implemented, the average time for MS/MS analysis of each of the n analytes can be estimated by the following equation:
| Equation 1 |
With 10 or more MS/MS scans implemented within each analysis cycle, the analysis efficiency of a miniature instrument could be comparable to or significantly higher than the current lab scale ion trap instruments.
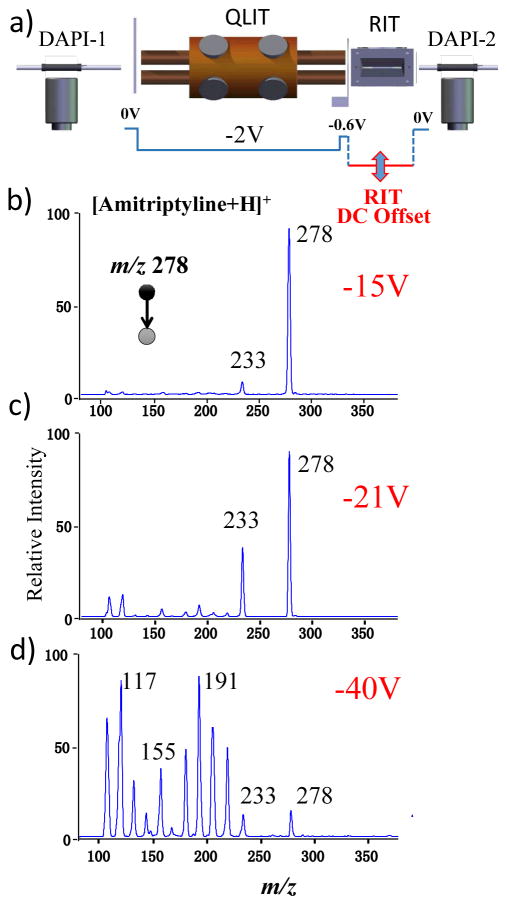
The fragmentation during mass selective transfer, as previously observed with the RIT-RIT configuration, was also explored with the QLIT-RIT in a controllable fashion. As shown in Figure 3a, a second DAPI was used and the manifold pressure was raised to 10 mtorr with a 10 ms opening immediately before the mass-selective axial transfer. As shown in Figure 3b–d for the protonated amitriptyline m/z 278, the degree of fragmentation during the mass-selective transfer could be well controlled by the difference in the DC float voltages of the QLIT and the RIT.
Figure 3.
a) Instrument setup for MS/MS with CID during mass selective ion transfer. MS/MS spectra recorded for mass-selective transfer of protonated amitriptyline m/z 278 at RIT DC offset at b) −15V, c) −21V and d) −40V. AC excitation for axial ejection from QLIT, 80 mV and 169 kHz, q = 0.45 in QLIT; q = 0.2 for trapping the ions in RIT.
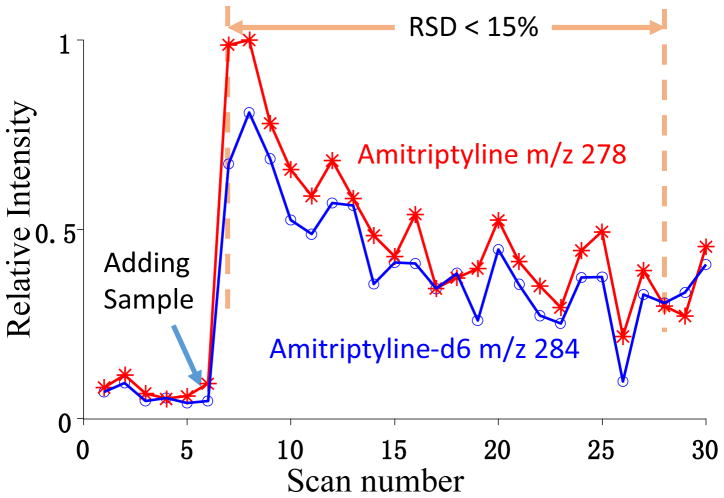
One additional significant advantage of performing multiple MS/MS analyses with a single pulse of ion introduction is the potential improvement of the precision in quantitative analysis. In the previous evaluation of the Mini 12 system of the DAPI-RIT configuration,4 we have shown that the stability of the signal is critical for the quantitation precision. Since the MS/MS analysis of the analyte and its internal standard (IS) are performed in two completely separated scans with an interval of 1–2s, the fluctuation of the ion current between the two ion introductions would result in large errors in the analyte-to-IS ratios (A/IS) measured. Using the multi-MS/MS analysis in a single scan, the analyte and IS ions can be collected at the same time and MS/MS analyzed sequentially with a time interval as short as 0.1 s.
To validate this concept, 40 μL methanol solution containing amitriptyline and the IS amitriptyline-d6, each at a concentration of 120 ng/mL, was dropped on a triangle paper substrate for paper spray ionization. For each scan cycle, all the ions were trapped in the QLIT; the protonated amitriptyline m/z 278 was first transferred to RIT and after 0.1 s the protonated amitriptyline m/z 284 was also transferred to RIT. MS analysis was then performed by the RIT and the intensities of m/z 278 and 284 were measured to calculate the A/IS ratios for each scan cycle. As shown in Figure 4, the ion signal intensity could vary significantly with the paper spray, due to the evaporation of the solvent.4, 21 However, the signal intensities of the analyte and IS were well tracing each other, since the analyte and IS ions were always sampled at the same time. A good precision in the A/IS ratio (RSD < 15%) were thereby obtained.
Figure 4.
The signal intensities measure by RIT for sequentially mass-selectively transferred amitriptyline m/z 278 and amitriptyline-d6, m/z 284. Paper spray of methanol solution with amitriptyline and amitriptyline-d6 at 120 ng/mL, spray voltage of 4.3 kV. AC excitation of 80 mV 169 kHz, q = 0.45, for mass-selective transfer from QLIT. q = 0.2 for the trapping the ions in RIT.
Conclusions
In this study, dual LIT configurations have been explored to enable multiple MS/MS processes with a single ion introduction. This development has a direct impact on the analytical performance of the MS systems using DAPIs, especially for miniature instruments coupled with ambient or atmospheric pressure ionization sources. The scan speed and the quantitation precision could be significantly improved. Efficiency in sample consumption can also be highly increased, which should also be attractive for both miniature and lab scale MS systems performing analyses of complex mixtures. An ideal configuration for implementing this concept should have the first LIT with a large trapping capacity to overcome the space charge effect; and the second LIT can be of relatively small size but with high resolution and mass accuracy for MS/MS analysis. In this study, the balanced RF field for LIT-1 enforced with a double phase RF and the symmetric trap geometry has been shown to be effective for improving the ion transfer efficiency. Further optimization might be achieved by applying a balanced RF field for LIT-2, along with locking the phases of the RFs applied on both traps and the AC for axial resonance ejection.
Supplementary Material
Acknowledgments
This work was supported by This work was supported by National Science Foundation (0847205-CHE), National Institute of General Medical Sciences (1R01GM106016) from the National Institutes of Health, and National Aeronautics and Space Administration (PIDDP NNX12AB16G).
References
- 1.Sleno L, Volmer DA. Ion activation methods for tandem mass spectrometry. Journal of Mass Spectrometry. 2004;39:1091–1112. doi: 10.1002/jms.703. [DOI] [PubMed] [Google Scholar]
- 2.Ouyang Z, Cooks RG. Miniature mass spectrometers. Annual Review of Analytical Chemistry. 2009;2:187–214. doi: 10.1146/annurev-anchem-060908-155229. [DOI] [PubMed] [Google Scholar]
- 3.Hendricks PI, Dalgleish JK, Shelley JT, Kirleis MA, McNicholas MT, Li LF, Chen TC, Chen CH, Duncan JS, Boudreau F, Noll RJ, Denton JP, Roach TA, Ouyang Z, Cooks RG. Autonomous in situ analysis and real-time chemical detection using a backpack miniature mass spectrometer: Concept, instrumentation development, and performance. Analytical Chemistry. 2014;86:2900–2908. doi: 10.1021/ac403765x. [DOI] [PubMed] [Google Scholar]
- 4.Li L, Chen TC, Ren Y, Hendricks PI, Cooks RG, Ouyang Z. Mini 12, miniature mass spectrometer for clinical and other applications—introduction and characterization. Analytical Chemistry. 2014;86:2909–2916. doi: 10.1021/ac403766c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cooks RG, Ouyang Z, Takats Z, Wiseman JM. Ambient mass spectrometry. Science. 2006;311:1566–1570. doi: 10.1126/science.1119426. [DOI] [PubMed] [Google Scholar]
- 6.Monge ME, Harris GA, Dwivedi P, Fernández FM. Mass spectrometry: Recent advances in direct open air surface sampling/ionization. Chemical Reviews. 2013;113:2269–2308. doi: 10.1021/cr300309q. [DOI] [PubMed] [Google Scholar]
- 7.Cooks RG, Busch KL, Glish GL. Mass spectrometry: Analytical capabilities and potentials. Science. 1983;222:273–291. doi: 10.1126/science.6353576. [DOI] [PubMed] [Google Scholar]
- 8.Gao L, Cooks RG, Ouyang Z. Breaking the pumping speed barrier in mass spectrometry: Discontinuous atmospheric pressure interface. Analytical Chemistry. 2008;80:4026–4032. doi: 10.1021/ac800014v. [DOI] [PubMed] [Google Scholar]
- 9.Gao L, Li G, Nie Z, Duncan J, Ouyang Z, Cooks RG. Characterization of a discontinuous atmospheric pressure interface. Multiple ion introduction pulses for improved performance. International Journal of Mass Spectrometry. 2009;283:30–34. [Google Scholar]
- 10.Xu W, Charipar N, Kirleis MA, Xia Y, Ouyang Z. Study of discontinuous atmospheric pressure interfaces for mass spectrometry instrumentation development. Analytical Chemistry. 2010;82:6584–6592. [PubMed] [Google Scholar]
- 11.Douglas DJ, Frank AJ, Mao D. Linear ion traps in mass spectrometry. Mass Spectrometry Reviews. 2005;24:1–29. doi: 10.1002/mas.20004. [DOI] [PubMed] [Google Scholar]
- 12.March RE, Todd JFJ. Quadrupole ion trap mass spectrometry. 2. John Wiley & Sons, Inc; Hoboken, Jew Jersey: 2005. [Google Scholar]
- 13.Pekar Second T, Blethrow JD, Schwartz JC, Merrihew GE, MacCoss MJ, Swaney DL, Russell JD, Coon JJ, Zabrouskov V. Dual-pressure linear ion trap mass spectrometer improving the analysis of complex protein mixtures. Analytical Chemistry. 2009;81:7757–7765. doi: 10.1021/ac901278y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guna M, Londry FA. Tandem ion trap design with enhanced mass analysis capabilities for large populations of ions. Analytical Chemistry. 2011;83:6363–6367. doi: 10.1021/ac201285n. [DOI] [PubMed] [Google Scholar]
- 15.Hager JW. A new linear ion trap mass spectrometer. Rapid Communications in Mass Spectrometry. 2002;16:512–526. doi: 10.1002/rcm.1020. [DOI] [PubMed] [Google Scholar]
- 16.Schwartz JC, Senko MW, Syka JEP. A two-dimensional quadrupole ion trap mass spectrometer. Journal of the American Society for Mass Spectrometry. 2002;13:659–669. doi: 10.1016/S1044-0305(02)00384-7. [DOI] [PubMed] [Google Scholar]
- 17.Ouyang Z, Wu GX, Song YS, Li HY, Plass WR, Cooks RG. Rectilinear ion trap: Concepts, calculations, and analytical performance of a new mass analyzer. Analytical Chemistry. 2004;76:4595–4605. doi: 10.1021/ac049420n. [DOI] [PubMed] [Google Scholar]
- 18.Gao L, Song QY, Patterson GE, Cooks RG, Ouyang Z. Handheld rectilinear ion trap mass spectrometer. Analytical Chemistry. 2006;78:5994–6002. doi: 10.1021/ac061144k. [DOI] [PubMed] [Google Scholar]
- 19.Gao L, Sugiarto A, Harper JD, Cooks RG, Zheng OY. Design and characterization of a multisource hand-held tandem mass spectrometer. Analytical Chemistry. 2008;80:7198–7205. doi: 10.1021/ac801275x. [DOI] [PubMed] [Google Scholar]
- 20.Wilm M, Mann M. Analytical properties of the nanoelectrospray ion source. Analytical Chemistry. 1996;68:1–8. doi: 10.1021/ac9509519. [DOI] [PubMed] [Google Scholar]
- 21.Ren Y, Wang H, Liu J, Zhang Z, McLuckey M, Ouyang Z. Analysis of biological samples using paper spray mass spectrometry: An investigation of impacts by the substrates, solvents and elution methods. Chromatographia. 2013;76:1339–1346. doi: 10.1007/s10337-013-2458-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.