Summary
Myelin sheaths provide critical functional and trophic support for axons in white matter tracts of the brain. Oligodendrocyte precursor cells (OPCs) have extraordinary metabolic requirements during development as they differentiate to produce multiple myelin segments, implying they must first secure adequate access to blood supply. However, mechanisms that coordinate myelination and angiogenesis are unclear. Here, we show that oxygen tension, mediated by OPC-encoded hypoxia-inducible factor (HIF) function, is an essential regulator of postnatal myelination. Constitutive HIF1/2α stabilization resulted in OPC maturation arrest through autocrine activation of canonical Wnt7a/7b. Surprisingly, such OPCs also show paracrine activity that induces excessive postnatal white matter angiogenesis in vivo, and directly stimulates endothelial cell proliferation in vitro. Conversely, OPC-specific HIF1/2α loss-of-function leads to insufficient angiogenesis in corpus callosum and catastrophic axon loss. These findings indicate that OPC-intrinsic HIF signaling couples postnatal white matter angiogenesis, axon integrity and the onset of myelination in mammalian forebrain.
Keywords: oligodendrocyte, myelin, Olig2, angiogenesis, hypoxia-inducible factor, Wnt signaling, CNS development, axonopathy
Introduction
Oligodendrocytes (OLs) are the myelinating cells of the central nervous system (CNS). Myelination enables rapid transmission of action potentials through saltatory conduction (Bradl and Lassmann, 2010), and OLs provide trophic support and maintain axon integrity (Funfschilling et al., 2012; Harris and Attwell, 2012; Lee et al., 2012; Rinholm et al., 2011). Developing oligodendrocyte precursor cells (OPCs) undergo as much as a 6500-fold increase in membrane area to provide myelin segments to multiple axons (Baron and Hoekstra, 2010; Chong et al., 2012), a process which entails extraordinary metabolic demands (Chrast et al., 2011; Harris and Attwell, 2012; Nave, 2010). Thus, OLs and OPCs require access to a rich vascular supply for nutritive and oxidative substrates. However, OLs are not known to regulate angiogenesis, and molecular mechanisms that might couple the timing of myelination to adequate blood supply during postnatal brain development are unknown.
Hypoxia-inducible factors (HIFs) are transcriptional mediators of the cellular response to hypoxia (Majmundar et al., 2010; Semenza, 2012), comprising a heterodimeric complex of an oxygen-sensitive subunit (HIF1α or HIF2a) with a constitutive subunit (HIF1β or HIF2β) (Hirose et al., 1996; Wang et al., 1995). In normoxic conditions, prolyl hydroxylase (PHD1–3) and von Hippel Lindau (VHL) target HIF1/2α for proteosomal degradation (Ivan et al., 2001; Jaakkola et al., 2001). Conversely, during hypoxia, stabilized HIF1/2α proteins bind HIF1β and translocate to the nucleus to activate gene targets by binding cis-acting motifs called hypoxia response elements (HREs) (Mazumdar et al., 2010; Patel and Simon, 2008).
Previous studies show that Wnt7a/7b function in embryonic neural precursors is essential for embryonic CNS angiogenesis (Daneman et al., 2009; Stenman et al., 2008). During development, the Wnt pathway is required for maturation of CNS blood vessels and the blood brain barrier (Liebner et al., 2008; Wang et al., 2012; Ye et al., 2009b), a process that involves vascular investment by pericytes and astrocytic end-feet (Daneman et al., 2010; Janzer and Raff, 1987). Robust CNS angiogenesis persists until postnatal day (P) 10 in mice, which coincides with myelination onset in the corpus callosum (Harb et al., 2013). The most active period of myelination in the postnatal human brain occurs during the first year of life, which correlates with increasing levels of blood flow and O2 (Franceschini et al., 2007; Kinney et al., 1988; Miller et al., 2012). Conversely, postnatal hypoxia results in delayed myelination (Ment et al., 1998; Silbereis et al., 2010; Tan et al., 2005; Weiss et al., 2004), in part, through activation of Wnt signaling, an inhibitor of OL differentiation (Fancy et al., 2011a; Fancy et al., 2011b; Ye et al., 2009a).
To better define molecular pathways that could integrate myelination and vascular supply, we hypothesized that oxygen levels directly regulate the differentiation of OLs. Here we show that OPC HIF1/2α activity inhibits myelination by inducing autocrine Wnt7a/7b signaling, which also has a novel paracrine role to promote Wnt-dependent vessel growth into developing postnatal white matter tracts. While constitutive HIF activation in OPCs caused striking hypervascularization throughout the brain, loss of OPC-encoded HIF1/2α function resulted in catastrophic loss of corpus callosum axons commencing at P4, when robust angiogenesis is taking place. Our findings establish a HIF—and by extension, oxygen— -dependent mechanism that is critical to precisely time the onset of myelination to environmental conditions that can adequately support the associated metabolic demands. Moreover, we show an unexpected role for OPCs as critical regulators of angiogenesis in the postnatal brain.
Results
Oxygen levels and cell-intrinsic VHL function regulate OPC differentiation and myelination
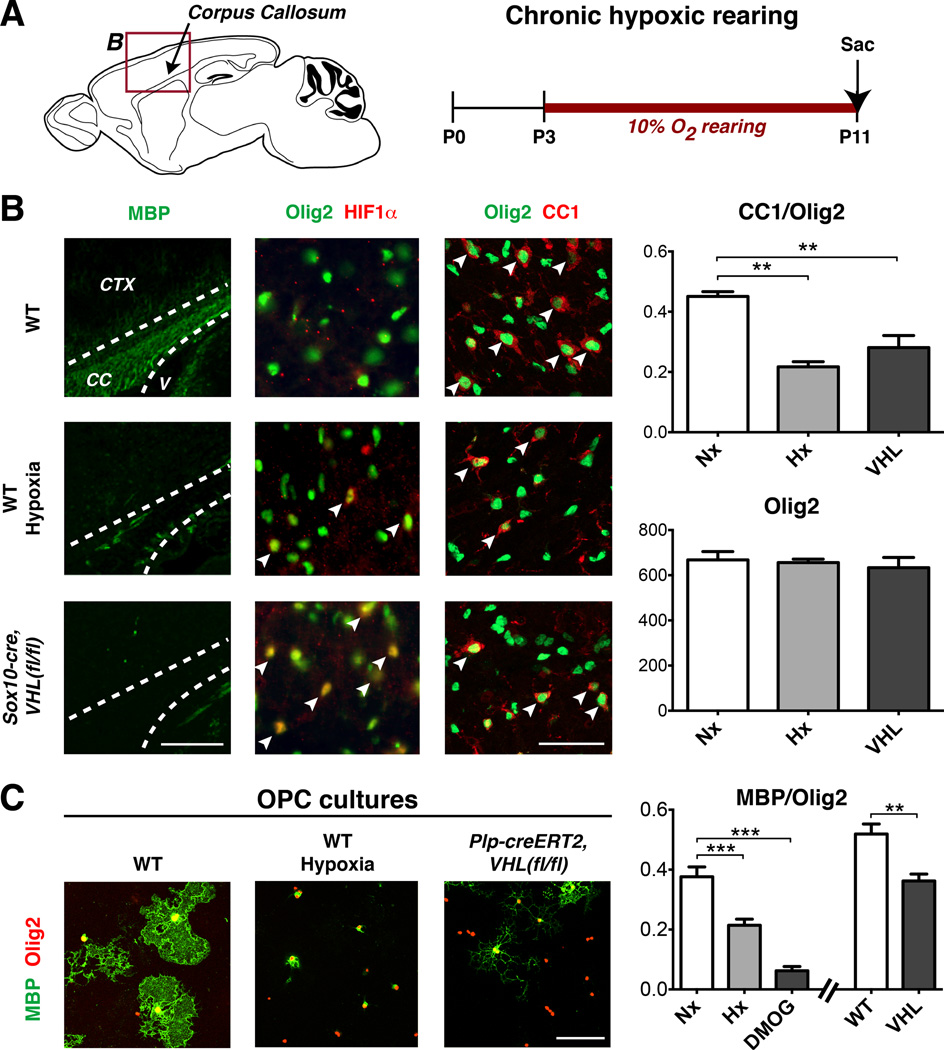
In mice, postnatal myelination in the corpus callosum and cerebellar white matter is initiated at about P7–9 and peaks at P15–21 (Tessitore and Brunjes, 1988). As shown (Figure 1A–B, Figure S1A–C), chronic exposure of neonatal mice to mild hypoxia (10% FiO2) from P3–11 resulted in hypomyelination and delayed OPC differentiation, without altering total OL lineage numbers (Olig2+). This was indicated by reduced expression of myelin basic protein (MBP) and cells expressing the mature lineage-specific marker CC1 (a.k.a., adenomatous polyposis coli, APC), consistent with previous findings (Weiss et al, 2004). Under such hypoxic conditions, we observed stabilized HIF1α proteins in white matter lysates and Olig2+ OPCs (Figure 1B, Figure S1D)
Figure 1. Oligodendrocyte-specific VHL deletion inhibits differentiation and myelination.
(A) Schematic of anatomical regions of corpus callosum (CC), cerebral cortex (CTX), and ventricle (V) presented in (B) and experimental timeline for chronic hypoxic rearing.
(B) Images showing hypomyelination, OL-lineage HIF1α expression, and OPC maturation arrest in CC of hypoxic WT mice or normoxic Sox10-cre, VHL(fl/fl) mice at P11. Arrowheads denote double-positive cells. Scale bar: 100µm (MBP), 50µm (Olig2).
(C) Immunopurified OPCs exposed to hypoxia or isolated from Plp-creERT2, VHL(fl/fl) mice show differentiation block. Scale bar: 100µm.
(For quantifications, mean+SEM; n≥3 experiments/genotype; **p<0.01, ***p<0.001; one-way ANOVA with Dunnett’s multiple comparison test)
See also Figure S1.
We next examined effects of cell-intrinsic HIF stabilization in OPCs. We targeted conditional knockout of a floxed VHL allele (Rankin et al, 2005) through intercrosses with Sox10-cre (Stolt et al., 2006), Olig1-cre (Lu et al., 2002) or tamoxifen-inducible PLP-creERT2 (Doerflinger et al., 2003) transgenic mice. As shown (Figure 1B), OPC-specific VHL conditional knockout by Sox10-cre resulted in HIF1α stabilization and severe OPC maturation arrest. We observed hypomyelination throughout the brain of Sox10-cre, VHL(fl/fl) mice (Figure 1B, Figure S1C), which displayed tremor, ataxia and failure to survive past weaning age (P21). It is possible that lethality resulted from VHL loss-of-function in the peripheral nervous system, which is also targeted by Sox10-cre (Stolt et al., 2006). However, Olig1-cre, VHL(fl/fl) mice showed a similar phenotype of hypomyelination and reduced viability past P10 (Figure S1E, data not shown). Together, these findings indicate that cell-intrinsic VHL function phenocopies the effects of hypoxia and is required for OPC maturation and myelination.
To further verify that effects of hypoxia on the OL lineage were direct, we purified OPCs by immunopanning from the neonatal brain for in vitro studies (Emery and Dugas, 2013). As shown (Figure 1C, Figure S1F–J), exposure to 2% oxygen or treatment with the HIF-stabilizing agent dimethyloxaloylglycine (DMOG) inhibited OPC maturation and myelin gene expression (MAG, MBP, CNPase). We found similar results in OPCs isolated from Plp-creERT2, VHL(fl/fl) mice following treatment with tamoxifen (Figure 1C). These findings show direct effects of oxygen levels on OPCs, and indicate that cell-autonomous HIF signaling causes maturation arrest.
Hypoxic effects on OPCs are mediated by HIF1/2α function
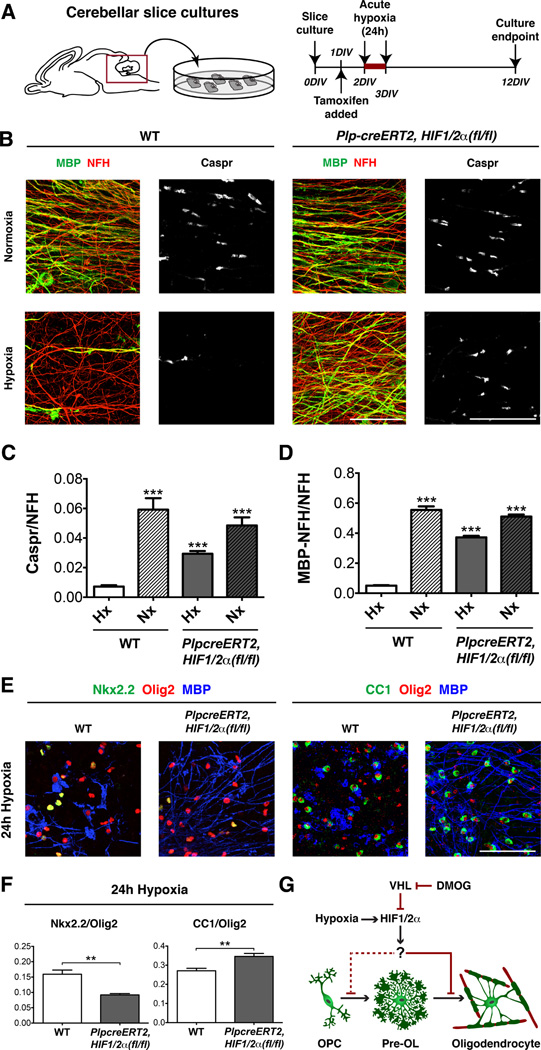
We next determined whether HIF1/2α function is required for hypoxia-induced hypomyelination. We crossed conditional HIF1α(fl/fl) and HIF2α(fl/fl) mutants to compound homozygosity (hereafter called HIF1/2α(fl/fl)) with PLP-creERT2 (Doerflinger et al., 2003). Given dramatic hypomyelination observed in the cerebellar white matter of Sox10-cre, VHL(fl/fl) mice (Figure S1C), we utilized a cerebellar explant culture assay suitable to quantify changes in postnatal myelination and compact myelin paranode formation (Fancy et al., 2011b; Yuen et al., 2013). We generated cerebellar explants from P0-P1 transgenic mice and added tamoxifen (which did not affect survival) to induce acute Cre-mediated excision of HIF1/2α in OLs (Figure 2A, Figure S2A). Although the Plp-creERT2 allele has been shown to have variable activity in vivo (Doerflinger et al., 2003), we observed about 85% of Olig2+ cells expressed a conditional (floxed) GFP reporter (Figure S2B). Subsequently, cultures were exposed to hypoxia (2% FiO2) for 24h and maintained for 12 days prior to analysis. As shown (Figure 2B–D, Figure S2C), hypoxia, and subsequent HIF activation, inhibited myelination and OPC differentiation in wild type cerebella, as shown by MBP staining as well as the ratio of Caspr paranode staining to NFH+ axons, and the increased ratio of Nkx2.2 (immature OPCs)/Olig2 (total OLs) double-positive cells (Fancy et al., 2011b). However, as shown (Figure 2B–F, Figure S2C–E), the degree of hypomyelination and OPC differentiation block was significantly reduced by deletion of HIF1/2α. Thus, hypoxia-induced hypomyelination and maturation arrest requires intact HIF function within OPCs (Figure 2G). Our findings do not rule out a role of other pathways (e.g., apoptosis inducing factor and AMP-activated protein kinase (Hardie et al., 2012; Joza et al., 2009)) in hypoxic regulation of OPCs.
Figure 2. OPC-encoded HIF1/2α function mediates hypoxia-induced hypomyelination.
(A) Schematic and timeline for cerebellar slice cultures (CSC) exposed to hypoxia.
(B) Removing HIF1/2α function in OLs significantly reduces hypoxia-induced hypomyelination in CSC. Scale bars: 25µm (Caspr), 50µm (MBP/NFH). n≥6 experiments/condition.
(C) Quantification of myelination in CSC.
(D) Additional quantification of myelination in CSC.
(E) Removing OPC HIF1/2α function significantly reduces hypoxia differentiation block in CSC. Scale bar: 100µm.
(F) Quantification of OL differentiation showing Nkx2.2/Olig2 (OPCs) numbers decreased and CC1/Olig2 (mature OLs) numbers increased. Data were analyzed by t-test and significant differences (**p<0.01) are shown.
(G) Model for HIF-induced OPC differentiation block.
(For quantifications in C and D, mean+SEM; **p<0.01, ***p<0.001; one-way ANOVA with Dunnett’s multiple comparison test)
See also Figure S2.
HIF stabilization in OPCs activates canonical Wnt signaling
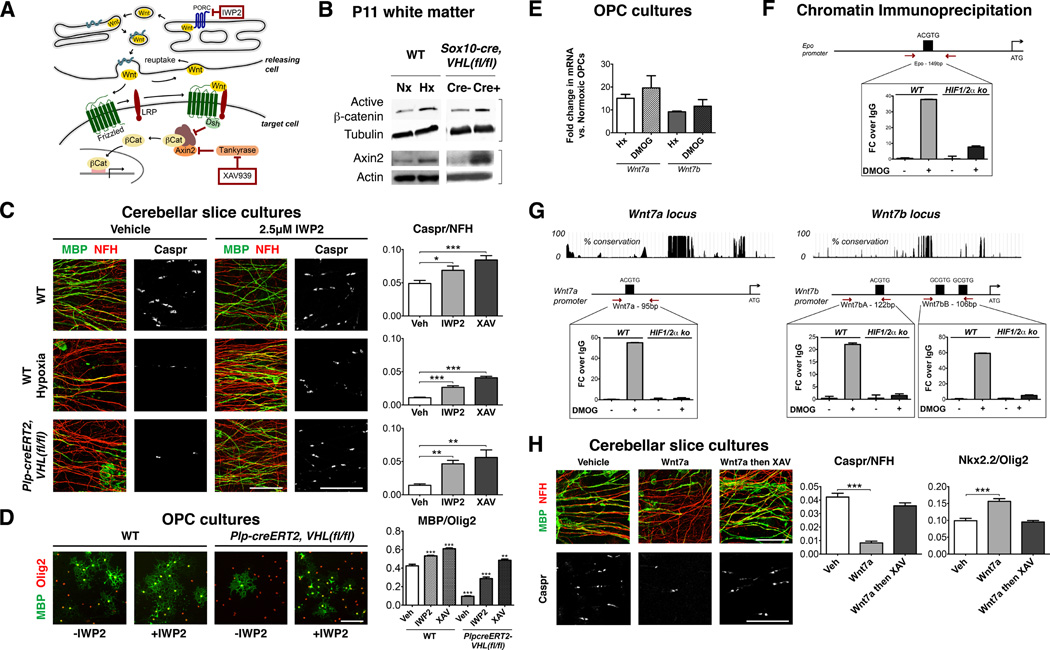
In OPCs, canonical Wnt signaling functions as a potent inhibitor of maturation (Fancy et al., 2009). We have further reported that hypoxia-induced hypomyelination in vitro can be normalized by treatment with XAV939 (Fancy et al., 2011b; Huang et al., 2009) (Figure 3A).
Figure 3. HIF stabilization in OPCs activates canonical Wnt signaling.
(A) Scheme showing Wnt signaling and inhibition of ligand secretion and canonical activity by porcupine inhibitor IWP2 and XAV939, which stabilizes Axin2 to promote β-catenin degradation.
(B) Western blots of P11 white matter demonstrating upregulation of activated β-catenin and Axin2 levels in WT mice reared in hypoxia and normoxic Sox10-cre, VHL(fl/fl) mice (n=3 animals/genotype).
(C) IWP2 prevents hypomyelination in CSC exposed to hypoxia or from Plp-creERT2, VHL(fl/fl) mice. Scale bars: 25µm (Caspr), 50µm (MBP/NFH).
(D) OPC maturation arrest in Plp-creERT2, VHL(fl/fl) OPCs is attenuated by IWP2 or XAV939. Scale bar: 100µm.
(E) Immunopurified OPCs cultured in hypoxic conditions or exposed to DMOG specifically upregulate Wnt7a and Wnt7b as shown by qRT-PCR (n=3).
(F) Positive control for ChIP analysis at Epo locus.
(G) Mouse HIF1/2α knockout and control embryonic fibroblasts cultured with/without DMOG (16h) assayed by ChIP. Following immunoprecipitation with antibodies against HIF1α or control (mouse IgG), DNA extracts were assessed by qRT-PCR. HIF1α bound to the Wnt7a locus at one HRE, and Wnt7b locus via two HREs. Binding was not observed in DMOG-treated HIF1/2α mutant cells, or non-DMOG-treated controls.
(H) Wnt7a proteins cause hypomyelination and OPC maturation arrest in CSC, which is reversed with XAV939. Scale bars: 25µm (Caspr), 50µm (MBP/NFH).
(For quantification in C, D, and H mean+SEM; n≥3 experiments; *p<0.05, **p<0.01, ***p<0.001; one-way ANOVA with Dunnett’s multiple comparison test)
See also Figure S3.
To determine if HIF signaling activates the Wnt pathway in white matter in vivo, we performed Western blot analysis of P11 corpus callosum lysates from wild type mice exposed to chronic hypoxia and normoxic Sox10-cre, VHL(fl/fl) conditional knockouts. As shown (Figure 3B, Figure S3A), we observed upregulation of the activated form of β-catenin, and the Wnt transcriptional targets Axin2, Notum and Naked1 compared to controls. Similar findings were obtained in DMOG-treated immunopurified OPC cultures (Figure S3B), indicating HIF stabilization activates canonical Wnt signaling in OPCs. As we used purified OPC cultures, these results also suggested that Wnt ligands produced by OPCs act in an autocrine fashion. To test this, we used IWP2, which inhibits Wnt ligand secretion by blocking porcupine function (Figure 3A, (Chen et al., 2009)). As shown (Figure 3C–D, Figure S3C), IWP2 was sufficient to reduce both hypomyelination and maturation arrest after hypoxia or OPC VHL loss-of-function. As a control for canonical Wnt pathway activity inhibition, we confirmed these effects with XAV939 (Figure 3A, C–D, Figure S3C–F). Thus, OPC HIF activation promotes the secretion of canonical Wnt ligand(s) that act in an autocrine manner to inhibit differentiation/myelination.
Evidence that Wnt7a and Wnt7b are direct HIF-inducible targets
Analysis of a database of genes expressed during OL development (Cahoy et al., 2008) revealed that Wnt4, Wnt7a and Wnt7b are expressed at high levels in OPCs and become down-regulated in mature OLs (Figure S3G). To further identify HIF targets, we performed qRT-PCR with primers for each of the 19 mammalian Wnt genes (Table S1) against mRNA from hypoxic or DMOG-treated OPCs. As shown (Figure 3E, Table S1), we observed upregulation of Wnt7a and Wnt7b but not other Wnt genes.
To determine if Wnt7a and/or Wnt7b are direct targets of HIF, we tested HRE (A/GTCTG) motifs proximal to the core promoters of these loci (see Extended Experimental Procedures) with chromatin immunoprecipitation (ChIP) in the presence/absence of DMOG to regulate HIF protein stability. Known HREs for Epo served as a positive control (Figure 3F), and HRE-negative upstream regions served as negative controls (Figure S3H). As shown (Figure 3G), anti-HIF1α antibody-mediated precipitation resulted in significant signal from putative Wnt7a or Wnt7b HREs and the Epo HRE in WT MEFs treated with DMOG, but not HIF1/2α double KO MEFs. These findings indicate HIF1α proteins bind to Wnt7a and Wnt7b HREs. Finally, we found that Wnt7a exposure resulted in OPC maturation arrest (increase in ratio of immature Nkx2.2/Olig2 cells) and hypomyelination that was rescued by XAV939 (Figure 3H; Figure S3I–J), consistent with roles as a HIF target downstream effector of canonical Wnt signaling in OPCs.
OPC HIF signaling promotes CNS angiogenesis and endothelial cell proliferation
Canonical Wnt signaling is essential for the formation of CNS vasculature in the embryo (Daneman et al., 2009; Stenman et al., 2008), and CNS angiogenesis persists postnatally through the sprouting and elongation of embryonically derived vessels during the first 10 days of murine life (Harb et al., 2013). Although angiogenic roles for OPCs are unprecedented, findings above raised the possibility that Wnt7a/7b also had paracrine roles in white matter.
We first investigated whether OPC HIF stabilization affected postnatal vascular development in vivo. As shown (Figure 4A–D, Figure S4A), vascular density of Sox10-cre, VHL(fl/fl) mice was significantly increased, as indicated by endothelial markers CD31, isolectin and Glut1. Similar findings were observed in Olig1-cre, VHL(fl/fl) mice (Figure S4B). Fate mapping for Sox10-cre and Olig1-cre drivers failed to show any contributions to endothelial or smooth muscle vascular cells (Table S3, Figure S4C–D). We did observe that 9% of white matter pericytes were fate mapped by Sox10-cre and Olig1-cre (Table S3 Figure S4C), so we cannot rule out a small contribution of these cells. Despite increased vessel density, we found normal investment by pericytes and astrocyte end-feet, and no evidence for blood-brain barrier leakage as assessed by lectin perfusion, fibrinogen staining outside of the vasculature, and absence of hemorrhage (Figure 4D, Figure S4E and data not shown).
Figure 4. HIF stabilization in OPCs promotes angiogenesis in vivo.
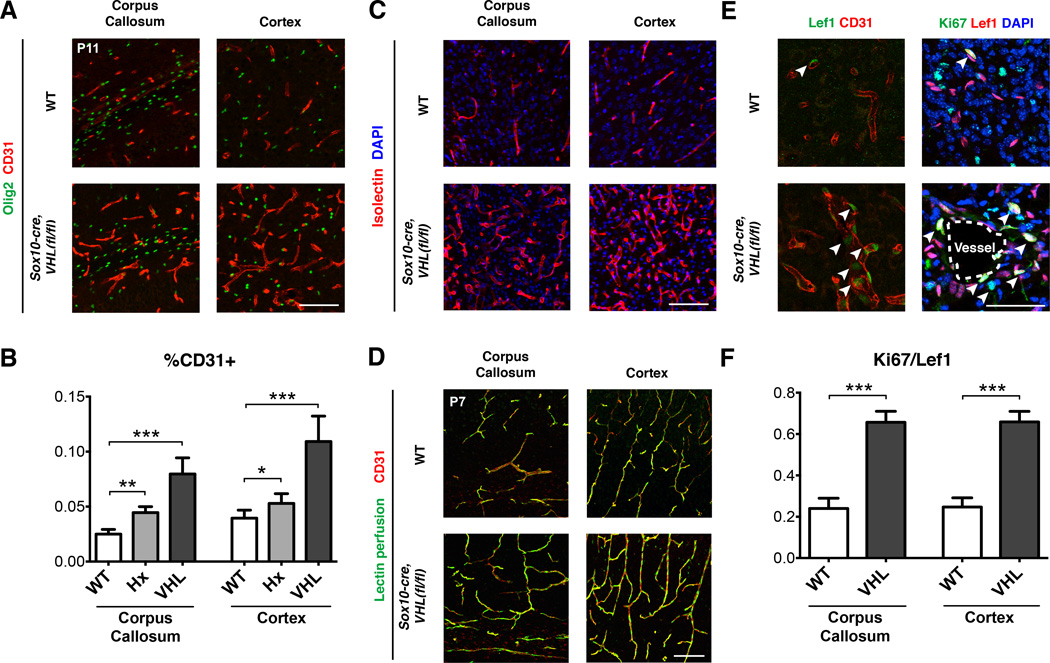
(A) Increased angiogenesis in Sox10-cre, VHL(fl/fl) mice as shown by expression of endothelial marker, CD31. Dense regions of Olig2 staining indicate white matter tracts in the corpus callosum. Scale bar: 100µm. n≥3 animals/genotype.
(B) Quantification of endothelial/vessel area (CD31+) demonstrates significant increases in hypoxic WT and normoxic Sox10-cre, VHL(fl/fl) mice. Data were analyzed by one-way ANOVA with Dunnett’s multiple comparison test, and significant differences (*p<0.05, **p<0.05, ***p<0.001) are shown.
(C) Endothelial marker Isolectin demonstrating increased angiogenesis in Sox10-cre, VHL(fl/fl) mice. Scale bar: 100µm.
(D) Isolectin perfusion in WT and Sox10-cre, VHL(fl/fl) mice indicating perfusion of blood vessels. Scale bar: 100µm.
(E) Increased Lef1 expression in endothelia of Sox10-cre, VHL(fl/fl) mice. Of these, the majority co-labeled with the proliferation marker, Ki67. Scale bar: 50µm. n≥3 animals/genotype.
(F) Quantification of Ki67+/Lef1+ endothelial cells in corpus callosum and cortex. Data were analyzed by t-test and significant differences (***p<0.001) are shown.
See also Figure S4, Table S1 and S3.
We next analyzed activation of endothelial Wnt signaling by expression of the transcriptional target, Lef1 (Eastman and Grosschedl, 1999; Huber et al., 1996; Porfiri et al., 1997). As shown (Figure 4E), we found robust induction of Lef1 in CD31+ endothelial cells of Sox10-cre, VHL(fl/fl) mutants; moreover, the majority of these co-stained with the proliferation marker Ki67 (Figure 4E–F), suggesting that Wnt signaling promoted vessel growth. Although members of the vascular endothelial growth factor (VEGF) family are HIF target genes expressed within the OL lineage during development (Figure S4F) (Cahoy et al., 2008), we found no evidence for increased VEGF-A expression in OPCs of Sox10-cre, VHL(fl/fl) mice in vivo (Figure S4G). Thus, HIF stabilization in normoxic OPCs promotes angiogenesis, Wnt signaling and endothelial proliferation in vivo.
OPCs directly promote angiogenesis in a Wnt-dependent manner
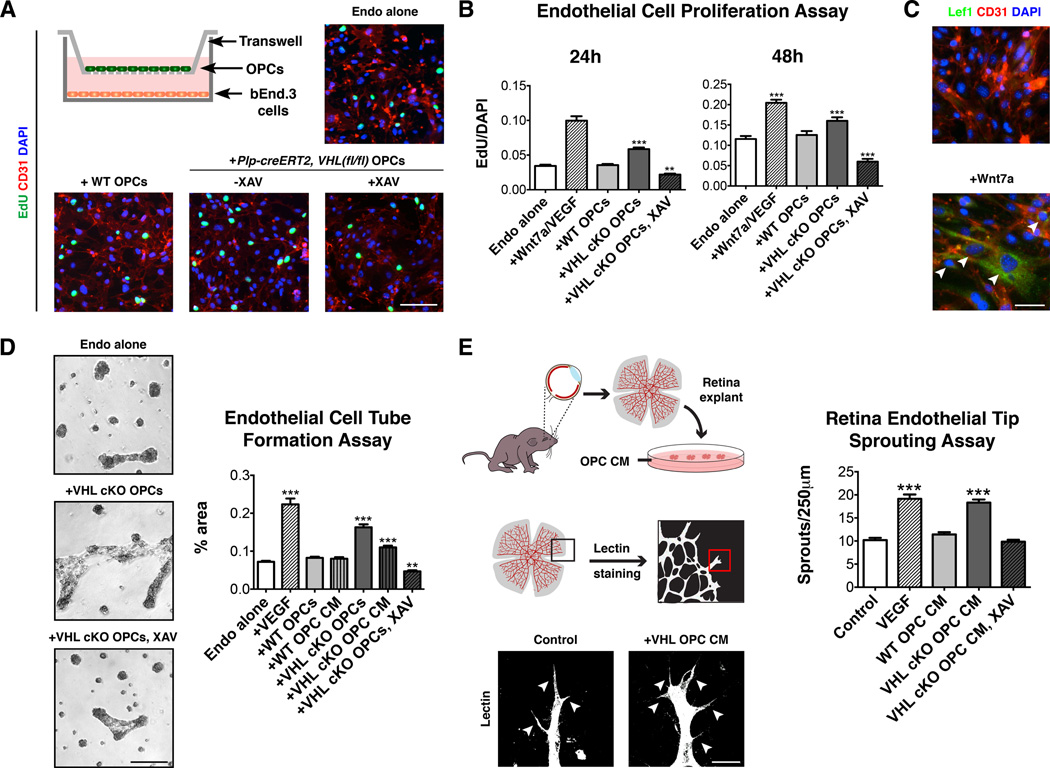
The results above do not address whether angiogenic effects of OPCs were direct and/or contact-mediated. As shown (Figure 5A–C, Figure S5A), addition of Wnt7a proteins to cultures of brain endothelial cell line bEnd.3 (ATCC) induced Lef1 expression and proliferation. To demonstrate direct effects of OPCs, we performed a transwell assay with bEnd.3 cells (Figure 5A), which allows exchange of diffusible factors but prevents cell-cell contact. Addition of soluble Wnt7a and/or VEGF proteins resulted in proliferation of bEnd.3 cells (Figure 5A–C, Figure S5A). We also observed these effects with tamoxifen-induced PLP-creERT2, VHL(fl/fl) OPCs (Figure 5A–C), which was inhibited by XAV939 (Figure 5A–C, Figure S5A–B). In contrast, VEGF inhibitor SU5416 did not inhibit OPC-induced endothelial proliferation (Figure S5C).
Figure 5. OPCs directly promote angiogenesis in a Wnt-dependent manner.
(A) Scheme showing transwell co-culture assay for OPCs and bEND.3 cells. OPCs from Plp-creERT2, VHL(fl/fl) mice induce endothelial cell proliferation in a Wnt-dependent manner. Scale bar: 100µm.
(B) Quantification of endothelial cell proliferation in transwell assay at 24h and 48h.
(C) Wnt7a treatment of bEND.3 cells induces Lef1 expression (arrowheads). Scale bar: 30µm.
(D) Transwell co-cultures of Plp-creERT2, VHL(fl/fl) OPCs and bEND.3 cells promotes endothelial cell tube formation in a Wnt-dependent manner. Scale bar: 500µm.
(E) Schematic showing retina endothelial tip sprouting assay. Conditioned medium from Plp-creERT2, VHL(fl/fl) OPCs promoted endothelial tip sprouting and filopodia extension in a Wnt-dependent manner. Scale bar: 25µm.
(For all quantifications mean+SEM; n≥3 experiments (A,B), n≥2 (D,E); **p<0.01, ***p<0.001; one-way ANOVA with Dunnett’s multiple comparison test)
See also Figure S5.
We next assessed endothelial tube formation of bEnd.3 cells in matrigel. As shown (Figure 5D), tamoxifen-induced PLP-creERT2, VHL(fl/fl) OPCs (or treatment with conditioned medium) promoted endothelial tube formation in a Wnt-dependent manner. Finally, we investigated direct effects of OPCs to promote endothelial tip sprouting of blood vessels in explants of neonatal mouse retina (Sawamiphak et al., 2010). We found that conditioned medium from VHL-deficient OPCs promoted retinal endothelial tip sprouting, and that such effects were inhibited by the addition of XAV939 (Figure 5E). Taken together, these results indicate OPCs directly induce angiogenesis in a non-contacted dependent, Wnt-mediated manner.
Oligodendrocyte HIF1/2 α function is essential for angiogenesis and integrity of corpus callosum
To explore OPC HIF functions in vivo, we intercrossed HIF1/2α(fl/fl) mice with Sox10-cre and Olig1-cre lines. Sox10-cre, HIF1/2α(fl/fl) only survived until P4–7. In contrast, Olig1-cre, HIF1/2α(fl/fl) mice were viable into adulthood as late as P90 (n=5), but exhibited foot clasping behavior (Figure S6A). The reasons for early lethality of Sox10-cre, HIF1/2α(fl/fl) mice compared to Olig1-cre, HIF1/2α(fl/fl) mice are unclear, but likely reflect differential targeting patterns to precursor cells of the CNS and PNS.
As shown (Figure 6A), histological analysis demonstrated dramatic loss of forebrain white matter tracts and presence of cysts in the corpus callosum at P4-P7. To resolve distinct and/or overlapping contributions of HIF1α versus HIF2α in this white matter phenotype, we analyzed single, compound and double mutant animals. As shown (Figure 6A,C, Table S2), in P4 double knockout Sox10-cre, HIF1/2α(fl/fl) and Olig1-cre, HIF1/2α(fl/fl) mice, we observed macroscopic and microscopic acellular cysts in the corpus callosum typically located at the boundary with adjacent grey matter structures (neocortex, striatum). In contrast, single HIF1 α or HIF2α mutants showed minimal effects, and compound mutants that were HIF1α(fl/fl);HIF2α(fl/+) or HIF1α(fl/+);HIF2α(fl/fl) showed only corpus callosum microcysts. Thus, OPC intrinsic HIF1α and HIF2α show partially overlapping yet essential functions in white matter development.
Figure 6. Oligodendrocyte HIF1/2α function is required for postnatal angiogenesis and maintenance of white matter integrity.
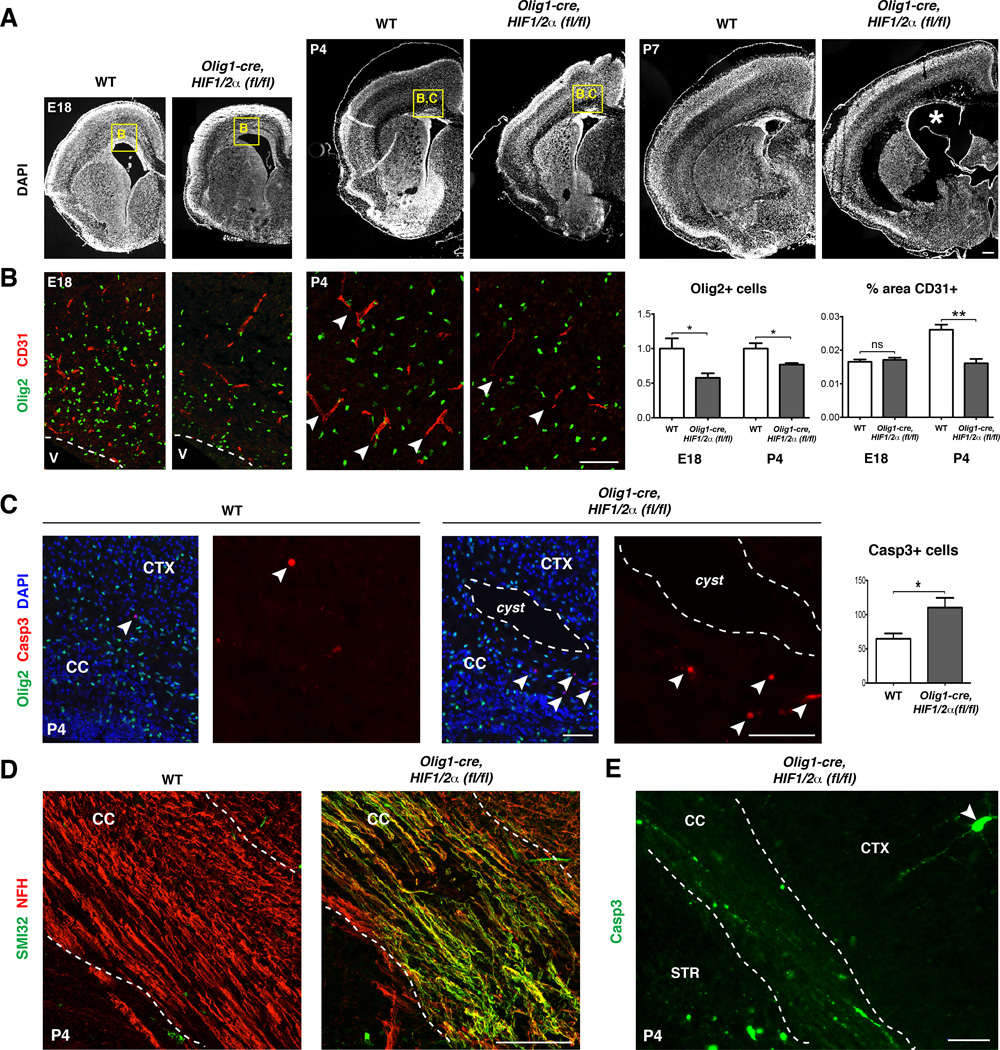
(A) DAPI stained sections at E18, P4, and P7 of mutant and control brains show white matter cysts (white asterisk) and dysgenesis by P7 in Olig1-cre, HIF1/2α(fl/fl) mice. n≥3 animals/genotype. Scale bar: 100µm.
(B) Olig2+ cells are reduced by ~40% compared to WT at E18 and ~15% at P4. Vessel density in SVZ at E18 is similar in WT and mutant mice, whereas Olig1-cre, HIF1/2α(fl/fl) mice show significantly decreased vessel density at P4 in corpus callosum. Data were analyzed by t-test and significant differences (*p<0.05, **p<0.01) are shown. Scale bar: 100µm.
(C) White matter cysts and increased apoptotic cells (Casp3+) in P4 in Olig1-cre, HIF1/2α(fl/fl) mice. Data were analyzed by t-test and the significant difference (*p<0.05) is shown. Scale bar: 100µm (merged), 50µm (Casp3)
(D) Widespread axonal damage, indicated by SMI32+ staining, observed at P4 throughout the corpus callosum of Olig1-cre, HIF1/2α(fl/fl) mice. Scale bar: 100µm.
(E) Robust Casp3 staining in axons of P4 Olig1-cre, HIF1/2α(fl/fl) corpus callosum. Note relative paucity of staining in cortex. Scale bar: 100µm.
See also Figure S6, Table S2 and S3.
In order to determine the basis for white matter loss, we assessed the ontogeny of OLs and the brain vasculature at E18, P4, and P7. While the brain of E18 Olig1-cre, HIF1/2α(fl/fl) mice had a grossly normal appearance and density of CD31+ endothelia (Figure 6A–B), dysgenesis of the forebrain white matter suggested abnormalities in OPC-induced angiogenesis with onset between P0-P7. Indeed, we observed that the density of CD31+ endothelia in corpus callosum showed a significant decrease at P4 in Olig1-cre, HIF1/2α(fl/fl) mice (Figure 6B). The P4 timepoint also showed high levels of cleaved Caspase3 (Casp3)+ apoptotic cells in the mutant white matter (Figure 6C), including activated CD68+ microglia/macrophages, Olig2+ and PDGFRα+ OPCs and GFAP+ astrocytes (Figure S6B). Despite this, numbers of Olig2+ cells in the white matter of Olig1-cre, HIF1/2α(fl/fl) mice were only about 15% diminished at P4 compared to WT, whereas we observed a 40% reduction at E18 (Figure 6B). Strikingly, the P4 mutant corpus callosum showed evidence of severe axonal damage as assessed by SMI32 and Casp3 expression (Figure 6D–E). Together, these findings indicate a sequence of deficient angiogenesis at P4, which leads to a general deterioration of white matter, resulting in acellular cysts by P7 (Figure 6A). Thus, combined HIF1/2α function in OPCs is necessary to promote postnatal white matter angiogenesis and maintain structural integrity of the corpus callosum.
HIF loss-of-function in OPCs is permissive for cortical vessel and projection neuron development
We next investigated the impact of OPC HIF deletion on cortical plate development. The mammalian neocortex is divided into six layers (Dugas-Ford et al., 2012). As shown in Figure 7A, although the cortical plate is thinner in Olig1-cre, HIF1/2α(fl/fl) mice at P7, its component layers are intact. In contrast to findings in white matter (Figure 6B), vessel density in the P4 cortex of Olig1-cre, HIF1/2α(fl/fl) mice was not significantly different than controls (Figure 7B). In addition, layer 2/3 neurons, marked by expression of SATB2 (Alcamo et al., 2008), as well as layer 5/6 neuron populations appeared to be preserved (Figure 7C).
Figure 7. Loss of OPC HIF1/2 α function is permissive for cortical development and angiogenesis.
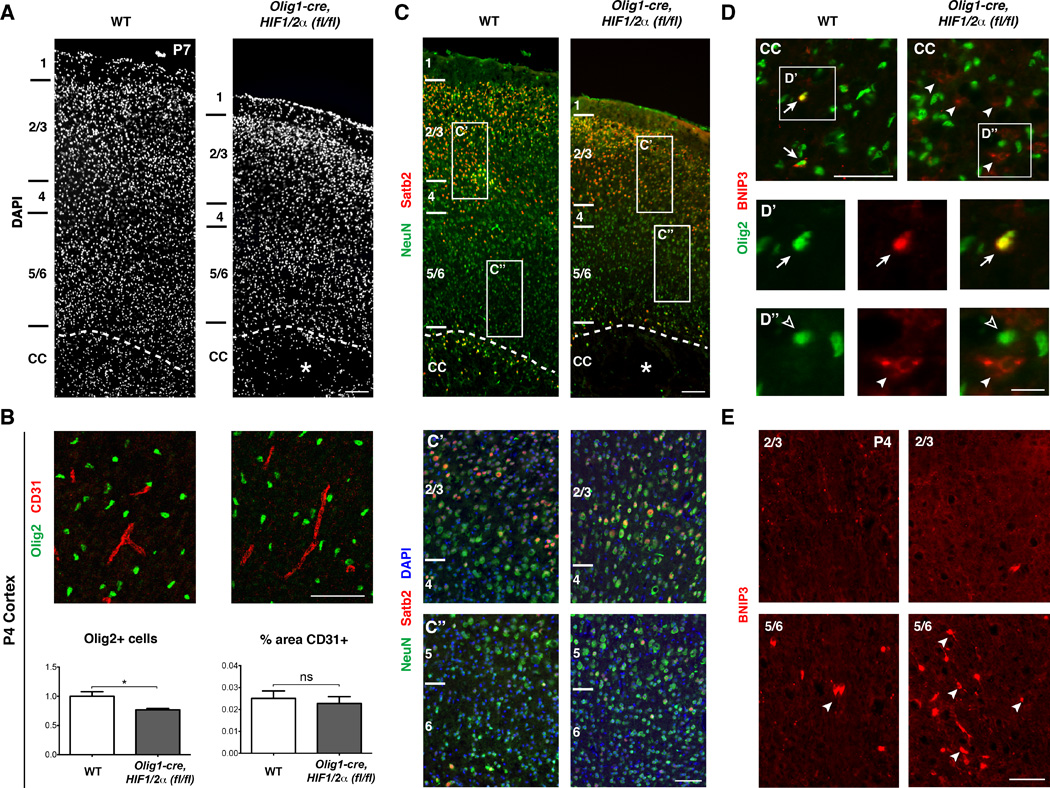
(A) DAPI stain of primary motor cortex in WT versus Olig1-cre, HIF1/2α(fl/fl) mice at P7 showing a thinner cortex in Olig1-cre, HIF1/2α(fl/fl) with the cortical layers and overall structure intact. Cortical layers are labeled to the left, and the asterisk denotes white matter cyst. n=3 animals/genotype. Scale bar: 200µm.
(B) OL numbers are reduced by approximately 23% in Olig1-cre, HIF1/2α(fl/fl) cortex, but vessel density (%CD31) is not statistically different. Data were analyzed by a two-tailed Student’s t-test and the significant difference (*p<0.05) is shown. n=3 animals/genotype. Scale bar: 100µm.
(C) Images of NeuN (green, pan-neuron marker), SatB2 (red, layer 2/3 callosal projection neurons), and DAPI providing further evidence that the cortex is grossly intact with ample numbers of callosal projection neurons. Note in higher magnification panels (C’ and C”) that cell density is grossly normal in Olig1-cre, HIF1/2α(fl/fl) cortex. Scale bars: 200µm; 100µm (insets).
(D) Images of the corpus callosum stained for BNIP3 (red) and Olig2 (green). In WT, BNIP3 is expressed in a subset of Olig2+ cells (arrows, D’ insets). In Olig1-cre, HIF1/2α(fl/fl) mice, while BNIP3 is not expressed in Olig2+ cells, aberrant expression of BNIP3 in non-Olig2+ cells (arrowheads, D” insets) is indicative of the general hypoxic microenvironment. Scale bars: 100µm; 20µm (insets).
(E) Images of BNIP3 staining in dorsal cortex (top row) and ventral cortex (bottom row). BNIP3 is enriched in ventral, but not dorsal cortex, suggesting selective hypoxia in grey matter regions adjacent to the corpus callosum, but not more dorsal areas. n=3 animals/genotype. Scale bar: 100µm.
We next used the HIF target BNIP3 (Bruick, 2000; Lee and Paik, 2006) as a physiologic readout of HIF pathway activity in WT and Olig1-cre, HIF1/2α(fl/fl) mutant animals. As shown (Figure 7D), BNIP3 is normally expressed at low levels in a subset of Olig2+ cells in the white matter of WT P4 mice. This indicates that a state of “physiological” hypoxia/HIF activation in normal white matter development, whereas cells in the cortex are mostly BNIP3-negative (Figure 7E). In contrast, we observed elevated numbers of BNIP3+ cells in the white matter and layers 5/6 of Olig1-cre, HIF1/2a(fl/fl) mice, indicating an abnormal state of hypoxia in these structures (Figure 7D–E); layer 2/3 neurons did not show increased BNIP3. As expected, we did not observe Olig2+/BNIP3+ cells in Olig1-cre, HIF1/2α(fl/fl) mice (Figure 7D). In summary, these findings suggest that deterioration of forebrain white matter tracts in Olig1-cre, HIF1/2α(fl/fl) is a primary—rather than a consequent—effect of deficient OPC-encoded HIF signaling on cortical plate projection neurons.
Discussion
Developing appropriate white matter blood flow is essential given the high metabolic demands of myelinating OLs and the axons they invest. Though classic papers have noted the anatomical relationship of OLs to blood vessels (Cammermeyer, 1960; Del Rio-Hortega, 2012), our results demonstrate OLs are critical regulators of postnatal CNS angiogenesis. We find that OPC-encoded HIF signaling coordinates the onset of postnatal myelination with establishment of adequate vasculature in the white matter through autocrine and paracrine Wnt activities, respectively (Figure S7).
Oxygen tension is a developmental regulator of postnatal myelination
Activity-dependent neuronal signals are thought to induce myelination (Demerens et al., 1996; Ishibashi et al., 2006; Stevens et al., 2002), and such coordination is important, in part, because myelin constrains axon outgrowth and synaptogenesis (Chong et al., 2012; Hu and Strittmatter, 2004). Our results suggest another level of regulation to ensure the presence of adequate blood supply and oxygen levels as a prerequisite for myelination to commence under appropriate physiological conditions. We propose an integrated HIF-regulated developmental mechanism (Figure S7) wherein OPCs that initially invest hypovascularized white matter are exposed to hypoxia, activate HIF signaling and produce Wnt ligands, which in turn trigger angiogenesis. With increased oxygen delivery, HIF signaling becomes downregulated, thus allowing for OPC maturation and myelination to take place. This dual mechanism helps ensure myelination will only proceed when blood supply is sufficient to meet attendant metabolic demands. In culture we could uncouple this relationship (by providing substrates and oxygen) and confirm HIF loss-of-function rescued hypoxia-induced hypomyelination. Thus, HIF function is necessary and sufficient for effects of oxygen levels on OPC maturation. Future studies are needed to determine whether HIF signaling also regulates OL development antenatally in the hypoxic intrauterine environment. We did observe that OPC numbers were deficient in Olig1-cre, HIF1/2α(fl/f) animals at E18 consistent with this possibility.
Autocrine Wnt signaling functions downstream of hypoxia/HIF in OPCs
Canonical Wnt signaling inhibits OPC maturation during development and in disease (Fancy et al., 2009; Fancy et al., 2011b; Ye et al., 2009a), and our studies show that HIF stabilization activates cell-autonomous Wnt production. HIF1α directly binds conserved HREs at the Wnt7a and Wnt7b loci, and stabilization of HIF in OPCs resulted in upregulation of Wnt7a/7b. Further studies are required to establish Wnt7a/7b as the specific downstream effectors of HIF signaling in OPCs, as other candidates (e.g. Wnt4, Wnt5a) are expressed in the OL lineage (Cahoy et al., 2008; Fancy et al., 2009). Although HIF stabilization in OPCs prevented postnatal maturation, we did not observe precocious myelination (e.g., with prenatal onset) in Olig1-cre, HIF1/2α(fl/fl) animals. This might suggest that downregulation of HIF signaling must also integrate with positive cues (e.g., axonal activity-dependent signals) for myelination to commence. Alternatively, it is possible that loss of HIF function results in the rapid death and removal of precociously maturing OLs.
OPCs regulate white matter angiogenesis through paracrine Wnt signaling
Postnatal forebrain angiogenesis is characterized by sprouting/ingrowth of blood vessels towards white matter regions from P0-P14 in mice (Harb et al., 2013; Sapieha, 2012). Conditional OPC knockout of HIF1/2α resulted in deficient angiogenesis at P4 and ensuing deterioration of large white matter tracts, such as corpus callosum by P7. Conversely, OPC HIF stabilization resulted in increased expression of the pro-angiogenic genes Wnt7a/7b and overproduction of blood vessels characterized by robust populations of Lef1+ endothelia. Although VEGF is expressed by neurons, astrocytes and microglia in response to hypoxia (Rosenstein et al., 2010), we did not observe VEGF induction by OPC-specific HIF stabilization. Our findings suggest dual functions for HIF-mediated Wnt signaling that couple OPC maturation and white matter vascular development. Although Wnt7a/7b are required for angiogenesis, their functions are dispensable after mature blood vessel structure is achieved (Daneman et al., 2009; Stenman et al., 2008); moreover, depletion of OLs in the adult brain (e.g. cuprizone-induced demyelination model) does not result in vascular abnormalities. These findings indicate roles of OPC and Wnt signaling in angiogenesis but not maintenance of mature vascular structure.
Oligodendrocyte HIF signaling is essential for developing white matter integrity
Mature OLs provide metabolic support for axons by supplying ATP, glycolytic substrates and nutrients (Funfschilling et al., 2012; Harris and Attwell, 2012; Lee et al., 2012; Rinholm et al., 2011). Here, we demonstrate that loss of HIF function in OPCs results in cell death, axon damage and the appearance of cysts in white matter at P4 followed by a catastrophic loss of axons at P7 in the corpus callosum. Preliminary analysis indicates this is also the case in white matter tracts throughout the forebrain, including internal capsule and striatum, whereas white matter tracts of the cerebellum and brainstem are preserved. These differences might reflect region-restricted roles for OPCs in white matter angiogenesis or, alternatively, regional variations in metabolic requirements of OPCs and/or the axons they invest. While our findings indicate that white matter deterioration from P4–7 results from inadequate vascular investment, HIF signaling within OLs might also produce trophic factors for axons. While such lesions may result from a failure of myelination, we think this is unlikely because the phenotype is observed in P7 corpus callosum before axons are normally myelinated.
Potential roles for oligodendrocytes in CNS injury
OLs are early responders to a broad spectrum of brain pathologies including demyelinating disorders (e.g., Multiple Sclerosis, MS), stroke and penetrating trauma (Chang et al., 2002; Hampton et al., 2004; Kuhlmann et al., 2008; Tanaka et al., 2001). The notion that OPCs might produce angiogenic factors to encourage revascularization of injured CNS tissue is consistent with recent studies (Cayre et al., 2013; Jiang et al., 2011; Pham et al., 2012). Our finding of cystic changes in the white matter of OPC HIF-null mice is reminiscent of periventricular leukomalacia, a condition observed in the brain of pre-term infants. White matter injury induced by experimental autoimmune encephalomyelitis results in local tissue hypoxia (Davies et al., 2013) and OL HIF1α expression has been reported in MS lesions (Aboul-Enein et al., 2003). Further studies are needed to determine roles for OPCs as mediators of vascular remodeling after white matter injury. In summary, our findings demonstrate that cell intrinsic HIF pathway function in OPCs couples postnatal myelination and angiogenesis during a critical window of early postnatal development.
Experimental Procedures
Animals
All experimental procedures were approved by the Institutional Animal Care and Use Committee and Laboratory Animal Resource Center at UCSF. Mouse colonies were maintained in accordance with NIH and UCSF guidelines. Sox10-cre (Stolt et al., 2006), Olig1-cre (Lu et al., 2002), Plp-CreERT2 (Doerflinger et al., 2003), VHL floxed (Rankin et al., 2005), HIF1α floxed (Ryan et al., 2000) and HIF2α floxed (Gruber et al., 2007) mice have been previously described.
Cerebellar Slice Cultures
Mouse explant cerebellar slice cultures were generated from P0-P1 mouse pups and cultured for 12 days in vitro (DIV). Tamoxifen (Sigma) was added to transgenic cultures at 1DIV and 3DIV. Hypoxic and DMOG cultures were exposed to hypoxia (2% FiO2) or DMOG (Sigma) between 2–3DIV. Factors were added after hypoxia or DMOG treatment, and replenished every other day. See Extended Experimental Procedures for more details.
qRT-PCR
RNA was isolated (Trizol extraction followed by RNeasy; Qiagen) from immunopurified OPC cultures and assayed for gene expression by SYBR-Green on a Lightcycler 480 (Roche).
Western blots
Protein was extracted using standard protocols (Kenney et al., 2003) and then detected by either an Amersham ECL luminescence kit (GE Healthcare) or by immunofluorescence using the Licor detection system (Licor, Inc.).
Chromatin Immunoprecipitation DNA binding assays
Chromatin IP for HIF1α was conducted using the Human/Mouse HIF-1α ExactaChIP Chromatin IP kit (R&D Systems) followed by qRT-PCR with primers flanking HREs in genomically conserved domains proximal to Wnt7a and Wnt7b core promoter regions. See Extended Experimental Procedures for more details.
OPC-endothelial cell transwell assays
Mouse immunopurified OPCs were plated on transwell inserts (Corning) and mouse brain endothelial cells (bEnd.3 cell line, ATCC CRL-2299) were plated on PDL-coated glass coverslips below. Proliferation was assessed with EdU labeling at 24h and 48h.
Endothelial tube formation assay
bEnd.3 cells were plated on a matrigel matrix (BD) and factors or transwell inserts with OPCs were added. Following incubation for 18 hours, endothelial cell tube formation was imaged under phase contrast.
Retinal explants
Retina from P4–5 CD1 mice were dissected and flat-mounted on Millicell inserts and allowed to recover for 2–4h after which factors or OPC conditioned medium were added. After 4–6h, explants were fixed and stained with Isolectin GS-IB4.
Statistical analyses
For all quantified data, mean + SEM values are presented. Statistical significance was determined using unpaired, 2-tailed Student’s T-tests, as well as one-way ANOVA with Dunnett’s multiple comparison test (GraphPad Prism).
Supplementary Material
Research Highlights.
Oxygen tension directly regulates oligodendrocyte maturation through HIF signaling.
Oligodendrocyte-encoded HIF activates Wnt signaling and angiogenesis in the brain.
Oligodendrocyte-driven angiogenesis is critical for axon/white matter integrity.
Acknowledgements
We are grateful to Emily Harrington and April Tenney for expert technical help, Matt Rasband, Nenad Sestan and Klaus Nave for discussions. We thank Andrew McMahon (USC, Los Angeles, CA) for genomic sequence information for Wnt7a and 7b loci, William Kaelin (Dana-Farber Cancer Institute, Boston, MA) for floxed VHL transgenic mice, and William Richardson (UCL, London, UK) for Sox10-cre transgenic mice. We also thank Nina Bauer, who drew all illustrations. T.J.Y. acknowledges a postdoctoral fellowship from the National Multiple Sclerosis Society (NMSS). J.C.S. acknowledges support from training grant T32 GM007449-36 from NIGMS and the Ruth Kirschstein NRSA fellowship F31 NS076254-03 from NINDS. This work was made possible by grants from NICHD (HD072544 to E.M.), NMSS (to D.H.R.) and NINDS (NS040511 to D.H.R.). D.H.R. is a HHMI Investigator.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions
T.J.Y. and J.C.S. performed all experiments and data analysis except the following. A.G. and S.M.C. assisted with immunostaining and genotyping. R.D., S.P.J.F., and E.M. provided advice on experimental design and reagents. H.Z. designed and optimized primers. T.J.Y., J.C.S., and D.H.R. designed all experiments and wrote the manuscript.
References
- Aboul-Enein F, Rauschka H, Kornek B, Stadelmann C, Stefferl A, Bruck W, Lucchinetti C, Schmidbauer M, Jellinger K, Lassmann H. Preferential loss of myelin-associated glycoprotein reflects hypoxia-like white matter damage in stroke and inflammatory brain diseases. J Neuropathol Exp Neurol. 2003;62:25–33. doi: 10.1093/jnen/62.1.25. [DOI] [PubMed] [Google Scholar]
- Alcamo EA, Chirivella L, Dautzenberg M, Dobreva G, Farinas I, Grosschedl R, McConnell SK. Satb2 regulates callosal projection neuron identity in the developing cerebral cortex. Neuron. 2008;57:364–377. doi: 10.1016/j.neuron.2007.12.012. [DOI] [PubMed] [Google Scholar]
- Baron W, Hoekstra D. On the biogenesis of myelin membranes: sorting, trafficking and cell polarity. FEBS Lett. 2010;584:1760–1770. doi: 10.1016/j.febslet.2009.10.085. [DOI] [PubMed] [Google Scholar]
- Bradl M, Lassmann H. Oligodendrocytes: biology and pathology. Acta Neuropathol. 2010;119:37–53. doi: 10.1007/s00401-009-0601-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruick RK. Expression of the gene encoding the proapoptotic Nip3 protein is induced by hypoxia. Proc Natl Acad Sci U S A. 2000;97:9082–9087. doi: 10.1073/pnas.97.16.9082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahoy JD, Emery B, Kaushal A, Foo LC, Zamanian JL, Christopherson KS, Xing Y, Lubischer JL, Krieg PA, Krupenko SA, et al. A transcriptome database for astrocytes, neurons, and oligodendrocytes: a new resource for understanding brain development and function. J Neurosci. 2008;28:264–278. doi: 10.1523/JNEUROSCI.4178-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cammermeyer J. Reappraisal of the perivascular distribution of oligodendrocytes. Am J Anat. 1960;106:197–231. doi: 10.1002/aja.1001060303. [DOI] [PubMed] [Google Scholar]
- Cayre M, Courtes S, Martineau F, Giordano M, Arnaud K, Zamaron A, Durbec P. Netrin 1 contributes to vascular remodeling in the subventricular zone and promotes progenitor emigration after demyelination. Development. 2013;140:3107–3117. doi: 10.1242/dev.092999. [DOI] [PubMed] [Google Scholar]
- Chang A, Tourtellotte WW, Rudick R, Trapp BD. Premyelinating oligodendrocytes in chronic lesions of multiple sclerosis. N Engl J Med. 2002;346:165–173. doi: 10.1056/NEJMoa010994. [DOI] [PubMed] [Google Scholar]
- Chen B, Dodge ME, Tang W, Lu J, Ma Z, Fan CW, Wei S, Hao W, Kilgore J, Williams NS, et al. Small molecule-mediated disruption of Wnt-dependent signaling in tissue regeneration and cancer. Nat Chem Biol. 2009;5:100–107. doi: 10.1038/nchembio.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong SY, Rosenberg SS, Fancy SP, Zhao C, Shen YA, Hahn AT, McGee AW, Xu X, Zheng B, Zhang LI, et al. Neurite outgrowth inhibitor Nogo-A establishes spatial segregation and extent of oligodendrocyte myelination. Proc Natl Acad Sci U S A. 2012;109:1299–1304. doi: 10.1073/pnas.1113540109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrast R, Saher G, Nave KA, Verheijen MH. Lipid metabolism in myelinating glial cells: lessons from human inherited disorders and mouse models. J Lipid Res. 2011;52:419–434. doi: 10.1194/jlr.R009761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daneman R, Agalliu D, Zhou L, Kuhnert F, Kuo CJ, Barres BA. Wnt/beta-catenin signaling is required for CNS, but not non-CNS, angiogenesis. Proc Natl Acad Sci U S A. 2009;106:641–646. doi: 10.1073/pnas.0805165106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daneman R, Zhou L, Kebede AA, Barres BA. Pericytes are required for blood-brain barrier integrity during embryogenesis. Nature. 2010;468:562–566. doi: 10.1038/nature09513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies AL, Desai RA, Bloomfield PS, McIntosh PR, Chapple KJ, Linington C, Fairless R, Diem R, Kasti M, Murphy MP, et al. Neurological deficits caused by tissue hypoxia in neuroinflammatory disease. Ann Neurol. 2013 doi: 10.1002/ana.24006. [DOI] [PubMed] [Google Scholar]
- Del Rio-Hortega P. Are the glia with very few processes homologous with Schwann cells? by Pio del Rio-Hortega. 1922. Clin Neuropathol. 2012;31:460–462. [PubMed] [Google Scholar]
- Demerens C, Stankoff B, Logak M, Anglade P, Allinquant B, Couraud F, Zalc B, Lubetzki C. Induction of myelination in the central nervous system by electrical activity. Proc Natl Acad Sci U S A. 1996;93:9887–9892. doi: 10.1073/pnas.93.18.9887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doerflinger NH, Macklin WB, Popko B. Inducible site-specific recombination in myelinating cells. Genesis. 2003;35:63–72. doi: 10.1002/gene.10154. [DOI] [PubMed] [Google Scholar]
- Dugas-Ford J, Rowell JJ, Ragsdale CW. Cell-type homologies and the origins of the neocortex. Proc Natl Acad Sci U S A. 2012;109:16974–16979. doi: 10.1073/pnas.1204773109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eastman Q, Grosschedl R. Regulation of LEF-1/TCF transcription factors by Wnt and other signals. Curr Opin Cell Biol. 1999;11:233–240. doi: 10.1016/s0955-0674(99)80031-3. [DOI] [PubMed] [Google Scholar]
- Emery B, Dugas JC. Purification of oligodendrocyte lineage cells from mouse cortices by immunopanning. Cold Spring Harb Protoc. 2013;2013:854–868. doi: 10.1101/pdb.prot073973. [DOI] [PubMed] [Google Scholar]
- Fancy SP, Baranzini SE, Zhao C, Yuk DI, Irvine KA, Kaing S, Sanai N, Franklin RJ, Rowitch DH. Dysregulation of the Wnt pathway inhibits timely myelination and remyelination in the mammalian CNS. Genes Dev. 2009;23:1571–1585. doi: 10.1101/gad.1806309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fancy SP, Chan JR, Baranzini SE, Franklin RJ, Rowitch DH. Myelin regeneration: a recapitulation of development? Annu Rev Neurosci. 2011a;34:21–43. doi: 10.1146/annurev-neuro-061010-113629. [DOI] [PubMed] [Google Scholar]
- Fancy SP, Harrington EP, Yuen TJ, Silbereis JC, Zhao C, Baranzini SE, Bruce CC, Otero JJ, Huang EJ, Nusse R, et al. Axin2 as regulatory and therapeutic target in newborn brain injury and remyelination. Nat Neurosci. 2011b;14:1009–1016. doi: 10.1038/nn.2855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franceschini MA, Thaker S, Themelis G, Krishnamoorthy KK, Bortfeld H, Diamond SG, Boas DA, Arvin K, Grant PE. Assessment of infant brain development with frequency-domain near-infrared spectroscopy. Pediatr Res. 2007;61:546–551. doi: 10.1203/pdr.0b013e318045be99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funfschilling U, Supplie LM, Mahad D, Boretius S, Saab AS, Edgar J, Brinkmann BG, Kassmann CM, Tzvetanova ID, Mobius W, et al. Glycolytic oligodendrocytes maintain myelin and long-term axonal integrity. Nature. 2012;485:517–521. doi: 10.1038/nature11007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber M, Hu CJ, Johnson RS, Brown EJ, Keith B, Simon MC. Acute postnatal ablation of Hif-2alpha results in anemia. Proc Natl Acad Sci U S A. 2007;104:2301–2306. doi: 10.1073/pnas.0608382104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampton DW, Rhodes KE, Zhao C, Franklin RJ, Fawcett JW. The responses of oligodendrocyte precursor cells, astrocytes and microglia to a cortical stab injury, in the brain. Neuroscience. 2004;127:813–820. doi: 10.1016/j.neuroscience.2004.05.028. [DOI] [PubMed] [Google Scholar]
- Harb R, Whiteus C, Freitas C, Grutzendler J. In vivo imaging of cerebral microvascular plasticity from birth to death. J Cereb Blood Flow Metab. 2013;33:146–156. doi: 10.1038/jcbfm.2012.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardie DG, Ross FA, Hawley SA. AMPK: a nutrient and energy sensor that maintains energy homeostasis. Nature reviews Molecular cell biology. 2012;13:251–262. doi: 10.1038/nrm3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris JJ, Attwell D. The energetics of CNS white matter. J Neurosci. 2012;32:356–371. doi: 10.1523/JNEUROSCI.3430-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirose K, Morita M, Ema M, Mimura J, Hamada H, Fujii H, Saijo Y, Gotoh O, Sogawa K, Fujii-Kuriyama Y. cDNA cloning and tissue-specific expression of a novel basic helix-loop-helix/PAS factor (Arnt2) with close sequence similarity to the aryl hydrocarbon receptor nuclear translocator (Arnt) Mol Cell Biol. 1996;16:1706–1713. doi: 10.1128/mcb.16.4.1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu F, Strittmatter SM. Regulating axon growth within the postnatal central nervous system. Semin Perinatol. 2004;28:371–378. doi: 10.1053/j.semperi.2004.10.001. [DOI] [PubMed] [Google Scholar]
- Huang SM, Mishina YM, Liu S, Cheung A, Stegmeier F, Michaud GA, Charlat O, Wiellette E, Zhang Y, Wiessner S, et al. Tankyrase inhibition stabilizes axin and antagonizes Wnt signalling. Nature. 2009;461:614–620. doi: 10.1038/nature08356. [DOI] [PubMed] [Google Scholar]
- Huber O, Korn R, McLaughlin J, Ohsugi M, Herrmann BG, Kemler R. Nuclear localization of beta-catenin by interaction with transcription factor LEF-1. Mech Dev. 1996;59:3–10. doi: 10.1016/0925-4773(96)00597-7. [DOI] [PubMed] [Google Scholar]
- Ishibashi T, Dakin KA, Stevens B, Lee PR, Kozlov SV, Stewart CL, Fields RD. Astrocytes promote myelination in response to electrical impulses. Neuron. 2006;49:823–832. doi: 10.1016/j.neuron.2006.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivan M, Kondo K, Yang H, Kim W, Valiando J, Ohh M, Salic A, Asara JM, Lane WS, Kaelin WG., Jr HIFalpha targeted for VHL-mediated destruction by proline hydroxylation: implications for O2 sensing. Science. 2001;292:464–468. doi: 10.1126/science.1059817. [DOI] [PubMed] [Google Scholar]
- Jaakkola P, Mole DR, Tian YM, Wilson MI, Gielbert J, Gaskell SJ, von Kriegsheim A, Hebestreit HF, Mukherji M, Schofield CJ, et al. Targeting of HIF-alpha to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science. 2001;292:468–472. doi: 10.1126/science.1059796. [DOI] [PubMed] [Google Scholar]
- Janzer RC, Raff MC. Astrocytes induce blood-brain barrier properties in endothelial cells. Nature. 1987;325:253–257. doi: 10.1038/325253a0. [DOI] [PubMed] [Google Scholar]
- Jiang L, Shen F, Degos V, Schonemann M, Pleasure SJ, Mellon SH, Young WL, Su H. Oligogenesis and Oligodendrocyte Progenitor Maturation Vary in Different Brain Regions and Partially Correlate with Local Angiogenesis after Ischemic Stroke. Transl Stroke Res. 2011;2:366–375. doi: 10.1007/s12975-011-0078-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joza N, Pospisilik JA, Hangen E, Hanada T, Modjtahedi N, Penninger JM, Kroemer G. AIF: not just an apoptosis-inducing factor. Annals of the New York Academy of Sciences. 2009;1171:2–11. doi: 10.1111/j.1749-6632.2009.04681.x. [DOI] [PubMed] [Google Scholar]
- Kenney AM, Cole MD, Rowitch DH. Nmyc upregulation by sonic hedgehog signaling promotes proliferation in developing cerebellar granule neuron precursors. Development. 2003;130:15–28. doi: 10.1242/dev.00182. [DOI] [PubMed] [Google Scholar]
- Kinney HC, Brody BA, Kloman AS, Gilles FH. Sequence of central nervous system myelination in human infancy. II. Patterns of myelination in autopsied infants. J Neuropathol Exp Neurol. 1988;47:217–234. doi: 10.1097/00005072-198805000-00003. [DOI] [PubMed] [Google Scholar]
- Kuhlmann T, Miron V, Cui Q, Wegner C, Antel J, Bruck W. Differentiation block of oligodendroglial progenitor cells as a cause for remyelination failure in chronic multiple sclerosis. Brain. 2008;131:1749–1758. doi: 10.1093/brain/awn096. [DOI] [PubMed] [Google Scholar]
- Lee H, Paik SG. Regulation of BNIP3 in normal and cancer cells. Molecules and cells. 2006;21:1–6. [PubMed] [Google Scholar]
- Lee Y, Morrison BM, Li Y, Lengacher S, Farah MH, Hoffman PN, Liu Y, Tsingalia A, Jin L, Zhang PW, et al. Oligodendroglia metabolically support axons and contribute to neurodegeneration. Nature. 2012;487:443–448. doi: 10.1038/nature11314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebner S, Corada M, Bangsow T, Babbage J, Taddei A, Czupalla CJ, Reis M, Felici A, Wolburg H, Fruttiger M, et al. Wnt/beta-catenin signaling controls development of the blood-brain barrier. J Cell Biol. 2008;183:409–417. doi: 10.1083/jcb.200806024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu QR, Sun T, Zhu Z, Ma N, Garcia M, Stiles CD, Rowitch DH. Common developmental requirement for Olig function indicates a motor neuron/oligodendrocyte connection. Cell. 2002;109:75–86. doi: 10.1016/s0092-8674(02)00678-5. [DOI] [PubMed] [Google Scholar]
- Majmundar AJ, Wong WJ, Simon MC. Hypoxia-inducible factors and the response to hypoxic stress. Mol Cell. 2010;40:294–309. doi: 10.1016/j.molcel.2010.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazumdar J, O'Brien WT, Johnson RS, LaManna JC, Chavez JC, Klein PS, Simon MC. O2 regulates stem cells through Wnt/beta-catenin signalling. Nat Cell Biol. 2010;12:1007–1013. doi: 10.1038/ncb2102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ment LR, Schwartz M, Makuch RW, Stewart WB. Association of chronic sublethal hypoxia with ventriculomegaly in the developing rat brain. Brain Res Dev Brain Res. 1998;111:197–203. doi: 10.1016/s0165-3806(98)00139-4. [DOI] [PubMed] [Google Scholar]
- Miller DJ, Duka T, Stimpson CD, Schapiro SJ, Baze WB, McArthur MJ, Fobbs AJ, Sousa AM, Sestan N, Wildman DE, et al. Prolonged myelination in human neocortical evolution. Proc Natl Acad Sci U S A. 2012;109:16480–16485. doi: 10.1073/pnas.1117943109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nave KA. Myelination and support of axonal integrity by glia. Nature. 2010;468:244–252. doi: 10.1038/nature09614. [DOI] [PubMed] [Google Scholar]
- Patel SA, Simon MC. Biology of hypoxia-inducible factor-2alpha in development and disease. Cell Death Differ. 2008;15:628–634. doi: 10.1038/cdd.2008.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pham LD, Hayakawa K, Seo JH, Nguyen MN, Som AT, Lee BJ, Guo S, Kim KW, Lo EH, Arai K. Crosstalk between oligodendrocytes and cerebral endothelium contributes to vascular remodeling after white matter injury. Glia. 2012;60:875–881. doi: 10.1002/glia.22320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porfiri E, Rubinfeld B, Albert I, Hovanes K, Waterman M, Polakis P. Induction of a beta-catenin-LEF-1 complex by wnt-1 and transforming mutants of beta-catenin. Oncogene. 1997;15:2833–2839. doi: 10.1038/sj.onc.1201462. [DOI] [PubMed] [Google Scholar]
- Rankin EB, Higgins DF, Walisser JA, Johnson RS, Bradfield CA, Haase VH. Inactivation of the arylhydrocarbon receptor nuclear translocator (Arnt) suppresses von Hippel-Lindau disease-associated vascular tumors in mice. Mol Cell Biol. 2005;25:3163–3172. doi: 10.1128/MCB.25.8.3163-3172.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinholm JE, Hamilton NB, Kessaris N, Richardson WD, Bergersen LH, Attwell D. Regulation of oligodendrocyte development and myelination by glucose and lactate. J Neurosci. 2011;31:538–548. doi: 10.1523/JNEUROSCI.3516-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenstein JM, Krum JM, Ruhrberg C. VEGF in the nervous system. Organogenesis. 2010;6:107–114. doi: 10.4161/org.6.2.11687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan HE, Poloni M, McNulty W, Elson D, Gassmann M, Arbeit JM, Johnson RS. Hypoxia-inducible factor-1alpha is a positive factor in solid tumor growth. Cancer Res. 2000;60:4010–4015. [PubMed] [Google Scholar]
- Sapieha P. Eyeing central neurons in vascular growth and reparative angiogenesis. Blood. 2012;120:2182–2194. doi: 10.1182/blood-2012-04-396846. [DOI] [PubMed] [Google Scholar]
- Sawamiphak S, Ritter M, Acker-Palmer A. Preparation of retinal explant cultures to study ex vivo tip endothelial cell responses. Nature protocols. 2010;5:1659–1665. doi: 10.1038/nprot.2010.130. [DOI] [PubMed] [Google Scholar]
- Semenza GL. Hypoxia-inducible factors in physiology and medicine. Cell. 2012;148:399–408. doi: 10.1016/j.cell.2012.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silbereis JC, Huang EJ, Back SA, Rowitch DH. Towards improved animal models of neonatal white matter injury associated with cerebral palsy. Dis Model Mech. 2010;3:678–688. doi: 10.1242/dmm.002915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenman JM, Rajagopal J, Carroll TJ, Ishibashi M, McMahon J, McMahon AP. Canonical Wnt signaling regulates organ-specific assembly and differentiation of CNS vasculature. Science. 2008;322:1247–1250. doi: 10.1126/science.1164594. [DOI] [PubMed] [Google Scholar]
- Stevens B, Porta S, Haak LL, Gallo V, Fields RD. Adenosine: a neuron-glial transmitter promoting myelination in the CNS in response to action potentials. Neuron. 2002;36:855–868. doi: 10.1016/s0896-6273(02)01067-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolt CC, Schlierf A, Lommes P, Hillgartner S, Werner T, Kosian T, Sock E, Kessaris N, Richardson WD, Lefebvre V, et al. SoxD proteins influence multiple stages of oligodendrocyte development and modulate SoxE protein function. Dev Cell. 2006;11:697–709. doi: 10.1016/j.devcel.2006.08.011. [DOI] [PubMed] [Google Scholar]
- Tan S, Drobyshevsky A, Jilling T, Ji X, Ullman LM, Englof I, Derrick M. Model of cerebral palsy in the perinatal rabbit. J Child Neurol. 2005;20:972–979. doi: 10.1177/08830738050200120801. [DOI] [PubMed] [Google Scholar]
- Tanaka K, Nogawa S, Ito D, Suzuki S, Dembo T, Kosakai A, Fukuuchi Y. Activation of NG2-positive oligodendrocyte progenitor cells during post-ischemic reperfusion in the rat brain. Neuroreport. 2001;12:2169–2174. doi: 10.1097/00001756-200107200-00025. [DOI] [PubMed] [Google Scholar]
- Tessitore C, Brunjes PC. A comparative study of myelination in precocial and altricial murid rodents. Brain Res. 1988;471:139–147. doi: 10.1016/0165-3806(88)90159-9. [DOI] [PubMed] [Google Scholar]
- Wang GL, Jiang BH, Rue EA, Semenza GL. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci U S A. 1995;92:5510–5514. doi: 10.1073/pnas.92.12.5510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Rattner A, Zhou Y, Williams J, Smallwood PM, Nathans J. Norrin/Frizzled4 signaling in retinal vascular development and blood brain barrier plasticity. Cell. 2012;151:1332–1344. doi: 10.1016/j.cell.2012.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss J, Takizawa B, McGee A, Stewart WB, Zhang H, Ment L, Schwartz M, Strittmatter S. Neonatal hypoxia suppresses oligodendrocyte Nogo-A and increases axonal sprouting in a rodent model for human prematurity. Exp Neurol. 2004;189:141–149. doi: 10.1016/j.expneurol.2004.05.018. [DOI] [PubMed] [Google Scholar]
- Ye F, Chen Y, Hoang T, Montgomery RL, Zhao XH, Bu H, Hu T, Taketo MM, van Es JH, Clevers H, et al. HDAC1 and HDAC2 regulate oligodendrocyte differentiation by disrupting the beta-catenin-TCF interaction. Nat Neurosci. 2009a;12:829–838. doi: 10.1038/nn.2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye X, Wang Y, Cahill H, Yu M, Badea TC, Smallwood PM, Peachey NS, Nathans J. Norrin, frizzled-4, and Lrp5 signaling in endothelial cells controls a genetic program for retinal vascularization. Cell. 2009b;139:285–298. doi: 10.1016/j.cell.2009.07.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuen TJ, Johnson KR, Miron VE, Zhao C, Quandt J, Harrisingh MC, Swire M, Williams A, McFarland HF, Franklin RJ, et al. Identification of endothelin 2 as an inflammatory factor that promotes central nervous system remyelination. Brain. 2013;136:1035–1047. doi: 10.1093/brain/awt024. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.