Abstract
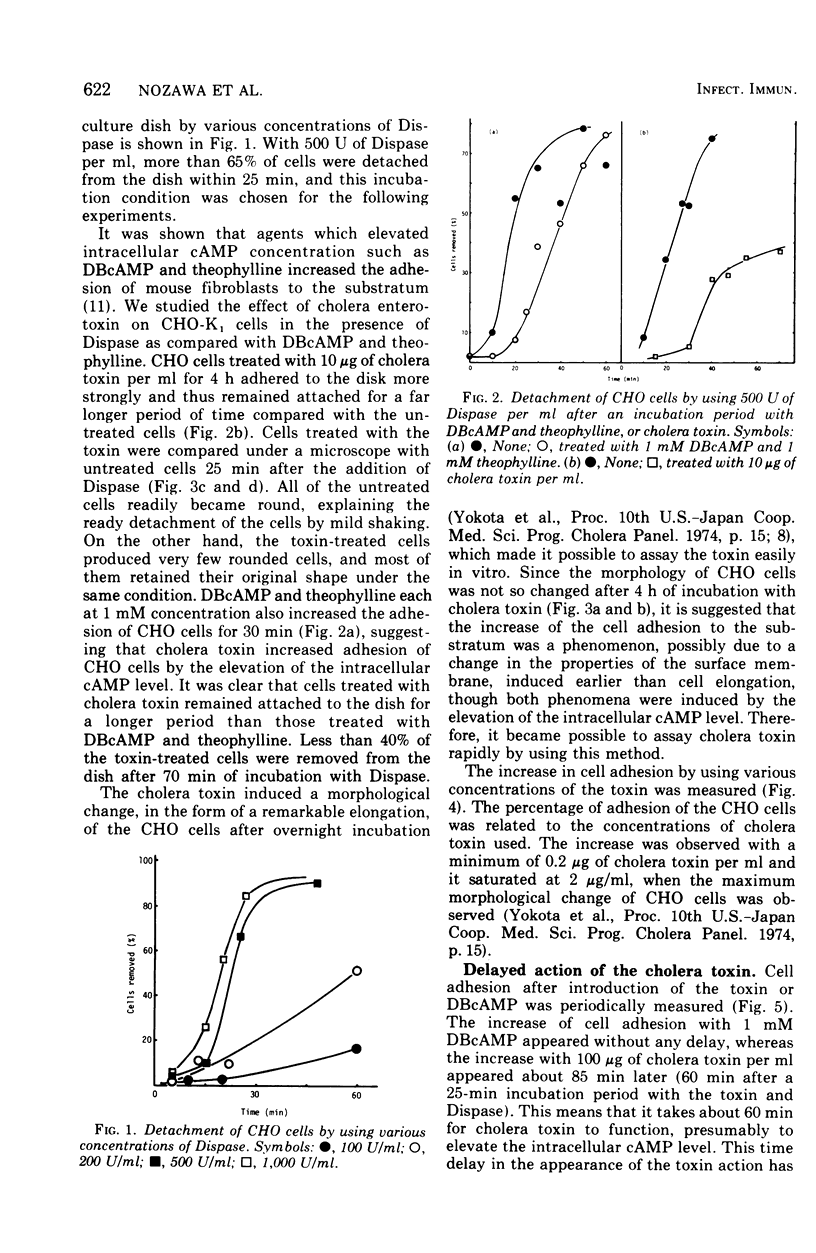
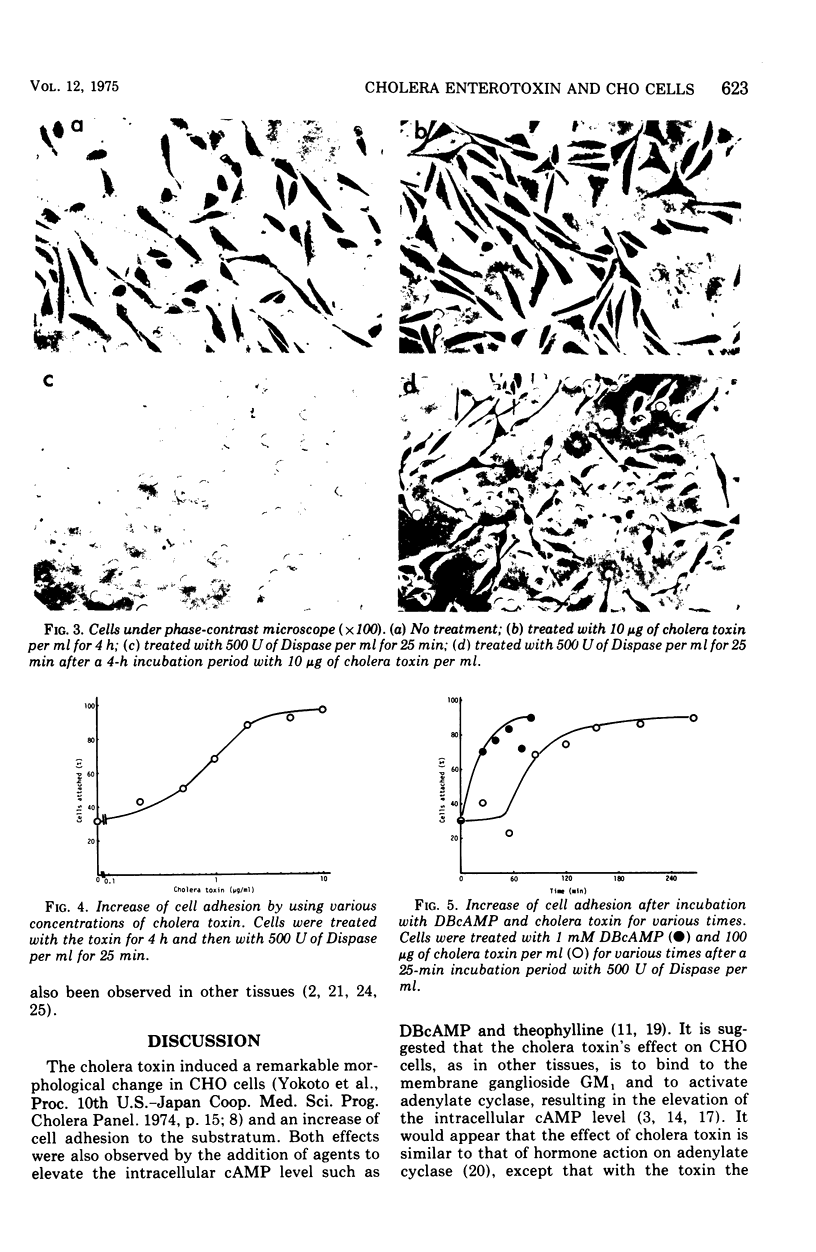
A new, simple assay method using Chinese hamster ovary cells was devised for cholera enterotoxin. The effect of the cholera toxin was measured by an increase in cell adhesion to the substratum. The increase was dependent on the concentration of the toxin used and was effective for a longer period of time than that of adhesion increased by dibutyryl cyclic adenosine monophosphate and theophyline. There was a delay of about 60 min before the increase in adhesion, using the toxin, appeared, whereas the increase caused by dibutyryl cyclic adenosine monophosphate appeared almost at once.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Craig J. P. Preparation of the vascular permeability factor of Vibrio cholerae. J Bacteriol. 1966 Sep;92(3):793–795. doi: 10.1128/jb.92.3.793-795.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuatrecasas P. Cholera toxin-fat cell interaction and the mechanism of activation of the lipolytic response. Biochemistry. 1973 Aug 28;12(18):3567–3577. doi: 10.1021/bi00742a033. [DOI] [PubMed] [Google Scholar]
- Cuatrecasas P. Gangliosides and membrane receptors for cholera toxin. Biochemistry. 1973 Aug 28;12(18):3558–3566. doi: 10.1021/bi00742a032. [DOI] [PubMed] [Google Scholar]
- Donta S. T., King M., Sloper K. Induction of steroidogenesis in tissue culture by cholera enterotoxin. Nat New Biol. 1973 Jun 20;243(129):246–247. doi: 10.1038/newbio243246a0. [DOI] [PubMed] [Google Scholar]
- Guerrant R. L., Brunton L. L., Schnaitman T. C., Rebhun L. I., Gilman A. G. Cyclic adenosine monophosphate and alteration of Chinese hamster ovary cell morphology: a rapid, sensitive in vitro assay for the enterotoxins of Vibrio cholerae and Escherichia coli. Infect Immun. 1974 Aug;10(2):320–327. doi: 10.1128/iai.10.2.320-327.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrant R. L., Chen L. C., Sharp G. W. Intestinal adenyl-cyclase activity in canine cholera: correlation with fluid accumulation. J Infect Dis. 1972 Apr;125(4):377–381. doi: 10.1093/infdis/125.4.377. [DOI] [PubMed] [Google Scholar]
- HAM R. G. CLONAL GROWTH OF MAMMALIAN CELLS IN A CHEMICALLY DEFINED, SYNTHETIC MEDIUM. Proc Natl Acad Sci U S A. 1965 Feb;53:288–293. doi: 10.1073/pnas.53.2.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollenberg M. D., Cuatrecasas P. Epidermal growth factor: receptors in human fibroblasts and modulation of action by cholera toxin. Proc Natl Acad Sci U S A. 1973 Oct;70(10):2964–2968. doi: 10.1073/pnas.70.10.2964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao F. T., Puck T. T. Genetics of somatic mammalian cells, VII. Induction and isolation of nutritional mutants in Chinese hamster cells. Proc Natl Acad Sci U S A. 1968 Aug;60(4):1275–1281. doi: 10.1073/pnas.60.4.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimberg D. V., Field M., Johnson J., Henderson A., Gershon E. Stimulation of intestinal mucosal adenyl cyclase by cholera enterotoxin and prostaglandins. J Clin Invest. 1971 Jun;50(6):1218–1230. doi: 10.1172/JCI106599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King C. A., Van Heyningen W. E. Deactivation of cholera toxin by a sialidase-resistant monosialosylganglioside. J Infect Dis. 1973 Jun;127(6):639–647. doi: 10.1093/infdis/127.6.639. [DOI] [PubMed] [Google Scholar]
- Kram R., Tomkins G. M. Pleiotypic control by cyclic AMP: interaction with cyclic GMP and possible role of microtubules. Proc Natl Acad Sci U S A. 1973 Jun;70(6):1659–1663. doi: 10.1073/pnas.70.6.1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Keefe E., Cuatrecasas P. Cholera toxin mimics melanocyte stimulating hormone in inducing differentiation in melanoma cells. Proc Natl Acad Sci U S A. 1974 Jun;71(6):2500–2504. doi: 10.1073/pnas.71.6.2500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce N. F., Carpenter C. C., Jr, Elliott H. L., Greenough W. B., 3rd Effects of prostaglandins, theophylline, and cholera exotoxin upon transmucosal water and electrolyte movement in the canine jejunum. Gastroenterology. 1971 Jan;60(1):22–32. [PubMed] [Google Scholar]
- Pierce N. F. Differential inhibitory effects of cholera toxoids and ganglioside on the enterotoxins of Vibrio cholerae and Escherichia coli. J Exp Med. 1973 Apr 1;137(4):1009–1023. doi: 10.1084/jem.137.4.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Professor G. Ya. Bei-Bienko. Nature. 1972 Mar 31;236(5344):247–248. doi: 10.1038/236247c0. [DOI] [PubMed] [Google Scholar]
- Puck T. T., Waldren C. A., Hsie A. W. Membrane dynamics in the action of dibutyryl adenosine 3':5'-cyclic monophosphate and testosterone on mammalian cells. Proc Natl Acad Sci U S A. 1972 Jul;69(7):1943–1947. doi: 10.1073/pnas.69.7.1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer D. E., Lust W. D., Sircar B., Goldberg N. D. Elevated concentration of adenosine 3':5'-cyclic monophosphate in intestinal mucosa after treatment with cholera toxin. Proc Natl Acad Sci U S A. 1970 Oct;67(2):851–856. doi: 10.1073/pnas.67.2.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp G. W., Hynie S. Stimulation of intestinal adenyl cyclase by cholera toxin. Nature. 1971 Jan 22;229(5282):266–269. doi: 10.1038/229266a0. [DOI] [PubMed] [Google Scholar]
- Sultzer B. M., Craig J. P. Cholera toxin inhibits macromolecular synthesis in mouse spleen cells. Nat New Biol. 1973 Aug 8;244(136):178–180. doi: 10.1038/newbio244178a0. [DOI] [PubMed] [Google Scholar]
- Vaughan M., Pierce N. F., Greenough W. B., 3rd Stimulation of glycerol production in fat cells by cholera toxin. Nature. 1970 May 16;226(5246):658–659. doi: 10.1038/226658a0. [DOI] [PubMed] [Google Scholar]
- Wolff J., Temple R., Cook G. H. Stimulation of steroid secretion in adrenal tumor cells by choleragen. Proc Natl Acad Sci U S A. 1973 Oct;70(10):2741–2744. doi: 10.1073/pnas.70.10.2741. [DOI] [PMC free article] [PubMed] [Google Scholar]



