Abstract
Dim-light vision is mediated by retinal rod cells. Rhodopsin (R), a G-protein-coupled receptor, switches to its active form () in response to absorbing a single photon and activates multiple copies of the G-protein transducin (G) that trigger further downstream reactions of the phototransduction cascade. The classical assumption is that R and G are uniformly distributed and freely diffusing on disk membranes. Recent experimental findings have challenged this view by showing specific R architectures, including RG precomplexes, nonuniform R density, specific R arrangements, and immobile fractions of R. Here, we derive a physical model that describes the first steps of the photoactivation cascade in spatiotemporal detail and single-molecule resolution. The model was implemented in the ReaDDy software for particle-based reaction-diffusion simulations. Detailed kinetic in vitro experiments are used to parametrize the reaction rates and diffusion constants of R and G. Particle diffusion and G activation are then studied under different conditions of R-R interaction. It is found that the classical free-diffusion model is consistent with the available kinetic data. The existence of precomplexes between inactive R and G is only consistent with the data if these precomplexes are weak, with much larger dissociation rates than suggested elsewhere. Microarchitectures of R, such as dimer racks, would effectively immobilize R but have little impact on the diffusivity of G and on the overall amplification of the cascade at the level of the G protein.
Introduction
In retinal rod cells, absorption of a photon by the visual G-protein-coupled receptor rhodopsin (R) initiates a cascade of biochemical reactions that eventually generates an electrical signal. A first stage of signal transduction and amplification is provided by the receptor-catalyzed nucleotide exchange in the rod G protein, transducin (G). R and G are located in disk membranes that fill the rod outer segment. Although R and G display fundamental similarities to other receptors and heterotrimeric Gαβγ proteins (1), the single-quantum detective function of the rod cell requires that both proteins have specific properties, including a very low basal activity to ensure low noise and a rapid and efficient sequential activation of multiple copies of the G protein by single activated molecules of the receptor. In the dark, the catalytic activity of rhodopsin is efficiently blocked by the covalently bound inverse agonist 11-cis-retinal. Light-induced cis/trans retinal isomerization triggers conformational changes in the receptor protein that culminate in an equilibrium between inactive Meta I (M1) and active Meta II () intermediates (2, 3). The G holoprotein is peripherally bound to the disk membrane by weak hydrophobic and ionic interactions. After the exchange of GDP for GTP in the -Gαβγ complex, the G protein dissociates and active Gα-GTP () binds to a cGMP phosphodiesterase in a noncatalytic, stoichiometric interaction. The active -phosphodiesterase complex rapidly hydrolyzes cGMP, leading to the closure of cGMP-dependent ion channels in the plasma membrane of the rod outer segment. Ca2+-dependent feedback leads to a delayed recovery of the dark concentration of cGMP. At the receptor level, deactivating proteins, namely, a receptor kinase and arrestin, bind to to mediate its phosphorylation and to cap it against further interaction with G. Deactivation by arrestin takes 0.2–0.3 s, thereby enabling sufficient G-protein activation. The whole system forms a closed signal-transduction module that transforms the input-signal photon into a transient receptor current that provides the electrical signal for the synapse of the rod (4).
Mathematical descriptions of the photocurrent response of retinal rod cells by a system of ordinary differential equations (ODEs) date back to the early seventies (5). Once the visual cascade was understood as a G-protein-coupled system, known partial steps were increasingly incorporated into schemes of differential equations describing signal transduction (6), eventually including downstream reactions (7, 8) and leading to a full simulation of the electrical response.
In the dim-light working range of the rod, each disk membrane receives only one or a few photon hits, triggering the whole cascade. To study these single-photon responses, the stochastic spatiotemporal nature of the system had to be incorporated into the simulations. The well-mixed assumption of ODE methods (i.e., ODEs have no concept of location, such as the position of the single activated rhodopsin) rendered these approaches not useful for our application. As a result, grid-based spatiotemporal chemical master equations (ST-CMEs) were used (9, 10), allowing for discrete particle numbers rather than concentrations and a higher spatial resolution of the disk membrane. However, this method relies on the well-mixed assumption being true for small subspaces (i.e., lattice boxes), in which the system is discretized. In a different approach, partial differential equations (PDEs) were used on the photoactivation cascade to achieve the same goal of a higher spatial resolution (11, 12, 13). The PDE approach is also useful for deriving general theoretical results, such as first passage times in specific geometries (14, 15, 16, 17). However, PDEs use concentrations of molecules and are therefore impractical when small particle numbers are important.
Recent experimental findings oppose the scenario of a well-mixed, free-diffusion, uniformly distributed disk membrane by reporting large rhodopsin clusters (18), immobile fractions of R (19), and special paracrystalline rhodopsin structures called racks of dimers (20).
The specific inhomogeneous architecture of these structures on all levels of detail together with the importance of individual molecules (a single triggers the cascade) goes beyond the limits of ST-CME and PDE methods. Individual molecules, arranged in their specific architectures, have been used in a Monte Carlo simulation to characterize the influence of such structures on the diffusion properties on the disk membrane (21). That study revealed a high impact of these structures on the diffusion but did not investigate their impact on reactions.
Such methods, which also treat reactions, are usually referred to as particle-based reaction-diffusion simulations (PBRD). Here, every molecule is represented individually in continuous space and time and its diffusion and reaction dynamics is simulated on a microscopic level. Individual molecules can be modeled as freely diffusing, as immobile obstacles, or in some trap potential. At the same time, reactions between particles can occur only upon physical encounter of reactive particles. In this way, complex interactions between particle mobility and reaction rates, such as inhibition of reactions due to spatial occlusion, can be investigated.
Covering all scenarios of rhodopsin architecture on a disk membrane requires single-particle resolution, particle diffusion, a simulation geometry, particle-particle interaction potentials, and reactions between particles. Other available tools (22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35) covered some but not all of these requirements. For this reason, the new PBRD package ReaDDy was developed (36). Using ReaDDy, we are able to test the effect of different supramolecular arrangements of R on molecule diffusivity and outcome of signal transduction at the same time.
We chose three representative cases to compare, namely, 1), the classic case, in which all particle species diffuse freely and are uniformly distributed; 2), the precomplex case, which is the same as case 1 but with an additional precomplex interaction between the inactive receptor and G (37); and 3), the rack case, where G diffuses freely and is uniformly distributed, but a large fraction of R is arranged in racks of dimers (20) and thereby rendered immobile (19).
For our model, a to our knowledge new set of kinetic rates of the G activation step was derived based on near-infrared light-scattering experiments using native disk membranes, as in a previous study (38). These rates give new insight into G-protein activation kinetics, revealing that the conformational changes leading from a receptor-G-protein encounter complex to an active complex and the GDP release are most probably the rate-limiting steps.
In using this set of kinetic rates in the particle-based simulation and combining it with the experimentally known kinetics of G-protein activation (38), we can assess the consistency of different R-architectures with experimental kinetics and give parameter ranges in which this consistency is provided.
The salient result of our study is that the supramolecular architecture has a surprisingly modest impact on the overall amplification of the cascade at the level of the G protein. It turns out that the diffusivity of the G-protein can override the influence of receptor architecture. An important exception is the case of precomplexes between R and G, which are only possible if they are formed very transiently. Considering the uniformity of the simulated signals, signal variability is moderate if there is only one fraction of receptor mobility present. Signal variability is increased by different fractions, i.e., mobile and immobile fractions of R, occurring at the same time (19).
Our derived model, at present containing the first steps of the photoactivation cascade including diffusion, crowding, molecular architecture, and detailed kinetics, can now serve as a building block for larger-scale physical simulations, e.g., of the entire signal cascade in a rod cell.
Materials and Methods
Kinetic parameter derivation and ODE modeling
Based on the reaction scheme in Fig. 1 (Eqs. 1–4) and the scenario (with or without precomplexes), the kinetic reaction rates are estimated from enzymatic parameters obtained in a previous study (38). These experiments were conducted at 22°C with purified disk membranes. Rhodopsin concentrations were [R] = 25,000 μm−2 and [] = 5.7 μm−2. G-protein activation ligand concentration was set to yield maximal production rates: [GDP]0 = 0 μm−2 and [GTP]0 = 3000 μm−2.
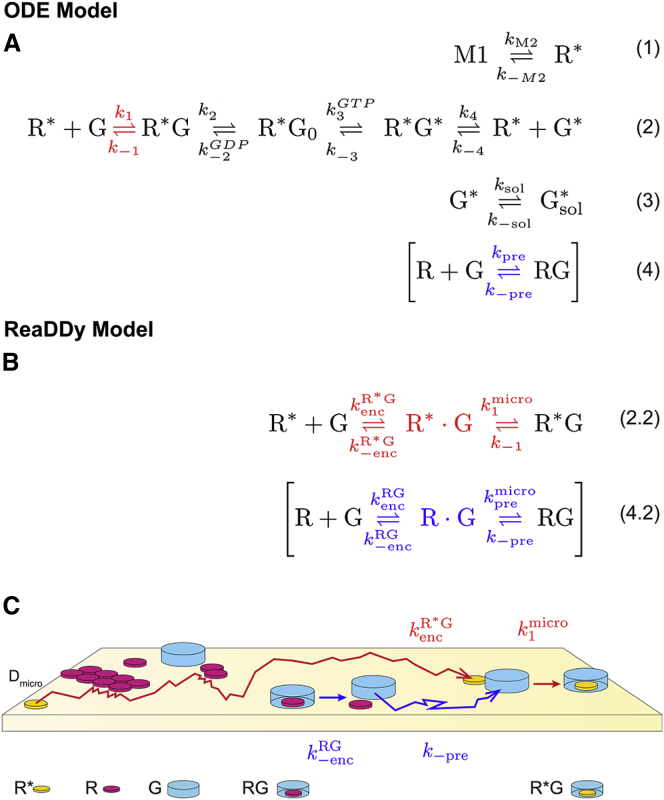
Figure 1.
Reaction kinetics at two levels of modeling detail. (A) The first steps of the photoactivation cascade consist of the following activation reactions: M1/ equilibrium (1); G-activation reactions, comprising formation of G complex, subsequent nucleotide exchange in G, and -complex dissociation (2); and dissociation of from the membrane (3). A nonproductive RG-complex formation (4) is added for the precomplex case. These reactions are used for the ODE model of the cascade. (B) Using particle-based reaction-diffusion (ReaDDy), bimolecular association reactions have to be split into the explicitly simulated diffusional encounter and the first-order transition from encounter complex to stable complex. This affects the G- and RG complex formation in (2.2) and (4.2) (red and blue). (C) Graphical representation of the microscopic diffusion and reaction components, illustrating space exclusions, molecular shape, and crowding. To see this figure in color, go online.
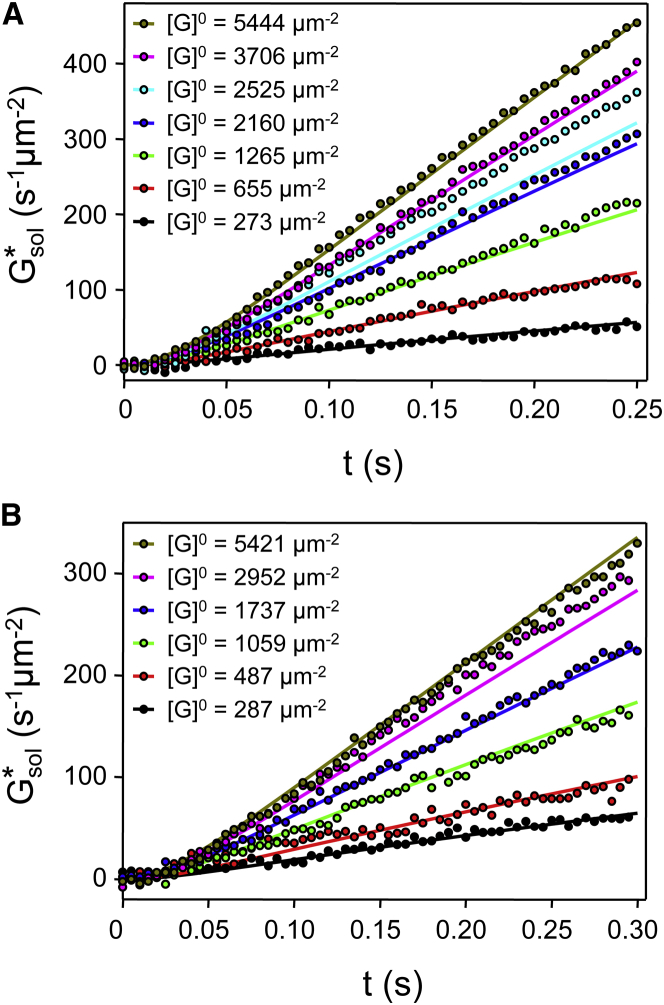
Reaction rates were then determined by fitting the ODE model (Fig. 1 A) to experimental traces of production over time (Fig. 2 A). To verify the results, another series of dissociation signals measured in the presence of 200 μM GTP (0 μM GDP, data taken from (38)) were simulated with both sets of rate constants. As seen in Fig. 2 B, the resulting traces are in good agreement with the experimental data (see Table 1 for the derived reaction rates and the Supporting Material for the details of rate derivation).
Figure 2.
ODE fit and modeling. (A) ODE fits (solid lines) to representative dissociation signals (circles; measuring conditions and rate constants as in Tables 1, S1, and S2). (B) ODE modeling (solid lines) of representative dissociation signals (200 μM GTP, 0 μM GDP; values taken from Heck and Hofmann (38)), with rate constants as in Table 1. Note that the two sets of rate constants (sets A and B in Table S4) yield essentially identical traces. To see this figure in color, go online.
Table 1.
Experimental parameters and derived reaction rates
| Parameter | Value | Description |
|---|---|---|
| Ta | 22°C | Temperature of the experiment |
| [R]a | 25,000 μm−2 | Rhodopsin density on disk vesicles |
| [], [M1]a,c | 5.7 μm−2 | Activated Rhodopsin density on disk vesicles |
| a,e | 273 μm−2 | Initial G density in first experiment |
| a,e | 1265 μm−2 | Initial G density in second experiment |
| a,e | 2525 μm−2 | Initial G density in third experiment |
| a,e | 5444 μm−2 | Initial G density in fourth experiment |
| [GDP]0a | 0 μm−2 | Initial GDP concentration in experiment |
| [GTP]0a | 3000 μm−2 | Initial GTP concentration in experiment |
| b | 35.4 s−1 | Rate of M1 to conversion of rhodopsin |
| b | 14.4 s−1 | Rate of -to-M1 conversion of rhodopsin |
| b | 0.36 μm2 s−1 | Rate of G-complex formation |
| d | 5000 s−1 | Microscopic rate of G-complex formation |
| b | 200 s−1 | Rate of G-complex dissociation |
| b | 600 s−1 | Rate of GDP release from G complex |
| = k−2 [GDP]b | 2.2 s−1μM−1 [GDP] | Rate of GDP uptake by G complex |
| = k3 [GTP]b | 2.6 s−1μM−1 [GTP] | Rate of GTP uptake by G complex |
| b | 600 s−1 | Rate of GTP release by complex |
| b | 60,000 s−1 | Rate of complex dissociation |
| b | 0 s−1 | Rate of complex formation |
| b | 10,000 s−1 | Rate of membrane dissociation |
| b | 0 s−1 | Rate of membrane binding |
| 1.67 μm2s−1 | Rate of RG-complex formation | |
| d | 108 s−1 | Microscopic rate of G-complex formation |
| 11,200 s−1 | Rate of RG-complex formation |
Experimental conditions in (38).
Rates were obtained via an ODE fitting procedure of experimental data (see methods and SI for details).
ODE simulations start with [M1] = 5.7 μm−2, ReaDDy simulations start with [] = 5.7 μm−2.
Rate required for ReaDDy simulations only. Rate applies for the complexation reaction, once the particles are closer than their reaction radii.
Concentrations used for both ReaDDy and ODE simulations. The ODE fit contained three additional experiments (see Table S2).
ReaDDy Model
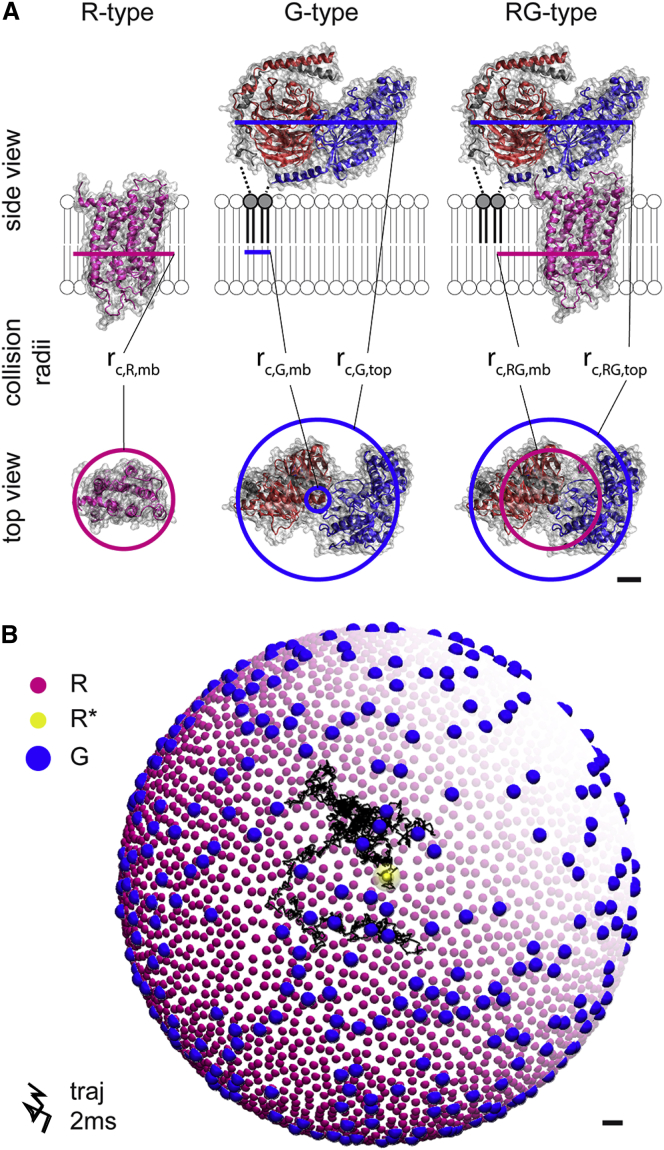
The detailed spatiotemporal model was set up and simulated with the particle-based reaction-diffusion simulation software ReaDDy, version 1.1 (36). All molecules are modeled as spherical particles. These particles collide with each other if they get closer than the sum of their collision radii (rc). These radii come in two types, rc,mb, for membrane internal collisions, and rc,sol, for the collisions of soluble parts of the molecules. A collision results in particle-particle repulsion, governed by a harmonic potential. If particles collide, such that the distance between them is smaller than the sum of their reaction radii (rr), a reaction can be triggered. All radii are taken from crystal structures (39, 40, 41, 42) (see Fig. 3 A). Due to the properties of the reference experiment (38), particle diffusion is confined to a spherical surface by applying a harmonic potential along the surface normal. The surface size (radius, r = 120 nm, and surface area, A = 0.18 μm2) was chosen such that it would host on average 1 , 4499 R, and 450 G (see Fig. 3 B). The Brownian dynamics are integrated with a time step of Δt = 20 ns. Typically, the total simulation time is 200 ms, corresponding to 107 time steps; on a standard CPU (Intel Xeon E5345@2.33 GHz), this would take ∼400 h. See the Supporting Material for a more detailed description of the ReaDDy model.
Figure 3.
ReaDDy model. (A) Collision radii of R-type, G-type, and RG-type particles were chosen based on crystal structures (R and , bovine 1U19 and 3PQR (40); G, bovine 1GOT (41); RG, 3SN6 (42)). Due to the different molecular shapes within and on the surface of the membrane, there are two types of collision radii: rc,mb for collisions within the membrane and rc,sol for collisions on the membrane surface (see Table 2 for radius parameters). (B) Disk-vesicle geometry with 4499 R (purple), 450 G (blue), and 1 (yellow) on a 0.18 μm2 surface. The diffusion trajectory (black line; and see inset) of is drawn for 2 ms. To see this figure in color, go online.
Microscopic modeling of diffusion and crowding
The simulation resolves all major disc membrane proteins explicitly and therefore, the diffusion constants used are those for a protein diffusing in a pure lipid membrane, D0. Collisions between particles lead to crowding effects: Depending on particle density, mobility, and size (43, 44, 45, 46), either normal diffusion but with a decreased diffusion constant, D, or anomalous diffusion with a time-dependent diffusion constant, D(t), is observed on long timescales (47, 48). Published values for rhodopsin and G protein correspond to such observed diffusion constants (19, 49, 50, 51, 52, 53, 54). D0 is first parametrized in the free-diffusion case by sampling and theoretical calculations (see Fig. S3 in the Supporting Material). The effect of rhodopsin architecture is investigated by comparing D0 and D.
Microscopic reaction modeling
In mass-action kinetics, bimolecular association rates, , incorporate both encounter complex formation due to diffusion (with rate kenc) and actual complex formation (with rate kmicro). Since the diffusion is simulated explicitly in the ReaDDy simulation, kmicro has to be derived from . This is necessary for G- and RG-complex formation reactions, resulting in Eqs. 2.2 and 4.2 in Fig. 1. (The other two bimolecular reactions involve the small, cytosolic molecules GDP and GTP that diffuse orders of magnitude faster than do membrane-bound proteins and are therefore considered unimolecular on our timescales). See the Supporting Material for derivation of the microscopic reaction rates and Table 2 for the resulting parameters.
Table 2.
Parameters of the ReaDDy model.
| Parameter | Value | Description |
|---|---|---|
| rvesicle | 120 nm | Radius of the simulated vesicle |
| Avesicle | 0.18 μm2 | Surface area of the simulated vesicle |
| nRa,b | 4499 | Number of rhodopsin particles on a vesicle |
| a,b | 1 | Number of activated rhodopsins on a vesicle |
| nG,1a | 49 | Initial number of Gs for first experiment simulation |
| nG,2a | 228 | Initial number of Gs for second experiment simulation |
| nG,3a,b | 454 | Initial number of Gs for third experiment simulation |
| nG,4a | 980 | Initial number of Gs for fourth experiment simulation |
| rc,R,mbc | 2.1 nm | Collision radius of R-type particles within the membrane |
| rr,Rc | 2.1 nm | Reaction radius of R-type particles |
| rc,G,mbd | 0.6 nm | Collision radius of G-type particles within the membrane |
| rc,G,sold | 3.4 nm | Collision radius of G-type particles on top of the membrane |
| rr,Gd | 3.0 nm | Reaction radius of G-type particles |
| rc,RG,mb | 2.1 nm | Collision radius of RG-type particles within the membrane |
| rc,RG,sol | 3.4 nm | Collision radius of RG-type particles on top of the membrane |
| kpote | 10kJ/mol/nm2 | Force constant of the repulsive particle-particle potential, Eq S8. |
| Δt | 20 ns | Integration timestep |
| f | 1.4 μm2/s | Diffusion constant of R in free lipid. |
| f | 2.0 μm2/s | Diffusion constant of G in free lipid. |
| f | 0.5 | Crowding factor in free diffusion at density [R]. |
| f | 0.7 μm2/s | Apparent diffusion constant of R in the free scenario. |
| f | 1.2 μm2/s | Apparent diffusion constant of G in the free scenario |
| f | 0.26 | Crowding factor in racks model at density [R]. |
| f | 0.4 μm2/s | Apparent diffusion constant of R in the racks scenario. |
| f | 0.8 μm2/s | Apparent diffusion constant of G in the racks scenario |
Results and Discussion
Free-diffusion model
We first derive a model assuming that both R and G are uniformly distributed and freely diffusing. This is the simplest assumption for the experimental preparation described in Heck and Hofmann (38), which used R and G on purified membranes.
First, the diffusion constants of R and G are parametrized. As shown in the work of Saxton (43), no percolation threshold exists if obstacles and tracers (both being rhodopsin in this case) diffuse at the same rate. In such cases, the mean-squared displacement of particles, , will start as , will show anomalous diffusion for a short period of time, and will finally display normal diffusion with (43, 44, 48, 56, 57, 58, 59) (see Klafter and Sokolov (60) for an introduction to anomalous diffusion). In our case, we operate at an occupied area fraction of , given a rhodopsin density of 25,000 μm−2. For this scenario, a crowding factor was calculated (43, 61). Our model reproduces these calculations, resulting in the diffusion parameters presented in Table 2.
Reactions in the model include the Meta1- equilibrium of light-activated receptor (Eq. 1), G activation reactions (Eq. 2) and membrane dissociation (Eq. 3). In this model, no precomplex reaction is included (Eq. 4). To get a comprehensive set of reaction rates, a set of experimental traces of different G concentrations (Table S2) was fitted simultaneously to an ODE model of the reaction scheme (Fig. 2). Two sets of reaction rates were obtained, and both fit the data (Table S4). The two sets are similar as to the rates of the initial G complex formation and dissociation (k1, k−1) and the final membrane dissociation (ksol). Once the G complex is formed, the rate-limiting step in production remains ambiguous. Placing the kinetic barrier on GDP release or the final -complex dissociation, both fit the experiments equally well (compare rate sets A and B in Table S4). It is widely accepted that GDP release is generally rate-limiting in G-protein-coupled receptor systems for nucleotide exchange reactions (see review by Johnston and Siderovsky (62)). We therefore select rate set A and use it for all further investigations and microscopic simulations.
The association of G and (by k1) is the only bimolecular reaction in our model that describes protein-protein interactions. Having parametrized all other reaction rates and model parameters, we can perform simulations with different values of , measure the resulting production, and choose the value that reproduces the experimental production rate (see the Supporting Material, especially Fig. S6, for the kmicro sampling setup). We observe that = 5000 s−1 matches experimental observations of 285 //s (for a setup of [G] = 2525 μm−2), leading to a reaction probability of 10−4 upon collision, given our chosen time discretization (see Eq. S12). The sampling reveals that the reaction does not operate at its diffusion limit, which is reached for > 106 s−1. In the limit, a production of 583 ± 86 //s is reached (see Fig. S2). At this point, GDP release is likely to become rate-limiting.
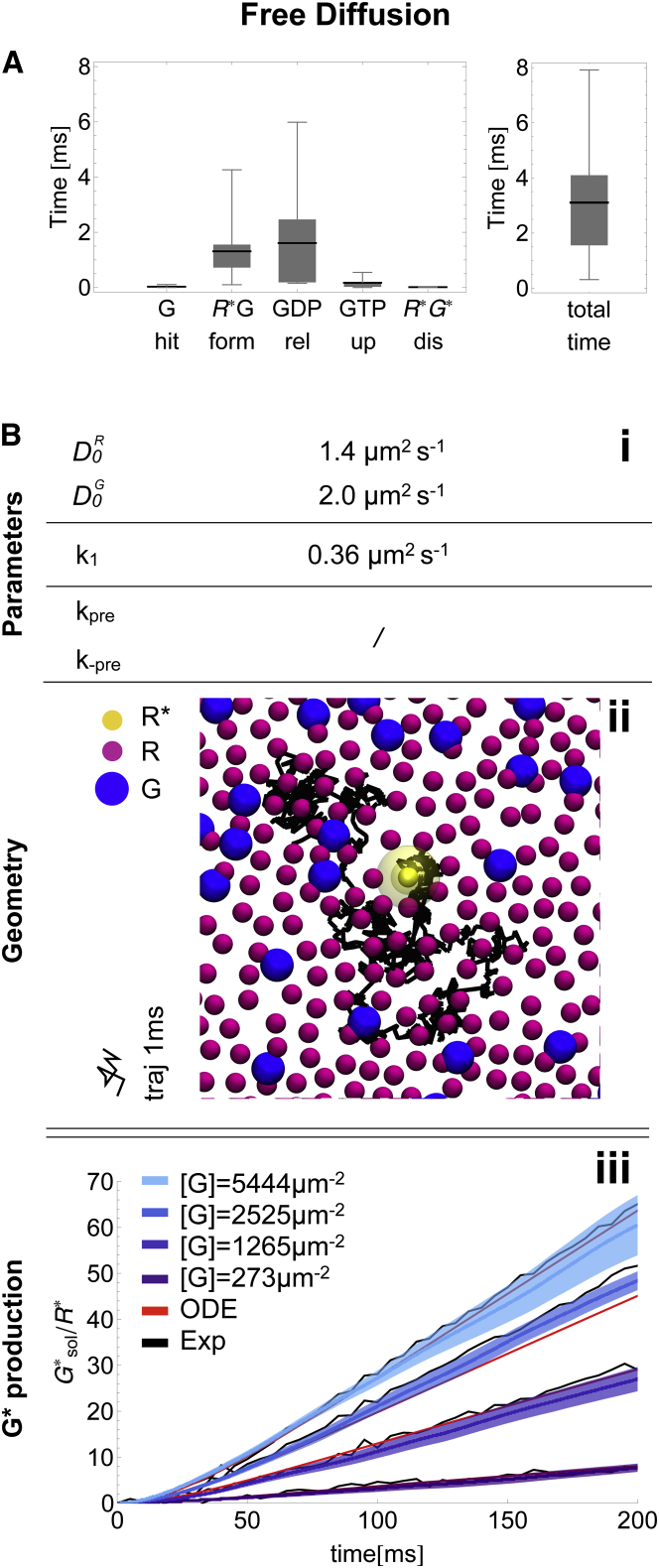
The microscopic model is now fully parametrized (see Tables 1 and 2). Simulation results are validated by comparing the kinetics of activated G-protein production () with both the ODE model and experiments. We observe that within statistical error, the model is consistent with experimental data for all concentrations (Fig. 4 A). The initial delay of the signals, observed in experiments, can be fully attributed to the Meta1-Meta2 equilibrium. The variability of the individual simulation trajectories is quite low, considering that they are consecutive single-molecule events of one activating multiple G. On the investigated timescale of 200 ms, the free-diffusion model does not exhibit significant spatial effects such as local G depletion around the location of . There is sufficient time for the system to equilibrate locally during G activation while the single active is in complex with G.
Figure 4.
Microscopic ReaDDy simulation of the free-diffusion scenario. (A) Box-whisker plot of mean times required for individual reaction steps in the G activation: hits the first free G (G hit), formation of the first G complex (G form), first GDP release (GDP rel), first GTP uptake (GTP up) and first dissociation (dis). The cumulative time of all individual steps is depicted on the righthand side. Shown are data from 12 simulations that ran for 10 ms. (B) Microscopic model. (i) Model parameters. (ii) Snapshot of the simulated geometry, containing R (purple), (yellow), and G (blue). Diffusion of is depicted for 1 ms. Note that all particles are freely diffusing, similar to the trajectory of . (iii) G activation over time. Compared are averages of 10 ReaDDy simulations (blue, standard error in light blue) with the ODE model (red) and the experimental data (black). To see this figure in color, go online.
We find a mean time of 3.11 ± 2.09 ms for the overall G activation at physiological [G] = 2525 μm−2 (Fig. 4 A). Note that this time does not include the Meta1-Meta2 equilibrium. Decomposing the mean cycle time into individual steps, we find that the first encounter between and G occurs very rapidly (0.03 ± 0.03 ms). The bottlenecks for G activation are the next two steps: the conformational change required to form the reactive G complex (1.31 ± 1.06 ms) and the subsequent GDP release (1.61 ± 1.82 ms). We can identify the complex formation as a bottleneck with high confidence, since the uncertainty of k1 is small (see Estimation of reaction rates in the Supporting Material). These two steps combined account for 94% of the average activation time. In the presence of millimolar GTP, the following GTP uptake and complex dissociation reactions occur on microsecond timescales (0.14 ± 0.11 ms and 0.02 ± 0.01 ms).
Let us compare our results with the free-diffusion limit. To probe this limit, all forward reaction rates were set to infinity and all backward reaction rates to zero (i.e., a collision event between and G immediately leads to and ). Under such conditions, we observe the initial production of 10,047 ± 331 //s (see Movie S1). This value is close to previous estimates (9590 //s after 10 ms of the flash, calculated from Lamb and Pugh (63)). In comparison, the measured activation rate is ∼300 //s (38). This emphasizes that in the free-diffusion setup, the output of the system is entirely determined by the series of chemical reactions or conformational transitions that take place during catalysis within the G complex.
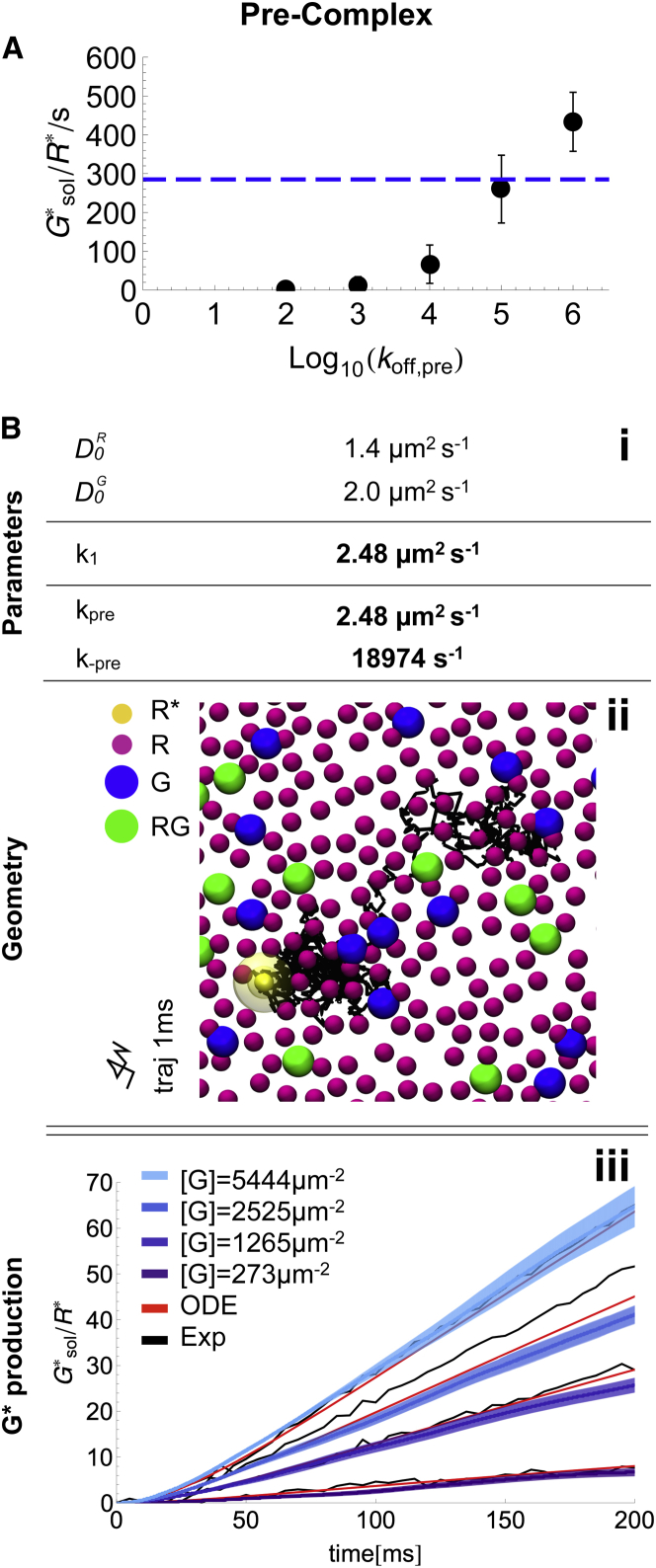
Existence of RG precomplexes
We next considered that inactive R and G could form a nonproductive precomplex (see Eq. 4 in the reaction model (Fig. 1)). Evidence for the existence of such precomplexes has been reported in several studies (21, 37, 64, 65, 66, 67, 68, 69), and it has been hypothesized that they are beneficial for G activation (37). Analogous to the formation of the reactive complex (Eqs. 2 and 2.2), formation of the inactive precomplex is a bimolecular reaction that needs to be separated into its contributions of diffusional encounter and microscopic reaction (Eq. 4.2).
In the presence of inactive precomplexes, both and must be parametrized. Generation of an ensemble of ReaDDy simulations to parametrize the two rates independently is computationally prohibitive. Therefore, we restrict ourselves to a limit analysis by setting both parameters to their diffusion limit, = = ∞. This limit is equivalent to setting the macroscopic association rates in the ODE model to their Smoluchowski rate: k1 = kpre = 1.67 μm2/s (see the Supporting Material for derivation). In the ReaDDy model, = = ∞ translates to a rate of 108 s−1 due to time discretization. Apart from including the precomplex reaction, the model setup is identical to the free-diffusion setup.
The only remaining undetermined parameter is the precomplex dissociation rate, k−pre, which together with the association rate determines the precomplex binding constant. We first consider using k−pre = 0.148/s, which was estimated from recent surface plasmon resonance measurements (37). Using this rate, the production is virtually zero and the kinetic model is thus inconsistent with the kinetic experimental data shown in Fig. 4. This is clear from the fact that k−pre = 0.148/s corresponds to precomplexes with a mean lifetime of 6.76 s—a factor of 3000 longer than the entire catalytic G activation found above. The situation is similar when the activated rhodopsin is precomplexed with G. Here, the first G gets activated faster, but owing to the fact that there is less G than R available, the system then becomes stuck while is waiting for a second free G. The time to reach a target () when multiple trap sites are around (R, to form a precomplex) has been studied by Saxton (70), who showed that traps always increase the mean time to reach the target, indicating that precomplexes cannot increase the production rate.
Precomplexes can only be consistent with measured kinetics if the precomplex is not too stable. ODE fitting reveals that the experimental production could only be reproduced with precomplex dissociation rates of at least k−pre > 11,200/s (Fig. 5 A and Supporting Material). This setting leads to a ratio of 20% free G to 80% RG precomplexes. Note that k−pre = 11,200/s is a lower bound to the dissociation rate; when the association rates k1 and kpre are smaller than their diffusion limits, the precomplex dissociation rate must be even larger to keep the overall production consistent with measured kinetics.
Figure 5.
Microscopic ReaDDy simulation of the pre-complex scenario. (A) Dependence of the production rate on the pre-complex off rate, given that the on rate is in the diffusion limit (simulations ran for 10 ms, depicted are mean and standard error). The dashed blue line indicates the experimentally found production rate. (B) Microscopic model: (i) model parameters, (bold) parameters different to the free diffusion scenario. (ii) Snapshot of the simulated geometry, containing R (purple), (yellow), G (blue) and RG-precomplex (green) particles. The diffusion of is depicted for 1ms. Note that all particles are freely diffusing similar to the trajectory of . (iii) G activation over time. Averages of 10 ReaDDy simulations (blue, with standard error (light blue)) are compared with the ODE model (red) and the experimental data (black). To see this figure in color, go online.
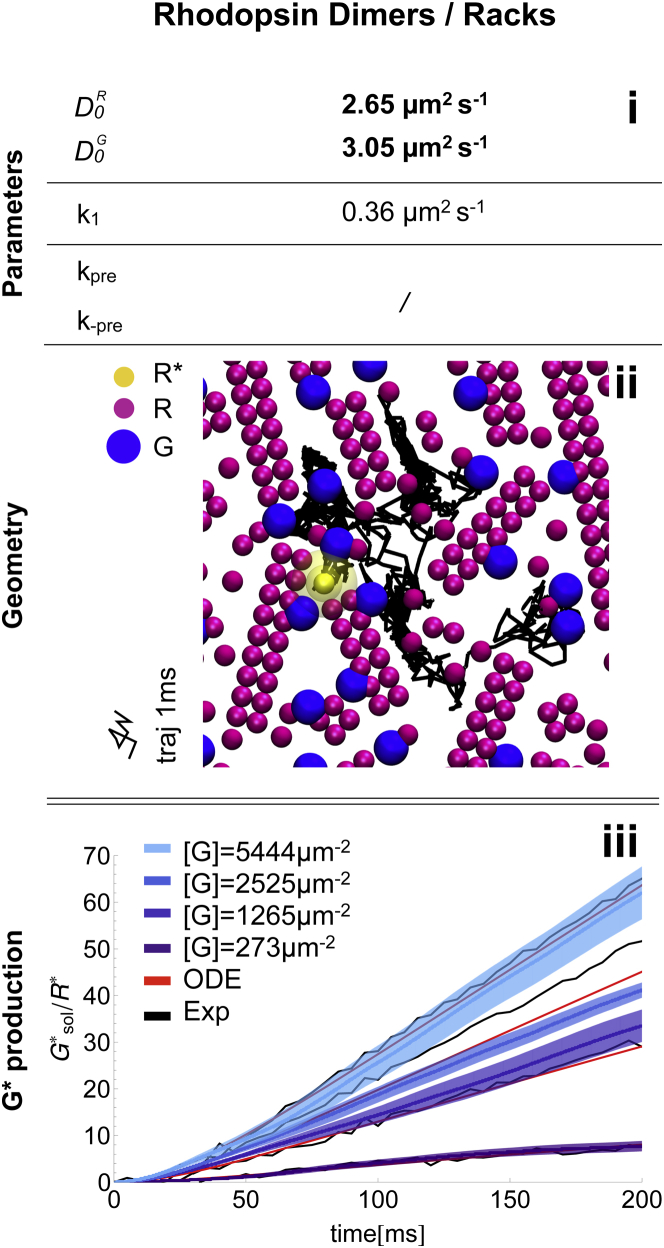
Existence of racks of rhodopsin dimers
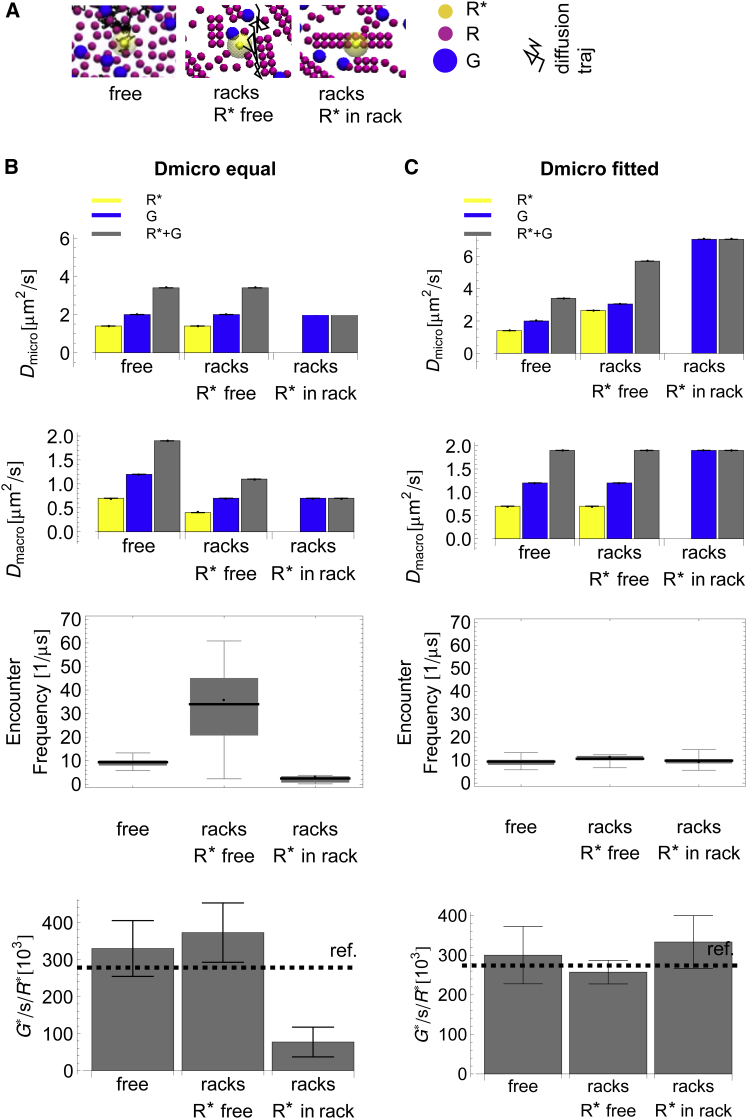
Using the microscopic model, we test the molecular mobility and production when R is arranged in rows of dimers. For the construction of a putative R arrangement, we combine experimental observations from Fotiadis et al. (20) (racks of rhodopsin dimers) and Govardovski et al. (19) (immobile rhodopsin fractions). Both observations are consistent, because supramolecular R structures would be expected to diffuse much more slowly than single free R molecules. In our model, 80% of the R molecules are immobilized and assigned to racks of different sizes (i.e., governed by a distribution derived from Fotiadis et al. (20) (see the Supporting Material), whereas individual rhodopsins and all G proteins are freely diffusing. Note, that diffusion of G through the racks is not possible in our simulation setup.
In this geometry, a photon could activate an immobilized R that is part of a rack or an R that is freely diffusing. Position and mobility of the single in the simulation is consequently modeled for these two cases (see Fig. 6 A and Fig. S8 C).
Figure 6.
Dependence of geometry on free lipid (D0) and apparent diffusion constant (D), encounter frequency, and production rate. (A) In the free-diffusion case (free), R (purple), (yellow), and G (blue) are all mobile. In the rack geometry, 80% of R is set as immobile in racks of dimers, whereas the other molecules diffuse freely. Here, can be part of the mobile fraction (racksfree) or be part of an immobile rack (racksin rack). (B and C) Dependency of D0 (first row), D (second row), -G encounter rate (third row), and production, given the geometries in A. Note the smaller scale of the y axis between the plots of free lipid and apparent diffusion constant. In B, the D0 of the free-diffusion case is imposed for all geometries. Consequently, the crowding effects of the geometry can be seen in the apparent diffusion constant and the resulting encounter and -production rates. In C, the crowding effects of the geometry are compensated by imposing higher free-lipid-diffusion constants on the particles (note that the diffusion of G is set to compensate the loss of diffusion contribution from the immobilized R in the in rack case). As a consequence, apparent diffusion, encounter frequency, and production are similar in all geometries. All data shown are averages and standard errors of six simulations per scenario. Simulation timescales are 100 μs for diffusion analysis, 1 ms for encounter analysis, and 10 ms for catalysis. To see this figure in color, go online.
The effects of immobile clustered obstacles on diffusion have been investigated in several theoretical studies (44, 57, 58, 71). It was shown by Saxton (43) that a percolation threshold, , exists when the occupied area fraction of obstacles, c, exceeds 0.332. If , the obstacles separate the diffusion-accessible space into smaller subspaces. In such a scenario, the observed diffusion coefficient, D, is distance-dependent and anomalous. In our model, the immobile obstacles occupy an area fraction of and the diffusion-accessible space is fully connected. The behavior of for this mixture of mobile and immobile obstacles was found to be similar to that in the free-diffusion case described above, leading to a crowding factor of (45). We reproduce these findings in our model and obtain = 0.42 μm2 s−1 and = 0.77 μm2 s−1. Note that a higher R density (e.g., the local density of 50,000 μm−2 reported in Fotiadis et al. (20)) would result in the system crossing the percolation threshold and likely showing anomalous diffusion.
It has been shown that obstacles can increase educt encounter rates (71). In our case, the G encounter rate was increased threefold relative to the free-diffusion case. However, this effect was countered by slower diffusion due to obstacle-related crowding. The two effects combined result in a production only slightly higher than that for free diffusion (Fig. 6 B, second column).
The effects are different when is part of an immobile rack (Fig. 6 B, third column). Now, is only accessible from one side and the G encounter rate is solely due to the mobility of G, leading consequently to an approximately fivefold decrease in encounters compared to the number observed in free diffusion. This results in an approximately threefold decrease in production. In effect, the racks geometry led to a slight increase in catalysis when is part of the mobile fraction and to a drastic decrease when is part of the immobile fraction.
In the next step, we reparametrized the free lipid diffusion constants, , of R and G such that the effective joint diffusion constant of the free-diffusion case was reached (, see Fig. 6 C, second row). For these cases, we observe the same encounter rates in all three scenarios, as well as the same production rate. We simulated the reparametrized rack case with the mobile for 200 ms and found a very good agreement with both the ODE model and the experimental data (Fig. 7 lower).
Figure 7.
Microscopic ReaDDy simulation of the racks of rhodopsin dimers scenario. (i) Model parameters, highlighting (bold) parameters different from the free-diffusion scenario. (ii) Snapshot of the simulated geometry, containing R (purple), (yellow), and G (blue). Note that only G, monomeric R, and particles are freely diffusing. R oligomers are considered immobile. (iii) G activation over time. Averages of 10 ReaDDy simulations (blue, with standard error in light blue) are compared with the ODE model (red) and the experimental data (black). To see this figure in color, go online.
In summary, racks of rhodopsin dimers are also consistent with available kinetic data when the free lipid diffusion constants are chosen to be slightly higher than the respective values predicted by theory. The confinement conditions of racks slightly increases production on short timescales if the activated receptor is mobile. If is part of a rack (i.e., it is immobile), the rack geometry decreases catalysis substantially, a reduction that can only be compensated by high G-diffusion constants. Although both cases, i.e., mobile and immobile, could be made consistent with kinetic data individually, the occurrence of both scenarios on the same disk membrane would pose a challenge. Given these results, fractions of mobile and immobile rhodopsin on the same disk simultaneously would lead to a higher variance of the output rate, compared to the case of a homogenous geometry.
Conclusion
We have presented a particle-based reaction-diffusion model for light-induced, rhodopsin-mediated activation of G-proteins. Although we have chosen rod cell phototransduction as an example, for which there is a large body of experimental data, the results should be applicable to G-protein activation in general. The kinetic parameters of the model were parametrized to fit observed effective diffusion constants (see Pugh and Lamb (49)) and the extensive G-activation kinetics measurements of Heck and Hofmann (38) via an ODE model. The model was implemented in and simulated with the particle-based reaction-diffusion software ReaDDy (36). The free-diffusion parametrization could quantitatively reproduce production during the first 200 ms after photoactivation of for a set of different initial G concentrations. The main observations for this model are as follows. 1), Diffusional G- encounter is fast, consistent with previous predictions that the typical production rates (on the order of several hundred per second) are well below the diffusion limit (which would be on the order of 10,000/s). 2), Due to the high protein density in the disk membrane, the mobility of R and G is reduced compared to diffusion in a pure lipid membrane (to 0.5 D0). 3), The conformational change required to form an active G complex after and G encounter is a bottleneck, requiring a lower millisecond timescale. 4), It is likely that GDP release from the G complex is the second bottleneck of the overall catalysis. Findings 3 and 4 define interesting targets for future investigations: With the advent of high-throughput computing technologies, such as special-purpose hardware (72), massive distribution computing (73, 74, 75), and Markov state models (76, 77, 78, 79), molecular dynamics simulations are now able to describe processes on the millisecond timescale in full atomistic detail. Investigation of the detailed mechanisms of the conformational change and the GDP release in would shed light on two steps that are important for the kinetics of phototransduction.
We have considered the existence of a nonproductive RG precomplex formation: . The existence of such precomplexes has been suggested by others (21, 37, 64, 65, 66, 67, 68, 69). Here, we found that the existence of precomplexes is consistent with our kinetic data if they are sufficiently transient. In particular, the precomplex dissociation rate needs to be on the order of 10,000 s−1 or larger. This bound arises from the fact that longer-lived precomplexes would arrest the system in an inactive state dominated by the waiting time of for G proteins stuck at inactive rhodopsins and would consequently reduce production to values incompatible with the present kinetic experimental data, a case referred to as inhibition by substrate depletion in enzyme kinetics. The existence of transient RG precomplexes must be compensated by increasing the rates in the rate-limiting steps described above. In particular, the conformational change could proceed somewhat faster, e.g., on the 100 μs timescale.
Please note that under no circumstances can the existence of precomplexes increase the production rate of , independent of whether the activated is initially precomplexed with a G or not (see Saxton (70) for a theoretical study on the effect of traps on the way to a target). If precomplexes exist, they may be due to side effects (since G has interactions that form a stable complex, it is reasonable that some of these interactions are also present in RG, forming transient complexes), but precomplexes may also play a role in the regulation of signal transduction, e.g., by limiting the output.
We have also considered the situation that rhodopsin forms rows of dimers (racks), as observed by Fotiadis et al. (20). One rhodopsin fraction (20%) consists of individual freely diffusing particles, and the other fraction (80%) is fixed in racks of dimers. In this model, each row of dimers is densely packed such that G cannot diffuse through it. This setup reduces the diffusional mobility of R and G by a factor of 2 more strongly than in the free-diffusion case (to 0.26D0), a behavior that has already been suggested in theoretical studies (61). If the single is considered to be not part of a rack, the encounter rate between and G is increased due to confinement compared to the free-diffusion case (also compare to Soula et al. (71)). This increase is compensated by the decreased long-range diffusion through racks. If is considered to be part of a rack, the lower accessibility of , its immobility, and the decrease in long-range diffusion together drastically decrease the -G encounter frequency, as well as formation. Note that our tested scenarios have an R density of 25,000 μm−2 and are therefore below the percolation threshold (i.e., the free diffusion space is fully connected). Higher R densities would likely partition the disc membrane and lead to anomalous diffusion (56, 59). In summary, as has been shown in experiments (80), the diffusion conditions and the microscopic environment on the disk membrane have a strong effect on the -G encounter frequency and therefore on the output of the cascade.
We find that all these effects can be compensated for if the free lipid diffusion constants (D0) are adapted to the geometry so as to reproduce published (49) apparent diffusion constants. Compensation is possible even if is immobilized completely. In this case, G-protein diffusion can make up for the encounter events alone if it is given a high free lipid diffusion constant (2.3). Such values might be possible (81), especially given that the slow membrane diffusion is likely only affecting the relatively small anchor of G-proteins.
Compensation is only possible for uniform conditions on the disk membrane. In a setup in which both scenarios coexist, i.e., fractions of free and immobile R on the disk membrane, it is difficult to compensate for the effects of both conditions at the same time. Such a situation could be experimentally detected by different formation rates for different individual photon events, depending on whether is freely diffusing or immobile.
Acknowledgments
This work was funded by Deutsche Forschungsgemeinschaft through SFB 740 to F.N., M.H., and K.P.H., a European Research Council starting grant (pcCell) to F.N., and a European Research Council advanced grant (TUDOR) to K.P.H.
Editor: Reinhard Lipowsky.
Footnotes
Five tables, eight figures, one movie and Supporting Methods are available at http://www.biophysj.org/biophysj/supplemental/S0006-3495(14)00690-0.
Contributor Information
Martin Heck, Email: martin.heck@charite.de.
Frank Noé, Email: frank.noe@fu-berlin.de.
Supporting Citations
References (82, 83, 84, 85, 86, 87, 88, 89) appear in the Supporting Material.
Supporting Material
References
- 1.Kruse A.C., Ring A.M., Kobilka B.K. Activation and allosteric modulation of a muscarinic acetylcholine receptor. Nature. 2013;504:101–106. doi: 10.1038/nature12735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hofmann K.P., Scheerer P., Ernst O.P. A G protein-coupled receptor at work: the rhodopsin model. Trends Biochem. Sci. 2009;34:540–552. doi: 10.1016/j.tibs.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 3.Elgeti M., Rose A.S., Heck M. Precision vs. flexibility in GPCR signaling. J. Am. Chem. Soc. 2013;135:12305–12312. doi: 10.1021/ja405133k. [DOI] [PubMed] [Google Scholar]
- 4.Hofmann K.P., Spahn C.M., Heinemann U. Building functional modules from molecular interactions. Trends Biochem. Sci. 2006;31:497–508. doi: 10.1016/j.tibs.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 5.Baylor D.A., Hodgkin A.L., Lamb T.D. The electrical response of turtle cones to flashes and steps of light. J. Physiol. 1974;242:685–727. doi: 10.1113/jphysiol.1974.sp010731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lamb T.D. Gain and kinetics of activation in the G-protein cascade of phototransduction. Proc. Natl. Acad. Sci. USA. 1996;93:566–570. doi: 10.1073/pnas.93.2.566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hamer R., Nicholas S., Tranchina D., Jarvinen J. Toward a unified model of vertebrate rod phototransduction. Vis. Neurosci. 2005;22:417–436. doi: 10.1017/S0952523805224045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Invergo B.M., Dell’Orco D., Bertranpetit J. A comprehensive model of the phototransduction cascade in mouse rod cells. Mol. Biosyst. 2014;10:1481–1489. doi: 10.1039/c3mb70584f. [DOI] [PubMed] [Google Scholar]
- 9.Lamb T.D. Stochastic simulation of activation in the G-protein cascade of phototransduction. Biophys. J. 1994;67:1439–1454. doi: 10.1016/S0006-3495(94)80617-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Felber S., Breuer H.P., Hofmann K.P. Stochastic simulation of the transducin GTPase cycle. Biophys. J. 1996;71:3051–3063. doi: 10.1016/S0006-3495(96)79499-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Andreucci D., Bisegna P., DiBenedetto E. Mathematical model of the spatio-temporal dynamics of second messengers in visual transduction. Biophys. J. 2003;85:1358–1376. doi: 10.1016/S0006-3495(03)74570-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Caruso G., Khanal H., DiBenedetto E. Mathematical and computational modelling of spatio-temporal signalling in rod phototransduction. Syst. Biol. 2005;152:119–137. doi: 10.1049/ip-syb:20050019. [DOI] [PubMed] [Google Scholar]
- 13.Caruso G., Bisegna P., DiBenedetto E. Modeling the role of incisures in vertebrate phototransduction. Biophys. J. 2006;91:1192–1212. doi: 10.1529/biophysj.106.083618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Straube R., Ward M.J. An asymptotic analysis of intracellular signaling gradients arising from multiple small compartments. SIAM J. Appl. Math. 2009;70:248–269. [Google Scholar]
- 15.Coombs D., Straube R., Ward M. Diffusion on a sphere with localized traps: mean first passage time, eigenvalue asymptotics, and Fekete points. SIAM J. Appl. Math. 2009;70:302–332. [Google Scholar]
- 16.Pillay S., Ward M.J., Kolokolnikov T. An asymptotic analysis of the mean first passage time for narrow escape problems: Part I: Two-dimensional domains. Multiscale Model. Simul. 2009;8:803–835. [Google Scholar]
- 17.Cheviakov A.F., Ward M.J., Straube R. An asymptotic analysis of the mean first passage time for narrow escape problems: Part II: The sphere. Multiscale Model. Simul. 2009;8:836–870. [Google Scholar]
- 18.Buzhynskyy N., Salesse C., Scheuring S. Rhodopsin is spatially heterogeneously distributed in rod outer segment disk membranes. J. Mol. Recognit. 2011;24:483–489. doi: 10.1002/jmr.1086. [DOI] [PubMed] [Google Scholar]
- 19.Govardovskii V.I., Korenyak D.A., Zueva L.V. Lateral diffusion of rhodopsin in photoreceptor membrane: a reappraisal. Mol. Vis. 2009;15:1717–1729. [PMC free article] [PubMed] [Google Scholar]
- 20.Fotiadis D., Liang Y., Palczewski K. Atomic-force microscopy: rhodopsin dimers in native disc membranes. Nature. 2003;421:127–128. doi: 10.1038/421127a. [DOI] [PubMed] [Google Scholar]
- 21.Dell’Orco D., Schmidt H. Mesoscopic Monte Carlo simulations of stochastic encounters between photoactivated rhodopsin and transducin in disc membranes. J. Phys. Chem. B. 2008;112:4419–4426. doi: 10.1021/jp709963f. [DOI] [PubMed] [Google Scholar]
- 22.Byrne M.J., Waxham M.N., Kubota Y. Cellular dynamic simulator: an event driven molecular simulation environment for cellular physiology. Neuroinformatics. 2010;8:63–82. doi: 10.1007/s12021-010-9066-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sanford C., Yip M.L., Parkinson J. Cell++—simulating biochemical pathways. Bioinformatics. 2006;22:2918–2925. doi: 10.1093/bioinformatics/btl497. [DOI] [PubMed] [Google Scholar]
- 24.Takahashi K., Tanase-Nicola S., ten Wolde P.R. Spatio-temporal correlations can drastically change the response of a MAPK pathway. Proc. Natl. Acad. Sci. USA. 2010;107:2473–2478. doi: 10.1073/pnas.0906885107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van Zon J.S., ten Wolde P.R. Green’s-function reaction dynamics: a particle-based approach for simulating biochemical networks in time and space. J. Chem. Phys. 2005;123:234910. doi: 10.1063/1.2137716. [DOI] [PubMed] [Google Scholar]
- 26.van Zon J.S.J., ten Wolde P.R.P. Simulating biochemical networks at the particle level and in time and space: Green’s function reaction dynamics. Phys. Rev. Lett. 2005;94:128103. doi: 10.1103/PhysRevLett.94.128103. [DOI] [PubMed] [Google Scholar]
- 27.Yachie-Kinoshita A., Nishino T., Tomita M. A metabolic model of human erythrocytes: practical application of the E-Cell Simulation Environment. J. Biomed. Biotechnol. 2010;2010:642420. doi: 10.1155/2010/642420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Klann M.T., Lapin A., Reuss M. Agent-based simulation of reactions in the crowded and structured intracellular environment: influence of mobility and location of the reactants. BMC Syst. Biol. 2011;5:71. doi: 10.1186/1752-0509-5-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Klann M., Koeppl H., Reuss M. Spatial modeling of vesicle transport and the cytoskeleton: the challenge of hitting the right road. PLoS ONE. 2012;7:e29645. doi: 10.1371/journal.pone.0029645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stiles J.R., Van Helden D., Salpeter M.M. Miniature endplate current rise times less than 100 microseconds from improved dual recordings can be modeled with passive acetylcholine diffusion from a synaptic vesicle. Proc. Natl. Acad. Sci. USA. 1996;93:5747–5752. doi: 10.1073/pnas.93.12.5747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kerr R.A., Bartol T.M., Stiles J.R. Fast Monte Carlo simulation methods for biological reaction-diffusion systems in solution and on surfaces. SIAM J. Sci. Comput. 2008;30:3126. doi: 10.1137/070692017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ridgway D., Broderick G., Ellison M.J. Coarse-grained molecular simulation of diffusion and reaction kinetics in a crowded virtual cytoplasm. Biophys. J. 2008;94:3748–3759. doi: 10.1529/biophysj.107.116053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Andrews S.S., Bray D. Stochastic simulation of chemical reactions with spatial resolution and single molecule detail. Phys. Biol. 2004;1:137–151. doi: 10.1088/1478-3967/1/3/001. [DOI] [PubMed] [Google Scholar]
- 34.Andrews S., Addy N., Brent R. Detailed simulations of cell biology with smoldyn 2.1. PLoS Computational Biol. 2010;6:e1000705. doi: 10.1371/journal.pcbi.1000705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gruenert G., Ibrahim B., Dittrich P. Rule-based spatial modeling with diffusing, geometrically constrained molecules. BMC Bioinformatics. 2010;11:307. doi: 10.1186/1471-2105-11-307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schöneberg J., Noé F. ReaDDy—a software for particle-based reaction-diffusion dynamics in crowded cellular environments. PLoS ONE. 2013;8:e74261. doi: 10.1371/journal.pone.0074261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dell’Orco D., Koch K.-W. A dynamic scaffolding mechanism for rhodopsin and transducin interaction in vertebrate vision. Biochem. J. 2011;440:263–271. doi: 10.1042/BJ20110871. [DOI] [PubMed] [Google Scholar]
- 38.Heck M., Hofmann K.P. Maximal rate and nucleotide dependence of rhodopsin-catalyzed transducin activation: initial rate analysis based on a double displacement mechanism. J. Biol. Chem. 2001;276:10000–10009. doi: 10.1074/jbc.M009475200. [DOI] [PubMed] [Google Scholar]
- 39.Okada T., Sugihara M., Buss V. The retinal conformation and its environment in rhodopsin in light of a new 2.2 Å crystal structure. J. Mol. Biol. 2004;342:571–583. doi: 10.1016/j.jmb.2004.07.044. [DOI] [PubMed] [Google Scholar]
- 40.Choe H.-W., Kim Y.J., Ernst O.P. Crystal structure of metarhodopsin II. Nature. 2011;471:651–655. doi: 10.1038/nature09789. [DOI] [PubMed] [Google Scholar]
- 41.Lambright D.G., Sondek J., Sigler P.B. The 2.0 Å crystal structure of a heterotrimeric G protein. Nature. 1996;379:311–319. doi: 10.1038/379311a0. [DOI] [PubMed] [Google Scholar]
- 42.Rasmussen S.G.F., DeVree B.T., Kobilka B.K. Crystal structure of the β2 adrenergic receptor-Gs protein complex. Nature. 2011;477:549–555. doi: 10.1038/nature10361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Saxton M.J. Lateral diffusion in an archipelago. The effect of mobile obstacles. Biophys. J. 1987;52:989–997. doi: 10.1016/S0006-3495(87)83291-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Saxton M.J. Lateral diffusion in an archipelago. Distance dependence of the diffusion coefficient. Biophys. J. 1989;56:615–622. doi: 10.1016/S0006-3495(89)82708-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Saxton M.J. Lateral diffusion in a mixture of mobile and immobile particles. A Monte Carlo study. Biophys. J. 1990;58:1303–1306. doi: 10.1016/S0006-3495(90)82470-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Saxton M.J. Lateral diffusion in an archipelago. Dependence on tracer size. Biophys. J. 1993;64:1053–1062. doi: 10.1016/S0006-3495(93)81471-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Metzler R., Nonnenmacher T.F. Space-and time-fractional diffusion and wave equations, fractional Fokker-Planck equations, and physical motivation. Chem. Phys. 2002;284:67–90. [Google Scholar]
- 48.Saxton M.J. A biological interpretation of transient anomalous subdiffusion. I. Qualitative model. Biophys. J. 2007;92:1178–1191. doi: 10.1529/biophysj.106.092619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pugh E.N., Jr., Lamb T.D. Amplification and kinetics of the activation steps in phototransduction. Biochim. Biophys. Acta. 1993;1141:111–149. doi: 10.1016/0005-2728(93)90038-h. [DOI] [PubMed] [Google Scholar]
- 50.Liebman P.A., Entine G. Lateral diffusion of visual pigment in photorecptor disk membranes. Science. 1974;185:457–459. doi: 10.1126/science.185.4149.457. [DOI] [PubMed] [Google Scholar]
- 51.Takezoe H., Yu H. Lateral diffusion of photopigments in photoreceptor disk membrane vesicles by the dynamic Kerr effect. Biochemistry. 1981;20:5275–5281. doi: 10.1021/bi00521a028. [DOI] [PubMed] [Google Scholar]
- 52.Gupta B.D., Williams T.P. Lateral diffusion of visual pigments in toad (Bufo marinus) rods and in catfish (Ictalurus punctatus) cones. J. Physiol. 1990;430:483–496. doi: 10.1113/jphysiol.1990.sp018303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang Q., Zhang X., Wensel T.G. Activation-dependent hindrance of photoreceptor G protein diffusion by lipid microdomains. J. Biol. Chem. 2008;283:30015–30024. doi: 10.1074/jbc.M803953200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Najafi M., Haeri M., Calvert P. Impact of signaling microcompartment geometry on GPCR dynamics in live retinal photoreceptors. J. Gen. Physiol. 2012;140:249–266. doi: 10.1085/jgp.201210818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Salom D., Lodowski D.T., Palczewski K. Crystal structure of a photoactivated deprotonated intermediate of rhodopsin. Proc. Natl. Acad. Sci. USA. 2006;103:16123–16128. doi: 10.1073/pnas.0608022103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Saxton M.J. Anomalous diffusion due to obstacles: a Monte Carlo study. Biophys. J. 1994;66:394–401. doi: 10.1016/s0006-3495(94)80789-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Javanainen M., Hammaren H., Vattulainen I. Anomalous and normal diffusion of proteins and lipids in crowded lipid membranes. Faraday Discuss. 2013;161:397–417. doi: 10.1039/c2fd20085f. discussion 419–459. [DOI] [PubMed] [Google Scholar]
- 58.Ehrig J., Petrov E.P., Schwille P. Near-critical fluctuations and cytoskeleton-assisted phase separation lead to subdiffusion in cell membranes. Biophys. J. 2011;100:80–89. doi: 10.1016/j.bpj.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Berry H., Chaté H. Anomalous diffusion due to hindering by mobile obstacles undergoing Brownian motion or Orstein-Ulhenbeck processes. Phys. Rev. E Stat. Nonlin. Soft Matter Phys. 2014;89:022708. doi: 10.1103/PhysRevE.89.022708. [DOI] [PubMed] [Google Scholar]
- 60.Klafter J., Sokolov I.M. Anomalous diffusion spreads its wings. Physics world. 2005;August:29–32. [Google Scholar]
- 61.Saxton M.J., Owicki J.C. Concentration effects on reactions in membranes: rhodopsin and transducin. Biochim. Biophys. Acta. 1989;979:27–34. doi: 10.1016/0005-2736(89)90519-1. [DOI] [PubMed] [Google Scholar]
- 62.Johnston C.A., Siderovski D.P. Receptor-mediated activation of heterotrimeric G-proteins: current structural insights. Mol. Pharmacol. 2007;72:219–230. doi: 10.1124/mol.107.034348. [DOI] [PubMed] [Google Scholar]
- 63.Lamb T.D., Pugh E.N., Jr. A quantitative account of the activation steps involved in phototransduction in amphibian photoreceptors. J. Physiol. 1992;449:719–758. doi: 10.1113/jphysiol.1992.sp019111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Alves I.D., Salgado G.F.J., Hruby V.J. Phosphatidylethanolamine enhances rhodopsin photoactivation and transducin binding in a solid supported lipid bilayer as determined using plasmon-waveguide resonance spectroscopy. Biophys. J. 2005;88:198–210. doi: 10.1529/biophysj.104.046722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dell’Orco D. A physiological role for the supramolecular organization of rhodopsin and transducin in rod photoreceptors. FEBS Lett. 2013;587:2060–2066. doi: 10.1016/j.febslet.2013.05.017. [DOI] [PubMed] [Google Scholar]
- 66.Fanelli F., Dell’Orco D. Rhodopsin activation follows precoupling with transducin: inferences from computational analysis. Biochemistry. 2005;44:14695–14700. doi: 10.1021/bi051537y. [DOI] [PubMed] [Google Scholar]
- 67.Fanelli F., Dell’orco D. Dark and photoactivated rhodopsin share common binding modes to transducin. FEBS Lett. 2008;582:991–996. doi: 10.1016/j.febslet.2008.02.041. [DOI] [PubMed] [Google Scholar]
- 68.Hamm H.E., Deretic D., Kohl B. Mechanism of action of monoclonal antibodies that block the light activation of the guanyl nucleotide-binding protein, transducin. J. Biol. Chem. 1987;262:10831–10838. [PubMed] [Google Scholar]
- 69.Kim T.Y., Uji-i H., Alexiev U. Monitoring the interaction of a single G-protein key binding site with rhodopsin disk membranes upon light activation. Biochemistry. 2009;48:3801–3803. doi: 10.1021/bi900308c. [DOI] [PubMed] [Google Scholar]
- 70.Saxton M.J. A biological interpretation of transient anomalous subdiffusion. II. Reaction kinetics. Biophys. J. 2008;94:760–771. doi: 10.1529/biophysj.107.114074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Soula H., Caré B., Berry H. Anomalous versus slowed-down Brownian diffusion in the ligand-binding equilibrium. Biophys. J. 2013;105:2064–2073. doi: 10.1016/j.bpj.2013.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shaw D.E., Maragakis P., Wriggers W. Atomic-level characterization of the structural dynamics of proteins. Science. 2010;330:341–346. doi: 10.1126/science.1187409. [DOI] [PubMed] [Google Scholar]
- 73.Shirts M., Pande V.S. COMPUTING: screen savers of the world unite! Science. 2000;290:1903–1904. doi: 10.1126/science.290.5498.1903. [DOI] [PubMed] [Google Scholar]
- 74.Harvey M.J., Giupponi G., Fabritiis G.D. ACEMD: accelerating biomolecular dynamics in the microsecond time scale. J. Chem. Theory Comput. 2009;5:1632–1639. doi: 10.1021/ct9000685. [DOI] [PubMed] [Google Scholar]
- 75.Eastman P., Friedrichs M.S., Pande V.S. OpenMM 4: a reusable, extensible, hardware independent library for high performance molecular simulation. J. Chem. Theory Comput. 2012;9:461–469. doi: 10.1021/ct300857j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Swope W.C., Pitera J.W., Suits F. Describing protein folding kinetics by molecular dynamics simulations: 1. Theory. J. Phys. Chem. B. 2004;108:6571–6581. [Google Scholar]
- 77.Schütte C., Fischer A., Deuflhard P. A direct approach to conformational dynamics based on hybrid Monte Carlo. J. Comput. Phys. 1999;151:146–168. [Google Scholar]
- 78.Noé F., Horenko I., Smith J.C. Hierarchical analysis of conformational dynamics in biomolecules: transition networks of metastable states. J. Chem. Phys. 2007;126:155102. doi: 10.1063/1.2714539. [DOI] [PubMed] [Google Scholar]
- 79.Noé F., Schütte C., Weikl T.R. Constructing the equilibrium ensemble of folding pathways from short off-equilibrium simulations. Proc. Natl. Acad. Sci. USA. 2009;106:19011–19016. doi: 10.1073/pnas.0905466106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Calvert P.D., Govardovskii V.I., Makino C.L. Membrane protein diffusion sets the speed of rod phototransduction. Nature. 2001;411:90–94. doi: 10.1038/35075083. [DOI] [PubMed] [Google Scholar]
- 81.Chang C.H., Takeuchi H., Ohnishi S. Lateral mobility of erythrocyte membrane proteins studied by the fluorescence photobleaching recovery technique. J. Biochem. 1981;90:997–1004. doi: 10.1093/oxfordjournals.jbchem.a133586. [DOI] [PubMed] [Google Scholar]
- 82.Segel I.H. John Wiley & Sons; New York: 1975. Enzyme Kinetics. [Google Scholar]
- 83.Kühn H., Bennett N., Chabre M. Interactions between photoexcited rhodopsin and GTP-binding protein: kinetic and stoichiometric analyses from light-scattering changes. Proc. Natl. Acad. Sci. USA. 1981;78:6873–6877. doi: 10.1073/pnas.78.11.6873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Heck M., Pulvermüller A., Hofmann K.P. Light scattering methods to monitor interactions between rhodopsin-containing membranes and soluble proteins. Methods Enzymol. 2000;315:329–347. doi: 10.1016/s0076-6879(00)15852-5. [DOI] [PubMed] [Google Scholar]
- 85.Parkes J.H., Liebman P.A. Temperature and pH dependence of the metarhodopsin I-metarhodopsin II kinetics and equilibria in bovine rod disk membrane suspensions. Biochemistry. 1984;23:5054–5061. doi: 10.1021/bi00316a035. [DOI] [PubMed] [Google Scholar]
- 86.Jäger S., Szundi I., Kliger D.S. Effects of pH on rhodopsin photointermediates from lumirhodopsin to metarhodopsin II. Biochemistry. 1998;37:6998–7005. doi: 10.1021/bi9728194. [DOI] [PubMed] [Google Scholar]
- 87.Seitz H.R., Heck M., Seelig A. Molecular determinants of the reversible membrane anchorage of the G-protein transducin. Biochemistry. 1999;38:7950–7960. doi: 10.1021/bi990298+. [DOI] [PubMed] [Google Scholar]
- 88.Kung C.E., Reed J.K. Microviscosity measurements of phospholipid bilayers using fluorescent dyes that undergo torsional relaxation. Biochemistry. 1986;25:6114–6121. [Google Scholar]
- 89.Erban R., Chapman S.J. Stochastic modelling of reaction-diffusion processes: algorithms for bimolecular reactions. Phys. Biol. 2009;6:046001. doi: 10.1088/1478-3975/6/4/046001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.