Abstract
The pathogenesis of nonalcoholic steatohepatitis (NASH) and inflammasome activation involves sequential hits. The inflammasome, which cleaves pro–interleukin-1β (pro–IL-1β) into secreted IL-1β, is induced by endogenous and exogenous danger signals. Lipo-polysaccharide (LPS), a toll-like receptor 4 ligand, plays a role in NASH and also activates the inflammasome. In this study, we hypothesized that the inflammasome is activated in NASH by multiple hits involving endogenous and exogenous danger signals. Using mouse models of methionine choline–deficient (MCD) diet–induced NASH and high-fat diet–induced NASH, we found up-regulation of the inflammasome [including NACHT, LRR, and PYD domains–containing protein 3 (NALP3; cryopyrin), apoptosis-associated specklike CARD-domain containing protein, pannexin-1, and pro–caspase-1] at the messenger RNA (mRNA) level increased caspase-1 activity, and mature IL-1β protein levels in mice with steatohepatitis in comparison with control livers. There was no inflammasome activation in mice with only steatosis. The MCD diet sensitized mice to LPS-induced increases in NALP3, pannexin-1, IL-1β mRNA, and mature IL-1β protein levels in the liver. We demonstrate for the first time that inflammasome activation occurs in isolated hepatocytes in steatohepatitis. Our novel data show that the saturated fatty acid (FA) palmitic acid (PA) activates the inflammasome and induces sensitization to LPS-induced IL-1β release in hepatocytes. Furthermore, PA triggers the release of danger signals from hepatocytes in a caspase-dependent manner. These hepatocyte-derived danger signals, in turn, activate inflammasome, IL-1β, and tumor necrosis factor α release in liver mononuclear cells.
Conclusion
Our novel findings indicate that saturated FAs represent an endogenous danger in the form of a first hit, up-regulate the inflammasome in NASH, and induce sensitization to a second hit with LPS for IL-β release in hepatocytes. Furthermore, hepatocytes exposed to saturated FAs release danger signals that trigger inflammasome activation in immune cells. Thus, hepatocytes play a key role in orchestrating tissue responses to danger signals in NASH.
Nonalcoholic fatty liver disease (NAFLD) is one of the most common liver diseases and affects more than one-third of the population of the Western world.1,2 The histopathological spectrum of NAFLD includes steatosis alone, steatosis with inflammation, and steatohepatitis with necroinflammation (with or without fibrosis).1 The last form, which is progressive, can lead to cirrhosis and even hepatocellular carcinoma.1 In 1998, the two-hit hypothesis of nonalcoholic steatohepatitis (NASH) pathogenesis was proposed. The initial step involves fat accumulation in the liver as a result of the excessive delivery of free fatty acids (FFAs) from the adipose tissue and an imbalance between lipid synthesis and export in hepatocytes.3 However, the role of fat accumulation as a component of the first hit and the implications for liver sensitization to further insults are not fully understood. Increased levels of saturated and monounsaturated FFAs have been reported in NASH patients4,5 and have been implicated in triggering inflammation and thus potentially serving as endogenous danger signals.6 Endotoxin or lipopolysaccharide (LPS), a bacterial wall component sensed by toll-like receptor 4 (TLR4), can act as a second hit and result in progressive liver injury. Increased plasma endotoxin levels have been detected in mice with methionine choline–deficient (MCD) steatohepatitis7 and in humans with NAFLD.8 Importantly, the fatty liver is highly sensitive to LPS, and a TLR4 deficiency has attenuated hepatic steatosis and inflammation in an animal model of NASH.9
Inflammasomes are major contributors to inflammation. They are large caspase-1–activating multiprotein complexes that sense both exogenous and endogenous danger signals through intracellular NOD-like receptors (NLRs).10 Among the three prototypes of inflammasomes, NACHT, LRR, and PYD domains–containing protein 3 (NALP3) is involved in sensing endogenous danger signals, including uric acid crystals and amyloid-β protein.11 In response to danger signals, NALP3 interacts with pro–caspase-1 through its adaptor molecule, apoptosis-associated speck-like CARD-domain containing protein (ASC), which leads to the activation of cas-pase-1. Active caspase-1 promotes the cleavage and, therefore, maturation of proinflammatory cytokines [pro–interleukin-1β (pro–IL-1β), pro–IL-18, and IL-33] to promote/sustain inflammation.10,11 Multiple studies have demonstrated that inflammasome activation is the result of two distinct signals: one that activates the transcription of pro–IL-1β, usually provided by TLR ligands, and another that mediates the assembly of the inflammasome.10,11 In some cells, such as macrophages, caspase-1 can be activated via the release of endogenous adenosine triphosphate (ATP), so a single dose of the TLR4 ligand LPS is sufficient to induce the prompt release of IL-1β.12 The expression and role of the inflammasome in Kupffer cells or hepatocytes in the liver have yet to be evaluated in NASH.
In this study, we postulated that fatty acids (FAs) may act as danger-associated molecular patterns (DAMPs), activate the inflammasome, and thus act as a first hit in steatohepatitis. We also tested the possibility that FAs may act differentially in liver parenchymal cells and immune cells and facilitate the release of other proinflammatory factors to induce inflammasome activation in a paracrine fashion; in the latter case, FAs may induce sensitization to LPS-induced inflammasome activation (a second hit) in cells initially insensitive to LPS. We thus employed mice with MCD-induced or high-fat diet (HFD)–induced steatohepatitis and leptin-deficient mice with steatosis to test the hypothesis that inflammasome activation occurs in NASH. In addition to the mouse models of NASH, we also evaluated human liver samples from patients with NASH.
Our novel data demonstrate that saturated FAs induce up-regulation of the inflammasome in hepatocytes and lead to sensitization to LPS-induced inflammasome activation and inflammatory injury. These results indicate novel mechanisms of persistent inflammation and identify possible new therapeutic targets for NASH.
Materials and Methods
Animal Studies
This study was approved by the Institutional Animal Use and Care Committee of the University of Massachusetts Medical School. Female C57Bl/6 wild-type mice that were 6 to 8 weeks old (n = 6–8 per group) were fed either an MCD diet for 5 weeks or an HFD for 4 weeks or 9 months. Control mice received either the same MCD diet supplemented with DL-methionine (3 g/kg) and choline bitartrate (2 g/kg; Dyets, Inc., Bethlehem, PA) or a regular rodent chow diet. We also used 9-week-old female mice that were leptin-deficient (i.e., ob/ob mice; B6.V-Lep ob/J, Jackson Laboratories) and an age- and sex-matched control group (C57Bl/6J). The presence of steatohepatitis was proven histologically in the MCD diet–fed mice, whereas fat deposition was proven with a liver triglyceride assay in the HFD-fed mice and the ob/ob mice. The TLR4 ligand LPS (Sigma, St. Louis, MO) was injected intraperitoneally [0.5 mg/kg of body weight for methionine choline–supplemented (MCS) mice and MCD mice and 12 µg for ob/ob mice].
Biochemical Analysis and Cytokine Measurements
Serum alanine aminotransferase levels were determined with a kinetic method (D-TEK, Bensalem, PA), and liver triglyceride levels were assessed with an L-type triglyceride H kit (Wako Chemicals USA, Inc., Richmond, VA). Serum tumor necrosis factor α (TNF-α) and IL-1β levels were determined with a BD cytometric bead array (BD Biosciences, Sparks, MD).
Histopathological Analysis
Sections of formalin-fixed livers were stained with hematoxylineosin, whereas optimal cutting temperature frozen samples were stained with Oil Red O; all slides were analyzed by microscopy. ImageJ and Microsuite (Olympus Soft Imaging Solutions GmbH, Münster, Germany) were used for imaging analysis at indicated magnifications on 20 high-power fields.
RNA Analysis
RNA was purified with the RNeasy kit (Qiagen Sciences, Germantown, MD) and with on-column DNA digestion. Complementary DNA was transcribed with a reverse-transcription system (Promega Corp., Madison, WI). Real-time quantitative polymerase chain reaction (qPCR) was performed with the iCycler (Bio-Rad Laboratories, Inc., Hercules, CA) and with SYBR Green as a detector of double-stranded DNA, as we described previously13; the primer sequences are shown in Supporting Table 1.
Caspase Activity Assay
Caspase-1 activity was determined in freshly prepared whole liver lysates with a colorimetric assay. The caspase-1 activity analysis was based on the cleavage of the WEHD-pNA (Trp-Glu-His-Asp-p-nitroanilide) substrate (R&D Systems, Minneapolis, MN).
Lactate Dehydrogenase (LDH) Release
The LDH assay (Sigma-Aldrich, St. Louis, MO) was used to measure the amount of cytoplasmic LDH released into the medium as an indicator of membrane integrity and cell viability.
In Vitro Experiments
Primary hepatocytes and liver mononuclear cells (LMNCs) were isolated by an enzyme-based tissue digestion method, as we described previously.14 Hepatocytes were plated onto collagen-coated plates and were stimulated with LPS (1000 ng/mL), palmitic acid (PA) coupled with bovine serum albumin (0.33 mM), or both with or without carbobenzoxy-valyl-alanyl-aspartyl-[O-methyl]-fluoromethylketone (ZVAD; 40 µM). Cell viability was evaluated by trypan blue staining. The purity of the cell population was assessed with qPCR.
The Hepa1–6 mouse hepatoma and RAW 264.7 mouse leukemic monocyte macrophage cell lines were maintained as described previously.15
Human Liver Samples
This study meets the ethical guidelines of the 1975 Declaration of Helsinki and was approved by the Committee for the Protection of Human Subjects in Research of the University of Massachusetts. All participants provided written consent for participation in the study.
Human liver tissue was obtained from biopsy samples from six patients with clinically and biopsy-proven NASH (two males and four females; age = 45 ± 8 years). The histological examination showed steatosis (<1/3 hepatocytes, n = 2; 1–2/3 hepatocytes, n = 3; and >2/3 hepatocytes, n = 1) with rare hepatocyte ballooning (0, n = 2, and <1/3 hepatocytes, n = 4) and inflammation with inflammatory scores of 1 to 4. Lobular inflammation was present in five patients. Fibrosis was not detected in any of the patients. Human liver tissue from patients infected with chronic hepatitis C (n = 5) were used as disease controls. Total RNA from normal human livers (n = 4) was purchased from OriGene Technologies (Rockville, MD)
Statistical Analysis
Statistical significance was determined with the nonparametric Kruskal-Wallis test and the Mann-Whitney test when appropriate. Data are presented as means and standard errors and are considered statistically significant at P ≤ 0.05.
Results
MCD Diet–Induced Steatohepatitis Is Associated With Increased IL-1β Production and NALP3 Inflammasome Activation in the Liver
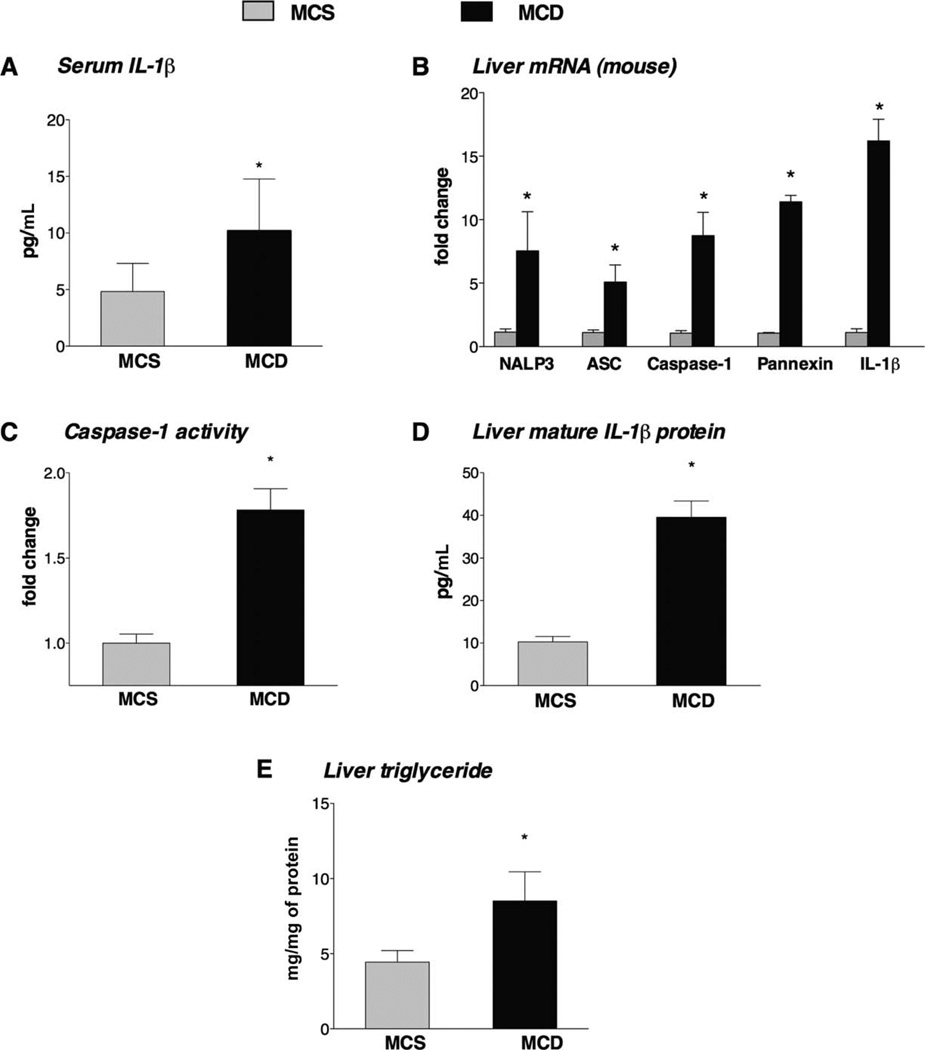
The MCD diet model of NASH is characterized by steatosis and prominent inflammation, which is indicated by an increased number of inflammatory cell infiltrates in the liver and elevated serum proinflammatory cytokine levels.9 The presence of steatohepatitis was histologically evaluated in the MCD diet–fed mice on the basis of the presence of steatosis and inflammatory cell infiltration.1 Here we found that among other proinflammatory cytokines,9 the levels of serum IL-1β (Fig. 1A) and hepatic IL-1β messenger RNA (mRNA; Fig. 1B) were significantly increased in the livers of MCD diet-fed mice in comparison with MCS controls. IL-1β is cleaved from pro–IL-1β by caspase-1, which is activated by the inflammasome complex.10,11 Thus, we tested the expression of inflammasome components (NALP3, caspase-1, and NALP adaptor molecule ASC) and found that all were up-regulated at the mRNA level in the livers of MCD diet–fed mice versus MCS diet–fed mice (Fig. 1B). The association of NALP3 with pro–caspase-1 via the adaptor molecule ASC results in auto-activation of caspase-1, which cleaves IL-1β.10 Caspase-1 activity was significantly increased in the livers of MCD diet–fed mice versus MCS controls (Fig. 1C). In agreement with the increased inflammasome expression and caspase-1 activation, the levels of mature IL-1β protein were increased in the livers of MCD diet–fed mice versus MCS controls (Fig. 1D). In addition to inflammation, there were increased triglyceride levels in the livers of MCD diet–fed mice, which indicated steatosis (Fig. 1E).
Fig. 1.
MCD diet–induced steatohepatitis is associated with increased IL-1β production and NALP3 inflammasome activation in the liver. C57Bl/6 mice were fed an MCD diet or an MCS diet for 5 weeks. (A) The serum IL-1β levels and (B) the liver mRNA fold changes for IL-1β and the NALP3 inflammasome complex (including NALP3, ASC, caspase-1, and pannexin-1) were determined. The functional activity of the inflammasome was evaluated by measurements of (C) the caspase-1 activity and (D) the mature IL-1β protein levels in the liver. (E) Liver triglycerides were measured (six mice per group). *P < 0.05 versus the MCS diet–fed mice.
Long-Term (but Not Short-Term) HFD Feeding Is Associated With Inflammasome Activation in the Liver
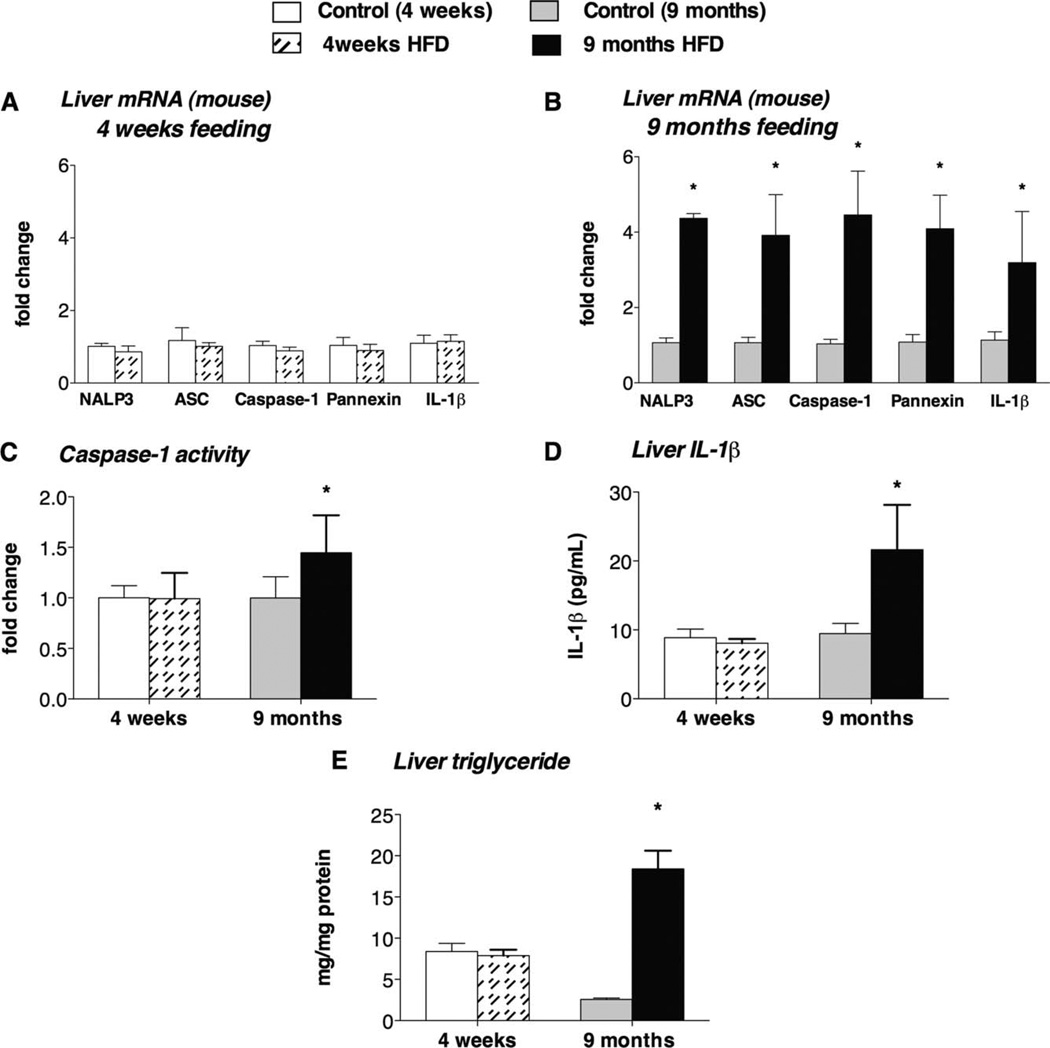
The spectrum of human NAFLD includes fatty liver disease and NASH. Although the MCD diet model induces NASH, an HFD results in steatosis after 4 weeks, and evidence of inflammation can be found after prolonged HFD feeding.16,17 In agreement with this, we observed an increase in liver TNF-α expression (Supporting Fig. 1) in livers only after 9 months of HFD feeding and not after 4 weeks. We found that 4 weeks of HFD feeding resulted in no increase in inflammasome expression (Fig. 2A), whereas 9 months of HFD feeding induced significant up-regulation of the NALP3 inflammasome complex (NALP3, ASC, caspase-1, pannexin-1, and IL-1β) at the mRNA level (Fig. 2B). Inflammasome activation was indicated by increased caspase-1 activity (Fig. 2C) and higher mature IL-1β protein levels in the liver in the 9-month HFD group but not in the 4-week HFD group in comparison with their corresponding controls (Fig. 2D). Increased liver triglyceride levels indicated fat accumulation with the MCD diet (Fig. 1E) and with 9 months of HFD feeding (Fig. 2E). There was no significant difference in liver triglyceride levels between the 4-week HFD group and the control group (Fig. 2E); notably, this control group had significantly higher triglyceride levels in comparison with the other control groups.
Fig. 2.
Long-term HFD diet–induced steatohepatitis is associated with increased IL-1β production and NALP3 inflammasome activation in the liver. C57Bl/6 mice were fed an HFD or a control diet for 4 weeks or 9 months. The liver mRNA fold changes for IL-1β and the NALP3 inflammasome complex (including NALP3, ASC, caspase-1, and pannexin-1) were determined after (A) 4 weeks or (B) 9 months of HFD feeding. The functional activity of the inflammasome was evaluated by measurements of (C) the caspase-1 activity and (D) the mature IL-1β protein levels in the liver. (E) Liver triglycerides were measured (six mice per group). *P < 0.05 versus the corresponding control group.
Liver steatosis without features of inflammation is also prominent in leptin-deficient (ob/ob) mice.18 We found no inflammasome activation in ob/ob mice versus their controls (Supporting Fig. 2), and an in vivo LPS challenge failed to induce accelerated inflammasome activation in ob/ob mice versus controls (Supporting Fig. 2).
Increased Inflammasome Expression in Human NASH
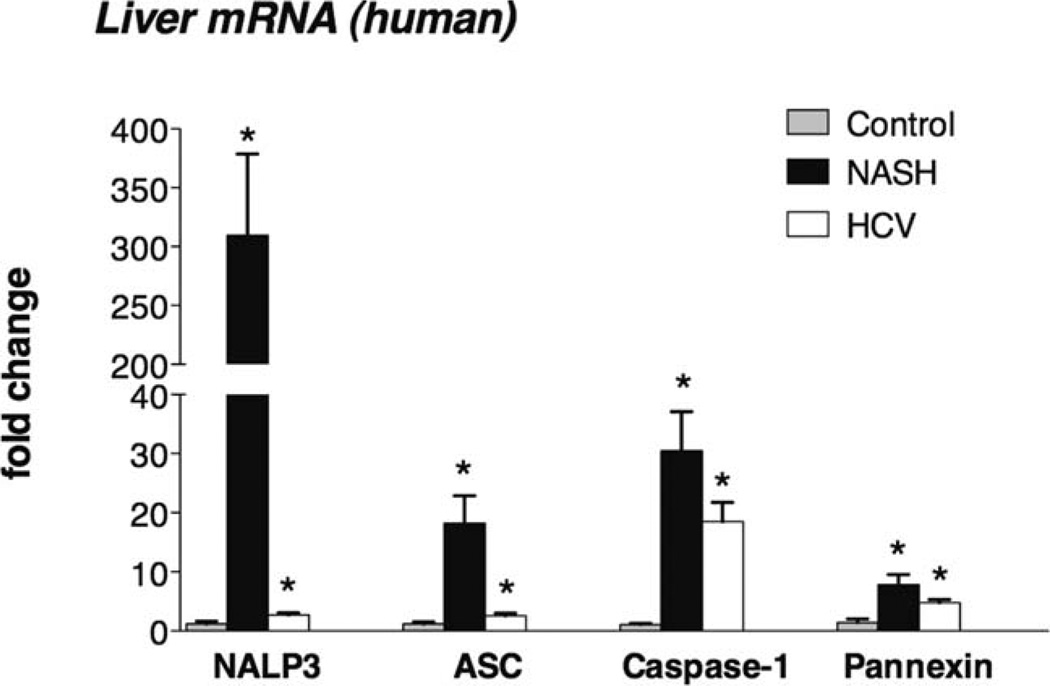
To validate observations from the mouse models, we next evaluated human livers. There was a significant increase in inflammasome gene expression (including NALP3, ASC, caspase-1, and pannexin-1) in the livers of NASH patients versus the livers of healthy controls (Fig. 3). This observation in human NASH corroborated the inflammasome activation in the mouse models of NASH. Liver samples from patients with chronic hepatitis C virus (HCV) infections also showed increased inflammasome expression, albeit to a lower extent than NASH livers (Fig. 3).
Fig. 3.
Increased inflammasome expression in human NASH. The mRNA expression of NALP3, ASC, caspase-1, and pannexin-1 was measured with qPCR in the livers of NASH patients, in commercially available, normal human livers (n = 4), and in liver samples from HCV-infected patients. *P < 0.05 versus the control.
LPS Induces Up-Regulation of the Inflammasome in the Liver
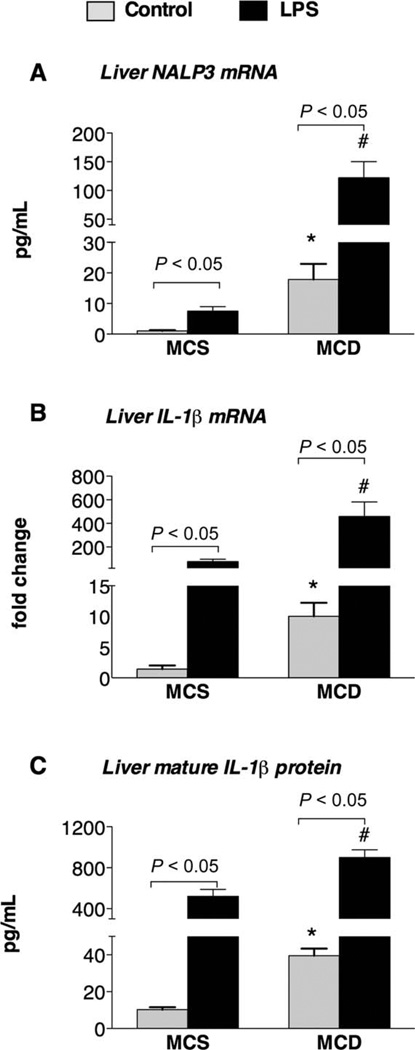
Inflammasome activation requires two signals, which usually consist of a combination of an endogenous danger signal and a TLR ligand.10–12 The pathogenesis of NASH has also been linked to two hits.3 It has been suggested that endotoxin (LPS), which is presumably gut-derived, usually acts as a potent second hit and aggravates liver injury.7–9 Here we tested whether the inflammasome could be further activated by TLR4/LPS in steatohepatitis. In vivo stimulation with the TLR4 ligand LPS led to up-regulation of the hepatic inflammasome components NALP3 and IL-1β at the mRNA level (Fig. 4A,B) and increased IL-1β protein levels in the liver (Fig. 4C) in both MCD diet–fed mice and MCS diet–fed mice. We also noted significantly higher induction of the inflammasome in MCD diet–fed mice versus MCS diet–fed mice after an LPS challenge. Altogether, these data suggest that NASH is associated with inflammasome activation and with sensitization to an LPS-induced up-regulation inflammasome function.
Fig. 4.
LPS induces the up-regulation of the inflammasome in the liver. C57Bl/6 mice were fed an MCD diet or an MCS diet for 5 weeks and were injected with LPS (0.5 mg/kg of body weight) intraperitone-ally for 2 hours. Hepatic mRNA expression for (A) NALP3 and (B) IL-1β was analyzed with qPCR, and (C) mature IL-1β protein levels were measured with an enzyme-linked immunosorbent assay (six mice per group). *P < 0.05 versus the MCS diet–fed mice at the baseline. #P < 0.05 versus the MCS diet–fed mice after LPS stimulation.
The Inflammasome Is Up-Regulated in Hepatocytes in NASH
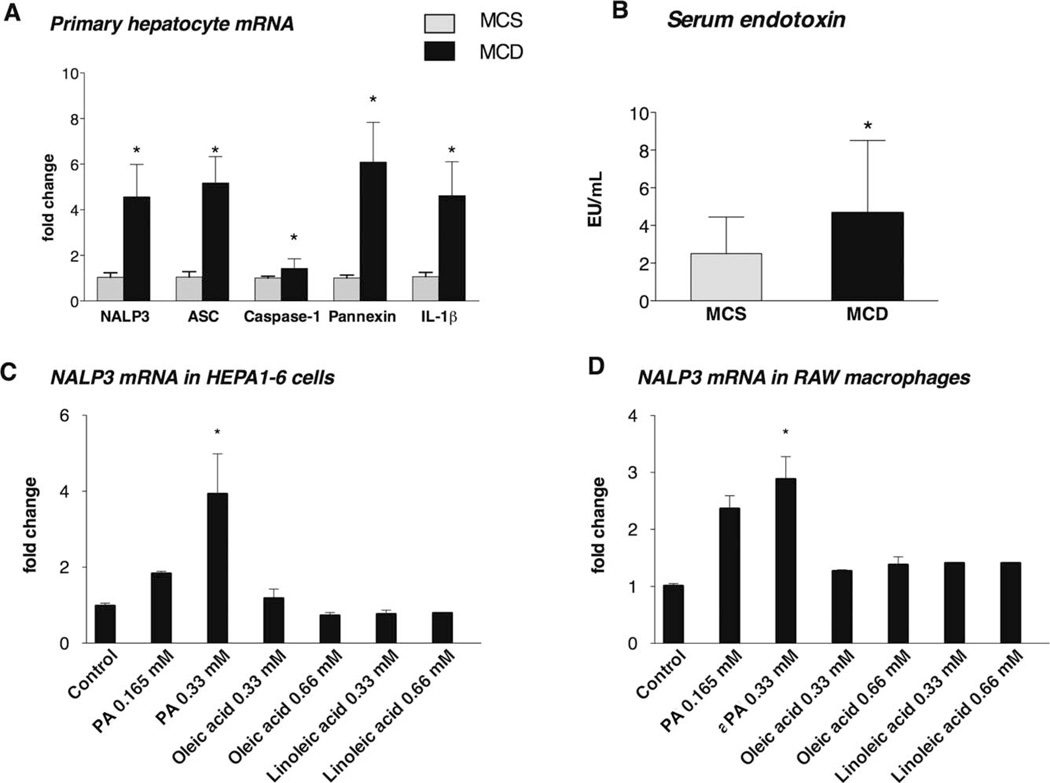
The liver is composed of both parenchymal cells (hepatocytes) and immune cells (macrophages, among others), and hepatocytes represent the majority of the cells. Inflammasome expression and activation have been studied mostly in innate immune cells11; to date, the expression and role of the inflammasome in parenchymal liver cells are largely unknown. Here we sought to evaluate whether inflammasome activation occurs in hepatocytes. We found that primary hepatocytes of MCD diet–fed mice had increased expression of NALP3, ASC, caspase-1, pannexin-1, and pro–IL-1β mRNA in comparison with controls (Fig. 5A). The purity of the primary hepatocyte isolates was confirmed by the high expression of albumin and the lack of inflammatory cell markers [CD11b (monocytes and macrophages), F4/80 (macrophages), CD11c (dendritic cells), and glial fibrillary acidic protein (stellate cells); see Supporting Fig. 2].
Fig. 5.
The inflammasome is up-regulated in hepatocytes in NASH. Saturated FAs (but not unsaturated FAs) induce NALP3 mRNA expression in hepatocyte and macrophage cell lines. C57Bl/6 mice were fed an MCD diet or an MCS diet for 5 weeks, and primary hepatocytes were isolated as described in the Materials and Methods section. (A) The hepatocyte mRNA fold changes of IL-1β and the NALP3 inflammasome complex (including NALP3, ASC, caspase-1, and pannexin-1) were determined. (B) Serum endotoxin data are presented as means and standard errors of the mean (four to six mice per group). (C) Hepa1–6 cells, which were used as prototypes for hepatocytes, and (D) RAW macrophages, which were used as prototypes for macrophages, were exposed to the saturated FA PA (0.165 or 0.33 mM) or unsaturated FAs such as oleic acid (0.33 or 0.66 mM) and linoleic acid (0.33 or 0.66 mM). NALP3 mRNA expression was analyzed with qPCR. *P < 0.05 versus the control.
FAs Induce Inflammasome Activation and Sensitization to LPS-Induced IL-1β Secretion in Hepato-cytes In Vitro
Both circulating FAs and gut-derived endotoxins (LPS) contribute to the pathogenesis of NASH.1–3,9 We found increased serum endotoxin levels in mice with steatohepatitis, and this suggests that gut-derived LPS, a TLR4 ligand, is present in this model of NASH (Fig. 5B). We also found that both the MCD diet and the HFD diet resulted in significant steatosis, which was indicated by increased hepatic triglyceride levels (Figs. 1E and 2E).
Taking into account that fatty livers had elevated expression of inflammasome components (Figs. 1 and 2) and that this process occurred in hepatocytes (Fig. 4), we next tested the effects of FAs and LPS on inflammasome expression in liver cells. An in vitro treatment revealed that PA, a saturated FA, induced increased expression of NALP3 mRNA in both Hepa1–6 cells (used as prototypes for hepatocytes; Fig. 5C) and RAW macrophages (used as prototypes for liver macrophages; Fig. 5D). In contrast, the unsaturated FAs oleic acid (Fig. 5D) and linoleic acid (Fig. 5E) failed to increase the mRNA expression of NALP3 in either Hepa1–6 cells or RAW macrophages. This suggests that the expression of constitutive inflammasome components is activated exclusively by saturated FAs.
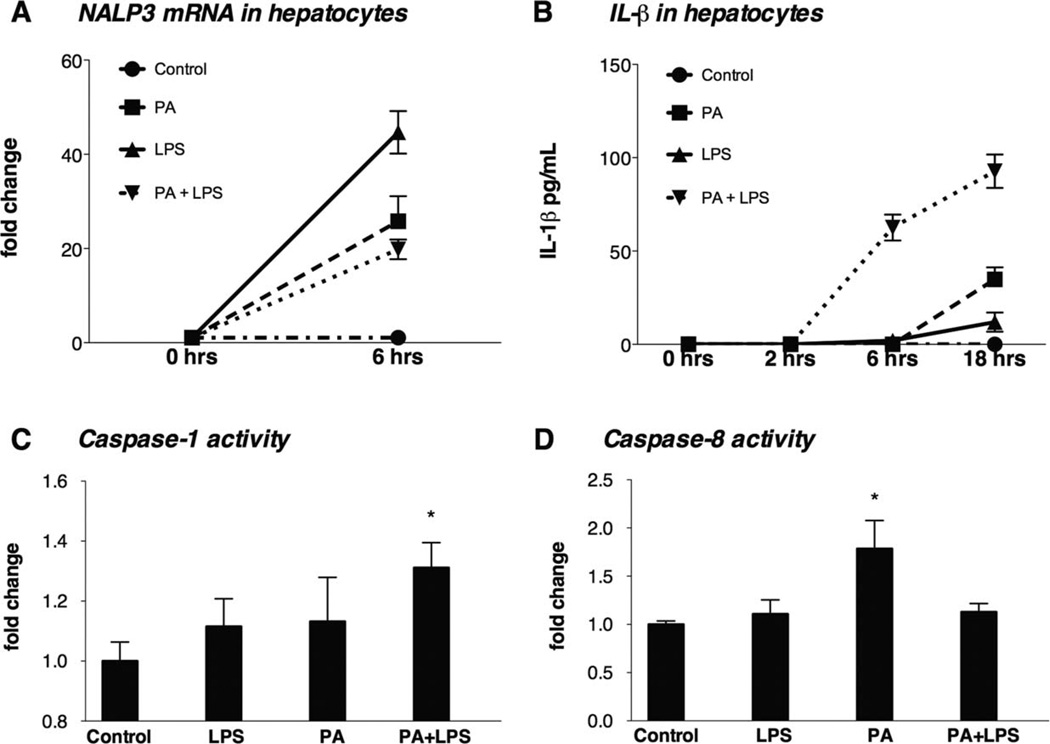
On the basis of this novel observation of the cell line, we sought to validate the results in primary hepatocytes. Murine primary hepatocytes were treated with PA, LPS, or both (18 hours of PA pretreatment followed by LPS). PA or LPS alone up-regulated NALP3 mRNA expression in hepatocytes (P < 0.01; Fig. 6A), and PA induced moderate increases in IL-1β protein secretion (Fig. 6B). Significantly higher levels and earlier production of IL-1β were seen in hepatocytes subjected to the PA pretreatment followed by LPS stimulation (P < 0.001) in comparison with hepatocytes subjected to the PA or LPS treatment alone, and this suggests sensitization in hepatocytes (Fig. 6B).
Fig. 6.
FAs induce inflammasome activation and sensitization to LPS-induced IL-1β secretions in hepatocytes in vitro that involve inflammasome-dependent (caspase-1) and inflammasome-independent (caspase-8–dependent) pathways. (A) Isolated hepatocytes from C57Bl/6 mice on a normal rodent diet were treated with PA (0.33 mM), LPS (1000 ng/mL), or both for 6 hours, and the NALP3 mRNA levels were determined with qPCR. (B) The IL-1β protein levels in the supernatants of hepatocytes were measured with an enzyme-linked immunosorbent assay after their exposure to PA (0.33 mM), LPS (1000 µg/mL), or both for 2, 6, or 18 hours. The activation of (C) inflammatory caspase-1 and (D) apoptotic caspase-8 was determined with an enzyme activity assay. *P < 0.05 versus the control.
Together, these results suggest that the saturated FA PA sensitizes the inflammasome to LPS-induced IL-1β release in hepatocytes.
IL-1β Production in Hepatocytes Occurs in an Inflammasome-Dependent (Caspase-1) Manner and in an Inflammasome-Independent (Caspase-8–De-pendent) Manner
To further evaluate the involvement of the inflammasome in IL-1β induction by PA and LPS in hepatocytes, we tested caspase-1 activation, which results in the cleavage of 45-kDa pro–caspase-1 into its enzymatically active form, a heterodimer of p20 and two p10 subunits.10 We found that PA did not initiate caspase-1 cleavage, whereas a pretreatment with PA followed by LPS stimulation resulted in significant caspase-1 activation in hepatocytes (Fig. 6C). This pattern of caspase-1 activation mirrored the release of IL-1β after the PA pretreatment and LPS stimulation (Fig. 6B), and this suggests that functional caspase-1 activation in hepatocytes requires signals from both saturated FAs and LPS.
The observation that PA alone induced IL-1β secretion (Fig. 6B) without extensive evidence of caspase-1 activation prompted us to evaluate alternative mechanisms for IL-1 cleavage in hepatocytes. Although pro–IL-1β cleavage is mostly a result of inflammasome-mediated caspase-1 activation, it can also be cleaved by caspase-8.19 Indeed, we found that PA20,21 (but not LPS) resulted in caspase-8 activation, and more importantly, caspase-8 activation was not increased by the combination of PA and LPS. These results suggest that caspase-8 could be involved in IL-1β cleavage in PA-treated hepatocytes (Fig. 6D).
PA-Treated Hepatocytes Transmit Danger Signals and Induce Inflammasome Activation in LMNCs
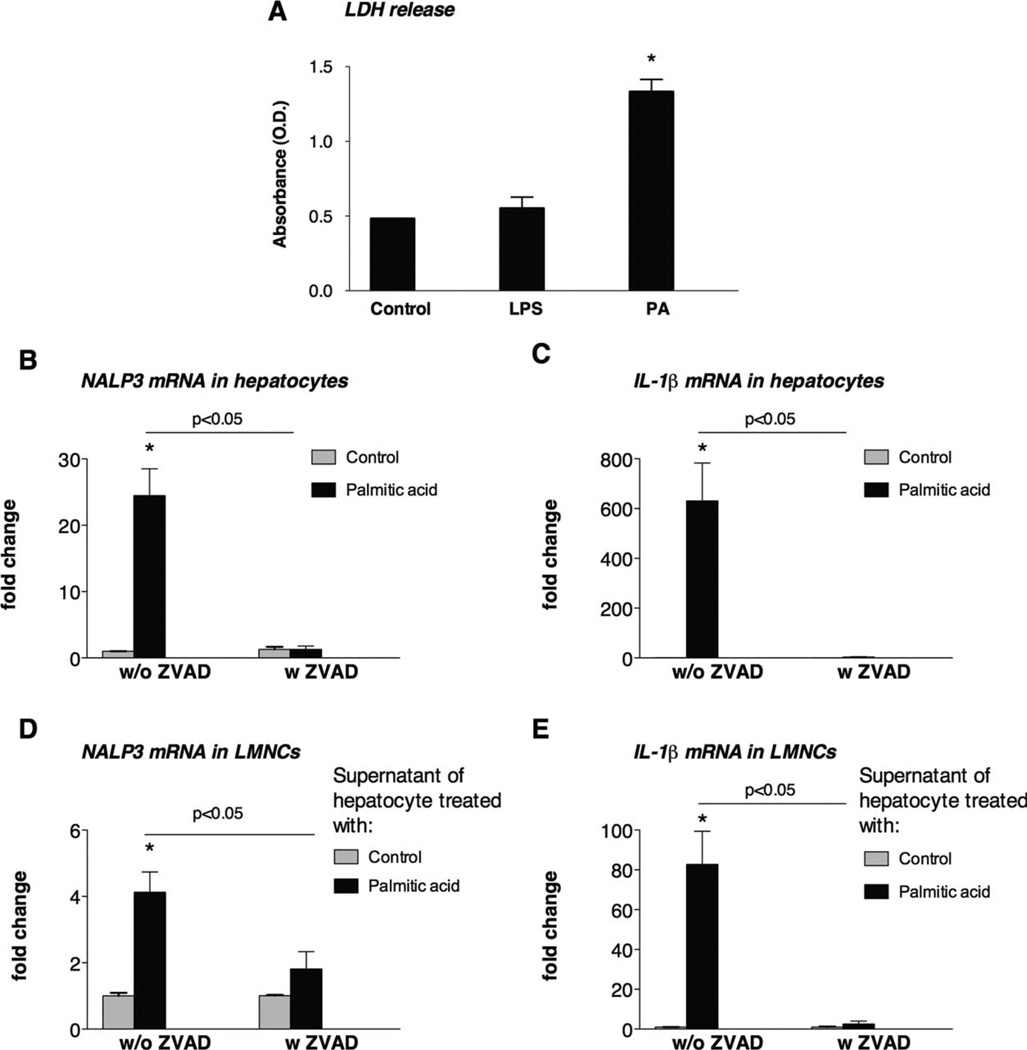
In addition to IL-1 cleavage, caspase-8 is also induced in apoptosis.22,23 Our observation of caspase-8 activation by PA (Fig. 6D) along with previous reports on the induction of apoptosis of hepatocytes by saturated FAs21–23 prompted us to evaluate the mechanistic link between inflammasome activation and cell death in NASH (Figs. 6D and 7A). Increased LDH release in hepatocytes after PA treatment indicated the induction of cell death (Fig. 7A). We determined that up-regulation of NALP3 and IL-1β mRNA by PA was caspase-dependent because these events were prevented by the addition of the pancaspase inhibitor ZVAD in hepatocytes (Fig. 7B,C). This observation also suggests that damage-associated molecules generated in apoptotic hepatocytes rather than PA itself could contribute to inflammasome activation.
Fig. 7.
PA-treated hepatocytes transmit danger signals and induce inflammasome activation in LMNCs. (A) Isolated hepatocytes from C57Bl/6 mice on a normal rodent diet were treated with PA (0.33 mM) or LPS (1000 ng/mL) in the presence or absence of the pancaspase inhibitor ZVAD (40 µM). LDH release as a marker of cell death was determined. The hepatocyte mRNA fold changes of (B) NALP3 and (C) IL-1β were analyzed with qPCR. Supernatants of hepatocytes that were treated with PA for 6 hours and then cultured in fresh media without PA were transferred to LMNCs. The mRNA expression of (D) NALP3 and (E) IL-1β was analyzed. *P < 0.05 versus the control.
To further evaluate the role of hepatocyte-derived damage-associated molecules in inflammasome activation and potential cross-talk between hepatocytes and mononuclear cells, we tested whether PA-treated hepatocytes could induce inflammasome activation in inflammatory cells. Hepatocytes were treated with PA for 6 hours and then were cultured in fresh media without PA. We found that these PA-free supernatants from PA-pretreated hepatocytes induced up-regulation of NALP3 (Fig. 7D) and IL-1β mRNA (Fig. 7E) in LMNCs; this suggests that FA-exposed hepatocytes can transfer activation to surrounding immune cells. The transmission of hepatocyte-derived danger signals to mononuclear cells was dependent on caspase activation in hepatocytes; this was suggested by the lack of LMNC activation with hepatocyte supernatants when ZVAD was added together with PA to hepatocytes (Fig. 7D,E).
These results suggest that hepatocytes are the first target of FAs and produce inflammasome-mediated danger signals, which in turn activate macrophages in a caspase-dependent manner.
Discussion
The pathogenesis of steatosis and inflammation in NASH has been linked to multiple mechanisms,3 such as oxidative stress, mitochondrial damage in hepatocytes, and gut-derived LPS.7–9 Inflammation is a response to cellular injury and pathogens, and it is triggered by endogenous and exogenous danger signals, respectively. NALPs, the receptor components of the inflammasome, sense endogenous danger signals, which up-regulate the inflammasome, a multiprotein complex involved in caspase-1–mediated IL-1 cleavage. Inflammasome activation is typically a result of two signals via TLR activation by exogenous or endogenous danger signals.10
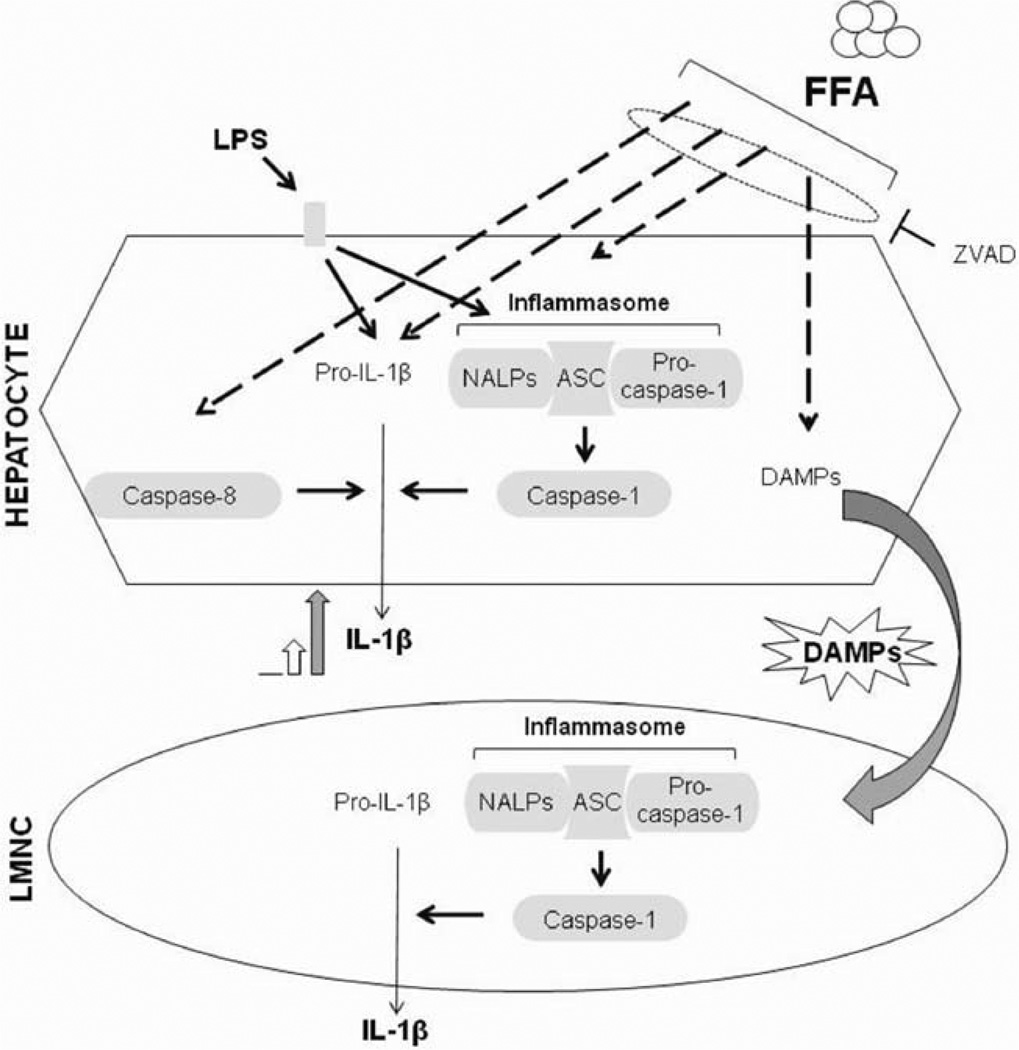
Here we report several findings related to the novel role of inflammasome activation in NASH. First, we describe up-regulation of the components of the NALP3 inflammasome (including NALP3, ASC, and pro–caspase-1) in mouse models of NASH and in human livers, and we demonstrate functional activation via caspase-1 activation and IL-1β production. Our data also suggest that inflammasome activation occurs in steatohepatitis and not in early steatosis in mice. Second, we report that although increased levels of circulating endotoxin likely contribute to inflammasome activation, exogenous LPS can amplify inflammasome activation and IL-1β secretion in steatohepatitis. Third, we demonstrate for the first time that inflammasome activation and IL-1β secretion occur in isolated hepatocytes in NASH. We reveal mechanistic insights into inflammasome activation and show that saturated FAs (but not unsaturated FAs) increase inflammasome expression and sensitize hepatocytes to IL-1β release by a second stimulus via TLR4 activation. Our novel data show that FAs not only up-regulate the inflammasome but also induce apoptosis and the release of danger signals in hepatocytes. Finally, we report for the first time that danger signals from FA-exposed hepatocytes induce inflammasome activation in LMNCs and demonstrate cross-talk between injured hepatocytes and inflammatory cells in NASH (Fig. 8).
Fig. 8.
Summary of inflammasome activation in NASH.
Both NALP3 and NALP1 are highly expressed in primary immune cells and in other cell types, including epithelial cells, neurons, and gonadal cells.24 Here we report that hepatocytes express NALPs. We have found that hepatocytes express the adaptor molecule ASC and the entire functional inflammasome machine and are capable of IL-1β production.
The elucidation of the triggering factors responsible for increased inflammasome expression and function in NASH is of emerging importance. FFAs can be recognized as endogenous danger molecules and induce inflammatory signaling and activation of nuclear factor kappa B and c-Jun N-terminal kinase–activator protein 1 pathways leading to cytokine and chemokine production.25,26 Although TLRs detect ligands either on the cell surface or in the lumen of the endoplasmatic reticulum,27 NLRs are intracellular cytoplasmic (NALP3) or nuclear (NALP1) sensors.24 We have found that saturated FAs induce up-regulation of pro–IL-1β and NALP3 in hepatocytes. Increased FFA levels have been reported in mice with MCD diet–induced,28 HFD-induced,29 or leptin deficiency–induced steatohepatitis30 and in human NAFLD patients with either steatosis or steatohepatitis.4,5 Although several reports have evaluated the FA profile and the ratio of saturated and un-saturated FAs in animal models28–30 and in human plasma in the setting of NASH,4,5 it is yet to be determined whether changes in the FA composition in the liver or serum correlate with steatosis or steatohepatitis. We speculate that saturated FAs in NASH may favor inflammasome activation, whereas a different composition of FFAs in simple steatosis may not trigger such events. These differences could be further amplified by the presence of additional signals such as LPS or danger signals from damaged hepatocytes.
Accumulating evidence shows that innate immune pathways are activated in metabolic syndrome and play a crucial role in the pathogenesis of NASH.31 Increased plasma levels of the TLR4 ligand LPS and enhanced susceptibility to LPS-induced liver damage have been observed.7–9 We found increased serum endotoxin levels in mice with steatohepatitis, which suggested the presence of an exogenous TLR ligand. We and others have shown that a TLR4 deficiency can prevent experimental NASH.9,32 The exogenous administration of LPS further increased IL-1β levels and inflammasome expression in livers with steatohepatitis; this suggests that the fatty liver is primed for LPS-induced inflammasome activation. This novel observation complements previous reports demonstrating that the fatty liver is sensitized to LPS-induced TNF-α production and LPS-induced liver damage.9 A TLR4 deficiency and the modulation of TLR4 pathways with probiotics altering intestinal flora and suppressing TLR-related responses improved liver injury and inflammation in NASH.9,32 More importantly, we identified an increased inflammasome function, which was indicated by the cleavage of pro–caspase-1 and increased IL-1 production, along with the increased expression of the inflammasome in our NASH model.
We have also demonstrated that saturated FAs contribute to the sensitization of LPS-induced IL-1β secretion in hepatocytes. It remains to be determined whether the effect of FAs on the inflammasome is direct or indirect and occurs via intermediate products of FFA metabolism or via FFA-induced cell death33 and the release of DAMP molecules. However, our finding that pancaspase inhibitor ZVAD can prevent FFA-induced inflammasome up-regulation suggests a role of lipotoxicity and endogenous danger molecules in this process.34,35 Saturated FAs (e.g., PA) are more toxic and apoptotic, whereas monounsaturated FAs (e.g., oleic acid) are lipogenic and protect against the apoptotic effects of saturated FAs in cell cultures.22 PA and LPS together lead to inflammasome and caspase-1 activation. In contrast, PA alone induces only caspase-8 activation without detectable inflammasome activation, and this suggests that caspase-8 is responsible for IL-1β cleavage in PA-treated hepatocytes. Caspase-8 has been shown to be an alternative for cleaving pro–IL-1β in macrophages in response to TLR3 and TLR4 stimulation.19 The caspase-1–independent release of IL-1β has also been reported in apoptosis induced by the Fas ligand in peritoneal immune cells.36 Here we demonstrate that danger signals released from damaged hepatocytes upon a saturated FA treatment trigger inflammasome activation in LMNCs.
Previous studies have shown an enhanced inflammatory response and liver injury with LPS in NASH.9 It is likely that in addition to gut-derived LPS, the levels of other danger signals from hepatocytes are also increased. A brief prestimulation with ATP leads to robust LPS-induced caspase-1 activation and IL-1β secretion in macrophages.37 Our data suggest that a sensitization to LPS-induced inflammasome activation and IL-1β secretion occurs in the fatty liver; IL-1β then can further amplify the inflammatory response through the IL-1 receptor. Finally, we cannot exclude the idea that in addition to FAs, alternative activators of the inflammasome such as ATP, monosodium urate crystals, and calcium pyrophosphate may contribute to inflammasome activation in the fatty liver.
In summary, we propose that the increased influx of saturated FAs to the liver leads to inflammasome activation, IL-1β cleavage, and inflammation. We have shown that saturated FAs induce hepatocyte apoptosis and the activation of caspase-8, which triggers the release of danger molecules. Altogether, these events synergize with circulating endotoxins, result in inflammasome activation in hepatocytes, create an amplification loop of inflammation by activating LMNCs, and induce liver injury. Our findings reveal novel mechanisms and suggest potential therapeutic strategies for NAFLD/NASH.
Supplementary Material
Acknowledgment
The authors greatly appreciate the collaboration of Jason Kim, who generously provided liver samples from HFD-fed mice.
This work was supported by grant DK075635 (Gyongyi Szabo), and the core resources supported by the Diabetes Endocrinology Research Center grant DK32520 were also used (Gyongyi Szabo is a member of the Diabetes Endocrinology Research Center of the University of Massachusetts).
Abbreviations
- ASC
apoptosis-associated speck-like CARD-domain containing protein
- ATP
adenosine triphosphate
- DAMP
danger-associated molecular pattern
- FA
fatty acid
- FFA
free fatty acid
- HCV
hepatitis C virus
- HFD
high-fat diet
- IL
interleukin
- LDH
lactate dehydrogenase
- LMNC
liver mononuclear cell
- LPS
lipopolysaccharide
- MCD
methionine choline–deficient
- MCS
methionine choline–supplemented
- mRNA
messenger RNA
- NAFLD
nonalcoholic fatty liver disease
- NALP
NACHT, LRR, and PYD domains–containing protein
- NASH
nonalcoholic steatohepatitis
- NLR
NOD-like receptor
- PA
palmitic acid
- qPCR
quantitative polymerase chain reaction
- TLR
toll-like receptor
- TNF-α
tumor necrosis factor α
- ZVAD
carbobenzoxy-valyl-alanyl-aspartyl-[O-methyl]-fluoromethylketone
Footnotes
Potential conflict of interest: Nothing to report.
Additional Supporting Information may be found in the online version of this article.
References
- 1.Tiniakos DG, Vos MB, Brunt EM. Nonalcoholic fatty liver disease: pathology and pathogenesis. Annu Rev Pathol. 2010;5:145–171. doi: 10.1146/annurev-pathol-121808-102132. [DOI] [PubMed] [Google Scholar]
- 2.Vuppalanchi R, Chalasani N. Nonalcoholic fatty liver disease and nonalcoholic steatohepatitis: selected practical issues in their evaluation and management. Hepatology. 2009;49:306–317. doi: 10.1002/hep.22603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Day CP, James OF. Steatohepatitis: a tale of two ‘hits’? Gastroenterology. 1998;114:842–845. doi: 10.1016/s0016-5085(98)70599-2. [DOI] [PubMed] [Google Scholar]
- 4.deAlmeida IT, Cortez-Pinto H, Fidalgo G, Rodrigues D, Camilo ME. Plasma total and free fatty acids composition in human non-alcoholic steatohepatitis. Clin Nutr. 2002;21:219–223. doi: 10.1054/clnu.2001.0529. [DOI] [PubMed] [Google Scholar]
- 5.Puri P, Wiest MM, Cheung O, Mirshahi F, Sargeant C, Min HK, et al. The plasma lipidomic signature of nonalcoholic steatohepatitis. Hepatology. 2009;50:1827–1838. doi: 10.1002/hep.23229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shi H, Kokoeva MV, Inouye K, Tzameli I, Yin H, Flier JS. TLR4 links innate immunity and fatty acid-induced insulin resistance. J Clin Invest. 2006;116:3015–3025. doi: 10.1172/JCI28898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cani PD, Amar J, Iglesias MA, Poggi M, Knauf C, Bastelica D, et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56:1761–1772. doi: 10.2337/db06-1491. [DOI] [PubMed] [Google Scholar]
- 8.Miele L, Valenza V, La Torre G, Montalto M, Cammarota G, Ricci R, et al. Increased intestinal permeability and tight junction alterations in non-alcoholic fatty liver disease. Hepatology. 2009;49:1877–1887. doi: 10.1002/hep.22848. [DOI] [PubMed] [Google Scholar]
- 9.Szabo G, Velayudham A, Romics L, Jr, Mandrekar P. Modulation of non-alcoholic steatohepatitis by pattern recognition receptors in mice: the role of toll-like receptors 2 and 4. Alcohol Clin Exp Res. 2005;29(Suppl):140S–145S. doi: 10.1097/01.alc.0000189287.83544.33. [DOI] [PubMed] [Google Scholar]
- 10.Yu HB, Finlay BB. The caspase-1 inflammasome: a pilot of innate immune responses. Cell Host Microbe. 2008;4:198–208. doi: 10.1016/j.chom.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 11.Martinon F, Mayor A, Tschopp J. The inflammasomes: guardians of the body. Ann Rev Immunol. 2009;27:229–265. doi: 10.1146/annurev.immunol.021908.132715. [DOI] [PubMed] [Google Scholar]
- 12.Netea MG, Nold-Petry CA, Nold MF, Joosten LA, Opitz B, van der Meer JH, et al. Differential requirement for the activation of the inflammasome for processing and release of IL-1beta in monocytes and macrophages. Blood. 2009;113:2324–2335. doi: 10.1182/blood-2008-03-146720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Velayudham A, Hritz I, Dolganiuc A, Mandrekar P, Kurt-Jones E, Szabo G. Critical role of toll-like receptors and the common TLR adaptor, MyD88, in the induction of granulomas and liver injury. J Hepatol. 2006;45:813–824. doi: 10.1016/j.jhep.2006.06.017. [DOI] [PubMed] [Google Scholar]
- 14.Hritz I, Mandrekar P, Velayudham A, Catalano D, Dolganiuc A, Kodys K, et al. The critical role of toll-like receptor (TLR) 4 in alcoholic liver disease is independent of the common TLR adapter MyD88. Hepatology. 2008;48:1224–1231. doi: 10.1002/hep.22470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Romics L, Dolganiuc A, Kodys K, Dreschler Y, Oak S, Velayudham A, et al. Selective priming to toll-like receptor 4 (TLR4), not TLR2, ligands by P. acnes involves up-regulation of MD-2 in mice. Hepatology. 2004;40:555–564. doi: 10.1002/hep.20350. [DOI] [PubMed] [Google Scholar]
- 16.Xu ZJ, Fan JG, Ding XD, Qiao L, Wang GL. Characterization of high fat diet-induced non-alcoholic steatohepatitis with fibrosis in rats. Dig Dis Sci. 2010;55:931–940. doi: 10.1007/s10620-009-0815-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ellett JD, Evans ZP, Atkinson C, Schmidt MG, Schnellmann RG, Chavin D. Toll-like receptor 4 is a key mediator of murine steatotic liver warm ischaemia/reperfusion injury. Liver Transpl. 2009;15:1101–1109. doi: 10.1002/lt.21782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schattenberg JM, Galle PR. Animal models of non-alcoholic steatohepatitis: of mice and men. Dig Dis. 2010;28:247–254. doi: 10.1159/000282097. [DOI] [PubMed] [Google Scholar]
- 19.Maelfait J, Vercammen E, Janssens S, Schotte P, Haegman M, Magez S, et al. Stimulation of toll-like receptor 3 and 4 induces interleukin-1β maturation by caspase-8. J Exp Med. 2008;205:1967–1973. doi: 10.1084/jem.20071632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cazanave SC, Gores G. Mechanisms and clinical implications of hepatocyte lipoapoptosis. Clin Lipidol. 2010;5:71–85. doi: 10.2217/clp.09.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ji J, Zhang L, Wang P, Mu YM, Zhu XY, Wu YY, et al. Saturated free fatty acid, palmitic acid, induces apoptosis in fetal hepatocytes in culture. Exp Toxicol Pathol. 2005;56:369–376. doi: 10.1016/j.etp.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 22.Ricchi M, Odoardi MR, Carulli L, Anzivino C, Ballestri S, Pinetti A, et al. Differential effect of oleic and palmitic acid on lipid accumulation and apoptosis in cultured hepatocytes. J Gastroenterol Hepatol. 2009;24:830–840. doi: 10.1111/j.1440-1746.2008.05733.x. [DOI] [PubMed] [Google Scholar]
- 23.Alkhouri N, Dixon LJ, Feldstein AE. Lipotoxicity in nonalcoholic fatty liver disease: not all lipids are created equal. Expert Rev Gastroenterol Hepatol. 2009;3:445–451. doi: 10.1586/egh.09.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kummer JA, Broekhuizen R, Everett H, Agostini L, Kuijk L, Martinon F, et al. Inflammasome components NALP1 and 3 show distinct but separate expression profiles in human tissues, suggesting a site-specific role in the inflammatory response. J Histochem Cytochem. 2007;55:443–452. doi: 10.1369/jhc.6A7101.2006. [DOI] [PubMed] [Google Scholar]
- 25.Joshi-Barve S, Barve SS, Amancherla K, Gobejishvili L, Hill D, Cave M, et al. Palmitic acid induces production of proinflammatory cytokine interleukin-8 from hepatocytes. Hepatology. 2007;46:823–830. doi: 10.1002/hep.21752. [DOI] [PubMed] [Google Scholar]
- 26.Malhi H, Bronk SF, Werneburg NW, Gores GJ. Free fatty acids induce JNK-dependent hepatocyte lipoapoptosis. J Biol Chem. 2006;281:12093–12101. doi: 10.1074/jbc.M510660200. [DOI] [PubMed] [Google Scholar]
- 27.Miyake K. Innate immune sensing of pathogens and danger signals by cell surface toll-like receptors. Semin Immunol. 2007;19:3–10. doi: 10.1016/j.smim.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 28.Rizki G, Arnaboldi L, Gabriella B, Yan J, Lee GS, Ng RK, et al. Mice fed a lipogenic methionine-choline-deficient diet develop hypermetabolism coincident with hepatic suppression of SCD-1. J Lipid Res. 2006;47:2280–2290. doi: 10.1194/jlr.M600198-JLR200. [DOI] [PubMed] [Google Scholar]
- 29.Oosterveer MH, vanDijk TH, Tietge UJF, Boer T, Havinga R, Stellaard F, et al. High fat feeding induces hepatic fatty acid elongation in mice. PLoS One. 2009;4:e6066. doi: 10.1371/journal.pone.0006066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cohen P, Miyazaki M, Socci ND, Hagge-Greenberg A, Liedtke W, Soukas AA, et al. Role of stearoyl-CoA desaturase-1 in leptin-mediated weight loss. Science. 2002;297:240–243. doi: 10.1126/science.1071527. [DOI] [PubMed] [Google Scholar]
- 31.Maher JJ, Leon P, Ryan JC. Beyond insulin resistance: innate immunity in nonalcoholic steatohepatitis. Hepatology. 2008;48:670–678. doi: 10.1002/hep.22399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rivera CA, Adegboyega P, van Rooijen N, Tagalicud A, Allman M, Wallace M. Toll-like receptor-4 signaling and Kupffer cells play pivotal role in the pathogenesis of non-alcoholic steatohepatitis. J Hepatol. 2007;47:571–579. doi: 10.1016/j.jhep.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Neuschwander-Tetri BA. Nontriglyceride hepatic lipotoxicity: the new paradigm for the pathogenesis of NASH. Curr Gastroenterol Rep. 2010;12:49–56. doi: 10.1007/s11894-009-0083-6. [DOI] [PubMed] [Google Scholar]
- 34.Jung Y, Diehl AM. Non-alcoholic steatohepatitis pathogenesis: role of repair in regulating the disease progression. Dig Dis. 2010;28:225–228. doi: 10.1159/000282092. [DOI] [PubMed] [Google Scholar]
- 35.Witek RP, Stone WC, Karaca FG, Syn WK, Pereira TA, Agboola KM, et al. Pan-caspase inhibitor VX-166 reduces fibrosis in an animal model of nonalcoholic steatohepatitis. Hepatology. 2009;50:1421–1430. doi: 10.1002/hep.23167. [DOI] [PubMed] [Google Scholar]
- 36.Miwa K, Asano M, Horai R, Iwakura Y, Nagata S, Suda T. Caspase 1-independent IL-1β release and inflammation induced by the apoptosis induced Fas ligand. Nat Med. 1998;4:1287–1292. doi: 10.1038/3276. [DOI] [PubMed] [Google Scholar]
- 37.Kahlenberg JM, Dubyak GR. Mechanisms of caspase-1 activation by P2×7 receptor mediated K+ release. Am J Physiol Cell Physiol. 2004;286:1100–1108. doi: 10.1152/ajpcell.00494.2003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.