Abstract
The melanocortin receptor (MCR) system has been studied extensively for its role in feeding and sexual behavior, but effects on social behavior have received little attention. α-MSH interacts with neural systems involved in sociality, including oxytocin, dopamine, and opioid systems. Acute melanotan-II (MTII), an MC3/4R agonist, potentiates brain oxytocin (OT) release and facilitates OT-dependent partner preference formation in socially monogamous prairie voles. Here we examined the long-term impact of early-life MCR stimulation on hypothalamic neuronal activity and social development in prairie voles. Male and female voles were given daily subcutaneous injections of 10 mg/kg MTII or saline between postnatal days (PND) 1-7. Neonatally-treated males displayed a reduction in initiated play fighting bouts as juveniles compared to control males. Neonatal exposure to MTII facilitated partner preference formation in adult females, but not males, after a brief cohabitation with an opposite-sex partner. Acute MTII injection elicited a significant burst of the immediate early gene EGR-1 immunoreactivity in hypothalamic OT, vasopressin, and corticotrophin releasing factor neurons, when tested in PND 6-7 animals. Daily neonatal treatment with 1 mg/kg of a more selective, brain penetrant MC4R agonist, PF44687, promoted adult partner preferences in both females and males compared with vehicle controls. Thus, developmental exposure to MCR agonists lead to a persistent change in social behavior, suggestive of structural or functional changes in the neural circuits involved in the formation of social relationships.
Keywords: early experience, oxytocin, social behavior, melanocortin receptor, melanotan-ii, prairie voles
Introduction
The melanocortin (MC) system has been studied extensively for its role in coordinating feeding (Poggioli et al., 1986), stress and anxiety (De Barioglio et al., 1991; Lu et al., 2003; Chaki and Okuyama, 2005) and sexual behavior (Argiolas et al., 2000; Rossler et al., 2006), among other physiological and behavioral processes (for review, see Wikberg et al., 2000; Mountjoy, 2010; Tao, 2010). However, the effects of melanocortin receptor activation on social behavior have received little attention. Alpha-melanocyte stimulating hormone (α–MSH) stimulates central OT release from rat hypothalamic slices, an effect that is blocked by an MC4 receptor (MC4R) antagonist (Sabatier et al., 2003). The MC4R also interacts with additional systems involved in the regulation of social behaviors, including dopamine (Lindblom et al., 2001), opioids (Alvaro et al., 1997), and corticotropin-releasing factor (CRF; Lu et al., 2003).
Recently, we have found that melanocortin signaling promotes social attachment in prairie voles (Modi et al., Unpublished results). The socially monogamous, biparental prairie vole (Microtus ochrogaster) exhibits a complex repertoire of social behaviors that have been associated with oxytocin, dopamine, opioid, and CRF signaling (Young and Wang, 2004; Aragona et al., 2006; Lim et al., 2007; Bosch et al., 2009; Burkett et al., 2011), and provides a excellent model to assess the neural underpinnings of social behavior (McGraw and Young, 2010). Pair bond formation is assessed using the partner preference test, in which a subject animal’s preference for a cohabitated partner or novel stranger animal is tested (Williams et al., 1992). In prairie voles, the partially brain penetrant MC3/4 agonist, melanotan-II (MTII), activates oxytocin (OT) neurons in the paraventricular nucleus of the hypothalamus (PVN), potentiates central OT release in response to a physiological stimulus (hypertonic saline), and facilitates partner preference formation (Modi et al., Unpublished results). These effects are thought to be mediated via MC4R as the selective, brain penetrant MC4R agonist, PF446687, also promotes partner preference formation (Modi et al., Unpublished results).
Adult prairie vole sociality is sensitive to early-life manipulations of parental care (Ahern and Young, 2009). One mechanism by which early social experience, in particular parent-infant interactions, may be translated into long-term behavioral alterations is through long-term organizational effects of neuropeptide activation through restructuring neural circuitry (Carter et al., 2009). For example, neonatal treatment with OT at birth impacts later socioemotional behaviors in prairie voles (Bales and Carter, 2003b; Kramer et al., 2003; Cushing et al., 2005; Bales et al., 2007). Interestingly, neonatal administration of α-MSH and ACTH-like peptides to rats leads to enhancements in adult attention to relevant stimuli (Champney et al., 1976), learning (Beckwith et al., 1977b; Acker et al., 1985), social contact in an open field (Beckwith et al., 1977a), and reductions in adult anxiety (Felszeghy et al., 1993). Early α-MSH treatment also impacts hypothalamic cytoskeletal proteins (Wu et al., 2006), hypothalamic dopamine neuron development (Egles et al., 1998), and neurite outgrowth (Joosten et al., 1996), thus leading to functional and structural changes in neural development. As MC agonists impact a variety of neural systems involved in sociality and acute MC4R stimulation promotes adult pair bonding, early MC stimulation may also stimulate encoding of early social information and lead to long-term organizational changes in social behavior.
Here, we investigated the effects of daily MTII and the more selective, small molecule MC4R agonist, PF446687 (Lansdell et al., 2010) injections during the first week of life on juvenile play and adult pair bonding in males and female prairie voles. We also assessed the impact of peripheral MTII on hypothalamic neuropeptide systems using the immediate early gene, early growth response factor-1, as a marker of neuronal activity. Daily neonatal treatment with MTII altered juvenile play behavior and both agonists enhanced adult social bonding, suggesting central MCR stimulation has long-lasting, organizational effects on social neural circuitry.
Materials and Methods
Animal care and handling
Subjects were laboratory-bred prairie voles, derived from a field-caught Illinois stock. The colony was maintained at 22°C and on a 14:10 h light:dark cycle with access to food (Purina high-fiber rabbit chow) and water ad libitum. Breeder housing consisted of large ventilated cages (34 × 30 × 19 cm) lined with bedding (bed-o-cob, Maumee, OH, USA). Subjects were not exposed to subsequent litters. Pups were weaned into same-sex same-treatment pairs or trios in smaller (30 × 18 × 19 cm) cages at PND21. From each litter, pups were randomly assigned to treatment groups and no more than 2 animals of the same sex and treatment group were used for each experiment. All procedures were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Emory University Institutional Animal Care and Use Committee. All efforts were made to minimize animal suffering, to reduce the number of animals used, and to utilize alternatives to in vivo techniques, if available.
Experimental design
The timelines of experiments and animal numbers can be found in Figure 1 and the legend. Experiments 1 and 3 investigated the behavioral consequences of daily nonselective MC agonist MTII or selective MC4R agonist PF446687 in males and females. Experiment 2 examined the impact of acute MTII injection on hypothalamic systems and plasma corticosterone.
Figure 1. Experimental design.
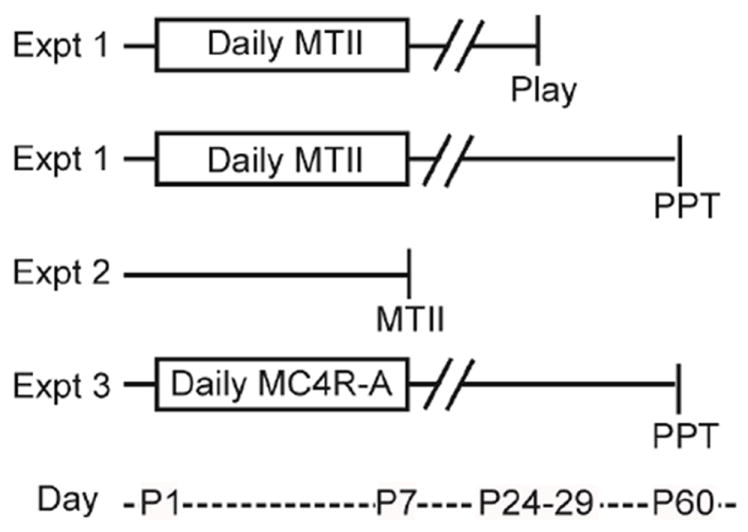
In Experiment 1, neonates were injected daily from PND1-7 with 10 mg/kg of melanotan-II (MTII) and either tested for juvenile play behavior (Sal, n = 11f, 16m; MTII, n = 11f, 15m) or adult partner preference (Sal, n = 15f, 10m; MTII, n = 18f, 12m). In Experiment 2, PND6-7 neonates were sacrificed 1-hr after an acute injection of 10mg/kg MTII or saline, or handling only (H, handled). Either immunohistochemistry (IHC) for hypothalamic neuropeptide activation (n = 6H, 4 sal, 4MTII) was performed or plasma corticosterone was assayed (n = 8H, 8sal, 9MTII). In experiment 3, neonates were injected daily with a melanocortin-4 agonist (MC4R-A, PF-446687, Pfizer) and tested for partner preference in adulthood (Vehicle, n = 9f, 9m; 0.1 mg/kg, n = 10f, 15m; 1 mg/kg, n = 9f, 15m). Animal numbers are outlined in tables on the right. F, female; M, male. PPT, Partner preference test.
Experiment 1. Effect of neonatal MTII on social behavior
Drug Administration
To determine the developmental effects of early-life MTII administration, neonatal prairie vole pups were subcutaneously injected daily with MTII acetate (Alpha diagnostics Intl, San Antonio, TX) or 0.9% saline vehicle (sal: Hospira, Lake Forest, IL) from PND1-7, with PND0 as the day of birth. Pups were toe clipped to identify treatment groups. Animals were injected with a sufficient volume of drug solution at a 0.08 mg/ml concentration to deliver 10 mg/kg using a 250 μl syringe (Hamilton, Reno, NV) with a 30 gauge needle. Volumes were adjusted for growth rate and ranged from 35 μl on day 1 to 65 μl on day 7, yielding doses roughly equivalent to the 10mg/kg previously shown to facilitate partner preference formation in adults (Modi et al., Unpublished results). Weights were taken from PND1-7 and at weaning.
Juvenile play and social exploration/affiliation
One cohort of animals was treated with MTII (n=11 females, 15 males) or saline (n=11 females, 16 males) and tested for juvenile social play between ages 24-29. As isolation increases play behavior in rodents (Panksepp and Beatty, 1980), subjects were singly housed and tested daily for 3 d after the first 24 hr isolation. Between 0700 and 1200 each day, subjects were paired with an age, weight, and sex-matched colony stimulus animal in a clean arena (37 × 31 × 19cm) and video-taped for play behavior for 10 min. The frequencies of juvenile play and social exploratory/affiliative behaviors were coded by a blind observer using Noldus Observer (Noldus, Sterling, VA). Coat color variations (see results) between treatment groups were not distinguishable in the video recordings. Behaviors were scored based on ethograms previously described (Gavish et al., 1983; Chau et al., 2008; Parent and Meaney, 2008; Veenema and Neumann, 2009; Wang et al., 2012; Branchi et al., 2013). Working definitions of the play and social exploratory/affiliative behaviors can be found in Table 1.
Table 1. Juvenile play ethogram.
The frequency of juvenile play and social exploration/affiliation with a novel conspecific was coded using the above working definitions of behaviors. Behaviors initiated by the subject animal over a 10min period for 3d were coded by a blind observer.
| Category | Behavior | Definition |
|---|---|---|
| Play | Aggressive grooming (AG) | one animal violently grabs at the fur of another and grooms, usually in the back region |
| Boxing (B) | both animals stand on the hind legs and use only their forepaws to attack repeatedly | |
| Chasing (C) | active following | |
| Pinning (Pi) | the action of one animal holding another down overhead and the other is typically on its back or supine | |
| Pouncing (Po) | jumping on another animal making contact with forepaws and a common play initiation behavior | |
| Pulling/biting (PB) | mouth to body contact | |
| Supine (Su) | subject on its back, commonly a result of physical attack or pinning or can be independently induced | |
| Wrestling/tackling (WT) | ventrum-to-ventrum embracing with biting without inflicting wounds | |
|
| ||
| Social Affiliation/Exploration | Allogrooming (Allo) | received or directed grooming to another subject |
| Genital investigation (GI) | Sniffing or grooming directed towards other animal’s genital region | |
| Huddling (H) | immobilized and in close contact | |
| Sniffing (Sn) | general sniffing the stimulus animal | |
| Social Contact (SC) | passive bodily contact | |
Partner preference test
A second cohort of males (n=12 MTII, 10 sal) and females (n=18 MTII, 15 sal) were tested for partner preference (PP). PP is a laboratory proxy for pair bond formation in which subjects spend significantly more time in immobile social contact with a previously cohabitated partner than with a novel stranger stimulus animal over a 3 hr testing period (Williams et al., 1992). Subjects (~65 d of age) were paired with sexually-experienced, vasectomized or ovariectomized opposite-sex stimulus animals in a clean cage. Stimulus animals were up to 1 yr of age. Females were videotaped throughout the first 6hr cohabitation to verify that no mating occurred. In contrast, males were paired with an ovariectomized female who was not primed with estradiol to prevent mating. The use of vasectomized males ensured a prevention of pregnancy in experimental females, eliminating any potential confounds of hormonal changes on neural neuropeptide or receptor expression.
For females 6 hr of cohabitation without mating is not typically sufficient to stimulate PP (Williams et al., 1992). However, 24 hrs of cohabitation even without mating can lead to PP. In contrast, in our laboratory, males require longer cohabitation times without mating in order to develop PP. For this reason, females and males were tested on different schedules at two different time points (6-, 24-hr for females; 24-, 48-hr for males) to maximize the likelihood of detecting either enhanced or impaired PP. Subjects were placed in a 3-chambered arena with “partner” and novel “stranger” stimuli tethered to either end. Time spent in immobile social contact (huddling) with each female during this 3 hr session was scored with an automated system (SocialScan 2.0, Clever Sys Inc., Reston, VA, USA) as previously described (Ahern et al., 2009). After behavior testing, brains were collected for analysis of OT receptor, vasopressin V1a receptor and OT mRNA. As no significant differences were detected in any of these neural variables, these data are not presented.
Experiment 2. Effect of neonatal MTII on immediate early gene expression (IEG) in peptidergic PVN neurons and corticosterone release
Procedure and tissue collection
As MTII stimulates IEG activity in the PVN of neonates (Glavas et al., 2007), we examined the neuropeptide phenotype of activated neurons of the PVN 60 min after MTII administration. We also assayed plasma corticosterone to examine the impact of MC activation of the HPA axis of neonates, as MTII induces corticosterone release in adults (Lu et al., 2003). At PND6-7, entire mixed-sex litters were removed from the nest and immediately subcutaneously injected with 65 μl of either saline (sal; n=4) or 10 mg/kg MTII (n=4), or not injected (handled, H; n=6). Pups were left isolated in a 30°C incubator (Water-jacketed warmer base, Thermocare, Incline Village, NV) for 1hr. Pups were deeply anesthetized with isoflourane, euthanized by rapid decapitation, and brains were removed and acrolein fixed. A second cohort of pups (n= 8 sal, 9 MTII, 8 H) was similarly injected and blood was collected for analysis of corticosterone response 1 hr after injection. Approximately equal numbers of each sex were used and combined in the analyses.
Brain processing and immunohistochemistry
Brains were removed and immersion fixed in 10 ml of 5% acrolein diluted in PBS for 3hr, followed by two 10 min washes in phosphate buffered saline (PBS, pH 7.4). Brains were transferred to 30% sucrose at 4 °C for at least 24 hr and sectioned on a microtome at 40 μM. Sections were stored in cryoprotectant at 4°C until immunohistochemical processing. Every third section was processed for Early Growth Response Protein-1 (EGR-1) and either OT, vasopressin (AVP) as described previously (Modi et al., Unpublished results) or corticotropin releasing factor (CRF). EGR-1 was chosen as a measure of neural activity as it has been reported to be a more sensitive marker of activity in the hypothalamus than Fos (Polston and Erskine, 1995). For immunofluorescence, sections were rinsed for 15 min in 0.1% sodium borohydride, pre-blocked for 1hr with normal goat serum, and then incubated for 47 hr at 4°C in primary antibody in PBS (pH 7.4) with 0.3% triton-X and 2% NGS (1:8,000 rabbit polyclonal anti-EGR1, sc-189, Santa Cruz Biotechnology, Santa Cruz, CA; 1:10,000 mouse monoclonal anti-OT, mAb5296, Millipore, Billerica, MA). For AVP-EGR1 double labeling, sections were incubated in primary for 46 hr (1:1,000 mouse monoclocal anti-VP, PS41, generously donated by Dr. H. Gainer, NIH, USA, (Ben-Barak et al., 1985)). Sections were washed in PBS and incubated in secondary Alexa Fluor conjugated antibodies for 3hr at 4°C (1:1,000 Alexa Fluor 568 for EGR-1 and Alexa Fluor 488 for OT or AVP, Life Technologies, Grand Island, NY). Tissue was mounted and cover slipped with Vectashield mounting medium with Dapi (Vector, Burlingame, CA), and Z-stack images were taken on a Leica confocal microscope at 40X magnification. Images were processed in Image J (NIH). EGR-1 positive nuclei were distinguished by red immunofluorescence, and OT or AVP cell bodies were labeled with green immunofluorescence.
CRF-EGR1 was processed with diaminobenzidine (DAB) immunostaining as the primary antibodies were produced in the same host. Sections were rinsed in 0.1% sodium borohydride for 15min, washed in PBS, rinsed in 0.05% hydrogen peroxide for 30min, washed in PBS-0.3% triton-X (PBST), preblocked in 5% NGS for 1hr, and incubated in primary antibody in PBST-2% NGS at 4°C for 45 hr (1:20,000 rabbit polyclonal anti-CRF, c-5348, Sigma-Aldrich, St. Louis, MO). Sections were then washed in PBST-2% NGS and incubated in biotinylated secondary in PBST-2% NGS (goat anti-rabbit, Vector, Burlingame, CA). An ABC kit (Vector, Burlingame, CA) was used to detect secondary binding, and a DAB kit was used for visualization (Vector, Burlingame, CA). Sections were washed and then incubated in anti-EGR-1 antibody for 42 hr at 4°C. EGR-1 was visualized with Nickel-DAB using the same kit. Sections were mounted, ethanol dehydrated, and coverslipped with Krystalon mounting media (Thermo Fisher Scientific, Waltham, MA). EGR1 nuclei were distinguished by purple nickel DAB staining, and CRF cell bodies by brown DAB staining.
The total number of EGR-1 double-labeled neurons in the PVN were counted in four to six bilateral sections for OT, two to four for AVP, and two to eleven for CRF from each subject. The total percentage of EGR-1 positive OT, AVP, or CRF cells was calculated across all PVN sections for each animal.
Blood collection and corticosterone assay
Plasma was assayed for corticosterone response to drug injection as previously described (Ahern et al., 2009). Approximately 100 μl trunk blood was collected with heparinized capillary tubes into EDTA-coated tubes with 5 μl aprotinin (Trysol, Fisher Scientific) to prevent protease activity. Blood was spun at 5000rpm for 5min and plasma was collected and stored at -20°C until assayed. Plasma samples were diluted 1:100 and assayed with a commercially available kit (MP Biomedicals, Orangeburg, NY) in triplicate (inter-assay CV, 7.2%, intra-assay CV, 10.3%). Assay services were provided by the Biomarkers Core Laboratory at the Yerkes National Primate Research Center. This facility is supported by the Yerkes National Primate Research Center base grant 2P51RR000165-51.
Experiment 3. Effect of Neonatal MC4R agonist PF446687 on Social Behavior
Procedure
To determine if behavioral effects of MTII are attributable to MC4R activation, pups were injected subcutaneously daily from PND 1-7 with the selective, highly brain penetrant MC4R agonist PF446687 (Lansdell et al., 2010, supplied by Pfizer Global Research and Development, Cambridge MA) as described for MTII. Neonates received vehicle (10% hydroxypropyl-cyclodextrin; Sigma-Aldrich, St. Louis, MO), 0.1mg/kg, or 1mg/kg PF446687.Animals were injected with a sufficient volume of drug solution at a 0.087 mg/ml concentration to deliver 1 mg/kg or 0.0087 mg/ml to deliver 0.1 mg/kg. Volumes ranged from 32 μl on day 1 to 60 μl on day 7. These doses are roughly equivalent to the 0.1 mg/kg and 1 mg/kg doses given to adults (Modi et al., Unpublished results) (see above).
Partner preference test
PP testing was performed as described above in males (n=9 veh, 15 0.1 mg/kg, 15 1 mg/kg PF44687) and females (n=9 veh, 10 0.1 mg.kg, 9 1 mg/kg PF446687). However, subjects (~70 d of age) were paired with intact partners rather than gonadectomized partners as we did not intend to analyze receptor expression levels following the procedure as in Experiment 1. Partners differed in age by up to 2 months. Female prairie voles are induced ovulators and take approximately 2 days to go into estrus and mate (Carter et al., 1988). Thus, female subjects likely did not mate for the entire 24hr while males paired with intact stimuli likely began mating toward the end of the 48hr cohabitation.
Statistical analyses
Data are presented as mean ± SEM, unless otherwise noted. Weights were analyzed with repeated measures (RM) ANOVA with day as a within-subjects factor and treatment as a between-subjects factor, followed by post-hoc Student’s t-tests. For play data, the frequency of each individual behavioral event initiated by the subject animal was summed across each 10 min period into either a “play” or “social exploratory/affiliative” category as described in Table 1. Statistical analyses for all behavioral outcomes were estimated with Linear Mixed Models in the PROC MIXED procedure of SAS, unless otherwise indicated. This procedure allowed us to control for the potentially dependent nature of individuals from the same litter by including “litter” as a random factor. A within-subject factor was included in the model for repeated measures. Individual play and social exploratory/affiliative behaviors were analyzed using a non-parametric Mann-Whitney U test as data failed to reach normality using the Kolomogoroc-Smirnov test. For PP data a Bonferroni correction was applied for multiple analyses within each sex (alpha = 0.025 for Exp 1; alpha = 0.017 for Exp 3). Immunohistochemistry results were analyzed with univariate ANOVAs with drug treatment as a factor and post-hoc Bonferroni corrected t-tests between the three treatments. All statistics were performed with SAS version 9.3 (SAS institute Inc., USA).
Results
Experiment 1. Effect of neonatal MTII on social behavior
Weight and coloration
MTII treated animals weighed significantly less than vehicle controls from PND 2-7 (Figure 2A; F1,31=17.25, p<0.001). Similar results were obtained from Cohort 1. MTII treatment impaired weight gain over time (time by treatment F6,186=14.92, p<0.001). Weights did not differ at weaning (p > 0.05; Figure 2B). Although not empirically quantified, MTII treated animals displayed darkened pigmentation as neonates (Figure 2C) and weanlings (Figure 2D), which was normalized in adults.
Figure 2. Daily neonatal MTII treatment reduced weight gain and induced darkened pigmentation.
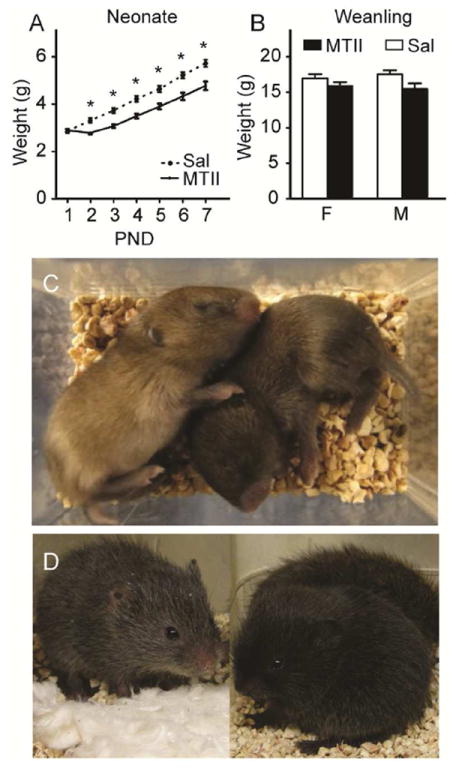
Prairie voles injected with MTII from PND1-7 weighed less than saline controls on PND2-7 (A), which was normalized by weaning at PND21 (B). Although not empirically quantified, daily MTII led to fur darkened pigmentation in neonates (C, left Sal, right MTII). Darkened pigmentation from early MTII injections was still observed at weaning (D, left Sal, right MTII). Asterisks in A indicate post-hoc Student’s t-tests with p<0.05
Juvenile play and social exploration/affiliation
Initiated play bouts increased over subsequent testing days (F2,144=6.81, p=0.002). A significant interaction between sex, treatment and time on initiated play bouts was detected (F2,135=3.22, p=0.043; Figure 3A). A significant increase in play from D1 to D3 was seen in saline-injected males (p=0.009), but not in MTII males. In contrast, MTII-treated (p=0.014), but not saline-treated, females displayed increased play from D1 to D3, but at no time point did MTII females differ from saline females in play. On the last day of testing, there was a main effect of drug (F1,37=4.97, p=0.032) and a sex by drug interaction (F1,37=4.32, p=0.045; Figure 3B). Neonatally MTII-treated males, but not females, initiated less play bouts compared with saline controls (p=0.008; Figure 3B). This effect was driven by a decrease in aggression-like wrestling/tackling (sal, 3.56 ± 0.97, MTII, 1.33 ± 0.61; U=65.5, exact p=0.030) and boxing (sal, 3.31 ± 0.71, MTII, 1.13 ± 0.39; U=54.5, exact p=0.008) in MTII males (Figure 3C). Thus, early treatment with MTII reduced male, but not female, juvenile play, but only those components involving aggressive-like behaviors. In contrast, MTII females initiated significantly more bouts of social exploratory/affiliative behaviors than did saline treated females (sex X treatment, F1,37=8.94, p=0.005; female MTII vs Sal, p=0.026), which was specifically driven by an increase in sniffing (sal, 2.45 ± 0.72, MTII, 5.64 ± 0.65; U=18.5, exact p=0.004; Figure 3D). There was no effect of MTII treatment on social exploratory/affiliative behavior in males.
Figure 3. Daily neonatal MTII reduced male juvenile play bouts.
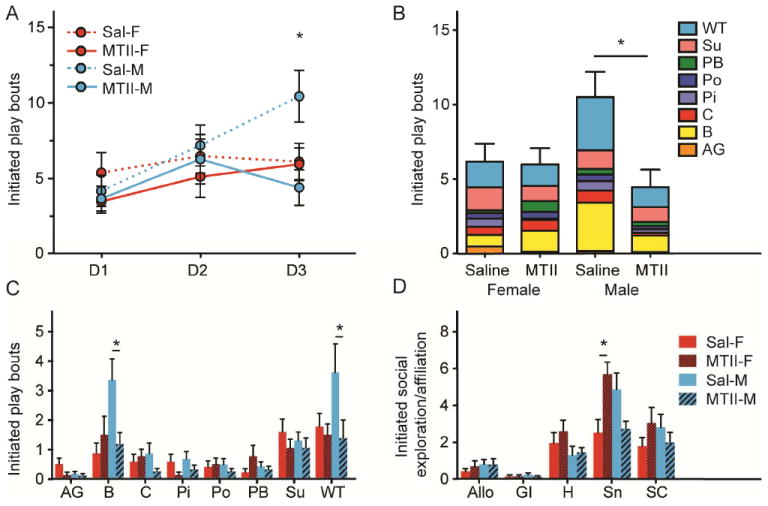
Between PND24-29, juvenile prairie voles injected with MTII or saline from PND1-7 were singly housed and tested for play behaviors for 10min with a novel untreated stimulus animal over a 3d period. Bouts of initiated play increased over time in saline males, but remained stable in MTII males (A). On the third testing day, MTII male play was significantly lower than saline male play (B), which was driven by a decrease in boxing and wrestling/tackling (C). MTII treated females displayed greater levels of sniffing than saline females on the third day of testing (D). Asterisks in A-B indicate post-hoc Student’s t-tests with p<0.05 and in C-D indicate Mann-Whitney U tests with p<0.05. AG, aggressive grooming; B, boxing; C, chasing; Pi, Pinning; Po, pouncing; PB, pulling/biting; Su, supine; WT, wrestling/tackling; Allo, allogrooming; GI, genital investigation; H, huddling; Sn, sniffing; SC, social contact.
Partner preference test
We detected a significant interaction between sex, treatment and stimulus animal on time spent in social contact (F1,92=4.63, p=0.034). Females that received neonatal MTII, but not saline, spent significantly more time with the partner than the stranger male after a 6hr non-mated cohabitation (Figure 4A; MTII, p<0.001, sal, p=0.74). After the full 24hr cohabitation, both experimental and control groups displayed a preference for the partner (Figure 4A; MTII p<0.001, Sal p=0.001). MTII treatment did not impact male partner preference, and neither experimental nor control groups spent significantly more time with the partner over the stranger at either point (Figure 4B).
Figure 4. Daily neonatal MTII facilitated adult female partner preference.
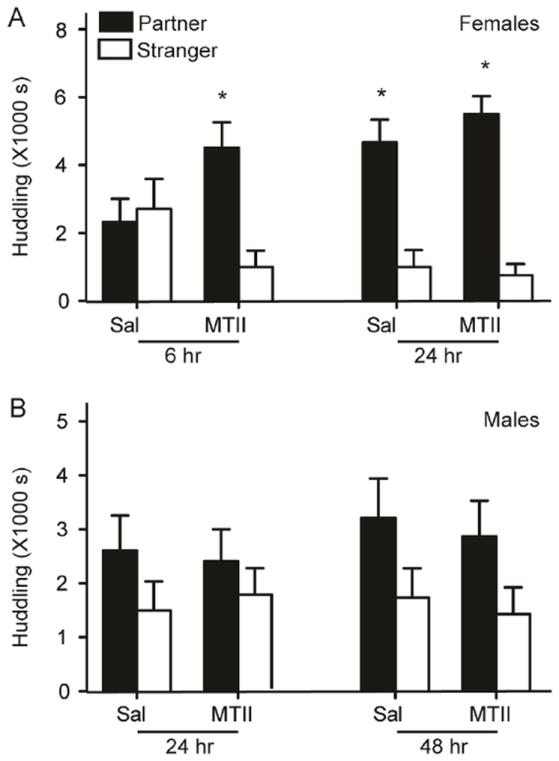
In females, early MTII treatment facilitated adult partner preference after an abbreviated 6 hr cohabitation without mating (A). MTII treatment had no effect on male partner preference after 24- or 48-hr of cohabitation with an unprimed female (B). Asterisk indicates planned Student’s t-tests with p<0.025.
Experiment 2. Effect of neonatal MTII on IEG expression in peptidergic neurons in PVN and corticosterone release
Activation of OT, AVP, and CRF neurons
The percent of peptidergic neurons immunopositive for EGR1 differed significantly between handled, saline, and MTII groups (OT, F2, 11=51.51, p<0.001; AVP, F2, 11=16.63, p<0.01; CRF, F2, 11=605.34, p<0.01; Figure 5A-J). A significant increase in EGR1 positive neurons for all neuropeptidergic populations was observed in the MTII group, in comparison to either saline or handled groups (p<0.005). Saline-injected neonates displayed a greater percentage of CRF-EGR1 cells (p<0.001), but not OT-EGR1 or AVP-EGR1, in comparison to handled controls.
Figure 5. MTII-induced neuropeptide activation in the PVN.
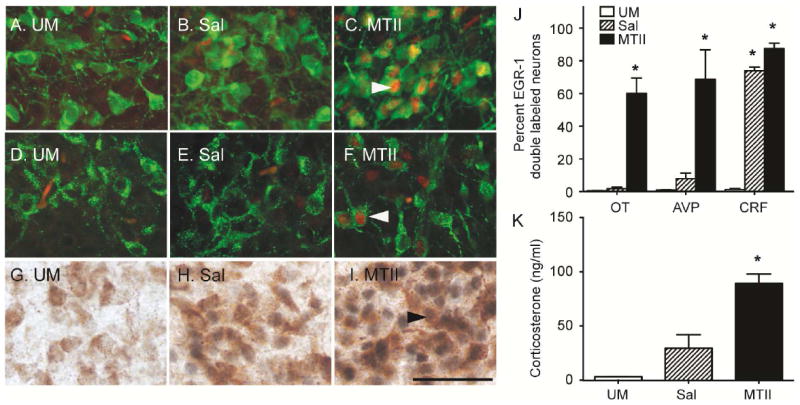
PND6-7 neonates were euthanized 1hr after an acute peripheral injection of 10mg/kg MTII and representative images of brains processed for immunohistochemistry for OT-EGR1 (A,B,C), AVP-EGR1 (D,E,F), and CRF-EGR1 (G,H,I) are shown. The left panel was handled (A,D,G), the middle was injected with saline (B,E,H), and the right received MTII (E,F,I). In fluorescent images, OT and AVP positive cell bodies are green and EGR1-labeled nuclei are red. In DAB processed slices, CRF cell bodies are brown, and EGR1 nuclei are black. MTII elicited significant activation in OT, AVP, and CRF neurons, whereas saline only activated CRF cells in comparison to handled controls (J). MTII injected neonates mounted a corticosterone response (K). H, handled. Color channels were adjusted in Image J (NIH) for fluorescent images. Asterisks indicate Student’s t-tests with p< 0.05. Scale bar represents 50 μM.
Corticosterone
MTII injected neonates displayed a significant increase in plasma corticosterone 1hr after the injection in both sexes (Figure 5K; F2,22=25.73, p<0.001; MTII vs H, p<0.001; MTII vs Sal, p=0.001, Sal vs H, p=0.201). There was no significant sex by treatment effect.
Experiment 3. Effect of neonatal selective MC4R agonist PF446687 on social behavior
Weight and coloration
PF446687 treated neonates had slowed weight gain over the first week of life (F6,312=2.88, p=0.001) but did not weigh significantly less than vehicle treated controls at any age (Figure 6A,B). Although we did not quantify pigmentation objectively, there was no obvious difference in pigmentation following daily PF446687 treatment.
Figure 6. Daily neonatal treatment with the specific MC4R agonist PF446687 facilitated partner preference in both sexes.
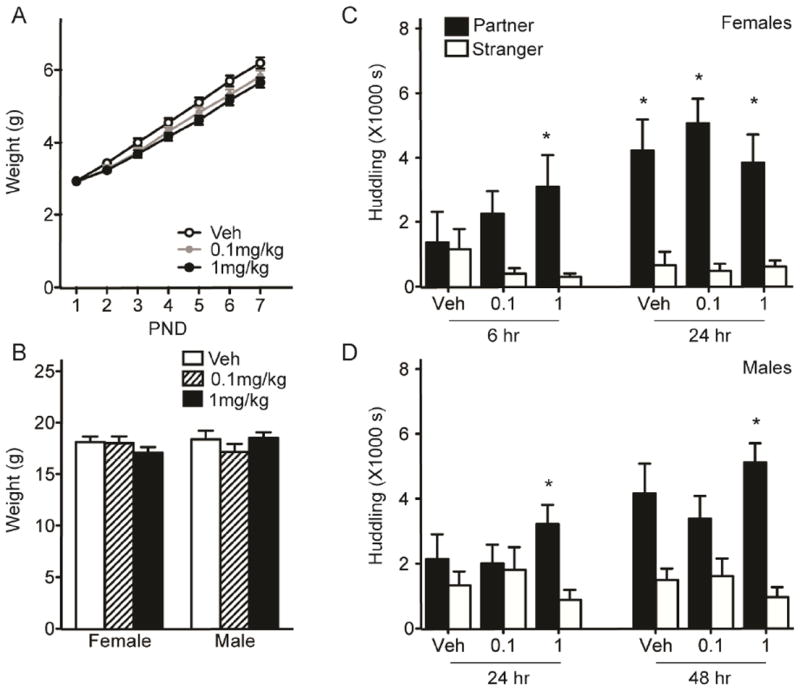
Daily injection with either 0.1mg/kg or 1mg/kg PF446687 did not lead to a significant weight loss in neonates (A) or juveniles (B) in comparison to vehicle (Veh)-injected controls. Prairie vole females injected with the either dose of PF446687 from PND1-7 spent significantly more time huddling with the partner over the stranger after an abbreviated 6hr cohabitation (C). Only those males injected with the high dose of PF446687 spent more time with the partner at both 24 and 48hr of cohabitation with an unprimed female (D). Asterisks indicate planned Student’s t-tests with p < 0.017.
Partner preference
Adult females neonatally treated with either the low (0.1 mg/kg) or high (1 mg/kg) dose of PF446687, but not the vehicle, spent significantly more time huddling with the partner over the stranger following a 6hr-cohabitation (Figure 6C; vehicle, p=0.85, 0.1mg/kg, p=0.02; 1mg/kg, p=0.001). Only the high dose remained significant after Bonferroni correction (alpha = 0.017). All groups displayed a preference after 24hr of cohabitation (vehicle, p=0.015; 0.1mg/kg, p<0.001; 1mg/kg, p=0.009). In males, 1mg/kg PF446687 treatment resulted in significantly more time spent huddling with the partner over the stranger at both 24- and 48-hour time points (Figure 6D; 24hr, p=0.006; 48hr, p<0.001). Vehicle and 0.1mg/kg PF446687 treated males did not show a significant preference at either time point.
Discussion
Developmental MC agonist administration impacted later juvenile and adult social behaviors. Daily peripheral injection (PND1-7) with the nonselective MC agonist MTII reduced later male, but not female, juvenile play behaviors (Figure 3). We previously found that an acute injection of MTII robustly facilitates partner preference, and this effect is blocked by pre-treatment with an OT antagonist (Modi et al., Unpublished results). Here, we found this same compound to lead to persistent changes in social bonding into adulthood (Figure 4). Females, but not males, given neonatal MTII displayed an enhancement in partner preference after an abbreviated non-mated cohabitation with an opposite-sex partner. PND6-7 neonates injected acutely with MTII mounted a corticosterone response and displayed robust hypothalamic EGR-1 activity in OT, AVP, and CRF neurons (Figure 5). As MTII is not specific to the MC4R, future experiments with antagonist treatments are needed to attribute behavioral and neural effects to the MC4R subtype. However, when given the more selective, highly brain penetrant MCR4 agonist PF446687, both sexes exhibited enhanced adult partner preference (Figure 6C,D), also suggesting that females are more sensitive to MC manipulation. Sex-specific behavioral effects of neonatal alpha-MSH administration have previously been reported (Beckwith et al., 1977a; Beckwith et al., 1977b). Activation of MC receptor subtypes other than MC4R may mask the effects of MC4R activation on partner preference in MTII-treated males. Although mating was not analyzed over the abbreviated cohabitation for PF446687 treated animals, it remains possible that early MC4R stimulation promoted adult sexual activity, however we did not detect enhanced mating in MTII treated females. Overall, the presence of persistent behavioral effects in adults resulting from neonatal MTII or PF446687 administration suggests that MCR activation in neonates developmentally alters the social brain circuitry, leading to an enhancement of adult social attachment.
MTII and PF446687 treatment also had differential effects on weight and pigmentation. MTII induced neonatal weight loss, likely via MC3 and MC4R (Huszar et al., 1997; Chen et al., 2000), and darkened pigmentation, via MC1R (Thody, 1999; Figure 1). PF446687 treated neonates did not weigh less than vehicle controls, but displayed slowed weight gain (Figure 6A). Although the MC4R system is critically involved in feeding, MC agonists do not always lead to weight loss (Wu et al., 2006; Muceniece et al., 2007). Additionally, multiple second messenger pathways activated from the same receptor or from different MCR4 agonists (Konda et al., 1994; Nickolls et al., 2005) or additional activation of the MC3R (Chen et al., 2000) may account for this discrepancy.
Saline-treated males displayed a trajectory of increased initiated play bouts over subsequent testing days, but saline-treated females displayed consistent levels throughout testing. Neonatal MTII-treated males fail to show this trajectory, and display significantly reduced play on the last day of testing compared to saline-treated males. A similar phenomenon is found in male rats with altered early care, as high levels of licking and grooming or a simulated tactile stimulation regime reduce juvenile play (Moore and Power, 1992; Parent and Meaney, 2008; Edelmann et al., 2013). In contrast, maternal separation increases rough and tumble play (Veenema and Neumann, 2009). Thus MTII treatment mimicked the effects of high levels of maternal nurturing. Social play during the juvenile period is part of the normal development of aggressive and sexual behaviors in males (Taylor, 1980; Spear and Brake, 1983) and is often higher in males than females (Poole and Fish, 1976; Thor and Holloway JR, 1983). MTII-treated females did not play more than saline females, although MTII treatment increased female play over time. Thus, neonatal MTII treatment differentially impacted male and female play. Of social exploratory/affiliative behaviors, MTII treated females displayed increased bouts of sniffing during.
It should be noted that isolation was necessary to achieve a significant amount of play in voles, as has previously been shown in mandarin voles (Wang et al., 2012) and rats (Panksepp and Beatty, 1980). Home-cage observations of prairie voles do not reveal any baseline sex differences in play (Chau et al., 2008), thus it is possible that the acute stress of isolation or the early stress of the saline injections lead to an increase in play. As isolation in voles increases basal corticosterone, PVN CRF levels, and AVP levels in the supraoptic nuclei, (Ruscio et al., 2007), it is possible that these systems act to promote play fighting. Indeed, play fighting in rats increases PVN CRF mRNA, and maternally separated rats display increased play fighting and increased PVN AVP mRNA (Veenema and Neumann, 2009). However, whether play is ethologically important for the social development of voles remains to be determined.
Developmental effects of neonatal alpha-MSH and ACTH have previously been reported. Daily (PND2-7) peripheral administration of alpha-MSH to rat pups improves adult male learning and visual discrimination (Beckwith et al., 1977b) and increases time spent in social contact in both sexes (Beckwith et al., 1977a). Melanocortins promote neuroplasticity and neurotrophin activity (Joosten et al., 1996; Xu et al., 2003; Shen et al., 2013), which may lead to persistent changes in neural architecture into adulthood. Indeed, alpha-MSH treatment for the first two weeks of life alters hypothalamic expression of cytoskeletal proteins involved in synaptic plasticity (Wu et al., 2006). MC4R stimulation also induces hypothalamic brain derived neurotropic factor release, which exerts effects on feeding and cardiovascular function, but may also position the system to regulate synaptic plasticity (Nicholson et al., 2007; Gomez-Pinilla et al., 2008). As pair bond formation requires social learning, attention to relevant social cues, and the drive to preferentially associate with a partner (Young and Wang, 2004), these changes in adult behavior would serve to promote social attachment.
An acute peripheral MTII injection in adult prairie voles results in IEG expression in OT neurons but not AVP neurons, suggesting a selective response (Modi et al., Unpublished results). Here, we found an acute injection of MTII robustly activated EGR-1 immunostaining in OT, AVP and CRF neurons in the PVN in prairie vole pups. Thus, neonatal MC stimulation has a more robust and general activation of hypothalamic neuropeptide systems, perhaps due to increased permeability of the blood brain barrier (Glavas et al., 2007). In adult mice, subsets of MC4R neurons in the PVN contain OT, CRF, and TRH mRNA, but no co-labeling with AVP mRNA was detected (Liu et al., 2003). However, alpha-MSH increases AVP release in hypothalamic explants (Dhillo et al., 2002). MTII has higher affinity for MC4R (Oosterom et al., 1999) and may not be penetrating the brain of adults at a level high enough to activate the subtype of MCRs on AVP neurons. It is also possible that neonates display a different developmental pattern of expression of MC receptors, suggestive of a functional difference in melanocortin systems during development.
One possible mechanism by which MC agonists are impacting socioemotional behavior is through the potentiation of the central OT system. Oxytocin decreases feeding (Arletti et al., 1989), promotes sexual behavior (Gorzalka and Lester, 1987), and induces the stretching, yawning, grooming response (Melis et al., 1986), thus may mediate some of the downstream anorexigenic and behavioral effects of MC stimulation (Sabatier, 2006). Activation of the OT system during early life, either through endogenous mechanisms or pharmacological interventions, can have a life-long impact on social behavior and neuroendocrine function (Carter et al., 2009; Keebaugh and Young, 2011). An injection of OT on the first day of life promotes adult partner preference in prairie voles (Bales and Carter, 2003a; Bales et al., 2007), thus our results are in line with an OT-dependent mechanism of MTII. Neonatal AVP may also modulate adult sociality, as daily postnatal treatment with AVP enhances later aggression in males prairie voles (Stribley and Carter, 1999). However, the long-term effects on partner preference formation are unknown. Neuropeptide signaling during infancy, specifically during parent-infant interactions (Caba et al., 2003), may assign a rewarding value to social stimulation and shape the neural networks that shape social bonding. The possibility that α-MSH may endogenously be released during nursing in neonatal animals and acts to prime OT neurons and promote early social attachment warrants investigation. We did not detect any effect of MTII treatment on later OT mRNA in the PVN or OTR or vasopressin 1a receptor binding in any brain region examined (data not shown), suggesting that early neuropeptide treatment may have led to changes in other neurotransmitter systems.
Prairie vole neonates mounted a corticosterone response and showed CRF neuron activation after MTII injection. However, saline injection alone induced significant CRF neuron activation, but not corticosterone release, as described previously in rats (Smith et al., 1997). Neonatal saline injection has been shown to have behavioral consequences in male prairie voles (Stribley and Carter, 1999; Bales and Carter, 2003a). It is possible that early CRF activation impaired control male pair bonding, and MTII did not rescue this behavior. Although in adulthood MCR activation may promote stress and anxiety (Lu et al., 2003; Liu et al., 2013), developmental effects may be act in the opposite direction. Neonatal POMC ablation leads to an anxiogenic phenotype in adulthood (Greenman et al., 2013), whereas neonate ACTH4-9 administration reduces later anxiety and depressive-like behavior (Felszeghy et al., 1993). In a phenomenon known as stress inoculation, a certain amount of activation of the stress axis early in development can have beneficial effects on emotionality later in life (Parker and Maestripieri, 2011). However, MTII acting directly on adrenal MC2R likely increases corticosterone release in comparison to the selective MC4R agonist PF446687, and the heightened stress activation may counteract effects on adult prosociality in MTII-treated male voles. Additionally, MC4R stimulation in the central amygdala leads to reductions in CRF mRNA (Brunson et al., 2001). It is therefore possible that the modulation of stress axis by MC4R underlies the observed changes in adult sociality.
MC4R are located in multiple limbic brain areas outside of the hypothalamus (see Mountjoy, 2010), including regions mediating sociality in prairie voles including the amygdala and prefrontal cortex (Modi et al., Unpublished results). In addition to impacting hypothalamic neuropeptide systems, MC4R signaling has been linked to dopaminergic and opioid-mediating reward and reinforcement systems, which are critically involved in pair bond formation (Burkett and Young, 2012). Alpha-MSH or MTII stimulates dopamine release in the ventral tegmental area through MC4R activation (Lindblom et al., 2001), alters dopamine receptor expression in the nucleus accumbens and tegmentum (Lindblom et al., 2002), modifies accumbal D1R signaling in response to stress (Lim et al., 2012), and influences hypothalamic dopamine neuron differentiation (Egles et al., 1998). The rewarding and addictive effects of cocaine and opiates are thought to be mediated by MC4R signaling (Alvaro et al., 1996; Alvaro et al., 1997; Hsu et al., 2005). The MC4R mediates a pleitropy of behavioral and neurobiological effects, and future studies will address the mechanism by which MC4R activation impacts sociality.
Conclusions and future directions
Our findings provide the first evidence that daily neonatal modulation of the melanocortin system enhances later social relationships. Early activation of the MC system led to persistent changes in juvenile and adult sociality, indicating that MCR stimulation has long-term effects on the development of social behavior. The effects of MC activation on both play and partner preference formation are in the opposite direction of early social deprivation, suggesting that perhaps MC activation mimics the neural impact of parental nurturing. MCR activation coordinates a variety of neurobiological and behavioral responses that are linked to social bond formation in prairie voles. Whether the MC system plays a role in the transduction of parental nurturing to prosocial neural development warrants further investigation. Given the enhancement of later social bonding, future experiments will assess the ability of MCR agonists to buffer against early life disruptions in parental care and rescue behavioral deficits in social attachment. Elucidation of the factors and mechanisms mediating normative social behavioral development can ultimately help to shed light on preventative strategies in the treatment of human disorders of the social domain.
highlights.
Daily neonatal melanotan II facilitates adult pair bonding in female prairie voles
Daily neonatal melanotan II reduces juvenile play in male, but not female voles
Acute melanotan II activates peptidergic neurons in neonatal prairie voles
Neonatal selective MC4R agonist promotes adult social attachment in both sexes
Acknowledgments
The authors would like to thank Pravina Fernandez, Rodrigo Triana del Rio, and Lorra Matthews for laboratory and animal care assistance, and Jamie LaPrairie and Kara Kittleberger for comments on the manuscript.
Funding and Disclosure
This work was support by NIH grant R01MH096983 and an Autism Speaks grant #7745 to LJY.CEB is supported by an NSF graduate research fellowship. Additional funding was provided by Office of Research Infrastructure Programs/OD P51OD11132 to YNPRC. LJY and MEM have applied for a patent (US20120108510 – Methods of improving behavioral therapies) for combining MCR agonists with behavioral therapies to enhance social cognition in psychiatric disorders. MEM is currently a fellow at Pfizer Pharmaceuticals. HW is supported by the Wenner-Gren Foundation
Abbreviations
- OT
Oxytocin
- OTR
Oxytocin receptor
- AVP
Vasopressin
- CRF
Corticotropin releasing hormone
- MC
Melanocortin
- MCR
Melanocortin Receptor
- PVN
Paraventricular nucleus of the hypothalamus
- BDNF
Brain-derived neurotrophic factor
- EGR-1
Early-growth factor
- MTII
Melanotan-II
- PP
partner preference
- IEG
immediate early gene
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Acker GR, Berran J, Strand FL. ACTH neuromodulation of the developing motor system and neonatal learning in the rat. Peptides. 1985;6(Suppl 2):41–49. doi: 10.1016/0196-9781(85)90133-0. [DOI] [PubMed] [Google Scholar]
- Ahern TH, Modi ME, Burkett JP, Young LJ. Evaluation of two automated metrics for analyzing partner preference tests. J Neurosci Methods. 2009;182:180–188. doi: 10.1016/j.jneumeth.2009.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahern TH, Young LJ. The impact of early life family structure on adult social attachment, alloparental behavior, and the neuropeptide systems regulating affiliative behaviors in the monogamous prairie vole (Microtus ochrogaster) Front Behav Neurosci. 2009;3:17. doi: 10.3389/neuro.08.017.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvaro JD, Tatro JB, Duman RS. Melanocortins and opiate addiction. Life Sci. 1997;61:1–9. doi: 10.1016/s0024-3205(97)00029-5. [DOI] [PubMed] [Google Scholar]
- Alvaro JD, Tatro JB, Quillan JM, Fogliano M, Eisenhard M, Lerner MR, Nestler EJ, Duman RS. Morphine down-regulates melanocortin-4 receptor expression in brain regions that mediate opiate addiction. Mol Pharmacol. 1996;50:583–591. [PubMed] [Google Scholar]
- Aragona BJ, Liu Y, Yu YJ, Curtis JT, Detwiler JM, Insel TR, Wang Z. Nucleus accumbens dopamine differentially mediates the formation and maintenance of monogamous pair bonds. Nat Neurosci. 2006;9:133–139. doi: 10.1038/nn1613. [DOI] [PubMed] [Google Scholar]
- Argiolas A, Melis MR, Murgia S, Schioth HB. ACTH- and alpha-MSH-induced grooming, stretching, yawning and penile erection in male rats: site of action in the brain and role of melanocortin receptors. Brain Res Bull. 2000;51:425–431. doi: 10.1016/s0361-9230(99)00270-1. [DOI] [PubMed] [Google Scholar]
- Arletti R, Benelli A, Bertolini A. Influence of oxytocin on feeding behavior in the rat. Peptides. 1989;10:89–93. doi: 10.1016/0196-9781(89)90082-x. [DOI] [PubMed] [Google Scholar]
- Bales KL, Carter CS. Developmental exposure to oxytocin facilitates partner preferences in male prairie voles (Microtus ochrogaster) Behav Neurosci. 2003a;117:854–859. doi: 10.1037/0735-7044.117.4.854. [DOI] [PubMed] [Google Scholar]
- Bales KL, Carter CS. Sex differences and developmental effects of oxytocin on aggression and social behavior in prairie voles (Microtus ochrogaster) Horm Behav. 2003b;44:178–184. doi: 10.1016/s0018-506x(03)00154-5. [DOI] [PubMed] [Google Scholar]
- Bales KL, van Westerhuyzen JA, Lewis-Reese AD, Grotte ND, Lanter JA, Carter CS. Oxytocin has dose-dependent developmental effects on pair-bonding and alloparental care in female prairie voles. Horm Behav. 2007;52:274–279. doi: 10.1016/j.yhbeh.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckwith BE, O’Quin RK, Petro MS, Kastin AJ, Sandman CA. The effects of neonatal injections of alpha-MSH on the open-field behavior of juvenile and adult rats. Physiol Psych. 1977a;5:295–299. [Google Scholar]
- Beckwith BE, Sandman CA, Hothersall D, Kastin AJ. Influence of neonatal injections of alpha-MSH on learning, memory and attention in rats. Physiol Behav. 1977b;18:63–71. doi: 10.1016/0031-9384(77)90095-6. [DOI] [PubMed] [Google Scholar]
- Ben-Barak Y, Russell JT, Whitnall MH, Ozato K, Gainer H. Neurophysin in the hypothalamo-neurohypophysial system. I. Production and characterization of monoclonal antibodies. J Neurosci. 1985;5:81–97. doi: 10.1523/JNEUROSCI.05-01-00081.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch OJ, Nair HP, Ahern TH, Neumann ID, Young LJ. The CRF system mediates increased passive stress-coping behavior following the loss of a bonded partner in a monogamous rodent. Neuropsychopharmacology. 2009;34:1406–1415. doi: 10.1038/npp.2008.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branchi I, Curley JP, D’Andrea I, Cirulli F, Champagne FA, Alleva E. Early interactions with mother and peers independently build adult social skills and shape BDNF and oxytocin receptor brain levels. Psychoneuroendocrinology. 2013;38:522–532. doi: 10.1016/j.psyneuen.2012.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunson KL, Khan N, Eghbal-Ahmadi M, Baram TZ. Corticotropin (ACTH) acts directly on amygdala neurons to down-regulate corticotropin-releasing hormone gene expression. Ann Neurol. 2001;49:304–312. [PMC free article] [PubMed] [Google Scholar]
- Burkett JP, Spiegel LL, Inoue K, Murphy AZ, Young LJ. Activation of mu-opioid receptors in the dorsal striatum is necessary for adult social attachment in monogamous prairie voles. Neuropsychopharmacology. 2011;36:2200–2210. doi: 10.1038/npp.2011.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkett JP, Young LJ. The behavioral, anatomical and pharmacological parallels between social attachment, love and addiction. Psychopharmacology (Berl) 2012;224:1–26. doi: 10.1007/s00213-012-2794-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caba M, Rovirosa MJ, Silver R. Suckling and genital stroking induces Fos expression in hypothalamic oxytocinergic neurons of rabbit pups. Brain Res Dev Brain Res. 2003;143:119–128. doi: 10.1016/s0165-3806(03)00064-6. [DOI] [PubMed] [Google Scholar]
- Carter CS, Boone EM, Pournajafi-Nazarloo H, Bales KL. Consequences of early experiences and exposure to oxytocin and vasopressin are sexually dimorphic. Dev Neurosci. 2009;31:332–341. doi: 10.1159/000216544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter CS, Witt DM, Thompson EG, Carlstead K. Effects of hormonal, sexual, and social history on mating and pair bonding in prairie voles. Physiol Behav. 1988;44:691–697. doi: 10.1016/0031-9384(88)90049-2. [DOI] [PubMed] [Google Scholar]
- Chaki S, Okuyama S. Involvement of melanocortin-4 receptor in anxiety and depression. Peptides. 2005;26:1952–1964. doi: 10.1016/j.peptides.2004.11.029. [DOI] [PubMed] [Google Scholar]
- Champney TF, Sahley TL, Sandman CA. Effects of neonatal cerebral ventricular injection of ACTH 4-9 and subsequent adult injections on learning in male and female albino rats. Pharmacol Biochem Behav. 1976;5:3–9. doi: 10.1016/0091-3057(76)90321-x. [DOI] [PubMed] [Google Scholar]
- Chau MJ, Stone AI, Mendoza SP, Bales KL. Is play behavior sexually dimorphic in monogamous species? Ethology. 2008;114:989–998. [Google Scholar]
- Chen AS, Marsh DJ, Trumbauer ME, Frazier EG, Guan XM, Yu H, Rosenblum CI, Vongs A, Feng Y, Cao L, Metzger JM, Strack AM, Camacho RE, Mellin TN, Nunes CN, Min W, Fisher J, Gopal-Truter S, MacIntyre DE, Chen HY, Van der Ploeg LH. Inactivation of the mouse melanocortin-3 receptor results in increased fat mass and reduced lean body mass. Nat Genet. 2000;26:97–102. doi: 10.1038/79254. [DOI] [PubMed] [Google Scholar]
- Cushing BS, Levine K, Cushing NL. Neonatal manipulation of oxytocin influences female reproductive behavior and success. Horm Behav. 2005;47:22–28. doi: 10.1016/j.yhbeh.2004.08.004. [DOI] [PubMed] [Google Scholar]
- De Barioglio SR, Lezcano N, Celis ME. Alpha MSH-induced excessive grooming behavior involves a GABAergic mechanism. Peptides. 1991;12:203–205. doi: 10.1016/0196-9781(91)90189-v. [DOI] [PubMed] [Google Scholar]
- Dhillo WS, Small CJ, Seal LJ, Kim MS, Stanley SA, Murphy KG, Ghatei MA, Bloom SR. The hypothalamic melanocortin system stimulates the hypothalamo-pituitary-adrenal axis in vitro and in vivo in male rats. Neuroendocrinology. 2002;75:209–216. doi: 10.1159/000054712. [DOI] [PubMed] [Google Scholar]
- Edelmann MN, Demers CH, Auger AP. Maternal touch moderates sex differences in juvenile social play behavior. PLoS One. 2013;8:e57396. doi: 10.1371/journal.pone.0057396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egles C, Rene F, Varon S, Louis JC, Felix JM, Schimchowitsch S. Differentiation of rat hypothalamic dopaminergic neurons is stimulated in vitro by target cells: the melanotrophs. Eur J Neurosci. 1998;10:1270–1281. doi: 10.1046/j.1460-9568.1998.00138.x. [DOI] [PubMed] [Google Scholar]
- Felszeghy K, Sasvari M, Nyakas C. Behavioral depression: opposite effects of neonatal dexamethasone and ACTH-(4-9) analogue (ORG 2766) treatments in the rat. Horm Behav. 1993;27:380–396. doi: 10.1006/hbeh.1993.1028. [DOI] [PubMed] [Google Scholar]
- Gavish L, Carter CS, Getz LL. Male-Female Interactions in Prairie Voles. Animal Behaviour. 1983;31:511–517. [Google Scholar]
- Glavas MM, Joachim SE, Draper SJ, Smith MS, Grove KL. Melanocortinergic activation by melanotan II inhibits feeding and increases uncoupling protein 1 messenger ribonucleic acid in the developing rat. Endocrinology. 2007;148:3279–3287. doi: 10.1210/en.2007-0184. [DOI] [PubMed] [Google Scholar]
- Gomez-Pinilla F, Vaynman S, Ying Z. Brain-derived neurotrophic factor functions as a metabotrophin to mediate the effects of exercise on cognition. Eur J Neurosci. 2008;28:2278–2287. doi: 10.1111/j.1460-9568.2008.06524.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorzalka BB, Lester GL. Oxytocin-induced facilitation of lordosis behaviour in rats is progesterone-dependent. Neuropeptides. 1987;10:55–65. doi: 10.1016/0143-4179(87)90089-8. [DOI] [PubMed] [Google Scholar]
- Greenman Y, Kuperman Y, Drori Y, Asa SL, Navon I, Forkosh O, Gil S, Stern N, Chen A. Postnatal ablation of POMC neurons induces an obese phenotype characterized by decreased food intake and enhanced anxiety-like behavior. Mol Endocrinol. 2013;27:1091–1102. doi: 10.1210/me.2012-1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu R, Taylor JR, Newton SS, Alvaro JD, Haile C, Han G, Hruby VJ, Nestler EJ, Duman RS. Blockade of melanocortin transmission inhibits cocaine reward. Eur J Neurosci. 2005;21:2233–2242. doi: 10.1111/j.1460-9568.2005.04038.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huszar D, Lynch CA, Fairchild-Huntress V, Dunmore JH, Fang Q, Berkemeier LR, Gu W, Kesterson RA, Boston BA, Cone RD, Smith FJ, Campfield LA, Burn P, Lee F. Targeted disruption of the melanocortin-4 receptor results in obesity in mice. Cell. 1997;88:131–141. doi: 10.1016/s0092-8674(00)81865-6. [DOI] [PubMed] [Google Scholar]
- Joosten EA, Verhaagh S, Martin D, Robe P, Franzen R, Hooiveld M, Doornbos R, Bar PR, Moonen G. Alpha-MSH stimulates neurite outgrowth of neonatal rat corticospinal neurons in vitro. Brain Research. 1996;736:91–98. doi: 10.1016/0006-8993(96)00700-7. [DOI] [PubMed] [Google Scholar]
- Keebaugh AC, Young LJ. Increasing oxytocin receptor expression in the nucleus accumbens of pre-pubertal female prairie voles enhances alloparental responsiveness and partner preference formation as adults. Horm Behav. 2011;60:498–504. doi: 10.1016/j.yhbeh.2011.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konda Y, Gantz I, DelValle J, Shimoto Y, Miwa H, Yamada T. Interaction of dual intracellular signaling pathways activated by the melanocortin-3 receptor. J Biol Chem. 1994;269:13162–13166. [PubMed] [Google Scholar]
- Kramer KM, Cushing BS, Carter CS. Developmental effects of oxytocin on stress response: single versus repeated exposure. Physiol Behav. 2003;79:775–782. doi: 10.1016/s0031-9384(03)00175-6. [DOI] [PubMed] [Google Scholar]
- Lansdell MI, Hepworth D, Calabrese A, Brown AD, Blagg J, Burring DJ, Wilson P, Fradet D, Brown TB, Quinton F, Mistry N, Tang K, Mount N, Stacey P, Edmunds N, Adams C, Gaboardi S, Neal-Morgan S, Wayman C, Cole S, Phipps J, Lewis M, Verrier H, Gillon V, Feeder N, Heatherington A, Sultana S, Haughie S, Martin SW, Sudworth M, Tweedy S. Discovery of a selective small-molecule melanocortin-4 receptor agonist with efficacy in a pilot study of sexual dysfunction in humans. J Med Chem. 2010;53:3183–3197. doi: 10.1021/jm9017866. [DOI] [PubMed] [Google Scholar]
- Lim BK, Huang KW, Grueter BA, Rothwell PE, Malenka RC. Anhedonia requires MC4R-mediated synaptic adaptations in nucleus accumbens. Nature. 2012;487:183–189. doi: 10.1038/nature11160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim MM, Liu Y, Ryabinin AE, Bai Y, Wang Z, Young LJ. CRF receptors in the nucleus accumbens modulate partner preference in prairie voles. Horm Behav. 2007;51:508–515. doi: 10.1016/j.yhbeh.2007.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindblom J, Kask A, Hagg E, Harmark L, Bergstrom L, Wikberg J. Chronic infusion of a melanocortin receptor agonist modulates dopamine receptor binding in the rat brain. Pharmacol Res. 2002;45:119–124. doi: 10.1006/phrs.2001.0913. [DOI] [PubMed] [Google Scholar]
- Lindblom J, Opmane B, Mutulis F, Mutule I, Petrovska R, Klusa V, Bergstrom L, Wikberg JE. The MC4 receptor mediates alpha-MSH induced release of nucleus accumbens dopamine. Neuroreport. 2001;12:2155–2158. doi: 10.1097/00001756-200107200-00022. [DOI] [PubMed] [Google Scholar]
- Liu H, Kishi T, Roseberry AG, Cai X, Lee CE, Montez JM, Friedman JM, Elmquist JK. Transgenic mice expressing green fluorescent protein under the control of the melanocortin-4 receptor promoter. J Neurosci. 2003;23:7143–7154. doi: 10.1523/JNEUROSCI.23-18-07143.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Garza JC, Li W, Lu XY. Melanocortin-4 receptor in the medial amygdala regulates emotional stress-induced anxiety-like behaviour, anorexia and corticosterone secretion. Int J Neuropsychopharmacol. 2013;16:105–120. doi: 10.1017/S146114571100174X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu XY, Barsh GS, Akil H, Watson SJ. Interaction between alpha-melanocyte-stimulating hormone and corticotropin-releasing hormone in the regulation of feeding and hypothalamo-pituitary-adrenal responses. J Neurosci. 2003;23:7863–7872. doi: 10.1523/JNEUROSCI.23-21-07863.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGraw LA, Young LJ. The prairie vole: an emerging model organism for understanding the social brain. Trends Neurosci. 2010;33:103–109. doi: 10.1016/j.tins.2009.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melis MR, Argiolas A, Gessa GL. Oxytocin-induced penile erection and yawning: site of action in the brain. Brain Research. 1986;398:259–265. doi: 10.1016/0006-8993(86)91485-x. [DOI] [PubMed] [Google Scholar]
- Modi ME, Inoue K, Barrett CE, Kittelberger KA, Smith DG, Landgraf R, Young LJ. Melanocortin receptor agonists enhance oxytocin-dependent partner preference formation in the prairie vole. doi: 10.1038/npp.2015.35. Unpublished results. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore CL, Power KL. Variation in maternal care and individual differences in play, exploration, and grooming of juvenile Norway rat offspring. Dev Psychobiol. 1992;25:165–182. doi: 10.1002/dev.420250303. [DOI] [PubMed] [Google Scholar]
- Mountjoy KG. Distribution and function of melanocortin receptors within the brain. Adv Exp Med Biol. 2010;681:29–48. doi: 10.1007/978-1-4419-6354-3_3. [DOI] [PubMed] [Google Scholar]
- Muceniece R, Zvejniece L, Vilskersts R, Liepinsh E, Baumane L, Kalvinsh I, Wikberg JE, Dambrova M. Functional evaluation of THIQ, a melanocortin 4 receptor agonist, in models of food intake and inflammation. Basic Clin Pharmacol Toxicol. 2007;101:416–420. doi: 10.1111/j.1742-7843.2007.00133.x. [DOI] [PubMed] [Google Scholar]
- Nicholson JR, Peter JC, Lecourt AC, Barde YA, Hofbauer KG. Melanocortin-4 receptor activation stimulates hypothalamic brain-derived neurotrophic factor release to regulate food intake, body temperature and cardiovascular function. J Neuroendocrinol. 2007;19:974–982. doi: 10.1111/j.1365-2826.2007.01610.x. [DOI] [PubMed] [Google Scholar]
- Nickolls SA, Fleck B, Hoare SR, Maki RA. Functional selectivity of melanocortin 4 receptor peptide and nonpeptide agonists: evidence for ligand-specific conformational states. J Pharmacol Exp Ther. 2005;313:1281–1288. doi: 10.1124/jpet.105.083337. [DOI] [PubMed] [Google Scholar]
- Oosterom J, Nijenhuis WA, Schaaper WM, Slootstra J, Meloen RH, Gispen WH, Burbach JP, Adan RA. Conformation of the core sequence in melanocortin peptides directs selectivity for the melanocortin MC3 and MC4 receptors. J Biol Chem. 1999;274:16853–16860. doi: 10.1074/jbc.274.24.16853. [DOI] [PubMed] [Google Scholar]
- Panksepp J, Beatty WW. Social deprivation and play in rats. Behav Neural Biol. 1980;30:197–206. doi: 10.1016/s0163-1047(80)91077-8. [DOI] [PubMed] [Google Scholar]
- Parent CI, Meaney MJ. The influence of natural variations in maternal care on play fighting in the rat. Dev Psychobiol. 2008;50:767–776. doi: 10.1002/dev.20342. [DOI] [PubMed] [Google Scholar]
- Parker KJ, Maestripieri D. Identifying key features of early stressful experiences that produce stress vulnerability and resilience in primates. Neurosci Biobehav Rev. 2011;35:1466–1483. doi: 10.1016/j.neubiorev.2010.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poggioli R, Vergoni AV, Bertolini A. ACTH-(1-24) and alpha-MSH antagonize feeding behavior stimulated by kappa opiate agonists. Peptides. 1986;7:843–848. doi: 10.1016/0196-9781(86)90104-x. [DOI] [PubMed] [Google Scholar]
- Polston EK, Erskine MS. Patterns of induction of the immediate-early genes c-fos and egr-1 in the female rat brain following differential amounts of mating stimulation. Neuroendocrinology. 1995;62:370–384. doi: 10.1159/000127027. [DOI] [PubMed] [Google Scholar]
- Poole TB, Fish J. An investigation of individual, age and sexual differences in the play of Rattus norvegicus (Mammalia: Rodentia) Journal of Zoology. 1976;179:249–259. [Google Scholar]
- Rossler AS, Pfaus JG, Kia HK, Bernabe J, Alexandre L, Giuliano F. The melanocortin agonist, melanotan II, enhances proceptive sexual behaviors in the female rat. Pharmacol Biochem Behav. 2006;85:514–521. doi: 10.1016/j.pbb.2006.09.023. [DOI] [PubMed] [Google Scholar]
- Ruscio MG, Sweeny T, Hazelton J, Suppatkul P, Sue Carter C. Social environment regulates corticotropin releasing factor, corticosterone and vasopressin in juvenile prairie voles. Horm Behav. 2007;51:54–61. doi: 10.1016/j.yhbeh.2006.08.004. [DOI] [PubMed] [Google Scholar]
- Sabatier N. alpha-Melanocyte-stimulating hormone and oxytocin: a peptide signalling cascade in the hypothalamus. J Neuroendocrinol. 2006;18:703–710. doi: 10.1111/j.1365-2826.2006.01464.x. [DOI] [PubMed] [Google Scholar]
- Sabatier N, Caquineau C, Dayanithi G, Bull P, Douglas AJ, Guan XM, Jiang M, Van der Ploeg L, Leng G. Alpha-melanocyte-stimulating hormone stimulates oxytocin release from the dendrites of hypothalamic neurons while inhibiting oxytocin release from their terminals in the neurohypophysis. J Neurosci. 2003;23:10351–10358. doi: 10.1523/JNEUROSCI.23-32-10351.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Y, Fu WY, Cheng EY, Fu AK, Ip NY. Melanocortin-4 receptor regulates hippocampal synaptic plasticity through a protein kinase A-dependent mechanism. J Neurosci. 2013;33:464–472. doi: 10.1523/JNEUROSCI.3282-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MA, Kim SY, van Oers HJ, Levine S. Maternal deprivation and stress induce immediate early genes in the infant rat brain. Endocrinology. 1997;138:4622–4628. doi: 10.1210/endo.138.11.5529. [DOI] [PubMed] [Google Scholar]
- Spear LP, Brake SC. Periadolescence: age-dependent behavior and psychopharmacological responsivity in rats. Dev Psychobiol. 1983;16:83–109. doi: 10.1002/dev.420160203. [DOI] [PubMed] [Google Scholar]
- Stribley JM, Carter CS. Developmental exposure to vasopressin increases aggression in adult prairie voles. Proc Natl Acad Sci U S A. 1999;96:12601–12604. doi: 10.1073/pnas.96.22.12601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao YX. The melanocortin-4 receptor: physiology, pharmacology, and pathophysiology. Endocr Rev. 2010;31:506–543. doi: 10.1210/er.2009-0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor GT. Fighting in juvenile rats and the ontogeny of agonistic behavior. J Comp Physiol Psychol. 1980;94:953–961. [Google Scholar]
- Thody AJ. alpha-MSH and the regulation of melanocyte function. Ann N Y Acad Sci. 1999;885:217–229. doi: 10.1111/j.1749-6632.1999.tb08679.x. [DOI] [PubMed] [Google Scholar]
- Thor DH, Holloway WR., JR Play-solicitation behavior in juvenile male and female rats. Animal Leaning & Behavior. 1983;11:173–178. [Google Scholar]
- Veenema AH, Neumann ID. Maternal separation enhances offensive play-fighting, basal corticosterone and hypothalamic vasopressin mRNA expression in juvenile male rats. Psychoneuroendocrinology. 2009;34:463–467. doi: 10.1016/j.psyneuen.2008.10.017. [DOI] [PubMed] [Google Scholar]
- Wang J, Tai F, Yan X, Yu P. Paternal deprivation alters play-fighting, serum corticosterone and the expression of hypothalamic vasopressin and oxytocin in juvenile male mandarin voles. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2012;198:787–796. doi: 10.1007/s00359-012-0748-8. [DOI] [PubMed] [Google Scholar]
- Wikberg JE, Muceniece R, Mandrika I, Prusis P, Lindblom J, Post C, Skottner A. New aspects on the melanocortins and their receptors. Pharmacol Res. 2000;42:393–420. doi: 10.1006/phrs.2000.0725. [DOI] [PubMed] [Google Scholar]
- Williams JR, Catania KC, Carter CS. Development of partner preferences in female prairie voles (Microtus ochrogaster): the role of social and sexual experience. Horm Behav. 1992;26:339–349. doi: 10.1016/0018-506x(92)90004-f. [DOI] [PubMed] [Google Scholar]
- Wu CS, Greenwood DR, Cooney JM, Jensen DJ, Tatnell MA, Cooper GJ, Mountjoy KG. Peripherally administered desacetyl alpha-MSH and alpha-MSH both influence postnatal rat growth and associated rat hypothalamic protein expression. Am J Physiol Endocrinol Metab. 2006;291:E1372–1380. doi: 10.1152/ajpendo.00480.2005. [DOI] [PubMed] [Google Scholar]
- Xu B, Goulding EH, Zang K, Cepoi D, Cone RD, Jones KR, Tecott LH, Reichardt LF. Brain-derived neurotrophic factor regulates energy balance downstream of melanocortin-4 receptor. Nat Neurosci. 2003;6:736–742. doi: 10.1038/nn1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young LJ, Wang Z. The neurobiology of pair bonding. Nat Neurosci. 2004;7:1048–1054. doi: 10.1038/nn1327. [DOI] [PubMed] [Google Scholar]


