Abstract
We report on the design and synthesis of molecules having E- and P-selectins blocking activity both in vitro and in vivo. The GlcNAc component of the selectin ligand sialyl LewisX was replaced by an acyclic tether that links two saccharide units. The minimization of intramolecular dipole–dipole interactions and the gauche effect would be at the origin of the conformational bias imposed by this acyclic tether. The stereoselective synthesis of these molecules, their biochemical and biological evaluations using surface plasmon resonance spectroscopy (SPR), and in vivo assays are described. Because the structure of our analogues differs from the most potent E-selectin antagonists reported, our acyclic analogues offer new opportunities for chemical diversity.
Keywords: Polysaccharide-based ligands, selectin antagonists, sialyl LewisX, surface plasmon resonance spectroscopy, carbohydrate recognition domain
The design of molecules mimicking natural ligands that interact with biologically relevant receptors is a widely used approach in medicinal chemistry. However, improving the potency of these natural molecules is challenging, particularly with polysaccharide compounds. These molecules are structurally complex and possess many stereocenters with different functionalities that complicate the identification of the pharmacophores involved in the binding to the receptor. Sialyl LewisX (1, sLeX), a sialylated and fucosylated tetrasaccharide, represents a particularly interesting target for the development of novel pharmaceutical agents and has not surprisingly been the subject of numerous medicinal studies (Figure 1).1−16 A sLeX antagonist (GMI-1070, 2) was recently shown to reverse vascular occlusions in sickle cell animal model and in preliminary clinical trials, when given intravenously (Figure 1).17
Figure 1.
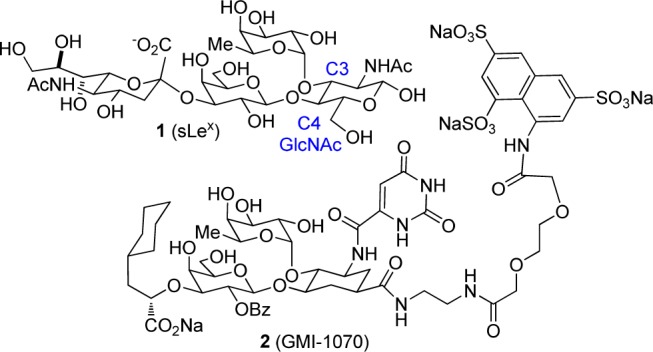
Sialyl LewisX (sLeX 1) and GMI-1070.17
sLeX is found on leukocytes at the terminus of P-selectin glycoprotein-1 ligand (PSGL-1) and E-selectin ligand-1 (ESL-1). E- and P-selectin proteins are expressed on the vascular walls responding to various inflammatory signals resulting from hypertension, atherosclerosis, and other traumas.18 The interactions between the vascular receptors and the sLeX ligands on the circulating cells induce the rolling of the leukocyte on the vascular walls, followed by their arrest and extravasation to inflammatory sites. Up-regulation of the β-2 integrin Mac-1 on the leukocytes surface after binding to vascular selectins would promote the aggregation of red blood cells (RBC) and eventually trigger the occlusion of small vessels.19 This is a particularly dangerous phenotype for sickle cell disease patients. Interestingly, E-selectins are also expressed by the bone marrow endothelial cells in the vascular hematopoietic stem cells (HSCs) niche.20 Binding to E-selectin seemingly induces HSC proliferation. Deletion of E-selectin in KO mice or blockade by GMI-1070 retarded their proliferation in this niche, allowing protection of the mice HSC from a systemic exposure to cytotoxic anticancer agents or irradiation. Enhanced survival relative to the control was observed in the recuperation phase of these animals. Another noteworthy biological role of selectin antagonists concerns their putative implication in reducing myocardial damage after percutaneous coronary intervention (PCI).21
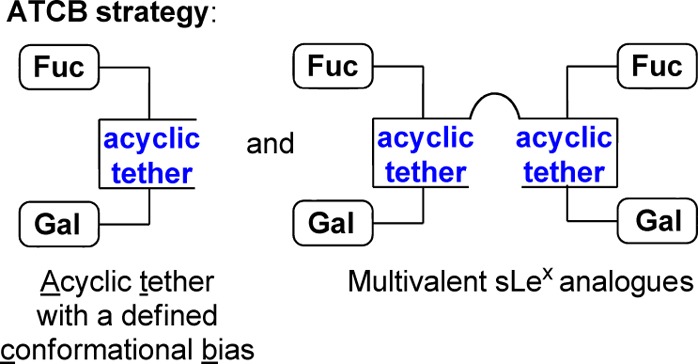
We have reported on the replacement of the sLeX GlucNAc saccharide unit with an acyclic tether possessing a conformational bias (ATCB strategy, Figure 2).22 A promising P-selectin antagonist with in vitro activity (3) was identified in this pilot study. X-ray and NMR analyses suggested that the acyclic l-tartrate methyl ester subunit of 3 (Figure 2) was orienting the two sugar moieties attached at C2 and C3 in a gauche conformation, similar to the one imposed by the GlcNAc unit of sLeX (Figure 1).22−24 This first study confirmed that the relative plasticity of our tether contrasts with the rigidity of cyclic tethers more generally employed, allowing a productive binding to the target receptors. A significant increase of activity was also observed when a benzoate group was introduced at C4 of the galactose subunit.
Figure 2.
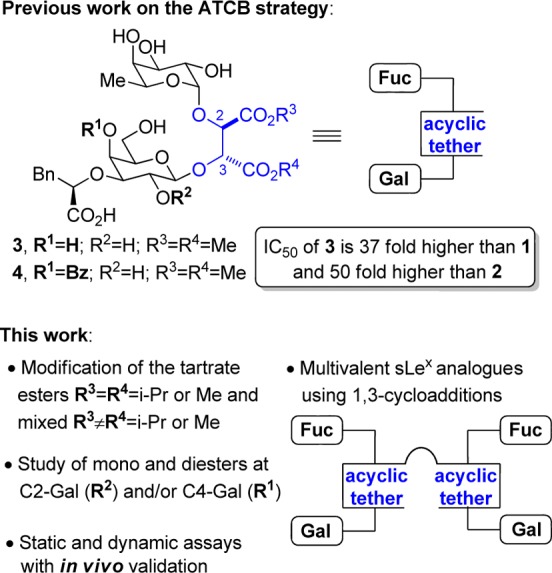
Acyclic tether with a defined conformational bias (ATCB strategy).
The present work aimed at improving the properties of our acyclic tether and at increasing the potency of the lead compound 3 that was identified previously. We hypothesized that bulkier ester groups could induce an orientation of the fucose and galactose sugar moieties to increase the binding to selectins. The impact of installing a benzoate group at the C2-galactose position (R2) was also examined. Other groups observed that this modification enhances significantly the potency of their sLeX analogues.7,9,16 Another avenue that we have begun to explore herein involves the preparation and biological evaluation of multivalent sLeX analogues (Figure 2).
Synthesis of sLeX Analogues with Acyclic Tethers
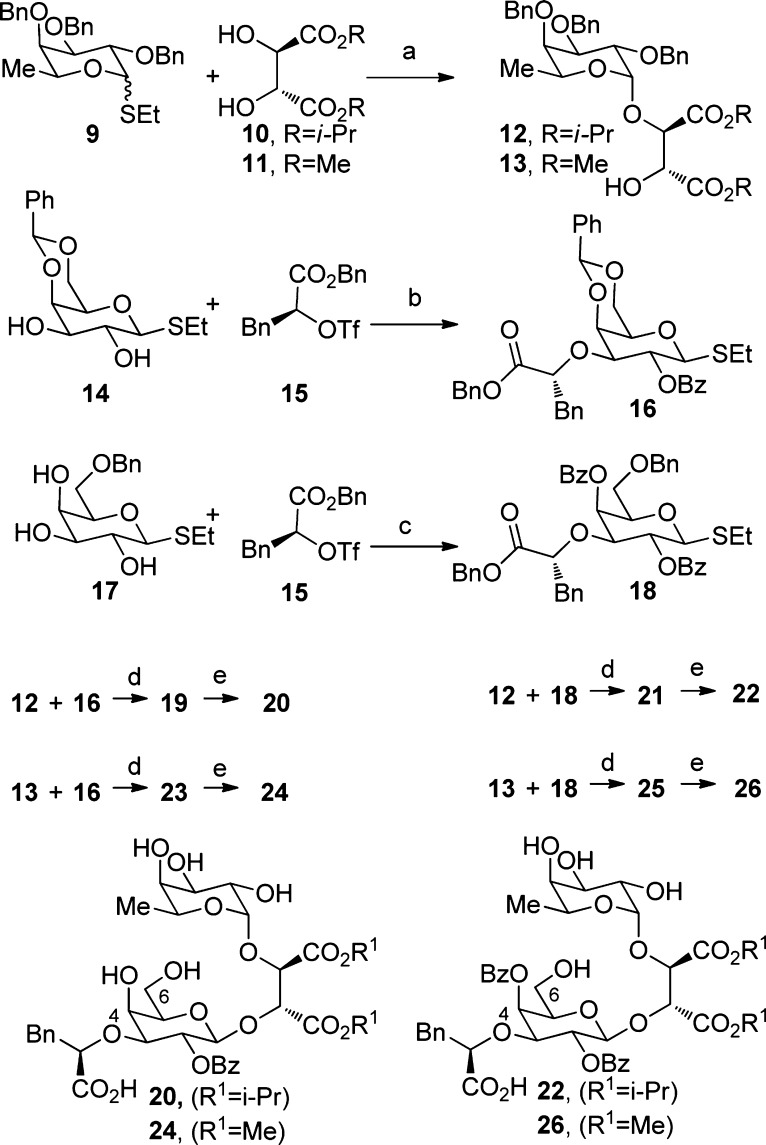
The first series of analogues was prepared by coupling the fucosides 12 and 13, bearing the acyclic tether, with galactoside donors 16 and 18 (Scheme 1). The former were prepared by adding l-tartrate ester 10 or 11 to perbenzylated thioethyl fucoside 9 in the presence of NIS (Scheme 1).25 The β-thioethyl galactoside with C4 and C6 hydroxyls protected by a benzylidene acetal was obtained by a regioselective C3 O-alkylation of 14 with triflate 15 using in situ formation of organotin acetals. The benzoate at C2 was then installed to give 16. A similar approach was employed from β-thioethyl galactoside 17 to generate 18. Both 16 and 18 were then coupled to 12 and 13 in the presence of NIS/TMSOTf at −30 °C.
Scheme 1. Synthesis of sLeX Analogues 20, 22, 24, and 26.
(a) NIS/CF3SO3H, CH2Cl2, −30 °C, 4 Å mol sieves (88% for 12 and 75% for 13); (b) i. Bu2SnO, MeOH, then CsF and 15 in THF; ii. BzCl, DMAP, DCM, 93% over 2 steps; (c) i. Bu2SnO, MeOH, then CsF and 15 in THF; ii. BzCl, DMAP, DCM, 70% over 2 steps; (d) NIS/TMSOTf, CH2Cl2, −30 °C, 4 Å mol sieves (60% for 19, 77% for 21, 67% for 23, and 72% for 25); (e) Pd/C, H2, dioxane (80% for 20, 65% for 22, 53% for 24, and 53% for 26).
The β-selectivities for these glycosylations are attributed to anchimeric assistance of the ester at C2.26 After debenzylation with Pd/C in the presence of H2, the targeted products 20, 22, 24, and 26 were obtained.
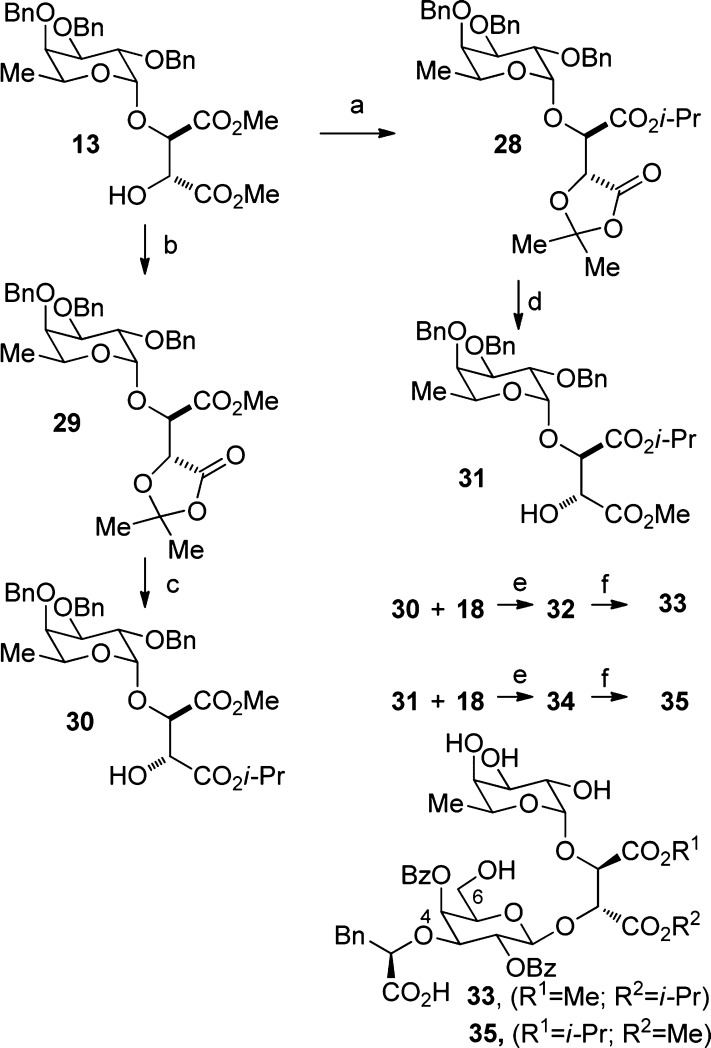
The selective differentiation of the tartrate esters was challenging (Scheme 2). A dioxolanone intermediate was prepared by hydrolyzing 13 with an NaOH solution and treating the resulting product with an excess of 2,2-dimethoxy propane and a catalytic amount of PTSA.27 The crude mixture was then dissolved in DMF and reacted with Cs2CO3 and isopropyl iodide to give 28. Hydrolysis of the latter with AcOH in water at 50 °C and treatment with TMSCH2N2 provided 31. Inverting the order of the esterification steps led to 30, the structure of which was confirmed by X-ray analysis of a para-nitrobenzoate derivative.28 Both 30 and 31 were then coupled with 18 using a mixture of TMSOTf/NIS in CH2Cl2 to generate 32 and 34. Removal of the four benzyl groups by hydrogenolysis yielded the final products 33 and 35.
Scheme 2. Synthesis of 33 and 35.
(a) i. NaOH solution (10%), THF, 25 °C; ii. 2,2-dimethoxy-propane, PTSA, DCM, 25 °C, no purification; iii. Cs2CO3, i-PrI, DMF, 25 °C, 56% over 3 steps; (b) i. NaOH solution (10%), THF, 25 °C; ii. 2,2-dimethoxypropane, PTSA, DCM, 25 °C; iii. TMSCH2N2, MeOH, 25 °C, 72% over 3 steps; (c) i. AcOH/H2O (80:20), 50 °C; ii. Cs2CO3, i-PrI, DMF, 25 °C, 59% over 2 steps; (d) i. AcOH/H2O (80:20), 50 °C; ii. TMSCH2N2, DCM, 25 °C, 66% over 2 steps; (e) TMSOTf, NIS, DCM, −25 °C, 81% for 32 and 75% for 34; (h) H2, Pd/C, dioxane, 23% for 33 and 20% for 35.
E- and P-Selectin Static Assays and P-Selectin Dynamic Assay
sLeX analogues were first evaluated in E- and P-selectin cell-based adhesion assays (static assay, Table 1).29 We also performed a more direct competition assay using surface plasmon resonance spectroscopy (SPR, dynamic assay, Table 1).22,30 The extracellular monomeric human PSGL-1 (hPSGL-1) fused with the Fc portion of a human IgG (rPSGL-Ig) was covalently attached to a sensor chip. A constant amount of soluble P-selectin with variable concentrations of one of our molecules was then injected in the flow cell. In this assay, the tested analogues compete with the carbohydrate motifs attached on the immobilized protein for binding to P-selectins (P-selectin dynamic assay).
Table 1. IC50 and Relative IC50 versus sLeX in E- and P-Selectin Static Assays and P-Selectin Dynamic Assay.
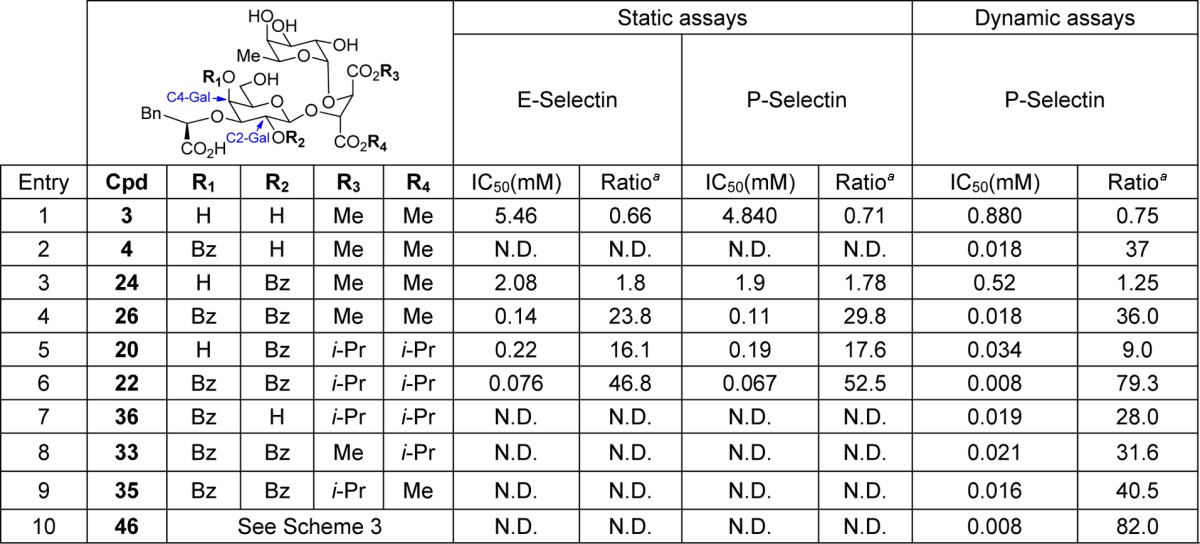
Ratio or relative IC50 = IC50(Cpd)/IC50(sLeX).
Biological evaluations of the analogues by static and dynamic assays are presented at Table 1. sLeX was used as a control in each assay. A ratio of the relative potency of the tested analogues and sLeX was calculated [IC50(Cpd)/IC50(sLeX)]. As reported in the previous pilot study, replacing the GlucNAc subunit by an acyclic tether provides molecular prototype 3 with an antagonist activity slightly lower than sLeX with both P- and E-selectin (entry 1). Compound 4, bearing a benzoate at the C4-Gal position, is 37 times more potent than sLeX (entry 2). We hypothesized that this improvement originates from a favorable interaction of the benzoate with the Tyr94 in the carbohydrate binding domain (CRD) of P-selectin.22
The potency of sLeX24 bearing a benzoate at C2 of galactose was next examined (entry 3, Table 1). Only a slight improvement of the potency was noted with 24, as compared to 3. The installation of benzoates at C2 and C4 provided a product (26) with high potency in the three assays (entry 4). Both 26 and 4 have, however, the same potency in the P-selectin binding assay, which indicates the importance of the benzoate at C4.
Di-isopropyl esters displayed improved IC50 in the static and dynamic assays. Compound 20 (entry 5) bearing a benzoate at C2-Gal was more potent than its dimethyl ester counterpart 24 (entry 5 versus entry 3). The dibenzoate derivative 22 (bearing diisopropyl esters) provided exciting results (entry 6). Potency ratios ranging from 47 to 79 were observed. We then sought to rationalize the increase of potency noted. As indicated by preliminary NMR spectroscopy experiments, the relative alignment of the fucose and galactose moieties was modified in the ground state conformation. Contrary to the methyl ester series, intramolecular nuclear Overhauser effect (NOE) interactions between the methyl of fucose and the methylene (C6) of galactose are now present, suggesting a proximal stacking of these units. These stacking interactions have been identified as important in the pioneering work of Thoma, Ernst, and others.4,9,12
We then prepared the C4–OBz 36 using the same sequence of reactions described in our previous study, changing only the esters (entry 2 versus entry 7). From this point on, only the P-selectin dynamic assay (SPR) was performed. No significant change of the potency was noted, a ratio of 28 being obtained for 36 (entry 7), as opposed to 37 for 4 (entry 2). This result shows the importance of the benzoate at C2 regarding the increase of potency induced by diisopropylester groups. The replacement of one of the isopropyls by a methyl was then evaluated. For each molecule 33 or 35 a significant reduction of potency was measured (entries 8 and 9). The variation of the nature of the esters and its replacement by other functionalities are avenues to be explored in a subsequent study.
In Vivo Evaluations of sLeX Analogues
We have begun the in vivo evaluation of our molecules. Leukocyte rolling flux was measured using intravital microscopy and tumor necrosis factor (TNFα) stimulated mouse cremaster. The monobenzoate di-isopropyl ester 20 dissolved in a saline solution was evaluated for its capacity to inhibit the decreased leukocyte rolling flux induced by TNFα. As seen in Figure 3, the addition of TNFα led to a decreased rolling velocity (B, red, versus A, red), which was not reversed by a subsequent saline control (B, green). Sialyl LewisX reversed the effect of TNFα (C, green). Similarly, a significant increase of rolling velocity was noted when analogue 20 was injected (D, green).
Figure 3.
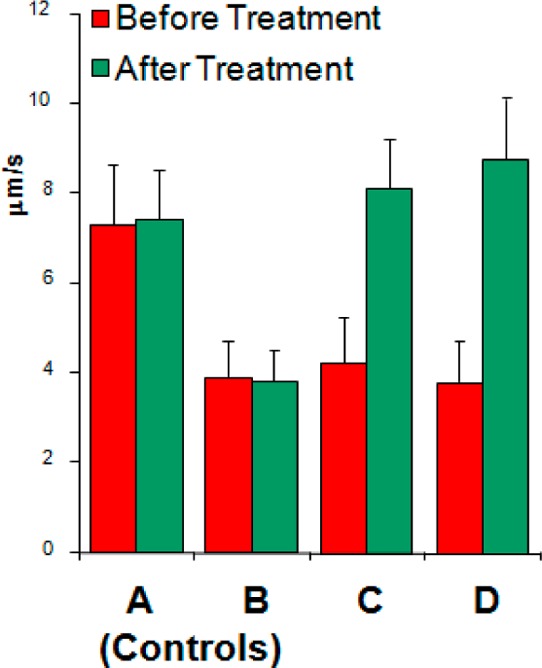
Control mice (red) were injected with 150 μL of saline (A) and 150 μL of saline containing 500 mg of rmTNFα (B–D). Results show rolling velocity of leukocytes before (red) and 10 min after the intrajugular injection of saline (B), sLeX (C), and 20 (D) at 100 mg/kg (green). Results are the averages of 5 readings per venule, 10 venules per mouse, and 5–6 mice per tested conditions (±SEM).
Multivalent sLeX Analogues
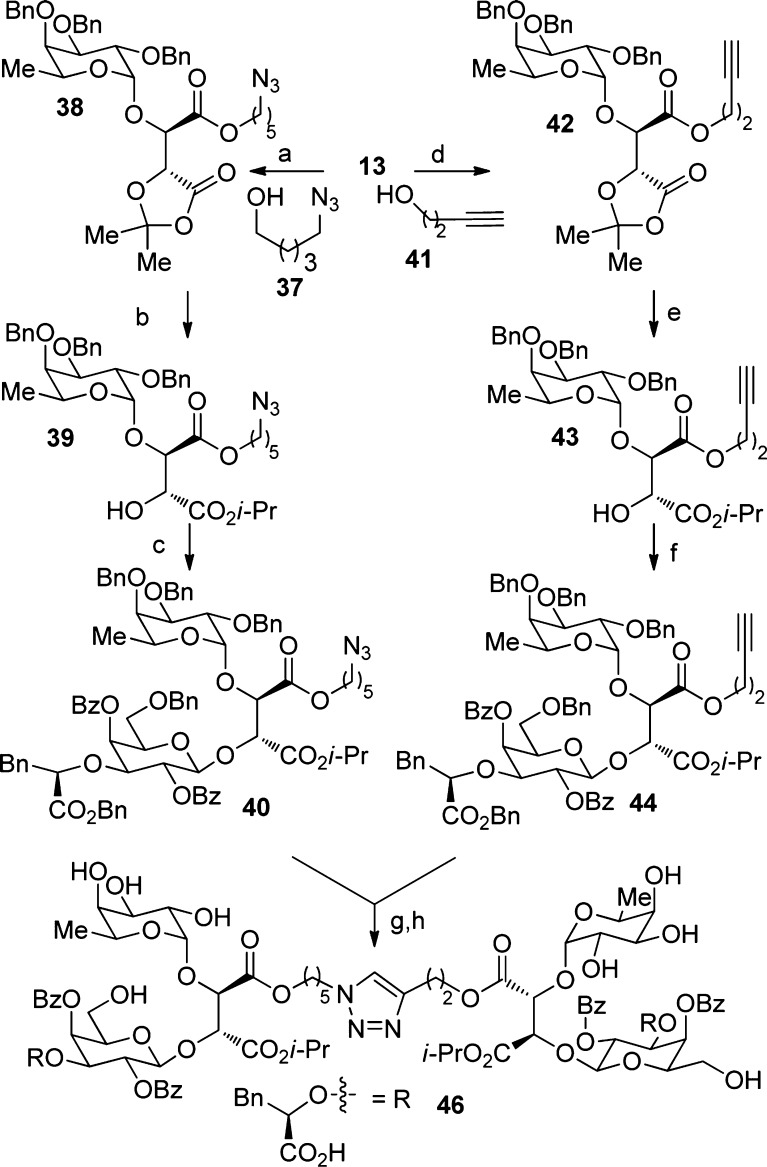
Multivalent ligands have attracted considerable attention in the carbohydrate community in the past decade.31−33 Divalent or trivalent ligands harboring sLeX have been previously synthesized; some showing increased potency.33−36 As illustrated in Scheme 3, we intended to prepare a bivalent ligand taking advantage of the ester groups on the acyclic tether moiety of our analogues to introduce other chemical entities. We planned to use a 1,3-dipolar cycloaddition to link two fragments by forming a triazole.37 The first fragment was generated from the acid 13 to which an azido pentanol was coupled. The corresponding ester 38 was then treated under acidic conditions to hydrolyze the dioxolanone. The free acid was then esterified to the isopropyl ester 39 and coupled to the dibenzoate donor to give the corresponding β-anomer 40. A similar reaction sequence was realized after adding the propargylic alcohol to the acid 13, which could be efficiently converted to 44. The azide 40 and alkyne 44 were then reacted in the presence of CuI and DIEA in THF at room temperature.37 The 1,3-triazole dimer was obtained in a 60% yield. The eight benzyl groups were then removed to give 46.
Scheme 3. Synthesis of Dimer 45.
(a) i. NaOH solution (10%), THF, 25 °C; ii. 2,2-dimethoxy-propane, PTSA, DCM, 25 °C, no purification; iii. 37, DCC, DMAP, DCM, 52% over 3 steps; (b) i. AcOH/H2O (80:20), 50 °C; ii. Cs2CO3, DMF, then i-PrI, 25 °C, 66% over 2 steps; (c) 18, NIS, TMSOTf, DCM, −25 °C, 69%; (d) i. NaOH solution (10%), THF, 25 °C; ii. 2,2-dimethoxy-propane, PTSA, DCM, 25 °C, no purification; iii. 41, DCC, DMAP, DCM, 25 °C, 53% over 3 steps; (e) i. AcOH/H2O (80:20), 50 °C; ii. Cs2CO3, DMF, then i-PrI, 25 °C, 58% over 2 steps; (f) 18, NIS, TMSOTf, DCM, −25 °C, 64%; (g) CuI, DIEA, THF, 25 °C, 60%; (h) Pd(OH)2, THF, 25 °C, 19%.
As seen in Table 1, our divalent ligand 46 showed a relative potency ratio of 82 in the P-selectin assay (entry 10, Table 1). Compared to analogue 35, this represented a more than 2-fold increase in potency (entry 9 versus entry 10). We are considering varying the length and the nature of the triazole tether chain by introducing different substituents to improve further this multivalent approach.
In conclusion, we have shown herein that by using an acyclic tether we were able to generate potent E- and P-selectin antagonists. The representative member of this series demonstrates in vivo activity in modifying the rolling of leukocytes induced by an inflammatory stimulus. We are now evaluating other acyclic tethers in order to probe the CRD of the selectin and to improve the resulting biological properties of this promising family of sLeX analogues.
Supporting Information Available
Details for surface plasmon resonace assays, methods of organic synthesis, and spectroscopic data of synthesized compounds. This material is available free of charge via the Internet at http://pubs.acs.org.
Funding for this research has been granted from Natural Sciences and Engineering Research Council (NSERC) and Fonds Québécois de la Recherche sur la Nature et les Technologies (FQRNT).
The authors declare no competing financial interest.
Supplementary Material
References
- Ragan J. A.; Cooper K. Synthesis of a galactose-fucose disaccharide mimic of sialyl Lewis X. Bioorg. Med. Chem. Lett. 1994, 4, 2563–2566. [Google Scholar]
- Prodger J. C.; Bamford M. J.; Gore P. M.; Holmes D. S.; Saez V.; Ward P. Synthesis of a novel analogue of sialyl Lewis X. Tetrahedron Lett. 1995, 36, 2339–2342. [Google Scholar]
- Prodger J. C.; Bamford M. J.; Bird M. I.; Gore P. M.; Holmes D. S.; Priest R.; Saez V. Mimics of the sialyl Lewis X tetrasaccharide. Replacement of the N-acetylglucosamine sugar with simple C2-symmetric 1,2-diols. Bioorg. Med. Chem. 1996, 4, 793–801. [DOI] [PubMed] [Google Scholar]
- Kolb H. C.; Ernst B. Development of tools for the design of selectin antagonists. Chem.—Eur. J. 1997, 3, 1571–1578. [Google Scholar]
- Kolb H. C.; Ernst B. Recent progresses in the glycodrug area. Pure Appl. Chem. 1997, 69, 1879–1884. [Google Scholar]
- Somers W. S.; Tang J.; Shaw G. D.; Camphausen R. T. Insights into the molecular basis of leukocyte tethering and rolling revealed by structures of P- and E-selectin bound to SLeX and PSGL-1. Cell 2000, 103, 467–479. [DOI] [PubMed] [Google Scholar]
- Thoma G.; Magnani J. L.; Patton J. T.; Ernst B.; Jahnke W. Preorganization of the bioactive conformation of sialyl LewisX analogues correlates with their affinity to E-selectin. Angew. Chem., Int. Ed. 2001, 40, 1941–1945. [PubMed] [Google Scholar]
- De Vleeschauwer M.; Vaillancourt M.; Goudreau N.; Guindon Y.; Gravel D. Design and synthesis of a new sialyl Lewis X mimetic: How selective are the selectin receptors?. Bioorg. Med. Chem. 2001, 11, 1109–1112. [DOI] [PubMed] [Google Scholar]
- Thoma G.; Bänteli R.; Jahnke W.; Magnani J. L.; Patton J. T. A readily available, highly potent E-selectin antagonist. Angew. Chem., Int. Ed. 2001, 40, 3644–3647. [DOI] [PubMed] [Google Scholar]
- Kaila N.; Thomas B. E. IV. Design and synthesis of sialyl LewisX mimics as E- and P-selectin inhibitors. Med. Res. Rev. 2002, 22, 566–601. [DOI] [PubMed] [Google Scholar]
- Hanessian S.; Mascitti V.; Rogel O. Synthesis of a potent antagonist of E-selectin. J. Org. Chem. 2002, 67, 3346–3354. [DOI] [PubMed] [Google Scholar]
- Thoma G.; Schwarzenbach F. Simplified sialyl LewisX analogues with improved E-selectin inhibition. Helv. Chim. Acta 2003, 86, 855–864. [Google Scholar]
- Kaila N.; Somers W. S.; Thomas B. E.; Thakker P.; Janz K.; DeBernardo S.; Tam S.; Moore W. J.; Yang R.; Wrona W.; Bedard P. W.; Crommie D.; Keith J. C.; Tsao D. H. H.; Alvarez J. C.; Ni H.; Marchese E.; Patton J. T.; Magnani J. L.; Camphausen R. T. Quinic acid derivatives as sialyl LewisX-mimicking selectin inhibitors: Design, synthesis, and crystal structure in complex with E-selectin. J. Med. Chem. 2005, 48, 4346–4357. [DOI] [PubMed] [Google Scholar]
- Titz A.; Marra A.; Cutting B.; Smieško M.; Papandreou G.; Dondoni A.; Ernst B. Conformational constraints: Nature does it best with sialyl LewisX. Eur. J. Org. Chem. 2012, 5534–5539. [Google Scholar]
- Schwizer D.; Patton J. T.; Cutting B.; Smieško M.; Wagner B.; Kato A.; Weckerle C.; Binder F. P. C.; Rabbani S.; Schwardt O.; Magnani J. L.; Ernst B. Pre-organization of the core structure of E-selectin antagonists. Chem.—Eur. J. 2012, 18, 1342–1351. [DOI] [PubMed] [Google Scholar]
- Egger J.; Weckerle C.; Cutting B.; Schwardt O.; Rabbani S.; Lemme K.; Ernst B. Nanomolar E-selectin antagonists with prolonged half-lives by a fragment-based approach. J. Am. Chem. Soc. 2013, 135, 9820–9828. [DOI] [PubMed] [Google Scholar]
- Chang J.; Patton J. T.; Sarkar A.; Ernst B.; Magnani J. L.; Frenette P. S. GMI-1070, a novel pan-selectin antagonist, reverses acute vascular occlusions in sickle cell mice. Blood 2010, 116, 1779–1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevilacqua M. P. Endothelial-leukocyte adhesion molecules. Annu. Rev. Immunol. 1993, 11, 767–804. [DOI] [PubMed] [Google Scholar]
- Hidalgo A.; Chang J.; Jang J.-E.; Peired A. J.; Chiang E. Y.; Frenette P. S. Heterotypic interactions enabled by polarized neutrophil microdomains mediate thromboinflammatory injury. Nat. Med. 2009, 15, 384–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler I. G.; Barbier V.; Nowlan B.; Jacobsen R. N.; Forristal C. E.; Patton J. T.; Magnani J. L.; Levesque J.-P. Vascular niche E-selectin regulates hematopoietic stem cell dormancy, self renewal and chemoresistance. Nat. Med. 2012, 18, 1651–1657. [DOI] [PubMed] [Google Scholar]
- Chelliah R.; Lucking A. J.; Tattersall L.; Daga S.; Beresford-Cleary N. J.; Cortas K.; Fox K. A. A.; Feuerstein G. Z.; Connolly T. M.; Newby D. E. P-selectin antagonism reduces thrombus formation in humans. J. Thromb. Haemostasis 2009, 7, 1915–1919. [DOI] [PubMed] [Google Scholar]
- Calosso M.; Charpentier D.; Vaillancourt M.; Bencheqroun M.; St-Pierre G.; Wilkes B. C.; Guindon Y. A new approach to explore the binding space of polysaccharide-based ligands: Selectin antagonists. ACS Med. Chem. Lett. 2012, 3, 1045–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinnbauer M.; Ernst B.; Wagner B.; Magnani J.; Benie A. J.; Peters T. Epitope mapping of sialyl Lewisx bound to E-selectin using saturation transfer difference NMR experiments. Glycobiology 2003, 13, 435–443. [DOI] [PubMed] [Google Scholar]
- Scheffler K.; Brisson J. R.; Weisemann R.; Magnani J. L.; Wong W. T.; Ernst B.; Peters T. Application of homonuclear 3D NMR experiments and 1D analogs to study the conformation of sialyl LewisX bound to E-selectin. J. Biomol. NMR 1997, 9, 423–436. [DOI] [PubMed] [Google Scholar]
- Zegelaar-Jaarsveld K.; van der Marel G. A.; van Boom J. H. Iodonium ion assisted synthesis of a common inner core trisaccharide fragment corresponding to the cell-wall phenolic glycolipid of Mycobacterium kansasii. Tetrahedron 1992, 48, 10133–10148. [Google Scholar]
- Veeneman G. H.; van Leeuwen S. H.; van Boom J. H. Iodonium ion promoted reactions at the anomeric centre. II An efficient thioglycoside mediated approach toward the formation of 1,2-trans linked glycosides and glycosidic esters. Tetrahedron Lett. 1990, 31, 1331–1334. [Google Scholar]
- Markert M.; Buchem I.; Krüger H.; Mahrwald R. A simple approach to 5,5′-bis(1,3-dioxolan-4-ones) of tartaric acids. Tetrahedron: Asymmetry 2004, 15, 803–806. [Google Scholar]
- Details are provided as Supporting Information.
- HL-60 cells were radiolabeled by adding [H3] thymidine in the incubation medium. Wells were precoated with goat anti-human1gG antibodies. E- or P-selectin 1gG were added. Radiolabeled cells were incubated in the presence of the immobilized selectins. After carefully washing the nonadhered cells, the radioactivity was measured. In the presence of putative selectin antagonist, the number of these radiolabeled cells will decrease in a dose-dependent way.
- Homola J. Present and future of surface plasmon resonance biosensors. Anal. Bioanal. Chem. 2003, 377, 528–539. [DOI] [PubMed] [Google Scholar]
- Roy R. Syntheses and some applications of chemically defined multivalent glycoconjugates. Curr. Opin. Struct. Biol. 1996, 6, 692–702. [DOI] [PubMed] [Google Scholar]
- Mammen M.; Choi S.-K.; Whitesides G. M. Polyvalent interactions in biological systems: Implications for design and use of multivalent ligands and inhibitors. Angew. Chem., Int. Ed. 1998, 37, 2754–2794. [DOI] [PubMed] [Google Scholar]
- Kiessling L. L.; Gestwicki J. E.; Strong L. E. Synthetic multivalent ligands in the exploration of cell-surface interactions. Curr. Opin. Chem. Biol. 2000, 4, 696–703. [DOI] [PubMed] [Google Scholar]
- Sprengard U.; Schudok M.; Schmidt W.; Kretzschmar G.; Kunz H. Multiple sialyl Lewisx N-glycopeptides: Effective ligands for E-selectin. Angew. Chem., Int. Ed. 1996, 35, 321–324. [Google Scholar]
- Kretzschmar G.; Sprengard U.; Kunz H.; Bartnik E.; Schmidt W.; Toepfer A.; Hörsch B.; Krause M.; Seiffge D. Oligosaccharide recognition by selectins: Synthesis and biological activity of multivalent sialyl lewis-X ligands. Tetrahedron 1995, 51, 13015–13030. [Google Scholar]
- Lin C.-H.; Shimazaki M.; Wong C.-H.; Koketsu M.; Juneja L. R.; Kim M. Enzymatic synthesis of a sialyl Lewis X dimer from egg yolk as an inhibitor of E-selectin. Biorg. Med. Chem. 1995, 3, 1625–1630. [DOI] [PubMed] [Google Scholar]
- Kolb H. C.; Finn M. G.; Sharpless K. B. Click chemistry: Diverse chemical function from a few good reactions. Angew. Chem., Int. Ed. 2001, 40, 2004–2021. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.