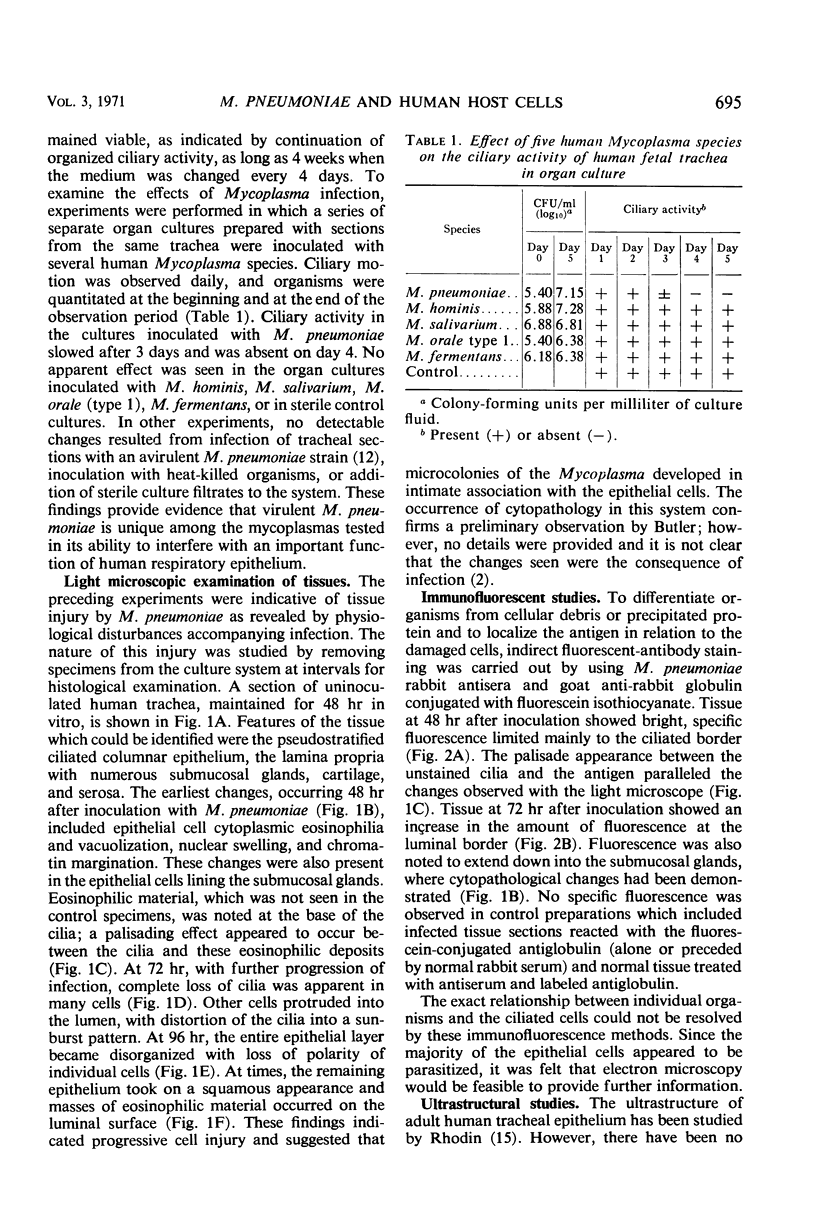
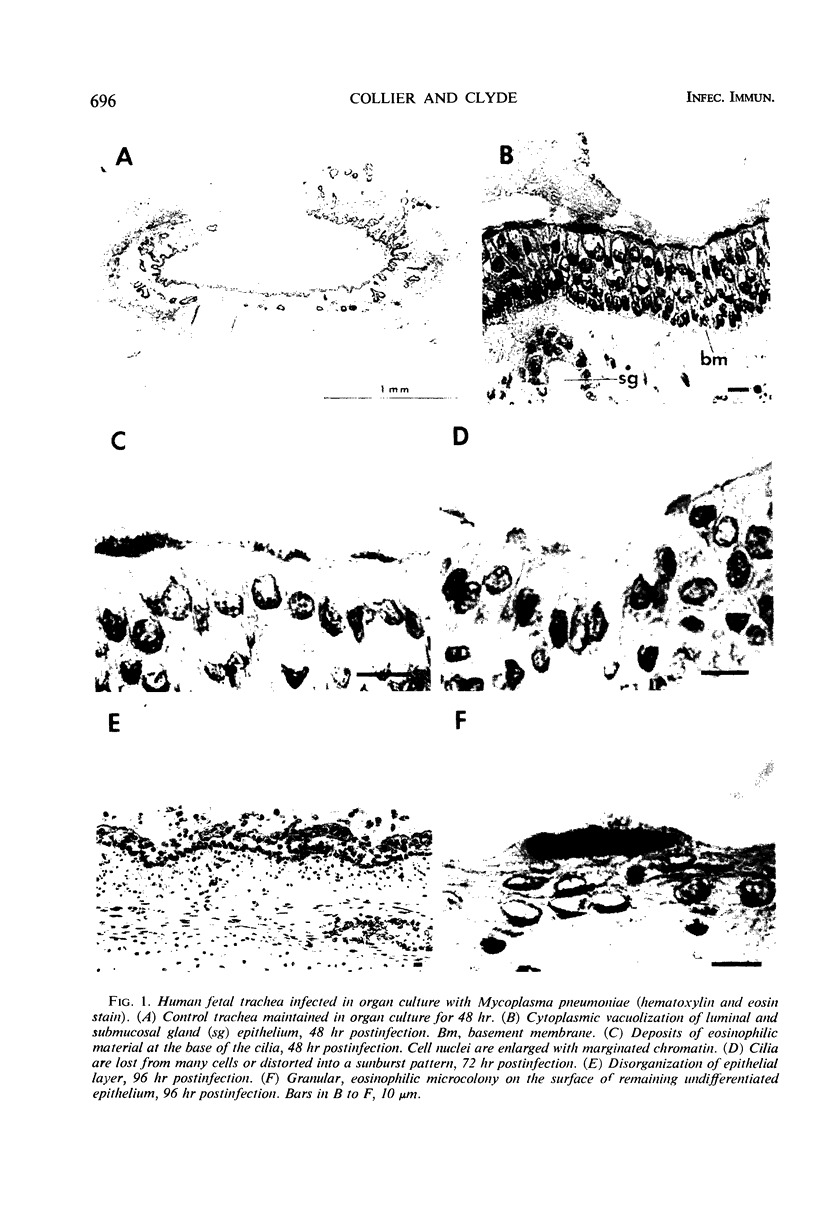
Abstract
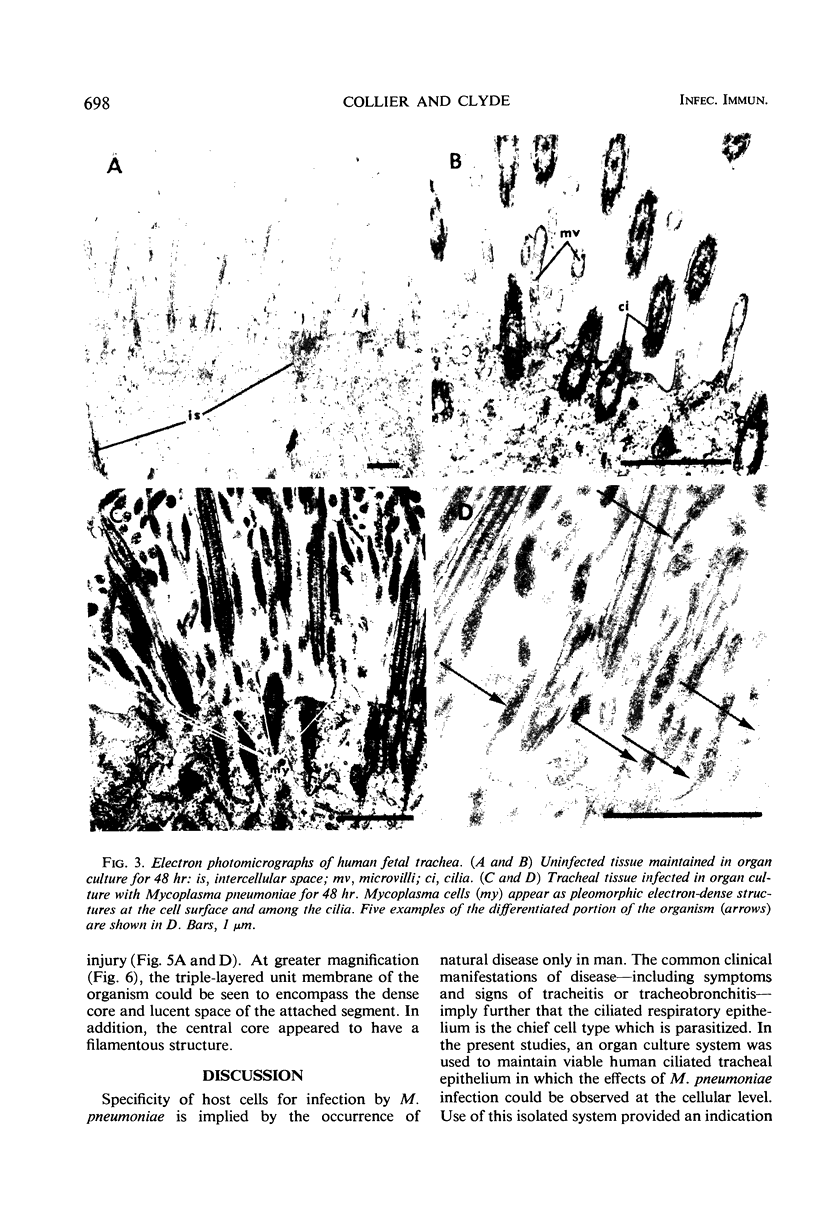
The interaction was studied between Mycoplasma pneumoniae and its natural host cell, the human respiratory epithelium. Organized, ciliated cells provided by fetal trachea in organ culture enabled examination of the host-parasite relationship by light, immunofluorescence, and electron microscopy. Impairment of cellular function was reflected by disorganization and loss of ciliary motion; this was associated with a sequence of cytopathological changes denoting progressive cell injury. The organisms were found concentrated on the luminal surface of ciliated epithelium and cells lining the submucosal glands. A differentiated portion of the Mycoplasma, consisting of an extension of the unit membrane containing an electron-dense core surrounded by a lucent space, served as the means of attachment to host cells. The findings suggest that the pathogenicity of M. pneumoniae depends upon intimate extracellular infection with production of functional and structural changes initiated by host cell membrane injury.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Biberfeld G., Biberfeld P. Ultrastructural features of Mycoplasma pneumoniae. J Bacteriol. 1970 Jun;102(3):855–861. doi: 10.1128/jb.102.3.855-861.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler M. Isolation and growth of mycoplasma in human embryo trachea cultures. Nature. 1969 Nov 8;224(5219):605–606. doi: 10.1038/224605a0. [DOI] [PubMed] [Google Scholar]
- Cherry J. D., Taylor-Robinson D. Growth and Pathogenesis of Mycoplasma mycoides var. capri in Chicken Embryo Tracheal Organ Cultures. Infect Immun. 1970 Oct;2(4):431–438. doi: 10.1128/iai.2.4.431-438.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen G., Somerson N. L. Mycoplasma pneumoniae: hydrogen peroxide secretion and its possible role in virulence. Ann N Y Acad Sci. 1967 Jul 28;143(1):85–87. doi: 10.1111/j.1749-6632.1967.tb27648.x. [DOI] [PubMed] [Google Scholar]
- Collier A. M., Clyde W. A., Jr, Denny F. W. Biologic effects of Mycoplasma pneumoniae and other mycoplasmas from man on hamster tracheal organ culture. Proc Soc Exp Biol Med. 1969 Dec;132(3):1153–1158. doi: 10.3181/00379727-132-34385. [DOI] [PubMed] [Google Scholar]
- Collier A. M., Clyde W. A., Jr, Denny F. W. Mycoplasma pneumoniae in hamster tracheal organ culture: immunofluorescent and electron microscopic studies. Proc Soc Exp Biol Med. 1971 Feb;136(2):569–573. doi: 10.3181/00379727-136-35313. [DOI] [PubMed] [Google Scholar]
- DONALD H. B., LIU C. Cytological studies of chick embryo cells infected with the virus of primary atypical pneumonia. Virology. 1959 Sep;9:20–29. doi: 10.1016/0042-6822(59)90097-2. [DOI] [PubMed] [Google Scholar]
- Furness G., Pipes F. J., McMurtrey M. J. Analysis of the life cycle of Mycoplasma pneumoniae by synchronized division and by ultraviolet and x irradiations. J Infect Dis. 1968 Feb;118(1):7–13. doi: 10.1093/infdis/118.1.7. [DOI] [PubMed] [Google Scholar]
- Hayflick L. Tissue cultures and mycoplasmas. Tex Rep Biol Med. 1965 Jun;23(Suppl):285+–285+. [PubMed] [Google Scholar]
- Kenny G. E. Heat-lability and organic solvent-solubility of mycoplasma antigens. Ann N Y Acad Sci. 1967 Jul 28;143(1):676–681. doi: 10.1111/j.1749-6632.1967.tb27713.x. [DOI] [PubMed] [Google Scholar]
- Kim K. S., Clyde W. A., Jr, Denny F. W. Physical properties of human Mycoplasma species. J Bacteriol. 1966 Jul;92(1):214–219. doi: 10.1128/jb.92.1.214-219.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipman R. P., Clyde W. A., Jr, Denny F. W. Characteristics of virulent, attenuated, and avirulent Mycoplasma pneumoniae strains. J Bacteriol. 1969 Nov;100(2):1037–1043. doi: 10.1128/jb.100.2.1037-1043.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniloff J., Morowitz H. J., Barrnett R. J. Ultrastructure and Ribosomes of Mycoplasma gallisepticum. J Bacteriol. 1965 Jul;90(1):193–204. doi: 10.1128/jb.90.1.193-204.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morowitz H. J., Maniloff J. Analysis of the life cycle of Mycoplasma gallisepticum. J Bacteriol. 1966 Apr;91(4):1638–1644. doi: 10.1128/jb.91.4.1638-1644.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodin J. A. The ciliated cell. Ultrastructure and function of the human tracheal mucosa. Am Rev Respir Dis. 1966 Mar;93(3 Suppl):1–15. doi: 10.1164/arrd.1966.93.3P2.1. [DOI] [PubMed] [Google Scholar]
- Sobeslavsky O., Prescott B., Chanock R. M. Adsorption of Mycoplasma pneumoniae to neuraminic acid receptors of various cells and possible role in virulence. J Bacteriol. 1968 Sep;96(3):695–705. doi: 10.1128/jb.96.3.695-705.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor-Robinson D., Manchee R. J. Spermadsorption and spermagglutination by mycoplasmas. Nature. 1967 Jul 29;215(5100):484–487. doi: 10.1038/215484a0. [DOI] [PubMed] [Google Scholar]
- Zucker-Franklin D., Davidson M., Thomas L. The interaction of mycoplasmas with mammalian cells. I. HeLa cells, neutrophils, and eosinophils. J Exp Med. 1966 Sep 1;124(3):521–532. doi: 10.1084/jem.124.3.521. [DOI] [PMC free article] [PubMed] [Google Scholar]