Abstract
How an individual responds to the environment depends upon both personal life history as well as inherited genetic and epigenetic factors from ancestors. Using a 2-hit, 3 generations apart model, we tested how F3 descendants of rats given in utero exposure to the environmental endocrine-disrupting chemical (EDC) vinclozolin reacted to stress during adolescence in their own lives, focusing on sexually dimorphic phenotypic outcomes. In adulthood, male and female F3 vinclozolin- or vehicle-lineage rats, stressed or nonstressed, were behaviorally characterized on a battery of tests and then euthanized. Serum was used for hormone assays, and brains were used for quantitative PCR and transcriptome analyses. Results showed that the effects of ancestral exposure to vinclozolin converged with stress experienced during adolescence in a sexually dimorphic manner. Debilitating effects were seen at all levels of the phenotype, including physiology, behavior, brain metabolism, gene expression, and genome-wide transcriptome modifications in specific brain nuclei. Additionally, females were significantly more vulnerable than males to transgenerational effects of vinclozolin on anxiety but not sociality tests. This fundamental transformation occurs in a manner not predicted by the ancestral exposure or the proximate effects of stress during adolescence, an interaction we refer to as synchronicity.
The legacy of exposure to environmental chemicals, including endocrine-disrupting chemicals (EDCs), has permanently altered the present and future health of humans and wildlife (1–4). Direct exposure to EDCs affects the life history of individuals and their descendants. Such context-dependent epigenetic modifications are not heritable per se because the germ cells are not affected. Other epigenetic modifications can affect the germline (germline-dependent) and thus manifest in each generation even in the absence of the causative agent (1–9). This is the case for several EDCs, notably, vinclozolin (10–12), bisphenol A (BPA; 13, 14), and tributyltin (15). Such transgenerational modifications affect all levels of biological organization, from gene regulation to behavioral interactions of conspecifics (13, 16–18).
Environmental and social stressors are an immediate and primary source of context-dependent epigenetic modifications (19–22). Chronic or excessive stress during sensitive periods such as prenatal development or the early postnatal period can predispose for disease and disorder later in life, a phenomenon known as the fetal basis of adult disease (23, 24). Later developmental periods, including adolescence, also have enduring effects that include neural remodeling, sensitivity to drugs of abuse, impaired learning and memory, and emotional disorders in adulthood (24–31).
Most neurobehavioral, neurological, and addiction disorders exhibit significant sex differences in relative risk level and severity. Women have higher levels of diseases and disorders such as Alzheimer's disease, dementia, major depressive disorder, posttraumatic stress disorders, and anxiety and panic disorders (32); whereas autism-spectrum disorder and attention deficit hyperactivity disorder predominate in men (33). Developmental sex differences in adrenal and reproductive hormones must play a role in their etiology because, in many instances, disorders manifest after adrenarche/puberty, fluctuate during menstrual cycles, and change prevalence/severity at menopause or during times of stress, suggesting involvement of adrenal and gonadal steroid hormones (34–38). Environmental exposures to compounds that disrupt these hormonal systems have had a major impact on health and are believed to be a contributing factor to the increased incidence of obesity, illness, and affective disorders in humans (1, 39, 40).
Vinclozolin is a commonly used fungicide with demonstrated antiandrogenic properties (41, 42). When administered to human cancer cell lines in vitro, it prevents the androgen receptor from binding androgen response elements, thereby inhibiting the androgen receptor's ability to act as a transcription factor (43). Vinclozolin administered by either intraperitoneal injection or orally is metabolized into two primary metabolites (M1 and M2), both of which are effective against preventing the androgen receptor from binding its targeted androgen response elements (42). Exposure to vinclozolin during organizational periods of embryonic development results in feminized males with reduced anogenital distance and hypospadias (41); as adults, these males exhibit a reduced number of testis cords, increased apoptosis of immature sperm, and decreasing sperm motility, all of which result in decreased fertility (44). Some aspects of this disease phenotype persist for up to 4 generations in the absence of further exposure (10).
Previous work from our laboratories reveal significant interactions of transgenerational vinclozolin effects inherited from the great-grandparents with stress experienced in adolescence (45). That work was limited to males, overlooking fundamental sex differences in reproductive investment, behavior, and survival. In addition to the individual effects of ancestral exposure to vinclozolin and stress during adolescence, we were particularly interested in the combination of these 2 events, a phenomenon we refer to as synchronicity. We demonstrate here that the sexes differ markedly in their responses to these 2 types of epigenetic modifications and that the synchronicity of ancestral exposure and proximate stress during one's own lifetime results in profound sex differences in the scope and nature of reactivity.
Materials and Methods
Animals
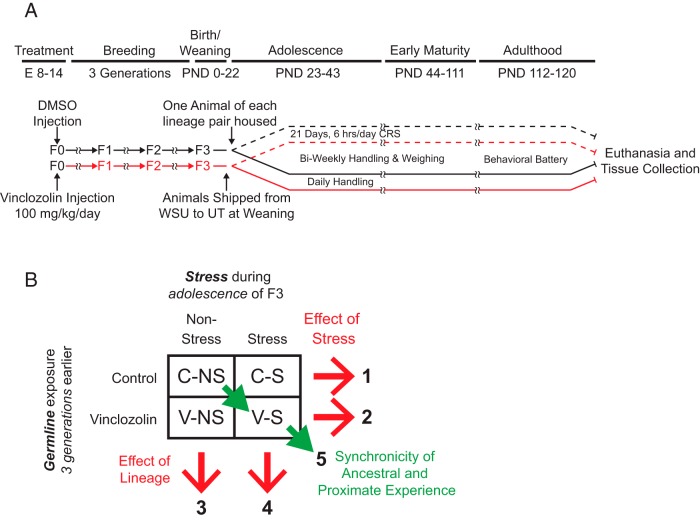
Male and female Sprague-Dawley rats, vinclozolin- and vehicle (dimethylsulfoxide)-lineage, were bred at Washington State University (10). An F0 generation of pregnant mothers (vinclozolin, n = 3; control, n = 2) was injected ip with vinclozolin (100 mg/kg/d) or dimethylsulfoxide (vehicle) on embryonic days 8 to 14 (Figure 1A). Subsequent generations were bred such that there was no sibling or cousin inbreeding. F3-generation pups with no chemical body burden were weaned at postnatal day (PND) 21, implanted sc with an identification microchip (AVID), and shipped to the University of Texas at Austin the following day. All animal protocols were approved at both Washington State University and the University of Texas at Austin. Upon arrival, animals were pair-housed with a same-sex individual of the opposing lineage (eg, a control-lineage male with a vinclozolin-lineage male). Cage-mates were all within 1 day separation in age. Dyads were housed in standard translucent polycarbonate rat cages (46 × 24 × 20.5 cm) with ad libitum access to tap water and standard rat chow (RMH 1800 from Purina) and environmental enrichment (7-cm diameter PVC pipe). The colony room was on a 14-hour light, 10-hour dark cycle with lights off at 8:30 am. Animals were handled and weighed twice a week (Supplemental Figure 1).
Figure 1.
Experimental model and categorization of effects of ancestral vinclozolin vs control lineage and CRS in rats. A, Experimental flowchart. Rats were exposed to vinclozolin or vehicle (dimethylsulfoxide) control 3 generations previously, and the F3 generation used for CRS during adolescence or no stress. All animals were then behaviorally tested as adults. B, Experimental groups and comparisons. Planned comparisons are shown, with comparisons 1, 3 and 5 the focal group of the study.
Chronic restraint stress in adolescence and behavioral testing in adulthood
Chronic restraint stress (CRS) during adolescence and behavioral testing of adults were conducted in a separate room as described in detail (45), transported in covered cages, and tested under dim red light or white light depending on the test. CRS was conducted for 6 hours daily for 21 days from PND 23 to 44 (Figure 1A).
Behavioral tests were conducted in a counterbalanced order and were administered to each individual beginning on PND 90. Behavioral tests consisted of open-field, sociability, and social novelty as described previously in earlier vinclozolin work (45) (Supplemental Methods). In addition, the light-dark box and elevated plus maze were added to provide more thorough behavioral characterization. The test for sociability was performed to measure social approach, anxiety, and exploration. The social novelty test immediately followed, enabling animals to differentiate between the now familiar conspecific and a novel, unfamiliar conspecific as a test for a rat's preference for social novelty (46, 47). The open-field test is a standard measure of anxiety, taking advantage of a rat's natural aversion to exploration of a central lit area and the preference to spend time around the edges (48, 49). Similarly, a light-dark box test measures anxiety-like behaviors and aversion to exploration in a brightly lit compared with a dark chamber (50). The elevated plus maze allows a rat to spend time in an enclosed runway compared with an open runway that is raised off the ground, again providing information about anxiety-like behaviors (51). Details for how these standard behaviors were performed are provided in Supplemental Methods. Stimulus rats used for social tests were naive, intact, same-sex, and age-matched Sprague-Dawley rats (Harlan). All behaviors were quantified using the video tracking system (ANY-maze) and apparatus from Stoelting. After all behavioral testing was completed, between PND 118 and 124, animals were euthanized by decapitation as per approved protocols.
Brain collection and selection of brain regions
The brain was quickly removed and processed as previously described (45). Briefly, each brain was split in the sagittal plane, with one sagittal half frozen for sectioning and cytochrome oxidase (CO) histochemistry and the other cut (2 mm) on a chilled rat brain matrix for later micropunching of specific nuclei for mRNA and transcriptome analyses. The following regions of the amygdala, hippocampus, and hypothalamus were the focus of this study, selected for their interconnectivity and functional relationships in the controls of the behaviors studied herein: basolateral amygdaloid nucleus (BLA), central amygdaloid nucleus (CeAmy), medial amygdaloid nucleus (MeAmy), anterior cortical amygdaloid nucleus (CoAmy), CA1 and the CA3 of the hippocampus, medial preoptic area (mPOA), ventromedial hypothalamic nucleus (VMN), and bed nucleus of the stria terminalis (BnST).
Brain metabolic activity
CO histochemistry, a measure of brain metabolic activity (52), was measured in 13 discrete neural nuclei (Supplemental Table 1). CO histochemistry is well-established as relating to overall metabolic history within a cell, as has been shown for learning and memory, sexual activity, and developmental experience (53). As published previously (45), in brief, fresh-frozen brain tissue was cryosectioned and mounted. Slices containing nuclei of interest were incubated in a heated bath containing 3,3′-diaminobenzidine (DAB) and saturated with oxygen. Cytochrome C within the tissue oxidizes DAB, which turns from clear to light brown. The slices were imaged (Javelin) using a constant-intensity light box (Northern Light R95) and the OD of defined nuclei was measured. The abundance of cytochrome C in brain tissue has been tightly linked to metabolic activity and therefore neuronal activity. Thus, the amount of DAB that is oxidized and its resulting OD is used as an index of metabolic history.
Gene expression analysis
Brain nuclei were punched from frozen coronal hemisections using a 1-mm diameter Palkovits punch. Ten regions were used for quantitative PCR (qPCR), and of these, 4 were used for further transcriptome analysis (Supplemental Table 1). RNA extraction, purification, and preparation for the cDNA reaction were performed based on standard procedures, as detailed in Supplemental Methods and as published previously (54). Gene expression analysis was performed using a 48-gene OD (qPCR) platform, the TaqMan low-density PCR array (TLDA) (Life Technologies) that enables detection of all 48 genes in microdissected brain regions of individual rats (54). The choice of genes for the qPCR panel was based on previous work from this laboratory and others and are listed in Table 1, with full gene names in Supplemental Table 2 (55, 56). Specific gene selection was based on a priori hypotheses about molecules involved in the neurobiological responses to EDCs, predicted sex differences, and relationships between selected genes and behaviors measured in the same rats. Selected genes fell in several functional groups including epigenetic modification, stress signaling, steroid hormones, and growth factors. TLDA qPCR results were quantified using a ViiA7 PCR machine (Life Technologies) as published (54). As a secondary level of analysis, transcriptome analysis was conducted on pooled samples in 4 of the brain regions (BLA, CeAmy, BnST, and CA3) using the Affymetric Rat Gene version 1.0 ST arrays, with all methods and analyses identical to those published by our laboratories (45) (Supplemental Methods).
Table 1.
Genes on the TLDA
| Functional Group | Genes |
|---|---|
| Housekeeping genes | 18S, Gapdh |
| Epigenetic modifiers | Dnmt1, Dnmt3a, Dnmt3b, Dnmt3l, Hdac1, Mbd2 |
| Stress signaling | Crh, Crh1, Gmeb2, Mc3r, Mcr4r, Mc5r, Nr3c1, Pomc |
| Steroidogenic enzymes | Cyp19a1, Hsd11b2, Srd5a1 |
| Sex steroid hormone receptors | Ar, Esr1, Esr2, Gnrhr, Gper, Pgr |
| Dopaminergic | Comt, Drd2, Drd4, Th |
| Serotonergic | Slc6a4 |
| Glutamatergic | Gria1, Gria2, Grik2, Grin1, Grin2a, Grin2b, Grin2c, Grin2d |
| GABAergic | Gad1, Gad2 |
| Neuropeptides and receptors | Avp, Avp1a, Kiss1, Kiss1r, Lepr, Oxt, Oxtr, Tac2 |
| Growth factors | Bdnf, Ctgr, Igf1, Igf1r, Igfbp2, Igfbp5, Negr1, Ptgds, S100a4, Tgfa, Tgfb1 |
| Transcription factors | Nfkb1, Nrf1, Per2 |
The genes measured by qPCR on a custom-designed TLDA are listed by functional group. Full gene names can be found in Supplemental Table 2.
Hormone assays
A terminal trunk blood sample was collected, centrifuged, and frozen for hormone analyses, each performed following manufacturers' protocols. The 3 sex steroid hormones (estradiol, progesterone, and testosterone) were selected based on previous work for their disruption by vinclozolin (45, 57, 58). Corticosterone was also measured because a primary dependent endpoint of this study was CRS, which affects adrenal glucocorticoid levels and is sexually dimorphic (59–61). For each assay, standards were processed in duplicate and samples in triplicate. In a few cases, outliers within a triplicate were removed using Grubb's test. Serum corticosterone was measured in 10 μL serum from individual rats in a single RIA (MP Biomedicals). The intra-assay coefficient of variation (CV) was 3%, and assay sensitivity was 25 ng/mL. Serum estradiol was measured in 200 μL serum in a single RIA (Beckman-Coulter). The intra-assay CV was 6.7%, and assay sensitivity was 5 pg/mL. Progesterone serum concentrations were determined via an enzyme immunoassay in 1 μL serum for females and 25 μL for males (Cayman Chemical). The intra-assay CV was 4.9%, and assay sensitivity was 7.8 pg/mL. Serum testosterone was measured by enzyme immunoassay (Cayman Chemical) using 200 μL serum for females and 6 μL for males. The intra-assay CV was 7.6%, and assay sensitivity was 3.9 pg/mL.
Statistics
Initial analyses of individual datasets were performed first, and this revealed that datasets had non-normal distributions and heterogeneous variance, determined by Shapiro-Wilk test for normality and Levene's test for the equality of variance, respectively. Therefore, Kruskal-Wallis nonparametric analysis was performed to determine a main effect of EDC lineage (vinclozolin vs control) and of stress (vs nonstress) for each endpoint. Subsequent comparisons within sex and between groups were performed via a pair-wise Mann-Whitney U tests between independent groups and appropriately controlled for using a Benjamini-Hochberg correction for multiple comparisons where appropriate. Functional landscape analysis of correlated traits was performed as per Ref. 62 to provide a visual representation of the relationships among multiple variables between groups of individuals, allowing for a more concise presentation of data. As such, this captures the nature of relationships pictorially. However, this analysis is in no way intended to replace individual statistical analyses. It is important to note that in the behavioral tests, each node within the landscape represents a composite of several measures, whereas the bar graphs represent an individual measure within a task. This is not the case for the functional landscapes of brain metabolism.
Results
Five sets of comparisons were performed as shown in Figure 1B: control nonstress (C-NS) vs control stress (C-S; comparison 1); C-NS vs vinclozolin nonstress (V-NS; comparison 3); and C-NS vs vinclozolin stress (V-S; comparison 5). Comparisons 1, 3, and 5 are considered first-order effects. Comparison 1 gives an index of stress vs nonstress in control rats; comparison 3 gives an index of control vs vinclozolin lineage in nonstressed rats; and comparison 5 represents the synchronicity of stress and vinclozolin compared with C-NS rats. For graphed data, statistical details are presented in the figures and tables; for data not shown, P values are indicated in the text.
Hormones and adrenal weight
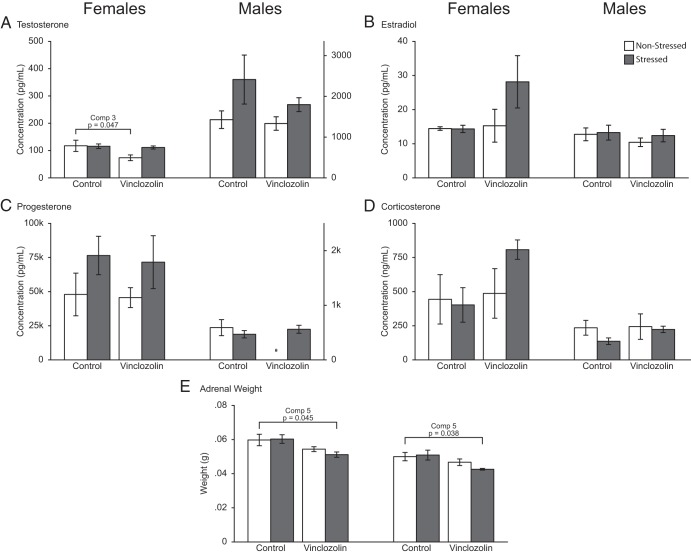
Stress and ancestral vinclozolin had effects on serum testosterone and progesterone in a sex-dependent manner (Figure 2). Estradiol was unaffected (Figure 2B). For testosterone, in the nonstressed females, vinclozolin-lineage rats had lower testosterone than control-lineage females (comparison 3, Figure 2A). Serum progesterone showed a main effect of stress in females only, with higher progesterone in stressed than nonstressed females of both lineages (Figure 2C). Finally, adrenal weight was measured because it is sexually dimorphic and has been shown to be affected by vinclozolin treatment (57, 63) and because CRS is well-established as affecting adrenal weight as we previously reported in an earlier study on males (45). In both sexes, there were main lineage effects, with adrenal weights lower in vinclozolin- than control-lineage rats (females P = .008 and males P = .025, Figure 2E). Furthermore, in both sexes, V-S rats had significantly lower adrenal weights compared with C-NS (comparison 5) counterparts.
Figure 2.
Serum testosterone (A), estradiol (B), progesterone (C), corticosterone (D), and adrenal weights (E) are shown. Significant pair-wise comparisons are shown within the figure. As main effects, progesterone (C) was increased in females due to CRS (P = .048), and adrenal weight was decreased in both females and males due to vinclozolin lineage (P = .008 and P = .025 respectively).
Behaviors
The Stoelting Any-Maze apparatuses allow for the observation and quantification of multiple measures within each specific behavioral test. Although numerous behaviors were quantified, the large number precludes presentation of every aspect. Therefore, the measure that best discriminated the experimental groups was chosen for purposes of graphic presentation and used in the functional landscapes.
Open-field, light-dark transition, and elevated plus maze behaviors were differentially affected in males and females by stress and lineage. Representative data of one individual component for each behavior are shown in Figure 3. For corner time in the open-field test, the synchronicity of stress and lineage (comparison 5) was affected in females, with V-S spending more time in corners compared with C-NS (Figure 3A). Corner time in males was affected only in the control lineage (comparison 1), with C-S showing lower corner time than C-NS.
Figure 3.
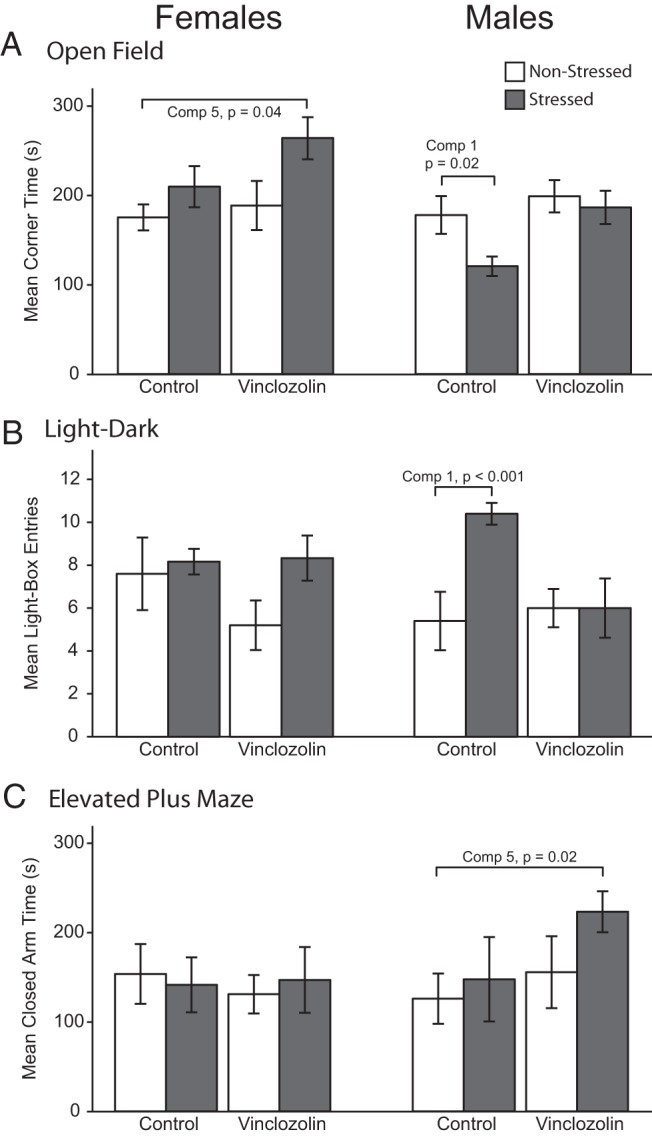
Behavioral measures of interest. A, Open-field test, with time spent in the corner of the arena indicating decreased exploration and increased anxiety. B, Light-dark box, with number of entries into the light box indicating increased exploration and decreased anxiety. C, Elevated plus maze, with time spent in the closed arm indicating decreased exploration and increased anxiety.
For the light-dark box, the number of entries into the light chamber was greater in male C-S than C-NS (comparison 1, Figure 3B). Vinclozolin-lineage males and females showed a delayed initial entry into the dark chamber, compared with control counterparts (data not shown; P < .01). Also, V-S males exhibited a longer latency to enter the dark chamber compared with C-NS males (comparison 5; data not shown, P = .03).
Elevated plus maze behavior was affected only in males (Figure 3C), with V-S spending more time in the closed arm than C-NS (comparison 5). Furthermore, V-S males spent more time in the closed arm of the elevated plus maze (P = .02), displayed a slower mean speed (P = .02), and covered a shorter distance (P = .02) than their C-NS counterparts (comparison 5, data not shown).
Behaviors in the sociability and social novelty test were also quantified (Supplemental Figure 2). In the test for sociability, both males and females preferred to associate with an animal as opposed to an empty cage (P < .001, Supplemental Figure 2A). In a test for social novelty, females preferred to spend more time with a novel animal as opposed to a familiar one (P = .005). Males showed no preference for social novelty (Supplemental Figure 2B).
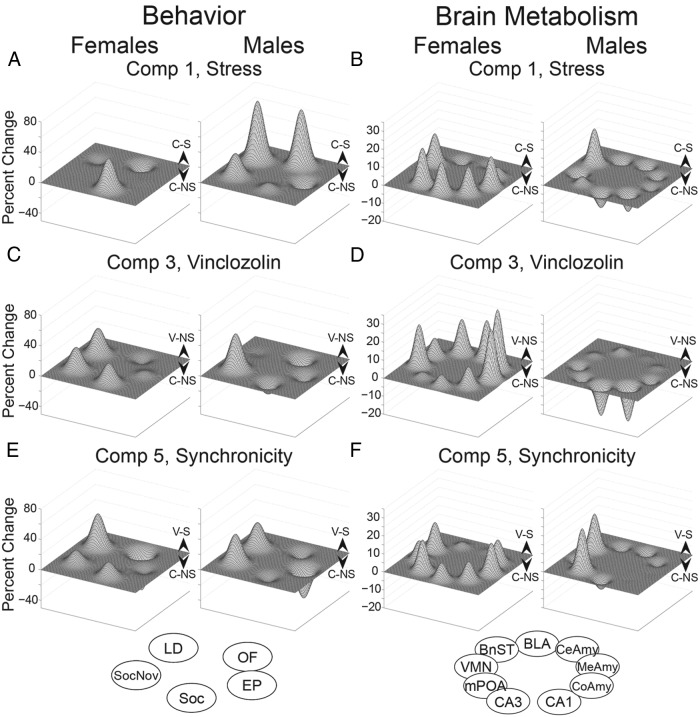
Functional landscape analysis was performed to provide an integrative perspective of behaviors related to anxiety, activity, and sociability for the first-order comparisons 1, 3, and 5 (Figures 4, A, C, and E, respectively). For comparison 1, there were few differences between C-S and C-NS females, whereas males differed for composite behaviors for light-dark and open-field tests. More effects of stress were seen in females compared with males for comparison 3. Finally, females and males were differentially affected in the synchronicity model of comparison 5.
Figure 4.
Functional landscapes of behaviors and brain metabolism (CO activity) are shown for comparison 1 (A and B), comparison 3 (C and D), and comparison 5 (E and F), respectively, in male and female rats. A relative increase for one group over the other is shown by the directionality and height of a peak or valley. For behaviors (A, C, and E), the clockwise nodes are: light-dark box (LD), elevated plus maze (EP), open-field (OF), sociability (SOC), and social novelty (SOCNOV). For brain metabolism (B, D, and F), the clockwise nodes shown are BLA, CeAmy, MeAmy, CoAmy, CA1 and CA3 of the hippocampus, mPOA, VMN, and BnST.
Brain metabolism
CO histochemistry was used as an index of brain metabolism (45, 52), and data are graphed as functional landscapes in Figure 4, B, D, and F (comparisons 1, 3, and 5, respectively). For comparison 1, which focuses on effects of stress in control rats (C-S vs C-NS; Figure 4B), C-S males showed increased metabolic activity compared with C-NS males in the BnST (P = .02), whereas females (C-S) showed increased activity compared with C-NS in the mPOA (P = .03) and BnST (P = .04). For comparison 3, effects of vinclozolin in nonstressed rats (C-NS vs V-NS; Figure 4D), C-NS males showed decreased activity compared with V-NS males in the CA1 and CA3 (P = .04 and P = .02), whereas V-NS females showed increased activity compared with C-NS females in the MeAmy (P = .04), CoAmy (P = .02), and VMN (P = .04).
Gene expression and transcriptome
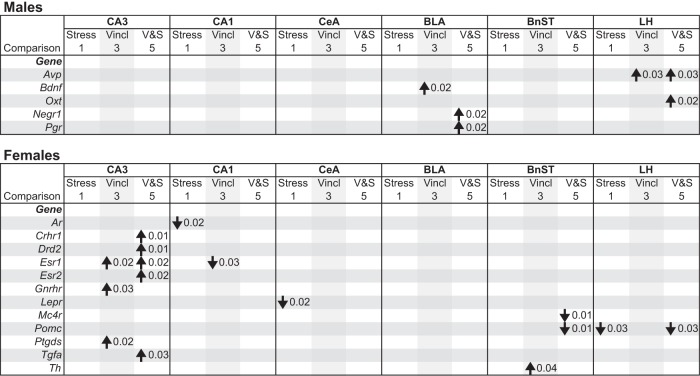
Brain micropunches were analyzed by TLDA, and the relative expression of 48 selected genes was analyzed (listed in Supplemental Table 2) in each of 10 selected brain regions (Supplemental Table 1). Results of affected genes are shown for the female CA3, CA1, and BnST (Figure 5), chosen because these regions had the most robust effects for comparisons 1, 3, and 5. A summary of identified genes and directionality of effect for these 3 comparisons is presented in Figure 6, showing sex- and region-specific differences in affected genes. Additional gene expression data are shown in Supplemental Figure 3 for the male BLA and for the male and female lateral hypothalamus.
Figure 5.
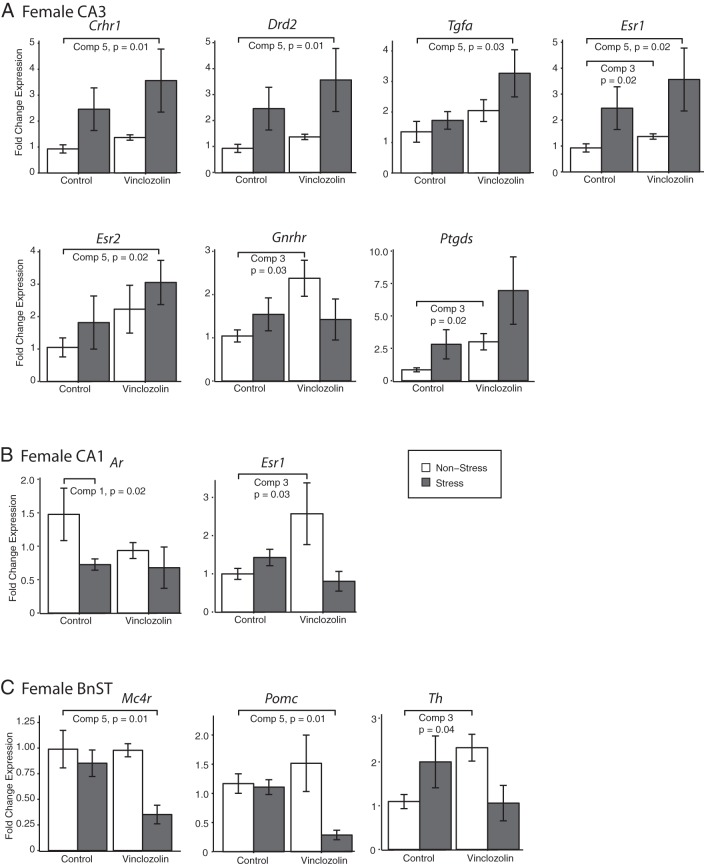
Gene expression results of affected genes identified by TLDA qPCR assays are shown for the female CA3 (A), CA1 (B), and BnST (C). A, In the CA3, 5 genes had higher expression in V-S than C-NS females (comparison 5) and 3 genes had higher expression in V-NS than C-NS females (comparison 3). B, In the CA1, Ar mRNA levels were lower in C-S than C-NS females (comparison 1) and Esr1 mRNA was higher in V-NS than C-S females (comparison 3). In the BnST, Mc4r and Pomc mRNA levels were lower in V-S than C-NS females (comparison 5) and for Th, mRNA levels were higher in V-NS than C-NS females (comparison 3).
Figure 6.
Gene Expression. Summary of mRNA expression of targeted genes, selected for their roles in social, affiliative, and anxiety-related behavioral tests and predicted to be regulated by endocrine disruptors and/or stress, for comparisons 1, 3, and 5. Six hypothalamic and hippocampal nuclei with significant gene expression effects are shown. Differences are shown relative to the C-NS group and are at least 2-fold in magnitude. Note that each region has unique gene expression changes by sex, treatment, and stress. Up arrows indicate a significant increase and down arrows represent a significant decrease relative to the C-NS group. LH, lateral hypothalamic nuclei; Vincl, vinclozolin.
In the female CA3 (Figure 5A), expression of a large number of genes was significantly different between V-S and C-NS (comparison 5): Crhr1, Drd2, Tgfa, Esr1, and Esr2 (gene abbreviations are listed in Supplemental Table 2). In all cases, expression was higher in V-S than C-NS. In addition, there was significantly higher expression of 3 genes in V-NS than C-NS (comparison 3): Esr1, Gnrhr, and Ptgds.
In the female CA1 (Figure 5B), expression of Ar was lower in C-S than C-NS (comparison 1), and for Esr1, expression was higher in V-NS than C-NS (comparison 3). Finally, 3 genes were affected in the female BnST (Figure 5C). Mc4r and Pomc were lower in V-S than C-NS females (comparison 5) and Th was higher in V-NS than C-NS females (comparison 3).
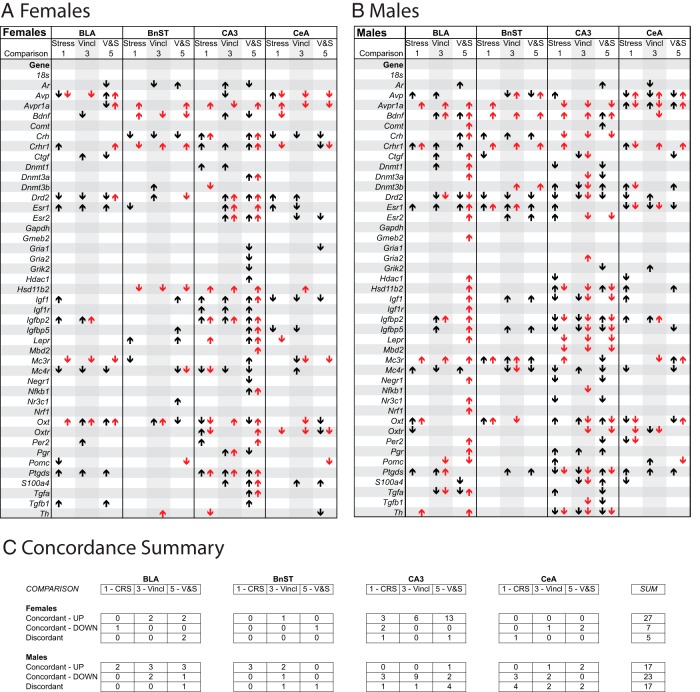
Previously published research on a similar experimental model has reported in-depth transcriptome analysis in response to ancestral vinclozolin (64) and CRS in males (45). In the current study, we performed transcriptome analysis in aliquots of RNA from the same samples used for TLDA in 4 selected brain nuclei: BLA, BnST, CeAmy, and CA3 (Supplemental Table 1) to compare with TLDA results (Figure 7). A cutoff of a 1.2-fold change (transcriptome) and a 1.5-fold change (TLDA) was applied for identified genes with the goal of supporting the results obtained from the more targeted TLDA analysis and to identify candidate networks for future analysis. Because samples were pooled for transcriptome analysis, but were individually assayed by TLDA, only general information about directionality of change with reference to C-NS can be drawn. Patterns of expression were very different between the sexes (Figure 7C). The TLDA analysis identified the female CA3 as having an overrepresented number of affected genes, especially for comparisons 3 and 5, an effect that was largely confirmed by transcriptome analysis, albeit with the caveat that samples were pooled for transcriptome analysis.
Figure 7.
TLDA and transcriptome comparisons. Correspondence between expression patterns in genes identified by PCR-based TLDA (black) and genome-wide transcriptome (gray) analysis is shown for affected genes in the 4 brain regions used for both TLDA and transcriptome (BLA, BnST, CeAmy, and CA3 of the hippocampus). Data are shown for females (A) and males (B). Columns show differences with C-NS individuals as the reference group. Up arrows indicate upregulation, whereas down arrows indicate downregulation. Cutoff criteria were >1.5-fold change (TLDA) and >1.2-fold change (transcriptome). C, Summary of concordance, defined as measures that were in the same direction (upregulated or downregulated). Discordant changes were those for which the 2 measures did not agree.
Discussion
Vinclozolin is a well-established endocrine disruptor when administered during critical developmental life stages. Due to its antiandrogenic properties, male offspring of dams treated with vinclozolin during pregnancy show a demasculinized morphological (reduced anogenital distance, nipple retention, ectopic testis, vaginal pouches, and agenesis of the prostate) and behavioral (failure to attain intromission or ejaculate with a receptive female) phenotype (42). Female rats display reduced anogenital distance (41); later in life, they exhibit uterine hemorrhaging and anemia during pregnancy and increased tumor formation (65).
In addition to its direct effects, vinclozolin was the first environmental EDC shown to have transgenerational effects (10, 16, 64, 66). Although another laboratory reported transgenerational actions of vinclozolin on expression of imprinted genes (12), other laboratories have not replicated these findings (67, 68), underscoring the need for more research and careful attention to experimental differences. However, inter- and transgenerational effects of other endocrine disruptors, such as BPA (13, 69), diethylstilbestrol (in mouse [70] and human [71]), and polychlorinated biphenyls (PCBs) (72) have been reported, adding further evidence that EDCs affect multiple generations, up to and beyond the F3 generation.
Previously, we showed that ancestral exposure to vinclozolin caused behavioral differences in the F3 male descendants when challenged with CRS (45). The present study was predicated on our understanding of fundamental sex differences in the developing nervous system governed by steroid exposure and functional outcomes on brain structure, neurochemistry, and numerous behaviors, both reproductive and otherwise (73). Although many effects of prenatal EDCs are sex-specific (74–77), little is known about sex-specific transgenerational EDC effects. This, together with observations that CRS elicits sex-specific responses in physiology and behavior, and expression of genes and proteins in related brain regions (78–80), led to our current work combining transgenerational effects of vinclozolin with CRS in males and females.
Behavioral effects of proximate adolescent stress and ancestral exposure to vinclozolin
In our study, males exposed to CRS in adolescence showed decreased anxiety behaviors in adulthood. Results of the open-field and light-dark box tests demonstrated greater exploration and decreased aversion to potentially dangerous and stressful situations. No such differences caused by CRS were found in females. These results suggest that males and females differ in the compensatory strategies acquired with early life stress.
By contrast, ancestral exposure to vinclozolin was associated with few behavioral effects in control (nonstressed) males or females, a finding that was surprising because previous work showed that ancestral vinclozolin increased anxiety behaviors in young females in an elevated plus maze and decreased anxiety behaviors of young males in a light-dark box (64). Differences between the laboratory environments (current work conducted at University of Texas Austin, past work at Washington State University) and age at testing may contribute to divergent experimental outcomes. Additionally, the limited number of animals used in the current study, derived from relatively few litters, likely plays a role in differences.
Other endocrine disruptors have been implicated in modifying anxiety responses. BPA consistently increases anxiety behavior in rats as measured by the elevated plus and open-field, regardless of sex, in F1 animals (81, 82). These results have been replicated in mice and extended to social behaviors and the light-dark box (83). Although BPA has a different mechanism of action, targeting different steroid hormone receptors, these results provide further support for transgenerational effects of EDCs on a suite of behaviors.
The combined effect of ancestral vinclozolin and proximate CRS, or their synchronicity, fell into 2 general behavioral outcomes: first, measures that were influenced by CRS that were abolished by vinclozolin, and second, those measures that neither CRS nor ancestral exposure to vinclozolin alone predicted. In the case of the open-field test, synchronicity was limited to females, with increased anxiety behavior in the V-S group compared with C-NS group. Synchronicity in males was seen in the elevated plus maze, again with increased levels in the V-S group. Neither of these outcomes could be predicted by either ancestral EDC exposure or CRS.
Social behavior was not strongly affected by vinclozolin or CRS, nor was there evidence of synchronicity. In the sociability test in which rats distinguished between a conspecific and an empty cage, both males and females showed increased time in the social chamber compared with the empty cage. A sex difference was seen in the social novelty test, where females showed a strong preference for the novel rat, whereas males spent equal time investigating a familiar and novel animal. No robust effects were seen with either lineage or CRS. These results are in contrast to our previous report where C-NS males showed a preference for social novelty (45), likely due to sample size.
Social behavior alterations have been noted for other EDCs such as BPA, which affects both directly exposed animals (F1) and transgenerational lineages (F3–F4), but there has been little investigation into the social effects of vinclozolin (69, 84). The effect of vinclozolin on social affiliation has been limited to the F1 generation, where vinclozolin increased juvenile play behavior in male rats (85). It is likely that social affiliation behavior contains aspects of anxiety and risk taking. Considering that stress has lasting effects on anxiety behavior in both sexes, it is interesting that investigation of the stimulus animals in the sociability and social novelty tests was not affected. Anxiety plays a role in social interactions, but not when stimulus animals are confined and cannot engage in complete tactile interactions. Because of this, it is possible to deconstruct interest and motivation to investigate conspecifics from sociality.
Metabolic brain activity
We found substantial effects of ancestral vinclozolin and CRS as determined by CO histochemistry, as well as substantial sex differences. Throughout the brain nuclei measured, females showed an increased metabolic profile due to stress and vinclozolin treatment, whereas males showed a decrease of lesser magnitude than the increase seen in females. Stress increases activity of the BnST in males and females, a brain area essential in the communication between the amygdala and hypothalamus, likely representing a long-term compensatory mechanism to deal with future stress. Ancestral vinclozolin exposure affects distinct but related groups of nuclei in males (hippocampal) and females (amygdaloid). These results demonstrate that males and females show a difference in how the activity of neural circuitry within each sex compensates for both ancestral and life challenges.
Gene expression
Gene expression results demonstrated sex differences in patterns of expression and regulation by vinclozolin ancestry and/or CRS, with far more effects in females, especially in CA3, CA1, and BnST. Of these, the CA3 of the female hippocampus were usually upregulated by vinclozolin. Among these were the estrogen receptors α and β that mediate effects of estradiol on neuronal activity in the hippocampus (86, 87). The Crhr1 and Drd2 receptors were also upregulated, genes that are involved in cognitive function and stress reactivity in the hippocampus (88, 89). Last, the growth factors Ptgds and Tgfa were upregulated, suggesting altered neuronal growth and remodeling (90, 91). Together, these expression changes suggest wholesale reprogramming of neurobiological gene expression, an effect that is manifested 3 generations later.
Previous work on EDCs has demonstrated effects on hippocampal gene expression, albeit mostly in the F1 generation. PCB exposure during gestation caused a delayed growth profile in the hippocampus indicated by dampened expression of neural proliferation genes (92). BPA affects the expression profile of neural proliferation genes multiple generations after exposure (93). Additionally, PCBs modulate neurotransmitter abundance in the hippocampus, including dopamine (94). Together, these results suggest that EDCs have particularly potent effects on the hippocampus and its growth and proliferation profile, with possible effects on memory, learning, and spatial navigation.
Far fewer gene expression effects were found in the male. In the BLA, 3 genes were upregulated in the vinclozolin lineage (V-NS or V-S) compared with C-NS. The BLA is involved in modulating fear and anxiety responses (95). Two of the 3 genes altered in the BLA are growth factors (Bdnf and Negr1). Increased growth factor expression in the BLA has been implicated in stress-induced structural plasticity, which may relate to the increased anxiety behaviors observed in V-S males.
Summary and conclusions
Animals and humans are exposed to a milieu of manmade and natural EDCs that can alter the trajectory of development and cause detrimental behavioral deficits through multiple generations. How an individual is able to respond to challenges during its lifetime can dictate the probability of reproductive success and survival. Here we show males and females differ in their behavioral response to these challenges. Identified alterations in metabolic history and gene expression may give clues as to the mechanisms responsible and provide targets for more focused analysis.
The process of life has a past and a present that, together, establish the parameters of future potential. Most experiments in this realm manipulate a single or limited number of factors to explore how challenges disrupt the individual. A large subset of these experiments have employed a 2-hit model fashioned after the classic organizational-activational paradigm from developmental biology in which a perturbation early in development is followed by a second perturbation, usually later in life, to explore the impact of these sequential events on physiological and/or behavioral parameters. Our studies are unlike these latter studies in that the separation between events is generations, not days or months within a lifetime. Our studies invite comparison with the classic nature-nurture problem or Gene X Environment studies. That is, vinclozolin exposure during embryonic life of the F1 generation becomes incorporated into the germline by epigenetic modifications, thereby becoming heritable or the nature, or G, component. In the third-generation offspring, who completely lack any body burden of vinclozolin, some individuals experience of CRS during adolescence that lead to epigenetic modifications that affect the nature and quality of later life; these are the nurture, or E, components.
The novelty of this study lies not in the nature of the effects (first-order or main effects of ancestral exposure to vinclozolin or CRS). Rather, it identifies how they transform the individual when they occur together (second-order effects or interactions and a third-order effect, synchronicity). Furthermore, the divergent effects seen among the sexes of F3 adults is dramatic, indicating that the consequences of global contamination and stressful experiences encountered by living descendants is likely to have its own specific risk for males and females for a given spectrum of adverse outcomes. Importantly, the gene expression patterns generally support the functional behavioral differences that were observed. The implications of these results for the protection of human health and endocrine-based questions target questions of morbidity and the quality of life.
Acknowledgments
We thank Richard Francis for his critique.
This work was supported by the National Science Foundation (to R.G.) and National Institutes of Health Grants ES020662 (to A.C.G.), ES012974 (to M.K.S.), and ES017538 and ES023254 (to D.C.).
Disclosure Summary: A.C.G., an author of this paper, is the editor-in-chief of Endocrinology. We claim no other conflict of interest.
Footnotes
- BLA
- basolateral amygdaloid nucleus
- BnST
- bed nucleus of the stria terminalis
- BPA
- bisphenol A
- CeAmy
- central amygdaloid nucleus
- C-NS
- control nonstress
- CO
- cytochrome oxidase
- CoAmy
- anterior cortical amygdaloid nucleus
- CRS
- chronic restraint stress
- C-S
- control stress
- CV
- coefficient of variation
- DAB
- 3,3′-diaminobenzidine
- EDC
- endocrine-disrupting chemical
- MeAmy
- medial amygdaloid nucleus
- mPOA
- medial preoptic area
- PCB
- polychlorinated biphenyl
- PND
- postnatal day
- qPCR
- quantitative PCR
- TLDA
- TaqMan low-density PCR array
- VMN
- ventromedial hypothalamic nucleus
- V-NS
- vinclozolin nonstress
- V-S
- vinclozolin stress.
References
- 1. Crews D, McLachlan JA. Epigenetics, evolution, endocrine disruption, health, and disease. Endocrinology. 2006;147(6 Suppl):S4–S10 [DOI] [PubMed] [Google Scholar]
- 2. Diamanti-Kandarakis E, Bourguignon JP, Giudice LC, et al. Endocrine-disrupting chemicals: an Endocrine Society scientific statement. Endocr Rev. 2009;30:293–342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Landrigan PJ. What causes autism? Exploring the environmental contribution. Curr Opin Pediatr. 2010;22(2):219–225 [DOI] [PubMed] [Google Scholar]
- 4. Landrigan PJ, Miodovnik A. Children's health and the environment: an overview. Mt Sinai J Med. 2011;78(1):1–10 [DOI] [PubMed] [Google Scholar]
- 5. Crews D. Epigenetics and its implications for behavioral neuroendocrinology. Front Neuroendocrinol. 2008;29(3):344–357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Whitehead A, Crawford DL. Variation within and among species in gene expression: raw material for evolution. Mol Ecol. 2006;15(5):1197–1211 [DOI] [PubMed] [Google Scholar]
- 7. Whitehead A, Crawford DL. Neutral and adaptive variation in gene expression. Proc Natl Acad Sci U S A. 2006;103(14):5425–5430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Whitehead A, Roach JL, Zhang S, Galvez F. Genomic mechanisms of evolved physiological plasticity in killifish distributed along an environmental salinity gradient. Proc Natl Acad Sci U S A. 2011;108(15):6193–6198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kidd KA, Blanchfield PJ, Mills KH, et al. Collapse of a fish population after exposure to a synthetic estrogen. Proc Natl Acad Sci U S A. 2007;104(21):8897–8901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Anway MD, Cupp AS, Uzumcu M, Skinner MK. Epigenetic transgenerational actions of endocrine disruptors and male fertility. Science. 2005;308(5727):1466–1469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Skinner MK, Manikkam M, Guerrero-Bosagna C. Epigenetic transgenerational actions of environmental factors in disease etiology. Trends Endocrinol Metab. 2010;21(4):214–222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Stouder C, Paoloni-Giacobino A. Transgenerational effects of the endocrine disruptor vinclozolin on the methylation pattern of imprinted genes in the mouse sperm. Reproduction. 2010;139(2):373–379 [DOI] [PubMed] [Google Scholar]
- 13. Wolstenholme JT, Edwards M, Shetty SR, et al. Gestational exposure to bisphenol A produces transgenerational changes in behaviors and gene expression. Endocrinology. 2012;153:3828–3838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Salian S, Doshi T, Vanage G. Impairment in protein expression profile of testicular steroid receptor coregulators in male rat offspring perinatally exposed to bisphenol A. Life Sci. 2009;85(1–2):11–18 [DOI] [PubMed] [Google Scholar]
- 15. Chamorro-García R, Sahu M, Abbey RJ, Laude J, Pham N, Blumberg B. Transgenerational inheritance of increased fat depot size, stem cell reprogramming, and hepatic steatosis elicited by prenatal exposure to the obesogen tributyltin in mice. Environ Health Perspect. 2013;121(3):359–366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Crews D, Gore AC, Hsu TS, et al. Transgenerational epigenetic imprints on mate preference. Proc Natl Acad Sci U S A. 2007;104(14):5942–5946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Crews D. The (bi)sexual brain. Science & Society Series on Sex and Science. EMBO Rep. 2012;13(9):779–784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kundakovic M, Gudsnuk K, Franks B, et al. Sex-specific epigenetic disruption and behavioral changes following low-dose in utero bisphenol A exposure. Proc Natl Acad Sci U S A. 2013;110(24):9956–9961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hunter RG, McEwen BS. Stress and anxiety across the lifespan: structural plasticity and epigenetic regulation. Epigenomics. 2013;5(2):177–194 [DOI] [PubMed] [Google Scholar]
- 20. Karatsoreos IN, McEwen BS. Resilience and vulnerability: a neurobiological perspective. F1000Prime Rep. 2013;5:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. McEwen BS. Brain on stress: how the social environment gets under the skin. Proc Natl Acad Sci U S A. 2012;109(Suppl 2):17180–17185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. McEwen BS. Neuroscience. Hormones and the social brain. Science. 2013;339(6117):279–280 [DOI] [PubMed] [Google Scholar]
- 23. Heindel JJ. The fetal basis of adult disease: Role of environmental exposures—introduction. Birth Defects Res A Clin Mol Teratol. 2005;73(3):131–132 [DOI] [PubMed] [Google Scholar]
- 24. Meaney MJ. Epigenetics and the biological definition of gene × environment interactions. Child Dev. 2010;81(1):41–79 [DOI] [PubMed] [Google Scholar]
- 25. Jankord R, Solomon MB, Albertz J, Flak JN, Zhang R, Herman JP. Stress vulnerability during adolescent development in rats. Endocrinology. 2011;152(2):629–638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Romeo RD. Adolescence: a central event in shaping stress reactivity. Dev Psychobiol. 2010;52(3):244–253 [DOI] [PubMed] [Google Scholar]
- 27. Romeo RD, Tang AC, Sullivan RM. Early life experiences: enduring behavioral, neurological, and endocrinological consequences. In: Hormones, Brain and Behavior. 2nd ed New York, NY: Elsevier; 2009 [Google Scholar]
- 28. McCormick CM, Mathews IZ. Adolescent development, hypothalamic-pituitary-adrenal function, and programming of adult learning and memory. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34(5):756–765 [DOI] [PubMed] [Google Scholar]
- 29. McCormick CM, Mathews IZ, Thomas C, Waters P. Investigations of HPA function and the enduring consequences of stressors in adolescence in animal models. Brain Cogn. 2010;72(1):73–85 [DOI] [PubMed] [Google Scholar]
- 30. McEwen BS. Stress, sex, and neural adaptation to a changing environment: mechanisms of neuronal remodeling. Ann N Y Acad Sci. 2010;1204(Suppl 1):E38–E59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wei L, Meaney MJ, Duman RS, Kaffman A. Affiliative Behavior Requires Juvenile, But Not Adult Neurogenesis. J Neurosci. 2011;31(40):14335–14345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Solomon MB, Herman JP. Sex differences in psychopathology: of gonads, adrenals and mental illness. Physiol Behav. 2009;97(2):250–258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Baron-Cohen S, Knickmeyer RC, Belmonte MK. Sex differences in the brain: implications for explaining autism. Science. 2005;310(5749):819–823 [DOI] [PubMed] [Google Scholar]
- 34. Goel N, Bale TL. Examining the intersection of sex and stress in modelling neuropsychiatric disorders. J Neuroendocrinol. 2009;21(4):415–420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Shors TJ. Significant life events and the shape of memories to come: a hypothesis. Neurobiol Learn Mem. 2006;85(2):103–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Weinstock M. Gender differences in the effects of prenatal stress on brain development and behaviour. Neurochem Res. 2007;32(10):1730–1740 [DOI] [PubMed] [Google Scholar]
- 37. Wood BL, Haque S, Weinstock A, Miller BD. Pediatric stress-related seizures: conceptualization, evaluation, and treatment of nonepileptic seizures in children and adolescents. Curr Opin Pediatr. 2004;16(5):523–531 [DOI] [PubMed] [Google Scholar]
- 38. Gillies GE, McArthur S. Estrogen actions in the brain and the basis for differential action in men and women: a case for sex-specific medicines. Pharmacol Rev. 2010;62(2):155–198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Grandjean P, Landrigan PJ. Developmental neurotoxicity of industrial chemicals. Lancet. 2006;368(9553):2167–2178 [DOI] [PubMed] [Google Scholar]
- 40. Landrigan PJ, Lambertini L, Birnbaum LS. A research strategy to discover the environmental causes of autism and neurodevelopmental disabilities. Environ Health Perspect. 2012;120(7):a258–a260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Gray LE, Jr, Ostby JS, Kelce WR. Developmental effects of an environmental antiandrogen: the fungicide vinclozolin alters sex differentiation of the male rat. Toxicol Appl Pharmacol. 1994;129(1):46–52 [DOI] [PubMed] [Google Scholar]
- 42. Kelce WR, Monosson E, Gamcsik MP, Laws SC, Gray LE. Environmental hormone disruptors: evidence that vinclozolin developmental toxicity is mediated by antiandrogenic metabolites. Toxicol Appl Pharmacol. 1994;126(2):276–285 [DOI] [PubMed] [Google Scholar]
- 43. Wong C, Kelce WR, Sar M, Wilson EM. Androgen receptor antagonist versus agonist activities of the fungicide vinclozolin relative to hydroxyflutamide. J Biol Chem. 1995;270(34):19998–20003 [DOI] [PubMed] [Google Scholar]
- 44. Uzumcu M, Suzuki H, Skinner MK. Effect of the anti-androgenic endocrine disruptor vinclozolin on embryonic testis cord formation and postnatal testis development and function. Reprod Toxicol. 2004;18(6):765–774 [DOI] [PubMed] [Google Scholar]
- 45. Crews D, Gillette R, Scarpino SV, Manikkam M, Savenkova MI, Skinner MK. Epigenetic transgenerational inheritance of altered stress responses. Proc Natl Acad Sci U S A. 2012;109(23):9143–9148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Moy SS, Nadler JJ, Perez A, et al. Sociability and preference for social novelty in five inbred strains: an approach to assess autistic-like behavior in mice. Genes Brain Behav. 2004;3(5):287–302 [DOI] [PubMed] [Google Scholar]
- 47. Tõnissaar M, Herm L, Eller M, Kõiv K, Rinken A, Harro J. Rats with high or low sociability are differently affected by chronic variable stress. Neuroscience. 2008;152(4):867–876 [DOI] [PubMed] [Google Scholar]
- 48. Klenerova V, Krejci I, Sida P, Hlinak Z, Hynie S. Modulary effects of oxytocin and carbetocin on stress-induced changes in rat behavior in the open-field. J Physiol Pharmacol. 2009;60(2):57–62 [PubMed] [Google Scholar]
- 49. Bouwknecht JA, Spiga F, Staub DR, Hale MW, Shekhar A, Lowry CA. Differential effects of exposure to low-light or high-light open-field on anxiety-related behaviors: Relationship to c-Fos expression in serotonergic and non-serotonergic neurons in the dorsal raphe nucleus. Brain Res Bull. 2007;72(1):32–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Bourin M, Hascoët M. The mouse light/dark box test. Eur J Pharmacol. 2003;463(1–3):55–65 [DOI] [PubMed] [Google Scholar]
- 51. Walf AA, Frye CA. The use of the elevated plus maze as an assay of anxiety-related behavior in rodents. Nat Protoc. 2007;2(2):322–328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Crews D, Rushworth D, Gonzalez-Lima F, Ogawa S. Litter environment affects behavior and brain metabolic activity of adult knockout mice. Front Behav Neurosci. 2009;3:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Sakata JT, Crews D, Gonzalez-Lima F. Behavioral correlates of differences in neural metabolic capacity. Brain Res Brain Res Rev. 2005;48(1):1–15 [DOI] [PubMed] [Google Scholar]
- 54. Walker DM, Kermath BA, Woller MJ, Gore AC. Disruption of Reproductive Aging in Female and Male Rats by Gestational Exposure to Estrogenic Endocrine Disruptors. Endocrinology. 2013;154(6):2129–2143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Siegel A. Anxiogenic effects of developmental bisphenol A exposure are associated with gene expression changes in the juvenile rat amygdala and mitigated by soy. PLoS One. 2012;7(9):e43890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Walker DM, Goetz BM, Gore AC. Dynamic Postnatal Developmental and Sex-Specific Neuroendocrine Effects of Prenatal Polychlorinated Biphenyls in rats. Mol Endocrinol. 2014;28(1):99–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Matsuura I, Saitoh T, Ashina M, et al. Evaluation of a two-generation reproduction toxicity study adding endpoints to detect endocrine disrupting activity using vinclozolin. J Toxicol Sci. 2005;30 Spec No:163–188 [DOI] [PubMed] [Google Scholar]
- 58. Loutchanwoot P, Wuttke W, Jarry H. Effects of a 5-day treatment with vinclozolin on the hypothalamo-pituitary-gonadal axis in male rats. Toxicology. 2008;243(1–2):105–115 [DOI] [PubMed] [Google Scholar]
- 59. Gądek-Michalska A, Spyrka J, Rachwalska P, Tadeusz J, Bugajski J. Influence of chronic stress on brain corticosteroid receptors and HPA axis activity. Pharmacol Rep. 2013;65(5):1163–1175 [DOI] [PubMed] [Google Scholar]
- 60. Hernandez ME, Martinez-Mota L, Salinas C, et al. Chronic stress induces structural alterations in splenic lymphoid tissue that are associated with changes in corticosterone levels in Wistar-Kyoto rats. Biomed Res Int. 2013;2013(3):1–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Kim JG, Jung HS, Kim KJ, Min SS, Yoon BJ. Basal blood corticosterone level is correlated with susceptibility to chronic restraint stress in mice. Neurosci Lett. 2013;555:137–142 [DOI] [PubMed] [Google Scholar]
- 62. Scarpino SV, Gillette R, Crews D. multiDimBio: an R package for the design, analysis, and visualization of systems biology experiments. arXiv preprint arXiv: 1404.0594. 2014. http://arxiv.org/abs/1404.0594
- 63. Dumm ME, Laken B, Ralli EP. Factors influencing adrenal weight and adrenal cholesterol in rats following stress. J Nutr. 1955;56(4):517–531 [DOI] [PubMed] [Google Scholar]
- 64. Skinner MK, Anway MD, Savenkova MI, Gore AC, Crews D. Transgenerational epigenetic programming of the brain transcriptome and anxiety behavior. PLoS One. 2008;3(11):e3745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Nilsson EE, Anway MD, Stanfield J, Skinner MK. Transgenerational epigenetic effects of the endocrine disruptor vinclozolin on pregnancies and female adult onset disease. Reproduction. 2008;135(5):713–721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Anway MD, Leathers C, Skinner MK. Endocrine Disruptor Vinclozolin Induced Epigenetic Transgenerational Adult-Onset Disease. Endocrinology. 2006;147(12):5515–5523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Schneider S, Kaufmann W, Buesen R, van Ravenzwaay B. Vinclozolin–the lack of a transgenerational effect after oral maternal exposure during organogenesis. Reprod Toxicol. 2008;25(3):352–360 [DOI] [PubMed] [Google Scholar]
- 68. Inawaka K, Kawabe M, Takahashi S, et al. Maternal exposure to anti-androgenic compounds, vinclozolin, flutamide and procymidone, has no effects on spermatogenesis and DNA methylation in male rats of subsequent generations. Toxicol Appl Pharmacol. 2009;237(2):178–187 [DOI] [PubMed] [Google Scholar]
- 69. Wolstenholme JT, Goldsby JA, Rissman EF. Transgenerational effects of prenatal bisphenol A on social recognition. Horm Behav. 2013;64(5):833–839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Newbold RR, Padilla-Banks E, Jefferson WN. Adverse effects of the model environmental estrogen diethylstilbestrol are transmitted to subsequent generations. Endocrinology. 2006;147(6 Suppl):S11–S7 [DOI] [PubMed] [Google Scholar]
- 71. Titus-Ernstoff L, Troisi R, Hatch EE, et al. Offspring of women exposed in utero to diethylstilbestrol (DES): a preliminary report of benign and malignant pathology in the third generation. Epidemiology. 2008;19(2):251–257 [DOI] [PubMed] [Google Scholar]
- 72. Steinberg RM, Walker DM, Juenger TE, Woller MJ, Gore AC. Effects of perinatal polychlorinated biphenyls on adult female rat reproduction: development, reproductive physiology, and second generational effects. Biol Reprod. 2008;78(6):1091–1101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Nugent BM, McCarthy MM. Epigenetic underpinnings of developmental sex differences in the brain. Neuroendocrinology. 2011;93:150–158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Gioiosa L, Parmigiani S, Saal vom FS, Palanza P. The effects of bisphenol A on emotional behavior depend upon the timing of exposure, age and gender in mice. Horm Behav. 2013;63(4):598–605 [DOI] [PubMed] [Google Scholar]
- 75. Lilienthal H, Heikkinen P, Andersson PL, Viluksela M. Sexually dimorphic behavior after developmental exposure to characterize endocrine-mediated effects of different non-dioxin-like PCBs in rats. Toxicology. 2013;311(1–2):52–60 [DOI] [PubMed] [Google Scholar]
- 76. Jones BA, Watson NV. Perinatal BPA exposure demasculinizes males in measures of affect but has no effect on water maze learning in adulthood. Horm Behav. 2012;61(4):605–610 [DOI] [PubMed] [Google Scholar]
- 77. Carbone S, Ponzo OJ, Gobetto N, et al. Antiandrogenic effect of perinatal exposure to the endocrine disruptor di-(2-ethylhexyl) phthalate increases anxiety-like behavior in male rats during sexual maturation. Horm Behav. 2013;63(5):692–699 [DOI] [PubMed] [Google Scholar]
- 78. Bowman RE, Beck KD, Luine VN. Chronic stress effects on memory: sex differences in performance and monoaminergic activity. Horm Behav. 2003;43(1):48–59 [DOI] [PubMed] [Google Scholar]
- 79. Kuipers SD, Trentani A, van der Zee EA, Boer den JA. Chronic stress-induced changes in the rat brain: role of sex differences and effects of long-term tianeptine treatment. Neuropharmacology. 2013;75:426–436 [DOI] [PubMed] [Google Scholar]
- 80. Lenglos C, Mitra A, Guèvremont G, Timofeeva E. Sex differences in the effects of chronic stress and food restriction on body weight gain and brain expression of CRF and relaxin-3 in rats. Genes Brain Behav. 2013;12(4):370–387 [DOI] [PubMed] [Google Scholar]
- 81. Patisaul HB, Bateman HL. Neonatal exposure to endocrine active compounds or an ERbeta agonist increases adult anxiety and aggression in gonadally intact male rats. Horm Behav. 2008;53(4):580–588 [DOI] [PubMed] [Google Scholar]
- 82. Diaz Weinstein S, Villafane JJ, Juliano N, Bowman RE. Adolescent exposure to Bisphenol-A increases anxiety and sucrose preference but impairs spatial memory in rats independent of sex. Brain Res. 2013;1529(C):56–65 [DOI] [PubMed] [Google Scholar]
- 83. Xu X, Hong X, Xie L, et al. Gestational and lactational exposure to bisphenol-A affects anxiety- and depression-like behaviors in mice. Horm Behav. 2012;62(4):480–490 [DOI] [PubMed] [Google Scholar]
- 84. Wolstenholme JT, Taylor JA, Shetty SR, Edwards M, Connelly JJ, Rissman EF. Gestational exposure to low dose bisphenol A alters social behavior in juvenile mice. PLoS One. 2011;6:e25448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Colbert NK, Pelletier NC, Cote JM, et al. Perinatal Exposure to Low Levels of the Environmental Antiandrogen Vinclozolin Alters Sex-Differentiated Social Play and Sexual Behaviors in the Rat. Environ Health Perspect. 2005;113(6):700–707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Smejkalova T, Woolley CS. Estradiol acutely potentiates hippocampal excitatory synaptic transmission through a presynaptic mechanism. J Neurosci. 2010;30(48):16137–16148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Huang GZ, Woolley CS. Estradiol acutely suppresses inhibition in the hippocampus through a sex-specific endocannabinoid and mGluR-dependent mechanism. Neuron. 2012;74(5):801–808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Wang XD, Chen Y, Wolf M, et al. Forebrain CRHR1 deficiency attenuates chronic stress-induced cognitive deficits and dendritic remodeling. Neurobiol Dis. 2011;42(3):300–310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Sakata K, Duke SM. Lack of BDNF expression through promoter IV disturbs expression of monoamine genes in the frontal cortex and hippocampus. Neuroscience. 2014;260(C):265–275 [DOI] [PubMed] [Google Scholar]
- 90. Nagata A, Suzuki Y, Igarashi M, et al. Human brain prostaglandin D synthase has been evolutionarily differentiated from lipophilic-ligand carrier proteins. Proc Natl Acad Sci U S A. 1991;88(9):4020–4024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Fallon J, Reid S, Kinyamu R, et al. In vivo induction of massive proliferation, directed migration, and differentiation of neural cells in the adult mammalian brain. Proc Natl Acad Sci U S A. 2000;97(26):14686–14691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Royland JE, Wu J, Zawia NH, Kodavanti PR. Gene expression profiles in the cerebellum and hippocampus following exposure to a neurotoxicant, Aroclor 1254: Developmental effects. Toxicol Appl Pharmacol. 2008;231(2):165–178 [DOI] [PubMed] [Google Scholar]
- 93. Jang YJ, Park HR, Kim TH, et al. High dose bisphenol A impairs hippocampal neurogenesis in female mice across generations. Toxicology. 2012;296(1–3):73–82 [DOI] [PubMed] [Google Scholar]
- 94. Honma T, Suda M, Miyagawa M, Wang RS, Kobayashi K, Sekiguchi S. Alteration of brain neurotransmitters in female rat offspring induced by prenatal administration of 16 and 64 mg/kg of 2,2′,4,4′,5,5′-hexachlorobiphenyl (PCB153). Ind Health. 2009;47(1):11–21 [DOI] [PubMed] [Google Scholar]
- 95. Campeau S, Davis M. Involvement of the central nucleus and basolateral complex of the amygdala in fear conditioning measured with fear-potentiated startle in rats trained concurrently with auditory and visual conditioned stimuli. J Neurosci. 1995;15(3 Pt 2):2301–2311 [DOI] [PMC free article] [PubMed] [Google Scholar]