Abstract
Recent studies have demonstrated an essential role of Gag-specific CD4+ T-cell responses for viral control in individuals infected with human immunodeficiency virus type 1. However, little is known about epitope specificities and functional roles of the Gag-specific helper T-cell responses in terms of vaccine-induced protection against a pathogenic retroviral challenge. We have previously demonstrated that immunization with Friend murine leukemia virus (F-MuLV) Gag proteins protects mice against the fatal Friend retrovirus (FV) infection. We report here the structure of a protective T helper cell (Th) epitope, (I)VTWEAIAVDPPP, identified in the p15 (MA) region of F-MuLV Gag. In mice immunized with the Th epitope-harboring peptide or a vaccinia virus-expressed native full-length MA protein, FV-induced early splenomegaly regressed rapidly. In these mice, FV-infected cells were eliminated within 4 weeks and the production of virus-neutralizing antibodies was induced rapidly after FV challenge, resulting in strong protection against the virus infection. Interestingly, mice immunized with the whole MA mounted strong CD4+ T-cell responses to the identified Th epitope, whereas mice immunized with mutant MA proteins that were not bound to the plasma membrane failed to mount efficient CD4+ T-cell responses, despite the presence of the Th epitope. These mutant MA proteins also failed to induce strong protection against FV challenge. These data indicate the importance of the properly processible MA molecule for CD4+ T-cell priming and for the resultant induction of an effective immune response against retrovirus infections.
Defining the immune mechanisms that facilitate resistance to viral infections is vital for the rational development of preventative and therapeutic modalities against virus-induced diseases. Substantial evidence indicates that virus-specific CD4+ T helper (Th) cells play a key role in the control of many different viral infections (reviewed in references 14 and 36). In mouse models, maintenance of CD8+ cytotoxic T-cell (CTL) responses and control of viremia have been demonstrated to depend on virus-specific CD4+ T cells during chronic viral infections (1, 28, 57, 62). In addition, cooperation between antigen-specific CD4+ T cells and neutralizing antibody (Ab)-producing B cells is required for long-term virus control in lymphocytic choriomeningitis virus infections (43, 53). With regard to immunosuppressive retrovirus infections, activation of virus-specific CTL responses alone is largely ineffective in inducing protection against simian immunodeficiency virus (SIV) infection (12, 49, 60). In contrast, adoptive transfer of autologous CD4+ T cells results both in the induction of virus-specific CTL responses and in the production of neutralizing Abs, with long-term anti-SIV control (56). Thus, the development and maintenance of functional CTL and B-cell responses that are aided by the activation of virus-specific CD4+ T cells might be required for effective protection against chronic virus infections. However, the precise nature of the virus-specific CD4+ T cells that contribute to effective antiviral immunity remains unclear. More recently, an inverse association between human immunodeficiency virus type 1 (HIV-1)-specific CD4+ T-cell responses and plasma viral load has been demonstrated in long-term nonprogressors and individuals treated with highly active antiretroviral therapy (22, 26, 42, 46, 47). Intriguingly, in such HIV-1-infected individuals, strong Gag-reactive CD4+ T-cell responses were detected in association with a high level of HIV-1-specific CTL responses.
The Gag protein of retroviruses is a major viral component and is relatively conserved in its structure among various isolates and between retroviruses of different host species in comparison with the Env protein. Broadly cross-reactive Th epitopes, as well as CTL epitopes, have been identified in conserved regions of retroviral Gag proteins (11, 29, 48, 58). Finally, by use of a mouse model of Friend retrovirus (FV) infection, it has been found that immunization with gag gene products induces CD4+ T-cell-mediated protective immunity (32), although the precise epitopes involved have not been identified. Given these observations, there is compelling evidence indicating that Gag-specific CD4+ T cells are effective in controlling retrovirus infections, and therefore they may be potential targets for the development of effective antiretrovirus vaccines.
FV is an immunosuppressive retrovirus complex that induces fatal erythroleukemia in adult immunocompetent mice. Since the cell surface receptors, intracellular signaling, and host factors controlling virus replication and host immune responses have been well characterized, infection with this retrovirus represents a useful model in which to study both acute and persistent viral infections, as well as virus-host interactions (reviewed in references 8 and 13). The replication-competent helper component of FV, Friend murine leukemia virus (F-MuLV), contains the immunological determinants necessary for anti-FV immune responses, while the replication-defective spleen focus-forming virus (SFFV) is required for the pathogenicity of FV complex in adult mice (21, 34). FV induces rapid splenomegaly because the SFFV envelope protein binds to the erythropoietin receptor on erythroid precursor cells, causing false proliferation signals. Susceptible animals develop acute and severe splenomegaly after FV inoculation, and unresolved infection leads to leukemic death within several weeks after challenge.
In order to understand and characterize the role of Gag-specific CD4+ T cells in protective immunity against retrovirus infections, we attempted here to identify a Th epitope in the MA protein of F-MuLV Gag and investigated the possible association of Gag-primed CD4+ T-cell responses with host protection. Furthermore, we examined structural features of the MA required for the induction of efficient cellular and humoral immune responses in vivo. The results provide new insights into different accessibilities for antigen presentation of the membrane-bound and unbound MA proteins and underscore their importance in vaccine development for retrovirus infections.
MATERIALS AND METHODS
Mice and virus.
Female C57BL/6 (B6) and BALB/c mice were purchased from Japan SLC, Inc. (Hamamatsu, Japan). A/WySnJ mice were originally purchased from The Jackson Laboratory (Bar Harbor, Maine). (B6 × A)F1 mice were bred and maintained at the animal facility, Kinki University School of Medicine, and these mice, aged 8 to 16 weeks at the time of immunization, were used for the experiments described below. A stock of B-tropic FV was originally given by Bruce Chesebro, Laboratory of Persistent Viral Diseases, National Institute of Allergy and Infectious Diseases (Hamilton, Mont.), and the stock used in the present study was prepared from infected BALB/c mice as a 20% spleen homogenate as described previously (31). For virus challenge, mice were injected in the tail vein with 1,500 spleen focus-forming units (SFFU) of FV complex in 0.5 ml of phosphate-buffered balanced salt solution (PBBS) containing 2% fetal bovine serum. After virus challenge, mice were observed daily, and the number of surviving mice was counted. The development of splenomegaly was monitored by palpation as described elsewhere (32). In some experiments, moribund mice were killed by cervical dislocation and spleen weights were measured to compare the results of palpation to actual spleen weights. Spleens weighing >0.5 g were consistently marked as palpable splenomegaly. All the animal experiments were approved and performed under relevant guidelines of the Japanese government and of Kinki University.
Construction of rVVs expressing the F-MuLV gag genes.
Recombinant vaccinia viruses (rVVs) were constructed by the standard homologous recombination method using transfer plasmids based on pSC11 (6). Fragments of a gag gene from an infectious molecular clone of F-MuLV, FB29 (39) (GenBank accession no. Z11128), that were cloned into rVV are shown in Fig. 1. An rVV, r9-28B, expressing the entire gPr80gag and Pr65gag proteins has been described previously (32). All fragments of MA were fused with a polyhistidine metal-binding peptide (His tag) at their C termini so that their expression could be visualized with an anti-His tag Ab. For construction of rVVs expressing the His tag-conjugated proteins, a derivative of pSC11 (pSC11-His) was newly generated by inserting a His tag sequence and the multiple cloning site from the pcDNA3.1/V5-His vector (Invitrogen Corp., Carlsbad, Calif.) into a StuI site of pSC11-SS as described elsewhere (17). All DNA fragments encoding portions of F-MuLV MA (Fig. 1) were synthesized by PCR using pairs of oligonucleotide primers with additional sequences to generate the restriction enzyme site at their 5′ ends. After digestion with the corresponding restriction enzymes, PCR-amplified fragments were inserted in frame into the multiple cloning site of pSC11-His, which allowed fusion of the MA gene fragments to the N-terminal end of the His tag. The resultant plasmids were used to generate rVV vMAs. An unmyristylated form of the MA in which the N-terminal glycine required for protein myristylation (44) was replaced with an alanine was created by site-directed mutagenesis. To introduce a glycine-to-alanine point mutation, the following oligonucleotide was used as the sense primer: 5′-CCCCGTCGACCATGGCCCAGGCTGTT-3′. The PCR product amplified with the mutagenic primer pair and with the plasmid harboring the whole F-MuLV gag gene (32) as the template was inserted into pSC11-His as described above to generate an rVV vMAmu. Nucleotide sequences of all the cloned DNA fragments were confirmed, and the protein expression from the newly constructed rVV was detected by Western blotting and/or immunofluorescent staining with an anti-His Ab (Santa Cruz Biotechnology, Santa Cruz, Calif.). A control rVV expressing the influenza virus hemagglutinin gene (vHA) has been described previously (51). As another control, an rVV, vHS1, expressing His tag-conjugated HS1, a hematopoietic-cell-specific intracellular molecule, was made by inserting a cDNA fragment encoding the N-terminal part (residues 1 to 204) of human HS1 (24) (GenBank accession no. H16663) into pSC11-His. Mice were inoculated with 107 PFU of an rVV via tail scratch (32), followed by an intravenous injection with the same amount of the identical virus 2 weeks later as a booster. Four weeks after the booster immunization, the mice were challenged with FV complex by intravenous inoculation.
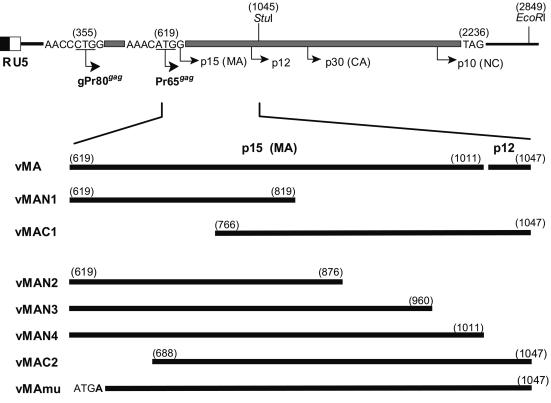
FIG. 1.
Schematic representation of the F-MuLV gag gene and strategies for construction of the rVVs expressing portions of the MA protein. Base numbers of the gag gene in parentheses are given according to the published sequence of F-MuLV FB29 (39).
Synthetic peptides and immunization.
Locations within MA and sequences of the peptides used in this study are shown in Fig. 6A and 7C. Overlapping 30-mer or 9- to 17-mer peptides covering the C-terminal half of the F-MuLV MA were ordered from QIAGEN K. K. (Tokyo, Japan). Lyophilized powder of each purified peptide was dissolved in Dulbecco's phosphate-buffered saline and emulsified with an equal volume of complete Freund's adjuvant (CFA; Difco, Detroit, Mich.). Mice were immunized once subcutaneously in the abdominal wall with multiple split doses for a total of 100 μl of emulsion containing 50 μg of a peptide; 4 weeks later, they were challenged with FV complex. Control mice were given the same amount of CFA emulsified with phosphate-buffered saline without any peptide.
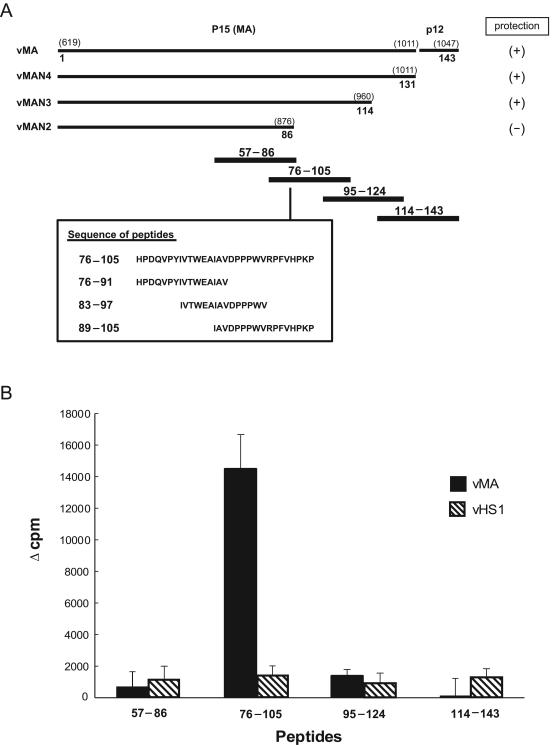
FIG. 6.
Identification of a T-cell-stimulating epitope in MA by use of synthetic peptides. (A) The position of each peptide tested is shown along the schematic representation of the truncated MA proteins used for the mapping experiment represented by Fig. 5. Numbers in parentheses are base positions in the gag gene; other numbers are amino acid positions starting from the initial methionine for Pr65gag. (B) Spleen T cells prepared from vMA- or vHS1-immunized mice at 3 weeks after immunization were cultured with one of the synthetic peptides shown (20 μM) and with syngeneic irradiated spleen cells as APC; their proliferative responses were measured by [3H]thymidine incorporation. Each result is the mean Δcpm for data obtained from five separate mice. Error bars, standard errors of the means. The experiments were performed twice with essentially identical results.
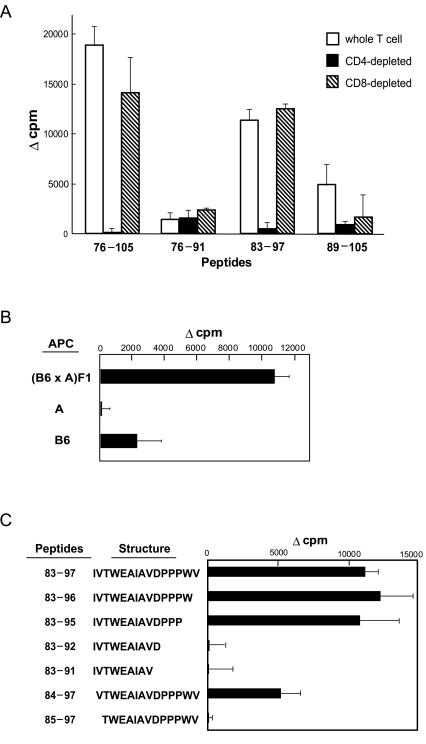
FIG. 7.
Fine specificities and MHC restriction of primed CD4+ T cells prepared from vMA-immunized mice. (A) Spleen T cells prepared from vMA-immunized mice were depleted of either CD4+ or CD8+ T cells by the magnetic cell sorting system. Unseparated T cells or purified T-cell subsets were incubated with each indicated peptide and with syngeneic spleen cells as APC, and antigen-specific proliferative responses were measured. Amino acid sequences of the peptides used here are shown in Fig. 6A. In this experiment, T cells from six immunized mice were pooled before the depletion of each subset. (B) T cells pooled from two to three vMA-immunized (B6 × A)F1 mice were stimulated with peptide 83-97 and irradiated spleen cells prepared from F1, B6, or A mice, and proliferative responses were analyzed as above. (C) T cells pooled from two vMA-immunized mice were stimulated with each indicated peptide and the syngeneic APC, and proliferative responses of T cells were measured. All data shown here are representative of two to four independent experiments with essentially identical results.
Flow cytometry.
Spleen tissue was dissociated in PBBS containing 2% fetal bovine serum, and a single-cell suspension was prepared as described elsewhere (16). Cells were incubated with 10 μg of anti-mouse CD16/CD32 (BD Biosciences PharMingen, San Diego, Calif.)/ml to prevent test Abs from binding to Fc receptors. For detection of erythroblasts infected with F-MuLV, spleen cells were incubated with R-phycoerythrin-conjugated TER-119 (BD Biosciences PharMingen) and biotinylated monoclonal Ab (MAb) 720 followed by fluorescein isothiocyanate-conjugated streptavidin (BD Biosciences PharMingen). TER-119 is specific for late erythroblasts and mature erythrocytes (23), and MAb 720 reacts specifically to F-MuLV gp70 but not to any other mouse retrovirus (45). Cells were also stained with isotype-matched control Abs. Dead cells were excluded from analyses by staining with 7-aminoactinomycin D (Beckman Coulter, Marseille, France), and viable cells were analyzed for specific staining with a FACScalibur (Becton Dickinson Immunocytometry Systems, Franklin Lakes, N.J.).
Infectious center assays.
Infectious center assays were performed as described previously (31). Briefly, spleen cell suspensions prepared from mice challenged with FV complex were serially diluted, plated in triplicate onto monolayers of Mus dunni cells, and then cocultured for 2 days. After fixation with methanol, F-MuLV-infected cell foci were stained with MAb 720, visualized by using the avidin-biotinylated peroxidase complex (ABC; Vector Laboratories, Burlingame, Calif.), and counted under a magnifier.
Assays for virus-neutralizing Abs.
Mice were bled of 100 μl from the retroorbital sinuses under ether anesthesia before immunization, at 2 weeks after the last immunization, and once a week after FV inoculation. The details of the assay for F-MuLV-neutralizing Abs have been described previously (31, 32).
Assays for T-cell proliferative responses.
Proliferative responses of T cells against MA peptides were analyzed at 3 weeks after immunization with each rVV. The assay method has been described elsewhere (15, 32). Briefly, nylon wool-passed T cells were prepared from the spleen, and irradiated (4,000 rads) syngeneic or parental spleen cells were used as antigen-presenting cells (APC). The T cells (5 × 105) were incubated with the APC (5 × 105) and each synthesized peptide (20 μM) in a total volume of 200 μl. Three days later, the cells were pulsed with [3H]thymidine (Amersham Biosciences, Piscataway, N.J.) added at 1.0 μCi per well; its uptake was measured 18 h later with a scintillation counter (Perkin-Elmer Applied Biosystems, Foster City, Calif.). All data are expressed as the mean difference in counts per minute (Δcpm, calculated as the average incorporation of [3H]thymidine by cultures stimulated with a peptide minus that by unstimulated cultures). In some experiments, proliferative responses of T-cell subsets were analyzed. Nylon wool-passed spleen T cells were incubated with an anti-CD4 or anti-CD8 MAb conjugated with magnetic microbeads and were passed through a separation column placed in a magnetic sorter I (Miltenyi Biotec GmbH, Bergisch Gladbach, Germany). The purity of the cell fractions was determined by flow cytometry after staining with appropriate fluorescence-labeled MAbs as described under “Flow cytometry” above. Each preparation contained less than 0.5% of the depleted cell type.
Western blotting.
For some mice immunized with an rVV, production of anti-MA Abs in sera was analyzed by immunoblotting. F-MuLV particles were purified from the culture supernatant of M. dunni cells chronically infected with FB29 as described previously (15, 33). The purified virus particles (8 μg/lane) were subjected to sodium dodecyl sulfate-polyacrylamide gradient gel (PAGEL AE6000; ATTO Corp., Tokyo, Japan) electrophoresis and transferred to a polyvinylidene difluoride membrane. After blocking with 10% skim milk, the membranes were incubated with a 1:10 dilution of each serum sample, followed by another incubation with a horseradish peroxidase-conjugated anti-mouse immunoglobulin (Ig) Ab (Zymed, South San Francisco, Calif.). MA proteins were visualized by using an enhanced chemiluminescence detection system (Amersham Biosciences) as described elsewhere (52). MAb 690, directed against F-MuLV MA (30), was used as a positive control for detection of blotted MA proteins.
Analysis of intracellular localization of His tag-conjugated MA proteins by confocal microscopy.
CV-1 cells were infected with a low titer of rVV and incubated overnight so that isolated infectious plaques were visible. The cells were fixed for 10 min in 3.7% formaldehyde, permeabilized by 0.4% Triton X-100, and blocked with 5% goat serum. His tag-conjugated MA proteins were stained with the anti-His Ab, followed by incubation with a fluorescein isothiocyanate-conjugated anti-rabbit Ig Ab (Southern Biotechnology, Birmingham, Ala.). The stained samples were scanned with an LSM 5 PASCAL laser confocal microscope (Carl Zeiss, Berlin, Germany).
Statistical analyses.
Survival data were expressed by the Kaplan-Meier method, and the Mantel-Haenszel log rank test was employed for comparison of survival curves using GraphPad Prism (GraphPad Software, Inc., San Diego, Calif.). Student's t test was used for comparison of data for T-cell proliferative responses and frequencies of spleen infectious centers between experimental groups.
RESULTS
Early protection against FV infection in mice immunized with F-MuLV MA.
The F-MuLV gag gene codes for two alternatively translated polyproteins, Pr65gag and gPr80gag (9). Pr65gag is the precursor to virion core structural proteins and is myristylated on the N-terminal glycine and proteolytically cleaved into four proteins (p15, p12, p30, and p10) during virion maturation. The glycosylated cell surface Gag protein, gPr80gag, contains the entire amino acid sequence of Pr65gag plus a leader sequence (Fig. 1). We previously showed that protective immune responses against FV infection mediated by CD4+ T cells were induced by immunization with the full-length gag gene products. Moreover, immunization with an rVV expressing full-length Pr65gag (positions 619 to 2849) or with an rVV expressing an N-terminal portion of Gag (positions 355 to 1047) representing the 5′ leader sequence, the entire MA, and a short N-terminal fragment of p12, elicited similarly efficient protection, suggesting that the major protective epitope might be located within a gag gene product encoded by the segment corresponding to positions 619 to 1047 (32). However, since it has been shown that two overlapping CTL epitopes are located within the leader peptide (7, 25, 54), it is not certain whether the MA protein alone can be protective.
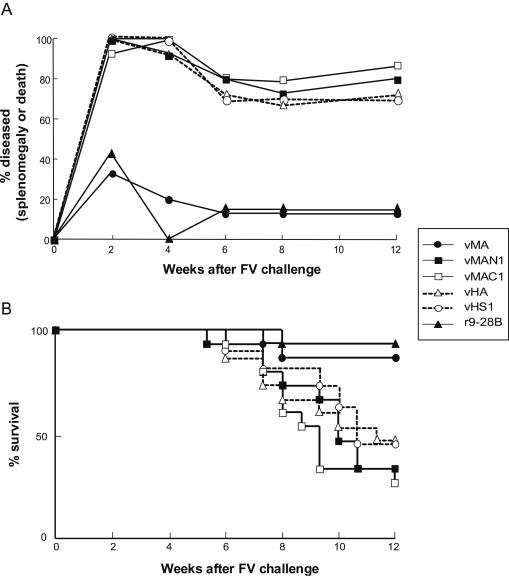
To further narrow the region containing the protective Th epitope, rVVs expressing full-length MA (vMA; positions 619 to 1047), the N-terminal half (vMAN1; positions 619 to 819), or the C-terminal half (vMAC1; positions 766 to 1047) were constructed (Fig. 1), and (B6 × A)F1 mice, which are susceptible to FV infection, were immunized twice with one of these rVVs before FV infection. As shown in Fig. 2, when immunized with the rVV expressing the entire Pr65gag/gPr80gag (r9-28B), more than 80% of the (B6 × A)F1 mice recovered from the initial development of splenomegaly by 6 weeks and survived longer than 12 weeks after inoculation with 1,500 SFFU of FV. On the other hand, all the control mice given the rVV expressing influenza virus HA or His tag-conjugated HS1 showed enlargement of the spleen shortly after the FV challenge, and about half of them died by 12 weeks postchallenge, results comparable to those observed for nonimmunized mice of the same strain. As expected, significant protection was observed in mice immunized with vMA, expressing the entire MA plus the short fragment of p12 but not the leader peptide, confirming the predicted existence of a protective epitope(s) in the MA region. Surprisingly, however, neither the N-terminal (vMAN1) nor the C-terminal (vMAC1) half of MA induced significant protection against FV infection. At 12 weeks postchallenge, the spleens of the surviving mice were weighed, and the results corresponded well with those of palpation, indicating that the individuals shown as diseased in Fig. 2A were indeed leukemic. The incidences of splenomegaly at 6 weeks after challenge were well correlated with the incidence of leukemic death or splenomegaly at 12 weeks after challenge. In addition, in the previous experiments performed with the same or similar mouse strains and ≥1,500 SFFU of FV, recovery from splenomegaly present at 6 to 7 weeks postchallenge has rarely been observed (31, 32). Therefore, in subsequent experiments, splenomegaly was monitored, as an indicator of the FV-induced disease, over a period of 6 weeks postchallenge. Furthermore, since mice immunized with vHA or vHS1 showed similar incidences of disease development, the rVV-expressing His tag-conjugated HS1 was subsequently used as the negative control.
FIG. 2.
Induction of protective immunity against FV infection with the MA region of F-MuLV Gag. Each group of (B6 × A)F1 mice (10 to 15 per group) was immunized twice with one of the rVVs shown. Four weeks after the second immunization, mice were injected intravenously with 1,500 SFFU of FV and then monitored for the development of splenomegaly and death. (A) The incidence of disease at each time point was calculated by adding the numbers of mice that had splenomegaly (>0.5 g) and mice that had died. (B) Survival curves of the groups of mice examined. Statistically significant differences (P < 0.05) between the upper two (▴, •) and the lower four survival curves were confirmed by the Mantel-Haenszel log rank test.
Kinetics of the development of early protective immunity in mice immunized with the MA proteins.
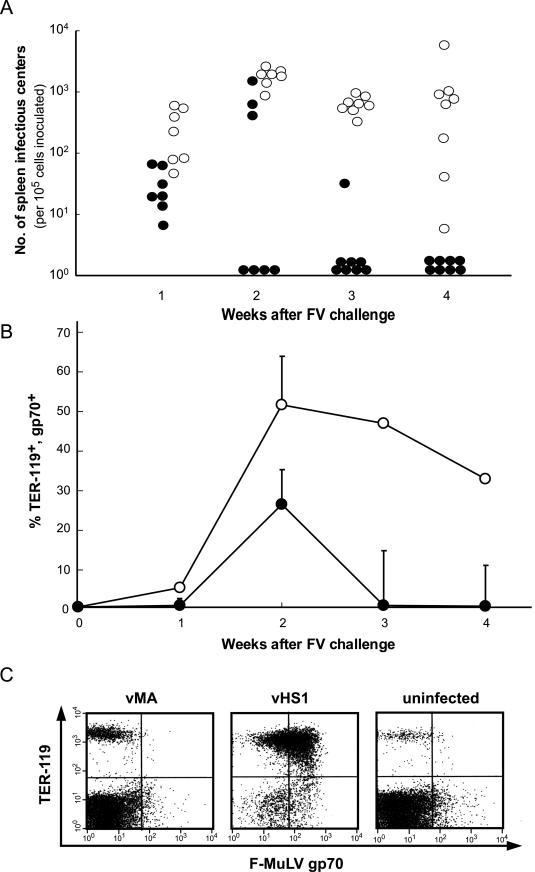
To elucidate how rapidly antiviral protection developed as a result of immunization with full-length MA, we prepared spleen cells from vMA-immunized mice at early time points after FV challenge and examined the numbers of virus-producing cells by infectious center assays (Fig. 3A). In both groups of mice, those immunized with vMA and those immunized with vHS1 (negative control), cells producing infectious F-MuLV particles were detected in the spleen at 1 week after FV challenge, but the average number of spleen infectious centers was significantly lower in vMA- than in vHS1-immunized mice (3.3 × 101 versus 2.7 × 102 per 105 nucleated cells, respectively). In control mice, spleens were dramatically enlarged, reaching a peak average weight of 1.96 g at 3 weeks postchallenge, and average numbers of spleen infectious centers increased and remained high through 4 weeks postchallenge. On the other hand, in vMA-immunized mice, the number of virus-producing cells rapidly decreased after 2 weeks postchallenge, eventually becoming undetectable at 4 weeks postchallenge.
FIG. 3.
Changes in the numbers of FV-infected cells in vMA-immunized mice detected by the infectious center assay (A) and fluorescence-activated cell sorter analyses (B and C). (B6 × A)F1 mice were immunized twice with vMA (•) or vHS1 (○), and 4 weeks later they were challenged with 1,500 SFFU of FV. A group of seven to eight mice was sacrificed at each of the indicated time points, and spleen cells were prepared for analysis. (A) Frequencies of spleen infectious centers were determined by infecting the indicator cells and staining infected foci with a MAb against F-MuLV gp70. Results are shown on a logarithmic scale. Significant differences (P < 0.05 by Student's t test) between the two groups were observed at all time points tested. (B) FV-infected erythroblasts in mice immunized with vMA (•) or vHS1 (○) were stained with a combination of MAb 720 and TER-119. Each data point represents the mean percentage of cells stained with both the MAbs ± the standard error of the mean. (C) Representative pattern of staining for each group of mice observed at 4 weeks postchallenge.
The spleen cells prepared as described above were also subjected to flow cytometric analyses using a combination of MAb 720 and TER-119. The latter marks late erythroblasts and mature red blood cells but not erythroid burst-forming units or CFU. The changes in the frequency of erythroblasts expressing F-MuLV gp70 were in good correlation with the changes in the numbers of spleen infectious centers (Fig. 3B), and the number of FV-infected cells in vMA-immunized mice was markedly reduced by 4 weeks postchallenge, as shown by the representative pattern of staining obtained at that time (Fig. 3C). These results suggested that immunization with vMA suppressed both viral replication and proliferation of FV-infected erythroid cells.
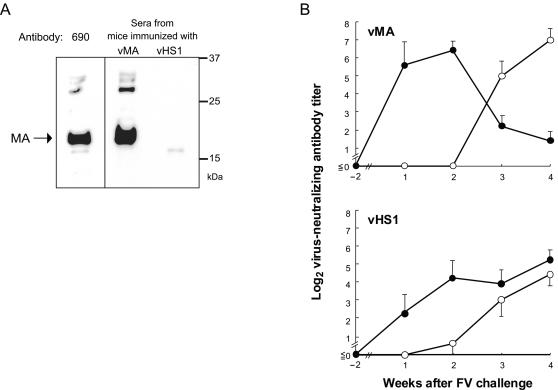
We next studied the kinetics of production of F-MuLV-neutralizing Abs in vMA-immunized and control mice. Before FV challenge, MA-reactive Abs were detected in sera obtained from vMA-immunized mice, but not in those obtained from vHS1-immunized mice, by Western blotting (Fig. 4A). Nevertheless, virus-neutralizing Abs were not detectable in any sera from vMA-immunized mice before challenge (Fig. 4B). After FV challenge, higher levels of virus-neutralizing IgM Abs were detected in vMA-immunized mice than in vHS1-immunized control mice. Further, IgM-to-IgG class switching of neutralizing Abs was observed in vMA-immunized mice at 3 weeks postchallenge, but neutralizing Abs remained IgM dominant in control mice during the observation period until 4 weeks postchallege.
FIG. 4.
Presence and titers of anti-MA and virus-neutralizing Abs in sera from vMA-immunized mice. Serum samples were collected from vMA- or vHS1-immunized mice at 2 weeks after the final immunization (−2) and at 1, 2, 3, and 4 weeks after FV challenge. (A) Detection of MA-reactive Abs in sera from immunized mice at 2 weeks after the final immunization but before challenge. In this experiment, serum samples from two to three mice were pooled and used for Western blot analysis. MAb 690, directed against F-MuLV MA, was used as a positive control. Data shown here are representative of two repeated experiments. (B) Titers of IgM (•) and IgG (○) F-MuLV-neutralizing Abs. Each data point is the mean titer from five separate serum samples ± the standard error of the mean, on a logarithmic scale.
Localization of protective epitopes in MA by expression of longer fragments.
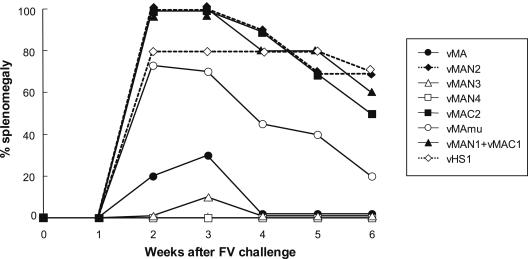
Since the results shown in Fig. 2 (in which neither the N-terminal nor the C-terminal half of MA induced resistance to FV infection) raised the question of whether two separate epitopes in MA might be necessary for full protection, we next attempted to immunize mice simultaneously with vMAN1 and vMAC1. However, contrary to our expectation, the combination of vMAN1 and vMAC1 was also unable to induce protective immunity (Fig. 5). Therefore, we decided to construct additional rVVs by deleting even shorter fragments from the vMA whose protective efficacy has been proven. Immunization with vMAN3 or vMAN4, which expressed MA with a short deletion in the C-terminal end or the entire MA without the p12 fragment, respectively, induced significant protection (Fig. 5). On the other hand, vMAN2, expressing MA with a longer C-terminal deletion, did not induce early regression of the splenomegaly that had developed immediately after FV challenge, indicating that at least one of the putative protective epitopes is localized between positions 876 and 960 of the F-MuLV gag gene, which encodes residues 86 to 114 of MA.
FIG. 5.
Localization of protective epitopes within the MA by using rVVs. The portions of F-MuLV MA expressed by the rVVs used are diagramed in Fig. 1. The ability of each rVV to induce protective immunity against FV infection was analyzed by immunizing (B6 × A)F1 mice (10 per group) and challenging them with FV. The development of splenomegaly over a 6-week period after challenge was observed as an indicator of FV-induced disease.
Although a longer segment of the N-terminal region of MA was included for comparison with vMAC1, vMAC2 did not induce significant protective immunity (Fig. 5). These results suggested the possibility that vMAC1 was ineffective not because of its lack of a protective epitope but because of inappropriate processing or presentation of an existing epitope. Since N-terminal myristylation is a common feature of retroviral MA that is necessary for stable association with the plasma membrane (4, 37, 44), an MA protein that is unmyristylated due to the lack of its N-terminal glycine is predicted not to bind efficiently to the plasma membrane and to remain primarily in the cytoplasm. As expected, immunization with vMAmu, expressing the whole MA protein in which the N-terminal glycine had been replaced with an alanine, was unable to prevent effectively the development of early splenomegaly in FV-infected mice (Fig. 5). Among mice immunized with vMAmu, 70% developed early splenomegaly by 2 weeks postchallenge, and half still carried an enlarged spleen until 4 weeks postchallenge. Thus, vMAmu appeared to show low immunogenicity in spite of possessing the full MA sequence, suggesting that myristylation of the MA protein at the N terminus might influence its immunogenicity.
Determination of a protective Th epitope with synthetic peptides.
Studies with the rVVs described above showed that a putative protective Th epitope should be present between residues 86 and 114 of MA. To identify the precise structure of the protective Th epitope, proliferative responses of T cells primed with vMA were analyzed by in vitro stimulation with overlapping 30-mer peptides covering the C-terminal half of MA, which should contain a protective epitope (Fig. 6A). Spleen T cells primed with the full-length MA protein showed proliferative responses only when stimulated with a peptide corresponding to residues 76 to 105 of MA, not when stimulated with any other peptide (Fig. 6B). When T cells from control mice immunized with vHS1 were stimulated, all the peptides tested induced only marginal levels of proliferation.
In a previous study, the T cells primed by immunization with Gag antigens were mainly CD4+ (32). Therefore, we next examined the antigen-specific proliferative responses of T-cell subsets. As expected, CD4-depleted T cells prepared from vMA-primed mice showed no proliferative response to stimulation with peptide 76-105, whereas CD8-depleted T cells proliferated at a level comparable to that of whole-spleen T cells (Fig. 7A). To further pursue the structure of the Th epitope, we additionally synthesized overlapping peptides of 15- to 17-mer lengths covering residues 76 to 105 (Fig. 6A) and analyzed their abilities to stimulate vMA-primed T cells. Whole T-cells proliferated in response to stimulation with a 15-mer peptide representing residues 83 to 97 (IVTWEAIAVDPPPWV) of MA; this proliferative response was totally abolished by the depletion of CD4+, but not of CD8+, T cells (Fig. 7). The peptide representing residues 76 to 91 was ineffective, and peptide 89-105 induced only marginal proliferation of vMA-primed T cells.
To analyze major histocompatibility complex (MHC) molecules involved in the presentation of the CD4+ T-cell epitope, T cells from the (B6 × A)F1 mice previously immunized with vMA were stimulated in vitro with the 15-mer peptide plus APC prepared from either B6, A, or F1 mice. The primed T cells showed an H-2b/a-restricted proliferative response, but both parental APC were ineffective at presenting the peptide (Fig. 7B), indicating the possibility that peptide 83-97 may be presented by either hybrid Ab/k or Eb/k class II molecules. To further investigate whether residues 83 to 97 (IVTWEAIAVDPPPWV) were the minimal structure for inducing the peptide-specific T-cell responses, several shorter peptides covering residues 83 to 97 were synthesized and tested for their abilities to stimulate vMA-primed T cells (Fig. 7C). The results showed that the 13-mer peptide spanning residues 83 to 95 (IVTWEAIAVDPPP) was sufficient to elicit vMA-primed T-cell responses of similar strength to those elicited by the 15-mer peptide. Elimination of the N-terminal I, as represented by peptide 84-97, significantly diminished the induced proliferative responses, and elimination of the two N-terminal residues from the 15-mer peptide totally abolished the stimulating potential. These results indicated that the 12-mer sequence VTWEAIAVDPPP, as the longest possibility, constituted the core structure of the T-cell epitope.
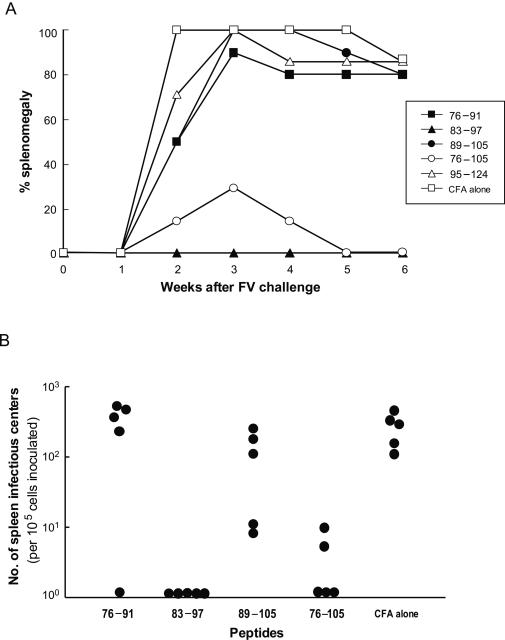
We next investigated whether immunization with the single Th epitope could induce effective protection against FV infection. Mice given a single immunization with either peptide 83-97 or peptide 76-105, but not those given any other peptide tested, showed a lack of development of splenomegaly or very rapid regression of the splenomegaly induced by FV challenge (Fig. 8A). In mice immunized with peptide 83-97 or 76-105, F-MuLV-producing cells in the spleen were either undetectable or detectable at very low frequencies at 4 weeks after FV challenge, as evaluated by infectious center assays (Fig. 8B). These results indicated that the Th epitope present between residues 83 and 97 of MA is sufficient to induce protective immune responses against FV infection.
FIG. 8.
Protection against FV infection induced by immunization with the Th epitope-harboring peptide alone. Mice (eight per group) were immunized once with 50 μg of one of the peptides shown or with CFA alone, followed by challenge with 1,500 SFFU of FV. (A) Splenomegaly over a 6-week period after challenge was observed as an indicator of FV-induced disease. (B) Frequencies of spleen infectious centers were determined at 4 weeks after FV challenge (five mice per group). The differences between CFA-injected and peptide 83-97- or 76-105-immunized mice were significant (P < 0.01 by Student's t test).
Correlation between intracellular localization and immunogenicity of mutant MA proteins.
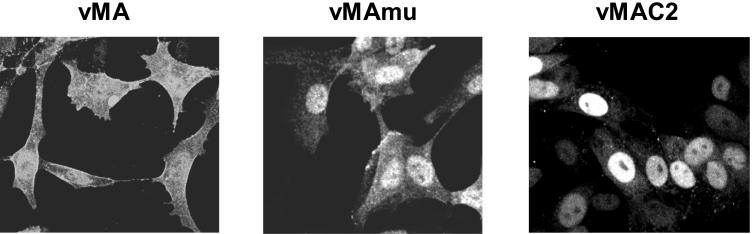
The results described above indicated again that vMAC1, which definitely carried the protective Th epitope, was nevertheless not protective, because of a reduced immunogenicity of the otherwise antigenic C-terminal portion of the MA protein. Substantial evidence suggests that the N-terminal region of retroviral MA, the amino-terminal glycine residue and basic sequences close downstream, is responsible for the targeting of MA to the plasma membrane (4, 37, 44, 61, 63). The results of the protection experiments with vMAC2 and vMAmu (Fig. 5) further suggested that the targeting of the MA molecule to different subcellular compartments might have an influence on their immunogenicity. To prove this assumption, we first examined the intracellular localization of the mutant MA proteins by staining them with an anti-His Ab. The native MA protein expressed by vMA infection was present throughout the cells in a diffuse distribution and also localized at the plasma membrane (Fig. 9), as expected. In contrast, the mutant form of full-length MA, which lacked the site of myristylation, expressed by vMAmu infection, was localized more prominently in the nucleus than in the cytoplasm. In addition, the fluorescent intensity in vMAmu-infected cells was relatively low at the edges of the cells in comparison with that in vMA-infected cells. Interestingly, the MA protein from which the N-terminal 24 residues had been deleted, expressed by vMAC2 infection, was localized largely in the nucleus, which implies that, in addition to the glycine residue, the N-terminal short region of F-MuLV MA (as shown for HIV-1 MA [61, 63]) is necessary for its localization at the plasma membrane. The mutant MA proteins expressed by vMAmu and vMAC2 were observed by fluorescence at levels comparable to that of the native MA protein expressed by vMA infection, and a quite similar result showing the almost equal level of their expression was also obtained by Western blot analysis (data not shown).
FIG. 9.
Intracellular localization of mutant MA proteins. CV-1 cells were infected with vMA, vMAmu, or vMAC2; 24 h later, they were fixed and permeabilized. His tag-conjugated MA proteins were visualized with an anti-His tag Ab. The experiments were performed three times with essentially identical results.
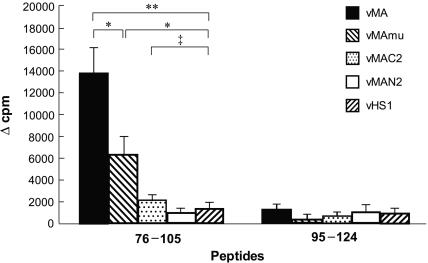
To confirm that these changes in intracellular localization of the MA protein do affect its immunogenicity, we next compared the abilities of these MA mutants to prime the antigen-specific CD4+ T cells (Fig. 10). T cells from vMAmu-immunized mice showed moderate proliferative responses when stimulated with the epitope-harboring peptide 76-105, but the response level was significantly lower than that of T cells taken from vMA-immunized mice. T cells prepared from vMAC2-immunized mice proliferated only marginally in response to stimulation with the Th peptide, showing no significant difference from the response of T cells from vHS1-immunized control mice. These results indicated that targeting of the MA protein to the plasma membrane might be critical for induction of efficient priming of MA-specific CD4+ T cells.
FIG. 10.
Different abilities of mutant and truncated MA proteins at priming CD4+ T cells. Proliferative responses of T cells obtained from mice immunized with each rVV were tested by stimulation with peptide 76-105. Each result is expressed as the mean Δcpm for data obtained from four to five mice. Error bars, standard errors of the means. The experiments were performed twice with essentially identical results. Statistically significant differences were observed by Student's t test (*, P < 0.05; **, P < 0.001). ‡, no significant difference (P > 0.05).
DISCUSSION
We have demonstrated here, for the first time, efficient protection against a pathogenic retrovirus infection through the priming of Gag-specific CD4+ T cells with an MA peptide. Immunization with the rVV expressing native MA alone was sufficient to protect mice from FV-induced disease development, and a protective epitope was present within residues 83 to 97 of MA. T cells primed in vivo with the native MA protein proliferated in vitro when stimulated with the minimal peptide 83-95 (IVTWEAIAVDPPP), and reactivity was completely abolished when CD4+ T cells were depleted, indicating that the protective epitope is recognized by CD4+ T cells. A hybrid class II molecule expressed on H-2b/a cells is required for the presentation of this epitope. Of note, this peptide is highly immunogenic, since a single vaccination with the epitope-harboring peptide was sufficient to protect mice against FV-induced disease. As in the previously demonstrated case of protection against FV infection induced by priming CD4+ T cells with an Env-derived single-epitope peptide (16, 31), multiple effector mechanisms might have been activated upon FV infection in mice immunized with the MA-derived peptide. In agreement with our results, it has been shown that HIV-specific CD4+ T cells in long-term nonprogressors are mainly directed against p17, the N-terminal HIV Gag protein analogous to F-MuLV MA (42). Thus, the high immunogenicity of MA might be a common feature among diverse retroviruses. In support of this, broadly reactive Th epitopes were also identified in the MA region by using macaques chronically infected with SIV (48). Taken together, these data suggest that MA might be a potential target for the development of effective antiretrovirus vaccines.
In all the mice immunized with native MA expressed from the rVV or with the Th epitope-harboring peptide alone, virus-producing cells became undetectable by 4 weeks after FV challenge. The immune effector functions exerted by the virus-specific CD4+ T cells might be very diverse (36), including helper functions provided for B- and CD8+ T-cell responses, production of antiviral cytokines, and direct cytolysis. Our results here have shown that the class switching of virus-neutralizing Abs from IgM to IgG after FV challenge is remarkably accelerated in vMA-immunized mice. MA-specific Abs were detected by Western blotting before FV challenge in the sera of mice immunized with the rVV expressing native MA, but they were incapable of neutralizing F-MuLV (Fig. 4). Thus, the presence of a dominant neutralizing epitope(s) within MA is unlikely. Since the neutralizing Abs were produced in vMA-immunized mice only after FV challenge, it is more likely that the MA-primed CD4+ T cells may have provided a helper function to Env-reactive B-cell responses. Alternatively, the production of Abs directed against the cell surface gPr80gag might have been facilitated after FV challenge by help from the MA-primed CD4+ T cells. Prevention of cell-to-cell transmission of retroviruses by anti-gPr80gag Abs has been demonstrated (41).
The virus-specific CD4+ T cells might also have been required for the maintenance of functional virus-specific CTL responses, as has been shown in other reports (1, 28, 40, 53). However, the mechanisms by which Gag-specific CD4+ T cells may fulfill this role is poorly understood. In HIV-1-infected individuals, enhanced CD4+ T-cell responses have been associated with a higher level of virus-specific CTL responses and lower viral loads (22, 46). In this regard, we and others (7, 25, 54) previously found the presence of overlapping CTL epitopes in the leader sequence upstream of the ATG start codon for Pr65gag, but not in the MA region, although it has not been determined whether these epitopes are protective or not. However, our previous work clearly demonstrated that the rVV expressing Pr65gag without the leader sequence was as effective as the rVV that expressed gPr80gag, indicating that the CTL epitope in the leader sequence is not a requisite for protection against FV infection. Thus, the CD4+ T cells primed with MA might have induced rapid responses of Gag-specific CTL, as well as Env-specific B cells, but the possible importance of Gag-specific CTL responses, if any, has yet to be identified.
Gag-specific CD4+ T cells may also have direct roles in the control of FV infection through their possible cytotoxic activities and production of antiviral cytokines. Direct cytotoxic activities of CD4+ T cells have been described in a number of viral infections (2, 19, 36, 59). A previous study with the FV-infected mouse model, in which direct cytotoxic activities of Env-specific CD4+ T cells were detected, also supported those observations (16). Among CD4+ T-cell clones established from HIV-1-infected individuals with vigorous Gag-specific responses, some displayed virus-specific cytotoxic activities (35, 55). Furthermore, CD4+ T cells have been shown to directly control virus replication by production of gamma interferon in FV infection (10, 18). Thus, CD4+ T cells primed with the MA protein might have contributed to the observed protection against FV infection through multiple effector functions.
The present study has also provided useful information on the structural requirements for effective priming of virus-specific CD4+ T-cell responses by the MA protein. T cells primed in vivo with native MA (vMA) proliferated when stimulated with the Th epitope-harboring peptide 76-105. In contrast, full-length MA lacking the N-terminal myristylation site (vMAmu) and the MA from which the N-terminal 24 residues had been deleted (vMAC2), despite carrying the whole Th epitope, induced only moderate or marginal T-cell responses, respectively, when used to prime T cells in vivo. Of note, their different abilities to elicit the CD4+ T-cell response were well correlated with their efficacies in inducing protection against FV infection in vivo. There was also a correlation between the observed degree of localization of the MA protein at the plasma membrane and its ability to elicit T-cell proliferation and immune protection: By the destruction of the myristylation site, the degree of localization of the MA protein at the plasma membrane was diminished, and the MA lacking the N-terminal 24 residues localized predominantly in the nucleus. These results indicate that the N-terminal region of F-MuLV MA, not just the myristylation site, is responsible for its subcellular localization. A highly basic domain between MA residues 17 and 31 in HIV-1, besides the myristylation signal, has been implicated in membrane binding of the Gag polyprotein (61, 63), and there is a corresponding basic region present between MA residues 17 and 34 in F-MuLV.
Efficient priming of CD4+ T cells by virally encoded proteins is dependent on sufficient levels of antigen expression and delivery of the protein-derived peptides to the MHC class II (MHC II) compartment. Although there is evidence for the activation of CD4+ T cells by viral-DNA-encoded proteins (20, 32, 36), the epitopes displayed on cytoplasmic proteins are usually presented by MHC class I (MHC I) molecules to CD8+ T cells. Epitopes presented by MHC II molecules to CD4+ T cells are mainly derived from extracellular “foreign” proteins taken into cells by endocytotic activities and then degraded in endosomal vesicles. Although retroviral MA is a cytoplasmic protein, there must be some mechanisms for MA to gain access to the cellular site of protein processing involved in peptide presentation on MHC II. It is possible that accumulation of MA at the plasma membrane may result in the formation of aggregates that might be engulfed into phagosomes by mechanisms similar to autophagocytosis. As another possibility, some portions of the MA molecule might be exposed on the outside of the viral envelope, since neutralizing Abs reactive to MA have been detected in some retrovirus infections (3, 5, 38, 50). In addition, there may be an alternative mechanism for the presentation of foreign antigens to CD4+ T cells, in which APC such as macrophages take up whole rVV-infected cells or their fragments by phagocytosis, and the degraded MA is presented on MHC II molecules through the conventional class II pathway. However, the last possibility is unlikely to be the main pathway for MHC II presentation of rVV-generated MA antigens, since the mutant MA proteins expressed by infection of vMAmu and vMAC2, which carried the Th epitope and were detected at a level comparable to that of the native MA expressed by vMA infection, nevertheless failed to induce strong enough CD4+ T-cell proliferation and full protection against FV infection. Therefore, the targeting of MA to the plasma membrane may provide this protein with efficient access to the cellular site for processing and presentation through MHC II pathways, which facilitates induction of the observed immune responses through more-efficient antigen-specific activation of CD4+ T cells. In support of this hypothesis, a recent study demonstrated that a chimeric HIV-1 p55gag protein forced to traffic to the MHC II compartment elicited strong cellular and humoral immune responses in immunized mice (27).
In summary, the results presented here provide compelling evidence that a retrovirus MA peptide is capable of inducing a strong CD4+ T-cell-mediated immune response, which results in effective protection against virus challenge. It will be interesting to design future studies to explore whether there are functional differences between Env-primed and Gag-primed CD4+ T cells, which may be of importance for the development of an effective antiretrovirus vaccine strategy. In addition, the finding that the binding of the MA protein to the plasma membrane is associated with its stronger immunogenicity may lead us to considerations of practical importance about the appropriate immunogenic forms of cytoplasmic proteins when they are considered as candidates for virus-based vaccines.
Acknowledgments
We thank M. Patrick Gorman for critical review of the manuscript and T. Yuasa for assistance in manuscript preparation.
This work was supported by grants from the Ministry of Education, Culture, Sports, Science and Technology and the Ministry of Health, Labor and Welfare of Japan and by the Japan Health Science Foundation.
REFERENCES
- 1.Battegay, M., D. Moskophidis, A. Rahemtulla, H. Hengartner, T. W. Mak, and R. M. Zinkernagel. 1994. Enhanced establishment of a virus carrier state in adult CD4+ T-cell-deficient mice. J. Virol. 68:4700-4704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bickham, K., C. Munz, M. L. Tsang, M. Larsson, J. F. Fonteneau, N. Bhardwaj, and R. Steinman. 2001. EBNA1-specific CD4+ T cells in healthy carriers of Epstein-Barr virus are primarily Th1 in function. J. Clin. Investig. 107:121-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boucher, C. A., W. J. Krone, J. Goudsmit, R. H. Meloen, P. H. Naylor, A. L. Goldstein, D. K. Sun, and P. S. Sarin. 1990. Immune response and epitope mapping of a candidate HIV-1 p17 vaccine HGP30. J. Clin. Lab. Anal. 4:43-47. [DOI] [PubMed] [Google Scholar]
- 4.Bryant, M., and L. Ratner. 1990. Myristoylation-dependent replication and assembly of human immunodeficiency virus 1. Proc. Natl. Acad. Sci. USA 87:523-527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buratti, E., S. G. Tisminetzky, P. D'Agaro, and F. E. Baralle. 1997. A neutralizing monoclonal antibody previously mapped exclusively on human immunodeficiency virus type 1 gp41 recognizes an epitope in p17 sharing the core sequence IEEE. J. Virol. 71:2457-2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chakrabarti, S., K. Brechling, and B. Moss. 1985. Vaccinia virus expression vector: coexpression of β-galactosidase provides visual screening of recombinant virus plaques. Mol. Cell. Biol. 5:3403-3409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen, W., H. Qin, B. Chesebro, and M. A. Cheever. 1996. Identification of a gag-encoded cytotoxic T-lymphocyte epitope from FBL-3 leukemia shared by Friend, Moloney, and Rauscher murine leukemia virus-induced tumors. J. Virol. 70:7773-7782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chesebro, B., M. Miyazawa, and W. J. Britt. 1990. Host genetic control of spontaneous and induced immunity to Friend murine retrovirus infection. Annu. Rev. Immunol. 8:477-499. [DOI] [PubMed] [Google Scholar]
- 9.Dickson, C., R. Eisenman, H. Fan, E. Hunter, amd N. Teich. 1982. Protein biosynthesis and assembly, p. 513-648. In N. T. R. Weiss, H. Varmus, and J. Coffin (ed.), RNA tumor viruses. Molecular biology of tumor viruses. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 10.Dittmer, U., K. E. Peterson, R. Messer, I. M. Stromnes, B. Race, and K. J. Hasenkrug. 2001. Role of interleukin-4 (IL-4), IL-12, and gamma interferon in primary and vaccine-primed immune responses to Friend retrovirus infection. J. Virol. 75:654-660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Durali, D., J. Morvan, F. Letourneur, D. Schmitt, N. Guegan, M. Dalod, S. Saragosti, D. Sicard, J. P. Levy, and E. Gomard. 1998. Cross-reactions between the cytotoxic T-lymphocyte responses of human immunodeficiency virus-infected African and European patients. J. Virol. 72:3547-3553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hanke, T., R. V. Samuel, T. J. Blanchard, V. C. Neumann, T. M. Allen, J. E. Boyson, S. A. Sharpe, N. Cook, G. L. Smith, D. I. Watkins, M. P. Cranage, and A. J. McMichael. 1999. Effective induction of simian immunodeficiency virus-specific cytotoxic T lymphocytes in macaques by using a multiepitope gene and DNA prime-modified vaccinia virus Ankara boost vaccination regimen. J. Virol. 73:7524-7532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hasenkrug, K. J., and B. Chesebro. 1997. Immunity to retroviral infection: the Friend virus model. Proc. Natl. Acad. Sci. USA 94:7811-7816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hogan, C. M., and S. M. Hammer. 2001. Host determinants in HIV infection and disease. Part 1. Cellular and humoral immune responses. Ann. Intern. Med. 134:761-776. [DOI] [PubMed] [Google Scholar]
- 15.Ishihara, C., M. Miyazawa, J. Nishio, and B. Chesebro. 1991. Induction of protective immunity to Friend murine leukemia virus in genetic nonresponders to virus envelope protein. J. Immunol. 146:3958-3963. [PubMed] [Google Scholar]
- 16.Iwanami, N., A. Niwa, Y. Yasutomi, N. Tabata, and M. Miyazawa. 2001. Role of natural killer cells in resistance against Friend retrovirus-induced leukemia. J. Virol. 75:3152-3163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Iwashiro, M., T. Kondo, T. Shimizu, H. Yamagishi, K. Takahashi, Y. Matsubayashi, T. Masuda, A. Otaka, N. Fujii, A. Ishimoto, M. Miyazawa, M. N. Robertson, B. Chesebro, and K. Kuribayashi. 1993. Multiplicity of virus-encoded helper T-cell epitopes expressed on FBL-3 tumor cells. J. Virol. 67:4533-4542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Iwashiro, M., K. Peterson, R. J. Messer, I. M. Stromnes, and K. J. Hasenkrug. 2001. CD4+ T cells and gamma interferon in the long-term control of persistent Friend retrovirus infection. J. Virol. 75:52-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jacobson, S., J. R. Richert, W. E. Biddison, A. Satinsky, R. J. Hartzman, and H. F. McFarland. 1984. Measles virus-specific T4+ human cytotoxic T cell clones are restricted by class II HLA antigens. J. Immunol. 133:754-757. [PubMed] [Google Scholar]
- 20.Jaraquemada, D., M. Marti, and E. O. Long. 1990. An endogenous processing pathway in vaccinia virus-infected cells for presentation of cytoplasmic antigens to class II-restricted T cells. J. Exp. Med. 172:947-954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kabat, D. 1989. Molecular biology of Friend viral erythroleukemia. Curr. Top. Microbiol. Immunol. 148:1-42. [DOI] [PubMed] [Google Scholar]
- 22.Kalams, S. A., S. P. Buchbinder, E. S. Rosenberg, J. M. Billingsley, D. S. Colbert, N. G. Jones, A. K. Shea, A. K. Trocha, and B. D. Walker. 1999. Association between virus-specific cytotoxic T-lymphocyte and helper responses in human immunodeficiency virus type 1 infection. J. Virol. 73:6715-6720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kina, T., K. Ikuta, E. Takayama, K. Wada, A. S. Majumdar, I. L. Weissman, and Y. Katsura. 2000. The monoclonal antibody TER-119 recognizes a molecule associated with glycophorin A and specifically marks the late stages of murine erythroid lineage. Br. J. Haematol. 109:280-287. [DOI] [PubMed] [Google Scholar]
- 24.Kitamura, D., H. Kaneko, Y. Miyagoe, T. Ariyasu, and T. Watanabe. 1989. Isolation and characterization of a novel human gene expressed specifically in the cells of hematopoietic lineage. Nucleic Acids Res. 17:9367-9379. [PMC free article] [PubMed] [Google Scholar]
- 25.Kondo, T., H. Uenishi, T. Shimizu, T. Hirama, M. Iwashiro, K. Kuribayashi, H. Tamamura, N. Fujii, R. Fujisawa, M. Miyazawa, and H. Yamagishi. 1995. A single retroviral Gag precursor signal peptide recognized by FBL-3 tumor-specific cytotoxic T lymphocytes. J. Virol. 69:6735-6741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Malhotra, U., M. M. Berrey, Y. Huang, J. Markee, D. J. Brown, S. Ap, L. Musey, T. Schacker, L. Corey, and M. J. McElrath. 2000. Effect of combination antiretroviral therapy on T-cell immunity in acute human immunodeficiency virus type 1 infection. J. Infect. Dis. 181:121-131. [DOI] [PubMed] [Google Scholar]
- 27.Marques, E. T., Jr., P. Chikhlikar, L. B. De Arruda, I. C. Leao, Y. Lu, J. Wong, J. S. Chen, B. Byrne, and J. T. August. 2003. HIV-1 p55Gag encoded in the lysosome-associated membrane protein-1 as a DNA plasmid vaccine chimera is highly expressed, traffics to the major histocompatibility class II compartment, and elicits enhanced immune responses. J. Biol. Chem. 278:37926-37936. [DOI] [PubMed] [Google Scholar]
- 28.Matloubian, M., R. J. Concepcion, and R. Ahmed. 1994. CD4+ T cells are required to sustain CD8+ cytotoxic T-cell responses during chronic viral infection. J. Virol. 68:8056-8063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McAdam, S., P. Kaleebu, P. Krausa, P. Goulder, N. French, B. Collin, T. Blanchard, J. Whitworth, A. McMichael, and F. Gotch. 1998. Cross-clade recognition of p55 by cytotoxic T lymphocytes in HIV-1 infection. AIDS 12:571-579. [DOI] [PubMed] [Google Scholar]
- 30.McAtee, F. J., and J. L. Portis. 1985. Monoclonal antibodies specific for wild mouse neurotropic retrovirus: detection of comparable levels of virus replication in mouse strains susceptible and resistant to paralytic disease. J. Virol. 56:1018-1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miyazawa, M., R. Fujisawa, C. Ishihara, Y. A. Takei, T. Shimizu, H. Uenishi, H. Yamagishi, and K. Kuribayashi. 1995. Immunization with a single T helper cell epitope abrogates Friend virus-induced early erythroid proliferation and prevents late leukemia development. J. Immunol. 155:748-758. [PubMed] [Google Scholar]
- 32.Miyazawa, M., J. Nishio, and B. Chesebro. 1992. Protection against Friend retrovirus-induced leukemia by recombinant vaccinia viruses expressing the gag gene. J. Virol. 66:4497-4507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miyazawa, M., M. Nose, M. Kawashima, and M. Kyogoku. 1987. Pathogenesis of arteritis of SL/Ni mice. Possible lytic effect of anti-gp70 antibodies on vascular smooth muscle cells. J. Exp. Med. 166:890-908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ney, P. A., and A. D. D'Andrea. 2000. Friend erythroleukemia revisited. Blood 96:3675-3680. [PubMed] [Google Scholar]
- 35.Norris, P. J., M. Sumaroka, C. Brander, H. F. Moffett, S. L. Boswell, T. Nguyen, Y. Sykulev, B. D. Walker, and E. S. Rosenberg. 2001. Multiple effector functions mediated by human immunodeficiency virus-specific CD4+ T-cell clones. J. Virol. 75:9771-9779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oxenius, A., R. M. Zinkernagel, and H. Hengartner. 1998. CD4+ T-cell induction and effector functions: a comparison of immunity against soluble antigens and viral infections. Adv. Immunol. 70:313-367. [DOI] [PubMed] [Google Scholar]
- 37.Pal, R., M. S. Reitz, Jr., E. Tschachler, R. C. Gallo, M. G. Sarngadharan, and F. D. Veronese. 1990. Myristoylation of Gag proteins of HIV-1 plays an important role in virus assembly. AIDS Res. Hum. Retrovir. 6:721-730. [DOI] [PubMed] [Google Scholar]
- 38.Papsidero, L. D., M. Sheu, and F. W. Ruscetti. 1989. Human immunodeficiency virus type 1-neutralizing monoclonal antibodies which react with p17 core protein: characterization and epitope mapping. J. Virol. 63:267-272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Perryman, S., J. Nishio, and B. Chesebro. 1991. Complete nucleotide sequence of Friend murine leukemia virus, strain FB29. Nucleic Acids Res. 19:6950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Picker, L. J., and V. C. Maino. 2000. The CD4+ T cell response to HIV-1. Curr. Opin. Immunol. 12:381-386. [DOI] [PubMed] [Google Scholar]
- 41.Pincus, S. H., R. Cole, R. Ireland, F. McAtee, R. Fujisawa, and J. Portis. 1995. Protective efficacy of nonneutralizing monoclonal antibodies in acute infection with murine leukemia virus. J. Virol. 69:7152-7158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pitcher, C. J., C. Quittner, D. M. Peterson, M. Connors, R. A. Koup, V. C. Maino, and L. J. Picker. 1999. HIV-1-specific CD4+ T cells are detectable in most individuals with active HIV-1 infection, but decline with prolonged viral suppression. Nat. Med. 5:518-525. [DOI] [PubMed] [Google Scholar]
- 43.Planz, O., S. Ehl, E. Furrer, E. Horvath, M. A. Brundler, H. Hengartner, and R. M. Zinkernagel. 1997. A critical role for neutralizing-antibody-producing B cells, CD4+ T cells, and interferons in persistent and acute infections of mice with lymphocytic choriomeningitis virus: implications for adoptive immunotherapy of virus carriers. Proc. Natl. Acad. Sci. USA 94:6874-6879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rein, A., M. R. McClure, N. R. Rice, R. B. Luftig, and A. M. Schultz. 1986. Myristylation site in Pr65gag is essential for virus particle formation by Moloney murine leukemia virus. Proc. Natl. Acad. Sci. USA 83:7246-7250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Robertson, M. N., M. Miyazawa, S. Mori, B. Caughey, L. H. Evans, S. F. Hayes, and B. Chesebro. 1991. Production of monoclonal antibodies reactive with a denatured form of the Friend murine leukemia virus gp70 envelope protein: use in a focal infectivity assay, immunohistochemical studies, electron microscopy and Western blotting. J. Virol. Methods 34:255-271. [DOI] [PubMed] [Google Scholar]
- 46.Rosenberg, E. S., M. Altfeld, S. H. Poon, M. N. Phillips, B. M. Wilkes, R. L. Eldridge, G. K. Robbins, R. T. D'Aquila, P. J. Goulder, and B. D. Walker. 2000. Immune control of HIV-1 after early treatment of acute infection. Nature 407:523-526. [DOI] [PubMed] [Google Scholar]
- 47.Rosenberg, E. S., J. M. Billingsley, A. M. Caliendo, S. L. Boswell, P. E. Sax, S. A. Kalams, and B. D. Walker. 1997. Vigorous HIV-1-specific CD4+ T cell responses associated with control of viremia. Science 278:1447-1450. [DOI] [PubMed] [Google Scholar]
- 48.Sarkar, S., V. Kalia, M. Murphey-Corb, and R. C. Montelaro. 2002. Detailed analysis of CD4+ Th responses to envelope and Gag proteins of simian immunodeficiency virus reveals an exclusion of broadly reactive Th epitopes from the glycosylated regions of envelope. J. Immunol. 168:4001-4011. [DOI] [PubMed] [Google Scholar]
- 49.Schmitz, J. E., M. J. Kuroda, S. Santra, V. G. Sasseville, M. A. Simon, M. A. Lifton, P. Racz, K. Tenner-Racz, M. Dalesandro, B. J. Scallon, J. Ghrayeb, M. A. Forman, D. C. Montefiori, E. P. Rieber, N. L. Letvin, and K. A. Reimann. 1999. Control of viremia in simian immunodeficiency virus infection by CD8+ lymphocytes. Science 283:857-860. [DOI] [PubMed] [Google Scholar]
- 50.Shang, F., H. Huang, K. Revesz, H. C. Chen, R. Herz, and A. Pinter. 1991. Characterization of monoclonal antibodies against the human immunodeficiency virus matrix protein, p17gag: identification of epitopes exposed at the surfaces of infected cells. J. Virol. 65:4798-4804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Smith, G. L., B. R. Murphy, and B. Moss. 1983. Construction and characterization of an infectious vaccinia virus recombinant that expresses the influenza hemagglutinin gene and induces resistance to influenza virus infection in hamsters. Proc. Natl. Acad. Sci. USA 80:7155-7159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tabata, N., M. Miyazawa, R. Fujisawa, Y. A. Takei, H. Abe, and K. Hashimoto. 2000. Establishment of monoclonal anti-retroviral gp70 autoantibodies from MRL/lpr lupus mice and induction of glomerular gp70 deposition and pathology by transfer into non-autoimmune mice. J. Virol. 74:4116-4126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Thomsen, A. R., J. Johansen, O. Marker, and J. P. Christensen. 1996. Exhaustion of CTL memory and recrudescence of viremia in lymphocytic choriomeningitis virus-infected MHC class II-deficient mice and B cell-deficient mice. J. Immunol. 157:3074-3080. [PubMed] [Google Scholar]
- 54.Uenishi, H., N. Iwanami, K. Kuribayashi, H. Tamamura, N. Fujii, T. Nakatani, T. Kawasaki, and H. Yamagishi. 1998. Overlapping epitopes of Friend murine leukemia virus gag-encoded leader sequence recognized by single cytotoxic T-lymphocyte clones. Immunol. Lett. 62:33-38. [DOI] [PubMed] [Google Scholar]
- 55.Venturini, S., D. E. Mosier, D. R. Burton, and P. Poignard. 2002. Characterization of human immunodeficiency virus type 1 (HIV-1) Gag- and Gag peptide-specific CD4+ T-cell clones from an HIV-1-seronegative donor following in vitro immunization. J. Virol. 76:6987-6999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Villinger, F., G. T. Brice, A. E. Mayne, P. Bostik, K. Mori, C. H. June, and A. A. Ansari. 2002. Adoptive transfer of simian immunodeficiency virus (SIV) naive autologous CD4+ cells to macaques chronically infected with SIV is sufficient to induce long-term nonprogressor status. Blood 99:590-599. [DOI] [PubMed] [Google Scholar]
- 57.von Herrath, M. G., M. Yokoyama, J. Dockter, M. B. Oldstone, and J. L. Whitton. 1996. CD4-deficient mice have reduced levels of memory cytotoxic T lymphocytes after immunization and show diminished resistance to subsequent virus challenge. J. Virol. 70:1072-1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wilson, C. C., B. Palmer, S. Southwood, J. Sidney, Y. Higashimoto, E. Appella, R. Chesnut, A. Sette, and B. D. Livingston. 2001. Identification and antigenicity of broadly cross-reactive and conserved human immunodeficiency virus type 1-derived helper T-lymphocyte epitopes. J. Virol. 75:4195-4207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yakushijin, Y., M. Yasukawa, and Y. Kobayashi. 1992. Establishment and functional characterization of human herpesvirus 6-specific CD4+ human T-cell clones. J. Virol. 66:2773-2779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yasutomi, Y., S. Koenig, R. M. Woods, J. Madsen, N. M. Wassef, C. R. Alving, H. J. Klein, T. E. Nolan, L. J. Boots, J. A. Kessler, E. A. Emini, A. J. Conley, and N. L. Letvin. 1995. A vaccine-elicited, single viral epitope-specific cytotoxic T lymphocyte response does not protect against intravenous, cell-free simian immunodeficiency virus challenge. J. Virol. 69:2279-2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yuan, X., X. Yu, T. H. Lee, and M. Essex. 1993. Mutations in the N-terminal region of human immunodeficiency virus type 1 matrix protein block intracellular transport of the Gag precursor. J. Virol. 67:6387-6394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zajac, A. J., J. N. Blattman, K. Murali-Krishna, D. J. Sourdive, M. Suresh, J. D. Altman, and R. Ahmed. 1998. Viral immune evasion due to persistence of activated T cells without effector function. J. Exp. Med. 188:2205-2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhou, W., L. J. Parent, J. W. Wills, and M. D. Resh. 1994. Identification of a membrane-binding domain within the amino-terminal region of human immunodeficiency virus type 1 Gag protein which interacts with acidic phospholipids. J. Virol. 68:2556-2569. [DOI] [PMC free article] [PubMed] [Google Scholar]