Abstract
The influence of astrocytes on synaptic function has been increasingly studied, owing to the discovery of both gliotransmission and morphological ensheathment of synapses. While astrocytes exhibit at best modest membrane potential fluctuations, activation of G-protein coupled receptors (GPCRs) leads to a prominent elevation of intracellular calcium which has been reported to correlate with gliotransmission. In this review, the possible role of astrocytic GPCR activation is discussed as a trigger to promote synaptic plasticity, by affecting synaptic receptors through gliotransmitters. Moreover, we suggest that volume transmission of neuromodulators could be a biological mechanism to activate astrocytic GPCRs and thereby to switch synaptic networks to the plastic mode during states of attention in cerebral cortical structures.
Keywords: acetylcholine, Gq signalling, IP3 receptors, d-serine, gamma oscillations
1. Introduction
With the advent of molecular genetics and cellular imaging techniques, our understanding of brain function has advanced substantially in the recent decade. Glial cell research has indisputably benefited from these techniques, as glial cells are generally electrically passive, and their dynamism resides most probably in biochemical and morphological changes. Among the glial cells, astrocytes occupy a significant proportion of the brain volume in mammals and are arguably the most numerous in primate cortical grey matter. The morphology of astrocytes is best described as an interface between vascular and neuronal networks. A typical protoplasmic astrocyte has a bushy organization of microprocesses that surround synapses and a few large processes that impinge on neighbouring vasculature (giant end-feet). For white matter fibrous astrocytes, the microprocesses extend around the nodal regions of myelinated axons. Such strategic positioning of astrocytes is indeed well matched with the classically supposed functions of astrocytes, including the clearance of synaptically released neurotransmitters, regulation of ionic concentrations and mediation of energy metabolism substrates. Since gliotransmission—the ability of astrocytes to secrete biochemical molecules to influence surrounding neurons—was discovered about two decades ago [1–3], astrocytes have been hypothesized to play active roles in neuronal network operations.
Membrane potential fluctuations recorded from the soma of mature astrocytes are quite modest (i.e. within several millivolts) at best. The resting membrane potential of a typical astrocyte is less than −80 mV, which is close to the reversal potential of potassium (K+). Astrocytes have an order-of-magnitude lower input resistance than pyramidal cells owing to K+ channels that are permeable at resting membrane potentials (e.g. TWIK-1, TREK-1 and Kir4.1) [4,5] as well as the existence of hemichannels and gap junctions. While these properties and the lack of active conductance make astrocytes electrophysiologically quiescent, astrocytes have been reported to have cytosolic calcium (Ca2+) elevations and intercellular Ca2+ waves [2]. These Ca2+ elevations occur without large membrane potential changes, because the Ca2+ is released from internal Ca2+ stores such as the endoplasmic reticulum (ER). Such cytosolic Ca2+ elevations in astrocytes have also been described in vivo in rodents [6]. Triggers that initiate astrocytic Ca2+ elevation are diverse, but common neurotransmitters and neuromodulators are potent agonists for astrocytic Ca2+ elevation through G-protein coupled receptors (GPCRs). One of the key questions in neuron–astrocyte interactions is whether astrocytic Ca2+ elevations play any role in brain operation, and to identify the circumstances under which such neuron–glia interactions occur. In this article, we focus on how subcortical neuromodulatory signals mediate astrocyte–neuron interactions in the context of synaptic plasticity in cerebral cortical structures.
2. Volume transmission versus synaptic transmission
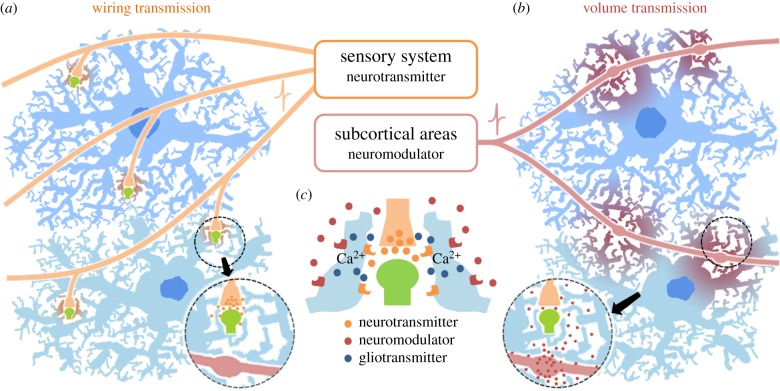
Chemical transmitters are released in two distinct transmission modes: wiring transmission and volume transmission (for classic reviews, see [7,8]). Wiring transmission is intercellular communication mediated via a physically defined connecting structure. Synaptic transmission is the primary mechanism of wiring transmission, and its primary feature is fast (millisecond-order) point-to-point communication. Glutamate and GABA are the predominant neurotransmitters for this in mammalian cortical structures. The potency and reliability of the synapse are the key determinants of information transmission. Astrocytic microprocesses that ensheath synapses are thought to increase the fidelity of synaptic transmission by rapid neurotransmitter clearance and insulation from other synapses [9].
Volume transmission is by non-synaptic release of neuromodulators diffusing through the extracellular space (ECS; figure 1), which is defined by an intricate and dense organization of synaptic and glial process morphology (for a review, see [10]). As a result, the manner of diffusion deviates significantly from free diffusion because of the tortuosity and limited volume fraction of the ECS. Subsequently, a relatively large number of cells sense neuromodulators via extrasynaptic receptors. In the cerebral cortex and hippocampus, volume-transmitted neuromodulators include acetylcholine and monoamines. The afferent fibres for neuromodulators are mainly of subcortical origin, and usually make asynaptic junctions in the cortex and hippocampus via terminal varicosities in stark contrast to glutamatergic and GABAergic innervation. For example, synaptic incidences are a mere 10–20% of the total varicosities for acetylcholine [11,12] and noradrenaline [13,14] and 20–30% for serotonin [15]. In addition to the complex ECS geometry, the true nature of ECS diffusion is complicated by the presence of diffusion obstacles (e.g. extracellular matrix and cell adhesion molecules) and active interference system (e.g. uptake by transporter or enzymatic degradation) [10,16]. Theoretical models and simulations have been compared with experimental data obtained by real-time iontophoresis or fluorescent macromolecule imaging [10].
Figure 1.
Wiring transmission versus volume transmission and their effects on astrocytes. (a) Wiring transmission targets designated synapses and produces localized responses in perisynaptic astrocytic processes. (b) In volume transmission, the neuromodulators diffuse into tortuous and convoluted ECS upon release from en passant varicosities. Such ECS diffusion results in activation of astrocytic GPCRs in larger areas than a synaptic component, resulting in synchronized and spatially spread astrocytic Ca2+ activities. (c) Volume transmission and synaptic transmission can occur simultaneously in brain states characterized by neuromodulator release, for instance, during attention.
As much as neurons receive this extrasynaptic neuromodulator transmission, astrocytes surrounding synapses are also the receivers. Serial reconstruction of the neuropil of rat hippocampal grey matter shows that glial processes occupy over 10% of all plasma membrane area [17]. This proportion is even higher when the analysis is confined to the extrasynaptic space, and thus glial surface represents a considerable target area for volume-transmitted neuromodulators. Moreover, astrocytes express receptors for subcortical neuromodulators [18].
Other remarkable differences between synaptic transmission and volume transmission are the time course and spatial range of signal transfer. While neurotransmitters travel 20–30 nm across the synaptic cleft, volume-transmitted neuromodulators travel on the scale of micrometres to reach their receptors. At the receptor end, ionotropic receptors dominate in glutamatergic and GABAergic synapses of the cerebral cortex and hippocampus, ensuring millisecond-order signal transmission. Extrasynaptic receptors of neurons include both ionotropic receptors and metabotropic GPCRs. Literature suggests that neuromodulator receptors in astrocytes are predominantly GPCRs that have a much slower signal transduction (at least hundreds of milliseconds) [19]. Therefore, while affecting many targets, volume transmission is not expected to provide temporally precise signal transmission. Considering the tight coupling of neuromodulatory systems and behavioural states, and the slow time course of GPCR signalling, elucidation of the significance of astrocytic activation by neuromodulators may yield a new insight into understanding neuronal information processing in distinct behavioural states.
3. Astrocytic response to neurotransmitters and neuromodulators
Astrocytes respond to neurotransmitters and neuromodulators through a wide variety of GPCRs. Their activations trigger production of inositol 1,4,5-triphosphate (IP3), which induces Ca2+ release from the ER. So far, several groups have reported that Ca2+ elevations in astrocytes lead to gliotransmission of glutamate, d-serine or ATP and in turn regulate neuronal activity and synaptic strength in brain slices [20–26]. d-Serine is an endogenous co-agonist of NMDA receptors (NMDARs), and several studies have suggested that astrocytes release d-serine by exocytosis [27–29]. d-Serine release from a single astrocyte can modulate neighbouring neuronal NMDAR currents [26], and basal astrocytic Ca2+ concentration and extracellular d-serine concentration are correlated [30]. Electron-microscopic analysis showed that glutamate and d-serine are localized in microvesicles near the ER within the perisynaptic processes of astrocytes [31], hinting at the significance of perisynaptic Ca2+ signalling in gliotransmission. Recent studies using high-resolution Ca2+ imaging of hippocampal slices suggested that astrocytic microprocesses respond to single synaptic activity with rapid and localized Ca2+ elevation [32,33]. Roles of gliotransmission from astrocytic processes in synaptic function have been studied in the hypothalamic nuclei, where the astrocytic coverage of synapses decreases during lactation. The availability of d-serine in the synapses was reduced in slices from lactating rats [34].
There is growing evidence that astrocyte-derived ATP, which was initially categorized as a paracrine messenger responsible for interglial propagation of Ca2+ waves [35–37], can regulate synaptic transmission [22,38]. Although several non-vesicular pathways have been identified, recent studies using transgenic mice selectively expressing a dominant-negative SNARE protein in astrocytes demonstrated the significance of vesicular release of astrocytic ATP [22,39]. Another study showed that electrical stimulation of excitatory input to the hypothalamus induces metabotropic glutamate receptor (mGluR)-dependent astrocytic Ca2+ elevation and release of ATP [40]. Notably, a rise in Ca2+ in the astrocyte compartments immediately adjacent to the postsynaptic neuron was necessary for ATP-mediated changes in synaptic transmission. Interestingly, noradrenaline application also led to astrocytic ATP release and similar synaptic transmission changes [41], implying that the astrocytes are capable of responding to and possibly integrating both neurotransmitters and neuromodulators (figure 1).
It has been shown that astrocytic Ca2+ signals in the adult brain are mediated by volume-transmitted neuromodulators. Upon electrical stimulation of locus coeruleus (LC; the sole source of noradrenergic input to cortex), astrocytes exhibit broad Ca2+ increases in somatosensory cortex [42]. Aversive stimulation, known to result in phasic LC activity, also led to widespread adrenergic astrocytic Ca2+ elevation throughout sensory cortex, which is more pronounced in awake conditions [43]. Acetylcholine also activates global astrocytic Ca2+ signalling in vivo. The predominant sources of cholinergic afferents for the cerebral cortex and hippocampus are the nucleus basalis of Meynert (NBM) and medial septum. We and others have demonstrated that stimulation of the respective cholinergic nuclei leads to muscarinic acetylcholine receptor (mAChR)-dependent astrocytic Ca2+ elevation in the cortex [44,45] and hippocampus [46]. Notably, NBM stimulation led to an increase in extracellular d-serine in the cortex of control mice, but not of mice lacking astrocyte-dominant IP3 receptors (IP3R2) [44]. Our results indicate that astrocytic Ca2+ responses by whisker or NBM stimulation differ in the following two aspects: (i) whisker stimulation induces mGluR-dependent weaker Ca2+ responses [47], whereas NBM stimulation produces mAChR-dependent robust responses; and (ii) while whisker-induced Ca2+ surges return to baseline even during stimulation, plateau Ca2+ increases persist throughout NBM stimulation.
GPCR signalling in astrocytes has also been suggested to regulate extracellular K+ [48,49], neurotransmitter uptake [50] and neurovascular coupling [51–53] (but also see [54,55]). On the other hand, some recent studies that use molecular genetics have challenged the validity of gliotransmission. Astrocytic expression of and subsequent activation of a foreign GPCR (MrgA1) to selectively induce astrocytic Ca2+ elevation [56,57] or genetic deletion of IP3R2s to diminish astrocytic Ca2+ elevations [57,58] did not result in a notable change in excitatory synaptic transmission in mouse hippocampal slices. This apparent contradiction may be due to the method used to stimulate astrocytes. For example, uncaging IP3 in MrgA1-positive astrocytes increased the frequency of glutamatergic miniature excitatory postsynaptic currents (mEPSCs) in nearby neurons [56]. A recent study further addressed this issue and showed that Ca2+ uncaging in astrocytes triggers glutamate release, whereas agonist activation of MrgA1, PAR-1 or purinergic receptors does not [49]. Moreover, astrocytic glutamate release can be mediated by channels [59,60], and Gq-coupled GPCRs may also have IP3-independent pathways [61]. Future investigation on neuromodulator-mediated Ca2+ signalling in astrocytic processes and their functional manipulation in vivo will advance our understanding of the role of astrocytes in normal brain function. Other key issues for future studies are to understand the functional significance of neuromodulator-driven global responses and neurotransmitter-driven individual localized transients and to identify the biological situation where these signalling modes are employed differentially or in synergy.
4. Neuromodulator activation and gamma oscillations
Distinct neuromodulators contribute to different modes of animals’ behavioural states. Likewise, animals’ behavioural states and neuronal population dynamics are tightly correlated. For instance, large amplitude slow waves (0.5–2 Hz) appear in the electroencephalogram (EEG) during deep sleep, whereas faster and lower amplitude patterns are seen in waking states. Gamma oscillations (30–100 Hz) appear during states of attention [62], and this rhythm is thought to bind neural representation of different sensory modalities [63]. Detailed cellular mechanisms underlying gamma oscillations are yet to be fully elucidated, but reciprocal interactions between excitatory pyramidal neurons and inhibitory interneurons, particularly parvalbumin positive fast-spiking basket cells, probably play a key role [64]. As attention is naturally related to cognitive processing and learning efficiency, synaptic plasticity is probably induced during the gamma oscillation state. Indeed, repetitive phase locked activity of neurons at a gamma frequency provides a situation favourable for spike-timing-dependent plasticity [65]. Moreover, this type of plasticity is enhanced by activation of mAChRs [65].
As neuromodulator release and EEG states are both highly correlated to an animal's behaviour, they should naturally be closely linked. As a matter of fact, the gamma states also coincide with release of neuromodulators. For instance, gamma oscillations are induced by electrical stimulation of the NBM in anaesthetized rats [66] or optogenetic stimulation of cholinergic neurons in the basal forebrain in awake mice [67]. Moreover, noradrenergic transmission has been shown to be crucial for waking gamma that appears shortly after gas anaesthesia wears off [68]. During awake and REM sleep periods, higher amounts of acetylcholine are released in the cortex and hippocampus than during slow wave sleep [69]. In accordance with cortical activation, cholinergic neurons in the basal forebrain increase their firing rates, and alter their firing mode from single spike to rhythmic bursting [70].
Exposure to enriched environments (EE) has been known to boost animals’ learning ability and its neural circuit remodelling effect has been studied for decades. We recently found that hippocampal gamma amplitude increases in rats raised in EE, which hints at a possible link between gamma oscillation and learning [71]. Increases of spine density and dendritic complexity are common effects of EE in the cortex and hippocampus. Although the mechanism for chronic gamma increase is most probably multifactorial, increased input to pyramidal cells is a conceivable factor, as gamma is a product of balanced excitatory and inhibitory synaptic input [72]. The requirement of NMDAR activation for chronic gamma enhancement [71] also suggests that a long-term potentiation (LTP)-like mechanism may be involved. Interestingly, GABAergic networks have also been reported to be altered by EE [73].
As well as neurotransmission, there are notable changes in neuromodulation after EE. Rats raised in EE after weaning show increased hippocampal and anterior cortical choline acetyltransferase activity after maze training [74]. Similarly, the concentration of released acetylcholine is higher when rats solve more difficult tasks [75]. The causal relationship between chronic gamma increase and neuromodulator systems is not resolved at this time. However, it is remarkable that many studies report enhanced LTP by neuromodulators including acetylcholine and noradrenaline, suggesting a permissive role of GPCR for synaptic plasticity and learning [76]. As described in §3, GPCRs are not only expressed in neurons. Given the existence and functional response of GPCRs in astrocytes, it is logical to ask whether activation of astrocytic GPCR has a role in synaptic plasticity in vivo.
5. Astrocytic modulation of synaptic plasticity during gamma states
As gamma states coincide with volume transmission of neuromodulators including acetylcholine and noradrenaline, astrocytic Ca2+ dynamics are more active during these states (§3). Recently, three independent studies have investigated the role of gamma-state-induced astrocytic Ca2+ elevation in synaptic plasticity. These studies were performed in the somatosensory cortex [44], visual cortex [45] and hippocampus [46]. In each of these studies, the respective cholinergic nucleus was stimulated while sensory stimuli or electrical afferent stimulation was presented to anaesthetized animals. As a result, long-lasting enhancements (more than 1 h) in stimulus-evoked potential or neuronal firing rate were observed. These effects were diminished in IP3R2 knockout (IP3R2-KO) mice, in which astrocytic large Ca2+ elevations are deficient, suggesting the causal relationship between the astrocytic Ca2+ elevation and induction of the synaptic plasticity.
NBM-evoked cortical gamma oscillations seem to be uninfluenced by astrocytic Ca2+, as the duration of gamma oscillations was similar between wild-type and IP3R2-KO mice [44]. Neuronal activity is temporally coordinated in gamma rhythms by NBM stimulation and this synchronization could be a prevailing mechanism of augmented synaptic plasticity [77]. However, the deficiency of NBM-associated cortical plasticity in IP3R2-KO mice strongly supports a role of astrocytic Ca2+ signalling in the synaptic plasticity.
In our investigation on plasticity in the somatosensory cortex, astrocytic Ca2+ activities were elevated during costimulation of whiskers and NBM. Similarly, the extracellular concentration of d-serine is elevated during NBM stimulation and returns to the baseline thereafter. Considering the major role of NMDARs in LTP [78], the lack of extracellular d-serine increase in IP3R2-KO mice suggests a pivotal role of astrocytic Ca2+ signalling during the induction phase of the synaptic plasticity. Chen et al. [45] showed that single unit activities in the visual cortex are enhanced when visual orientation stimuli are combined with NBM stimulation. Importantly, the neuronal response is enhanced only for the orientation paired with NBM stimulation. As the orientation tunings of individual synapses are intermingled in mouse primary visual cortex [79], this result advocates the importance of sensory input as the determinant for specificity of plasticity. Further investigation on spatio-temporal relationship of active astrocytes and augmented synapses should characterize the effective range of gliotransmission.
While the synaptic plasticity in our study is NMDAR-dependent, a study by Navarrete et al. [46] investigated cholinergically augmented hippocampal CA3–CA1 plasticity in the presence of an NMDAR blocker. Their results suggest that glutamate acts as the gliotransmitter affecting neuronal mGluRs to express presynaptic plasticity, whereas a recent paper suggests other interpretations such as transient change of extracellular ionic composition [48]. These differences suggest that the molecular mechanisms of astrocyte-assisted synaptic plasticity may be diverse, but the common denominator of all in vivo experiments is the activation of cholinergic volume transmission (figure 2). Notably, similar hippocampal plasticity was evoked when medial septum stimulation was replaced by tail pinch [46], and atropine could largely, but not completely, block the field response potentiation, suggesting that other neuromodulators such as noradrenaline could also be involved. Additionally, in prolonged gamma states, astrocytes can secrete cytokines including S100B and influence network synchronization [80] and synaptic plasticity [81].
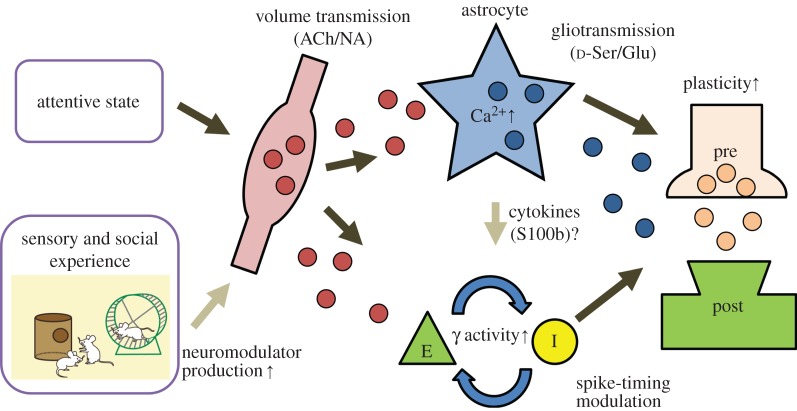
Figure 2.
Schematic diagram for neuron–astrocyte interaction in the context of gamma-state-induced synaptic plasticity. Attentive states drive volume transmission of subcortical neuromodulators which in turn activates neuronal gamma oscillations and astrocytic gliotransmission to establish a state for synaptic plasticity induction. Sensory and social experience enhances neuromodulator production and gliotransmission of cytokines enhances gamma oscillations, although the exact mechanism remains to be elucidated. ACh, acetylcholine; NA, noradrenaline; E, excitatory neuron; I, inhibitory neuron.
6. Concluding remarks
We have discussed a possible role of cortical astrocytes as an element enhancing cortical plasticity via gliotransmission. Volume transmission of subcortical neuromodulators serves as the drive for activation of astrocytes and gamma oscillations. Gamma oscillations appear during attentive states and provide temporally synchronized activation of groups of neurons (i.e. cell assembly), association of which will lead to formation of memory and learning. Considering the astrocytic expression of functional GPCRs for neuromodulators and the tight relationship between the subcortical neuromodulator system and the cognitive states of an animal, the framework of this model is sound. Recently, multiple groups have shown that the cholinergic system can mediate such a mechanism in rodent cortex and hippocampus [44–46,82]. Remarkably, noradrenergic transmission is reported to provide a dominant drive for astrocytic Ca2+ elevations during awake states [43]. Cholinergic volume transmission may provide an additional input to enhance Ca2+ elevations in astrocytes during attention. Indeed, a synergistic effect of acetylcholine and noradrenaline in synaptic plasticity has been described [83,84]. It is conceivable that similar operating principles are in effect in extracortical areas. For instance, the basal ganglia system is under the strong control of dopaminergic innervation, whereas the cerebellar cortex receives significant serotonergic and noradrenergic innervations. Molecular and physiological investigations on the heterogeneity of astrocytes will be important to understand the regional operational characteristics of astrocyte–neuron interactions. Response to neuromodulators is widespread across the astrocytic syncytium owing to the nature of volume transmission, and possibly owing to interastrocytic Ca2+ wave propagation [85]. Synaptic activity-driven elevation of focal Ca2+ rise in astrocytes [32,33] may provide us an additional mechanism to promote synaptic efficacy.
Acknowledgements
The authors thank Drs Yuki Oe and Kentaroh Takagaki for critical reading of the manuscript.
References
- 1.Parpura V, Basarsky TA, Liu F, Jeftinija K, Jeftinija S, Haydon PG. 1994. Glutamate-mediated astrocyte–neuron signalling. Nature 369, 744–747. ( 10.1038/369744a0) [DOI] [PubMed] [Google Scholar]
- 2.Nedergaard M. 1994. Direct signaling from astrocytes to neurons in cultures of mammalian brain cells. Science 263, 1768–1771. ( 10.1126/science.8134839) [DOI] [PubMed] [Google Scholar]
- 3.Hassinger TD, Guthrie PB, Atkinson PB, Bennett MV, Kater SB. 1996. An extracellular signaling component in propagation of astrocytic calcium waves. Proc. Natl Acad. Sci. USA 93, 13 268–13 273. ( 10.1073/pnas.93.23.13268) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhou M, Xu G, Xie M, Zhang X, Schools GP, Ma L, Kimelberg HK, Chen H. 2009. TWIK-1 and TREK-1 are potassium channels contributing significantly to astrocyte passive conductance in rat hippocampal slices. J. Neurosci. 29, 8551–8564. ( 10.1523/JNEUROSCI.5784-08.2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Djukic B, Casper KB, Philpot BD, Chin L-S, McCarthy KD. 2007. Conditional knock-out of Kir4.1 leads to glial membrane depolarization, inhibition of potassium and glutamate uptake, and enhanced short-term synaptic potentiation. J. Neurosci. 27, 11 354–11 365. ( 10.1523/JNEUROSCI.0723-07.2007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hirase H, Qian L, Bartho P, Buzsaki G. 2004. Calcium dynamics of cortical astrocytic networks in vivo. PLoS Biol. 2, E96 ( 10.1371/journal.pbio.0020096) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Agnati LF, Zoli M, Strömberg I, Fuxe K. 1995. Intercellular communication in the brain: wiring versus volume transmission. Neuroscience 69, 711–726. ( 10.1016/0306-4522(95)00308-6) [DOI] [PubMed] [Google Scholar]
- 8.Zoli M, Agnati LF. 1996. Wiring and volume transmission in the central nervous system: the concept of closed and open synapses. Prog. Neurobiol. 49, 363–380. ( 10.1016/0301-0082(96)00020-2) [DOI] [PubMed] [Google Scholar]
- 9.Nedergaard M, Verkhratsky A. 2012. Artifact versus reality: how astrocytes contribute to synaptic events. Glia 60, 1013–1023. ( 10.1002/glia.22288) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sykova E, Nicholson C. 2008. Diffusion in brain extracellular space. Physiol. Rev. 88, 1277–1340. ( 10.1152/physrev.00027.2007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mechawar N, Cozzari C, Descarries L. 2000. Cholinergic innervation in adult rat cerebral cortex: a quantitative immunocytochemical description. J. Comp. Neurol. 428, 305–318. () [DOI] [PubMed] [Google Scholar]
- 12.Umbriaco D, Watkins KC, Descarries L, Cozzari C, Hartman BK. 1994. Ultrastructural and morphometric features of the acetylcholine innervation in adult rat parietal cortex: an electron microscopic study in serial sections. J. Comp. Neurol. 348, 351–373. ( 10.1002/cne.903480304) [DOI] [PubMed] [Google Scholar]
- 13.Séguéla P, Watkins KC, Geffard M, Descarries L. 1990. Noradrenaline axon terminals in adult rat neocortex: an immunocytochemical analysis in serial thin sections. Neuroscience 35, 249–264. ( 10.1016/0306-4522(90)90079-J) [DOI] [PubMed] [Google Scholar]
- 14.Cohen Z, Molinatti G, Hamel E. 1997. Astroglial and vascular interactions of noradrenaline terminals in the rat cerebral cortex. J. Cereb. Blood Flow Metab. 17, 894–904. ( 10.1097/00004647-199708000-00008) [DOI] [PubMed] [Google Scholar]
- 15.Umbriaco D, Garcia S, Beaulieu C, Descarries L. 1995. Relational features of acetylcholine, noradrenaline, serotonin and GABA axon terminals in the stratum radiatum of adult rat hippocampus (CA1). Hippocampus 5, 605–620. ( 10.1002/hipo.450050611) [DOI] [PubMed] [Google Scholar]
- 16.Vizi ES, Fekete A, Karoly R, Mike A. 2010. Non-synaptic receptors and transporters involved in brain functions and targets of drug treatment. Br. J. Pharmacol. 160, 785–809. ( 10.1111/j.1476-5381.2009.00624.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mishchenko Y, Hu T, Spacek J, Mendenhall J, Harris KM, Chklovskii DB. 2010. Ultrastructural analysis of hippocampal neuropil from the connectomics perspective. Neuron 67, 1009–1020. ( 10.1016/j.neuron.2010.08.014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hösli E, Hösli L. 1993. Receptors for neurotransmitters on astrocytes in the mammalian central nervous system. Prog. Neurobiol. 40, 477–506. ( 10.1016/0301-0082(93)90019-O) [DOI] [PubMed] [Google Scholar]
- 19.Agulhon C, Petravicz J, McMullen AB, Sweger EJ, Minton SK, Taves SR, Casper KB, Fiacco TA, McCarthy KD. 2008. What is the role of astrocyte calcium in neurophysiology? Neuron 59, 932–946. ( 10.1016/j.neuron.2008.09.004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fellin T, Pascual O, Gobbo S, Pozzan T, Haydon PG, Carmignoto G. 2004. Neuronal synchrony mediated by astrocytic glutamate through activation of extrasynaptic NMDA receptors. Neuron 43, 729–743. ( 10.1016/j.neuron.2004.08.011) [DOI] [PubMed] [Google Scholar]
- 21.Fiacco TA, McCarthy KD. 2004. Intracellular astrocyte calcium waves in situ increase the frequency of spontaneous AMPA receptor currents in CA1 pyramidal neurons. J. Neurosci. 24, 722–732. ( 10.1523/JNEUROSCI.2859-03.2004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pascual O, et al. 2005. Astrocytic purinergic signaling coordinates synaptic networks. Science 310, 113–116. ( 10.1126/science.1116916) [DOI] [PubMed] [Google Scholar]
- 23.Serrano A, Haddjeri N, Lacaille JC, Robitaille R. 2006. GABAergic network activation of glial cells underlies hippocampal heterosynaptic depression. J. Neurosci. 26, 5370–5382. ( 10.1523/JNEUROSCI.5255-05.2006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Perea G, Araque A. 2007. Astrocytes potentiate transmitter release at single hippocampal synapses. Science 317, 1083–1086. ( 10.1126/science.1144640) [DOI] [PubMed] [Google Scholar]
- 25.Jourdain P, et al. 2007. Glutamate exocytosis from astrocytes controls synaptic strength. Nat. Neurosci. 10, 331–339. ( 10.1038/nn1849) [DOI] [PubMed] [Google Scholar]
- 26.Henneberger C, Papouin T, Oliet SH, Rusakov DA. 2010. Long-term potentiation depends on release of D-serine from astrocytes. Nature 463, 232–236. ( 10.1038/nature08673) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schell MJ, Molliver ME, Snyder SH. 1995. D-serine, an endogenous synaptic modulator: localization to astrocytes and glutamate-stimulated release. Proc. Natl Acad. Sci. USA 92, 3948–3952. ( 10.1073/pnas.92.9.3948) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mothet JP, Pollegioni L, Ouanounou G, Martineau M, Fossier P, Baux G. 2005. Glutamate receptor activation triggers a calcium-dependent and SNARE protein-dependent release of the gliotransmitter D-serine. Proc. Natl Acad. Sci. USA 102, 5606–5611. ( 10.1073/pnas.0408483102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang Y, Ge W, Chen Y, Zhang Z, Shen W, Wu C, Poo M, Duan S. 2003. Contribution of astrocytes to hippocampal long-term potentiation through release of D-serine. Proc. Natl Acad. Sci. USA 100, 15 194–15 199. ( 10.1073/pnas.2431073100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shigetomi E, Jackson-Weaver O, Huckstepp RT, O'Dell TJ, Khakh BS. 2013. TRPA1 channels are regulators of astrocyte basal calcium levels and long-term potentiation via constitutive D-serine release. J. Neurosci. 33, 10 143–10 153. ( 10.1523/JNEUROSCI.5779-12.2013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bergersen LH, et al. 2011. Immunogold detection of l-glutamate and d-serine in small synaptic-like microvesicles in adult hippocampal astrocytes. Cereb. Cortex 22, 1690–1697. ( 10.1093/cercor/bhr254) [DOI] [PubMed] [Google Scholar]
- 32.Di Castro MA, Chuquet J, Liaudet N, Bhaukaurally K, Santello M, Bouvier D, Tiret P, Volterra A. 2011. Local Ca2+ detection and modulation of synaptic release by astrocytes. Nat. Neurosci. 14, 1276–1284. ( 10.1038/nn.2929) [DOI] [PubMed] [Google Scholar]
- 33.Panatier A, Vallee J, Haber M, Murai KK, Lacaille JC, Robitaille R. 2011. Astrocytes are endogenous regulators of basal transmission at central synapses. Cell 146, 785–798. ( 10.1016/j.cell.2011.07.022) [DOI] [PubMed] [Google Scholar]
- 34.Panatier A, Theodosis DT, Mothet JP, Touquet B, Pollegioni L, Poulain DA, Oliet SH. 2006. Glia-derived D-serine controls NMDA receptor activity and synaptic memory. Cell 125, 775–784. ( 10.1016/j.cell.2006.02.051) [DOI] [PubMed] [Google Scholar]
- 35.Cotrina ML, Lin JH, Alves-Rodrigues A, Liu S, Li J, Azmi-Ghadimi H, Kang J, Naus CC, Nedergaard M. 1998. Connexins regulate calcium signaling by controlling ATP release. Proc. Natl Acad. Sci. USA 95, 15 735–15 740. ( 10.1073/pnas.95.26.15735) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guthrie PB, Knappenberger J, Segal M, Bennett MV, Charles AC, Kater SB. 1999. ATP released from astrocytes mediates glial calcium waves. J. Neurosci. 19, 520–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Newman EA. 2001. Propagation of intercellular calcium waves in retinal astrocytes and Müller cells. J. Neurosci. 21, 2215–2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang JM, Wang HK, Ye CQ, Ge W, Chen Y, Jiang ZL, Wu CP, Poo MM, Duan S. 2003. ATP released by astrocytes mediates glutamatergic activity-dependent heterosynaptic suppression. Neuron 40, 971–982. ( 10.1016/S0896-6273(03)00717-7) [DOI] [PubMed] [Google Scholar]
- 39.Lalo U, Palygin O, Rasooli-Nejad S, Andrew J, Haydon PG, Pankratov Y. 2014. Exocytosis of ATP from astrocytes modulates phasic and tonic inhibition in the neocortex. PLoS Biol. 12, e1001747 ( 10.1371/journal.pbio.1001747) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gordon GRJ, Baimoukhametova DV, Hewitt SA, Rajapaksha WR, Fisher TE, Bains JS. 2005. Norepinephrine triggers release of glial ATP to increase postsynaptic efficacy. Nat. Neurosci. 8, 1078–1086. ( 10.1038/nn1498) [DOI] [PubMed] [Google Scholar]
- 41.Gordon GR, Iremonger KJ, Kantevari S, Ellis-Davies GC, MacVicar BA, Bains JS. 2009. Astrocyte-mediated distributed plasticity at hypothalamic glutamate synapses. Neuron 64, 391–403. ( 10.1016/j.neuron.2009.10.021) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bekar LK, He W, Nedergaard M. 2008. Locus coeruleus alpha-adrenergic-mediated activation of cortical astrocytes in vivo. Cereb. Cortex 18, 2789–2795. ( 10.1093/cercor/bhn040) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ding F, O'Donnell J, Thrane AS, Zeppenfeld D, Kang H, Xie L, Wang F, Nedergaard M. 2013. α1-Adrenergic receptors mediate coordinated Ca2+ signaling of cortical astrocytes in awake, behaving mice. Cell Calcium 54, 387–394. ( 10.1016/j.ceca.2013.09.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Takata N, Mishima T, Hisatsune C, Nagai T, Ebisui E, Mikoshiba K, Hirase H. 2011. Astrocyte calcium signaling transforms cholinergic modulation to cortical plasticity in vivo. J. Neurosci. 31, 18 155–18 165. ( 10.1523/JNEUROSCI.5289-11.2011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen N, Sugihara H, Sharma J, Perea G, Petravicz J, Le C, Sur M. 2012. Nucleus basalis-enabled stimulus-specific plasticity in the visual cortex is mediated by astrocytes. Proc. Natl Acad. Sci. USA 109, E2832–E2841. ( 10.1073/pnas.1206557109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Navarrete M, Perea G, Fernandez de Sevilla D, Gómez-Gonzalo M, Núñez A, Martín ED, Araque A. 2012. Astrocytes mediate in vivo cholinergic-induced synaptic plasticity. PLoS Biol. 10, e1001259 ( 10.1371/journal.pbio.1001259) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang X, Lou N, Xu Q, Tian GF, Peng WG, Han X, Kang J, Takano T, Nedergaard M. 2006. Astrocytic Ca2+ signaling evoked by sensory stimulation in vivo. Nat. Neurosci. 9, 816–823. ( 10.1038/nn1703) [DOI] [PubMed] [Google Scholar]
- 48.Wang F, Smith NA, Xu Q, Fujita T, Baba A, Matsuda T, Takano T, Bekar L, Nedergaard M. 2012. Astrocytes modulate neural network activity by Ca2+-dependent uptake of extracellular K+. Sci. Signal. 5, ra26 ( 10.1126/scisignal.2002334) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang F, Smith NA, Xu Q, Goldman S, Peng W, Huang JH, Takano T, Nedergaard M. 2013. Photolysis of caged Ca2+ but not receptor-mediated Ca2+ signaling triggers astrocytic glutamate release. J. Neurosci. 33, 17 404–17 412. ( 10.1523/JNEUROSCI.2178-13.2013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hertz L, Lovatt D, Goldman SA, Nedergaard M. 2010. Adrenoceptors in brain: cellular gene expression and effects on astrocytic metabolism and [Ca2+]i. Neurochem. Int. 57, 411–420. ( 10.1016/j.neuint.2010.03.019) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mulligan SJ, MacVicar BA. 2004. Calcium transients in astrocyte endfeet cause cerebrovascular constrictions. Nature 431, 195–199. ( 10.1038/nature02827) [DOI] [PubMed] [Google Scholar]
- 52.Takano T, Tian GF, Peng W, Lou N, Libionka W, Han X, Nedergaard M. 2006. Astrocyte-mediated control of cerebral blood flow. Nat. Neurosci. 9, 260–267. ( 10.1038/nn1623) [DOI] [PubMed] [Google Scholar]
- 53.Bekar LK, Wei HS, Nedergaard M. 2012. The locus coeruleus-norepinephrine network optimizes coupling of cerebral blood volume with oxygen demand. J. Cereb. Blood Flow Metab. 32, 2135–2145. ( 10.1038/jcbfm.2012.115) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nizar K, et al. 2013. In vivo stimulus-induced vasodilation occurs without IP3 receptor activation and may precede astrocytic calcium increase. J. Neurosci. 33, 8411–8422. ( 10.1523/JNEUROSCI.3285-12.2013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Takata N, Nagai T, Ozawa K, Oe Y, Mikoshiba K, Hirase H. 2013. Cerebral blood flow modulation by basal forebrain or whisker stimulation can occur independently of large cytosolic Ca2+ signaling in astrocytes. PLoS ONE 8, e66525 ( 10.1371/journal.pone.0066525) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fiacco TA, Agulhon C, Taves SR, Petravicz J, Casper KB, Dong X, Chen J, McCarthy KD. 2007. Selective stimulation of astrocyte calcium in situ does not affect neuronal excitatory synaptic activity. Neuron 54, 611–626. ( 10.1016/j.neuron.2007.04.032) [DOI] [PubMed] [Google Scholar]
- 57.Agulhon C, Fiacco TA, McCarthy KD. 2010. Hippocampal short- and long-term plasticity are not modulated by astrocyte Ca2+ signaling. Science 327, 1250–1254. ( 10.1126/science.1184821) [DOI] [PubMed] [Google Scholar]
- 58.Petravicz J, Fiacco TA, McCarthy KD. 2008. Loss of IP3 receptor-dependent Ca2+ increases in hippocampal astrocytes does not affect baseline CA1 pyramidal neuron synaptic activity. J. Neurosci. 28, 4967–4973. ( 10.1523/JNEUROSCI.5572-07.2008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Woo DH, et al. 2012. TREK-1 and Best1 channels mediate fast and slow glutamate release in astrocytes upon GPCR activation. Cell 151, 25–40. ( 10.1016/j.cell.2012.09.005) [DOI] [PubMed] [Google Scholar]
- 60.Sasaki T, Beppu K, Tanaka KF, Fukazawa Y, Shigemoto R, Matsui K. 2012. Application of an optogenetic byway for perturbing neuronal activity via glial photostimulation. Proc. Natl Acad. Sci. USA 109, 20 720–20 725. ( 10.1073/pnas.1213458109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Agulhon C, Boyt KM, Xie AX, Friocourt F, Roth BL, McCarthy KD. 2013. Modulation of the autonomic nervous system and behaviour by acute glial cell Gq protein-coupled receptor activation in vivo. J. Physiol. 591, 5599–5609. ( 10.1113/jphysiol.2013.261289) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jensen O, Kaiser J, Lachaux J-P. 2007. Human gamma-frequency oscillations associated with attention and memory. Trends Neurosci. 30, 317–324. ( 10.1016/j.tins.2007.05.001) [DOI] [PubMed] [Google Scholar]
- 63.Singer W, Gray CM. 1995. Visual feature integration and the temporal correlation hypothesis. Annu. Rev. Neurosci. 18, 555–586. ( 10.1146/annurev.ne.18.030195.003011) [DOI] [PubMed] [Google Scholar]
- 64.Cardin JA, Carlen M, Meletis K, Knoblich U, Zhang F, Deisseroth K, Tsai LH, Moore CI. 2009. Driving fast-spiking cells induces gamma rhythm and controls sensory responses. Nature 459, 663–667. ( 10.1038/nature08002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wespatat V, Tennigkeit F, Singer W. 2004. Phase sensitivity of synaptic modifications in oscillating cells of rat visual cortex. J. Neurosci. 24, 9067–9075. ( 10.1523/JNEUROSCI.2221-04.2004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Metherate R, Cox CL, Ashe JH. 1992. Cellular bases of neocortical activation: modulation of neural oscillations by the nucleus basalis and endogenous acetylcholine. J. Neurosci. 12, 4701–4711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pinto L, Goard MJ, Estandian D, Xu M, Kwan AC, Lee S-H, Harrison TC, Feng G, Dan Y. 2013. Fast modulation of visual perception by basal forebrain cholinergic neurons. Nat. Neurosci. 16, 1857–1863. ( 10.1038/nn.3552) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Constantinople CM, Bruno RM. 2011. Effects and mechanisms of wakefulness on local cortical networks. Neuron 69, 1061–1068. ( 10.1016/j.neuron.2011.02.040) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Marrosu F, Portas C, Mascia MS, Casu MA, Fà M, Giagheddu M, Imperato A, Gessa GL. 1995. Microdialysis measurement of cortical and hippocampal acetylcholine release during sleep–wake cycle in freely moving cats. Brain Res. 671, 329–332. ( 10.1016/0006-8993(94)01399-3) [DOI] [PubMed] [Google Scholar]
- 70.Lee MG, Hassani OK, Alonso A, Jones BE. 2005. Cholinergic basal forebrain neurons burst with theta during waking and paradoxical sleep. J. Neurosci. 25, 4365–4369. ( 10.1523/JNEUROSCI.0178-05.2005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shinohara Y, Hosoya A, Hirase H. 2013. Experience enhances gamma oscillations and interhemispheric asymmetry in the hippocampus. Nat. Commun. 4, 1652 ( 10.1038/ncomms2658) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Atallah BV, Scanziani M. 2009. Instantaneous modulation of gamma oscillation frequency by balancing excitation with inhibition. Neuron 62, 566–577. ( 10.1016/j.neuron.2009.04.027) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Donato F, Rompani SB, Caroni P. 2013. Parvalbumin-expressing basket-cell network plasticity induced by experience regulates adult learning. Nature 504, 272–276. ( 10.1038/nature12866) [DOI] [PubMed] [Google Scholar]
- 74.Park GAS, Pappas BA, Murtha SM, Ally A. 1992. Enriched environment primes forebrain choline acetyltransferase activity to respond to learning experience. Neurosci. Lett. 143, 259–262. ( 10.1016/0304-3940(92)90278-F) [DOI] [PubMed] [Google Scholar]
- 75.Pych JC, Chang Q, Colon-Rivera C, Haag R, Gold PE. 2014. Acetylcholine release in the hippocampus and striatum during place and response training. Learn. Mem. 12, 564–572. ( 10.1101/lm.33105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gu Q. 2002. Neuromodulatory transmitter systems in the cortex and their role in cortical plasticity. Neuroscience 111, 815–835. ( 10.1016/S0306-4522(02)00026-X) [DOI] [PubMed] [Google Scholar]
- 77.Weinberger NM, Miasnikov AA, Bieszczad KM, Chen JC. 2013. Gamma band plasticity in sensory cortex is a signature of the strongest memory rather than memory of the training stimulus. Neurobiol. Learn. Mem. 104, 49–63. ( 10.1016/j.nlm.2013.05.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bliss TV, Collingridge GL. 1993. A synaptic model of memory: long-term potentiation in the hippocampus. Nature 361, 31–39. ( 10.1038/361031a0) [DOI] [PubMed] [Google Scholar]
- 79.Jia H, Rochefort NL, Chen X, Konnerth A. 2010. Dendritic organization of sensory input to cortical neurons in vivo. Nature 464, 1307–1312. ( 10.1038/nature08947) [DOI] [PubMed] [Google Scholar]
- 80.Sakatani S, Seto-Ohshima A, Shinohara Y, Yamamoto Y, Yamamoto H, Itohara S, Hirase H. 2008. Neural-activity-dependent release of S100B from astrocytes enhances kainate-induced gamma oscillations in vivo. J. Neurosci. 28, 10 928–10 936. ( 10.1523/JNEUROSCI.3693-08.2008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Nishiyama H, Knopfel T, Endo S, Itohara S. 2002. Glial protein S100B modulates long-term neuronal synaptic plasticity. Proc. Natl Acad. Sci. USA 99, 4037–4042. ( 10.1073/pnas.052020999) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.López-Hidalgo M, Salgado-Puga K, Alvarado-Martínez R, Medina AC, Prado-Alcalá RA, García-Colunga J. 2012. Nicotine uses neuron–glia communication to enhance hippocampal synaptic transmission and long-term memory. PLoS ONE 7, e49998 ( 10.1371/journal.pone.0049998) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bear MF, Singer W. 1986. Modulation of visual cortical plasticity by acetylcholine and noradrenaline. Nature 320, 172–176. ( 10.1038/320172a0) [DOI] [PubMed] [Google Scholar]
- 84.Bröcher S, Artola A, Singer W. 1992. Agonists of cholinergic and noradrenergic receptors facilitate synergistically the induction of long-term potentiation in slices of rat visual cortex. Brain Res. 573, 27–36. ( 10.1016/0006-8993(92)90110-U) [DOI] [PubMed] [Google Scholar]
- 85.Kuga N, Sasaki T, Takahara Y, Matsuki N, Ikegaya Y. 2011. Large-scale calcium waves traveling through astrocytic networks in vivo. J. Neurosci. 31, 2607–2614. ( 10.1523/JNEUROSCI.5319-10.2011) [DOI] [PMC free article] [PubMed] [Google Scholar]