Background: Human RPE65 has a lower retinol isomerohydrolase activity compared with chicken RPE65.
Results: Two point mutations and one short fragment substitution with counterparts of chicken RPE65 substantially enhanced the enzymatic activity of human RPE65.
Conclusion: The activity of RPE65 is determined by a few key residues.
Significance: The highly active human RPE65 mutant can be used to improve RPE65 gene therapy.
Keywords: Enzyme Kinetics, Retina, Retinal Metabolism, Retinoid, Vitamin A
Abstract
RPE65 is the retinoid isomerohydrolase that converts all-trans-retinyl ester to 11-cis-retinol, a key reaction in the retinoid visual cycle. We have previously reported that cone-dominant chicken RPE65 (cRPE65) shares 90% sequence identity with human RPE65 (hRPE65) but exhibits substantially higher isomerohydrolase activity than that of bovine RPE65 or hRPE65. In this study, we sought to identify key residues responsible for the higher enzymatic activity of cRPE65. Based on the amino acid sequence comparison of mammalian and other lower vertebrates' RPE65, including cone-dominant chicken, 8 residues of hRPE65 were separately replaced by their counterparts of cRPE65 using site-directed mutagenesis. The enzymatic activities of cRPE65, hRPE65, and its mutants were measured by in vitro isomerohydrolase activity assay, and the retinoid products were analyzed by HPLC. Among the mutants analyzed, two single point mutants, N170K and K297G, and a double mutant, N170K/K297G, of hRPE65 exhibited significantly higher catalytic activity than WT hRPE65. Further, when an amino-terminal fragment (Met1–Arg33) of the N170K/K297G double mutant of hRPE65 was replaced with the corresponding cRPE65 fragment, the isomerohydrolase activity was further increased to a level similar to that of cRPE65. This finding contributes to the understanding of the structural basis for isomerohydrolase activity. This highly efficient human isomerohydrolase mutant can be used to improve the efficacy of RPE65 gene therapy for retinal degeneration caused by RPE65 mutations.
Introduction
Retinal pigment epithelium (RPE)2-specific 65-kDa protein (RPE65) is essential for metabolism of vitamin A in the eye and for maintenance of normal vision. It is the enzyme that catalyzes isomerization of all-trans-retinyl ester to 11-cis-retinol (11cROL), the precursor of the chromophore of visual pigments (see Fig. 1) (1–3). Mutations of RPE65 are associated with inherited retinal dystrophies such as Leber's congenital amarousis and retinitis pigmentosa (4–17). Previous RPE65 gene replacement therapy in RPE65 null mutants of dog and mouse models displayed promising effects on retinal degeneration (18–23). However, human RPE65 (hRPE65) has lower specific activity than other retinoid-processing enzymes (24–26), and a high abundance of RPE65 in the RPE (11 μg/eye in bovine) is thus required to generate sufficient 11-cis-retinoid for normal vision (1, 27). This demand for high RPE65 levels limits the efficacy of RPE65 gene therapy, and a mutant with increased activity levels would circumvent this limitation.
FIGURE 1.
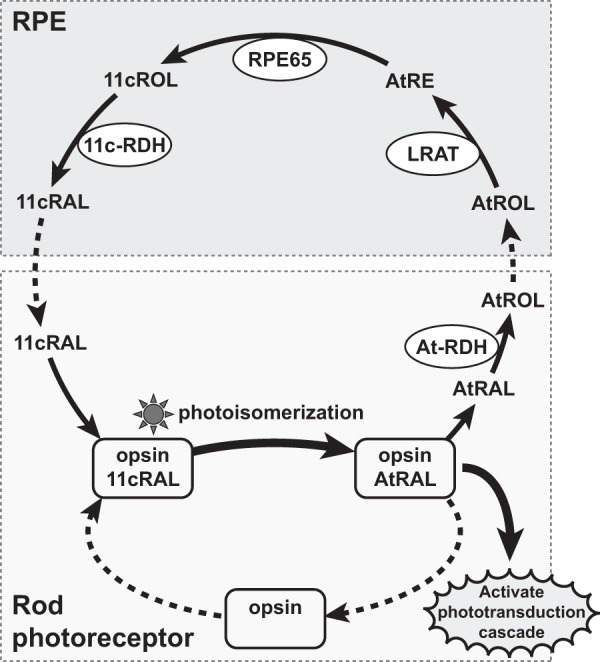
Diagram of the visual cycle. The chromophore, 11-cis-retinal (11cRAL), in visual pigments is isomerized to all-trans-retinal (atRAL) by light, which triggers the phototransduction cascade. atRAL is then released from opsin and reduced to all-trans-retinol (atROL) by an all-trans-retinol dehydrogenase (At-RDH). All-trans-retinol in photoreceptors is transported to the RPE and esterified to all-trans-retinyl ester (atRE) by lecithin retinol acyltransferase (LRAT). All-trans-retinyl ester is converted to 11cROL by the isomerohydrolase RPE65 and further oxidized to 11-cis-retinal by an 11-cis-retinol dehydrogenase (11c-RDH). Finally, regenerated 11-cis-retinal is transported back to photoreceptors and binds to opsin protein to form visual pigment.
Several vectors for gene delivery, such as adenovirus (28, 29), recombinant adeno-associated virus (19, 29–33), lentivirus (29, 34–36), plasmid incorporated in nanoparticles (37), and plasmid DNA with electroporation (38, 39) have been used to deliver intact DNA (or reporter genes) to the ocular tissues. Particularly, gene delivery into the subretinal space using adenovirus and lentiviral vectors expressing GFP showed widely distributed GFP expression (29). Recent human clinical trials using recombinant adeno-associated virus expressing WT hRPE65 showed encouraging results; the initial trials showed modest improvements of vision in patients with retinitis pigmentosa and Leber's congenital amarousis (40–43). However, no present attempts for RPE65 gene therapy have successfully generated full vision recovery despite successful gene delivery. This may be due to low enzymatic activity of hRPE65, meaning that enhancement of hRPE65 enzymatic activity should be considered as an alternative approach.
We have previously reported that RPE65 in cone-dominant chicken has a substantially higher isomerohydrolase activity compared with that in rod-dominant mammals (44). We have cloned chicken RPE65 (cRPE65) and expressed it in the same heterologous system as that for hRPE65. Recombinant cRPE65 showed significantly higher specific isomerohydrolase activity than that of hRPE65 when the activities were normalized by RPE65 protein levels (44), suggesting that RPE65 from cone-dominant chicken is a more efficient isomerohydrolase, in comparison with mammalian RPE65, probably to meet the higher demand for 11-cis-retinal regeneration in the cone-dominant retina. Chicken and human RPE65 proteins share 90% sequence identity (50 different amino acid residues) at the amino acid level (44). We hypothesized that some of the divergent amino acid residues in cRPE65 were responsible for its higher isomerohydrolase activity.
In this study, we substituted the potential key residues in hRPE65 with their counterparts of cRPE65 and evaluated the isomerohydrolase activities of the generated hRPE65 mutants.
EXPERIMENTAL PROCEDURES
Prediction of Candidate Residues Responsible for Higher Enzymatic Activity of cRPE65
In order to predict the key residues responsible for the higher enzymatic activity and/or protein levels of RPE65 in cone-dominant species, RPE65 amino acid sequences from chicken (accession number NP_990215), zebrafinch (Taeniopygia guttata, XP_002187720), and American chameleon (Anolis carolinensis, XP_003225885) were aligned with other known RPE65 sequences: human (accession number NP_000320), bovine (NP_776878), dog (NP_001003176), rat (NP_446014), mouse (NP_084263), newt (Q8AXN9), salamander (Q9YI25), and clawed frog (NP_001080269).
Site-directed Mutagenesis
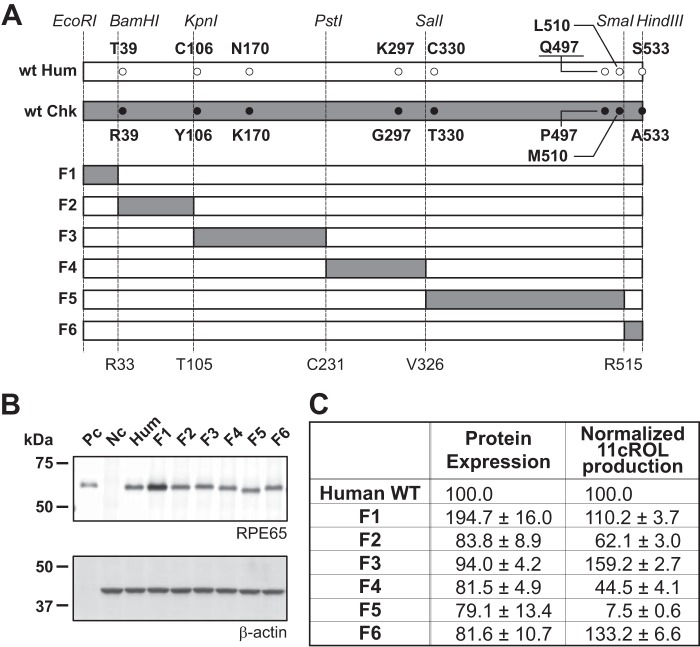
WT hRPE65 and cRPE65 cDNAs were subcloned into cloning vectors as described previously (1, 44). The candidate key residues in hRPE65 were replaced by their counterparts in cRPE65 using the QuikChange site-directed mutagenesis kit (Stratagene, La Jolla, CA), following the manufacturer's protocol. The introduced mutations were confirmed by sequencing from both strands using an ABI-3730 DNA sequencer (Applied Biosystems, Foster City, CA) and subcloned into an expression vector, pcDNA3.1(−) (Invitrogen). Following the sequence confirmation, the expression constructs were purified by a QIAfilter Maxi Prep kit (Qiagen, Valencia, CA). Further, the hRPE65 and cRPE65 cDNA were individually subcloned into pUC18. To generate six restriction fragments of hRPE65 and cRPE65, unique restriction enzyme sites were introduced without changing the amino acid sequence (see Fig. 5A). Each fragment of hRPE65 was replaced by its counterpart of cRPE65 using the introduced restriction enzyme sites. All primer sets used in this study are summarized in Table 1.
FIGURE 5.
Schematic diagram of chimeric human RPE65 mutants and their enzymatic activities. A, the positions of candidate sites in each fragment and specific restriction sites are presented in the diagram (white bar, hRPE65; gray bar, cRPE65; F1–F6, chimeric hRPE65 replaced with the corresponding cRPE65 fragment). B, expression levels of hRPE65 and chimeras were measured by Western blot analysis (Pc, 2.5 μg of bovine RPE microsomal protein; Hum, 20 μg of total cellular protein from hRPE65), and protein levels of RPE65 were semiquantified by densitometry. C, enzymatic activities were measured by the in vitro isomerohydrolase assay and quantified using generated 11-cis-[3H]retinol and normalized by their relative RPE65 protein levels (C).
TABLE 1.
Primer sets in this study
| Primer | Sequence |
|---|---|
| Hum T39R-Fwd | 5′-CCCCCTCTGGCTCCGCGGCAGTCTCCTTC-3′ |
| Hum T39R-Rev | 5′-GAAGGAGACTGCCGCGGAGCCAGAGGGGG-3′ |
| Hum N170K-Fwd | 5′-GGTTGATCTTTGCAAGTATGTCTCTGTC-3′ |
| Hum N170K-Rev | 5′-GACAGAGACATACTTGCAAAGATCAACC-3′ |
| Hum C330T-Fwd | 5′-GATTGTGGATCTCTGCACCTGGAAAGGATTTG-3′ |
| Hum C330T-Rev | 5′-CAAATCCTTTCCAGGTGCAGAGATCCACAATC-3′ |
| Hum Q497P-Fwd | 5′-GCCCAGGAGCAGGACCAAAGCCTGCTTATC-3′ |
| Hum Q497P-Rev | 5′-GATAAGCAGGCTTTGGTCCTGCTCCTGGGC-3′ |
| Hum C106Y-Fwd | 5′-CAGAATTTGGCACCTATGCTTTCCCAGATCCC-3′ |
| Hum C106Y-Rev | 5′-GGGATCTGGGAAAGCATAGGTGCCAAATTCTG-3′ |
| Hum K297G-Fwd | 5′-GCTGACAAAAAAAGGGGAAAGTACCTCAATAATAAATACAG-3′ |
| Hum K297G-Rev | 5′-CTGTATTTATTATTGAGGTACTTTCCCCTTTTTTTGTCAGC-3′ |
| Hum L510M-Fwd | 5′-CTGAATGCCAAGGACATGAGTGAAGTTGCCCGG-3′ |
| Hum L510M-Rev | 5′-CCGGGCAACTTCACTCATGTCCTTGGCATTCAG-3′ |
| Hum S533A-Fwd | 5′-GGACTGTTCAAAAAAGCTTGAGCATACTCCAGCAAGC-3′ |
| Hum S533A-Rev | 5′-GCTTGCTGGAGTATGCTCAAGCTTTTTTGAACAGTCC-3′ |
| Hum KpnI-Fwd | 5′-CAGAATTTGGTACCTGTGCTTTCCCAG-3′ |
| Hum KpnI-Rev | 5′-CTGGGAAAGCACAGGTACCAAATTCTG-3′ |
| Hum SalI-Fwd | 5′-GGGTTTCTGATTGTCGACCTCTGCTGCTGG-3′ |
| Hum SalI-Rev | 5′-CCAGCAGCAGAGGTCGACAATCAGAAACCC-3′ |
| ChkPstI-Fwd | 5′-GCAGTTCCCCTGCAGTGACAGATTTAAG-3′ |
| ChkPstI-Rev | 5′-CTTAAATCTGTCACTGCAGGGGAACTGC-3′ |
| Chk SmaI-Fwd | 5′-GTGAAGTGGCCCGGGCAGAAGTGGAGG-3′ |
| Chk SmaI-Rev | 5′-CCTCCACTTCTGCCCGGGCCACTTCAC-3′ |
Plasmid Transfection
Constructed plasmids expressing WT hRPE65 and cRPE65 and hRPE65 mutants were purified using a QIAfilter Maxi Prep kit (Qiagen, Valencia, CA) and transfected into 293A-LRAT cells, a cell line stably expressing human LRAT (45), using Fugene 6 transfection reagent (Roche Applied Science) or polyethyleneimine (PEI; Polysciences, Inc. (Warrington, PA)) (1 mg/ml, pH 7.4, 2:1 PEI/DNA ratio), and the culture media were replaced at 6 h following the transfection. At 48 h post-transfection, cells were harvested by a cell scraper and rinsed twice with ice-cold PBS. Protein levels and enzymatic activities of WT hRPE65 and its mutants were confirmed by Western blot analyses and an in vitro isomerohydrolase activity assay.
Western Blot Analysis
Total cellular protein concentrations were measured using a Bradford assay (46). Equal amounts of total cellular proteins (20 μg) of 293A-LRAT cells expressing WT hRPE65 and cRPE65 and of hRPE65 mutants and bovine RPE microsomal proteins (2.5 μg) as a positive control were resolved by electrophoresis through 8% Tris-glycine SDS-polyacrylamide gel and electrotransferred onto an Immobilon PVDF membrane (Millipore, Billerica, MA) unless specified. The membrane was blocked with 5% (w/v) nonfat dry milk in TBST (Tris-buffered saline with 0.1% Tween 20) for 30 min and subsequently incubated overnight at 4 °C with a 1:1,000 dilution of an anti-RPE65 polyclonal antibody (27) to identify the key residues and a 1:50,000 dilution of an anti-β-actin monoclonal antibody (Sigma-Aldrich). We used another RPE65 antibody (44) to compare the expression levels of cRPE65 and chimeric mutants of RPE65 to avoid the immunoreactivity difference on fragment 3. After four washes with TBST, the membrane was incubated in a light-shielding container for 1.5 h with a 1:25,000 dilution of goat anti-mouse IgG conjugated with DyLight-549 and goat anti-rabbit IgG conjugated with DyLight-649 (Pierce), and the bands were detected using the FluorChem Q imaging system (ProteinSimple, Santa Clara, CA). The signal intensities were semiquantified by densitometry using AlphaView SA software (ProteinSimple) and averaged from at least three independent experiments.
Isomerohydrolase Activity Assay
293A-LRAT cells were separately transfected with plasmids expressing WT human and chicken RPE65 and hRPE65 mutants. 293A-LRAT cells expressing red fluorescence protein (RFP) were used as a negative control. Cells were lysed by sonication in a reaction buffer (10 mm 1,3-bis(tris(hydroxymethyl)-methylamino) propane, pH 8.0, 100 mm NaCl). All-trans-[11,12-3H]retinol (1 mCi/ml, 45.5 Ci/mmol; American Radiolabeled Chemicals, Inc., St. Louis, MO) in N,N-dimethyl formamide was used as the substrate for the isomerohydrolase assay. For each reaction, total cellular proteins (125 μg) were added into 200 μl of reaction buffer (10 mm 1,3-bis(tris(hydroxymethyl)-methylamino) propane, pH 8.0, 100 mm NaCl) containing 0.2 μm all-trans-retinol, 1% BSA, and 25 μm cellular retinal aldehyde-binding protein (47). The reaction was stopped, and retinoids were extracted with 300 μl of cold methanol and 300 μl of hexane. The generated retinoids were analyzed by normal phase HPLC as described previously (1). The peak of each retinoid isomer was identified based on its characteristic retention time and absorption spectrum of retinoid standards. Isomerohydrolase activity was calculated from the area of the 11cROL peak using Radiomatic 610TR software (PerkinElmer Life Sciences) with synthetic 11-cis-[3H]retinol as the standard. To minimize the variation of the transfection efficiency, all in vitro activity assays of the mutants were conducted side-by-side with WT hRPE65, and the catalytic activities were expressed as values relative to that of WT hRPE65 unless specified.
RESULTS
Comparison of Expression and Enzymatic Activities of Human and Chicken RPE65
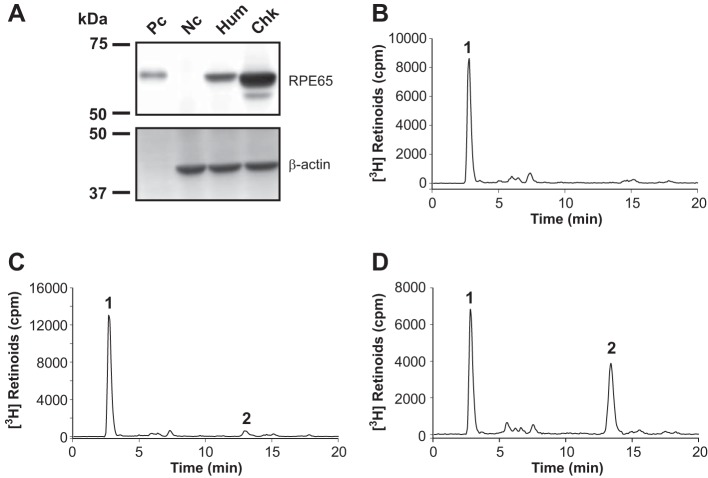
Plasmids expressing RFP (negative control), hRPE65, and cRPE65 were separately transfected into the 293A-LRAT cells and cultured for 48 h. The expression levels and enzymatic activities of hRPE65 and cRPE65 were verified by Western blot analysis (Fig. 2A) and an in vitro isomerohydrolase assay. Both hRPE65 and cRPE65, but not negative control protein, RFP, converted all-trans-retinyl ester into 11cROL, the product of isomerohydrolase (Fig. 2, B–D). cRPE65 produced substantially higher levels of 11cROL than that of hRPE65 after the normalization to its RPE65 protein level, indicating a higher enzymatic activity of cRPE65, consistent with our previous report (44).
FIGURE 2.
Expression and enzymatic activities of RPE65 from humans and chicken. Plasmids expressing WT hRPE65, cRPE65, and red fluorescence protein (RFP, negative control) were separately transfected into 293A-LRAT cells. Expression levels and enzymatic activities of hRPE65 and cRPE65 were measured by Western blot analysis (A, Pc, bovine RPE microsomal protein (2.5 μg); Nc, RFP; Hum, hRPE65; Chk, cRPE65; 20 μg each) and an in vitro isomerohydrolase activity assay, respectively. All-trans-[3H]retinol (0.2 μm) was incubated with 125 μg of total cellular protein from the cells expressing RFP (B), hRPE65 (C), and cRPE65 (D) for 2 h, and the generated retinoids were analyzed by HPLC. Peak 1, retinyl esters; peak 2, 11cROL.
Prediction of Key Residues Responsible for Increased Isomerohydrolase Activity of cRPE65
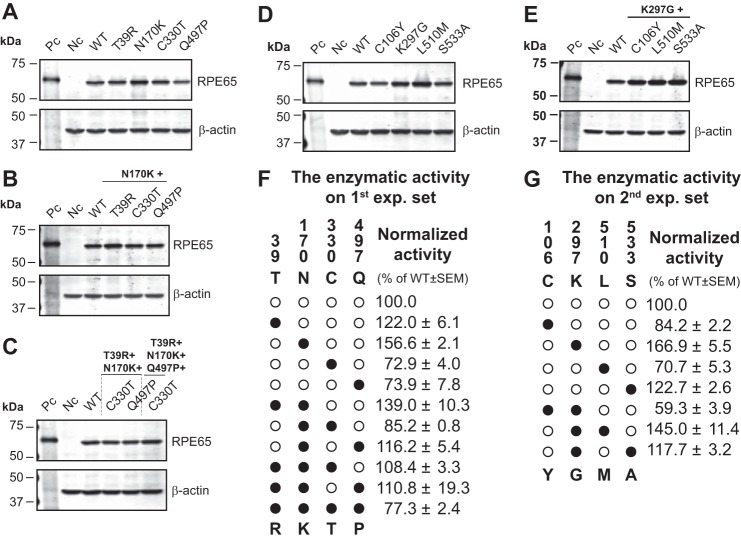
Based on an amino acid sequence alignment of vertebrate RPE65, we selected 8 residues as the candidates that may be responsible for the difference in the isomerohydrolase activity between hRPE65 and cRPE65 (Fig. 3). We divided the candidate residues into two groups in this study: Group A, residues that were found to be identical in the three cone-dominant species but were substituted in other selected vertebrates (Thr39, Cys106, Asn170, Cys330, and Gln497 in hRPE65); Group B, residues that were conserved in lower vertebrates but diverse in mammalians (Lys297, Leu510, and Ser533 in hRPE65). In order to evaluate the contribution of these residues to isomerohydrolase activity, a series of point mutations of the 8 candidate residues (in Groups A and B) in hRPE65 were generated by site-directed mutagenesis. For in vitro enzyme activity assays, we first examined the effect of 4 residues (Thr39, Asn170, Cys330, and Gln497 in hRPE65) in Group A as the first experimental set, and the remaining residues (1 residue in Group A (Cys106) and 3 residues in Group B (Lys297, Leu510, and Ser533)) were studied as the second experimental set.
FIGURE 3.
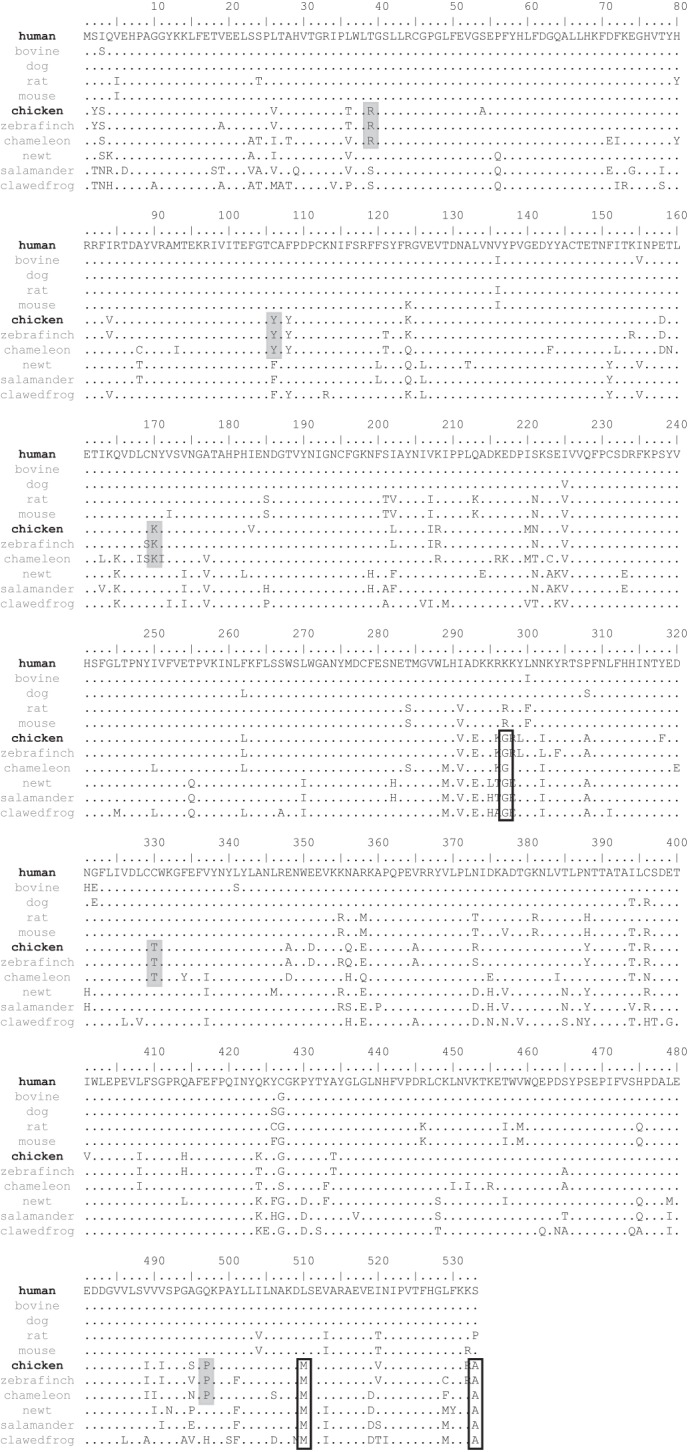
Amino acid sequence alignment of RPE65 from different species. RPE65 sequences from the human, bovine, dog, rat, mouse, chicken, zebrafinch, American chameleon, Japanese fireberry newt, tiger salamander, and clawed frog were aligned. Amino acid residues identical to those of hRPE65 are indicated with dots. Residues conserved in the cone-dominant species are indicated by gray boxes (Group A). Residues conserved in the lower vertebrates are signified by white boxes (Group B).
Impacts of Site-directed Point Mutations on Protein Levels and Catalytic Activities of RPE65
Plasmids expressing hRPE65 and cRPE65 and the site-directed mutants of hRPE65 were separately transfected into 293A-LRAT cells, and the transfected cells were cultured for 48 h. Protein expression was confirmed by Western blot analysis (Table 2), and the same batches of total cellular proteins were used for the in vitro isomerohydrolase activity assay. For all of the hRPE65 mutants containing single, double, and multiple mutations, expression levels of RPE65 were comparable (Fig. 4, A–E, Table 2), whereas the two single point mutants N170K and K297G exhibited 1.6- and 1.7-fold higher enzymatic activity, respectively, than WT hRPE65 after normalization by total RPE65 expression levels (Fig. 4, F and G). Furthermore, the tested double, triple, or multiple mutants with N170K (or K297G) in each experimental sets did not further enhance catalytic activity of RPE65. These results suggest that mutations of N170K and K297G in hRPE65 may be important for increasing its enzymatic activity.
TABLE 2.
Expression levels of generated RPE65 mutants in this study
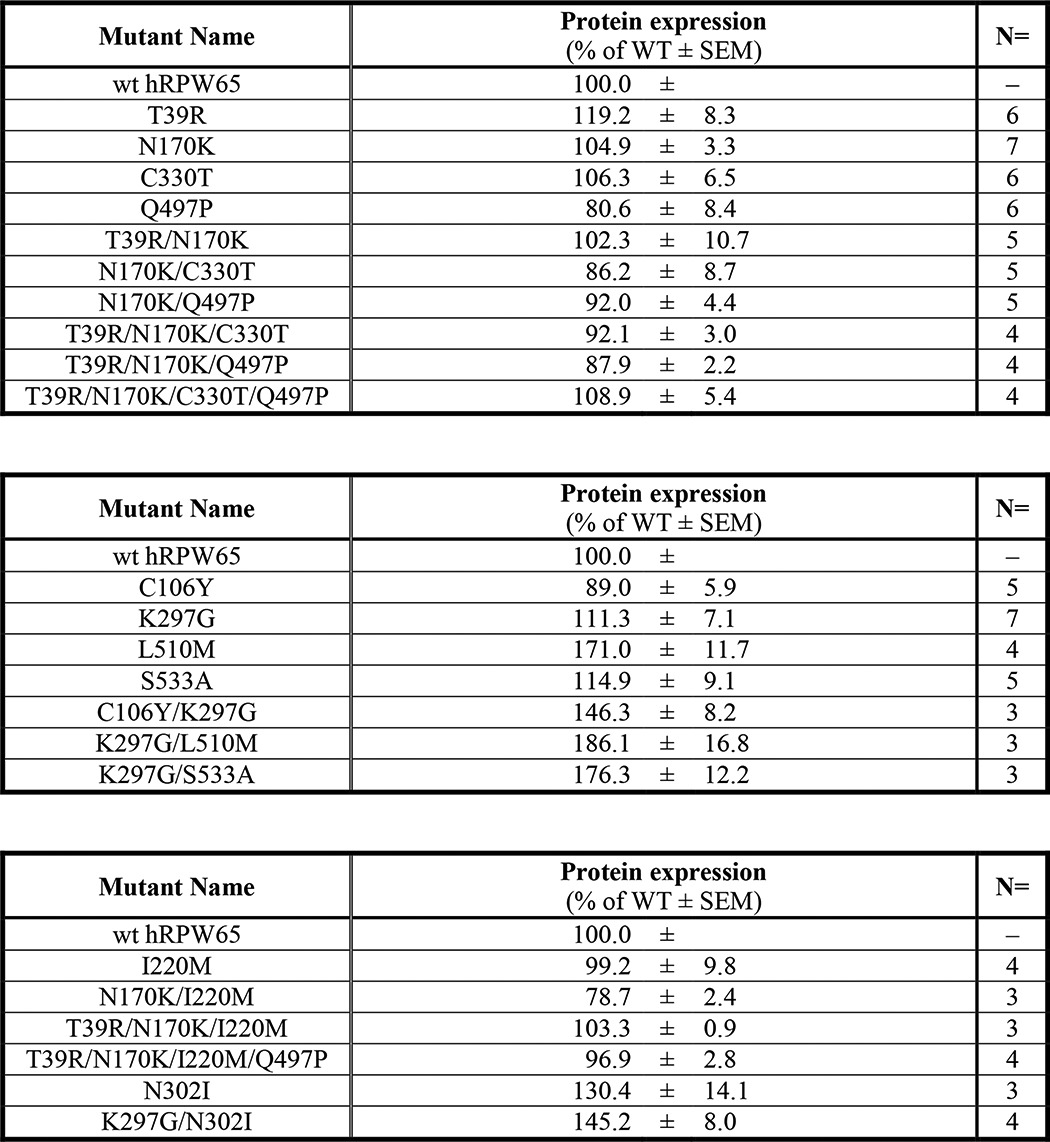
FIGURE 4.
Impacts of site-directed mutations on isomerohydrolase activity and protein level of RPE65. Plasmids expressing WT hRPE65 and cRPE65, and the indicated hRPE65 mutants were separately transfected into 293A-LRAT cells and cultured for 48 h. A–D, an equal amount of total proteins from cell lysates (20 μg) was used for Western blotting using an antibody specific for RPE65, with an anti-β-actin antibody as loading control (A, single; B, double; C, triple and quadruple mutants in the first experimental set; D, single; E, double mutants in the second experimental set. Pc, positive control (bovine RPE microsomal protein, 2.5 μg); Nc, negative control (red fluorescence protein); WT, WT hRPE65). RPE65 levels were semiquantified by densitometry and normalized by β-actin levels. F and G, in vitro activity assays were performed with the same batch of samples of Western blot analyses. Isomerohydrolase activities were quantified by generated 11-cis-[3H]retinol from three independent measurements and normalized by their relative RPE65 protein levels. Values were expressed as relative activity (percentage of WT hRPE65 activity, mean ± S.E., n = 3). In the enzymatic activity tables (F and G), human (○) and chicken (●) residues are indicated as white and black circles, respectively. The vertical numbers indicate the positions of candidate residues. Letters above and below the panel in the tables (F and G) represent hRPE65 and cRPE65 amino acid residues at the indicated positions, respectively (e.g. ●●○● in the table of the first experimental set (F) indicates a triple mutant (T39R/N170K/Q497P) of hRPE65).
Construction of Chimeric Human RPE65 and Its Impacts on Its Protein Levels and Catalytic Activity
To further improve the catalytic activity of hRPE65, we constructed chimeric RPE65 by replacing a peptide fragment of hRPE65 with the counterpart of cRPE65 (Fig. 5A, F1–F6). At 48 h post-transfection, the cells were harvested for Western blot analyses and in vitro isomerohydrolase assays. Western blot analysis showed that a F1 chimeric mutant (replaced fragment 1) displayed a higher protein level of RPE65 than did WT hRPE65 and other chimeric mutants (Fig. 5, B and C). Interestingly, F1 and F3 chimeric mutants showed ∼1.5–2-fold higher catalytic activity than did WT hRPE65, whereas the F2, F4, and F5 chimeric mutants substantially decreased the catalytic activities of hRPE65. Following the normalization by RPE65 protein levels, the F1 chimeric mutant showed 11cROL production (110.2% of WT hRPE65) comparable with that of WT hRPE65, suggesting that the enhanced catalytic activity of the F1 chimera was probably due to the increased protein level of the F1 RPE65 chimera (Fig. 5, B and C). It should be noted that the F3 chimera displayed higher normalized enzymatic activity than WT hRPE65, probably due to N170K mutation (159.2% of WT hRPE65). This is well correlated with the result of the N170K single mutation (see Fig. 3).
Generation of Superisomerohydrolase (sIMH) by Combination of Highly Active Mutants
Finally, we combined the three highly active mutants identified from the series of site-directed point mutants and the chimeric mutants. As we examined earlier, two single mutants (N170K and K297G) showed significantly higher 11cROL production than did WT hRPE65 (Fig. 6B), although their expression levels were similar to that of WT hRPE65 (Fig. 6A, Table 2). As shown in Fig. 6, a double mutant (N170K/K297G) of hRPE65 demonstrated 3.2-fold higher 11cROL production than WT hRPE65. In addition, a combination of the F1 chimera with the N170K mutations showed even higher protein levels (2.2-fold) and 11cROL production (2.2-fold) than that of N170K single mutant. Finally, after combining all of these three mutations, the 11cROL production of a combined mutant, F1/N170K/K297G (sIMH), was further increased to 4.4-fold of that of WT hRPE65, which is comparable with the activity of WT cRPE65 (5.8-fold in the same assay; Fig. 6B). These results indicate that the three mutations are responsible for the higher activity of cRPE65.
FIGURE 6.
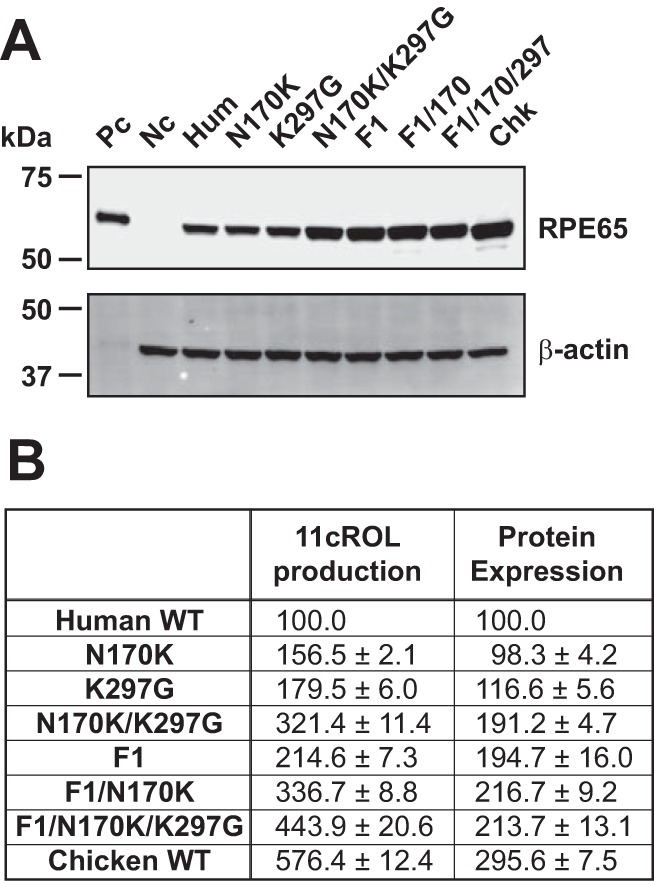
Impacts of the site-directed mutations and fragment replacement with those of cRPE65 on protein levels and enzymatic activities of hRPE65. The identified site-directed mutants in this study (N170K and K297G) and the F1 chimera were combined to produce sIMH (F1/N170K/K297G). The identified point mutants and the F1 chimera of RPE65 were expressed in 293A-LRAT cells. The cells were harvested 48 h after the transfection, and protein levels of RPE65 were confirmed by Western blot analyses (A). Enzymatic activities were measured by the in vitro isomerohydrolase assay and quantified by generated 11-cis-[3H]retinol (B). Isomerohydrolase activities were quantified by generated 11-cis-[3H]retinol (pmol/h) and presented as a percentage of WT hRPE65 activity (mean ± S.E., n = 3).
DISCUSSION
It has been well established that RPE65 is an essential enzyme for the normal vision because RPE65 deficiency causes blindness and retina dystrophies, such as Leber's congenital amarousis or retinitis pigmentosa (4–17). In order to restore the visual function in patients with RPE65 deficiency, RPE65 gene replacement therapy has been performed (40–43). However, none of the gene replacement trials have fully restored the visual function in RPE65-deficient patients, although the gene delivery itself was successful (29). The unsatisfactory efficacy may be ascribed to the weak catalytic activity of RPE65 (kcat = 1.45 × 10−4 s−1) (24) in comparison with other retinoid-processing enzymes (25, 26). This hypothesis is supported by the observation that endogenous RPE65 exists at high levels in the RPE (11 μg/eye in bovine), probably to compensate for its relatively low activity (27). It is likely that current RPE65 gene delivery strategies cannot achieve such a high protein level of RPE65. These studies suggest that enhancement of catalytic activity of the delivered hRPE65 may be a means to improve the efficacy of RPE65 gene therapies.
The present study represents the first approach to improve the catalytic activity of hRPE65. Previously, we have shown that RPE65 from cone-dominant chicken has substantially higher isomerohydrolase activity than that of RPE65 from rod-dominant mammalians (44). This finding suggests that there is a potential to improve catalytic activity of hRPE65 by replacing some key residues with those of cRPE65. In order to identify the key residues responsible for the higher enzymatic activity in cRPE65, three cone-dominant species (two avian and one diurnal-type reptile) were selected as the template for amino acid sequence comparison. Among the divergent residues, 8 amino acid residues were selected as candidates in this study. The residues Glu158, Lys208, and Leu408 in hRPE65 were not selected for the study because their substitutions preserve physico-chemical properties in other selected vertebrates (Asp158, Arg208, and Ile408). Although Phe108 is conserved in cone-dominant avian and reptile species, it was also conserved in the clawed frog. Therefore, we did not select the Phe108 residue for the mutagenesis study. It is noteworthy that some residue replacements that did not fulfill the above-mentioned criteria of residue selection (I220M and N302I) did not substantially increase the catalytic activity of hRPE65 even in combinations with other mutations (N170K/I220M, T39R/N170K/I220M, and T39R/N170K/I220M/Q497P) (Table 3).
TABLE 3.
Isomerohydrolase activities on I220M and N302I mutant series of hRPE65
In vitro activity assays of the indicated mutants were performed as described under “Experimental Procedures,” and isomerohydrolase activities were expressed as relative activity following the normalization by RPE65 levels (percentage of WT hRPE65 activity, mean ± S.E., n = 3).
| Mutant name | Normalized activity (percentage of WT ± S.E.) |
|---|---|
| % | |
| WT hRPE65 | 100 |
| I220M | 62.0 ± 11.0 |
| N170K/I220M | 31.1 ± 7.8 |
| T39R/N170K/I220M | 109.7 ± 4.2 |
| T39R/N170K/I220M/Q497P | 108.6 ± 10.4 |
| N302I | 124.8 ± 13.3 |
| N302I/K297G | 107.7 ± 6.9 |
Among the tested mutants, we successfully identified two point mutations, N170K and K297G, which significantly increased 11cROL production compared with that of WT hRPE65. The double mutant that combined these mutations (N170K/K297G) showed a further enhanced 11cROL production (3.2-fold that of WT RPE65) of hRPE65. Furthermore, 11cROL production of this double mutant was even more enhanced by the replacement of the F1 fragment with that of cRPE65 (Met1–Arg33; containing 3 divergent residues: S2Y, I3S, and L26V). As a result, this superisomerohydrolase mutant (sIMH; F1/N170K/K297G) showed an approximately 4.4-fold higher 11cROL production than did WT hRPE65 under the same experimental conditions. Production of 11cROL by sIMH was near the level of WT cRPE65 (5.8-fold that of WT hRPE65).
As further confirmation of enhanced enzymatic activity of sIMH, we evaluated catalytic efficiencies of WT hRPE65 and sIMH (Fig. 7). sIMH showed a Vmax value ∼3-fold higher than that of WT hRPE65 (Fig. 7, B and C). It is noteworthy that the estimated concentration of substrate, retinyl esters, in the mouse RPE is ∼500 μm (48–50), which is much higher than Km for both WT RPE65 and sIMH (Fig. 7, B and C). In this case, Km is not an important factor because the enzyme is saturated by substrate at this concentration.
 |
Km can be neglected in the formula when S ≫ Km.
FIGURE 7.
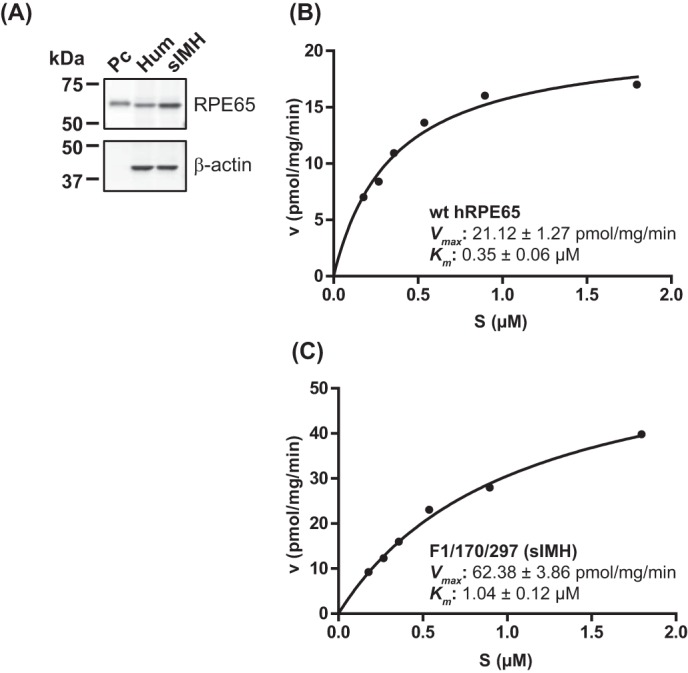
Analyses of catalytic efficiencies of WT hRPE65 and sIMH. Adenoviruses expressing hRPE65 and sIMH were prepared as described previously (1, 45). WT hRPE65 and sIMH were separately expressed in 293A (without LRAT) cells for 24 h at a multiplicity of infection of 100, and their expression was evaluated by Western blot analysis (A). Various concentrations of the substrate in liposome were incubated with total cellular protein lysates (125 μg) in the in vitro enzyme assays as described previously (24, 52), and Km and Vmax of WT hRPE65 (B) and sIMH (C) were calculated through a Michaelis-Menten plot of generated 11cROL.
The rate of the enzyme reaction is actually equal to Vmax in the RPE, meaning that Vmax of RPE65 plays a major role in physiological conditions (Fig. 7, B and C).
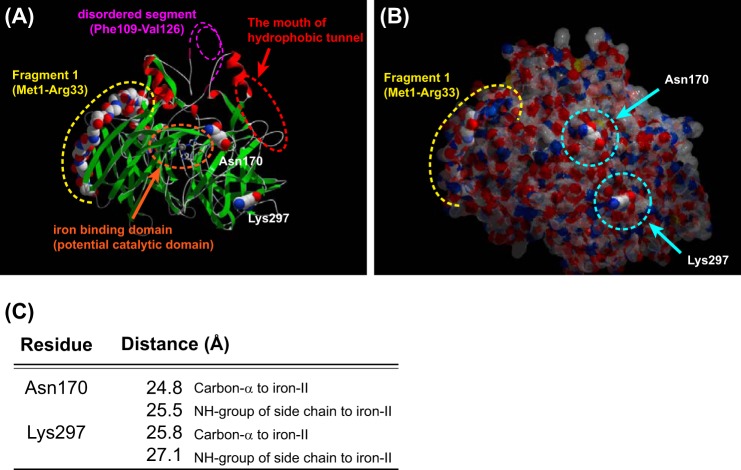
To understand the potential contribution of the identified key residues to higher catalytic activity of cRPE65, we analyzed the three-dimensional structure of bovine RPE65 (Protein Data Bank entry 3FSN) by the SwissPDB Viewer version 4.01 (51) and displayed the results by POV-Ray version 3.61. In the three-dimensional model, both Asn170 and Lys297 residues are localized on the surface of the RPE65 molecule (Fig. 8, A and B) and distant from the co-factor iron in the RPE65 catalytic site (Fig. 8C), suggesting that these two residues are unlikely to directly participate in the catalysis of the substrate. We speculate that these residue substitutions might improve RPE65 membrane association for more efficient substrate intake and/or product (11cROL) release. Alternatively, these two residues might contribute to proper folding of RPE65 to achieve its active conformation upon association with the membrane. Interestingly, the F1 chimera also revealed a significant improvement of the catalytic activity of hRPE65 due to increased protein levels of RPE65. Unlike the other two residue substitutions, the F1 chimera in which only the F1 fragment is replaced with that of cRPE65 showed a catalytic activity similar to that of WT hRPE65 after normalization by RPE65 protein level. The F1 fragment contains 33 amino acids and only 3 residues that differ between hRPE65 and cRPE65. These residues in the F1 fragment might contribute to proper folding to enhance protein stability of the hRPE65 mutant. Another possibility is the contribution of the nucleotide sequence difference. Among the 99 base pairs encoding the 33 amino acids in the F1 fragment, there are 19 different nucleotides between hRPE65 and cRPE65. These nucleotide substitutions might improve the stability of the hRPE65 mRNA or contribute to more efficient codon usage, leading to higher levels of expression.
FIGURE 8.
Locations of Asn170 and Lys297 and the F1 fragment in an RPE65 three-dimensional structure model. The locations of two identified key residues and the F1 fragment are shown in the three-dimensional model based on the crystal structure of bovine RPE65 (Protein Data Bank entry 3FSN). The iron binding site, within the catalytic domain, is indicated by an orange dotted circle. The disordered segment (Phe109–Val126), which contains a palmitoylated Cys residue (Cys112), is shown by a pink dotted line. The entrance of the hydrophobic tunnel containing an active site is indicated by a red dotted circle. The location of the F1 fragment is indicated by the yellow dotted line. Both Asn170 and Lys297 residues are located on the surface of the protein (A and B) and more than 20 Å distant from iron(II) in the potential catalytic domain (C).
In summary, this study generated a highly active hRPE65 mutant, termed sIMH, by replacing only 5 residues with those of cRPE65. This sIMH displayed higher catalytic activity (4.4-fold higher than WT hRPE65) after normalization by the total cellular protein levels. Although further studies are necessary to clarify how these mutations increase the catalytic activity and protein level of RPE65, the sIMH obtained in this study can be used for the next generation of RPE65 gene replacement therapy and may be more effective than WT hRPE65 in the treatment of retinal dystrophies. These results will also contribute to the understanding of structural basis for the isomerohydrolase activity of RPE65.
Note Added in Proof
Table 2 was inadvertently omitted from the Papers in Press version.
This work was supported, in whole or in part, by National Institutes of Health Grants EY012231, EY018659, EY019309, and GM104934. This work was also supported by a grant from the International Retinal Research Foundation and Oklahoma Center for the Advancement of Science and Technology (OCAST) Grants HR12-103 and HR13-076.
- RPE
- retinal pigment epithelium
- RPE65
- RPE-specific 65-kDa protein
- 11cROL
- 11-cis-retinol
- cRPE65
- chicken RPE65
- hRPE65
- human RPE65
- sIMH
- superisomerohydrolase.
REFERENCES
- 1. Moiseyev G., Chen Y., Takahashi Y., Wu B. X., Ma J. X. (2005) RPE65 is the isomerohydrolase in the retinoid visual cycle. Proc. Natl. Acad. Sci. U.S.A. 102, 12413–12418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Redmond T. M., Poliakov E., Yu S., Tsai J. Y., Lu Z., Gentleman S. (2005) Mutation of key residues of RPE65 abolishes its enzymatic role as isomerohydrolase in the visual cycle. Proc. Natl. Acad. Sci. U.S.A. 102, 13658–13663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jin M., Li S., Moghrabi W. N., Sun H., Travis G. H. (2005) Rpe65 is the retinoid isomerase in bovine retinal pigment epithelium. Cell 122, 449–459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dharmaraj S. R., Silva E. R., Pina A. L., Li Y. Y., Yang J. M., Carter C. R., Loyer M. K., El-Hilali H. K., Traboulsi E. K., Sundin O. K., Zhu D. K., Koenekoop R. K., Maumenee I. H. (2000) Mutational analysis and clinical correlation in Leber congenital amaurosis. Ophthalmic Genet. 21, 135–150 [PubMed] [Google Scholar]
- 5. Gal A., Gu S., Bremser D., Lorenz B., Apfelstedt-Sylla E., Zrenner E., Gerding H., Denton M. J., Thompson D. A. (1998) Mutation spectrum of RPE65 in autosomal recessive childhood-onset severe retinal dystrophy. Invest. Ophthalmol. Vis. Sci. 39, S901 [Google Scholar]
- 6. Gu S. M., Thompson D. A., Srikumari C. R., Lorenz B., Finckh U., Nicoletti A., Murthy K. R., Rathmann M., Kumaramanickavel G., Denton M. J., Gal A. (1997) Mutations in RPE65 cause autosomal recessive childhood-onset severe retinal dystrophy. Nat. Genet. 17, 194–197 [DOI] [PubMed] [Google Scholar]
- 7. Lorenz B., Gyürüs P., Preising M., Bremser D., Gu S., Andrassi M., Gerth C., Gal A. (2000) Early-onset severe rod-cone dystrophy in young children with RPE65 mutations. Invest. Ophthalmol. Vis. Sci. 41, 2735–2742 [PubMed] [Google Scholar]
- 8. Lotery A. J., Namperumalsamy P., Jacobson S. G., Weleber R. G., Fishman G. A., Musarella M. A., Hoyt C. S., Héon E., Levin A., Jan J., Lam B., Carr R. E., Franklin A., Radha S., Andorf J. L., Sheffield V. C., Stone E. M. (2000) Mutation analysis of 3 genes in patients with Leber congenital amaurosis. Arch. Ophthalmol. 118, 538–543 [DOI] [PubMed] [Google Scholar]
- 9. Marlhens F., Griffoin J. M., Bareil C., Arnaud B., Claustres M., Hamel C. P. (1998) Autosomal recessive retinal dystrophy associated with two novel mutations in the RPE65 gene. Eur. J. Hum. Genet. 6, 527–531 [DOI] [PubMed] [Google Scholar]
- 10. Morimura H., Fishman G. A., Grover S. A., Fulton A. B., Berson E. L., Dryja T. P. (1998) Mutations in the RPE65 gene in patients with autosomal recessive retinitis pigmentosa or leber congenital amaurosis. Proc. Natl. Acad. Sci. U.S.A. 95, 3088–3093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pittler S. J., Miller B. E., Simovich M. J. (1999) A homozygous Y144D change in the RPE65 gene in a patient with Leber congenital amaurosis and keratoconus. Invest. Ophthalmol. Vis. Sci. 40, S471 [Google Scholar]
- 12. Simovich M. J., Miller B., Ezzeldin H., Kirkland B. T., McLeod G., Fulmer C., Nathans J., Jacobson S. G., Pittler S. J. (2001) Four novel mutations in the RPE65 gene in patients with Leber congenital amaurosis. Hum. Mutat. 18, 164. [DOI] [PubMed] [Google Scholar]
- 13. Thompson D. A., Gyürüs P., Fleischer L. L., Bingham E. L., McHenry C. L., Apfelstedt-Sylla E., Zrenner E., Lorenz B., Richards J. E., Jacobson S. G., Sieving P. A., Gal A. (2000) Genetics and phenotypes of RPE65 mutations in inherited retinal degeneration. Invest. Ophthalmol. Vis. Sci. 41, 4293–4299 [PubMed] [Google Scholar]
- 14. Thompson D. A., Lorenz B., Gyurus P., Gao J., Hanna D. B., Andrassi M. (1999) RPE65 mutations in childhood-onset severe retinal degenerations. Invest. Ophthalmol. Vis. Sci. 40, S575 [Google Scholar]
- 15. Wada Y., Nakazawa M., Abe T., Fuse N., Tamai M. (2000) Clinical variability of patients associated with gene mutations of visual cycle protein: arrestin, RPE65 and RDH5 genes. Invest. Ophthalmol. Vis. Sci. 41, S617 [Google Scholar]
- 16. Marlhens F., Bareil C., Griffoin J. M., Zrenner E., Amalric P., Eliaou C., Liu S. Y., Harris E., Redmond T. M., Arnaud B., Claustres M., Hamel C. P. (1997) Mutations in RPE65 cause Leber's congenital amaurosis. Nat. Genet. 17, 139–141 [DOI] [PubMed] [Google Scholar]
- 17. Yzer S., van den Born L. I., Schuil J., Kroes H. Y., van Genderen M. M., Boonstra F. N., van den Helm B., Brunner H. G., Koenekoop R. K., Cremers F. P. (2003) A Tyr368His RPE65 founder mutation is associated with variable expression and progression of early onset retinal dystrophy in 10 families of a genetically isolated population. J. Med. Genet. 40, 709–713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Acland G. M., Aguirre G. D., Ray J., Zhang Q., Aleman T. S., Cideciyan A. V., Pearce-Kelling S. E., Anand V., Zeng Y., Maguire A. M., Jacobson S. G., Hauswirth W. W., Bennett J. (2001) Gene therapy restores vision in a canine model of childhood blindness. Nat. Genet. 28, 92–95 [DOI] [PubMed] [Google Scholar]
- 19. Narfström K., Katz M. L., Bragadottir R., Seeliger M., Boulanger A., Redmond T. M., Caro L., Lai C. M., Rakoczy P. E. (2003) Functional and structural recovery of the retina after gene therapy in the RPE65 null mutation dog. Invest. Ophthalmol. Vis. Sci. 44, 1663–1672 [DOI] [PubMed] [Google Scholar]
- 20. Lai C. M., Yu M. J., Brankov M., Barnett N. L., Zhou X., Redmond T. M., Narfstrom K., Rakoczy P. E. (2004) Recombinant adeno-associated virus type 2-mediated gene delivery into the Rpe65−/− knockout mouse eye results in limited rescue. Genet. Vaccines Ther. 2, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bennicelli J., Wright J. F., Komaromy A., Jacobs J. B., Hauck B., Zelenaia O., Mingozzi F., Hui D., Chung D., Rex T. S., Wei Z., Qu G., Zhou S., Zeiss C., Arruda V. R., Acland G. M., Dell'Osso L. F., High K. A., Maguire A. M., Bennett J. (2008) Reversal of blindness in animal models of leber congenital amaurosis using optimized AAV2-mediated gene transfer. Mol. Ther. 16, 458–465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chen Y., Moiseyev G., Takahashi Y., Ma J. X. (2006) RPE65 gene delivery restores isomerohydrolase activity and prevents early cone loss in Rpe65−/− mice. Invest. Ophthalmol. Vis. Sci. 47, 1177–1184 [DOI] [PubMed] [Google Scholar]
- 23. Acland G. M., Aguirre G. D., Bennett J., Aleman T. S., Cideciyan A. V., Bennicelli J., Dejneka N. S., Pearce-Kelling S. E., Maguire A. M., Palczewski K., Hauswirth W. W., Jacobson S. G. (2005) Long-term restoration of rod and cone vision by single dose rAAV-mediated gene transfer to the retina in a canine model of childhood blindness. Mol. Ther. 12, 1072–1082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nikolaeva O., Takahashi Y., Moiseyev G., Ma J. X. (2009) Purified RPE65 shows isomerohydrolase activity after reassociation with a phospholipid membrane. FEBS J. 276, 3020–3030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gallego O., Belyaeva O. V., Porté S., Ruiz F. X., Stetsenko A. V., Shabrova E. V., Kostereva N. V., Farrés J., Parés X., Kedishvili N. Y. (2006) Comparative functional analysis of human medium-chain dehydrogenases, short-chain dehydrogenases/reductases and aldo-keto reductases with retinoids. Biochem. J. 399, 101–109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Belyaeva O. V., Korkina O. V., Stetsenko A. V., Kim T., Nelson P. S., Kedishvili N. Y. (2005) Biochemical properties of purified human retinol dehydrogenase 12 (RDH12): Catalytic efficiency toward Retinoids and C-9 aldehydes and effects of cellular retinol-binding protein type I (CRBPI) and cellular retinaldehyde-binding protein (CRALBP) on the oxidation and reduction of retinoids. Biochemistry 44, 7035–7047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ma J. X., Zhang J., Othersen K. L., Moiseyev G., Ablonczy Z., Redmond T. M., Chen Y., Crouch R. K. (2001) Expression, purification, and MALDI analysis of RPE65. Invest. Ophthalmol. Vis. Sci. 42, 1429–1435 [PubMed] [Google Scholar]
- 28. Bennett J., Wilson J., Sun D., Forbes B., Maguire A. (1994) Adenovirus vector-mediated in vivo gene transfer into adult murine retina. Invest. Ophthalmol. Vis. Sci. 35, 2535–2542 [PubMed] [Google Scholar]
- 29. Zhang S. H., Wu J. H., Wu X. B., Dong X. Y., Liu X. J., Li C. Y., Qian-Huang (2008) Distinctive gene transduction efficiencies of commonly used viral vectors in the retina. Curr. Eye Res. 33, 81–90 [DOI] [PubMed] [Google Scholar]
- 30. Weber M., Rabinowitz J., Provost N., Conrath H., Folliot S., Briot D., Chérel Y., Chenuaud P., Samulski J., Moullier P., Rolling F. (2003) Recombinant adeno-associated virus serotype 4 mediates unique and exclusive long-term transduction of retinal pigmented epithelium in rat, dog, and nonhuman primate after subretinal delivery. Mol. Ther. 7, 774–781 [DOI] [PubMed] [Google Scholar]
- 31. Yokoi K., Kachi S., Zhang H. S., Gregory P. D., Spratt S. K., Samulski R. J., Campochiaro P. A. (2007) Ocular gene transfer with self-complementary AAV vectors. Invest. Ophthalmol. Vis. Sci. 48, 3324–3328 [DOI] [PubMed] [Google Scholar]
- 32. Auricchio A., Kobinger G., Anand V., Hildinger M., O'Connor E., Maguire A. M., Wilson J. M., Bennett J. (2001) Exchange of surface proteins impacts on viral vector cellular specificity and transduction characteristics: the retina as a model. Hum. Mol. Genet. 10, 3075–3081 [DOI] [PubMed] [Google Scholar]
- 33. Pang J. J., Lauramore A., Deng W. T., Li Q., Doyle T. J., Chiodo V., Li J., Hauswirth W. W. (2008) Comparative analysis of in vivo and in vitro AAV vector transduction in the neonatal mouse retina: effects of serotype and site of administration. Vision Res. 48, 377–385 [DOI] [PubMed] [Google Scholar]
- 34. Yáñez-Munoz R. J., Balaggan K. S., MacNeil A., Howe S. J., Schmidt M., Smith A. J., Buch P., MacLaren R. E., Anderson P. N., Barker S. E., Duran Y., Bartholomae C., von Kalle C., Heckenlively J. R., Kinnon C., Ali R. R., Thrasher A. J. (2006) Effective gene therapy with nonintegrating lentiviral vectors. Nat. Med. 12, 348–353 [DOI] [PubMed] [Google Scholar]
- 35. Bemelmans A. P., Kostic C., Crippa S. V., Hauswirth W. W., Lem J., Munier F. L., Seeliger M. W., Wenzel A., Arsenijevic Y. (2006) Lentiviral gene transfer of RPE65 rescues survival and function of cones in a mouse model of Leber congenital amaurosis. PLoS Med. 3, e347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Miyoshi H., Takahashi M., Gage F. H., Verma I. M. (1997) Stable and efficient gene transfer into the retina using an HIV-based lentiviral vector. Proc. Natl. Acad. Sci. U.S.A. 94, 10319–10323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Farjo R., Skaggs J., Quiambao A. B., Cooper M. J., Naash M. I. (2006) Efficient non-viral ocular gene transfer with compacted DNA nanoparticles. PLoS One 1, e38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kachi S., Oshima Y., Esumi N., Kachi M., Rogers B., Zack D. J., Campochiaro P. A. (2005) Nonviral ocular gene transfer. Gene Ther. 12, 843–851 [DOI] [PubMed] [Google Scholar]
- 39. Johnson C. J., Berglin L., Chrenek M. A., Redmond T. M., Boatright J. H., Nickerson J. M. (2008) Technical brief: subretinal injection and electroporation into adult mouse eyes. Mol. Vis. 14, 2211–2226 [PMC free article] [PubMed] [Google Scholar]
- 40. Maguire A. M., Simonelli F., Pierce E. A., Pugh E. N., Jr., Mingozzi F., Bennicelli J., Banfi S., Marshall K. A., Testa F., Surace E. M., Rossi S., Lyubarsky A., Arruda V. R., Konkle B., Stone E., Sun J., Jacobs J., Dell'Osso L., Hertle R., Ma J. X., Redmond T. M., Zhu X., Hauck B., Zelenaia O., Shindler K. S., Maguire M. G., Wright J. F., Volpe N. J., McDonnell J. W., Auricchio A., High K. A., Bennett J. (2008) Safety and efficacy of gene transfer for Leber's congenital amaurosis. N. Engl. J. Med. 358, 2240–2248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bainbridge J. W., Smith A. J., Barker S. S., Robbie S., Henderson R., Balaggan K., Viswanathan A., Holder G. E., Stockman A., Tyler N., Petersen-Jones S., Bhattacharya S. S., Thrasher A. J., Fitzke F. W., Carter B. J., Rubin G. S., Moore A. T., Ali R. R. (2008) Effect of gene therapy on visual function in Leber's congenital amaurosis. N. Engl. J. Med. 358, 2231–2239 [DOI] [PubMed] [Google Scholar]
- 42. Cideciyan A. V., Aleman T. S., Boye S. L., Schwartz S. B., Kaushal S., Roman A. J., Pang J. J., Sumaroka A., Windsor E. A., Wilson J. M., Flotte T. R., Fishman G. A., Heon E., Stone E. M., Byrne B. J., Jacobson S. G., Hauswirth W. W. (2008) Human gene therapy for RPE65 isomerase deficiency activates the retinoid cycle of vision but with slow rod kinetics. Proc. Natl. Acad. Sci. U.S.A. 105, 15112–15117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hauswirth W. W., Aleman T. S., Kaushal S., Cideciyan A. V., Schwartz S. B., Wang L., Conlon T. J., Boye S. L., Flotte T. R., Byrne B. J., Jacobson S. G. (2008) Treatment of Leber congenital amaurosis due to RPE65 mutations by ocular subretinal injection of adeno-associated virus gene vector: short-term results of a phase I trial. Hum. Gene Ther. 19, 979–990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Moiseyev G., Takahashi Y., Chen Y., Kim S., Ma J. X. (2008) RPE65 from cone-dominant chicken is a more efficient isomerohydrolase compared with that from rod-dominant species. J. Biol. Chem. 283, 8110–8117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Takahashi Y., Moiseyev G., Chen Y., Ma J. X. (2005) Identification of conserved histidines and glutamic acid as key residues for isomerohydrolase activity of RPE65, an enzyme of the visual cycle in the retinal pigment epithelium. FEBS Lett. 579, 5414–5418 [DOI] [PubMed] [Google Scholar]
- 46. Bradford M. M. (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72, 248–254 [DOI] [PubMed] [Google Scholar]
- 47. Crabb J. W., Chen Y., Goldflam S., West K., Kapron J. (1998) Methods for producing recombinant human cellular retinaldehyde-binding protein. Methods Mol. Biol. 89, 91–104 [DOI] [PubMed] [Google Scholar]
- 48. Jeon C. J., Strettoi E., Masland R. H. (1998) The major cell populations of the mouse retina. J. Neurosci. 18, 8936–8946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Gao H., Hollyfield J. G. (1992) Aging of the human retina. Differential loss of neurons and retinal pigment epithelial cells. Invest. Ophthalmol. Vis. Sci. 33, 1–17 [PubMed] [Google Scholar]
- 50. Ruiz A., Mark M., Jacobs H., Klopfenstein M., Hu J., Lloyd M., Habib S., Tosha C., Radu R. A., Ghyselinck N. B., Nusinowitz S., Bok D. (2012) Retinoid content, visual responses, and ocular morphology are compromised in the retinas of mice lacking the retinol-binding protein receptor, STRA6. Invest. Ophthalmol. Vis. Sci. 53, 3027–3039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Guex N., Peitsch M. C. (1997) SWISS-MODEL and the Swiss-PdbViewer: an environment for comparative protein modeling. Electrophoresis 18, 2714–2723 [DOI] [PubMed] [Google Scholar]
- 52. Takahashi Y., Moiseyev G., Chen Y., Farjo K., Nikolaeva O., Ma J. X. (2011) An enzymatic mechanism for generating the precursor of endogenous 13-cis-retinoic acid in the brain. FEBS J. 278, 973–987 [DOI] [PMC free article] [PubMed] [Google Scholar]