Abstract
trans-Sialidase (TS) enzymes catalyze the transfer of sialyl (Sia) residues from Sia(α2-3)Gal(β1-x)-glycans (sialo-glycans) to Gal(β1-x)-glycans (asialo-glycans). Aiming to apply this concept for the sialylation of linear and branched (Gal)nGlc oligosaccharide mixtures (GOS) using bovine κ-casein-derived glycomacropeptide (GMP) as the sialic acid donor, a kinetic study has been carried out with three components of GOS, i.e., 3′-galactosyl-lactose (β3′-GL), 4′-galactosyl-lactose (β4′-GL), and 6′-galactosyl-lactose (β6′-GL). This prebiotic GOS is prepared from lactose by incubation with suitable β-galactosidases, whereas GMP is a side-stream product of the dairy industry. The trans-sialidase from Trypanosoma cruzi (TcTS) was expressed in Escherichia coli and purified. Its temperature and pH optima were determined to be 25°C and pH 5.0, respectively. GMP [sialic acid content, 3.6% (wt/wt); N-acetylneuraminic acid (Neu5Ac), >99%; (α2-3)-linked Neu5Ac, 59%] was found to be an efficient sialyl donor, and up to 95% of the (α2-3)-linked Neu5Ac could be transferred to lactose when a 10-fold excess of this acceptor substrate was used. The products of the TcTS-catalyzed sialylation of β3′-GL, β4′-GL, and β6′-GL, using GMP as the sialic acid donor, were purified, and their structures were elucidated by nuclear magnetic resonance spectroscopy. Monosialylated β3′-GL and β4′-GL contained Neu5Ac connected to the terminal Gal residue; however, in the case of β6′-GL, TcTS was shown to sialylate the 3 position of both the internal and terminal Gal moieties, yielding two different monosialylated products and a disialylated structure. Kinetic analyses showed that TcTS had higher affinity for the GL substrates than lactose, while the Vmax and kcat values were higher in the case of lactose.
INTRODUCTION
trans-Sialidases (TS; EC 3.2.1.18) are unique enzymes that naturally perform the transfer of sialyl residues from one sialo-glycan to the terminal Gal residue of another asialo-glycan. These enzymes have been found mainly in members of the Trypanosoma genus, most notably in Trypanosoma cruzi and Trypanosoma brucei, the causative agents of Chagas' disease and sleeping sickness, respectively (1). Other examples are TS of Trypanosoma congolense (2) and bioengineered TS from Trypanosoma rangeli (3). TS are structurally related to bacterial and eukaryotic exo-sialidases and belong to glycoside hydrolase family 33 (http://www.cazy.org). T. cruzi trans-sialidase (TcTS) was the first TS identified (4) and remains the best studied. TcTS is glycosylphosphatidylinositol (GPI) anchored to the cell surface of T. cruzi cells (5), where its main physiological role is to scavenge sialic acid and transfer it to its extracellular mucins (6). This feature is important for host cell adhesion and subsequent host invasion (7), and in later stages it helps to evade and even compromise the host immune response (8). The genome sequence of T. cruzi revealed a large number of genes encoding both inactive and active TcTS isoforms (9), which emphasized its importance in general survival and pathogenicity of the pathogen. TrcTS611/2 is a DNA clone from T. cruzi encoding the active N-terminal catalytic protein domain but lacking the highly immunogenic C-terminal part, called shed acute-phase antigen (SAPA) repeats (10, 11). This clone was used to construct pTrcTS611/2 (12), which has been used in almost all subsequent biochemical and structural studies of heterologously produced TcTS.
The TcTS crystal structure has been reported (13), and subsequent mutational studies have resulted in the elucidation of its catalytic mechanism. TcTS-mediated trans-sialylation was shown to proceed via a ping-pong mechanism (14, 15). In the first step, a relatively long-lived sialo-enzyme intermediate is formed through a covalent bond to the nucleophile Tyr342 (16). Asp59 then acts as the acid/base catalyst (14), and nucleophilic attack of the hydroxyl group at C-3 of β-galactose (β-Gal) ultimately leads to coupling of sialic acid to this natural acceptor substrate and release of the sialylated product. Two highly flexible hydrophobic residues, Tyr119 and Trp312, are important in acceptor binding and mediate its correct positioning for efficient sialyl transfer through stacking interactions (13). Hydrolysis may occur in the absence of suitable β-Gal acceptor substrates, with water acting as the acceptor substrate and resulting in the release of sialic acid. The enzyme has a narrow and hydrophobic catalytic cleft which may help to exclude water from the catalytic center, minimizing hydrolysis and facilitating trans-glycosylation (13, 17, 18).
TcTS exhibits large substrate promiscuity; various glycoproteins, oligosaccharides, and even artificial compounds can be used as sialic acid donor substrates. It has strict regio-selectivity, however, for donor substrates possessing sialic acid bound via an (α2-3) linkage to a terminal β-Gal (19, 20); both N-acetylneuraminic acid (Neu5Ac) and N-glycolylneuraminic acid (Neu5Gc) can be transferred (20, 21). Various compounds possessing a terminal β-galactopyranose can be used as acceptor substrates, and due to its retaining mechanism, products formed by TcTS have a terminal Sia(α2-3)Gal(β1) unit (20). The many studies on the donor and acceptor substrate specificities of TcTS have been reviewed (1), and mutagenesis (22) and modeling studies (23, 24) have appeared.
Due to its importance in host cell invasion and pathogenicity, TcTS has attracted substantial medical attention, and great strides have been made to discover inhibitors that may serve as therapeutics against the parasite (1, 25). The unique sialic acid-transferring capabilities of TcTS also have been noticed by glycobiologists and biochemists and, using mainly 2-O-(4-methylumbelliferyl)-α-N-acetylneuraminic acid (4MU-Neu5Ac), 2-O-(p-nitrophenyl)-α-N-acetylneuraminic acid (pNP-Neu5Ac), and Neu5Ac(α2-3)lactose (3′-SL) as donor substrates, the enzyme has been widely exploited in the chemo-enzymatic synthesis of various sialylated glycoconjugates (1). Because of their anti-infective and immunostimulating properties (26, 27), as well as their possible role in cognitive development (28, 29), sialylated oligosaccharides, especially 3′-SL, have the strong interest of the dairy industry for applications in the infant food sector. To upgrade the TcTS-mediated production of 3′-SL from lactose and a suitable, low-cost sialyl donor substrate, several protocols have been reported and patented. Attention has been paid especially to the use of the glycoproteins fetuin (30) and κ-casein-derived glycomacropeptide (31, 32), both rich in (α2-3)-linked sialic acid and available in relatively large amounts at relatively low cost, as sialyl donor substrates.
One of the most successful prebiotics today is GOS, galactooligosaccharide mixtures of degrees of polymerization (DP) 2 to 8, prepared by incubation of lactose with specific β-galactosidases (33). Depending on the enzyme used, GOS contains an array of linear and branched (Gal)nGlc oligosaccharides with different substitution patterns (S. S. van Leeuwen, B. J. H. Kuipers, L. Dijkhuizen, and J. P. Kamerling, unpublished data). In view of the reported functions of both GOS and sialylated oligosaccharides, it is hypothesized that partial sialylation of GOS will generate mixtures of compounds that combine the different properties, meaning novel products with broad functionalities. In the context of our studies focused on the TcTS-mediated production of mixtures of GOS and sialylated GOS using Vivinal GOS as the sialyl acceptor substrate and bovine κ-casein-derived glycomacropeptide (GMP) as the sialyl donor substrate (34, 35), it is of the utmost importance to get insight into the efficiency and specificity of the (α2-3) sialylation of terminal Gal(β1-2), Gal(β1-3), Gal(β1-4), and Gal(β1-6) residues in GOS. In this study, we present kinetic details of the sialylation reaction of TcTS with three commercially available representative structures of GOS, human milk, and cow milk, namely, 3′-galactosyl-lactose (β3′-GL), 4′-galactosyl-lactose (β4′-GL), and 6′-galactosyl-lactose (β6′-GL).
MATERIALS AND METHODS
Bacterial strains, plasmids, and chemicals.
Escherichia coli BL21(DE3) (Invitrogen) and plasmid pTrcTS611/2, a gift of A. C. C. Frasch (Buenos Aires, Argentina) (12), were used for the heterologous expression of TcTS. 2-O-(4-Methylumbelliferyl)-α-N-acetylneuraminic acid (4MU-Neu5Ac), N-acetylneuraminyl(α2-3)lactose (3′-SL), N-acetylneuraminic acid (Neu5Ac), galactosyl(β1-3)lactose (β3′-GL), galactosyl(β1-4)lactose (β4′-GL), and galactosyl(β1-6)lactose (β6′-GL) (both Gal and Glc have the d configuration) were obtained from Carbosynth Ltd. (Berkshire, United Kingdom). 4-Methylumbelliferone and lactose were obtained from Sigma (St. Louis, MO). κ-Casein-derived glycomacropeptide (GMP) was provided by FrieslandCampina Research (Wageningen, The Netherlands).
TcTS production and purification.
E. coli BL21(DE3) carrying pTrcTS611/2 was grown overnight at 37°C with agitation (220 rpm) in Luria-Bertani (LB) broth containing 100 μg/ml ampicillin, diluted 1:100, and incubated under the same conditions until an optical density at 600 nm (OD600) of 0.6 to 0.8 was reached. Cells were induced to overexpress recombinant protein by adding isopropyl thio-β-d-galactopyranoside (0.1 mM), followed by incubation at 18°C with agitation (220 rpm) for 4 h. Cells were harvested by centrifugation (15 min, 5,000 × g) and frozen (−80°C) until needed. Lysis was achieved by resuspending the cell pellet in bacterial protein extraction reagent (B-PER; Thermo Scientific, Breda, The Netherlands) according to the manufacturer's protocol. Cell debris was removed by ultracentrifugation (20 min, 40,000 × g) at 4°C. The obtained cell extract then was subjected to Ni2+ affinity chromatography using His select (Sigma). After 1.5 h of binding at 4°C on a rotary shaker, the protein was consecutively washed with Tris-HCl buffer (50 mM, pH 8.0) containing NaCl (0.3 M) and imidazole (5 and 30 mM) prior to elution with 200 mM imidazole in the same buffer. The purified enzyme was washed and concentrated in Tris-HCl (50 mM, pH 8.0) using an Amicon Ultra 50K filter (Millipore, Amsterdam, The Netherlands). The purity of the enzyme was assessed on SDS-PAGE, and protein concentration was measured with Bradford reagent (Bio-Rad, Veenendaal, The Netherlands) and bovine serum albumin as a standard.
Enzyme kinetics.
Reaction mixtures of 100 μl containing 1 mM 4MU-Neu5Ac (donor), 1 mM lactose (acceptor), and 100 ng TcTS enzyme were incubated in a buffered solution for 10 min at various temperatures. Reactions were stopped by adding 95% dimethyl sulfoxide, and trans-sialylation activity was determined by quantitatively measuring the formation of 3′-SL using high-pH anion-exchange chromatography with pulsed amperometric detection (HPAEC-PAD; CarboPac PA-1). The optimal temperature of TcTS was determined by performing incubations between 15 and 50°C at pH 7.5. The optimal pH for trans-sialylation activity was measured within the pH range of 4.0 to 9.0, using 50 mM Na-citrate (pH 4.0 to 5.5), 50 mM HEPES (pH 6.0 to 7.0), and 50 mM Tris (pH 7.5 to 9.0) or a 1:1:1 mixture of these buffers at pH 4.0 to 9.0.
To determine kinetic parameters of TcTS with GMP and 4MU-Neu5Ac as donors and lactose as the acceptor substrate, 100 ng TcTS, 5 mM lactose, and various concentrations of donor substrates in 100 μl 50 mM Na-citrate (pH 5.0) were incubated for 15 min at 25°C. Reactions were stopped by dilution with dimethyl sulfoxide, and trans-sialylation activity was determined by quantitative measurement of the amount of 3′-SL using HPAEC-PAD (CarboPac PA-1).
Kinetic analyses of TcTS with β3′-GL, β4′-GL, and β6′-GL as acceptors were performed in 25 μl 50 mM Na-citrate (pH 5.0) containing 10 mM 4MU-Neu5Ac (donor), various concentrations of acceptor substrates, and 20 ng TcTS for 5 min at 25°C. trans-Sialylation activity was determined by quantifying 4-methylumbelliferone release using fluorescence spectroscopy on a Fluostar Galaxy instrument (BMG Laboratories) with excitation and emission at 390 nm and 450 nm, respectively. Reactions were quenched by diluting the reaction mixture 10-fold, such that the final mixture contained 50% methanol and 50 mM Tris, pH 9.0. The obtained activity values were plotted against the used substrate concentrations and followed Michaelis-Menten kinetics. Km and Vmax were determined by nonlinear regression analysis using SigmaPlot 12.0. Under the conditions used, hydrolysis was negligible, since no free Neu5Ac was detected by HPAEC-PAD.
High-pH anion-exchange chromatography analysis.
Analyses of oligosaccharides were carried out by HPAEC on a Dionex ICS-3000 system (Thermo Scientific) equipped with a CarboPac PA-1 guard column (50 by 4 mm) and a CarboPac PA-1 column (250 by 4 mm). For detection, a pulsed amperometric detector with a gold working electrode and an Ag/AgCl pH reference electrode were used. The system was run with a gradient of 30 to 600 mM sodium acetate in 0.1 M NaOH (1 ml/min). All chromatograms were analyzed using Chromeleon 6.8 chromatography data system software (Thermo Scientific).
Preparative isolation of isomeric monosialylated oligosaccharides was performed on a Dionex ICS-5000 system equipped with a CarboPac PA-1 guard column (50 by 4 mm) and a CarboPac PA-1 column (250 by 9 mm), using 36 mM sodium acetate in 0.1 M NaOH (3 ml/min) as the eluent (isocratic runs).
Analysis of sialic acid content and linkage type of κ-casein-derived GMP.
To determine the total sialic acid content of GMP, the sample was incubated with 0.1 M HCl for 1 h at 80°C, neutralized with an appropriate volume of 1 M NaOH, and subjected to HPAEC-PAD analysis. For quantification of released Neu5Ac and Neu5Gc, we made use of calibration curves of known concentrations of both sialic acids. Following the same analysis protocol, the ratio of (α2-3)- to (α2-6)-linked sialic acid in GMP was determined by comparing the release of sialic acid by linkage-type nonspecific sialidase A (Prozyme, Hayward, CA) and (α2-3)-specific sialidase S (Prozyme). Enzymatic incubations were carried out according to the manufacturer's protocols.
Isolation of sialylated galactosyl-lactose products by anion-exchange chromatography.
Reaction mixtures containing GMP [67.5 mg/ml; 5 mM (α2-3)-linked Neu5Ac], galactosyl-lactose (β3′-GL, β4′-GL, or β6′-GL; 2 mM), and TcTS (1 μg/ml) were incubated for 24 h at 25°C and pH 5.0. The progress of the reaction was monitored by analyzing samples on HPAEC-PAD. Incubation mixtures were passed over an Amicon Ultra 15-ml filter (10,000-nominal-molecular-weight-limit membrane) (Millipore) according to the manufacturer's protocol and applied to Carbograph solid-phase extraction (SPE) columns (300 mg; Grace, Breda, The Netherlands). After washing with 0.1% trifluoroacetic acid, oligosaccharide mixtures were eluted with 25% acetonitrile containing 0.1% trifluoroacetic acid. Combined eluents were evaporated under a stream of nitrogen, and residues were lyophilized from water. Finally, oligosaccharide mixtures were separated on a 1-ml Resource Q (GE Healthcare, Uppsala, Sweden) column attached to an Äkta Performance fast protein liquid chromatography (FPLC) system (GE Healthcare) using a gradient of 0 to 0.5 M NaCl at a flow rate of 1 ml/min. Sialylated oligosaccharides were isolated based on UV detection at 214 nm.
MALDI-TOF-MS.
Matrix-assisted laser desorption ionization–time-of-flight mass spectrometry (MALDI-TOF-MS) experiments were performed on an Axima mass spectrometer (Shimadzu Kratos Inc., Manchester, United Kingdom) operating with a nitrogen laser (337 nm, 3-ns pulse width) and an accelerating voltage of 20 kV. All measurements were recorded in the negative-ion mode, using the reflector mode for monosialylated galactosyl-lactoses and the linear mode for disialylated galactosyl-lactose. Samples (1 μl) were mixed in 1:1 ratios with 1 mg/ml 2′,4′,6′-trihydroxyacetophenone (THAP; Sigma) in 50% acetonitrile containing 10 mM ammonium citrate. Sample spots were vacuum dried to prevent crystal formation. Calibration was performed with native α-, β-, and γ-cyclodextrins (Fluka, Buchs, Switzerland) using norharmane (Sigma) as the matrix according to the manufacturer's protocol.
NMR spectroscopy.
All one-dimensional 1H NMR, two-dimensional 1H-1H, and two-dimensional 13C-1H correlation spectra were recorded at a probe temperature of 298 K on a Varian Inova 600 spectrometer (NMR Center, University of Groningen). Samples were exchanged twice with D2O (99.9 atom% D; Cambridge Isotope Laboratories, Inc., Andover, MA) with intermediate lyophilization and then dissolved in 650 μl D2O. Proton and carbon chemical shifts are expressed in ppm in reference to internal acetone (δ 1H 2.225; δ 13C 31.08). One-dimensional 600-MHz 1H NMR spectra were recorded with a 5,000-Hz spectral width at 16,000 complex data points, using a WET1D pulse to suppress the HOD signal. 1H-1H correlation spectroscopy (COSY) spectra were recorded in 256 increments at 4,000 complex data points with a spectral width of 5,000 Hz. 1H-1H total correlation spectroscopy (TOCSY) spectra were recorded using MLEV17 mixing sequences with 50- and 150-ms spin-lock times. 13C-1H heteronuclear single quantum coherence (HSQC) spectra were recorded with a width of 5,000 Hz in the t2 direction and 10,000 Hz in the t1 direction. 1H-1H rotating-frame Overhauser enhancement spectroscopy (ROESY) spectra with a mixing time of 300 ms were recorded in 200 increments of 4,000 complex data points with a spectral width of 5,000 Hz. All spectra were processed using MestReNova 5.3 (Mestrelabs Research SL, Santiago de Compostella, Spain) using Whittaker Smoother baseline correction.
RESULTS AND DISCUSSION
General characteristics of the produced recombinant TcTS in the transfer of Neu5Ac from 4MU-Neu5Ac to lactose.
Heterologously produced trans-sialidase (TcTS) was purified to near homogeneity by His-tagged affinity chromatography, showing a single band of the expected size of around 76 kDa on a reducing SDS-PAGE gel (calculated using the Clone Manager program). To verify earlier reports on the trans-sialidase activity of TcTS, including kinetic parameters, various incubations of the enzyme with 1 mM 4MU-Neu5Ac and 1 mM lactose, followed by N-acetylneuraminyl(α2-3)lactose (3′-SL) quantification by HPAEC-PAD, were carried out. The temperature optimum for TcTS activity was 25°C (Fig. 1A), which is similar to that previously reported for the enzyme heterologously produced in E. coli (36), while the native enzyme produced in trypomastigotes showed optimal activity at 13°C (20). The optimal pH for TcTS activity was pH 5.0 (Fig. 1B). Previous studies reported maximal TcTS activities between pHs 6 and 7 (37, 38), at neutral pH (36, 39), and at pH 7.9 (20). These differences may be explained by the different methods that were used to measure trans-sialylation activity (e.g., different substrates, buffers, and incubation temperatures). Glycosylation also may account for the difference in pH optima between TcTS produced in bacterial and that in eukaryotic cell lines, since the TcTS amino acid sequence contains two potential glycosylation sites, of which at least one was shown to be occupied after expression in lepidopteran cells (40). To exclude any effects of the chemical composition of the buffers on the enzyme, its activity over the range of pH 4.5 to 9.0 was measured in a cocktail composed of all three buffers used in a 1:1:1 ratio. The highest activity was observed at pH 5.0 to 5.5 (data not shown), excluding that the enzyme had a preference for one of the buffer components. TcTS is known to have a relatively low thermal stability (36). Indeed, preincubation of the enzyme for 30 min at a temperature of 40°C and higher resulted in strong loss of activity (Fig. 1C). The addition of 1 mg/ml of bovine serum albumin (BSA) to the enzyme solution improved the thermostability and resulted in an overall increase of trans-sialylation activity (Fig. 1C). Heterologously produced TcTS in its pure form previously proved to be labile, while its activity was retained in crude E. coli lysates (41). The presence of stabilizing proteins, such as BSA, may assist in proper folding of TcTS, resulting in the higher activity of the enzyme. Interestingly, BSA had no effect on TcTS activity when the enzyme was incubated with the κ-casein-derived glycomacropeptide (GMP) as the sialic acid donor (data not shown).
FIG 1.
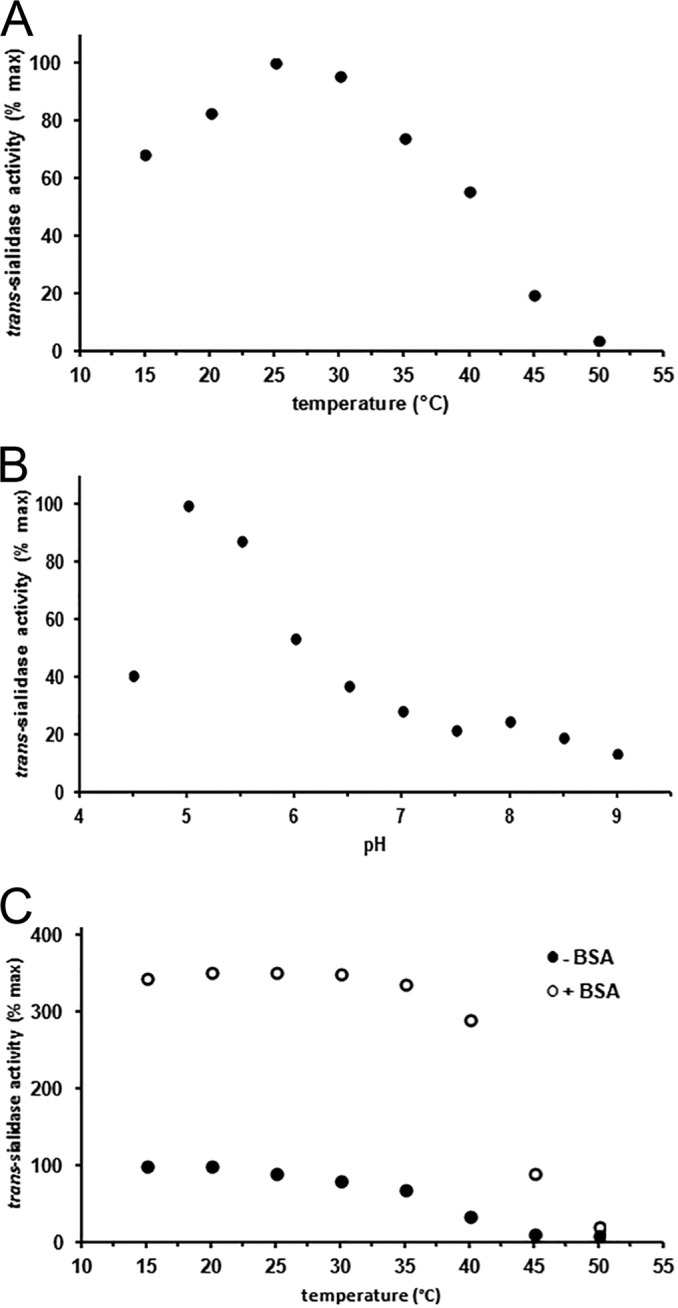
Incubations of 100-μl samples, containing 1 mM 4MU-Neu5Ac, 1 mM lactose, and 100 ng TcTS. The buffers used were Na-citrate (pH 4.5 to 5.5), HEPES (pH 6.0 to 7.0), and Tris-HCl (pH 7.5 to 9.0). Conversion analyses were carried out by HPAEC-PAD. All data represent averages from duplicate experiments, and the 100% value represents 1.0 μmol · min−1 · mg−1 (A) and 4.0 μmol · min−1 · mg−1 (B and C). (A) Temperature-dependent activity of TcTS was determined over the range of 15 to 50°C at pH 7.5. (B) Optimal pH for trans-sialidase activity of TcTS in the pH range 4.5 to 9.0 at 25°C. (C) Heat stability/residual activity of TcTS, determined by preincubating the enzyme at a range of 15 to 50°C for 30 min in the presence (open circles) or absence (closed circles) of 1 mg/ml bovine serum albumin (BSA); analysis was done at pH 5.0 and 25°C.
Sialic acid characteristics of the κ-casein-derived glycomacropeptide preparation.
GMP obtained by chymosin digestion of κ-casein was selected as the sialic acid donor for the decoration of GOS with sialic acid. The major O-glycans of GMP include Neu5Ac(α2-3)Gal(β1-3)GalNAc, Gal(β1-3)[Neu5Ac(α2-6)]GalNAc, and Neu5Ac(α2-3)Gal(β1-3)[Neu5Ac(α2-6)]GalNAc (42, 43, 44); only Neu5Ac(α2-3)-containing components will act as the donor. Total sialic acid analysis by HPAEC-PAD of the used GMP preparation yielded a content of 3.6% ± 0.04% (wt/wt), corresponding to 0.12 mmol/g dry matter, of which the vast majority (>99%) was N-acetylneuraminic acid (Neu5Ac), and the remaining fraction (<1%) was N-glycolylneuraminic acid (Neu5Gc). By comparing the amount of sialic acid released by the linkage-type nonspecific sialidase A and the (α2-3)-specific sialidase S, the percentage of (α2-3)-linked Neu5Ac in GMP was determined to be 59% (±2%).
Kinetic parameters for the sialylation of lactose with GMP as the sialic acid donor.
Various concentrations of GMP and lactose were incubated with TcTS at 25°C and pH 5.0, and the product 3′-SL was quantified by HPAEC-PAD to determine the maximal sialic acid transfer efficiency. When using a 10-fold excess of lactose (10 mM) to donor [GMP, 13.5 mg/ml; 1 mM (α2-3)-linked Neu5Ac], up to 95% of the (α2-3)-linked Neu5Ac could be transferred to the acceptor. Conversely, using a 10-fold excess of donor [GMP, 135 mg/ml; 10 mM (α2-3)-linked Neu5Ac] resulted in a maximal sialylation of acceptor substrate (1 mM lactose) of 100%. TcTS displays Michaelis-Menten kinetics; the affinity for GMP was determined with various donor concentrations and a fixed concentration of lactose (acceptor). For comparison, 4MU-Neu5Ac was included in the kinetic analysis. The affinity and Vmax of TcTS were higher for GMP than for the artificial substrate 4MU-Neu5Ac, with Km values of 0.18 mM (α2-3)-linked Neu5Ac in GMP and 1.65 mM in 4MU-Neu5Ac and Vmax values of 3.20 and 1.48 μmol · min−1 · mg−1, respectively. Earlier reported Km values were 0.8 and 2.5 mM for 3′-SL and 4MU-Neu5Ac as donors, respectively (20).
Galactosyl-lactoses as acceptor substrates in the trans-sialylation reaction using GMP as the donor.
3′-Galactosyl-lactose [Gal(β1-3)Gal(β1-4)Glc; β3′-GL], 4′-galactosyl-lactose [Gal(β1-4)Gal(β1-4)Glc; β4′-GL], and 6′-galactosyl-lactose [Gal(β1-6)Gal(β1-4)Glc; β6′-GL] were tested as acceptor substrates in 100-μl reaction mixtures containing 2 mM acceptor, 5 mM (α2-3)-linked Neu5Ac (67.5 mg/ml GMP), and 500 ng TcTS (25°C, pH 5.0). HPAEC-PAD analysis of the three incubation mixtures after 1 h showed in each case, besides the GL acceptor, the clear presence of a new peak with a longer retention time than that of the GL (Fig. 2). The conversion of the acceptor substrates after 1 h was estimated to be between 20 and 25% (based on the disappearance of the acceptor using a calibration curve). The three fractions were isolated from the incubation mixtures via anion-exchange chromatography on Resource Q and analyzed by MALDI-TOF-MS. All three fractions showed a pseudomolecular ion [M-H]− at m/z = 795.26, corresponding to monosialylated (Neu5Ac) GL (Fig. 3).
FIG 2.
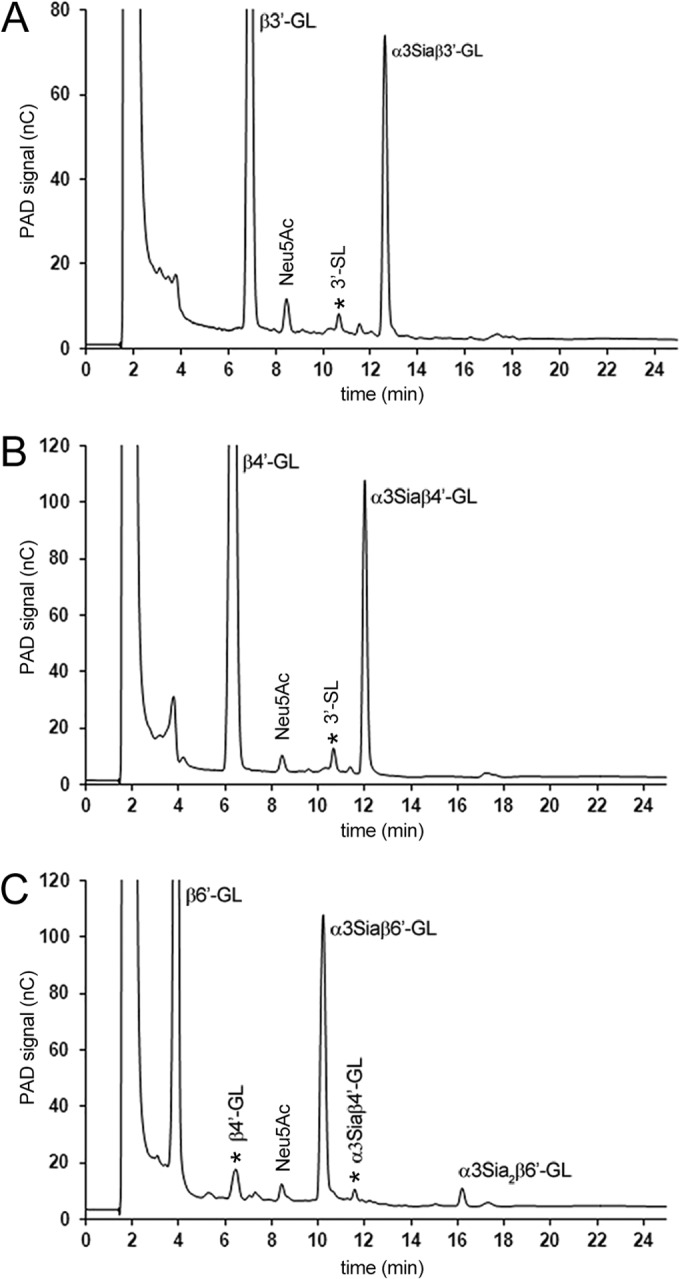
HPAEC-PAD profiles of TcTS reaction mixtures with β′3-GL (t = 7 min) (A), β′4-GL (t = 6 min) (B), and β′6-GL (t = 4 min) (C) as acceptor substrates and GMP as the sialic acid donor after 1 h of incubation at pH 5.0 and 25°C. All three reactions show a single main sialylated product with retention times of 12.5, 12.0, and 10.5 min for α3Siaβ′3-GL, α3Siaβ′4-GL, and α3Siaβ′6-GL, respectively. In the case of β′6-GL, a disialylated product (α3Sia2β′6-GL) was observed at a retention time of 16 min. Contaminants are indicated by an asterisk.
FIG 3.
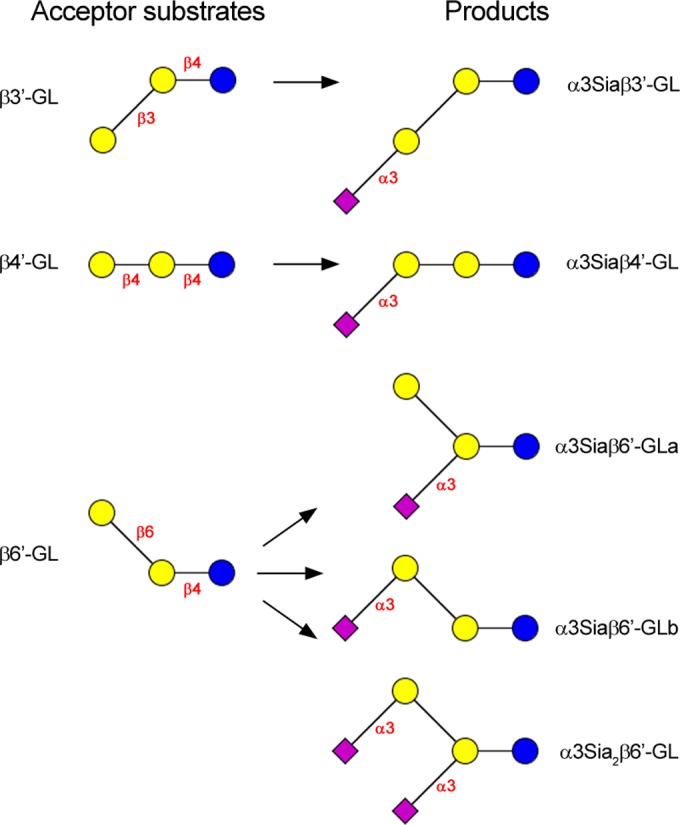
Schematic presentation of the (α2-3)-sialylation of galactosyl(β1-3)lactose (β3′-GL), galactosyl(β1-4)lactose (β4′-GL), and galactosyl(β1-6)lactose (β6′-GL) using heterologously produced trans-sialidase. Coding system: glucose (Glc), blue circle; galactose (Gal), yellow circle; N-acetylneuraminic acid (Neu5Ac), pink square.
Closer inspection of the HPAEC profile of the β6′-GL incubation showed a second new and smaller peak at a higher retention time than that of the monosialylated β6′-GL (Fig. 2C). Therefore, the incubations of β3′-GL, β4′-GL, and β6′-GL with GMP and TcTS were repeated and monitored for 24 h. In the case of β3′-GL and β4′-GL, no additional product formation was seen, but in the case of β6′-GL the second peak had increased. Subsequent separation of the β6′-GL incubation mixture on Resource Q (see Fig. S1 in the supplemental material) and isolation afforded two charged fractions in the mono and disialo range (molar ratio, 70:30; based on the UV detection of C=O in Neu5Ac at 214 nm, taking into account the number of Neu5Ac units [one or two] in the detected molecules). MALDI-TOF MS analysis of the fraction of higher retention time revealed a pseudomolecular ion, [M-H]−, at m/z = 1,086.36, in accordance with a disialylated β6′-GL component (Fig. 3). Such a double sialylation has been described earlier for Gal(β1-6)Gal(β1-OMe) using TcTS and αNeu5Ac p-nitrophenyl glycoside as the donor (45), illustrating that in specific structural situations, TcTS also is able to transfer (α2-3)-linked Neu5Ac to internal Gal residues. Based on the latter data, it is logical to assume that the product of higher retention time should be Neu5Ac(α2-3)Gal(β1-6)[Neu5Ac(α2-3)]Gal(β1-4)Glc, whereas the monosialylated β6′-GL fraction should contain two products, i.e., Neu5Ac(α2-3)Gal(β1-6)Gal(β1-4)Glc and Gal(β1-6)[Neu5Ac(α2-3)]Gal(β1-4)Glc (Fig. 3). Interestingly, these results are in agreement with the recent finding that Gal(β1-O(CH2)7CH3) and Gal(β1-4)GlcNAc(β1-O(CH2)7CH3, modified at Gal C-2 or C-4 (H, F, OCH3, NH2), greatly diminished or even prevented sialylation with TcTS and α-Neu5Ac p-nitrophenyl glycoside as the donor, while those modified at Gal C-6 (H, F, OCH3, and NH2) were well tolerated as acceptor substrates (25).
NMR analysis of sialylated β3′-GL, β4′-GL, and β6′-GL.
For a more detailed structural analysis of the products by NMR spectroscopy, suitable amounts of the various sialylated galactosyl-lactoses were prepared. In the following discussion of the NMR data, the sequence of each GL is represented by C-B-A, with C as the terminal Gal unit, B as the internal Gal unit, and A as the reducing Glc unit; D stands for Neu5Ac(α2-3).
The 1H NMR spectrum of monosialylated β3′-GL (Table 1 and Fig. 3; also see Fig. S2 in the supplemental material) showed anomeric signals at δ 5.223 (Aα H-1), δ 4.664 (Aβ H-1), δ 4.513 (B H-1), and δ 4.689 (C H-1). The D H-3a and H-3e signals at δ 1.802 and δ 2.764, respectively, are indicative of a Neu5Ac(α2-3) residue (46). The 1H NMR spectrum is identical to that reported earlier for Neu5Ac(α2-3)Gal(β1-3)Gal(β1-4)Glc (α3Siaβ3′-GL), found in the acidic fraction of colostrum/milk of several mammalian species, including Bactrian camel (47), red kangaroo (48), and koala (49).
TABLE 1.
1H chemical shifts of structural-reporter-group signals of monosaccharide residues present in the galactosyl-lactoses (C-B-A) β3′-GL, β4′-GL, and β6′-GL and their sialylated (D) products
| H atom | Shift fora: |
|||||||
|---|---|---|---|---|---|---|---|---|
| β3′-GL | α3Siaβ3′-GL | β4′-GL | α3Siaβ4′-GL | β6′-GL | α3Siaβ6′-GLa | α3Siaβ6′-GLb | α3Sia2β6′-GL | |
| Aα-1 | 5.226 | 5.223 | 5.225 | 5.220 | 5.223 | 5.217 | 5.228 | 5.222 |
| Aβ-1 | 4.667 | 4.664 | 4.664 | 4.663 | 4.667 | 4.662 | 4.660 | 4.656 |
| B-1 | 4.513 | 4.513 | 4.486 | 4.473 | 4.483 | 4.535 | 4.462 | 4.545 |
| C-1 | 4.614 | 4.689 | 4.603 | 4.660 | 4.460 | 4.470 | 4.528 | 4.514 |
| B-3 | 3.84b | ND | 3.77b | ND | 3.66 | 4.125 | 3.67 | 4.126 |
| C-3 | 3.67b | 4.114 | 3.66b | 4.107 | 3.67 | 3.66 | 4.100 | 4.093 |
| B-4 | 4.198 | 4.192 | 4.193 | 4.204 | 3.940 | 3.986 | 3.98 | 3.997 |
| C-4 | 3.922 | 3.950 | 3.904 | 3.931 | 3.974 | 3.914 | 3.95 | 3.942 |
| B-6a | 3.81b | ND | 3.80b | ND | 4.079 | 4.037 | 4.073 | 4.044 |
| B-6b | 3.76b | ND | 3.77b | ND | 3.93 | 3.92 | 3.93 | 3.90 |
| D-3a | 1.802 | 1.796 | 1.810 | 1.815 | 1.812 | |||
| D-3e | 2.764 | 2.762 | 2.756 | 2.755 | 2.752 | |||
| D-NAc | 2.029 | 2.029 | 2.030 | 2.031 | 2.028 | |||
Values are in ppm relative to the signal of internal acetone (δ 2.225). Shifts were determined at 298 K, and D2O was used. ND, not determined.
Chemical shifts derived from two-dimensional NMR experiments (our unpublished data).
In the 1H NMR spectrum of monosialylated β4′-GL (Table 1 and Fig. 3; also see Fig. S3 in the supplemental material), anomeric signals are observed at δ 5.220 (Aα H-1), δ 4.663 (Aβ H-1), δ 4.473 (B H-1), and δ 4.660 (C H-1). The Neu5Ac(α2-3) residue is reflected by the D H-3a and H-3e signals at δ 1.796 and δ 2.762, respectively (46). Going from β4′-GL to monosialylated β4′-GL (Table 1; also see Fig. S3), the sialylation of O-3 of the terminal Gal residue in β4′-GL is evident from the strong downfield shift of C H-3 (δ 3.66→δ 4.107). Summarizing the NMR and MS data, the structure of monosialylated β4′-GL is Neu5Ac(α2-3)Gal(β1-4)Gal(β1-4)Glc (α3Siaβ4′-GL). β4′-GL had been tested earlier as an acceptor substrate, showing incorporation of Neu5Ac, but no structural and kinetic details have been presented (20).
The 1H NMR spectrum of monosialylated β6′-GL (Resource Q fraction 1) showed anomeric doublets at δ 5.228, δ 5.218, δ 4.665, δ 4.659, δ 4.539, δ 4.526, δ 4.472, and δ 4.460, indicating the presence of more distinct monosaccharide units than can be explained by a single monosialylated trisaccharide structure. Indeed, using an isocratic HPAEC elution program, the monosialylated β6′-GL product could be separated on CarboPac PA-1 into two components (peak ratio, 55:45), monosialylated β6′-GLa (fraction 1a) and monosialylated β6′-GLb (fraction 1b). Both components were isolated via HPAEC for further NMR analysis.
The 1H NMR spectrum of monosialylated β6′-GLa (HPAEC fraction 1a) (Table 1 and Fig. 3; also see Fig. S4B in the supplemental material) showed anomeric signals at δ 5.217 (Aα H-1), δ 4.662 (Aβ H-1), δ 4.535 (B H-1), and δ 4.470 (C H-1). The Neu5Ac H-3a and H-3e signals at δ 1.810 and δ 2.756, respectively, confirmed the presence of a Neu5Ac(α2-3) residue (46). Using two-dimensional 1H-1H COSY, 1H-1H TOCSY, and 1H-1H ROESY spectra, in combination with two-dimensional 13C-1H HSQC spectra, all 1H and 13C chemical shifts could be assigned (see Table S1 in the supplemental material) and interpreted, affording the structure Gal(β1-6)[Neu5Ac(α2-3)]Gal(β1-4)Glc (α3Siaβ6′-GLa). See the supplemental material for a detailed description of the NMR analysis.
In the 1H NMR spectrum of monosialylated β6′-GLb (HPAEC fraction 1b) (Table 1 and Fig. 3; see Fig. S4C in the supplemental material), anomeric signals are detected at δ 5.228 (Aα H-1), δ 4.660 (Aβ H-1), δ 4.528 (C H-1), and δ 4.462 (B H-1). The Neu5Ac H-3a and H-3e signals at δ 1.815 and δ 2.755, respectively, confirmed the presence of a Neu5Ac(α2-3) residue (46). Using two-dimensional NMR spectroscopy as carried out for α3Siaβ6′-GLa, all 1H and 13C chemical shifts could be assigned (see Table S1) and interpreted, leading to the structure Neu5Ac(α2-3)Gal(β1-6)Gal(β1-4)Glc (α3Siaβ6′-GLb). See the supplemental material for a detailed description of the NMR analysis.
The 1H NMR spectrum of disialylated β6′-GL (Resource Q fraction 2) (Table 1 and Fig. 3; also see Fig. S4D in the supplemental material) showed anomeric signals at δ 5.222 (Aα H-1), δ 4.656 (Aβ H-1), δ 4.514 (C H-1), and δ 4.545 (B H-1). The Neu5Ac H-3a and H-3e signals at δ 1.812 and δ 2.752, respectively, with twice the intensity of α3Siaβ6′-GLa and α3Siaβ6′-GLb, confirmed the presence of two Neu5Ac(α2-3) residues (46). Using two-dimensional NMR spectroscopy, as carried out for the monosialylated β6′-GL components, all 1H and 13C chemical shifts were determined (see Table S1) and interpreted, yielding the structure Neu5Ac(α2-3)Gal(β1-6)[Neu5Ac(α2-3)]Gal(β1-4)Glc (α3Sia2β6′-GL). See the supplemental material for a detailed description of the NMR analysis.
Kinetic parameters for the sialylation of galactosyl-lactoses with 4MU-Neu5Ac as the sialic acid donor.
To gain further insight into TcTS acceptor specificity in relation to the use of GOS as an acceptor, the kinetic properties of the enzyme with the three galactosyl-lactose acceptor substrates, β3′-GL, β4′-GL, and β6′-GL, with 4MU-Neu5Ac as the donor substrate, were determined. The trans-sialylation activity was quantified by measuring the release of 4MU via fluorescence spectroscopy. Under the chosen conditions (see Materials and Methods), hydrolysis activity was negligible, since no free sialic acid was detected by HPAEC-PAD. All reactions followed Michaelis-Menten kinetics for trans-sialylation activity. The calculated Km values were much lower for β3′-GL, β4′-GL, and β6′-GL than for lactose (Table 2). Conversely, the Vmax determined for lactose was higher than that determined for β3′-GL, β4′-GL, and β6′-GL (Table 2). The kcat value for lactose, being similar to that reported previously (15), was higher than the kcat values for the GL substrates. The higher affinity of the GL substrates compared to that for lactose suggests stronger binding of the former to TcTS, e.g., with stronger and/or more interactions with amino acid residues in the acceptor binding site. Because of its higher kcat value, the catalytic reaction is more efficient in the case of lactose. Because of the short incubation time in these experiments, the disialylation of β6′-GL still can be neglected.
TABLE 2.
Kinetic parameters of TcTS with 4MU-Neu5Ac as donor substrate and β3′-GL, β4′-GL, β6′-GL, and lactose as acceptor substratesa
| Acceptor substrate | Km (mM) | Vmax (μmol · min−1 · mg−1) | kcat (s−1) | kcat/Km (mM−1 · s−1) |
|---|---|---|---|---|
| 3′-Galactosyl-lactose | 0.06 | 13.9 | 3.7 | 62.0 |
| 4′-Galactosyl-lactose | 0.32 | 14.0 | 3.4 | 10.5 |
| 6′-Galactosyl-lactose | 0.13 | 12.5 | 3.0 | 23.3 |
| Lactose | 0.79 | 22.9 | 4.8 | 6.1 |
The data represent averages from triplicate experiments (for details, see Materials and Methods).
Conclusions.
The recombinant trans-sialidase enzyme TcTS is capable of transferring sialic acid from GMP to β3′-GL, β4′-GL, and β6′-GL substrates. With β6′-GL, this resulted in the formation of three differently sialylated products, α3Siaβ6′-GLa, α3Siaβ6′-GLb, and α3Sia2β6′-GL. The finding of the latter three products makes it clear that the β6′-GL substrate has the flexibility for the enzyme to couple sialic acid to the 3 positions of both galactose moieties. In β3′-GL, the O-3 position of the internal Gal residue is already substituted, yielding α3Siaβ3′-GL, with Neu5Ac only coupled at the terminal Gal unit. In the case of β4′-GL, the three-dimensional structure most likely leads to steric hindrance in the carbohydrate-enzyme interaction for sialylation of the internal Gal residue, yielding α3Siaβ4′-GL with Neu5Ac only coupled at the terminal Gal unit. 2′-Galactosyl-lactose (β2′-GL) was not available for testing, but it has been demonstrated that incubation of Gal(β1-2)Gal(β1-6)[Gal(β1-4)]GlcNAc(β1-SPh) with TcTS and αNeu5Ac p-nitrophenyl glycoside afforded Neu5Ac(α2-3)Gal(β1-2)Gal(β1-6)[Neu5Ac(α2-3)Gal(β1-4)]GlcNAc(β1-SPh) (50). Interestingly, the TcTS-catalyzed selective transfer of Neu5Ac from 3′-SL as the donor led to the sialylation of only one terminal Gal residue in Gal(β1-3)[Gal(β1-2)]Gal(β1-OBn), yielding Neu5Ac(α2-3)Gal(β1-3)[Gal(β1-2)]Gal(β1-OBn) (51, 52).
The TcTS enzyme was shown to have higher affinity for all three oligosaccharides than lactose, while the catalytic efficiency was higher for lactose. It is clear that enzymatic synthesis of sialylated galacto-oligosaccharides using TcTS will lead to a large ensemble of mono- and disialylated compounds, and that GMP, a waste whey product of cheese production, is an excellent sialyl donor that is abundantly available and relatively cheap. The data presented here provide a firm basis for our research aiming to produce GOS (acceptor) decorated with sialic acid using bovine κ-casein-derived GMP as the donor in TcTS-catalyzed reactions (34, 35; M. H. Wilbrink, G. A. ten Kate, S. S. van Leeuwen, P. Sanders, G. J. Gerwig, E. Sallomons, J. A. Hage, L. Dijkhuizen, and J. P. Kamerling, unpublished data).
Supplementary Material
ACKNOWLEDGMENTS
We thank A. C. C. Frasch (Universidad Nacional General San Martin, Buenos Aires, Argentina) for the gift of plasmid pTrcTS611/1.
This project was jointly financed by the European Union, European Regional Development Fund and The Ministry of Economic Affairs, Agriculture and Innovation, Peaks in the Delta, the Municipality of Groningen, the Provinces of Groningen, Fryslân, and Drenthe, as well as by the Dutch Carbohydrate Competence Center (CCC WP6).
Footnotes
Published ahead of print 25 July 2014
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AEM.01465-14.
REFERENCES
- 1.Schauer R, Kamerling JP. 2011. The chemistry and biology of trypanosomal trans-sialidases: virulence factors in Chagas disease and sleeping sickness. ChemBioChem 12:2246–2264. 10.1002/cbic.201100421 [DOI] [PubMed] [Google Scholar]
- 2.Tiralongo E, Schrader S, Lange H, Lemke H, Tiralongo J, Schauer R. 2003. Two trans-sialidase forms with different sialic acid transfer and sialidase activities from Trypanosoma congolense. J. Biol. Chem. 278:23301–23310. 10.1074/jbc.M212909200 [DOI] [PubMed] [Google Scholar]
- 3.Paris G, Ratier L, Amaya MF, Nguyen T, Alzari PM, Frasch ACC. 2005. A sialidase mutant displaying trans-sialidase activity. J. Mol. Biol. 345:923–934. 10.1016/j.jmb.2004.09.031 [DOI] [PubMed] [Google Scholar]
- 4.Previato JO, Andrade AFB, Pessolani MCV, Mendonça-Previato L. 1985. Incorporation of sialic acid into Trypanosoma cruzi macromolecules. A proposal for a new metabolic route. Mol. Biochem. Parasitol. 16:85–96. 10.1016/0166-6851(85)90051-9 [DOI] [PubMed] [Google Scholar]
- 5.Agusti R, Couto AS, Campetella OE, Frasch ACC, de Lederkremer RM. 1997. The trans-sialidase of Trypanosoma cruzi is anchored by two different lipids. Glycobiology 7:731–735. 10.1093/glycob/7.6.731 [DOI] [PubMed] [Google Scholar]
- 6.Schenkman S, Ferguson MAJ, Heise N, Cardoso de Almeida ML, Mortara RA, Yoshida N. 1993. Mucin-like glycoproteins linked to the membrane by glycosylphosphatidylinositol anchor are the major acceptors of sialic acid in a reaction catalyzed by trans-sialidase in metacyclic forms of Trypanosoma cruzi. Mol. Biochem. Parasitol. 59:293–303. 10.1016/0166-6851(93)90227-O [DOI] [PubMed] [Google Scholar]
- 7.Schenkman S, Eichinger D. 1993. Trypanosoma cruzi trans-sialidase and cell invasion. Parasitol. Today 9:218–222. 10.1016/0169-4758(93)90017-A [DOI] [PubMed] [Google Scholar]
- 8.Tomlinson S, Raper J. 1998. Natural human immunity to trypanosomes. Parasitol. Today 14:354–359. 10.1016/S0169-4758(98)01295-2 [DOI] [PubMed] [Google Scholar]
- 9.El-Sayed NM, Myler PJ, Bartholomeu DC, Nilsson D, Aggarwal G, Tran AN, Ghedin E, Worthey EA, Delcher AL, Blandin G, Westenberger SJ, Caler E, Cerqueira GC, Branche C, Haas B, Anupama A, Arner E, Aslund L, Attipoe P, Bontempi E, Bringaud F, Burton P, Cadag E, Campbell DA, Carrington M, Crabtree J, Darban H, da Silveira JF, de Jong P, Edwards K, Englund PT, Fazelina G, Feldblyum T, Ferella M, Frasch AC, Gull K, Horn D, Hou L, Huang Y, Kindlund E, Klingbeil M, Kluge S, Koo H, Lacerda D, Levin MJ, Lorenzi H, Louie T, Machado CR, McCulloch R, McKenna A, Mizuno Y, Mottram JC, Nelson S, Ochaya S, Osoegawa K, Pai G, Parsons M, Pentony M, Pettersson U, Pop M, Ramirez JL, Rinta J, Robertson L, Salzberg SL, Sanchez DO, Seyler A, Sharma R, Shetty J, Simpson AJ, Sisk E, Tammi MT, Tarleton R, Teixeira S, Van Aken S, Vogt C, Ward PN, Wickstead B, Wortman J, White O, Fraser CM, Stuart KD, Andersson B. 2005. The genome sequence of Trypanosoma cruzi, etiologic agent of Chagas disease. Science 309:409–415. 10.1126/science.1112631 [DOI] [PubMed] [Google Scholar]
- 10.Parodi AJ, Pollevick GD, Mautner M, Buschiazzo A, Sanchez DO, Frasch ACC. 1992. Identification of the gene(s) coding for the trans-sialidase of Trypanosoma cruzi. EMBO J. 11:1705–1710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cremona ML, Sánchez DO, Frasch ACC, Campetella O. 1995. A single tyrosine differentiates active and inactive Trypanosoma cruzi trans-sialidases. Gene 160:123–128. 10.1016/0378-1119(95)00175-6 [DOI] [PubMed] [Google Scholar]
- 12.Buschiazzo A, Campetella O, Frasch ACC. 1997. Trypanosoma rangeli sialidase: cloning, expression and similarity to T. cruzi trans-sialidase. Glycobiology 7:1167–1173. 10.1093/glycob/7.8.1167 [DOI] [PubMed] [Google Scholar]
- 13.Buschiazzo A, Amaya MF, Cremona ML, Frasch AC, Alzari PM. 2002. The crystal structure and mode of action of trans-sialidase, a key enzyme in Trypanosoma cruzi pathogenesis. Mol. Cell 10:757–768. 10.1016/S1097-2765(02)00680-9 [DOI] [PubMed] [Google Scholar]
- 14.Amaya MF, Watts AG, Damager I, Wehenkel A, Nguyen T, Buschiazzo A, Paris G, Frasch AC, Withers SG, Alzari PM. 2004. Structural insights into the catalytic mechanism of Trypanosoma cruzi trans-sialidase. Structure 12:775–784. 10.1016/j.str.2004.02.036 [DOI] [PubMed] [Google Scholar]
- 15.Damager I, Buchini S, Amaya MF, Buschiazzo A, Alzari P, Frasch AC, Watts A, Withers SG. 2008. Kinetic and mechanistic analysis of Trypanosoma cruzi trans-sialidase reveals a classical ping-pong mechanism with acid/base catalysis. Biochemistry 47:3507–3512. 10.1021/bi7024832 [DOI] [PubMed] [Google Scholar]
- 16.Watts AG, Damager I, Amaya ML, Buschiazzo A, Alzari P, Frasch AC, Withers SG. 2003. Trypanosoma cruzi trans-sialidase operates through a covalent sialyl-enzyme intermediate: Tyrosine is the catalytic nucleophile. J. Am. Chem. Soc. 125:7532–7533. 10.1021/ja0344967 [DOI] [PubMed] [Google Scholar]
- 17.Buschiazzo A, Tavares GA, Campetella O, Spinelli S, Cremona ML, París G, Amaya MF, Frasch ACC, Alzari PM. 2000. Structural basis of sialyltransferase activity in trypanosomal sialidases. EMBO J. 19:16–24. 10.1093/emboj/19.1.16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Demir O, Roitberg AE. 2009. Modulation of catalytic function by differential plasticity of the active site: case study of Trypanosoma cruzi trans-sialidase and Trypanosoma rangeli sialidase. Biochemistry 48:3398–3406. 10.1021/bi802230y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vandekerckhove F, Schenkman S, Pontes de Carvalho L, Tomlinson S, Kiso M, Yoshida M, Hasegawa A, Nussenzweig V. 1992. Substrate specificity of the Trypanosoma cruzi trans-sialidase. Glycobiology 2:541–548. 10.1093/glycob/2.6.541 [DOI] [PubMed] [Google Scholar]
- 20.Scudder P, Doom JP, Chuenkova M, Manger ID, Pereira MEA. 1993. Enzymatic characterization of β-d-galactoside α2,3-trans-sialidase from Trypanosoma cruzi. J. Biol. Chem. 268:9886–9891 [PubMed] [Google Scholar]
- 21.Agustí R, Giorgi ME, de Lederkremer RM. 2007. The trans-sialidase from Trypanosoma cruzi efficiently transfers α-(2→3)-linked N-glycolylneuraminic acid to terminal β-galactosyl units. Carbohydr. Res. 342:2465–2469. 10.1016/j.carres.2007.07.018 [DOI] [PubMed] [Google Scholar]
- 22.Paris G, Cremona ML, Amaya MF, Buschiazzo A, Giambiagi S, Frasch ACC, Alzari PM. 2001. Probing molecular function of trypanosomal sialidases: Single point mutations can change substrate specificity and increase hydrolytic activity. Glycobiology 11:305–311. 10.1093/glycob/11.4.305 [DOI] [PubMed] [Google Scholar]
- 23.Pierdominici-Sottile G, Roitberg AE. 2011. Proton transfer facilitated by ligand binding. An energetic analysis of the catalytic mechanism of Trypanosoma cruzi trans-sialidase. Biochemistry 50:836–842. 10.1021/bi101648z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mitchell FL, Neres J, Ramraj A, Raju RK, Hillier IH, Vincent MA, Bryce RA. 2013. Insights into the activity and specificity of Trypanosoma cruzi trans-sialidase from molecular dynamics simulations. Biochemistry 52:3740–3751. 10.1021/bi301112p [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harrison JA, Kartha KPR, Fournier EJL, Lowary TL, Malet C, Nilsson UJ, Hindsgaul O, Schenkman S, Naismith JH, Field RA. 2011. Probing the acceptor substrate binding site of Trypanosoma cruzi trans-sialidase with systematically modified substrates and glycoside libraries. Org. Biomol. Chem. 9:1653–1660. 10.1039/c0ob00826e [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bode L. 2006. Recent advances on structure, metabolism, and function of human milk oligosaccharides. J. Nutr. 136:2127–2130 [DOI] [PubMed] [Google Scholar]
- 27.Boehm G, Stahl B. 2007. Oligosaccharides from milk. J. Nutr. 137:847S–849S [DOI] [PubMed] [Google Scholar]
- 28.Wang B, McVeagh P, Petocz P, Brand-Miller J. 2003. Brain ganglioside and glycoprotein sialic acid in breastfed compared with formula-fed infants. Am. J. Clin. Nutr. 78:1024–1029 [DOI] [PubMed] [Google Scholar]
- 29.Wang B, Yu B, Karim M, Hu H, Sun Y, McGreevy P, Petocz P, Held S, Brand-Miller J. 2007. Dietary sialic acid supplementation improves learning and memory in piglets. Am. J. Clin. Nutr. 85:561–569 [DOI] [PubMed] [Google Scholar]
- 30.Lee SG, Shin DH, Kim BG. 2002. Production of sialyloligosaccharides by trans-sialidase catalyzed reaction using fetuin as a sialic acid donor. Enzyme Microb. Technol. 31:742–746. 10.1016/S0141-0229(02)00212-0 [DOI] [Google Scholar]
- 31.Pelletier M, Barker WA, Hakes DJ, Zopf DA. 16 March 2004. Methods for producing sialyloligosaccharides in a dairy source. US patent 6706497B2
- 32.Holck J, Larsen DM, Michalak M, Li H, Kjaerulff L, Kirpekar F, Gotfredsen CH, Forssten S, Ouwehand AC, Mikkelsen JD, Meyer AS. 2014. Enzyme catalysed production of sialylated human milk oligosaccharides and galactooligosaccharides by Trypanosoma cruzi trans-sialidase. New Biotechnol. 31:156–165. 10.1016/j.nbt.2013.11.006 [DOI] [PubMed] [Google Scholar]
- 33.Van Leusen E, Torringa E, Groenink P, Kortleve P, Geene R, Schoterman M, Klarenbeek B. 2014. Industrial applications of galacto-oligosaccharides, p 470–491 In Moreno FJ, Sanz ML. (ed), Food oligosaccharides: production, analysis and bioactivity. Wiley-Blackwell, Weinheim, Germany [Google Scholar]
- 34.Wilbrink MH, ten Kate GA, Sanders P, Sallomons E, Klarenbeek B, Hage H, van Vuure CA, Verdonk JMAJ, Dijkhuizen L, Kamerling JP. 2013. Enzymatic decoration of galacto-oligosaccharides with sialic acid, p 246 Abstr. 10th Carbohydr. Bioeng. Meet., Prague, Czech Republic [Google Scholar]
- 35.Sallomons E, Wilbrink MH, Sanders P, Kamerling JP, van Vuure CA, Hage JA. June 2013. Methods for providing sialylated oligosaccharides. Patent WO 2013/085384 A1
- 36.Ribeirão M, Pereira-Chioccola VL, Eichinger D, Rodrigues MM, Schenkman S. 1997. Temperature differences for trans-glycosylation and hydrolysis reaction reveal an acceptor binding site in the catalytic mechanism of Trypanosoma cruzi trans-sialidase. Glycobiology 7:1237–1246. 10.1093/glycob/7.8.1237 [DOI] [PubMed] [Google Scholar]
- 37.Schenkman S, Jiang MS, Hart GW, Nussenzweig V. 1991. A novel cell surface trans-sialidase of Trypanosoma cruzi generates a stage-specific epitope required for invasion of mammalian cells. Cell 65:1117–1125. 10.1016/0092-8674(91)90008-M [DOI] [PubMed] [Google Scholar]
- 38.Schenkman S, Pontes de Carvalho L, Nussenzweig V. 1992. Trypanosoma cruzi trans-sialidase and neuraminidase activities can be mediated by the same enzymes. J. Exp. Med. 175:567–575. 10.1084/jem.175.2.567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schrader S, Tiralongo E, Paris G, Yoshino T, Schauer R. 2003. A nonradioactive 96-well plate assay for screening of trans-sialidase activity. Anal. Biochem. 322:139–147. 10.1016/j.ab.2003.07.016 [DOI] [PubMed] [Google Scholar]
- 40.Marchal I, Cerutti M, Mir AM, Juliant S, Devauchelle G, Cacan R, Verbert A. 2001. Expression of a membrane-bound form of Trypanosoma cruzi trans-sialidase in baculovirus-infected insect cells: a potential tool for sialylation of glycoproteins produced in the baculovirus-insect cells system. Glycobiology 11:593–603. 10.1093/glycob/11.7.593 [DOI] [PubMed] [Google Scholar]
- 41.Turnbull WB, Harrison JA, Kartha KPR, Schenkman S, Field RA. 2002. Observations on chemical and enzymatic approaches to α-2,3-sialylated octyl β-lactoside. Tetrahedron 58:3207–3216. 10.1016/S0040-4020(02)00265-X [DOI] [Google Scholar]
- 42.Van Halbeek H, Dorland L, Vliegenthart JFG, Fiat AM, Jolles P. 1980. A 360-MHz 1H-NMR study of three oligosaccharides isolated from cow κ-casein. Biochim. Biophys. Acta 623:295–300. 10.1016/0005-2795(80)90257-3 [DOI] [PubMed] [Google Scholar]
- 43.Holland JW, Deeth HC, Alewood PF. 2005. Analysis of O-glycosylation site occupancy in bovine κ-casein glycoforms separated by two-dimensional gel electrophoresis. Proteomics 5:990–1002. 10.1002/pmic.200401098 [DOI] [PubMed] [Google Scholar]
- 44.Holland JW, Deeth HC, Alewood PF. 2006. Resolution and characterisation of multiple isoforms of bovine κ-casein by 2-DE following a reversible cysteine-tagging enrichment strategy. Proteomics 6:3087–3095. 10.1002/pmic.200500780 [DOI] [PubMed] [Google Scholar]
- 45.Singh S, Scigelova M, Hallberg ML, Howarth OW, Schenkman S, Crout DHG. 2000. Synthesis of sialyloligosaccharides using the trans-sialidase from Trypanosoma cruzi: novel branched and di-sialylated products from digalactoside acceptors. J. Chem. Soc. Chem. Commun. 2000:1013–1014. 10.1039/B002302G [DOI] [Google Scholar]
- 46.Vliegenthart JFG, Kamerling JP. 2007. 1H NMR structural-reporter-group concepts in carbohydrate analysis, p 133–191 In Kamerling JP, Boons GJ, Lee YC, Suzuki A, Taniguchi N, Voragen AGJ. (ed), Comprehensive glycoscience–from chemistry to systems biology, vol 2 Elsevier, Amsterdam, The Netherlands [Google Scholar]
- 47.Fukuda K, Yamamoto A, Ganzorig K, Khuukhenbaatar J, Senda A, Saito T, Urashima T. 2010. Chemical characterization of the oligosaccharides in Bactrian camel (Camelus bactrianus) milk and colostrum. J. Dairy Sci. 93:5572–5587. 10.3168/jds.2010-3151 [DOI] [PubMed] [Google Scholar]
- 48.Anraku T, Fukuda K, Saito T, Messer M, Urashima T. 2012. Chemical characterization of acidic oligosaccharides in milk of the red kangaroo (Macropus rufus). Glycoconj. J. 29:147–156. 10.1007/s10719-012-9372-7 [DOI] [PubMed] [Google Scholar]
- 49.Urashima T, Taufik E, Fukuda R, Nakamura T, Fukuda K, Saito T, Messer M. 2013. Chemical characterization of milk oligosaccharides of the koala (Phascolarctos cinereus). Glycoconj. J. 30:801–811. 10.1007/s10719-013-9484-8 [DOI] [PubMed] [Google Scholar]
- 50.Van Well RM, Collet BYM, Field RA. 2008. Synthesis of mucin glycans from the protozoon parasite Trypanosoma cruzi. Synlett 14:2175–2177. 10.1055/s-2008-1078249 [DOI] [Google Scholar]
- 51.Agusti R, Mendoza VM, Gallo-Rodriguez C, de Lederkremer RM. 2005. Selective sialylation of 2,3-di-O-(β-d-galactopyranosyl)-d-galactose catalyzed by Trypanosoma cruzi trans-sialidase. Tetrahedron Asymmetry 16:541–551. 10.1016/j.tetasy.2004.11.075 [DOI] [Google Scholar]
- 52.Mendoza VM, Agusti R, Gallo-Rodriguez C, de Lederkremer RM. 2006. Synthesis of the O-linked pentasaccharide in glycoproteins of Trypanosoma cruzi and selective sialylation by recombinant trans-sialidase. Carbohydr. Res. 341:1488–1497. 10.1016/j.carres.2006.03.033 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


