Abstract
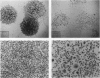
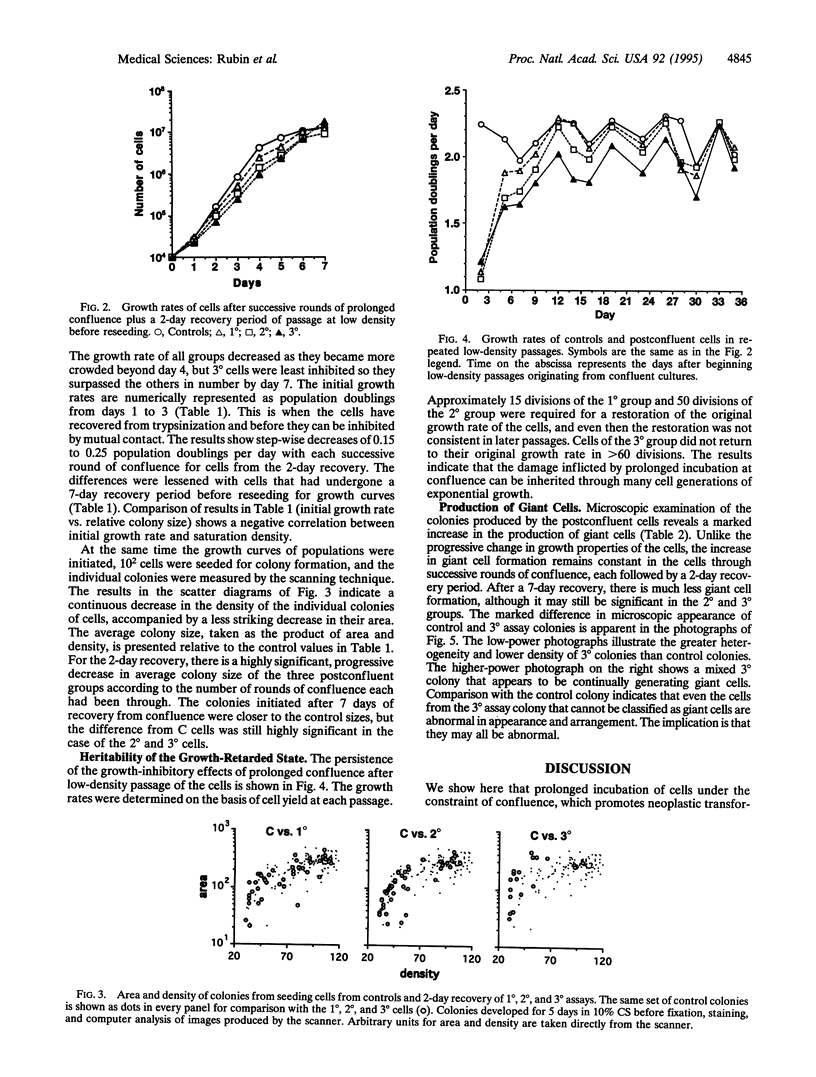
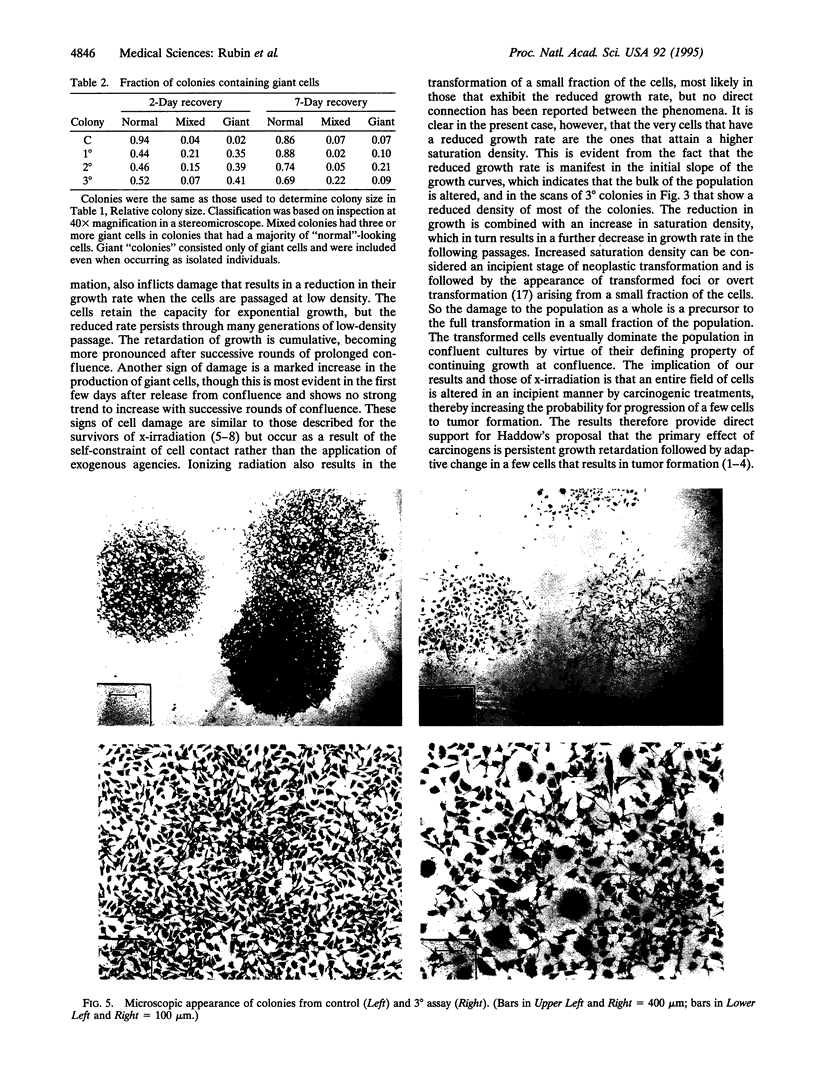
Prolonged incubation of NIH 3T3 cells under the growth constraint of confluence results in the death of some cells in a manner suggestive of apoptosis. Successive rounds of prolonged incubation at confluence of the surviving cells produce increasing neoplastic transformation in the form of increments in saturation density and transformed focus formation. Cells from the postconfluent cultures are given a recovery period of various lengths to remove the direct inhibitory effect of confluence before their growth properties are studied. It is found that with each round of confluence the exponential growth rate of the cells at low densities gets lower and the size of isolated colonies of the same cells shows a similar progressive reduction. The decreased growth rate of cells from the third round of confluence persists for > 60 generations of growth at low density. The proportion of colonies containing giant cells is much higher after a 2-day recovery from confluence than after a 7-day recovery. Retardation of growth at low density and increased saturation density appear to be two sides of the same coin: both occur in the entire population of cells and precede the formation of transformed foci. We propose that the slowdown in growth and the formation of giant cells result from heritable damage to the cells, which in turn drives their transformation. Similar results have been reported for the survivors of x-irradiation and of treatment with chemical carcinogens and are associated with the aging process in animals. We suggest that these changes result from free radical damage to membrane lipids with particular damage to lysosomes. Proteases and nucleases would then be released to progressively modify the growth behavior and genetic stability of the cells toward autonomous proliferation.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALLISON A. C., MALLUCCI L. UPTAKE OF HYDROCARBON CARCINOGENS BY LYSOSOMES. Nature. 1964 Sep 5;203:1024–1027. doi: 10.1038/2031024b0. [DOI] [PubMed] [Google Scholar]
- Brunk U., Ericsson J. L., Pontén J., Westermark B. Residual bodies and "aging" in cultured human glia cells. Effect of entrance into phase 3 and prolonged periods of confluence. Exp Eye Res. 1973 Apr;79(1):1–14. [PubMed] [Google Scholar]
- Cameron I. L. Cell proliferation and renewal in aging mice. J Gerontol. 1972 Apr;27(2):162–172. doi: 10.1093/geronj/27.2.162. [DOI] [PubMed] [Google Scholar]
- Chang W. P., Little J. B. Delayed reproductive death in X-irradiated Chinese hamster ovary cells. Int J Radiat Biol. 1991 Sep;60(3):483–496. doi: 10.1080/09553009114552331. [DOI] [PubMed] [Google Scholar]
- Chow M., Yao A., Rubin H. Cellular epigenetics: topochronology of progressive "spontaneous" transformation of cells under growth constraint. Proc Natl Acad Sci U S A. 1994 Jan 18;91(2):599–603. doi: 10.1073/pnas.91.2.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DESAI I. D., SAWANT P. L., TAPPEL A. L. PEROXIDATIVE AND RADIATION DAMAGE TO ISOLATED LYSOSOMES. Biochim Biophys Acta. 1964 May 11;86:277–285. doi: 10.1016/0304-4165(64)90054-6. [DOI] [PubMed] [Google Scholar]
- Friedman E., Urmacher C., Winawer S. A model for human colon carcinoma evolution based on the differential response of cultured preneoplastic, premalignant, and malignant cells to 12-O-tetradecanoylphorbol-13-acetate. Cancer Res. 1984 Apr;44(4):1568–1578. [PubMed] [Google Scholar]
- Hansen R. J., Switzer R. L., Hinze H., Holzer H. Effects of glucose and nitrogen source on the levels of proteinases, peptidases, and proteinase inhibitors in yeast. Biochim Biophys Acta. 1977 Jan 24;496(1):103–114. doi: 10.1016/0304-4165(77)90119-2. [DOI] [PubMed] [Google Scholar]
- Hockenbery D. M., Oltvai Z. N., Yin X. M., Milliman C. L., Korsmeyer S. J. Bcl-2 functions in an antioxidant pathway to prevent apoptosis. Cell. 1993 Oct 22;75(2):241–251. doi: 10.1016/0092-8674(93)80066-n. [DOI] [PubMed] [Google Scholar]
- JONES H. B. Demographic consideration of the cancer problem. Trans N Y Acad Sci. 1956 Feb;18(4):298–333. doi: 10.1111/j.2164-0947.1956.tb00453.x. [DOI] [PubMed] [Google Scholar]
- Kennedy A. R., Cairns J., Little J. B. Timing of the steps in transformation of C3H 10T 1/2 cells by X-irradiation. Nature. 1984 Jan 5;307(5946):85–86. doi: 10.1038/307085a0. [DOI] [PubMed] [Google Scholar]
- Kennedy A. R., Fox M., Murphy G., Little J. B. Relationship between x-ray exposure and malignant transformation in C3H 10T1/2 cells. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7262–7266. doi: 10.1073/pnas.77.12.7262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuroki T., Drevon C. Inhibition of chemical transformation in C3H/10T1/2 cells by protease inhibitors. Cancer Res. 1979 Jul;39(7 Pt 1):2755–2761. [PubMed] [Google Scholar]
- Messadi D. V., Billings P., Shklar G., Kennedy A. R. Inhibition of oral carcinogenesis by a protease inhibitor. J Natl Cancer Inst. 1986 Mar;76(3):447–452. [PubMed] [Google Scholar]
- Quigley J. P. Phorbol ester-induced morphological changes in transformed chick fibroblasts: evidence for direct catalytic involvement of plasminogen activator. Cell. 1979 May;17(1):131–141. doi: 10.1016/0092-8674(79)90301-5. [DOI] [PubMed] [Google Scholar]
- Rubin A. L., Yao A., Rubin H. Relation of spontaneous transformation in cell culture to adaptive growth and clonal heterogeneity. Proc Natl Acad Sci U S A. 1990 Jan;87(1):482–486. doi: 10.1073/pnas.87.1.482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin H. Incipient and overt stages of neoplastic transformation. Proc Natl Acad Sci U S A. 1994 Dec 6;91(25):12076–12080. doi: 10.1073/pnas.91.25.12076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin H. Uniqueness of each spontaneous transformant from a clone of BALB/c 3T3 cells. Cancer Res. 1988 May 1;48(9):2512–2518. [PubMed] [Google Scholar]
- Rubin H., Xu K. Evidence for the progressive and adaptive nature of spontaneous transformation in the NIH 3T3 cell line. Proc Natl Acad Sci U S A. 1989 Mar;86(6):1860–1864. doi: 10.1073/pnas.86.6.1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SINCLAIR W. K. X-RAY-INDUCED HERITABLE DAMAGE (SMALL-COLONY FORMATION) IN CULTURED MAMMALIAN CELLS. Radiat Res. 1964 Apr;21:584–611. [PubMed] [Google Scholar]
- Seymour C. B., Mothersill C., Alper T. High yields of lethal mutations in somatic mammalian cells that survive ionizing radiation. Int J Radiat Biol Relat Stud Phys Chem Med. 1986 Jul;50(1):167–179. doi: 10.1080/09553008614550541. [DOI] [PubMed] [Google Scholar]
- Shipley G. D., Ham R. G. Improved medium and culture conditions for clonal growth with minimal serum protein and for enhanced serum-free survival of Swiss 3T3 cells. In Vitro. 1981 Aug;17(8):656–670. doi: 10.1007/BF02628401. [DOI] [PubMed] [Google Scholar]
- Smith G. J., Bell W. N., Grisham J. W. Clonal analysis of the expression of multiple transformation phenotypes and tumorigenicity by morphologically transformed 10T1/2 cells. Cancer Res. 1993 Feb 1;53(3):500–508. [PubMed] [Google Scholar]
- Wyllie A. H., Kerr J. F., Currie A. R. Cell death: the significance of apoptosis. Int Rev Cytol. 1980;68:251–306. doi: 10.1016/s0074-7696(08)62312-8. [DOI] [PubMed] [Google Scholar]
- Yao A., Rubin H. A critical test of the role of population density in producing transformation. Proc Natl Acad Sci U S A. 1994 Aug 2;91(16):7712–7716. doi: 10.1073/pnas.91.16.7712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao A., Rubin H. Automatic enumeration and characterization of heterogeneous clonal progression in cell transformation. Proc Natl Acad Sci U S A. 1993 Nov 15;90(22):10524–10528. doi: 10.1073/pnas.90.22.10524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zubenko G. S., Park F. J., Jones E. W. Genetic properties of mutations at the PEP4 locus in Saccharomyces cerevisiae. Genetics. 1982 Dec;102(4):679–690. doi: 10.1093/genetics/102.4.679. [DOI] [PMC free article] [PubMed] [Google Scholar]