Abstract
The default mode network (DMN) has been largely studied by imaging, but not yet by neurodynamics, using electroencephalography (EEG) functional connectivity (FC). mindfulness meditation (MM), a receptive, non-elaborative training is theorized to lower DMN activity. We explored: (i) the usefulness of EEG-FC for investigating the DMN and (ii) the MM-induced EEG-FC effects. To this end, three MM groups were compared with controls, employing EEG-FC (–MPC, mean phase coherence). Our results show that: (i) DMN activity was identified as reduced overall inter-hemispheric gamma MPC during the transition from resting state to a time production task and (ii) MM-induced a state increase in alpha MPC as well as a trait decrease in EEG-FC. The MM-induced EEG-FC decrease was irrespective of expertise or band. Specifically, there was a relative reduction in right theta MPC, and left alpha and gamma MPC. The left gamma MPC was negatively correlated with MM expertise, possibly related to lower internal verbalization. The trait lower gamma MPC supports the notion of MM-induced reduction in DMN activity, related with self-reference and mind-wandering. This report emphasizes the possibility of studying the DMN using EEG-FC as well as the importance of studying meditation in relation to it.
Keywords: default mode network, mindfulness meditation, functional connectivity, mean phase coherence, electroencephalography
INTRODUCTION
This article focuses on two inter-related topics. The first is the identification of the electroencephalographic functional connectivity (EEG-FC) signature of the default mode network (DMN). The DMN, mostly studied by functional magnetic resonance imaging (fMRI), is a task-inhibited network active during resting state and largely related with mind-wandering (Gusnard et al., 2001; Raichle et al., 2001; Buckner et al., 2008;) and self-referential activity (Northoff et al., 2006), which includes the medial prefrontal cortex (mPFC), posterior cingulate cortex (PCC), inferior parietal lobule (IPL), medial temporal lobe (MTL) and lateral temporal cortex (LTC) (Buckner et al., 2008). The second topic of this report is the study of EEG-FC alterations following mindfulness meditation (MM) practice and their possible relation with the DMN. MM, as practiced in the West and as studied in research paradigms, is a technique of remaining aware and noticing the salient features of present experience while refraining from evaluative processes, conceptual elaboration and mind wandering (Kabat-Zinn, 2003). Previous research has indicated that MM may engage mechanism for vigilance, monitoring and cognitive control (Lutz et al., 2008; Zeidan et al., 2010; Hölzel et al., 2011b). Thus, it was expected and indeed found that it would result in decreases of activity in areas of DMN, specifically in mPFC, which has been linked to self-referential narrative thinking and valuation.
In this report we build on a recent publication on DMN activation during MM, in which we reported two major findings (Berkovich-Ohana et al., 2012). The first was the identification of DMN activity as a reduction in gamma power within frontal-midline regions during a time-production (TP) task compared with resting state. The choice of the TP task to index a reduction in DMN activity was based on previous work showing that timing systematically activates several cortical regions, including: (i) attentional regions, such as the right parietal and dorsolateral prefrontal cortex (DLPFC) (reviewed by Walsh, 2003; Oliveri et al., 2009; Wittmann, 2009), consistent with a great body of behavioral studies showing that attention is mandatory for accurate timing (reviewed by Brown, 1997); (ii) the supplementary motor area (SMA) (Coull and Nobre, 1998; Ferrandez et al., 2003; Coull, 2004; Macar et al., 2004) and (iii) the anterior insular cortex (Craig, 2002, 2009; Wittmann et al., 2010). The right parietal and SMA regions are part of the dorsal attention network (Corbetta et al., 2008), comprising a task-activated network, also named the ‘extrinsic system’, suggested to be antagonistic to the task-inactivated DMN (Fox et al., 2005; Golland et al., 2007; Tian et al., 2007) and the DLPFC and anterior insular cortex are part of the frontoparietal control system, interposed between the ‘intrinsic’ and ‘extrinsic’ systems (Vincent et al., 2008) and possibly adjudicating between these two potentially competing brain systems (Vincent et al., 2008; Spreng et al., 2010; Smallwood et al., 2012). To conclude, the TP task is related with the task-activated network and the frontoparietal control system, hence with DMN deactivation. The second finding was that MM practitioners exhibited reduced frontal gamma power, related to DMN activity, as a trait (long term) effect. Nolfe (2011) called attention to the need to expand this study to include functional connectivity, which is precisely the aim of this work.
There is a growing effort to establish the relationship between the DMN-related blood oxygenated level dependent (BOLD) fMRI signal and electrophysiology (Broyd et al., 2009). Most DMN-EEG studies investigated either spectral power (Chen et al., 2008; Berkovich-Ohana et al., 2012) or its correlation with the fMRI BOLD signal (Laufs et al., 2003; Mantini et al., 2007; Meltzer et al., 2007; Scheeringa et al., 2008). Accumulating evidence suggests that while DMN activity is generally negatively correlated with frontal and midline theta (4–8 Hz) power (Meltzer et al., 2007; Scheeringa et al., 2008), the activity in the prefrontal area of the DMN, which is strongly related to self-reference, is manifested in the gamma (25–45 Hz) power band (Mantini et al., 2007; Chen et al., 2008; Berkovich-Ohana et al., 2012). Additionally, the posterior regions of the DMN, including parietal-occipital midline cortex, were found to be related positively with alpha (8–13 Hz) power (Knyazev et al., 2011). The manifestation of DMN in EEG-FC has not been reported yet, excluding one conference paper (Chen, 2007), relating DMN activity with higher theta and alpha coherence in the frontal area.
Various meditation techniques were shown to induce changes in EEG-FC (summarized by Cahn and Polich, 2006; Ivanovski and Malhi, 2007). Several meditation studies reported increased FC as both state and trait effects (Cahn and Polich, 2006), the majority being Transcendental Meditation (TM) studies reporting increased state coherence in the theta–alpha range, along two axes: bilateral frontal (Dillbeck and Bronson, 1981; Farrow and Hebert, 1982; Travis and Wallace, 1999; Travis, 2001; Hebert et al., 2005) and frontal-posterior (Aftanas and Golocheikine, 2001, 2002). Similar trait effects were found in TM practitioners during rest or cognitive tasks (Orme-Johnson and Haynes, 1981; Dillbeck and Vesely, 1986; Travis, 1991). Very few meditation studies investigated FC in other forms of meditation. These include reports of state increased coherence in the bilateral frontal theta–alpha range during Zen practice (Murata et al., 2004), and increased frontal and frontal-posterior theta FC during Sahaja Yoga meditation (Aftanas and Golosheykin, 2001). Several studies also reported gamma FC alterations. Lutz et al. (2004) studied loving–kindness–compassion meditation in proficient Tibetan practitioners, and reported increased state and trait synchrony. In contrast, Faber et al. (2004) found in a Zen practitioner, a state reduction in gamma coherence. In contrast to the generally increased EEG-FC in various meditation forms and in line with Faber et al. (2004), Lehmann et al. (2012) recently reported reduced state FC for five meditation traditions (Tibetan Buddhists, QiGong, Sahaja Yoga, Ananda Marga Yoga and Zen), for all frequency bands (delta to gamma). This short review reveals ambiguity in the EEG-FC effect of meditation in general as well as a complete lack of studies reporting FC during or following MM practice.
Here, we employ our unique sample of MM practitioners, including three levels of expertise and a suitable control group (Berkovich-Ohana et al., 2012) to explore: (i) the usefulness of EEG-FC for the investigation of a subtle shift in DMN activity; (ii) what the MM-induced trait and state EEG-FC effects are as a function of MM expertise and whether they reflect DMN activity changes. Based on the reports relating DMN activity to EEG as well as on the meditation literature, we focused our analyses on the theta, alpha and gamma bands. Similar to our previous work, DMN activity was identified as a change in FC in a TP task compared to resting state, and hemispheric asymmetry was further considered. In relation to the two questions raised, we tested the following hypotheses based on our previous report and the surveyed literature: (i) EEG –FC would be useful in the study of the DMN, possibly seen as decreased frontal-midline gamma FC and increased frontal theta–alpha FC and (ii) MM practitioners would exhibit lower activity in DMN-related EEG-FC as a trait.
METHODS
Participants and procedure
Participants were 36 MM practitioners (all practice within the Theravada tradition), divided into three groups: short, intermediate and long-term (ST, IT and LT, respectively, n = 12 each), with varying expertise (mean of ∼900, ∼2570 and ∼7500 h, respectively) and 12 age-matched controls, all right-handed. The research was approved by the institutional ethical committee and informed consent was obtained from participants.
EEG was recorded for 5 min during resting state (RS), a TP task, and a meditation (MED) session of 15 min, all conditions eyes-closed (additional tasks were excluded from this report). For a detailed exposition of the TP task and its analysis, see Glicksohn and Hadad (2012). Briefly, the participants produced target durations (4, 8, 16 and 32 s) by pressing a button. Participants were not supervised as to their strategy and counting was used in >95%. The TP analyses and results have been reported elsewhere (Berkovich-Ohana et al., 2011, 2012).
At the end of the recording session, participants were questioned as to their experiences during the different tasks, by a semi-structured interview. Specifically, the meditation questionnaire comprised several questions, including: (i) did you fall asleep during the meditation session; (ii) what was the meditation depth, compared to your averaged ordinary meditative experience (1, very low; 10, very high) and (iii) what was the percentage of ‘free-from-thought’ time during the session.
EEG analysis
EEG was recorded with a 65-channel geodesic sensor net (EGI), sampled at 500 Hz and referenced to the vertex (Cz), with analog 0.1–200 Hz band-pass filtering. Alternating current line noise was removed by a 50 Hz digital notch filter. Impedance was usually kept under 40 kΩ, well below the 200 kΩ limit for accurate signal acquisition with this system (Ferree et al., 2001). The data were referenced offline to average reference and then visually screened for artifacts. Whenever electrodes were affected in a widespread distribution by artifacts, data were excluded. Whenever several channels exhibited a noisy recording due to local high impedance (>40 kΩ, mean of three electrodes per epoch, in ∼10% of the epochs), the corrected values were off-line calculated using spherical spline interpolation (Perrin et al., 1989).
For each condition, the first 16 non-overlapping, artifact-free epochs of 2.048 s were extracted for further analysis. For MED, epochs were extracted from the middle (between 5 and 10 min from the start) to enable ‘settling’ in the condition. From the TP task, all epochs were taken from the period prior to pressing the button, thus excluding the possibility of a motor artifact.
Cortically induced gamma activity is known to be at possible risk of contamination with electromyographic (EMG) muscular activity (Whitham et al., 2007), or saccade-related spike potentials (SP) due to eye movements (Yuval-Greenberg and Deouell, 2009). To minimize this risk, we used four steps for caution: (i) our entire report deals with eyes-closed conditions, whereas the SP is elicited at the onset of small saccades which occur during eyes-open fixation (Martinez-Conde et al., 2004); (ii) given that the EMG peaks at 70–80 Hz (Cacioppo et al., 1990), we used much lower frequencies (25–45 Hz); (iii) we excluded all the circumference electrodes, closest to eyes, neck and face muscles (Figure 1) and (iv) we assessed the reliability for all electrodes included in this study using the coefficient of variation (CV) of log gamma power, which can be viewed as a measure of pattern stability (Fingelkurts et al., 2006). This analysis revealed high internal consistency reliability during RS, MED and TP (detailed in Berkovich-Ohana et al., 2012).
Fig. 1.
Electrode net configuration and ROIs used for MPC calculations (right) as well as intra- (top left) and inter- (bottom left) hemispheric long-range connections used.
There are different ways of assessing phase synchronization between a pair of EEG signals (for a review, see Pereda et al., 2005). We used the mean phase coherence (MPC) index (Mormann et al., 2000). MPC is a measure of how the relative phase is distributed over the unit circle. If two signals are phase synchronized, the relative phase will occupy a small portion of the circle and MPC is high and vice versa (Pereda et al., 2005). Thus, MPC is close to 0 for uncorrelated signals, whereas it approaches 1 if there is strong phase synchronization. For further mathematical details about the method, see Bhattacharya and Petsche (2001, 2005), Pereda et al. (2005) and Reiterer et al. (2009). The application of MPC analysis is not a common practice, therefore, we provide more explanatory comments as well as a comparison of our results with the well-known coherence analysis showing a very high consistency between the two methods (see Supplementary Data).
As MPC is independent of frequency, the raw EEG signal was filtered into the different frequency bands prior to the calculation of MPC values for each electrode pair [i.e. (65 × 64) / 2 = 2080 MPC values for each epoch]. The MPC cannot be supposed to be normally distributed as its values are bounded within the interval [0, 1], hence, we used the Fisher z-transformation for statistical comparisons. In order to reduce the dimensionality of the data, nine regions of interest (ROIs) were defined (10 intra-hemisphere connections and 16 inter-hemisphere connections; Figure 1): left and right frontal (F), central (C), temporal (T), parietal-occipital (PO) and midline (M). The mean MPC between different ROIs was calculated as the mean z-transformed MPC value of all the possible pairwise connections between the two ROIs.
Statistical analyses
Analyses of variance (ANOVAs) were performed on the ROI mean z-transformed MPC values. The first two were designed to answer the question of DMN-related changes in MPC, testing the inter-hemisphere (inter-HEM) and intra-hemisphere (intra-HEM) connections, respectively, with one grouping factor (C, ST-MM, IT-MM and LT-MM) and with repeated measures on condition (RS and TP), band (theta, alpha and gamma), hemisphere (used only in the intra-HEM ANOVA) and connections (16 inter-HEM or 10 intra-HEM), applying the Greenhouse–Geisser corrected P-values.
To answer the question of the trait effect of MM expertise level on MPC, we used two additional ANOVAs, with inter-and intra-HEM connections, respectively. These ANOVAs were conducted only on RS, with a grouping factor and repeated measures on band (used only in the intra-HEM ANOVA), hemisphere and connections (as above).
Finally, to answer the question of state effects in the MM groups, we compared only the MPC values of the MM groups. We did not include any ‘meditation’ state for the control group, to avoid any procedure that would not be a just comparison with MM. Thus, two ANOVAs were conducted with one grouping factor (three MM groups) and repeated measures on condition (RS and MED), band, hemisphere (as above) and connections (as above).
Although statistically significant results were reported for ROIs only, we added topographic distributions of pairwise electrode MPC values (Figures 2B and 3B) to visualize the more precise distribution of FC on the scalp. For post hoc hypotheses we used t-tests (asterisk in the figures indicating respective P-values, adopting a minimal value of P < 0.01 due to multiple tests) as well as Pearson correlations.
Fig. 2.
(A) Mean z-MPC within the intra-HEM connections in the three bands during resting state (RS; left) and the time production (TP; right) task. *P < 0.01. (B) Topographic distribution of mean z-MPC values over all subjects (n = 48) for individual intra-HEM connections, within the three bands, theta (top), alpha (middle) and gamma (bottom).
Fig. 3.
(A) Mean z-MPC group differences during the resting state: the group · band · hemisphere interaction. (B) Topographic distribution of mean z-MPC values over the different groups for individual intra-HEM electrode connections within the three bands, theta (top), alpha (middle) and gamma (bottom).
RESULTS
First-person reports
None of the participants reported sleeping during the meditation session. The reported meditation depth was generally above the average score (of 5), indicating a relatively deep meditative state and was similar for the three MM groups: 5.7 ± 1.7, 6.4 ± 1.8 and 6.9 ± 2.2, for the ST, IT and LT-MM, respectively [F(2,35) = 1.11; MSE (mean square error) = 3.7, P = ns]. The percentage of ‘free-from-thought’ time during the session was significantly different for the three MM groups, rising with expertise: 57.8 ± 22.9, 67.7 ± 29.8 and 86.2 ± 18.3, for the ST, IT and LT-MM, respectively [F(2,35) = 3.91; MSE = 568, P < 0.05]. There was no significant correlation between meditation depth and ‘free-from-thought’ percentages. This could be expected, as meditative depth is scaled compared with the ordinary meditation experience, which varies between participants markedly, and shares no common scale between participants. In contrast, percentages of time shares a common time scale. Additionally, there was no significant correlation between ‘free-from-thought’ percentages and MPC, which survived the correction for multiple comparisons. These results provide an independent evaluation of relatively deep and ‘free-from-thought’ laboratory-induced meditative experiences.
DMN-related MPC changes
In the first ANOVA testing inter-HEM MPC changes in the transition from RS to TP, neither main effect for condition nor any of its interactions were uncovered. Turning to the second ANOVA testing intra-HEM FC, we found a significant condition × band interaction [F(2,68) = 3.19; MSE = 0.131, P < 0.05], whereas gamma MPC decreased during TP compared to RS, alpha MPC somewhat increased (Figure 2). Post hoc t-tests indicated only a significant decrease in gamma MPC during the TP compared with RS [t = −2.88, df = 44, P < 0.01]. This suggests that DMN-related MPC could be generally seen within the gamma band. In addition, we noted three other interactions of interest: The first was a significant band × hemisphere interaction [F(2,68) = 4.11; MSE = 0.026, P < 0.05], with alpha MPC being generally higher and gamma MPC slightly lower in the right hemisphere (relative to the left). The second was a significant band × connections interaction [F(18,612) = 9.32; MSE = 0.012, P < 0.001]: the connections between the frontal and other ROIs (F-T, F-PO and F-M) showed highest alpha and lowest gamma MPC, whereas the C-T connections showed highest theta and gamma MPC and lowest alpha MPC. The third was a hemisphere × connections interaction [F(9,306) = 2.78; MSE = 0.015, P < 0.05] stemming from lower MPC between the Temporal ROI and others (T-C, T-PO and T-M) in the left hemisphere. As to the question of group differences, we found a significant group × hemisphere × connections interaction [F(27,306) = 1.94; MSE = 0.015, P < 0.05] stemming from: (i) higher MPC in the left hemisphere between the F-M and T-PO connections in the C and ST-MM groups compared to the IT-MM and LT-MM groups and (ii) higher MPC in the right hemisphere between the F-PO and PO-M connections in the C and ST-MM groups compared with the IT-MM and LT-MM groups. The group interaction did not include condition, hence we conclude that there are no group differences related to the way the DMN deactivates during the transition from RS to a TP task, which was the main question tested here.
MM trait effects in MPC
In the third ANOVA testing the trait effect of MM expertise within inter-HEM MPC during RS, we found, as before, no interesting effects. In contrast, looking at intra-HEM trait differences between the C and the various MM groups (fourth ANOVA), we uncovered two main effects: (i) band [F(2,68) = 38.76; MSE = 0.108, P < 0.001], with alpha MPC values being the highest and gamma MPC the lowest, as before; (ii) connections [F(9,306) = 14.15; MSE = 0.024, P < 0.001], with PO-M exhibiting the highest value and F-C and T-M the lowest values.
We further uncovered three significant two-way interactions. The first was a band × hemisphere interaction [F(2,68) = 3.77; MSE = 0.019, P < 0.05]: as before, whereas alpha MPC was generally higher in the right, gamma MPC was higher in the left hemisphere. The second was a band × connections interaction [F(18,612) = 3.36; MSE = 0.012, P < 0.001], whereas alpha MPC was generally higher compared to theta MPC, this situation is reversed for C-T. The third was a hemisphere × connections interaction [F(9,306) = 2.63; MSE = 0.011, P < 0.05]: MPC values for the connections C-T, T-M and C-PO were highest in the right and C-M MPC was highest in the left hemisphere.
More importantly, there was a significant group × band × hemisphere interaction [F(6,68) = 2.73; MSE = 0.019, P < 0.05] (Figure 3). It should be emphasized that the exact profile of the decrease in MPC over groups is different, as we did not find a group main effect. Thus, we discuss each band and hemisphere separately. In the theta band, we see right greater than left MPC in the control group compared with the MM practitioners, irrespective of expertise. In the alpha band, the MM practitioners exhibit a gradient of lower MPC values compared to controls seen in the left hemisphere, whereas their right hemisphere values generally exceed those of the control group. A post hoc ANOVA designed to compare alpha MPC asymmetry scores (mean left minus right alpha MPC) between the MM and C groups yielded a significant increase in asymmetry toward right hemisphere in the MM [F(1,47) = 6.113; MSE = 0.004, P = 0.01]. In the gamma band, we see a lower MPC value only in the LT-MM compared with controls, especially in the left hemisphere. A post hoc Pearson correlation between mean left gamma MPC and expertise (years) yielded a significant negative correlation [r = −0.462, P < 0.005, n = 36].
MM state effects in MPC
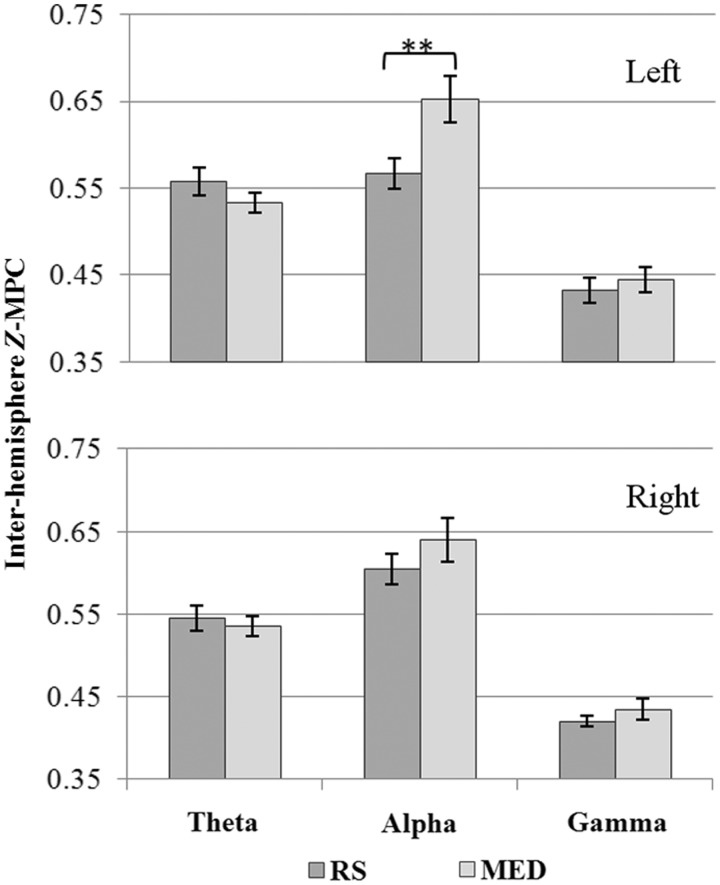
In the fifth ANOVA testing the state effect of MM within intra-HEM MPC, we uncovered a significant condition × band × hem interaction [F(2,50) = 3.26; MSE = 0.021 , P < 0.05], depicted in Figure 4. As can be clearly seen, gamma and theta MPC were largely unchanged. Although alpha MPC was high and of equal magnitude in both the left and right hemispheres during MED, it was only during RS that we see MPC R > L asymmetry. We also observed significant band × connections (P < 0.01) and condition × band (P < 0.05) interactions as well as connections and band main effects (both at P < 0.001). For the inter-HEM (sixth ANOVA) we found a significant band × connections interaction as well as connections and band main effects (all at P < 0.001), but no interaction or main effect of condition. Interestingly, we did not find a main effect, or any interaction with group, or any correlation between left alpha MPC during MED with expertise level, thus there were no group differences or expertise effects as a state effect.
Fig. 4.
Mean z-MPC differences between RS and MED for MM. The Condition · Band interaction for the left-HEM connections (top) and right-HEM connections (bottom). **P < 0.005.
DISCUSSION
Studying the DMN by means of EEG-FC
Our findings reveal that DMN deactivation during the TP compared with RS was manifested by a significantly lower intra-HEM gamma MPC, irrespective of group. Additionally, there was a significant condition × band interaction wherein alpha MPC increased during TP (Figure 2), which might be related to an increase in executive control function, manifested in alpha FC (Sauseng et al., 2005).
Importantly, the reduction in gamma MPC resembles the previously reported decrease in gamma power in the same paradigm and groups (Berkovich-Ohana et al., 2012). This is in line with our hypothesis, based on several studies reporting power and FC to co-vary in various cognitive domains, such as sleep (Dumermuth and Lehmann, 1981), language processing (Bastiaansen and Hagoort, 2006) and meditation (Travis, 2001; Travis et al., 2002). It is also worth mentioning here that the fact that we report a reduction during the attentionally demanding TP task, and not an increase in gamma FC, strengthens our claim that our results represent cognitive processes, rather than muscular artifacts (see ‘Methods’ section for discussion of gamma band artifacts).
The decreased gamma and increased alpha FC during the transition from RS to TP could be due to the reorganization of resources in frontal areas associated with the TP task. Particularly, it may index the transition of the control/executive system from coupling with the task-deactivated DMN, possibly reflected within the gamma band, toward coupling with the task-activated network, possibly reflected within the alpha band. This proposition is based on a recent hypothesis, suggesting that the control system could work in conjunction with both perceptual information from the task-activated network and autobiographical information provided by the DMN to selectively reinforce either internal or external trains of thought (Smallwood et al., 2012). Hence, the following could be a plausible scenario: During the RS, the DLPFC, a region systematically related with timing (Walsh, 2003; Oliveri et al., 2009; Wittmann, 2009), which is also a component of the control system (Vincent et al., 2008), was coupled with the DMN, as previously reported (Christoff et al., 2009). However, during the transition to TP, the DLPFC decoupled from the DMN and coupled with the right parietal and SMA regions, which are part of the dorsal attention network (Corbetta et al., 2008) comprising the task-activated network and are also systematically reported to be involved in timing processes (Coull and Nobre, 1998; Ferrandez et al., 2003; Coull, 2004; Macar et al., 2004). Obviously, further work which includes localization would be necessary in order to investigate such a proposition. However, it is detailed here to emphasize how the reorganization of resources in frontal areas might explain the reported results during the transition from RS to TP.
Our results are, however, in disagreement with the only report thus far of DMN-related FC (Chen, 2007), relating DMN activity with higher theta and alpha coherence in the frontal area. A possible explanation for the discrepancy with our results could be the difference in paradigm. Chen (2007) considered the transition from an eyes-closed to an eyes-open condition as a DMN-deactivation task. However, as extrinsic and extrinsic activities are thought to be antagonistic (Fox et al., 2005, Golland et al., 2007), DMN deactivates the more the ‘extrinsic’ task is demanding (Soddu et al., 2009). Merely opening the eyes will undoubtedly lower alpha activity, as they indeed reported, but could still count as a resting state condition. In fact, an eyes-open resting state condition is customarily tested against external demanding tasks in DMN imaging studies (e.g. Golland et al., 2007; Preminger et al., 2010). Our study bypasses this obstacle by engaging the participant in an attention-demanding task (TP).
The fingerprint of DMN activity in gamma FC shown here is also in accord with recent studies exploring the electrophysiological correlates of the DMN with intracerebral EEG (Jerbi et al., 2010; Ossandón et al., 2011). These studies showed that DMN areas displayed transient suppressions of gamma power during demanding task performance. Our results emphasize the usefulness of non-invasive EEG measures, specifically gamma activity, for the study of the DMN.
Trait and state MPC changes following MM practice
In this study we report an MM-related trait decrease in FC, irrespective of expertise or band, as seen in the significant group × band × hemisphere interaction (Figure 3). Additionally, we report a state-related increase in alpha MPC (Figure 4). Following is a discussion of each frequency band separately.
Generally, the reported results show large heterogeneity. It should be noted here that establishing a reliable signature of a specific meditation technique is notoriously difficult. Not only are techniques different form each other (even within the Theravada tradition) and have different stages both of the overall progress and of an individual session, but also individual participants develop over the course of their practice their own strategies of getting to desired meditation states. The heterogeneity of results here seems to point to this.
Theta band
We did not find a state effect in the theta band. Moreover, our results indicate that following MM practice, irrespective of proficiency, there is a decrease in right theta MPC compared with the control group. This is in contrast with other studies investigating various forms of meditation (summarized by Cahn and Polich, 2006; Ivanovski and Malhi, 2007), including Sahaj Samadhi (SS) state effects (Aftanas and Golocheikine, 2001; Baijal and Srinivasan, 2010) and TM state (Gaylord et al., 1989) and trait effects (Orme-Johnson and Haynes, 1981; Dillbeck and Vesely, 1986; Travis, 1991). Yet, it is in some agreement with two other studies reporting meditation-induced state decreased theta in five different techniques (Baijal and Srinivasan, 2010; Lehmann et al., 2012). The explanation for this discrepancy may lie in the meditative practice employed, as different techniques are known to show different characteristics (Dunn et al., 1999; Lutz et al., 2008; Travis and Shear, 2010).
A plausible explanation for the decrease in right theta MPC in MM practitioners compared with controls might be a different right-lateralized proportion of gray to white matter between groups. In the control group, RS theta FC is higher in the right hemisphere (Figure 3), as reported in the literature (Swenson and Tucker, 1983), and attributed to a greater proportion of white matter in the right hemisphere, rendering its organization more diffuse in general and leading to a greater interconnectedness among regions. Thus, the MM-related trait reduction in right theta MPC could indicate a higher proportion of gray to white matter in the right hemisphere. Indeed, MM practitioners were shown to exhibit higher gray matter volume in the right insula and hippocampus, regions related to interoceptive awareness and emotion regulation, respectively (e.g. Hölzel et al., 2007, 2011a; 2011b; Luders et al., 2012).
Alpha band
As a state effect, we found an increase in left hemisphere alpha MPC (Figure 4). This result is in accord with previous research, both on TM and Sahaj Samadhi (Farrow and Hebert, 1982; Travis and Wallace, 1999; Travis, 2001; Hebert et al., 2005) as well as Zen meditation (Murata et al., 2004).
Interestingly, the MM groups exhibited in the left hemisphere a trait decrease in MPC, while their right hemisphere values generally exceeded those of the control group, rendering their alpha MPC significantly asymmetric toward stronger right hemisphere connectivity compared with controls (Figure 3). This result is in contrast with the literature, where increases in trait alpha coherence are commonly found following meditation (reviewed by Cahn and Polich, 2006). Yet, given that the majority of these are TM studies (Orme-Johnson & Haynes, 1981; Dillbeck and Vesely, 1986; Travis, 1991), they are categorically different from MM (Lutz et al., 2008).
Although an increase in the right lateralization of alpha coherence was previously related to increased anxiety (Swenson and Tucker, 1983), this interpretation for the asymmetrical alpha MPC in the MM practitioners has low plausibility, as MM has been shown to reduce anxiety (reviewed by Chambers et al., 2009; Rubia, 2009).
The significant right asymmetry in MM practitioners compared with controls might be related to heightened creativity, as the left hemisphere was found to present lower synchrony than the right in artists, compared with non-artists (Bhattacharya and Petsche, 2002, 2005). This asymmetry was interpreted as supporting the ‘significance of the right hemisphere for metaphors, imagination, expressiveness and emotional memories’ (Bhattacharya and Petsche, 2002). Our results support the suggestion that meditation induces a transition toward a more symbolic and creative mode of cognition, as a trait effect (Glicksohn, 1998; Horan, 2009).
Gamma band
We did not find a gamma state effect, in contrast to the ground-breaking study of Lutz et al. (2004), reporting increased state-related gamma FC. The discrepancy with this report might stem from the expertise (experts in the other study vs non-experts in this report) or difference in technique (compassion meditation vs MM). Following this argument, a more comparable meditation technique would be Zen, another open monitoring technique (Lutz et al., 2008). Faber et al. (2004) studied one Zen practitioner (expertise not specified) and reported state-related decrease in gamma coherence. Obviously, further research is warranted, as the current literature on state gamma FC is scarce and ambiguous.
Turning to the gamma FC trait effects, we found a decrease compared with controls in the LT-MM, especially in the left hemisphere (Figure 3). As gamma MPC has been found to reflect DMN activity in this study (previous section), we interpret this finding as lower DMN activity during RS, thus a trait, for the MM practitioners compared with controls, possibly indicative of lower self-reference (Northoff et al., 2006) and mind-wandering (Smallwood and Smith, 2006; Smallwood et al., 2007; Christoff et al., 2009), as hypothesized. This result is in accord with accumulating evidence from fMRI studies, showing reduced DMN activity during meditation. Specifically, mindfulness-induced reduction in activity was found in anterior and posterior midline DMN regions, including the mPFC, PCC and precuneus (Farb et al., 2007; Pagnoni et al., 2008; Brewer et al., 2011; Ives-Deliperi et al., 2011), which are particularly linked with spontaneous self-generated mental activity, i.e. streams of thoughts, episodic memory, mind wandering and self-related processing (Gusnard et al., 2001; Northoff et al., 2006; Smallwood and Schooler, 2006; Mason et al., 2007; Sajonz et al., 2010; Kim, 2012) as well as the lateral temporal cortices, including the angular gyrus (Pagnoni et al., 2008), particularly linked with semantic and conceptual processing (Binder et al., 1999; Buckner et al., 2008). However, while some studies indicate that meditation training may reduce the DMN activity, others show an opposite effect, of meditation-induced increased activity within the DMN, both within the frontal region of mPFC (Hölzel et al., 2007) as well as in posterior regions, including right PCC and precuneus (Tang et al., 2009). Additionally, increased resting state functional connectivity within the DMN was reported (Jang et al., 2011; Taylor et al., 2013) as well as between DMN regions and attention (Hasenkamp and Barsalou, 2012), control (Brewer et al., 2011) and sensory (Kilpatrick et al., 2011) networks. Finally, anatomical studies reported increased gray matter concentration in the PCC (Hölzel et al., 2011b). Taken together, studies indicate that meditation training alters the DMN activity, connectivity and anatomy, but the directions of change vary between studies (Tang and Posner, 2013).
Additionally, our results indicate a negative correlation between MM expertise and left gamma FC, in accord with previous studies where meditation expertise positively correlated with task performance (Chan and Woollacott, 2007; Cahn and Polich, 2009). The left lateralization of the MM-induced lower gamma MPC gives support to the suggestion that ‘most systems of meditation alter consciousness by inhibiting cognitive functions associated with the dominant or left cortical hemisphere’ (Earle, 1984). We further suggest that it might be related to lower internal verbalization and conceptual processing, reduced with increasing expertise, based on the following arguments: (i) various techniques of meditation are designed purposely to avoid logical and verbal reactions (Bishop et al., 2004; Scola and David, 2011); (ii) there is ample evidence on the relation between the left hemisphere, verbalization and concept formation, accumulating from split-brain (Gazzaniga, 1989, 2000), structural (Fine et al., 2009) and EEG (Davidson et al., 1990) studies and (iii) neuronal synchronization in the gamma frequency is related to manipulation of semantic information when found within the left hemisphere in general (Bastiaansen and Hagoort, 2006) and in left DMN regions in particular (Binder et al., 1999).
CONCLUSIONS
We summarize this study with two conclusions: (i) EEG-FC could indeed be useful to study DMN activity and (ii) MM-induced EEG-FC effects show reduced trait DMN activity. We also report lateralized effects, which suggest lower internal verbalization and heightened creativity. This report emphasizes the possibility of studying the DMN using EEG as well as highlighting the importance of studying meditation in relation to it (Nolfe, 2011).
SUPPLEMENTARY DATA
Supplementary data are available at SCAN online.
Conflict of Interest
None declared.
Supplementary Material
Acknowledgments
We thank Dr Zoran Josipovic and Dr Yi-Yuan Tang for suggesting very helpful comments to the previous version of this manuscript.
The research was supported by the Mind and Life Institute Francisco J. Varela Research Award 6546 and a grant from the Bial Foundation (27/10).
REFERENCES
- Aftanas LI, Golocheikine SA. Human anterior and frontal midline theta and lower alpha reflect emotionally positive state and internalized attention: high-resolution EEG investigation of meditation. Neuroscience Letters. 2001;310:57–60. doi: 10.1016/s0304-3940(01)02094-8. [DOI] [PubMed] [Google Scholar]
- Aftanas LI, Golocheikine SA. Non-linear dynamic complexity of the human EEG during meditation. Neuroscience Letters. 2002;330:143–6. doi: 10.1016/s0304-3940(02)00745-0. [DOI] [PubMed] [Google Scholar]
- Baijal S, Srinivasan N. Theta activity and meditative states: spectral changes during concentrative meditation. Cognitive Processing. 2010;11:31–8. doi: 10.1007/s10339-009-0272-0. [DOI] [PubMed] [Google Scholar]
- Bastiaansen M, Hagoort P. Oscillatory neuronal dynamics during language comprehension. Progress in Brain Research. 2006;159:179–96. doi: 10.1016/S0079-6123(06)59012-0. [DOI] [PubMed] [Google Scholar]
- Berkovich-Ohana A, Glicksohn J, Goldstein A. Temporal cognition changes following mindfulness, but not transcendental meditation practice. In: Algom D, Zakay D, Chajut E, Shaki E, Mama Y, Shakuf V, editors. Fechner Day 2011: Proceedings of the 27th Annual Meeting of the International Society for Psychophysics. Raanana, Israel: 2011. pp. 245–50. [Google Scholar]
- Berkovich-Ohana A, Glicksohn J, Goldstein A. Mindfulness-induced changes in gamma band activity – implications for the default mode network, self-reference and attention. Clinical Neurophysiology. 2012;123:700–10. doi: 10.1016/j.clinph.2011.07.048. [DOI] [PubMed] [Google Scholar]
- Bhattacharya J, Petsche H. Enhanced phase synchrony in the electroencephalograph band for musicians while listening to music. Physical Review E. 2001;64:12902. doi: 10.1103/PhysRevE.64.012902. [DOI] [PubMed] [Google Scholar]
- Bhattacharya J, Petsche H. Shadows of artistry: cortical synchrony during perception and imagery of visual art. Cognitive Brain Research. 2002;13:179–86. doi: 10.1016/s0926-6410(01)00110-0. [DOI] [PubMed] [Google Scholar]
- Bhattacharya J, Petsche H. Drawing on mind’s canvas: Differences in cortical integration patterns between artists and non-artists. Human Brain Mapping. 2005;26:1–14. doi: 10.1002/hbm.20104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder JR, Frost JA, Hammeke TA, Bellgowan PSF, Rao SM, Cox RW. Conceptual processing during the conscious resting state: a functional MRI study. Journal of Cognitive Neuroscience. 1999;11:80–93. doi: 10.1162/089892999563265. [DOI] [PubMed] [Google Scholar]
- Bishop SR, Lau M, Shapiro S, et al. Mindfulness: A proposed operational definition. Clinical Psychology. 2004;11:230–41. [Google Scholar]
- Brewer JA, Worhunsky PD, Gray JR, Tang YY, Weber J, Kober H. Meditation experience is associated with differences in default mode network activity and connectivity. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:20254–9. doi: 10.1073/pnas.1112029108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown SW. Attentional resources in timing: Interference effects in concurrent temporal and nontemporal working memory tasks. Perception & Psychophysics. 1997;59:1118–40. doi: 10.3758/bf03205526. [DOI] [PubMed] [Google Scholar]
- Broyd SJ, Demanuele C, Debener S, Helps SK, James CJ, Sonuga-Barke EJS. Default-mode brain dysfunction in mental disorders: a systematic review. Neuroscience & Biobehavioral Reviews. 2009;33:279–96. doi: 10.1016/j.neubiorev.2008.09.002. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Andrews-Hanna J, Schachter D. The brain’s default network: Anatomy, function, and relevance to disease. In: Kingstone A, Miller MB, editors. The Year in Cognitive Neuroscience 2008. Annals of the New York Academy of Sciences. New York: New York Academy of Sciences; 2008. pp. 1–38. [DOI] [PubMed] [Google Scholar]
- Cacioppo JT, Tassinary LG, Fridlund AJ. The skeletomotor system. In: Tassinary L, editor. Principles of Psychophysiology. New York: Cambridge University Press; 1990. pp. 325–84. [Google Scholar]
- Cahn BR, Polich J. Meditation states and traits: EEG, ERP, and neuroimaging studies. Psychological Bulletin. 2006;132:180–211. doi: 10.1037/0033-2909.132.2.180. [DOI] [PubMed] [Google Scholar]
- Chambers R, Gullone E, Allen NB. Mindful emotion regulation: An integrative review. Clinical Psychology Review. 2009;23:560–72. doi: 10.1016/j.cpr.2009.06.005. [DOI] [PubMed] [Google Scholar]
- Chan D, Woollacott M. Effects of level of meditation experience on attentional focus: is the efficiency of executive or orientation networks improved? The Journal of Alternative and Complementary Medicine. 2007;13:651–8. doi: 10.1089/acm.2007.7022. [DOI] [PubMed] [Google Scholar]
- Chen ACN. Joint meeting of the 6th international symposium on noninvasive functional source imaging of the brain and heart and the international conference on functional biomedical imaging (NFSI-ICFBI) Hangzhou, China: Curran Associations; 2007. EEG default modenetwork in the human brain: spectral field power, coherence, topology, and current source imaging; pp. 215–8. [Google Scholar]
- Chen ACN, Feng WJ, Zhao HX, Yin YL, Wang PP. EEG default mode network in the human brain: spectral regional field powers. NeuroImage. 2008;41:561–74. doi: 10.1016/j.neuroimage.2007.12.064. [DOI] [PubMed] [Google Scholar]
- Christoff K, Gordon AM, Smallwood J, Smith R, Schooler JW. Experience sampling during fMRI reveals default network and executive system contributions to mind wandering. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:8719–24. doi: 10.1073/pnas.0900234106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M, Patel G, Shulman GL. The reorienting system of the human brain: from environment to theory of mind. Neuron. 2008;58:306–24. doi: 10.1016/j.neuron.2008.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coull JT. fMRI studies of temporal attention: allocating attention within, or towards, time. Cognitive Brain Research. 2004;21:216–26. doi: 10.1016/j.cogbrainres.2004.02.011. [DOI] [PubMed] [Google Scholar]
- Coull JT, Nobre AC. Where and when to pay attention: the neural systems for directing attention to spatial locations and to time intervals as revealed by both PET and fMRI. Journal of Neuroscience. 1998;18:7426–35. doi: 10.1523/JNEUROSCI.18-18-07426.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig AD. How do you feel? Interoception: the sense of the physiological condition of the body. Nature Reviews Neuroscience. 2002;3:655–66. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- Craig AD. How do you feel–now? The anterior insula and human awareness. Nature Reviews Neuroscience. 2009;10:59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- Davidson RJ, Chapman JP, Chapman LJ, Henriques JB. Asymmetrical brain electrical activity discriminates between psychometrically-matched verbal and spatial cognitive tasks. Psychophysiology. 1990;27:528–43. doi: 10.1111/j.1469-8986.1990.tb01970.x. [DOI] [PubMed] [Google Scholar]
- Dillbeck MC, Bronson EC. Short-term longitudinal effects of the Transcendental Meditation technique on EEG power and coherence. International Journal of Neuroscience. 1981;14:147–51. doi: 10.3109/00207458108985827. [DOI] [PubMed] [Google Scholar]
- Dillbeck MC, Vesely SA. Participation in the Transcendental Meditation program and frontal EEG coherence during concept learning. International Journal of Neuroscience. 1986;29:45–55. doi: 10.3109/00207458608985634. [DOI] [PubMed] [Google Scholar]
- Dumermuth G, Lehmann D. EEG power and coherence during non-REM and REM phases in humans in all-night sleep analyses. European Neurology. 1981;20:429–34. doi: 10.1159/000115274. [DOI] [PubMed] [Google Scholar]
- Dunn BR, Hartigan JA, Mikulas WL. Concentration and mindfulness meditations: Unique forms of consciousness? Applied Psychophysiology and Biofeedback. 1999;24:147–65. doi: 10.1023/a:1023498629385. [DOI] [PubMed] [Google Scholar]
- Earle JB. Cerebral laterality and meditation: A review of the literature. In: Shapiro DH, Walsh R, editors. Meditation: Classic and Contemporary Perspectives. New York, USA: Aldine Publishing; 1984. pp. 396–414. [Google Scholar]
- Faber PL, Lehmann D, Gianotti LRR, Kaelin M, Pascual-Marqui RD. Meeting of the International Society for Neuronal Regulation. Winterthur, Switzerland: 2004. Scalp and intracerebral (LORETA) theta and gamma EEG coherence in meditation. [Google Scholar]
- Farb NAS, Segal ZV, Mayberg H, Bean J, McKeon D, Fatima Z, et al. Attending to the present: mindfulness meditation reveals distinct neural modes of self-reference. Social Cognitive and Affective Neuroscience. 2007;2:313–22. doi: 10.1093/scan/nsm030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrow JT, Hebert JR. Breath suspension during the Transcendental Meditation technique. Psychosomatic Medicine. 1982;44:133–53. doi: 10.1097/00006842-198205000-00001. [DOI] [PubMed] [Google Scholar]
- Ferrandez A-M, Hugueville L, Lehéricy S, Poline J-B, Marsault C, Pouthas V. Basal ganglia and supplementary motor area subtend duration perception: an fMRI study. NeuroImage. 2003;19:1532–44. doi: 10.1016/s1053-8119(03)00159-9. [DOI] [PubMed] [Google Scholar]
- Ferree TC, Luu P, Russell GS, Tucker DM. Scalp electrode impedance, infection risk, and EEG data quality. Clinical Neurophysiology. 2001;112:536–44. doi: 10.1016/s1388-2457(00)00533-2. [DOI] [PubMed] [Google Scholar]
- Fine EM, Delis DC, Dean D, et al. Left frontal lobe contributions to concept formation: A quantitative MRI study of performance on the Delis–Kaplan Executive Function System Sorting Test. Journal of Clinical and Experimental Neuropsychology. 2009;31:624–31. doi: 10.1080/13803390802419017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fingelkurts AA, Fingelkurts AA, Ermolaev VA, Kaplan AY. Stability, reliability and consistency of the compositions of brain oscillations. International Journal of Psychophysiology. 2006;59:116–26. doi: 10.1016/j.ijpsycho.2005.03.014. [DOI] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:9673–8. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaylord C, Orme-Johnson D, Travis F. The effects of the Transcendental Mediation technique and progressive muscle relaxation on EEG coherence, stress reactivity, and mental health in black adults. International Journal of Neuroscience. 1989;46:77–86. doi: 10.3109/00207458908991618. [DOI] [PubMed] [Google Scholar]
- Gazzaniga MS. Organization of the human brain. Science. 1989;245:947–52. doi: 10.1126/science.2672334. [DOI] [PubMed] [Google Scholar]
- Gazzaniga MS. Cerebral specialization and interhemispheric communication: Does the corpus callosum enable the human condition? Brain. 2000;123:1293–326. doi: 10.1093/brain/123.7.1293. [DOI] [PubMed] [Google Scholar]
- Glicksohn J. States of consciousness and symbolic cognition. Journal of Mind and Behavior. 1998;19:105–18. [Google Scholar]
- Glicksohn J, Hadad Y. Sex differences in time production revisited. Journal of Individual Differences. 2012;33:35–42. [Google Scholar]
- Golland Y, Bentin S, Gelbard H, et al. Extrinsic and intrinsic systems in the posterior cortex of the human brain revealed during natural sensory stimulation. Cerebral Cortex. 2007;17:766–77. doi: 10.1093/cercor/bhk030. [DOI] [PubMed] [Google Scholar]
- Gusnard DA, Akbudak E, Shulman GL, Raichle ME. Medial prefrontal cortex and self-referential mental activity: relation to a default mode of brain function. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:4259–64. doi: 10.1073/pnas.071043098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasenkamp W, Barsalou LW. Effects of meditation experience on functional connectivity of distributed brain networks. Frontiers in Human Neuroscience. 2012;6:1–14. doi: 10.3389/fnhum.2012.00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebert R, Lehmann D, Tan G, Travis F, Arenander A. Enhanced EEG alpha time-domain phase synchrony during Transcendental Meditation: implications for cortical integration theory. Signal Processing. 2005;85:2213–32. [Google Scholar]
- Hölzel BK, Carmody J, Vangel M, et al. Mindfulness practice leads to increases in regional brain gray matter density. Psychiatry Research: Neuroimaging. 2011a;191:36–43. doi: 10.1016/j.pscychresns.2010.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hölzel BK, Lazar SW, Gard T, Schuman-Olivier Z, Vago DR, Ott U. How does Mindfulness meditation work? Proposing mechanisms of action from a conceptual and neural perspective. Perspectives on Psychological Science. 2011b;6:537–59. doi: 10.1177/1745691611419671. [DOI] [PubMed] [Google Scholar]
- Hölzel BK, Ott U, Hempel H, et al. Differential engagement of anterior cingulate and adjacent medial frontal cortex in adept meditators and non-meditators. Neuroscience Letters. 2007;421:16–21. doi: 10.1016/j.neulet.2007.04.074. [DOI] [PubMed] [Google Scholar]
- Horan R. The neuropsychological connection between creativity and meditation. Creativity Research Journal. 2009;21:199–222. [Google Scholar]
- Ivanovski B, Malhi GS. The psychological and neurophysiological concomitants of mindfulness forms of meditation. Acta Neuropsychiatrica. 2007;19:76–91. doi: 10.1111/j.1601-5215.2007.00175.x. [DOI] [PubMed] [Google Scholar]
- Ives-Deliperi VL, Solms M, Meintjes EM. The neural substrates of mindfulness: An fMRI investigation. Social Neuroscience. 2011;6:231–42. doi: 10.1080/17470919.2010.513495. [DOI] [PubMed] [Google Scholar]
- Jang J, Jung WH, Kang DH, et al. Increased default mode network connectivity associated with meditation. Neuroscience Letters. 2011;487:358–62. doi: 10.1016/j.neulet.2010.10.056. [DOI] [PubMed] [Google Scholar]
- Jerbi K, Vidal JR, Ossandon T, et al. Exploring the electrophysiological correlates of the default-mode network with intracerebral EEG. Frontiers in Systems Neuroscience. 2010;4:1–9. doi: 10.3389/fnsys.2010.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabat-Zinn J. Mindfulness-based interventions in context: past, present, and future. Clinical Psychology: Science & Practice. 2003;10:144–56. [Google Scholar]
- Kilpatrick LA, Suyenobu BY, Smith SR, et al. Impact of mindfulness-based stress reduction training on intrinsic brain connectivity. NeuroImage. 2011;56:290–298. doi: 10.1016/j.neuroimage.2011.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H. A dual-subsystem model of the brain’s default network: Self-referential processing, memory retrieval processes and autobiographical memory retrieval. NeuroImage. 2012;61:966–77. doi: 10.1016/j.neuroimage.2012.03.025. [DOI] [PubMed] [Google Scholar]
- Knyazev GG, Slobodskoj-Plusnin JY, Bocharov AV, Pylkova LV. The default mode network and EEG alpha oscillations: An independent component analysis. Brain Research. 2011;1402:67–79. doi: 10.1016/j.brainres.2011.05.052. [DOI] [PubMed] [Google Scholar]
- Lachaux JP, Rodriguez E, Martinerie J, Varela FJ. Measuring phase synchrony in brain signals. Human Brain Mapping. 1999;8:194–208. doi: 10.1002/(SICI)1097-0193(1999)8:4<194::AID-HBM4>3.0.CO;2-C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laufs H, Krakow K, Sterzer P, et al. Electroencephalographic signatures of attentional and cognitive default modes in spontaneous brain activity fluctuations at rest. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:11053–8. doi: 10.1073/pnas.1831638100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann D, Faber PL, Tei S, Pascual-Marqui RD, Milz P, Kochi K. Reduced functional connectivity between cortical sources in five meditation traditions detected with lagged coherence using EEG tomography. NeuroImage. 2012;60:1574–86. doi: 10.1016/j.neuroimage.2012.01.042. [DOI] [PubMed] [Google Scholar]
- Luders E, Kurth F, Mayer EA, Toga AW, Narr KL, Gaser C. The unique brain anatomy of meditation practitioners: alterations in cortical gyrification. Frontiers in Human Neuroscience. 2012;6:1–9. doi: 10.3389/fnhum.2012.00034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz A, Greischar LL, Rawlings NB, Ricard M, Davidson RJ. Long-term meditators self-induce high-amplitude gamma synchrony during mental practice. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:16369–73. doi: 10.1073/pnas.0407401101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz A, Slagter HA, Dunne JD, Davidson RJ. Attention regulation and monitoring in meditation. Trends in Cognitive Sciences. 2008;12:163–9. doi: 10.1016/j.tics.2008.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macar F, Anton JL, Bonnet M, Vidal F. Timing functions of the supplementary motor area: an event-related fMRI study. Cognitive Brain Research. 2004;21:206–15. doi: 10.1016/j.cogbrainres.2004.01.005. [DOI] [PubMed] [Google Scholar]
- Mantini D, Perrucci MG, Del Gratta C, Romani GL, Corbetta M. Electrophysiological signatures of resting state networks in the human brain. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:13170–5. doi: 10.1073/pnas.0700668104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Conde S, Macknik SL, Hubel DH. The role of fixational eye movements in visual perception. Nature Reviews Neuroscience. 2004;5:229–40. doi: 10.1038/nrn1348. [DOI] [PubMed] [Google Scholar]
- Mason MF, Norton MI, Van Horn JD, Wegner DM, Grafton ST, Macrae CN. Wandering minds: the default network and stimulus-independent thought. Science. 2007;315:393–5. doi: 10.1126/science.1131295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meltzer JA, Negishi M, Mayes LC, Constable RT. Individual differences in EEG theta and alpha dynamics during working memory correlate with fMRI responses across subjects. Clinical Neurophysiology. 2007;118:2419–36. doi: 10.1016/j.clinph.2007.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mormann F, Lehnertz K, David P, Elger C. Mean phase coherence as a measure for phase synchronization and its application to the EEG of epilepsy patients. Physica D. 2000;144:358–69. [Google Scholar]
- Murata T, Takahashi T, Hamada T, et al. Individual trait anxiety levels characterizing the properties of Zen meditation. Neuropsychobiology. 2004;50:189–94. doi: 10.1159/000079113. [DOI] [PubMed] [Google Scholar]
- Nolfe G. EEG and meditation. Clinical Neurophysiology. 2011;123:631–2. doi: 10.1016/j.clinph.2011.08.016. [DOI] [PubMed] [Google Scholar]
- Northoff G, Heinzel A, De Greck M, Bermpohl F, Dobrowolny H, Panksepp J. Self-referential processing in our brain–a meta-analysis of imaging studies on the self. NeuroImage. 2006;31:440–57. doi: 10.1016/j.neuroimage.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Oliveri M, Koch G, Caltagirone C. Spatial–temporal interactions in the human brain. Experimental Brain Research. 2009;195:489–97. doi: 10.1007/s00221-009-1834-1. [DOI] [PubMed] [Google Scholar]
- Orme-Johnson DW, Haynes CT. EEG phase coherence, pure consciousness, creativity, and TM—Sidhi experiences. International Journal of Neuroscience. 1981;13:211–7. doi: 10.3109/00207458108985804. [DOI] [PubMed] [Google Scholar]
- Ossandón T, Jerbi K, Vidal JR, et al. Transient suppression of broadband gamma power in the default-mode network is correlated with task complexity and subject performance. Journal of Neuroscience. 2011;31:14521–30. doi: 10.1523/JNEUROSCI.2483-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagnoni G, Cekic M, Guo Y. “Thinking about Not-Thinking”: Neural correlates of conceptual processing during Zen meditation. PLoS One. 2008;3:3083. doi: 10.1371/journal.pone.0003083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereda E, Quiroga RQ, Bhattacharya J. Nonlinear multivariate analysis of neurophysiological signals. Progress in Neurobiology. 2005;77:1–37. doi: 10.1016/j.pneurobio.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Perrin F, Pernier J, Bertrand O, Echallier JF. Spherical splines for scalp potential and current density mapping. Electroencephalography and Clinical Neurophysiology. 1989;72:184–7. doi: 10.1016/0013-4694(89)90180-6. [DOI] [PubMed] [Google Scholar]
- Preminger S, Harmelech T, Malach R. Stimulus-free thoughts induce differential activation in the human default network. NeuroImage. 2010;54:1692–702. doi: 10.1016/j.neuroimage.2010.08.036. [DOI] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:676–82. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiterer S, Pereda E, Bhattacharya J. Measuring second language proficiency with EEG synchronization: how functional cortical networks and hemispheric involvement differ as a function of proficiency level in second language speakers. Second Language Research. 2009;25:77–106. [Google Scholar]
- Rubia K. The neurobiology of meditation and its clinical effectiveness in psychiatric disorders. Biological Psychology. 2009;82:1–11. doi: 10.1016/j.biopsycho.2009.04.003. [DOI] [PubMed] [Google Scholar]
- Sajonz B, Kahnt T, Margulies DS, et al. Delineating self-referential processing from episodic memory retrieval: Common and dissociable networks. NeuroImage. 2010;50:1606–17. doi: 10.1016/j.neuroimage.2010.01.087. [DOI] [PubMed] [Google Scholar]
- Sauseng P, Klimesch W, Schabus M, Doppelmayr M. Fronto-parietal EEG coherence in theta and upper alpha reflect central executive functions of working memory. International Journal of Psychophysiology. 2005;57:97–103. doi: 10.1016/j.ijpsycho.2005.03.018. [DOI] [PubMed] [Google Scholar]
- Scheeringa R, Bastiaansen M, Petersson KM, Oostenveld R, Norris DG, Hagoort P. Frontal theta EEG activity correlates negatively with the default mode network in resting state. International Journal of Psychophysiology. 2008;67:242–51. doi: 10.1016/j.ijpsycho.2007.05.017. [DOI] [PubMed] [Google Scholar]
- Scola MD, David A. The hemispheric specialization of the human brain and its application to psychoanalytic principles. Jefferson Journal of Psychiatry. 2011;2:2–11. [Google Scholar]
- Smallwood J, Brown K, Baird B, Schooler JW. Cooperation between the default mode network and the frontal-parietal network in the production of an internal train of thought. Brain Research. 2012;1428:60–70. doi: 10.1016/j.brainres.2011.03.072. [DOI] [PubMed] [Google Scholar]
- Smallwood J, McSpadden M, Schooler JW. The lights are on but no one’s home: Meta-awareness and the decoupling of attention when the mind wanders. Psychonomic Bulletin & Review. 2007;14:527–33. doi: 10.3758/bf03194102. [DOI] [PubMed] [Google Scholar]
- Smallwood J, Schooler JW. The restless mind. Psychological Bulletin. 2006;132:946–58. doi: 10.1037/0033-2909.132.6.946. [DOI] [PubMed] [Google Scholar]
- Soddu A, Boly M, Nir Y, et al. Reaching across the abyss: recent advances in functional magnetic resonance imaging and their potential relevance to disorders of consciousness. Progress in Brain Research. 2009;177:261–74. doi: 10.1016/S0079-6123(09)17718-X. [DOI] [PubMed] [Google Scholar]
- Spreng RN, Stevens WD, Chamberlain JP, Gilmore AW, Schacter DL. Default network activity, coupled with the frontoparietal control network, supports goal-directed cognition. NeuroImage. 2010;53:303–317. doi: 10.1016/j.neuroimage.2010.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swenson RA, Tucker DM. Multivariate analysis of EEG coherence: Stability of the metric, individual differences in patterning and response to arousal. Biological Psychology. 1983;17:59–75. doi: 10.1016/0301-0511(83)90066-2. [DOI] [PubMed] [Google Scholar]
- Tang YY, Posner MI. Special issue on mindfulness neuroscience. Social Cognitive and. Affective Neuroscience. 2013;8:1–3. doi: 10.1093/scan/nss104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang YY, Ma Y, Fan Y, et al. Central and autonomic nervous system interaction is altered by short-term meditation. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:8865–71. doi: 10.1073/pnas.0904031106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor VA, Daneault V, Grant J, et al. Impact of meditation training on the default mode network during a restful state. Social Cognitive and Affective Neuroscience. 2013;8:4–14. doi: 10.1093/scan/nsr087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian L, Jiang T, Liu Y, et al. The relationship within and between the extrinsic and intrinsic systems indicated by resting state correlational patterns of sensory cortices. NeuroImage. 2007;36:684–90. doi: 10.1016/j.neuroimage.2007.03.044. [DOI] [PubMed] [Google Scholar]
- Travis F. Autonomic and EEG patterns distinguish transcending from other experiences during Transcendental Meditation practice. International Journal of Psychophysiology. 2001;42:1–9. doi: 10.1016/s0167-8760(01)00143-x. [DOI] [PubMed] [Google Scholar]
- Travis FT. Eyes open and TM EEG patterns after one and after eight years of TM practice. Psychophysiology. 1991;28:S58. [Google Scholar]
- Travis F, Shear J. Focused attention, open monitoring and automatic self-transcending: Categories to organize meditations from Vedic, Buddhist and Chinese traditions. Consciousness and Cognition. 2010;19:1110–8. doi: 10.1016/j.concog.2010.01.007. [DOI] [PubMed] [Google Scholar]
- Travis F, Tecce J, Arenander A, Wallace RK. Patterns of EEG coherence, power, and contingent negative variation characterize the integration of transcendental and waking states. Biological Psychology. 2002;61:293–319. doi: 10.1016/s0301-0511(02)00048-0. [DOI] [PubMed] [Google Scholar]
- Travis F, Wallace RK. Autonomic and EEG patterns during eyes-closed rest and Transcendental Meditation (TM) practice: the basis for a neural model of TM practice. Consciousness and Cognition. 1999;8:302–18. doi: 10.1006/ccog.1999.0403. [DOI] [PubMed] [Google Scholar]
- Tucker DM, Roth DL, Bair TB. Functional connections among cortical regions: topography of EEG coherence. Electroencephalography and Clinical Neurophysiology. 1986;63:242–50. doi: 10.1016/0013-4694(86)90092-1. [DOI] [PubMed] [Google Scholar]
- Vincent JL, Kahn I, Snyder AZ, Raichle ME, Buckner RL. Evidence for a frontoparietal control system revealed by intrinsic functional connectivity. Journal of Neurophysiology. 2008;100:3328–42. doi: 10.1152/jn.90355.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh V. A theory of magnitude: common cortical metrics of time, space and quantity. Trends in Cognitive Sciences. 2003;7:483–8. doi: 10.1016/j.tics.2003.09.002. [DOI] [PubMed] [Google Scholar]
- Whitham EM, Pope KJ, Fitzgibbon SP, et al. Scalp electrical recording during paralysis: quantitative evidence that EEG frequencies above 20 Hz are contaminated by EMG. Clinical Neurophysiology. 2007;118:1877–88. doi: 10.1016/j.clinph.2007.04.027. [DOI] [PubMed] [Google Scholar]
- Wittmann M. The inner experience of time. Philosophical Transactions of the Royal Society B: Biological Sciences. 2009;364:1955–67. doi: 10.1098/rstb.2009.0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittmann M, Simmons AN, Aron JL, Paulus MP. Accumulation of neural activity in the posterior insula encodes the passage of time. Neuropsychologia. 2010;48:3110–20. doi: 10.1016/j.neuropsychologia.2010.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuval-Greenberg S, Deouell LY. The broadband-transient induced gamma-band response in scalp EEG reflects the execution of saccades. Brain Topography. 2009;22:3–6. doi: 10.1007/s10548-009-0077-6. [DOI] [PubMed] [Google Scholar]
- Zeidan F, Johnson SK, Diamond BJ, David Z, Goolkasian P. Mindfulness meditation improves cognition: Evidence of brief mental training. Consciousness and Cognition. 2010;19:597–605. doi: 10.1016/j.concog.2010.03.014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.