Abstract
Immunotoxins are a group of protein-based therapeutics, basically comprising two functional moieties: one is the antibody or antibody Fv fragment that allows the immunotoxin to bind specifically to target cells; another is the plant or bacterial toxin that kills the cells upon internalization. Immunotoxins have several unique features which are superior to conventional chemotherapeutics, including high specificity, extraordinary potency, and no known drug resistance. Development of immunotoxins evolves with time and technology, but significant progress has been achieved in the past 20 years after introduction of recombinant DNA technique and generation of the first single-chain variable fragment of monoclonal antibodies. Since then, more than 1,000 recombinant immunotoxins have been generated against cancer. However, most success in immunotoxin therapy has been achieved against hematological malignancies, several issues persist to be significant barriers for effective therapy of human solid tumors. Further development of immunotoxins will largely focus on the improvement of penetration capability to solid tumor mass and elimination of immunogenicity occurred when given repeatedly to patients. Promising strategies may include construction of recombinant antibody fragments with higher binding affinity and stability, elimination of immunodominant T- and B-cell epitopes of toxins, modification of immunotoxins with macromolecules like poly(ethylene glycol) and liposomes, and generation of immunotoxins with humanized antibody fragments and human endogenous cytotoxic enzymes. In this paper, we briefly reviewed the evolution of immunotoxin development and then discussed the challenges of immunotoxin therapy for human solid tumors and the potential strategies we may seek to overcome the challenges.
Keywords: Recombinant immunotoxin, cancer therapy, solid tumor, challenges, strategies
Introduction
In the last decade of the 19th century, Paul Ehrlich postulated that if a compound could be made that selectively targeted against a disease-causing organism, then a toxin for that organism could be delivered along with the agent of selectivity and he further created the term “antibody” for such products (1, 2). With great efforts of about one century, scientists have not only confirmed the presence of antibody but also unraveled its structure and function. Introduction of hybridoma technology by Kohler and Milstein in 1975 made it possible to produce monoclonal antibodies (MAbs) in a large scale (3). More recent development of recombinant DNA techniques provoked the interest of scientists to generate engineered antibody fragments as well as cytotoxic toxins (4, 5). Because of these technical achievements, antibody-based therapy has become one of the fastest growing fields in tumor therapy in recent years (6, 7).
In general, naked MAbs are rarely potent enough against cancer by themselves, they are more often used through linking cytotoxic chemical drugs or protein toxins. The former is usually called antibody-drug conjugates (ADCs), while the latter is known as immunotoxins (ITs). ADCs are generated by conjugating a MAb with a cytotoxic drug through a crosslinking reagent, while ITs are prepared by chemically conjugating an antibody or genetically fusing fragments of an antibody with a toxin, mostly from plants or bacteria such as ricin, saporin, Pseudomonas exotoxin A (PE), and Diphtheria toxin (DT) (8–10). Both ADCs and ITs are designed based on the concept that selective accumulation of cytotoxic agents at the tumor site and within the tumor cells can be achieved through the antibody specificity by targeting a specific antigen highly expressed by tumor cells, thereby improving therapeutic efficacy, while minimizing side effects induced by cytotoxic agents (11, 12). Since there have already had many excellent reviews on various aspects of ADCs, we focused in this review on the progress of IT development, and the major challenges we are facing and the potential strategies we may seek in the IT therapy of human solid tumors.
Evolution of IT Development
ITs are basically composed of two functional moieties: one is a MAb or Fv portions of an antibody; another is a plant or bacterial toxin. MAbs are known to be the most specific agent against an antigen expressed by cancer cells, while the toxin part is among the most potent agents against cancer cells. One single IT molecule can inactivate over 200 ribosomes or elongation factor-2 molecules per minute and is potent enough to kill a cell as compared to 104–105 molecules of a chemotherapeutic drug that are needed to kill one cell (13).
Development of ITs evolves with time and technology (5). The first generation of ITs was generated by coupling a native toxin with a MAb through a crosslinking reagent that forms disulfide bonds between the toxin and antibody moieties. However, native toxins induce severe side effects when given to humans due to their non-specific binding to normal cells. Native toxins are commonly composed of three domains: one is the receptor binding or cell recognition domain that enables the toxin to bind to the cell surface; one is the translocation domain that helps translocation of the A chain into cytosol; and the third one is the catalytic domain (also called activity domain or A chain) that exerts cytotoxic effects on cells upon translocation to the cytosol compartment (14, 15). The binding domains of different toxins recognize various receptors ubiquitiously on normal cells. The non-specific binding compromises the specificity of ITs, and induces severe systemic side effects. Thereby, toxins were deglycosylated and the binding domain was deleted when conjugated to MAbs, which led to the development of second generation of ITs. As expected, this approach significantly reduces the non-specific toxicities of ITs, allowing more ITs to be given to humans. Although the results were encouraging, some problems for the second generation ITs persisted, including: 1) poor stability due to the chemical crosslinking between antibody and toxin moieties; 2) heterogeneous composition and reduced binding affinity caused by the random conjugation; 3) poor penetration to solid tumor mass because of the large molecular size (>190 kDa); 4) immunogenicity; and 5) limited production (5, 16).
To improve the pharmacokinetics and reduce the side effects of ITs, great efforts have then been made to generate the third generation ITs which is called recombinant ITs (RITs). Development of RITs is driven by the ability to genetically design and express the antibody fragments and toxins with recombinant DNA techniques (17–19). Generally speaking, development of RITs involves two critical steps: 1) design and construct the recombinant antibody fragments and mutated PE or DT; and 2) expression and purification of the constructed products.
Regarding the expression of RITs, yeast, bacteria, CHO cells, and insect cells are the systems most frequently used (20–22). Each system has its unique features, but the most critical requirements for an expression system are the capability to properly fold complex proteins like RITs with multiple domains, and resistance to the toxin moiety. Cheap, fast, and easy to produce and purify the products is one more requirement. Bacterial systems are generally resistant to toxins and they are currently more widely used to generate RITs. A major limitation is that bacterial systems lack the ability to efficiently fold complex proteins. RITs with multiple domains must be denatured and refolded ex vivo to recover the binding capability and bioactivity. This also limits the yield of RIT production using bacterial systems. Toxin-resistant cell lines such as CHO and HEK293T are also used to produce RITs, but it is labor-intensive and time-consuming to select and characterize toxin-resistant cells (23). High cost for production is another issue for cell lines. Yeasts, like Pichia pastoris (P. pastoris), could grow in a simple, inexpensive medium with a high growth rate in either a shake flask or a fermenter, making it suitable for both small and large scale production. Importantly, P. pastoris is capable of properly folding RITs (20, 21). Similar to mammalian cell lines, P. pastoris is sensitive to toxins, thus it is also essential to select toxin-resistant strains.
Since the first report on generation of variable domain fragments in 1988, more than 1,000 RITs have been developed with different systems and development of RITs is becoming one of the fast-growing fields in cancer therapy (24, 25). This is also due to the superior features of RITs over the first two generations. First, RITs have a much smaller molecular size, which permits them penetrating into the deeper region of solid tumors. Second, RITs exhibit a more desirable pharmacokinetics with reduced immunogenicity and off-target toxicity. Third, application of engineered expression systems allows large-scale production of RITs more cost-effectively, eliminating the concern on production yield for clinical use. However, there are still some challenges we have to face, especially when RITs are used to treat human solid tumors and among them are the limited penetration capability and immunogenicity of RITs.
Limited Penetration Capability
1. Molecular size, binding affinity, and binding-site barrier
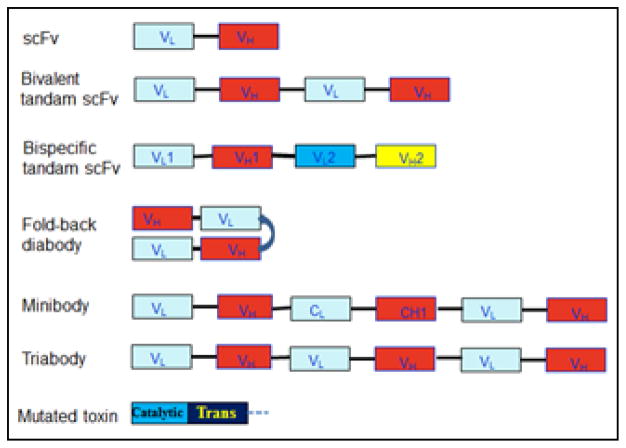
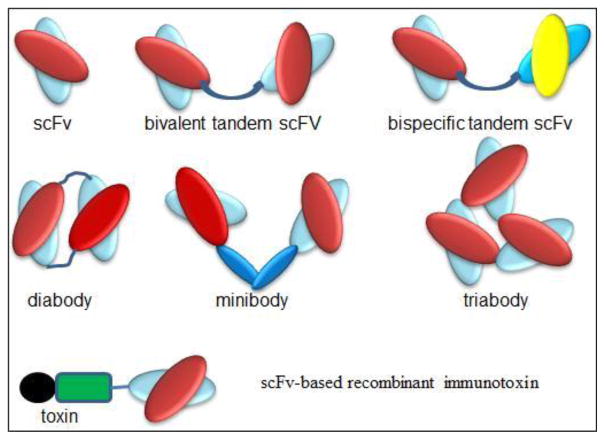
Antibodies share a relatively uniform and well-characterized protein structure. They are typically composed of two large heavy chains and two small light chains, presenting a “Y”-shape. One characteristic is that antibodies have a small and extremely variable region at the two tips of “Y” that allows millions of antibodies possessing different and specific antigen binding sites (26, 27). Therefore, the smallest engineered fragment of antibodies that retains the original binding site is the scFv (25–30 kDa), which consists of a variable heavy domain (VH) and a variable light domain (VL) joined by a linker of 10 to 25 amino acid peptide (Fig. 1 and 2). The peptide linker is usually rich in glycine for flexibility and serine or threonine for solubility. Therefore, the smallest RITs generated currently are those containing one scFv. Due to the small size (~60 kDa), these small RITs exhibit markedly improved penetration capability to solid tumor mass, but they are also cleared quickly from bloodstream (t1/2 = ~20 min) (28). They also have a low binding affinity due to the monovalency. The outcome is low tumor uptake and low therapeutic efficacy. More desirable pharmacokinetics has been achieved by constructing RITs using bivalent or divalent scFv (tandem scFv and diabody, 50–60 kDa), and scFv-fusion proteins (minibody, 80 kDa; scFv-Fc, 105 kDa) (29–30). Bivalent scFv is engineered by linking two scFvs with a peptide linker. This has been achieved with two formats: one is bivalent tandem scFv which is generated when the two scFvs form a single peptide chain; and another is bivalent scFv diabody which is generated by avoiding dimerization of the VH and VL domains from one scFv through a short linker (about five amino acids), while forcing the two scFvs to dimerize by using a long linker (about 15 amino acids) (Fig. 2). Bivalent scFv RITs have a high binding affinity close to full antibodies, which are due to the increase of bivalent binding fraction and a decrease of the equilibrium dissociation constant (KD). Under most conditions for bivalent scFv binding, two measurable KD exist with one for monovalent and the other for bivalent binding. The overall binding affinity of an antibody fragment is determined by the fraction of bivalent binding. Increasing the bivalent binding fraction is one approach to enhance the binding affinity, which can be achieved by optimize the primary and secondary structures (27). Wang et al. have compared the binding affinity among different formats and shown that the bivalent fold-back format of RITs is several folds higher than bivalent tandem and monovalent scFv formats (31). Bivalent scFv RITs also have a longer circulation time (t1/2 = ~40 min) than scFv RITs, but is still much shorter than antibody-toxin conjugates (t1/2 = 4–8 hours or more). Other formats such as triabodies, tetrabodies and scFv-Fc have also been produced, but these formats are less commonly used to construct RITs because the benefit from increased binding affinity could be compromised by the increased molecular size. An alternative development is bispecific tandem scFv which is generated by linking two scFvs from two antibodies targeting different antigens (32–34). The therapeutic benefit of bispecific RITs is still unclear.
Figure 1.
Constructs of different formats of recombinant antibody fragments and mutated toxin with deletion of the binding domain. VL and VH are the variable domains of light and heavy chains, respectively. scFv: single-chain fragment variable; Trans: translocation domain of toxin.
Figure 2.
Carton structures of different formats of recombinant antibody fragments and representative scFv-based immunotoxin.
As pointed out above, limited transvascular diffusion of RITs into solid tumors represents a major problem for cancer therapy. Similar to naked antibodies, penetration of RITs into tumors is a process of diffusion, which is greatly affected by the molecular size and binding affinity of RITs as well as by the properties of antigen (density, distribution, and internalization rate). Decreased penetration rate following binding with antigens is referred to as the “binding-site barrier” (35–37). In general, smaller RITs and those with higher binding affinity have better penetration capabilities. Some studies have shown that the binding-site barrier could be overcome by increasing the dose, but the off-target toxicity will increase too. In this respect, increasing the stability and circulating half-life of a RIT by optimizing its structure offers an approach to enhance the penetration and accumulation of RITs in tumors.
2. Modifications of RITs with macromolecules
Successful delivery of drugs has been achieved with PEGylated liposomes, polymeric micelles, lipoplexes, and polyplexes (38, 39). One successful example is the liposomal doxorubicin (Doxil), the first FDA-approved nanodrug (40). Success of Doxil is based on several unrelated principles including prolonged circulation time and avoidance of the reticuloendothelial system due to the use of PEGylated liposomes, and high and stable remote loading of doxorubicin driven by a transmembrane ammonium sulfate gradient, which also allows for drug release at tumor site. Because of the enhanced permeability and retention (EPR) effect in tumors, Doxil is passively targeted to tumors and its doxorubicin is released and becomes available to tumor cells. The EPR effect is based on the fact that the tumor vasculature is “leaky”, with an effective pore size of 200 nm to 600 nm in diameter in the endothelial lining of blood vessels (41, 42). The EPR effect allows for extravasation and accumulation of macromolecules in the interstitial space of tumors. Such accumulation is additionally affacted by the virtual lack of a lymphatic system, responsible for the drainage of macromolecules from normal tissues. Such strategies have also been used to extend the half-life of RITs. Wang et al. have reported that a PEGylated chimeric toxin composed of transforming growth factor-α and PE exhibited an improvement in its circulation time and a decrease in its immunogenicity (43). Studies by Tsutsumi et al. have also shown that PEGylation of the RIT (anti-Tac(Fv)-PE38, LMB-2) improved its antitumor activity and reduced its toxicity and immunogenicity (44). PEGylation and coating may reduce opsonization of RITs by blood proteins, prevent interaction with blood components, and minimize the uptake by the reticuloendothelial system (45).
PEGylation or other coatings, however, may hinder drug release and drug interaction with target cells, which can be an obstacle in the realization of therapeutic response (39, 45). Attempts have been made to avoid this situation by means of shedding (i.e. a loss of the coating after arrival at the target site --- extracellular or intracellular release) (46–48). Shedding has been designed with various strategies and one effective strategy is the use of a pH-sensitive functional group as a linker between the coating and its anchor by taking advantage of the low extracellular pH (as low as 6.0) in tumors. The pH-sensitive functional group such as diorthoester, orthoester, vinyl ether, phosphoramidate, hydrazone, and thiopropionate undergoes protonation in the low pH environment, leading to hydrolysis of the sensitive bond and therefore to collapse of the particles (38, 39). Although coating and shedding approach has been well tested for delivery of chemotherapeutic drugs, there are no reports for its use in RIT therapy.
Immunogenicity
1. Immunogenicity of antibody fragment and toxin moieties
IT therapy has been used successfully in patients with some types of hematological malignancies. Although easy access to tumor cells is a major factor, less human immunoreaction is another factor that is critical for the success. These patients typically have a severely compromised immune system because of the disease and previous chemotherapy. However patients with solid tumors often have a fairly healthy immune system. When RITs are given repeatedly to these patients, immunoreaction including neutralizing antibodies develops inevitably. Such a response does not necessarily cause severe side effects, but it may lead to a loss of the RIT efficacy and/or result in neutralization of the endogenous counterpart of patients (11, 49). Human immunoreaction is a major reason to stop repeated administration of RITs.
Both of the two components of RITs are immunogenic to humans, but the neutralizing antibodies are formed in patients mostly against the toxin moiety, occasionally against the mouse scFv when a mouse antibody sequence is used to construct the RITs. Reduced immunogenicity of antibody fragments is largely due to the removal of Fc region. A major intrinsic factor for the antibody’s immunogenicity is the presence of carbohydrate side chains attached to the antibody via glycosylation sites conferred by the amino acid sequence of the light and heavy chain Fv regions (50, 51).
PE and DT are the two toxins commonly used to construct RITs (15, 52, 53). PE is a 613 amino acid protein (66 kDa) originally produced by the bacterium Pseudomonas aeruginosa. A unique feature of PE is its resistance to various mutations without compromising its cytotoxicity. This characteristic enables PE to be modified genetically to raise its stability and lower its immunogenicity. To this end, various mutated versions of PE have been generated, and the 38 kDa and 40 kDa fragments (named PE38 and PE40, respectively) are the versions most frequently used in RIT construction. DT is a 535 amino acid protein (62 kDa) secreted by Corynebacterium diphtheria. Similar to PE, DT also has three functional domains, but organized in the reverse order. Except for the truncated fragments of DT486, DT389 and DT390, one modification of DT involves substitution of two amino acids at positions 390 and 525 in the C-terminal region, which results in a new molecule crossreacting material-107 (CRM-107) (54, 55). A major benefit of these mutated versions of PE and DT is the reduced non-specific toxicity to humans. For example, PE40 has been shown to be more than 100-fold less toxic than the native PE, and CRM-107 reduces the non-specific binding of native DT by 8000-fold, thus increasing the toxin’s tumor-specificity of 10,000-fold (13, 55). However, the immunogenicity of these mutated toxins remains to be an issue although reduced significantly. An associated issue is that the immunogenecity to humans is determined only in clinical trials or after product launch and there is still a debate on the suitability of animal models for immunogenicity prediction during drug development because of the species-specificity of the immune response (56, 57). Most studies have shown that conventional animal models over-estimate immunogenicity in patients, making them unsuitable to predict immunogenicity in patients. However, animal models are increasingly used for selected immunogenicity studies.
2. Efforts on minimizing immunogenicity and next generation of RITs
To mitigate the immunogenicity, several strategies have been tested. Efforts to decrease antibody responses to ITs with cyclophosphamide, cyclosporine, or rituximab have been unsuccessful (58, 59). Interestingly, combined use of RITs with immune-modulating agents has been shown to be an approach to reduce the generation of neutralizing antibodies. Studies by Mossoba et al. have shown that induction treatment with pentostatin and cyclophosphamide before IT therapy effectively prevents the formation of neutralizing antibodies in mice and patients treated with a RIT called SS1P, which is further improved by maintenance pentostatin and cyclophosphamide therapy (60–62). Pentostatin and cyclophosphamide are two agents that could severely deplete host B and T immune cells with relative sparing of host myeloid cells, and without activity in mesothelioma. Elimination of immunodominant T- and B-cell epitopes is another strategy under studies to reduce the toxin immunogenicity (63–65). Pastan et al. have identified seven major B-cell epitope groups with 13 subgroups by using 60 monoclonal antibodies against PE38 and by mutating large surface-exposed residues to alanine (65). It has been shown that deletion of the specific hydrophilic amino acids from PE38 protein has significantly reduced its immunogenicity but still retain its full cytotoxic activity (65).
The immuogenicity problem is also addressed by developing the next or fourth generation of RITs through using humanized or human antibody fragments and human endogenous cytotoxic enzymes such as RNase, Granzyme B, and death-associated protein kinase 2 (DAPK2) (66–68). RNase is a type of nuclease, playing critical roles in the maturation of RNA molecules as well as clearance of cellular RNA that is no longer required. Granzyme B is an immune defense protein that is secreted from the cytotoxic granules of activated cytotoxic T-cells and natural killer cells. Following the perforin-dependent translocation of Granzyme B into the cytoplasm of target cells, a proteolytic cascade can be initiated, which leads to target cell undergoing apoptosis. DAPK2 is an enzyme that belongs to the serine/threonine protein kinase family and functions as a positive mediator of apoptosis. Overproduction of DAPK2 protein has been shown to induce cell apoptosis. It is highly expected that the immunogenicity of fourth generation RITs will be minimized, but studies are still very limited and the therapeutic efficacy needs to be determined.
Summary
Reviewing various clinical and preclinical studies, there is no doubt that RITs are one of the most promising methods for cancer therapy. At present, limited penetration capability into solid tumors and immunogenecity represent the two major barriers or challenges for RIT therapy of human solid tumors. Others such as vascular leak syndrome and hepatotoxicity are observed as dose-limiting side effects in some patients, but both are relatively rare (69, 70). Vascular leak syndrome is characterized by increased vascular permeability accompanied by extravasation of fluids and proteins, resulting in interstitial edema and organ failure. Although the pathogenesis of vascular damage is poorly understood, RITs is considered to bind with endothelium to induce a direct toxic effect or activate leukocytes to induce inflammatory cascades and disrupt endothelial cell integrity of normal blood vessels. Hepatotoxicity is a common side effect for PE-based RITs, presumably attributed to TNF-alpha release of Kupffer cells following binding with PE (71). Optimizing the inherent relationship between amino acid sequence, structure, and function will be at the heart of further optimized engineering of the antibody fragment moiety, while full humanization of the RITs will be a key for elimination of the immunogenicity.
References
- 1.Gensini GF, Conti AA, Lippi D. The contributions of Paul Ehrlich to infectious disease. J Infect. 2007;54(3):221–4. doi: 10.1016/j.jinf.2004.05.022. [DOI] [PubMed] [Google Scholar]
- 2.Winau F, Westphal O, Winau R. Paul Ehrlich--in search of the magic bullet. Microbes Infect. 2004;6(8):786–9. doi: 10.1016/j.micinf.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 3.Kohler G, Milstein C. Continuous cultures of fused cells secreting antibody of prede ned speci city. Nat. 1975;256:495–7. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- 4.Niv R, Cohen CJ, Denkberg G, Segal D, Reiter Y. Antibody engineering for targeted therapy of cancer: recombinant Fv-immunotoxins. Curr Pharm Biotechnol. 2001;2(1):19–46. doi: 10.2174/1389201013378824. [DOI] [PubMed] [Google Scholar]
- 5.Pastan I, Hassan R, FitzGerald DJ, Kreitman RJ. Immunotoxin treatment of cancer. Annu Rev Med. 2007;58:221–37. doi: 10.1146/annurev.med.58.070605.115320. [DOI] [PubMed] [Google Scholar]
- 6.Sapra P, Shor B. Monoclonal antibody-based therapies in cancer: advances and challenges. Pharmacol Ther. 2013;138:452–69. doi: 10.1016/j.pharmthera.2013.03.004. [DOI] [PubMed] [Google Scholar]
- 7.Dillman RO. Cancer immunotherapy. Cancer Biother Radiopharm. 2011;26:1–64. doi: 10.1089/cbr.2010.0902. [DOI] [PubMed] [Google Scholar]
- 8.Dosio F, Brusa P, Cattel L. Immunotoxins and anticancer drug conjugate assemblies: the role of the linkage between components. Toxins (Basel) 2011;3:848–83. doi: 10.3390/toxins3070848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Becker N, Benhar I. Antibody-based immunotoxins for the treatment of cancer. Antibodies. 2012;1:39–69. [Google Scholar]
- 10.Janthur WD, Cantoni N, Mamot C. Drug conjugates such as antibody drug conjugates (ADCs), immunotoxins and immunoliposomes challenge daily clinical practice. Int J Mol Sci. 2012;13:16020–45. doi: 10.3390/ijms131216020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Madhumathi J, Verma RS. Therapeutic targets and recent advances in protein immunotoxins. Curr Opin Microbiol. 2012;15:300–9. doi: 10.1016/j.mib.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 12.Risberg K, Fodstad O, Andersson Y. Immunotoxins: a promising treatment modality for metastatic melanoma? The Ochsner J. 2012;10:193–9. [PMC free article] [PubMed] [Google Scholar]
- 13.Li YM, Hall WA. Targeted toxins in brain tumor therapy. Toxins (Basel) 2010;2(11):2645–62. doi: 10.3390/toxins2112645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pastan I, Hassan R, Fitzgerald DJ, Kreitman RJ. Immunotoxin therapy of cancer. Nat Rev Cancer. 2006;6:559–65. doi: 10.1038/nrc1891. [DOI] [PubMed] [Google Scholar]
- 15.Adkins I, Holubova J, Kosova M, Sadilkova L. Bacteria and their toxins tamed for immunotherapy. Curr Pharm Biotechnol. 2012;13:1446–73. doi: 10.2174/138920112800784835. [DOI] [PubMed] [Google Scholar]
- 16.Litvak-Greenfeld D, Benhar I. Risks and untoward toxicities of antibody-based immunoconjugates. Adv Drug Deliv Rev. 2012;64:1782–99. doi: 10.1016/j.addr.2012.05.013. [DOI] [PubMed] [Google Scholar]
- 17.Pranchevicius MC, Vieira TR. Production of recombinant immunotherapeutics for anticancer treatment: The role of bioengineering. Bioengineered. 2013;4(5):305–12. doi: 10.4161/bioe.24666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Govindan SV, Goldenberg DM. Designing immunoconjugates for cancer therapy. Expert Opin Biol Ther. 2012;12(7):873–90. doi: 10.1517/14712598.2012.685153. [DOI] [PubMed] [Google Scholar]
- 19.Zhang F, Shan L, Liu Y, Neville D, Woo JH, Chen Y, Korotcov A, Lin S, Huang S, Sridhar R, Liang W, Wang PC. An anti-PSMA bivalent immunotoxin exhibits specificity and efficacy for prostate cancer imaging and therapy. Adv Healthc Mater. 2013;2:736–44. doi: 10.1002/adhm.201200254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu YY, Woo JH, Neville DM. Overexpression of an anti-CD3 immunotoxin increases expression and secretion of molecular chaperone BiP/Kar2p by Pichia pastoris. Appl Environ Microbiol. 2005;71:5332–40. doi: 10.1128/AEM.71.9.5332-5340.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu YY, Woo JH, Neville DM. Targeted introduction of a diphtheria toxin resistant mutation into the chromosomal EF-2 locus of Pichia pastoris and expression of immunotoxin in the EF-2 mutants. Protein Expr Purif. 2003;30:262–74. doi: 10.1016/s1046-5928(03)00129-3. [DOI] [PubMed] [Google Scholar]
- 22.Wan L, Zeng L, Chen L, Huang Q, Li S, Lu Y, Li Y, Cheng J, Lu X. Expression, purification, and refolding of a novel immunotoxin containing humanized single-chain fragment variable antibody against CTLA4 and the N-terminal fragment of human perforin. Protein Expr Purif. 2006;48(2):307–13. doi: 10.1016/j.pep.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 23.Liu YY, Gordienko I, Mathias A, Ma S, Thompson J, Woo JH, Neville DM., Jr Expression of an anti-CD3 single-chain immunotoxin with a truncated diphtheria toxin in a mutant CHO cell line. Protein Expr Purif. 2000;19:304–11. doi: 10.1006/prep.2000.1255. [DOI] [PubMed] [Google Scholar]
- 24.Bird RE, Hardman KD, Jacobson JW, Johnson S, Kaufman BM, Lee SM, Lee T, Pope SH, Riordan GS, Whitlow M. Single-chain antigen-binding proteins. Science. 1988;242:423–6. doi: 10.1126/science.3140379. [DOI] [PubMed] [Google Scholar]
- 25.Huston JS, Levinson D, Mudgett-Hunter M, Tai MS, Novotny J, Margolies MN, Ridge RJ, Bruccoleri RE, Haber E, Crea R, Oppermann H. Protein engineering of antibody binding sites: Recovery of specific activity in an anti-digoxin single-chain Fv analogue produced in Escherichia coli. Proc Natl Acad Sci USA. 1988;85:5879–83. doi: 10.1073/pnas.85.16.5879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Holliger P, Hudson PJ. Engineered antibody fragments and the rise of single domains. Nat Biotechnol. 2005;23:1126–36. doi: 10.1038/nbt1142. [DOI] [PubMed] [Google Scholar]
- 27.Olafsen T, Wu AM. Antibody vectors for imaging. Semin Nucl Med. 2010;40:167–81. doi: 10.1053/j.semnuclmed.2009.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bühler P, Wetterauer D, Gierschner D, Wetterauer U, Beile UE, Wolf P. Influence of structural variations on biological activity of anti-PSMA scFv and immunotoxins targeting prostate cancer. Anticancer Res. 2010;30:3373–9. [PubMed] [Google Scholar]
- 29.Thompson J, Stavrou S, Weetall M, Hexham JM, Digan ME, Wang Z, Woo JH, Yu Y, Mathias A, Liu YY, Ma S, Gordienko I, Lake P, Neville DM., Jr Improved binding of a bivalent single-chain immunotoxin results in increased efficacy for in vivo T-cell depletion. Protein Eng. 2001;14:1035–41. doi: 10.1093/protein/14.12.1035. [DOI] [PubMed] [Google Scholar]
- 30.Nelson Al. Antibody fragments: hope and hype. MAbs. 2010;2:77–83. doi: 10.4161/mabs.2.1.10786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang Z, Kim GB, Woo JH, Liu YY, Mathias A, Stavrou S, Neville DM., Jr Improvement of a recombinant anti-monkey anti-CD3 diphtheria toxin based immunotoxin by yeast display affinity maturation of the scFv. Bioconjug Chem. 2007;18:947–55. doi: 10.1021/bc0603438. [DOI] [PubMed] [Google Scholar]
- 32.May C, Sapra P, Gerber HP. Advances in bispecific biotherapeutics for the treatment of cancer. Biochem Pharmacol. 2012;84(9):1105–12. doi: 10.1016/j.bcp.2012.07.011. [DOI] [PubMed] [Google Scholar]
- 33.Reichert JM. Bispecific antibodies and ADCs: Once and future kings? MAbs. 2011;3(4):329–30. doi: 10.4161/mabs.3.4.16589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Frankel AE, Woo JH. Bispecific immunotoxins. Leuk Res. 2009;33(9):1173–4. doi: 10.1016/j.leukres.2009.03.037. [DOI] [PubMed] [Google Scholar]
- 35.Saga T, Neumann RD, Heya T, Sato J, Kinuya S, Le N, Paik CH, Weinstein JN. Targeting cancer micrometastases with monoclonal antibodies: a binding-site barrier. Proc Natl Acad Sci USA. 1995;92(19):8999–9003. doi: 10.1073/pnas.92.19.8999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Weinstein JN, van Osdol W. Early intervention in cancer using monoclonal antibodies and other biological ligands: micropharmacology and the “binding site barrier”. Cancer Res. 1992;52(9 Suppl):2747s–51s. [PubMed] [Google Scholar]
- 37.Juweid M, Neumann R, Paik C, Perez-Bacete MJ, Sato J, van Osdol W, Weinstein JN. Micropharmacology of monoclonal antibodies in solid tumors: direct experimental evidence for a binding site barrier. Cancer Res. 1992;52(19):5144–53. [PubMed] [Google Scholar]
- 38.Drummond DC, Zignani M, Leroux J. Current status of pH-sensitive liposomes in drug delivery. Prog Lipid Res. 2000;39:409–60. doi: 10.1016/s0163-7827(00)00011-4. [DOI] [PubMed] [Google Scholar]
- 39.Romberg B, Hennink WE, Storm G. Sheddable coatings for long-circulating nanoparticles. Pharm Res. 2008;25:55–71. doi: 10.1007/s11095-007-9348-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Barenholz Y. Doxilreg;-the first FDA-approved nano-drug: lessons learned. J Control Release. 2012;160:117–34. doi: 10.1016/j.jconrel.2012.03.020. [DOI] [PubMed] [Google Scholar]
- 41.Maeda H, Nakamura H, Fang J. The EPR effect for macromolecular drug delivery to solid tumors: Improvement of tumor uptake, lowering of systemic toxicity, and distinct tumor imaging in vivo. Adv Drug Deliv Rev. 2013;65:71–9. doi: 10.1016/j.addr.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 42.Waite CL, Roth CM. Nanoscale drug delivery systems for enhanced drug penetration into solid tumors: current progress and opportunities. Crit Rev Biomed Eng. 2012;40:21–41. doi: 10.1615/critrevbiomedeng.v40.i1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang QC, Pai LH, Debinski W, FitzGerald DJ, Pastan I. Polyethylene glycol-modified chimeric toxin composed of transforming growth factor alpha and Pseudomonas exotoxin. Cancer Res. 1993;53:4588–94. [PubMed] [Google Scholar]
- 44.Tsutsumi Y, Onda M, Nagata S, Lee B, Kreitman RJ, Pastan I. Site-specific chemical modification with polyethylene glycol of recombinant immunotoxin anti-Tac(Fv)-PE38 (LMB-2) improves antitumor activity and reduces animal toxicity and immunogenicity. Proc Natl Acad Sci USA. 2000;97:8548–53. doi: 10.1073/pnas.140210597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Buse J, El-Aneed A. Properties, engineering and applications of lipid-based nanoparticle drug-delivery systems: current research and advances. Nanomed. 2010;5:1237–60. doi: 10.2217/nnm.10.107. [DOI] [PubMed] [Google Scholar]
- 46.Lu T, Wang Z, Ma Y, et al. Influence of polymer size, liposomal composition, surface charge, and temperature on the permeability of pH-sensitive liposomes containing lipid-anchored poly(2-ethylacrylic acid) Int J Nanomed. 2012;7:4917–26. doi: 10.2147/IJN.S35576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wehunt MP, Winschel CA, Khan AK, Guo TL, Abdrakhmanova GR, Sidorov V. Controlled drug-release system based on pH-sensitive chloride-triggerable liposomes. J Liposome Res. 2013;23:37–46. doi: 10.3109/08982104.2012.727423. [DOI] [PubMed] [Google Scholar]
- 48.Leite EA, Souza CM, Carvalho-Júnior AD, Coelho LG, Lana AM, Cassali GD, Oliveira MC. Encapsulation of cisplatin in long-circulating and pH-sensitive liposomes improves its antitumor effect and reduces acute toxicity. Int J Nanomed. 2012;7:5259–69. doi: 10.2147/IJN.S34652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kelly RJ, Sharon E, Pastan I, Hassan R. Mesothelin-targeted agents in clinical trials and in preclinical development. Mol Cancer Ther. 2012;11:517–25. doi: 10.1158/1535-7163.MCT-11-0454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Harding Fiona A, corresponding author, Stickler Marcia M, Razo Jennifer, DuBridge Robert B. The immunogenicity of humanized and fully human antibodies: Residual immunogenicity resides in the CDR regions. MAbs. 2010;2(3):256–65. doi: 10.4161/mabs.2.3.11641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Arnold JN, Wormald MR, Sim RB, Rudd PM, Dwek RA. The impact of glycosylation on the biological function and structure of human immunoglobulins. Annu Rev Immunol. 2007;25:21–50. doi: 10.1146/annurev.immunol.25.022106.141702. [DOI] [PubMed] [Google Scholar]
- 52.Antignani A, FitzGerald D. Immunotoxins: The Role of the Toxin. Toxins (Basel) 2013;5(8):1486–502. doi: 10.3390/toxins5081486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bernardes N, Chakrabarty AM, Fialho AM. Engineering of bacterial strains and their products for cancer therapy. Appl Microbiol Biotechnol. 2013;97(12):5189–99. doi: 10.1007/s00253-013-4926-6. [DOI] [PubMed] [Google Scholar]
- 54.Riedel CJ, Muraszko KM, Youle RJ. Diphtheria toxin mutant selectively kills cerebellar Purkinje neurons. Proc Natl Acad Sci USA. 1990;87(13):5051–5. doi: 10.1073/pnas.87.13.5051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Greenfield L, Johnson VG, Youle RJ. Mutations in diphtheria toxin separate binding from entry and amplify immunotoxin selectivity. Science. 1987;238:536–9. doi: 10.1126/science.3498987. [DOI] [PubMed] [Google Scholar]
- 56.Brinks V, Jiskoot W, Schellekens H. Immunogenicity of therapeutic proteins: the use of animal models. Pharm Res. 2011;28:2379–85. doi: 10.1007/s11095-011-0523-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bussiere JL. Animal models as indicators of immunogenicity of therapeutic proteins in humans. Dev Biol (Basel) 2003;112:135–9. [PubMed] [Google Scholar]
- 58.Selvaggi K, Saria EA, Schwartz R, Vlock DR, Ackerman S, Wedel N, Kirkwood JM, Jones H, Ernstoff MS. Phase I/II study of murine monoclonal antibody-ricin A chain (Xomazyme-Mel) immunoconjugate plus cyclosporine A in patients with metastatic melanoma. J Immunother. 1993;13:201–7. doi: 10.1097/00002371-199304000-00007. [DOI] [PubMed] [Google Scholar]
- 59.Hassan R, Williams-Gould J, Watson T, Pai-Scherf L, Pastan I. Pretreatment with rituximab does not inhibit the human immune response against the immunogenic protein LMB-1. Clin Cancer Res. 2004;10:16–8. doi: 10.1158/1078-0432.ccr-1160-3. [DOI] [PubMed] [Google Scholar]
- 60.Mossoba ME, Onda M, Taylor J, Massey PR, Treadwell S, Sharon E, Hassan R, Pastan I, Fowler DH. Pentostatin plus cyclophosphamide safely and effectively prevents immunotoxin immunogenicity in murine hosts. Clin Cancer Res. 2011;17(11):3697–705. doi: 10.1158/1078-0432.CCR-11-0493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hassan R, Miller AC, Sharon E, Thomas A, Reynolds JC, Ling A, Kreitman RJ, Miettinen MM, Steinberg SM, Fowler DH, Pastan I. Major cancer regressions in mesothelioma after treatment with an anti-mesothelin immunotoxin and immune suppression. Sci Transl Med. 2013;5(208):208ra147. doi: 10.1126/scitranslmed.3006941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Weldon JE, Xiang L, Zhang J, Beers R, Walker DA, Onda M, Hassan R, Pastan I. A recombinant immunotoxin against the tumor-associated antigen mesothelin reengineered for high activity, low off-target toxicity, and reduced antigenicity. Mol Cancer Ther. 2013;12:48–57. doi: 10.1158/1535-7163.MCT-12-0336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Liu W, Onda M, Lee B, Hassan R, Xiang L, Pastan I. Recombinant immunotoxin engineered for low immunogenicity and antigenicity by identifying and silencing human B-cell epitopes. Proc Natl Acad Sci USA. 2012;109:11782–7. doi: 10.1073/pnas.1209292109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Onda M, Nagata S, FitzGerald DJ, Beers R, Fisher RJ, Vincent JJ, Lee B, Nakamura M, Hwang J, Kreitman RJ, Hassan R, Pastan I. Characterization of the B cell epitopes associated with a truncated form of Pseudomonas exotoxin (PE38) used to make immunotoxins for the treatment of cancer patients. J Immunol. 2006;177:8822–34. doi: 10.4049/jimmunol.177.12.8822. [DOI] [PubMed] [Google Scholar]
- 65.Pastan I, Onda M, Weldon J, Fitzgerald D, Kreitman R. Immunotoxins with decreased immunogenicity and improved activity. Leuk Lymphoma. 2011;52 (Suppl 2):87–90. doi: 10.3109/10428194.2011.573039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Weidle UH, Georges G, Brinkmann U. Fully human targeted cytotoxic fusion proteins: new anticancer agents on the horizon. Cancer Genomics Proteomics. 2012;9(3):119–33. [PubMed] [Google Scholar]
- 67.Mathew M, Verma RS. Humanized immunotoxins: a new generation of immunotoxins for targeted cancer therapy. Cancer Sci. 2009;100(8):1359–65. doi: 10.1111/j.1349-7006.2009.01192.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rosenblum MG, Barth S. Development of novel, highly cytotoxic fusion constructs containing granzyme B: unique mechanisms and functions. Curr Pharm Des. 2009;15(23):2676–92. doi: 10.2174/138161209788923958. [DOI] [PubMed] [Google Scholar]
- 69.Liu XY, Pop LM, Schindler J, Vitetta ES. Immunotoxins constructed with chimeric, short-lived anti-CD22 monoclonal antibodies induce less vascular leak without loss of cytotoxicity. MAbs. 2012;4:57–68. doi: 10.4161/mabs.4.1.18348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Smallshaw JE, Ghetie V, Rizo J, Fulmer JR, Trahan LL, Ghetie MA, Vitetta ES. Genetic engineering of an immunotoxin to eliminate pulmonary vascular leak in mice. Nat Biotechnol. 2003;21:387–91. doi: 10.1038/nbt800. [DOI] [PubMed] [Google Scholar]
- 71.Rosenblum MG, Shawver LK, Marks JW, Brink J, Cheung L, Langton-Webster B. Recombinant immunotoxins directed against the c-erb-2/HER2/neu oncogene product: in vitro cytotoxicity, pharmacokinetics, and in vivo efficacy studies in xenograft models. Clin Cancer Res. 1999;5:865–74. [PubMed] [Google Scholar]