Abstract
Although voltage-gated sodium channels are known to be deployed along experimentally demyelinated axons, the molecular identities of the sodium channels expressed along axons in human demyelinating diseases such as multiple sclerosis (MS) have not been determined. Here we demonstrate changes in the expression of sodium channels in demyelinated axons in MS, with Nav1.6 confined to nodes of Ranvier in controls but with diffuse distribution of Nav1.2 and Nav1.6 along extensive regions of demyelinated axons within acute MS plaques. Using triple-labeled fluorescent immunocytochemistry, we also show that Nav1.6, which is known to produce a persistent sodium current, and the Na+/Ca2+ exchanger, which can be driven by persistent sodium current to import damaging levels of calcium into axons, are colocalized with β-amyloid precursor protein, a marker of axonal injury, in acute MS lesions. Our results demonstrate the molecular identities of the sodium channels expressed along demyelinated and degenerating axons in MS and suggest that coexpression of Nav1.6 and Na+/Ca2+ exchanger is associated with axonal degeneration in MS.
Keywords: demyelinating diseases, action potential conduction, axonal degeneration
Nine genes encode distinct voltage-gated sodium channels (Nav1.1–Nav1.9) with a common motif but with different amino acid sequences and physiological characteristics (1). Nav1.6 is the major sodium channel, which is clustered at the nodes of Ranvier (2), although Nav1.2 is also present at some nodes (3, 4). However, the identity of sodium-channel isoforms that are present along demyelinated axons in disorders such as multiple sclerosis (MS) has not been established. In MS, the loss of myelin produces failure of axonal action-potential conduction that is associated with clinical exacerbations, but axonal conduction can recover as a result of expression of new sodium channels along demyelinated axons, providing a substrate for remission of clinical deficits (5). Axonal degeneration also occurs in MS, contributes to persistent neurological deficits (6–9), and may involve persistently activated sodium channels that drive injurious reverse Na+/Ca2+ exchange (10, 11). Blocking of sodium channels prevents axonal degeneration within white matter tracts in a variety of disease models (11–17), including a model of MS, experimental autoimmune encephalomyelitis (EAE) (18, 19).
During the development of myelinated CNS tracts, Nav1.2 channels [which are also present along unmyelinated axons (3, 20, 21)] are initially expressed along premyelinated axons, with a transition to clusters of Nav1.6 at mature nodes of Ranvier (3, 22). In dysmyelinated axons from Shiverer mice, Nav1.2 channels are retained, and Nav1.6 is not expressed (3, 23), and in axons from Plp/–mice, which myelinate normally and then lose their myelin, there is a loss of Nav1.6 clustering and increased expression of Nav1.2 (24). Electrophysiological (25), cytochemical (26), and immunocytochemical (27–29) studies using pan-specific sodium-channel antibodies demonstrate a higher-than-normal density of sodium channels in chronically demyelinated axons but do not reveal the isoforms of the channels. A 4-fold increase in saxitoxin-binding sites in demyelinated white matter from MS patients also suggests the deployment of new Na channels (30) but gives no clues about the channel isoform(s) that are expressed.
Recent studies have demonstrated up-regulated expression of Nav1.2 and Nav1.6 along extensive regions of demyelinated axons in EAE (4, 31). Nav1.2 channels produce rapidly activating and inactivating currents (32–34) and appear to support action-potential conduction, which occurs before myelination (35, 36), suggesting that newly produced Nav1.2 channels can support conduction in demyelinated axons. Nav1.6 channels, on the other hand, produce a persistent current in addition to rapidly activating and inactivating currents (33, 37, 38), and Nav1.6 is coexpressed together with the Na+/Ca2+ exchanger (NCX) along degenerating axons in EAE (31).
In this study, we present an analysis of Nav1.6 and Nav1.2 sodium channels, and of the NCX, in white matter from the human CNS, obtained postmortem from patients with secondary progressive MS, and from control subjects with no neurological disease. We demonstrate that there are changes in the pattern of expression of sodium channels along demyelinated CNS axons in MS, with expression of Nav1.6 confined to nodes of Ranvier in control white matter and with diffuse expression of both Nav1.2 and Nav1.6 along extensive regions of demyelinated axons in MS. We also show that Nav1.6 (but not Nav1.2), coexpressed with the NCX, is associated with axonal injury in MS.
Materials and Methods
MS Tissue. Postmortem cervical spinal cord and optic nerve tissue, acquired by means of a rapid protocol from patients with disabling secondary progressive MS (n = 7; 46.1 ± 6.5 yr, mean disease duration 14.0 ± 3.7 yr) and from controls (n = 6; 66.4 ± 6.0 yr) with no neurological disease, was obtained from the NeuroResource tissue bank (Institute of Neurology, London) (39); 1-cm3 tissue blocks were placed in OCT mounting medium (Lamb, London) on cork discs, then gently stirred for 9 s in isopentane precooled in liquid nitrogen before storage in airtight containers at –80°C. All tissue analyzed was characterized by oil red O and hematoxylin staining of 10 μm sections taken in triplicate, i.e., immediately before, in the middle, and immediately after serial sections cut from each tissue block. Acute MS lesions with ongoing or recent demyelination were identified on the basis of the presence of substantial numbers (graded as ≥3 on a 0–5 scale) of oil red O-positive macrophages, containing neutral lipids resulting from myelin breakdown (40).
Immunocytochemistry. Tissue sections were processed for immunocytochemistry as described (4, 31). Briefly, sections were incubated simultaneously or in combination with anti-myelin basic protein (MBP) mouse IgG (1:4,000; Sternberger Monoclonals, Lutherville, MD), anti-β-amyloid precursor protein (β-APP) mouse IgG (1:100; Chemicon), anti-Caspr mouse IgG [1:500; provided by M. Rasband, University of Connecticut, Farmington (36)] or anti-NCX mouse IgM [NCX1 isoform, shown to be expressed in white matter axons (41)] (1:200; RDI, Flanders, NJ), anti-phosphorylated neurofilament (SMI-31, 1:20,000; Sternberger), anti-nonphosphorylated neurofilament (SMI-32, 1:20,000; Sternberger Monoclonals), and rabbit polyclonal antibodies to Nav1.6 (residues 1042–1061; 1:100; Alomone, Jerusalem) or Nav1.2 (residues 467–485; 1:100; Alomone). Sections were then washed in PBS, incubated with appropriate secondary antibodies, comprising goat anti-rabbit IgG-Cy3 (1:2,000; Amersham Biosciences), goat anti-mouse IgG-Alexa Fluor 488 (1:1,000; Molecular Probes), goat anti-mouse IgM-Alexa Fluor 488 (1:1,000; Molecular Probes), and goat anti-mouse IgG-Cy5 (1:200, Rockland, Gilbertsville, PA), in blocking solution for 3 h, washed in PBS, and mounted. Biotinylated Ricinus communis agglutinin 1 (1:200; Vector Laboratories) was used as a microglial/macrophage marker (42) and reacted with streptavidin-Cy5 (1:350; Amersham Biosciences) for detection. Control experiments, which included the omission of primary or secondary antibodies, showed no staining (data not shown).
Tissue Analysis. For analysis of sections, multiple representative images were accrued by confocal microscopy with a Nikon Eclipse E600 microscope. Analysis was confined to images in which axons were sectioned longitudinally as evident from the presence of linear (presumably demyelinated) axonal profiles with diffuse sodium-channel immunoreactivity, running for >20–30 μm along the fiber tract within the plane of single sections.
In contrast to control white matter in which there was focal expression of Nav1.6 and absence of Nav1.2 at nodes of Ranvier (delineated by Caspr immunolabeling), we observed multiple extensive regions of diffuse Nav1.2 and/or Nav1.6 sodium-channel immunoreactivity along demyelinated (demonstrated by the lack of MBP immunostaining) axons (Figs. 1 and 2); neurofilament staining confirmed the axonal identity of these profiles (Fig. 2). As an index of the frequency of axonal profiles, we counted the number of these profiles that displayed diffuse regions of immunostaining for either Nav1.2 or Nav1.6, extending >10 μm in length (therefore excluding nodal foci of immunostaining) as described (31). For quantification, a target line extending across the width of the image and perpendicular to the axis of the nerve fibers was overlaid on 10–18 randomly selected images (each 300 × 300 μm) per subject, and axonal profiles (>10-μm length) with sodium-channel immunostaining that intersected the target line were counted. This approach was used in preference to expressing data per unit area because it negates the chance of duplicating quantification for a given axon as it moves in and out the tissue plane. An estimate of the number of injured axons per mm3 can be extrapolated from the data assuming a section thickness of 10 μm and a window of 9 × 104 μm2 (300 μm × 300 μm) for each image oriented perpendicular to the axis of the fiber tract. To facilitate the identification of immunopositive profiles and remove observer bias, quantitative microdensitometry was performed by using iplab image processing software (Scanalytics, Fairfax, VA) (4). Signal intensities were obtained by manually outlining profiles (10- to 15-μm length) and, using iplab, we integrated densitometry function to calculate mean signal intensities for the outlined areas. A profile was identified as a detectable linear outline extending for >10 μm within the plane of section and as immunopositive if it displayed an optical intensity at least twice that of background levels. A minimum of 400 profiles per subject (control, n = 3; MS, n = 4) were identified (total 1,628 profiles examined in MS lesions) from multiple images. Statistical analysis was performed using the Student t test and the χ2 test. The quantitative data presented represent the mean number of axonal profiles with diffuse Nav1.2 or Nav1.6 immunostaining ± SEM per 600 μm of target in control and MS spinal cord.
Fig. 1.
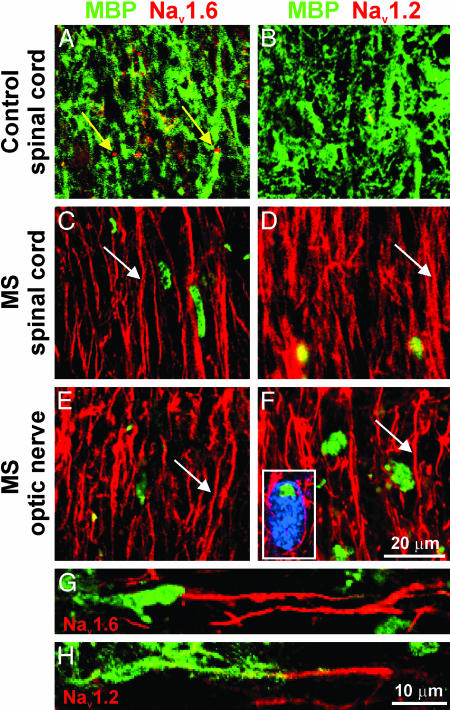
Nav1.6 and Nav1.2 sodium channels are expressed along extensive regions of demyelinated axons in MS. Shown are representative images demonstrating sections of white matter from spinal cord (A–D) and optic nerve (E and F) immunostained for MBP (green) as a marker of myelination and for sodium channels Nav1.6 (A, C, and E; red) and Nav1.2 (B, D, and F; red). Control spinal cord white matter (A and B) demonstrates robust MBP immunostaining consistent with myelinated axons and does not display axonal profiles with diffuse (>10 μm) sodium-channel immunostaining. Small foci of Nav1.6 immunostaining (A; yellow arrows) are present, consistent with the focal distribution of Nav1.6 at nodes of Ranvier in control spinal cord white matter, whereas Nav1.2 immunostaining is absent (B). Within acute MS lesions from spinal cord (C and D) and optic nerve (E and F), there is significant demyelination as evident by marked attenuation of MBP immunostaining (residual foci of MBP immunostaining represent intracellular MBP products within macrophages, identified with Ricinus communis agglutinin 1 labeling; blue, F Inset). Multiple axonal profiles within these lesions display diffuse sodium-channel immunostaining (extending in many axons for >20 μm; C, D, E, and F, white arrows) for Nav1.6 (C and E) and Nav1.2 (D and F). (G and H) Shown is the edge of active spinal cord plaques in MS and diffuse sodium-channel immunostaining for Nav1.6 (G; red) and Nav1.2 (H; red) along regions of axons where MBP immunostaining (green) is absent or markedly attenuated.
Fig. 2.
Nav1.6 and Nav1.2 immunostaining in human control CNS and in MS. Shown are representative digital images of sections of postmortem spinal cord white matter from control (A and B) and MS (C–L) patients, immunostained to show Nav1.6 (red), Nav1.2 (red), Caspr (green), and neurofilaments (blue). In control white matter (A) and in normal-appearing white matter (NAWM) in MS tissue (C), Nav1.6 is localized at nodes of Ranvier and is bounded by Caspr without appreciable overlap, whereas Nav1.2 is not detectable (B and D). Within MS plaques, linear axonal profiles with continuous Nav1.6 (E) and Nav1.2 (F) immunostaining are present. In some instances an extensive zone of Nav1.6 (G) or Nav1.2 (H) immunostaining is bounded by Caspr, without overlap. Colocalization of Nav1.6 (I) and Nav1.2 (J) with neurofilament immunostaining (SMI 31/32; K and L; blue) further establishes the identity of these profiles as axons.
Results
Nav1.6 and Nav1.2 Sodium Channels Are Expressed Along Demyelinated Axons in Acute MS Plaques. Control white matter from patients with no neurological disease displayed abundant staining for MBP (Fig. 1 A and B) and a pattern of expression of Nav1.6 and Nav1.2 similar to the pattern in rodents. An antibody recognizing Caspr, an integral constituent of paranodal junctions that is believed to participate in demarcation of ion channel domains at nodes of Ranvier (43, 44), was used to delineate nodal regions. Examination of control spinal cord and optic nerve demonstrated focal Nav1.6 immunostaining at nodes of Ranvier. The expression of Nav1.6 was confined to nodal regions, and the nodal foci of Nav1.6 immunostaining were bounded by Caspr without evidence of overlap, consistent with previous reports in rodents (2, 45) (Fig. 2 A). Very few (<1%) nodes of Ranvier demonstrated Nav1.2 immunostaining.
White matter from control spinal cords showed diffuse nonnodal (>10-μm length) Nav1.2 immunolabeling of a small number of axons consistent with immunolabeling along unmyelinated fibers, which are known to express Nav1.2 (3, 20, 21), with a total of 10.4 ± 1.2 axon profiles with diffuse Nav1.2 immunostaining per 600-μm target. Only a very small number of axon profiles displayed diffuse Nav1.6 immunostaining (2.2 ± 1.6 axon profiles per 600-μm target; Fig. 3); these did not express NCX or β-APP (data not shown) and are likely to represent nonmyelinated fibers, where Nav1.6 is known to be expressed (46). Very low levels of NCX were detected at a small number of control nodes.
Fig. 3.
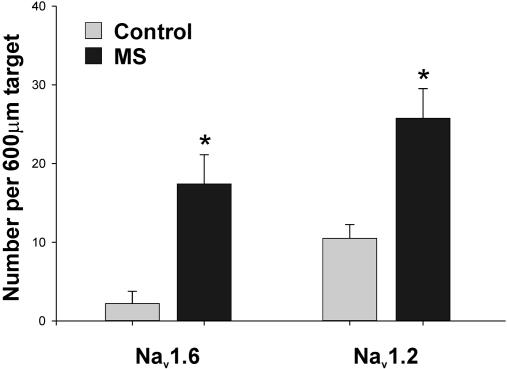
Increased number of axons with extensive Nav1.6 and Nav1.2 immunostaining in MS spinal cord white matter. This histogram demonstrates a significant increase in the number of axons displaying diffuse sodium-channel immunostaining extending >10 μm along the fiber axis in MS spinal cord lesions. *, P < 0.05 compared with controls.
Acute MS lesions, identified by the presence of substantial numbers of oil red O-positive macrophages (40), displayed a distinctly different pattern of sodium-channel expression. Within MS lesions, there was a significant increase in the number of presumably demyelinated axons running along the fiber tract in regions of attenuated MBP immunostaining (Fig. 1 C–F), which display extensive (>10 μm) sodium-channel immunostaining. Fig. 2 I–L illustrates staining of these profiles for neurofilaments, establishing their identity as axons. Neurofilament staining occasionally demonstrated axonal tortuosity or swelling suggestive of injury, but this was not used to identify or quantitate injured axons, which were definitively identified by staining for β-APP (see below). In some cases, the extensive zone of Nav1.6 or Nav1.2 staining was bounded by Caspr (Fig. 2 G and H), which further confirmed the identity of the profile as an axon. As at normal nodes, there was no overlap between Nav1.6 and Caspr immunostaining (Fig. 2 G and H). There was an 8-fold increase in the number of axons with diffuse Nav1.6 immunostaining (17.4 ± 3.7 per 600-μm target; P < 0.05 compared with controls) and a 2.5-fold increase in the number of axons with Nav1.2 immunostaining (25.8 ± 3.8 per 600-μm target; P < 0.05 compared with controls) within MS lesions in the spinal cord (Fig. 3). Similar changes were observed in the optic nerve of MS subjects (data not shown) where, importantly, unmyelinated fibers are not normally present (35).
Nodes of Ranvier within normal-appearing white matter from MS subjects appeared to display an arrangement similar to controls, with nodal foci of Nav1.6 immunostaining bounded by Caspr (Fig. 2 C and D); a quantitative analysis of nodal immunoreactivity in normal-appearing white matter was not carried out.
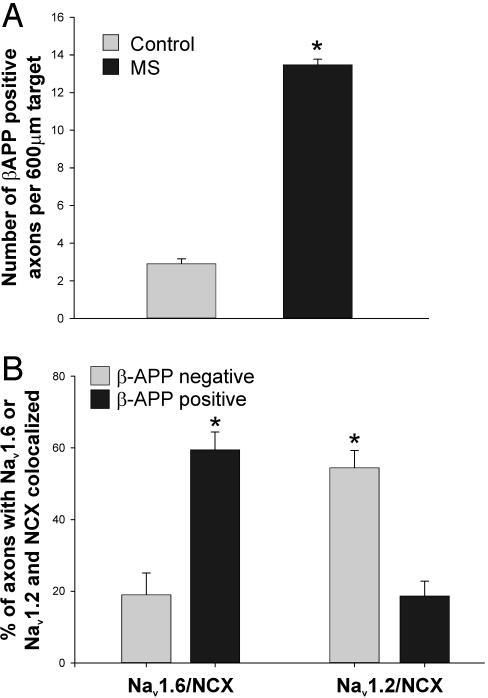
Nav1.6 Is Associated with Axonal Injury in MS. Studies in EAE (31) indicate that expression of Nav1.6, but not Nav1.2, over extended regions is associated with axonal injury. To delineate the relationship of Nav1.6 compared with Nav1.2 sodium-channel expression and axonal injury in MS, their colocalization with β-APP, a marker of axonal injury (7, 47, 48), was examined in spinal cord sections. Consistent with previous studies (7, 47, 48), we demonstrated evidence of axonal injury in acute MS lesions, with a 5-fold increase in the number of β-APP-positive axons (13.5 ± 0.3 per 600-μm target in MS, compared with 2.9 ± 0.3 per 600-μm target in controls; P < 0.001) (Fig. 4A). These β-APP-immunopositive axons in MS tended to express Nav1.6 over extensive regions. Diffuse Nav1.6 sodium-channel immunostaining was expressed in 82.3 ± 3.2% (n = 4 patients; 425 axons) of β-APP-immunopositive axons. In contrast, only 21.7 ± 4.7% (n = 4 patients; 309 axons, P < 0.001) of β-APP-immunopositive axons expressed diffuse Nav1.2 sodium-channel immunostaining. Assuming a section thickness of 10 μm, our results suggest the presence of 7,500 injured axons per mm3 of tissue within these acute lesions, similar to the value (11,000/mm3) reported by Trapp et al. (7).
Fig. 4.
(A) Increased β-APP expression in acute MS lesions. This histogram illustrates a significant increase in the number of axonal profiles that are β-APP-positive in MS. *, P < 0.001 compared with controls. (B) NCX and Nav1.6 are coexpressed in β-APP-positive axons in MS. Triple immunolabeling was used to determine the proportion of β-APP-positive axons, and β-APP-negative axons, that coexpress NCX and Nav1.6, or NCX and Nav1.2, over extensive regions. The proportion of axons that coexpress Nav1.6 and NCX is significantly higher in β-APP-positive axons than in β-APP-negative axons. *, P < 0.005.
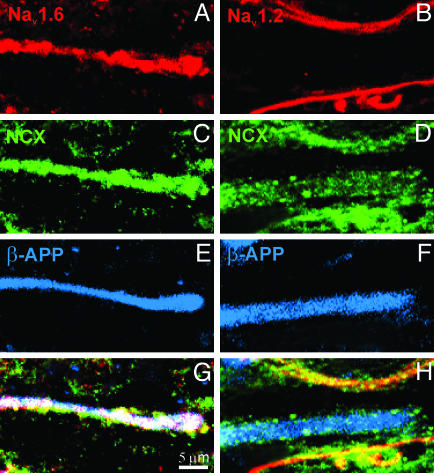
Nav1.6 and NCX Are Colocalized Within β-APP-Positive Axons in Acute MS Lesions. Electrophysiological studies indicate that reverse operation of the NCX, driven by a persistent sodium influx through sodium channels, can lead to a deleterious accumulation of Ca2+, resulting in degeneration of axons within white matter (10, 11). We have recently demonstrated that Nav1.6 is colocalized with NCX in β-APP-positive axons in EAE (31). Therefore, we asked whether a similar pattern was present in MS lesions, using triple-label immunocytochemistry to colocalize β-APP with Nav1.6 and NCX, or with Nav1.2 and NCX. β-APP-positive axons tended to coexpress Nav1.6 and NCX (Fig. 5). The percentage of β-APP-positive axons that displayed extensive regions of Nav1.6/NCX immunolabeling (i.e., that displayed both Nav1.6 and NCX) was 59.4 ± 5.0% (n = 4 patients, 425 axons), significantly greater than the percentage of β-APP-negative axons that displayed Nav1.6/NCX immunolabeling (18.9 ± 6.1%, n = 4 patients, 256 axons; P < 0.005) (Figs. 4B and 5). In contrast Nav1.2 and NCX tended to be coexpressed in β-APP-negative axons; only 18.7 ± 4.1% of β-APP-positive axons coexpressed Nav1.2 and NCX (n = 4 patients, 309 axons), whereas 56.4 ± 4.8% of β-APP-negative axons coexpressed Nav1.2 and NCX (n = 4 patients, 638 axons; P < 0.005) (Figs. 4B and 5). Thus the majority of β-APP-positive axons in MS display extensive regions where both Nav1.6 and NCX are present, whereas the majority of β-APP-negative axons coexpress Nav1.2 and NCX.
Fig. 5.
β-APP-positive spinal cord axons coexpress NCX and Nav1.6 over extensive regions in acute MS lesions. Digital images demonstrate axons in MS spinal cord white matter immunostained for β-APP (E and F; blue), sodium channel Nav1.6 (A; red) or Nav1.2 (B; red), and NCX (C and D; green). G and H correspond to merged images (white). A, C, E, and G show coexpression of Nav1.6 and NCX within axons displaying β-APP, a marker of axonal injury. In contrast, B, D, F, and H demonstrate NCX-immunopositive staining but an absence of Nav1.2 immunostaining within β-APP-positive axons, and coexpression of NCX and Nav1.2 within β-APP-negative axons.
Discussion
In this study we identify the sodium channels that are expressed along axons within MS lesions. We show that, whereas Nav1.6 is expressed focally at nodes of Ranvier in control white matter, two sodium-channel isoforms, Nav1.2 and Nav1.6, are expressed along extensive regions of demyelinated axons from acute MS lesions. We also demonstrate the selective colocalization of Nav1.6 and the NCX within axons expressing β-APP, a marker of axonal injury, in MS.
Although lacking in molecular specificity, earlier studies demonstrated that, whereas action-potential generation is confined to the nodal zones where sodium channels are clustered in normal myelinated and acutely demyelinated axons (49–52), some demyelinated axons develop a continuous mode of action-potential conduction, which is supported by a more diffuse distribution of channels (25). Early morphological studies using cytochemical (26) and immunocytochemical labeling methods with pan-specific antibodies, which do not distinguish between subtypes of sodium channels (27–29), demonstrated higher-than-normal densities of sodium channels along extensive regions of demyelinated axons, consistent with the development of continuous conduction. A recent study using subtype-specific antibodies showed that Nav1.2 and Nav1.6 are distributed diffusely along extensive regions of demyelinated axons in EAE (4).
Based on their physiological properties, it would be expected that Nav1.2 and Nav1.6, which both produce rapidly activating and inactivating currents (32–34), would both support action-potential generation. Consistent with this, Nav1.6 is the predominant sodium channel at nodes of Ranvier (2), whereas Nav1.2 is expressed along premyelinated CNS axons (3, 22), which are known to conduct action potentials (35, 36). There is evidence suggesting that some sodium channels, when colocalized with the NCX, can contribute to axonal degeneration. Studies in the optic nerve have demonstrated that sustained sodium influx through sodium channels can drive reverse Na+/Ca2+ exchange that triggers Ca2+-mediated axonal degeneration (11). Block of sodium channels and of the NCX prevents white matter axon degeneration after a variety of insults (11–15) including injury produced by NO (16, 17), which is present at increased concentrations within MS lesions. In EAE, the sodium-channel blockers phenytoin (18) and flecainide (19) have a protective effect, preventing the degeneration of CNS axons, maintaining axonal conduction, and improving clinical outcome.
Several lines of evidence suggest that Nav1.6 contributes to the persistent current that drives reverse Na+/Ca2+ exchange in injured axons in MS, as suggested in the model illustrated in Fig. 6. Myelinated axons, which are sensitive to injury produced by reverse Na+/Ca2+ exchange driven by a persistent Na current (10, 11), express Nav1.6 at higher levels than other sodium channels (2, 4). In contrast, dysmyelinating CNS axons, which express Nav1.2 rather than Nav1.6, (3, 23), are substantially less sensitive than myelinated axons to this type of injury (53). Nav1.6 channels produce a persistent current in addition to a transient current (33, 37), and the persistent current produced by Nav1.6 is much larger than the persistent current produced by Nav1.2 (33). Herzog et al. (38) performed patch-clamp analysis on dorsal root ganglion neurons expressing recombinant Nav1.6 channels and detected persistent Nav1.6 currents in all cells that were studied. The present results from MS, like recent studies in EAE (31), show an association between expression of Nav1.6, but not Nav1.2, and axonal degeneration; Nav1.6 and the NCX were both, in fact, detectable and colocalized in 60% of β-APP-labeled axons in MS but were found to be coexpressed in <20% of axons that were β-APP-negative. We observed extensive regions of coexpression of Nav1.6 and NCX often extending for >50 μm; however, it was not possible to follow single axons for the extent of the entire lesion, and although we predict that the colocalization of Nav1.6 and NCX induces a focal injury, we cannot exclude a more diffuse process. It might be argued that diffuse Nav1.6 immunostaining along extensive regions of axons in MS is due to a damming up of an intracellular pool of Nav1.6 channels as a result of impaired axoplasmic transport associated with demyelination or axonal injury, rather than insertion of Nav1.6 channels along the demyelinated axon membrane. However, this argument is not supported by our observations, which indicate that regions of Nav1.6 expression along demyelinated axons are bounded by Caspr without overlap, as they are in normal myelinated axons (43, 44). Thus we propose that these diffusely expressed Nav1.6 channels are inserted within the axon membrane and contribute to the model proposed in Fig. 6.
Fig. 6.
Proposed mechanism of axonal injury by means of coexpression of Nav1.6 and NCX. The model suggests that Nav1.6 sodium channels are up-regulated (1) and expressed along some demyelinated axons, where they produce persistent sodium current (2). The persistent sodium current can drive reverse sodium/calcium exchange (3) and accumulation of intraaxonal calcium (4), triggering injurious secondary cascades and axonal injury.
As in our earlier studies in EAE (31), we observed extensive regions of expression of Nav1.6 in >80% of β-APP-positive axons and coexpression of Nav1.6 and NCX in a majority of β-APP-positive axons in MS, but were unable to detect these extensive zones of coexpression in the remaining 40% of β-APP-positive axons. Moreover, we observed coexpression of Nav1.6 and NCX in 18% of β-APP-negative axons. Although we are unable to explain these apparent discrepancies, it should be noted that sodium influx via voltage-gated sodium channels occurs relatively early in the axonal injury cascade (11), possibly earlier than β-APP can be detected, and that, as axonal injury proceeds and axonal protein molecules are degraded, Nav1.6 and NCX levels may fall below detectable levels (31). Irrespective of this point, the present findings show a high level of coexpression of Nav1.6 and NCX, but not of Nav1.2 and NCX, in injured axons in MS.
In the absence of more complete clinical histories, it is not possible to correlate the changes that we have observed in demyelinated axons in MS with clinical status; future studies, in which clinically symptomatic lesions are compared with clinically silent ones, may permit such correlation. We cannot comment, on the basis of the present results, on the mechanisms that determine whether a given axon will express Nav1.6 or Nav1.2; neurotrophic factors play a role in regulating neuronal sodium-channel expression (54–56), but other factors may also be involved. Irrespective of this, our results provide a demonstration of molecular plasticity along demyelinated axons in the human CNS in MS, in which the pattern of sodium-channel expression is altered compared with normal myelinated axons. Our results indicate, specifically, that Nav1.2 and Nav1.6 are expressed along demyelinated axons in MS and suggest that the presence of these two channel isoforms may affect axonal function in different ways.
Acknowledgments
M.J.C. thanks Medical Director General, U.K., for support. We thank Dr. Matthew Rasband for the generous gift of Caspr antibody and critical appraisal of the manuscript, and Lynda Tyrell, Pamela Zwinger, and BartToftness for excellent technical assistance. This work was supported in part by grants from the National Multiple Sclerosis Society and the Medical Research Service and Rehabilitation Research Service, Department of Veteran Affairs, and by gifts from the Paralyzed Veterans of America, the United Spinal Association, and the Nancy Davis Foundation.
Abbreviations: MS, multiple sclerosis; MBP, myelin basic protein; β-APP, β-amyloid precursor protein; NCX, Na+/Ca+ exchanger; EAE, experimental autoimmune encephalomyelitis.
References
- 1.Goldin, A. L., Barchi, R. L., Caldwell, J. H., Hofmann, F., Howe, J. R., Hunter, J. C., Kallen, R. G., Mandel, G., Meisler, M. H., Netter, Y. B., et al. (2000) Neuron 28, 365–368. [DOI] [PubMed] [Google Scholar]
- 2.Caldwell, J. H., Schaller, K. L., Lasher, R. S., Peles, E. & Levinson, S. R. (2000) Proc. Natl. Acad. Sci. USA 97, 5616–5620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boiko, T., Rasband, M. N., Levinson, S. R., Caldwell, J. H., Mandel, G., Trimmer, J. S. & Matthews, G. (2001) Neuron 30, 91–104. [DOI] [PubMed] [Google Scholar]
- 4.Craner, M. J., Lo, A. C., Black, J. A. & Waxman, S. G. (2003) Brain 126, 1552–1561. [DOI] [PubMed] [Google Scholar]
- 5.Waxman, S. G. (1998) N. Engl. J. Med. 338, 323–325. [DOI] [PubMed] [Google Scholar]
- 6.Ferguson, B., Matyszak, M. K., Esiri, M. M. & Perry, V. H. (1997) Brain 120, 393–399. [DOI] [PubMed] [Google Scholar]
- 7.Trapp, B. D., Peterson, J., Ransohoff, R. M., Rudick, R., Mork, S. & Bo, L. (1998) N. Engl. J. Med. 338, 278–285. [DOI] [PubMed] [Google Scholar]
- 8.Lovas, G., Szilagyi, N., Majtenyi, K., Palkovits, M. & Komoly, S. (2000) Brain 123, 308–317. [DOI] [PubMed] [Google Scholar]
- 9.Bjartmar, C., Kidd, G., Mork, S., Rudick, R. & Trapp, B. D. (2000) Ann. Neurol. 48, 893–901. [PubMed] [Google Scholar]
- 10.Stys, P. K., Sontheimer, H., Ransom, B. R. & Waxman, S. G. (1993) Proc. Natl. Acad. Sci. USA 90, 6976–6980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stys, P. K., Waxman, S. G. & Ransom, B. R. (1992) J. Neurosci. 12, 430–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stys, P. K., Ransom, B. R. & Waxman, S. G. (1992) J. Neurophysiol. 67, 236–240. [DOI] [PubMed] [Google Scholar]
- 13.Imaizumi, T., Kocsis, J. D. & Waxman, S. G. (1998) Brain Res. 779, 292–296. [DOI] [PubMed] [Google Scholar]
- 14.Agrawal, S. K. & Fehlings, M. G. (1996) J. Neurosci. 16, 545–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rosenberg, L. J., Teng, Y. D. & Wrathall, J. R. (1999) J. Neurosci. 19, 6122–6133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garthwaite, G., Goodwin, D. A., Batchelor, A. M., Leeming, K. & Garthwaite, J. (2002) Neuroscience 109, 145–155. [DOI] [PubMed] [Google Scholar]
- 17.Kapoor, R., Davies, M., Blaker, P. A., Hall, S. M. & Smith, K. J. (2003) Ann. Neurol. 53, 174–180. [DOI] [PubMed] [Google Scholar]
- 18.Lo, A. C., Saab, C. Y., Black, J. A. & Waxman, S. G. (2003) J. Neurophysiol. 90, 3566–3571. [DOI] [PubMed] [Google Scholar]
- 19.Bechtold, D. A., Kapoor, R. & Smith, K. J. (2004) Ann. Neurol. 55, 607–616. [DOI] [PubMed] [Google Scholar]
- 20.Westenbroek, R. E., Merrick, D. K. & Catterall, W. A. (1989) Neuron 3, 695–704. [DOI] [PubMed] [Google Scholar]
- 21.Gong, B., Rhodes, K. J., Bekele-Arcuri, Z. & Trimmer, J. S. (1999) J. Comp. Neurol. 412, 342–352. [PubMed] [Google Scholar]
- 22.Kaplan, M. R., Cho, M. H., Ullian, E. M., Isom, L. L., Levinson, S. R. & Barres, B. A. (2001) Neuron 30, 105–119. [DOI] [PubMed] [Google Scholar]
- 23.Westenbroek, R. E., Noebels, J. L. & Catterall, W. A. (1992) J. Neurosci. 12, 2259–2267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rasband, M. N., Kagawa, T., Park, E. W., Ikenaka, K. & Trimmer, J. S. (2003) J. Neurosci. Res. 73, 465–470. [DOI] [PubMed] [Google Scholar]
- 25.Bostock, H. & Sears, T. A. (1978) J. Physiol. 280, 273–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Foster, R. E., Whalen, C. C. & Waxman, S. G. (1980) Science 210, 661–663. [DOI] [PubMed] [Google Scholar]
- 27.Novakovic, S. D., Levinson, S. R., Schachner, M. & Shrager, P. (1998) Muscle Nerve 21, 1019–1032. [DOI] [PubMed] [Google Scholar]
- 28.England, J. D., Gamboni, F. & Levinson, S. R. (1991) Brain Res. 548, 334–337. [DOI] [PubMed] [Google Scholar]
- 29.England, J. D., Gamboni, F., Levinson, S. R. & Finger, T. E. (1990) Proc. Natl. Acad. Sci. USA 87, 6777–6780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moll, C., Mourre, C., Lazdunski, M. & Ulrich, J. (1991) Brain Res. 556, 311–316. [DOI] [PubMed] [Google Scholar]
- 31.Craner, M. J., Hains, B. C., Lo, A. C., Black, J. A. & Waxman, S. G. (2004) Brain 127, 294–303. [DOI] [PubMed] [Google Scholar]
- 32.Auld, V. J., Goldin, A. L., Krafte, D. S., Marshall, J., Dunn, J. M., Catterall, W. A., Lester, H. A., Davidson, N. & Dunn, R. J. (1988) Neuron 1, 449–461. [DOI] [PubMed] [Google Scholar]
- 33.Smith, M. R., Smith, R. D., Plummer, N. W., Meisler, M. H. & Goldin, A. L. (1998) J. Neurosci. 18, 6093–6102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stuhmer, W., Conti, F., Suzuki, H., Wang, X. D., Noda, M., Yahagi, N., Kubo, H. & Numa, S. (1989) Nature 339, 597–603. [DOI] [PubMed] [Google Scholar]
- 35.Foster, R. E., Connors, B. W. & Waxman, S. G. (1982) Brain Res. 255, 371–386. [DOI] [PubMed] [Google Scholar]
- 36.Rasband, M. N., Peles, E., Trimmer, J. S., Levinson, S. R., Lux, S. E. & Shrager, P. (1999) J. Neurosci. 19, 7516–7528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Burbidge, S. A., Dale, T. J., Powell, A. J., Whitaker, W. R., Xie, X. M., Romanos, M. A. & Clare, J. J. (2002) Mol. Brain Res. 103, 80–90. [DOI] [PubMed] [Google Scholar]
- 38.Herzog, R. I., Cummins, T. R., Ghassemi, F., Dib-Hajj, S. D. & Waxman, S. G. (2003) J. Physiol. 551, 741–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Newcombe, J. & Cuzner, M. L. (1993) J. Neural Transm. Suppl. 39, 155–163. [PubMed] [Google Scholar]
- 40.Li, H., Newcombe, J., Groome, N. P. & Cuzner, M. L. (1993) Neuropathol. Appl. Neurobiol. 19, 214–223. [DOI] [PubMed] [Google Scholar]
- 41.Steffensen, I., Waxman, S. G., Mills, L. & Stys, P. K. (1997) Brain Res. 776, 1–9. [DOI] [PubMed] [Google Scholar]
- 42.Bagasra, O., Michaels, F. H., Zheng, Y. M., Bobroski, L. E., Spitsin, S. V., Fu, Z. F., Tawadros, R. & Koprowski, H. (1995) Proc. Natl. Acad. Sci. USA 92, 12041–12045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Einheber, S., Zanazzi, G., Ching, W., Scherer, S., Milner, T. A., Peles, E. & Salzer, J. L. (1997) J. Cell Biol. 139, 1495–1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bhat, M. A., Rios, J. C., Lu, Y., Garcia-Fresco, G. P., Ching, W., St. Martin, M., Li, J., Einheber, S., Chesler, M., Rosenbluth, J., et al. (2001) Neuron 30, 369–383. [DOI] [PubMed] [Google Scholar]
- 45.Arroyo, E. J., Xu, T., Grinspan, J., Lambert, S., Levinson, S. R., Brophy, P. J., Peles, E. & Scherer, S. S. (2002) J. Neurosci. 22, 1726–1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Black, J. A., Renganathan, M. & Waxman, S. G. (2002) Brain Res. Mol. Brain Res. 105, 19–28. [DOI] [PubMed] [Google Scholar]
- 47.Bitsch, A., Schuchardt, J., Bunkowski, S., Kuhlmann, T. & Bruck, W. (2000) Brain 123, 1174–1183. [DOI] [PubMed] [Google Scholar]
- 48.Kuhlmann, T., Lingfeld, G., Bitsch, A., Schuchardt, J. & Bruck, W. (2002) Brain 125, 2202–2212. [DOI] [PubMed] [Google Scholar]
- 49.Rasminsky, M. & Sears, T. A. (1972) J. Physiol. 227, 323–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ritchie, J. M. & Rogart, R. B. (1977) Proc. Natl. Acad. Sci. USA 74, 211–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Waxman, S. G. (1977) Arch. Neurol. (Chicago) 34, 585–589. [DOI] [PubMed] [Google Scholar]
- 52.Shrager, P. (1989) Brain Res. 483, 149–154. [DOI] [PubMed] [Google Scholar]
- 53.Waxman, S. G., Davis, P. K., Black, J. A. & Ransom, B. R. (1990) Ann. Neurol. 28, 335–340. [DOI] [PubMed] [Google Scholar]
- 54.Dib-Hajj, S. D., Black, J. A., Cummins, T. R., Kenney, A. M., Kocsis, J. D. & Waxman, S. G. (1998) J. Neurophysiol. 79, 2668–2676. [DOI] [PubMed] [Google Scholar]
- 55.Toledo-Aral, J. J., Brehm, P., Halegoua, S. & Mandel, G. (1995) Neuron 14, 607–611. [DOI] [PubMed] [Google Scholar]
- 56.Boucher, T. J., Okuse, K., Bennett, D. L., Munson, J. B., Wood, J. N. & McMahon, S. B. (2000) Science 290, 124–127. [DOI] [PubMed] [Google Scholar]