Abstract
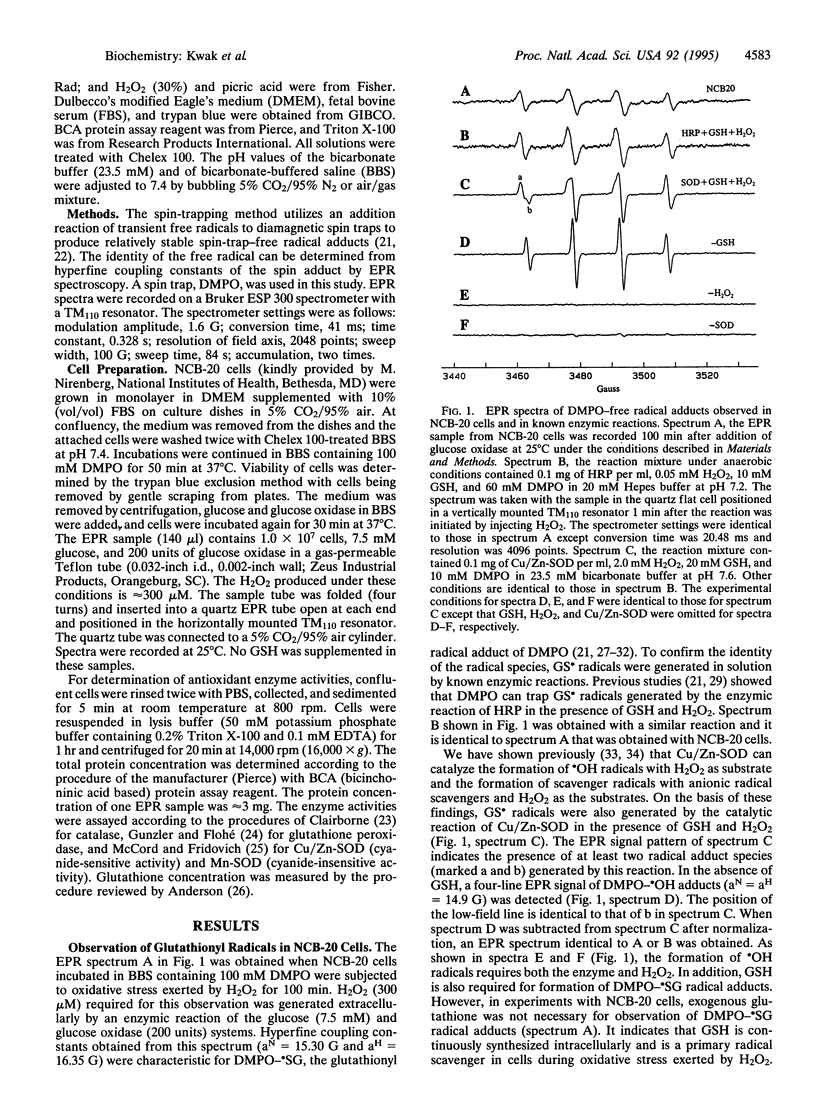
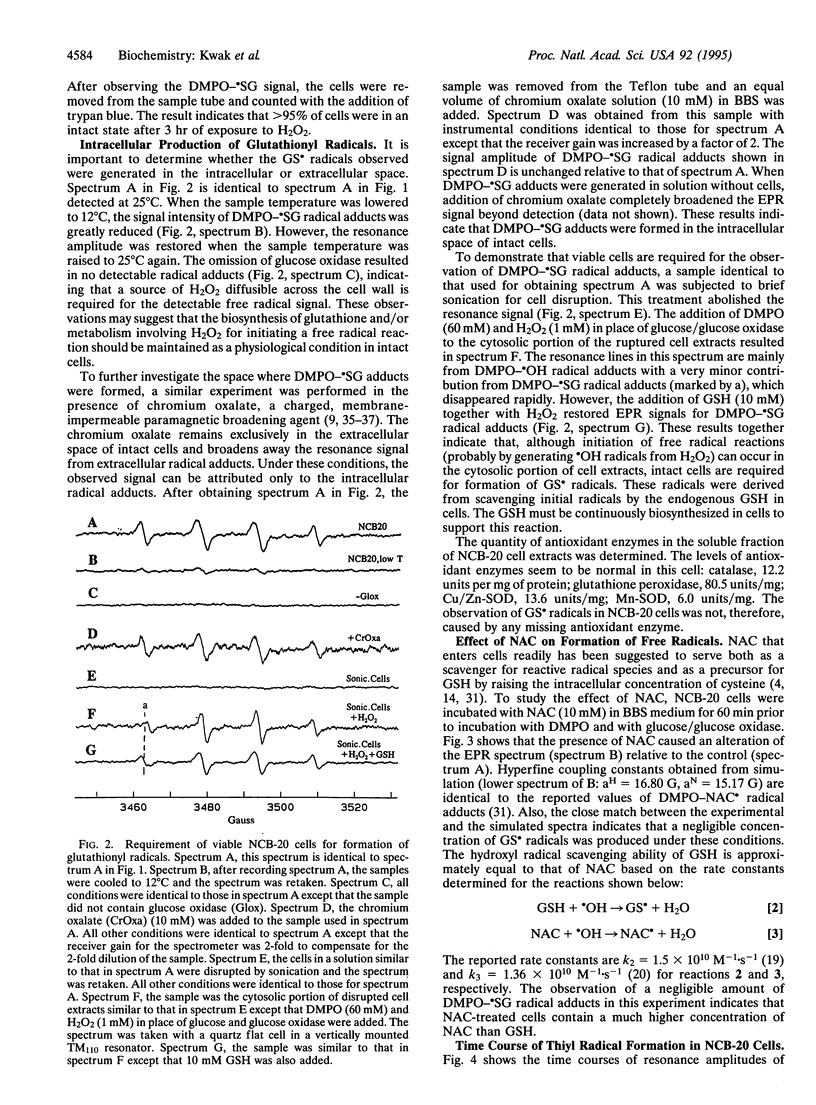
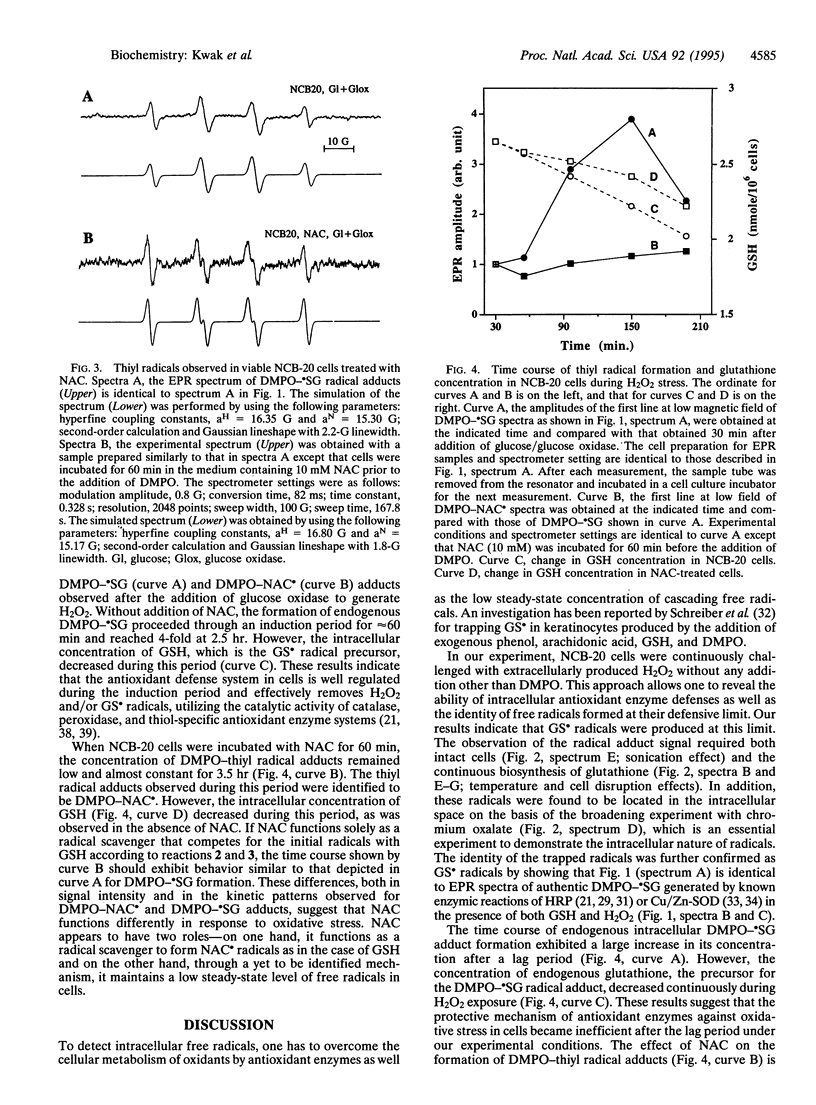
We report the detection of endogenous intracellular glutathionyl (GS.) radicals in the intact neuroblastoma cell line NCB-20 under oxidative stress. Spin-trapping and electron paramagnetic resonance (EPR) spectroscopic methods were used for monitoring the radicals. The cells incubated with the spin trap 5,5-dimethyl-1-pyrroline 1-oxide (DMPO) were challenged with H2O2 generated by the enzymic reaction of glucose/glucose oxidase. These cells exhibit the EPR spectrum of the GS. radical adduct of DMPO (DMPO-.SG) without exogenous reduced glutathione (GSH). The identity of this radical adduct was confirmed by observing hyperfine coupling constants identical to previously reported values in in vitro studies, which utilized known enzymic reactions, such as horseradish peroxidase and Cu/Zn superoxide dismutase, with GSH and H2O2 as substrates. The formation of the GS. radicals required viable cells and continuous biosynthesis of GSH. No significant effect on the resonance amplitude by the addition of a membrane-impermeable paramagnetic broadening agent indicated that these radicals were located inside the intact cell. N-Acetyl-L-cysteine (NAC)-treated cells produced NAC-derived free radicals (NAC.) in place of GS. radicals. The time course studies showed that DMPO-.SG formation exhibited a large increase in its concentration after a lag period, whereas DMPO-NAC. formation from NAC-treated cells did not show this sudden increase. These results were discussed in terms of the limit of antioxidant enzyme defenses in cells and the potential role of the GS. radical burst in activation of the transcription nuclear factor NF-kappa B in response to oxidative stress.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aruoma O. I., Halliwell B., Hoey B. M., Butler J. The antioxidant action of N-acetylcysteine: its reaction with hydrogen peroxide, hydroxyl radical, superoxide, and hypochlorous acid. Free Radic Biol Med. 1989;6(6):593–597. doi: 10.1016/0891-5849(89)90066-x. [DOI] [PubMed] [Google Scholar]
- Berg S. P., Nesbitt D. M. Chromium oxalate: a new spin label broadening agent for use with thylakoids. Biochim Biophys Acta. 1979 Dec 6;548(3):608–615. doi: 10.1016/0005-2728(79)90068-9. [DOI] [PubMed] [Google Scholar]
- Chae H. Z., Kim I. H., Kim K., Rhee S. G. Cloning, sequencing, and mutation of thiol-specific antioxidant gene of Saccharomyces cerevisiae. J Biol Chem. 1993 Aug 5;268(22):16815–16821. [PubMed] [Google Scholar]
- Chen K., Glockner J. F., Morse P. D., 2nd, Swartz H. M. Effects of oxygen on the metabolism of nitroxide spin labels in cells. Biochemistry. 1989 Mar 21;28(6):2496–2501. doi: 10.1021/bi00432a022. [DOI] [PubMed] [Google Scholar]
- Davies M. J., Forni L. G., Shuter S. L. Electron spin resonance and pulse radiolysis studies on the spin trapping of sulphur-centered radicals. Chem Biol Interact. 1987 Feb;61(2):177–188. doi: 10.1016/0009-2797(87)90038-x. [DOI] [PubMed] [Google Scholar]
- Duh E. J., Maury W. J., Folks T. M., Fauci A. S., Rabson A. B. Tumor necrosis factor alpha activates human immunodeficiency virus type 1 through induction of nuclear factor binding to the NF-kappa B sites in the long terminal repeat. Proc Natl Acad Sci U S A. 1989 Aug;86(15):5974–5978. doi: 10.1073/pnas.86.15.5974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harman L. S., Carver D. K., Schreiber J., Mason R. P. One- and two-electron oxidation of reduced glutathione by peroxidases. J Biol Chem. 1986 Feb 5;261(4):1642–1648. [PubMed] [Google Scholar]
- Kennedy C. H., Pryor W. A., Winston G. W., Church D. F. Hydroperoxide-induced radical production in liver mitochondria. Biochem Biophys Res Commun. 1986 Dec 30;141(3):1123–1129. doi: 10.1016/s0006-291x(86)80160-7. [DOI] [PubMed] [Google Scholar]
- Kim K., Kim I. H., Lee K. Y., Rhee S. G., Stadtman E. R. The isolation and purification of a specific "protector" protein which inhibits enzyme inactivation by a thiol/Fe(III)/O2 mixed-function oxidation system. J Biol Chem. 1988 Apr 5;263(10):4704–4711. [PubMed] [Google Scholar]
- Lai C. S., Froncisz W., Hopwood L. E. An evaluation of paramagnetic broadening agents for spin probe studies of intact mammalian cells. Biophys J. 1987 Oct;52(4):625–628. doi: 10.1016/S0006-3495(87)83253-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCord J. M., Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J Biol Chem. 1969 Nov 25;244(22):6049–6055. [PubMed] [Google Scholar]
- Meister A., Anderson M. E. Glutathione. Annu Rev Biochem. 1983;52:711–760. doi: 10.1146/annurev.bi.52.070183.003431. [DOI] [PubMed] [Google Scholar]
- Meister A. Glutathione-ascorbic acid antioxidant system in animals. J Biol Chem. 1994 Apr 1;269(13):9397–9400. [PubMed] [Google Scholar]
- Meyer M., Schreck R., Baeuerle P. A. H2O2 and antioxidants have opposite effects on activation of NF-kappa B and AP-1 in intact cells: AP-1 as secondary antioxidant-responsive factor. EMBO J. 1993 May;12(5):2005–2015. doi: 10.1002/j.1460-2075.1993.tb05850.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mãrtensson J., Meister A., Mrtensson J. Glutathione deficiency decreases tissue ascorbate levels in newborn rats: ascorbate spares glutathione and protects. Proc Natl Acad Sci U S A. 1991 Jun 1;88(11):4656–4660. doi: 10.1073/pnas.88.11.4656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mårtensson J., Jain A., Stole E., Frayer W., Auld P. A., Meister A. Inhibition of glutathione synthesis in the newborn rat: a model for endogenously produced oxidative stress. Proc Natl Acad Sci U S A. 1991 Oct 15;88(20):9360–9364. doi: 10.1073/pnas.88.20.9360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintiliani M., Badiello R., Tamba M., Esfandi A., Gorin G. Radiolysis of glutathione in oxygen-containing solutions of pH7. Int J Radiat Biol Relat Stud Phys Chem Med. 1977 Aug;32(2):195–202. doi: 10.1080/09553007714550891. [DOI] [PubMed] [Google Scholar]
- Roederer M., Staal F. J., Raju P. A., Ela S. W., Herzenberg L. A., Herzenberg L. A. Cytokine-stimulated human immunodeficiency virus replication is inhibited by N-acetyl-L-cysteine. Proc Natl Acad Sci U S A. 1990 Jun;87(12):4884–4888. doi: 10.1073/pnas.87.12.4884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross D. Glutathione, free radicals and chemotherapeutic agents. Mechanisms of free-radical induced toxicity and glutathione-dependent protection. Pharmacol Ther. 1988;37(2):231–249. doi: 10.1016/0163-7258(88)90027-7. [DOI] [PubMed] [Google Scholar]
- Ross D., Norbeck K., Moldéus P. The generation and subsequent fate of glutathionyl radicals in biological systems. J Biol Chem. 1985 Dec 5;260(28):15028–15032. [PubMed] [Google Scholar]
- Samuni A., Carmichael A. J., Russo A., Mitchell J. B., Riesz P. On the spin trapping and ESR detection of oxygen-derived radicals generated inside cells. Proc Natl Acad Sci U S A. 1986 Oct;83(20):7593–7597. doi: 10.1073/pnas.83.20.7593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreck R., Rieber P., Baeuerle P. A. Reactive oxygen intermediates as apparently widely used messengers in the activation of the NF-kappa B transcription factor and HIV-1. EMBO J. 1991 Aug;10(8):2247–2258. doi: 10.1002/j.1460-2075.1991.tb07761.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber J., Foureman G. L., Hughes M. F., Mason R. P., Eling T. E. Detection of glutathione thiyl free radical catalyzed by prostaglandin H synthase present in keratinocytes. Study of co-oxidation in a cellular system. J Biol Chem. 1989 May 15;264(14):7936–7943. [PubMed] [Google Scholar]
- Staal F. J., Roederer M., Herzenberg L. A., Herzenberg L. A. Intracellular thiols regulate activation of nuclear factor kappa B and transcription of human immunodeficiency virus. Proc Natl Acad Sci U S A. 1990 Dec;87(24):9943–9947. doi: 10.1073/pnas.87.24.9943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadtman E. R. Protein oxidation and aging. Science. 1992 Aug 28;257(5074):1220–1224. doi: 10.1126/science.1355616. [DOI] [PubMed] [Google Scholar]
- Toledano M. B., Leonard W. J. Modulation of transcription factor NF-kappa B binding activity by oxidation-reduction in vitro. Proc Natl Acad Sci U S A. 1991 May 15;88(10):4328–4332. doi: 10.1073/pnas.88.10.4328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellner V. P., Anderson M. E., Puri R. N., Jensen G. L., Meister A. Radioprotection by glutathione ester: transport of glutathione ester into human lymphoid cells and fibroblasts. Proc Natl Acad Sci U S A. 1984 Aug;81(15):4732–4735. doi: 10.1073/pnas.81.15.4732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yim M. B., Chae H. Z., Rhee S. G., Chock P. B., Stadtman E. R. On the protective mechanism of the thiol-specific antioxidant enzyme against the oxidative damage of biomacromolecules. J Biol Chem. 1994 Jan 21;269(3):1621–1626. [PubMed] [Google Scholar]
- Yim M. B., Chock P. B., Stadtman E. R. Copper, zinc superoxide dismutase catalyzes hydroxyl radical production from hydrogen peroxide. Proc Natl Acad Sci U S A. 1990 Jul;87(13):5006–5010. doi: 10.1073/pnas.87.13.5006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yim M. B., Chock P. B., Stadtman E. R. Enzyme function of copper, zinc superoxide dismutase as a free radical generator. J Biol Chem. 1993 Feb 25;268(6):4099–4105. [PubMed] [Google Scholar]


