Abstract
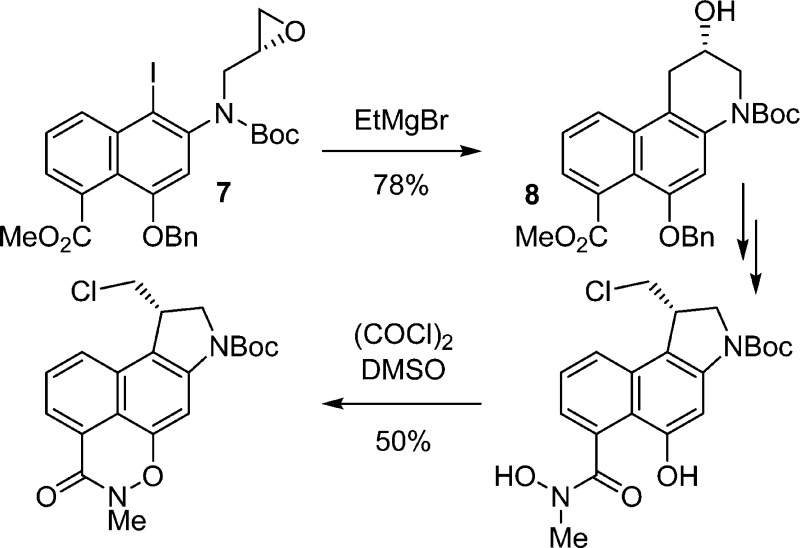
A short, asymmetric synthesis of a cyclic N-acyl O-amino phenol duocarmycin prodrug subject to reductive activation based on the simplified 1,2,9,9a-tetrahydrocyclopropa[c]benz[e]indol-4-one (CBI) DNA alkylation subunit is described. A key element of the approach entailed treatment of iodo-epoxide 7, prepared by N-alkylation of 6 with (S)-glycidal 3-nosylate, with EtMgBr at room temperature to directly provide the optically pure alcohol 8 in 78% yield (99% ee) derived from an effective metal–halogen exchange and subsequent regioselective intramolecular 6-endo-tet cyclization. Following O-debenzylation, introduction of a protected N-methylhydroxamic acid, direct trannannular spirocyclization, and subsequent stereoelectronically controlled acid-catalyzed cleavage of the resulting cyclopropane (HCl), further improvements in a unique intramolecular cyclization with N–O bond formation originally introduced for formation of the reductively labile prodrug functionality are detailed.
Introduction
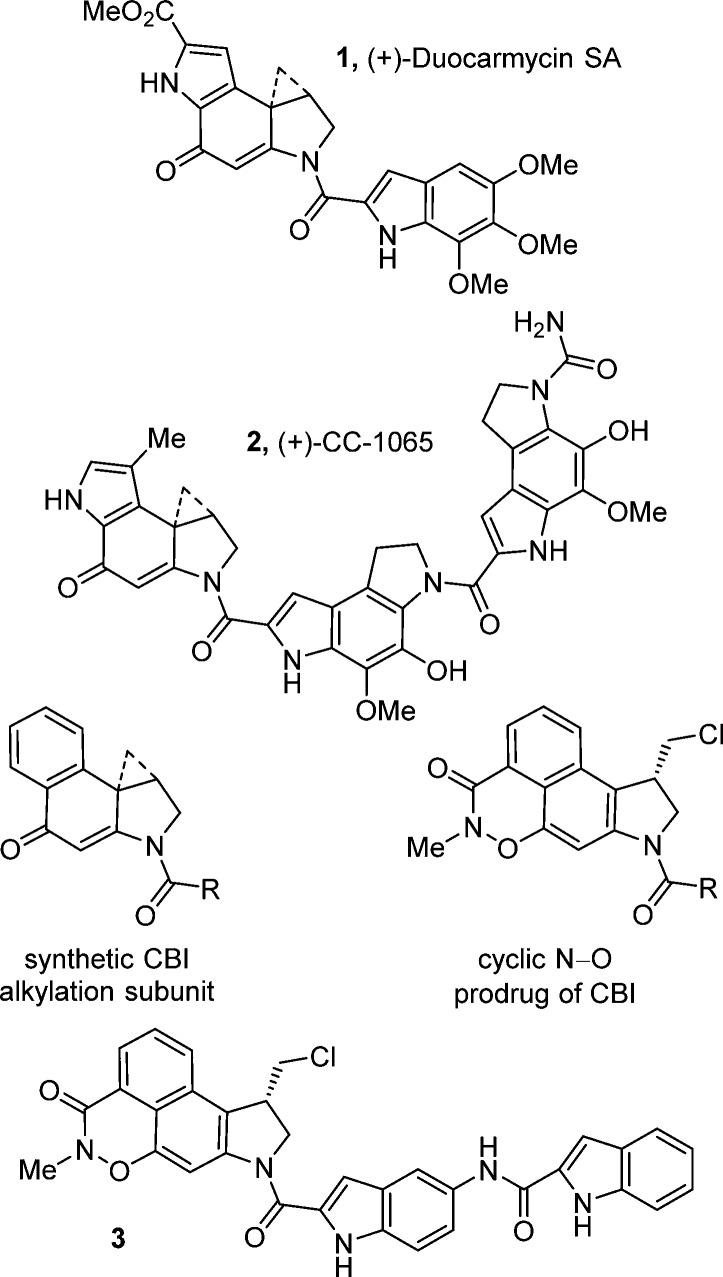
Duocarmycin SA (1)1 and CC-1065 (2)2 are the two most widely recognized members of a class of exceptionally potent naturally occurring antitumor compounds that also include duocarmycin A3 and yatakemycin4 (Figure 1). Each of these natural products has been shown to derive its antitumor properties from its ability to alkylate DNA in a sequence-selective manner,5 undergoing a stereoelectronically controlled adenine N3 alkylation within the minor groove at defined locations within 4–5 base pair A–T rich sites.6 Extensive studies conducted with the natural products, their synthetic unnatural enantiomers,7 and a systematic series of key analogues have defined a range of fundamental features that control their DNA alkylation selectivity, efficiency, and catalysis,8 providing a detailed understanding of the relationships between structure, reactivity, and biological activity.
Figure 1.
Structure of (+)-duocarmycin SA, (+)-CC-1065, CBI, the cyclic N–O prodrug of the CBI alkylation subunit, and prodrug 3.
Recently, we reported the synthesis and examination of both acyclic9 and cyclic10N-acyl O-amino phenol derivatives as members of a unique class of reductively cleaved prodrugs of the duocarmycin family of natural products.11 These prodrugs were explored with analogues incorporating the synthetically more accessible 1,2,9,9a-tetrahydrocyclopropa[c]benz[e]indol-4-one (CBI) alkylation subunit12,13 (Figure 1) and with the intention of attenuating the extraordinary potency of the compounds. The expectation was that they may be chemically tuned for cleavage selectively within hypoxic tumor environments that have intrinsically higher intracellular concentrations of reducing nucleophiles. The most recent of these, a class of cyclic N-acyl O-amino phenol prodrugs,10 were designed to liberate the free drug without the release of an extraneous group. In vivo evaluation of the most stable of these latter prodrugs, 3, showed that it exhibited extraordinary antitumor efficacy in a simple tumor model (T/C > 1500, L1210; 6/10 one year survivors) substantially exceeding that of the free drug, that its therapeutic window of activity was much larger than that of the free drug, and yet that it displayed a potency in vivo that approached the free drug.10 These studies indicate that prodrug 3 may benefit from either its controlled slow release of the free drug or its preferential intracellular reductive cleavage.
In a continuation of these studies and prompted by the need for improved access to the materials, herein we report an asymmetric synthesis of 3, avoiding the late-stage chiral phase resolution of our initial synthesis and improving key elements of the approach including a unique cyclization with N–O bond formation for introduction of the reductively labile prodrug functionality.
Results and Discussion
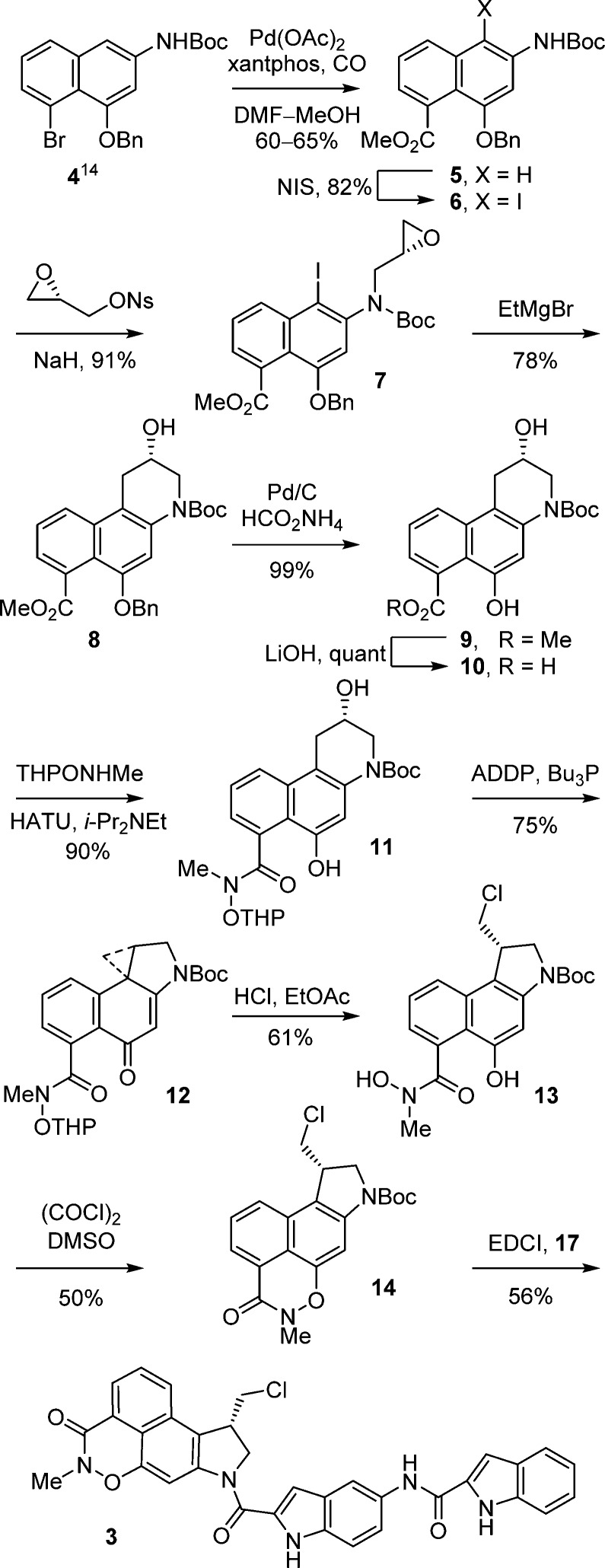
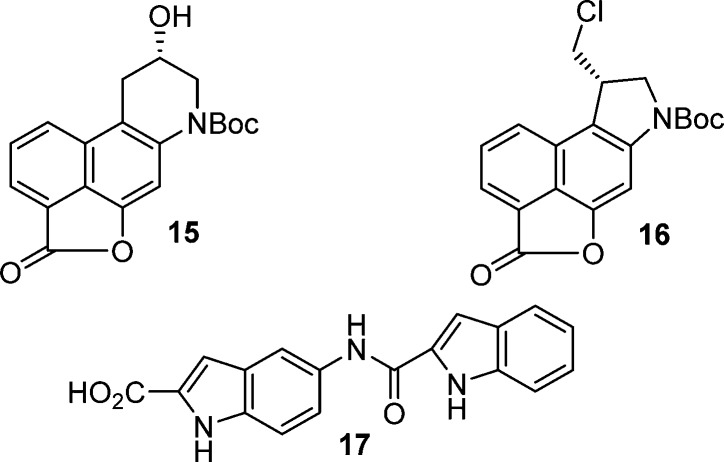
Palladium(0)-catalyzed carbonylation of 4,14 in DMF–MeOH (2:1, 0.13 M, 0.1 equiv of Pd(OAc)2, 0.2 equiv of xantphos, 1 equiv of K2CO3, CO atm, 100 °C, 17 h), provided methyl ester 5 in good conversion (60–65%) (Scheme 1). Regioselective iodination of 5 (2 equiv of N-iodosuccinimide (NIS), cat. HOAc, toluene, 25 °C, 17 h, 82%) followed by N-alkylation of 6 with (S)-glycidal 3-nosylate (99% ee) with clean SN2 displacement of the nosylate (1.15 equiv, 1.5 equiv of NaH, DMF, 0–25 °C, 5 h, 91%) set the stage for a key cyclization. Treatment of iodo-epoxide 7 with EtMgBr at room temperature directly provided the optically pure alcohol 8 in 78% yield derived from selective metal–halogen exchange and subsequent intramolecular 6-endo-tet cyclization. Analogous to observations made in an asymmetric synthesis of CBI itself,13c formation of the aryl Grignard reagent by metal–halogen exchange (2.0 equiv of EtMgBr, 23 °C) is followed by the rapid intramolecular epoxide ring opening to give near exclusively 8, the result of intramolecular 6-endo versus 5-exo addition to the epoxide with only detection of trace amounts of the isomeric product (>13:1, <5%). A similar but technically more demanding protocol, entailing metal–halogen exchange (i-PrMgCl, THF, −40 °C, 20 min) followed by transmetalation with CuI–PBu3 (−78 °C, 1 h),15 also provided 8 (−40 °C, 30 min, 68%) in comparable conversions. O-Debenzylation of 8, accomplished by transfer hydrogenolysis (cat. 10% Pd/C, 10 equiv of HCO2NH4, THF–MeOH, 25 °C, 1 h, 99%), followed by methyl ester hydrolysis (5 equiv of LiOH, THF–MeOH–H2O 3:3:1, 70 °C, 4 h, quant.), provided carboxylic acid 10. Coupling of 10 (1.5 equiv of (1-[bis(dimethylamino)methylene]-1H-1,2,3-triazolo[4,5-b]pyridinium 3-oxide hexafluorophosphate (HATU), 1.5 equiv of i-Pr2NEt, 25 °C, 18 h, 90%) with N-methylhydroxylamine protected as its tetrahydropyranyl (THP) derivative (20, prepared as detailed in the Experimental Section) provided intermediate 11 functionalized for N–O bond formation yet permitted subsequent spirocyclization and HCl addition to the activated cyclopropane and could be readily deprotected without competitive N-Boc deprotection or N–O bond cleavage. Lactone 15 was observed in the reaction mixture, could be isolated and characterized, and converts to product 11 when subjected to the reaction conditions, indicating that 15 can serve as an intermediate in the generation of 11 (Figure 2). Direct transannular Ar-3′ spirocyclization upon Mitsunobu activation of the secondary alcohol 11 (5 equiv of 1,1′-(azodicarbonyl)dipiperidine (ADDP), 5 equiv of Bu3P, THF, 23 °C, 1 h, 75%) provided 12. Subsequent treatment of 12 with 4 N HCl in EtOAc (−78 °C, 2 h, 61–78%) afforded 13 derived from a stereoelectronically controlled regioselective cyclopropane cleavage and acid-catalyzed THP deprotection without competitive N-Boc deprotection provided that the reaction was carried out and quenched at low temperature. The key oxazinone was closed with N–O bond formation upon exposure of 13 to Swern oxidation conditions (3 equiv of (COCl)2, 6 equiv of DMSO, CH2Cl2), yielding 14 (50%) in an improved yield relative to our original report.10 More careful control of the reaction conditions, especially the reaction temperature (−78 °C, 30 min and −15 °C, 2 h vs −78 to −10 °C, 2 h) served to substantially improve the conversion of 13 to 14 (50 vs 20%) originally reported.10 As disclosed in our original work,10 this represents a new and unique method for intramolecular formation of an N–O bond and presumably entails activation of the hydroxamic acid alcohol as its dimethyl sulfoxonium cation for subsequent intramolecular phenol displacement. Additionally and in the conversion of 12 to 13, the lactone byproduct 16 (19%) was also isolated, and it was occasionally detected in the conversion of 13 to 14 as a minor byproduct but not quantitated.
Scheme 1. Asymmetric Synthesis of 14 and 3.
Figure 2.
Structures of 15–17.
The optical purity of 14 was established by chiral phase HPLC (Chiralcel OD column, 0.46 × 25 cm, 5% i-PrOH/hexane) and established to be 99% ee, reflecting the optical purity of the starting (S)-glycidal 3-nosylate (99% ee) (see Figure S1, Supporting Information). As described previously,10 acid-catalyzed N-Boc deprotection of 14 (4 N HCl, EtOAc, 25 °C, 15 min) and immediate coupling of the resulting HCl salt with 17 (3 equiv of 1-ethyl-3-(3-(dimethylamino)propyl)carbodiimide (EDCI), DMF, 25 °C, 20 h, 56%) afforded 3.
Conclusions
An effective, improved, and asymmetric synthesis of a reductively activated cyclic N-acyl O-amino phenol duocarmycin prodrug based on the simplified 1,2,9,9a-tetrahydrocyclopropa[c]benz-[e]indol-4-one (CBI) DNA alkylation subunit is described. Its use in furthering the preclinical exploration of such analogues of the natural products is in progress and will be disclosed in due course.
Experimental Section
Methyl 8-(Benzyloxy)-6-((tert-butyloxycarbonyl)amino)-1-naphthoate (5)
A solution of 4 (500 mg, 1.17 mmol) in a 2:1 mixture of DMF–CH3OH (0.13 M) was treated with Pd(OAc)2 (26.2 mg, 0.117 mmol), xantphos (135 mg, 0.234 mmol), and K2CO3 (162 mg, 1.17 mmol) under N2. CO gas was bubbled through the solution, and the reaction vessel atmosphere was exchanged with CO. The reaction vessel was sealed, after which the mixture was heated to 100 °C and stirred for 17 h. The reaction mixture was cooled to room temperature and filtered. The filtrate was diluted with EtOAc, washed with H2O and saturated aqueous NaCl, and dried over Na2SO4. The solvent was concentrated, and the residue was purified by flash chromatography (10–20% EtOAc/hexanes gradient elution) to give 5 (286 mg, 60%) as an orange solid: mp 178–179 °C; 1H NMR (CDCl3, 600 MHz) δ 7.73 (dd, J = 8.4, 1.2 Hz, 1H), 7.48 (d, J = 7.2 Hz, 2H), 7.4–7.39 (m, 3H), 7.37 (t, J = 7.2 Hz, 1H), 7.26 (dd, J = 7.2, 1.2 Hz, 1H), 7.10 (s, 1H), 6.62 (s, 1H), 5.14 (s, 2H), 3.31 (s, 3H), 1.55 (s, 9H); 13C NMR (CDCl3, 150 MHz) δ 171.6, 154.7, 152.7, 137.0, 135.9, 135.4, 129.9, 128.95, 128.92 (2C), 128.7 (2C), 128.6, 126.1, 123.5, 118.1, 107.4, 100.5, 81.0, 71.4, 51.9, 28.5 (3C); IR (film) νmax 1717, 1543, 1240, 1155 cm–1; ESI-TOF HRMS m/z 408.1808 (M + H+, C24H25NO5 requires 408.1805).
Methyl 8-(Benzyloxy)-6-((tert-butyloxycarbonyl)amino)-5-iodo-1-naphthoate (6)
A suspension of 5 (2.12 g, 5.20 mmol) and NIS (2.23 g, 10.4 mmol) in toluene (124 mL) was treated with acetic acid (1.1 mL) under Ar in the dark. The reaction flask was wrapped with aluminum foil and stirred at 25 °C in the dark for 17 h. The reaction mixture was poured into H2O and extracted with EtOAc. The organic layer was washed with saturated aqueous NaCl, dried over Na2SO4, and concentrated. The residue was purified by flash chromatography (10–25% EtOAc/hexanes gradient elution) to give 6 (2.28 g, 82%) as a pale tan solid: mp 172 °C; 1H NMR (CDCl3, 500 MHz) δ 8.16–8.14 (m, 2H), 7.51–7.48 (m, 3H), 7.42 (t, J = 8.0 Hz, 2H), 7.38–7.35 (m, 2H), 7.31 (dd, J = 7.0, 1.0 Hz, 1H), 5.19 (s, 2H), 3.24 (s, 3H), 1.58 (s, 9H); 13C NMR (CDCl3, 150 MHz) δ 207.1, 171.1, 153.4, 152.5, 135.5, 134.3, 131.6, 130.5, 129.4 (2C), 128.7 (2C), 127.1, 125.2, 124.0, 118.6, 100.2, 81.6, 71.7, 52.0, 31.1, 28.5 (3C); IR (film) νmax 2928, 1732, 1620, 1497, 1364, 1279, 1229, 1155 cm–1; ESI-TOF HRMS m/z 534.0769 (M + H+, C24H24INO5 requires 534.0772).
Methyl (R)-8-(Benzyloxy)-6-((tert-butyloxycarbonyl)(oxiran-2-ylmethyl)amino)-5-iodo-1-naphthoate (7)
A solution of 6 (2.28 g, 4.27 mmol) and (S)-glycidal 3-nosylate (99% ee, 1.44 g, 5.55 mmol) in DMF (40 mL) was cooled to 0 °C and treated with NaH (60% dispersion in mineral oil, 256 mg, 6.41 mmol). The reaction mixture was stirred at 0 °C for 2 h, after which it was warmed to room temperature and stirred for 3 h. The solution was poured into ice-cold H2O and extracted with EtOAc. The organic layer was washed with H2O and saturated aqueous NaCl, dried over Na2SO4, and concentrated. The residue was purified by flash chromatography (5–20% EtOAc/hexanes gradient elution) to provide 7 (2.3 g, 91%) as a pale orange solid and as a mixture of rotamers (1:1): mp 105–106 °C; 1H NMR (acetone-d6, 600 MHz) δ 8.36 (t, J = 7.5 Hz, 1H), 7.71–7.67 (m, 1H), 7.54–7.53 (m, 3H), 7.44–7.42 (m, 2H), 7.39 (d, J = 7.5 Hz, 1H), 7.30 (s, 0.5H), 7.20 (s, 0.5H), 5.32 (s, 2H), 4.05 (ddd, J = 13.8, 13.8, 3.6 Hz, 1H), 3.46 (s, 1.5H), 3.42 (s, 1.5H), 3.36–3.27 (m, 2H), 2.67 (dd, J = 4.5, 4.5 Hz, 0.5H), 2.62 (dd, J = 4.5, 4.5 Hz, 0.5H), 2.41–2.37 (m, 1H), 1.30 (s, 4.5H), 1.29 (s, 4.5H); 13C NMR (acetone-d6, 150 MHz) δ 170.89, 170.85, 155.8, 155.7, 154.3, 154.2, 145.8, 145.4, 136.98, 136.93, 136.40, 136.34, 135.19, 135.16, 132.1, 129.76, 129.66, 129.50, 129.48, 129.35, 129.31, 129.28, 128.69, 128.67, 122.1, 110.5, 110.4, 96.9, 96.1, 80.93, 80.88, 72.3, 72.2, 53.4, 52.30, 52.25, 52.18, 52.13, 50.4, 50.0, 46.6, 46.2, 28.5 (3C); IR (film) νmax 2927, 1713, 1701, 1365, 1278, 1152 cm–1; [α]27D +9 (c 0.1, acetone); ESI-TOF HRMS m/z 590.1035 (M + H+, C27H28INO6 requires 590.1034).
4-(tert-Butyl) 7-Methyl (S)-6-(Benzyloxy)-2-hydroxy-2,3-dihydrobenzo[f]quinoline-4,7(1H)-dicarboxylate (8)
A solution of 7 (250 mg, 0.424 mmol) in anhydrous THF (2.4 mL) was treated with EtMgBr (0.94 mL, 0.848 mmol, 0.9 M in THF) at 0 °C. The reaction mixture was stirred at room temperature for 1 h, after which the reaction was quenched with the addition of saturated aqueous NH4Cl, diluted with EtOAc, washed with H2O and saturated aqueous NaCl, and dried over Na2SO4. The solvent was removed under reduced pressure, and the residue was purified by PTLC (50% EtOAc/hexanes elution) to provide 8 (153 mg, 78%) as a white amorphous solid: mp 60–61 °C; 1H NMR (acetone-d6, 600 MHz) δ 7.98 (dd, J = 8.7, 1.2 Hz, 1H), 7.56–7.53 (m, 3H), 7.45–7.42 (m, 3H), 7.38 (t, J = 7.2 Hz, 1H), 7.34 (dd, J = 7.2, 1.2 Hz, 1H), 5.21 (dd, J = 13.2, 10.8 Hz, 2H), 4.30–4.28 (m, 2H), 3.96 (dd, J = 12.0, 3.0 Hz, 1H), 3.59 (dd, J = 12.6, 7.2 Hz, 1H), 3.40–3.37 (m, 1H), 3.37 (s, 3H), 2.94 (dd, J = 16.8, 6.0 Hz, 1H), 1.52 (s, 9H); 13C NMR (acetone-d6, 150 MHz) δ 171.6, 154.7, 152.5, 138.1, 137.6, 134.3, 131.9, 129.6 (2C), 129.4 (2C), 129.2, 126.9, 125.1, 124.6, 120.1, 115.2, 107.0, 81.5, 72.0, 65.1, 52.0, 51.0, 34.3, 28.6 (3C); IR (film) νmax 3430, 2929, 1695, 1366, 1247, 1151, 1084 cm–1; [α]30D +40 (c 0.1, acetone); ESI-TOF HRMS m/z 464.2065 (M + H+, C27H29NO6 requires 464.2068).
4-(tert-Butyl) 7-Methyl (S)-2,6-dihydroxy-2,3-dihydrobenzo[f]quinoline-4,7(1H)-dicarboxylate (9)
A solution of 8 (160 mg, 0.345 mmol) in 9:1 mixture of THF–CH3OH (0.03 M) was treated with ammonium formate (210 mg, 3.45 mmol) under Ar, after which 10% Pd/C (73 mg, 0.069 mmol) was added. The reaction mixture was stirred at 25 °C for 1 h. The reaction mixture was filtered through Celite and concentrated under reduced pressure. The residue was purified by PTLC (50% EtOAc/hexanes elution) to give 9 as a pale yellow amorphous solid. The product was triturated with hexane, providing 9 (128 mg, 99%) as a pale yellow amorphous solid: mp 39–40 °C; 1H NMR (acetone-d6, 600 MHz) δ 9.22 (br, 1H), 7.96 (dd, J = 8.7, 0.9 Hz, 1H), 7.52 (dd, J = 8.7, 6.9 Hz, 1H), 7.37 (dd, J = 6.9, 0.9 Hz, 1H), 7.34 (s, 1H), 4.28–4.26 (m, 1H), 3.96 (dd, J = 12.6, 3.0 Hz, 1H), 3.85 (s, 3H), 3.56 (dd, J = 12.6, 7.8 Hz, 1H), 3.36 (dd, J = 10.8, 6.0 Hz, 1H), 2.91 (dd, J = 10.8, 6.0 Hz, 1H), 1.51 (s, 9H); 13C NMR (acetone-d6, 150 MHz) δ 172.1, 154.6, 151.0, 138.2, 134.5, 131.7, 126.4, 125.3, 124.2, 119.7, 113.8, 109.2, 81.3, 65.2, 52.5, 50.9, 34.3, 28.5 (3C); IR (film) νmax 3305, 2929, 1693, 1389, 1252, 1153, 1083 cm–1; [α]27D +35 (c 0.1, acetone); ESI-TOF HRMS m/z 374.1596 (M + H+, C20H23NO6 requires 374.1598).
(S)-4-(tert-Butoxycarbonyl)-2,6-dihydroxy-1,2,3,4-tetrahydrobenzo[f]quinoline-7-carboxylic Acid (10)
Compound 9 (123 mg, 0.329 mmol) was dissolved in a 3:3:1 mixture of THF–CH3OH–H2O (0.03 M). LiOH–H2O (69 mg, 1.65 mmol) was added, and the reaction mixture was stirred at 70 °C for 4 h. The reaction mixture was acidified to pH 1 with aqueous 1 N HCl, after which it was diluted with EtOAc. The organic layer was separated, washed with H2O and saturated aqueous NaCl, and dried over NasSO4. The organic extract was concentrated to give 10 (118 mg, 100%) as a pale tan solid: mp 102–104 °C; 1H NMR (acetone-d6, 600 MHz) δ 9.55 (br, 1H), 8.03 (dd, J = 8.4, 1.2 Hz, 1H), 7.64 (dd, J = 7.2, 1.2 Hz, 1H), 7.54 (dd, J = 8.4, 7.2 Hz, 1H), 7.36 (s, 1H), 4.27 (br, 1H), 3.96 (dd, J = 12.6, 2.4 Hz, 1H), 3.56 (dd, J = 12.6, 7.5 Hz, 1H), 3.38 (dd, J = 16.5, 5.7 Hz, 1H), 2.93 (dd, J = 16.5, 5.7 Hz, 1H), 1.52 (s, 9H); 13C NMR (acetone-d6, 150 MHz) δ 173.1, 154.7, 151.4, 138.4, 134.8, 131.3, 126.5, 126.3, 126.1, 120.0, 113.9, 110.1, 81.3, 65.2, 50.9, 34.5, 28.6 (3C); IR (film) νmax 3299, 2929, 1681, 1392, 1256, 1156 cm–1; [α]26D +34 (c 0.1, acetone); ESI-TOF HRMS m/z 360.1443 (M + H+, C19H21NO6 requires 360.1442).
tert-Butyl (2S)-2,6-Dihydroxy-7-(methyl((tetrahydro-2H-pyran-2-yl)oxy)carbamoyl)-2,3-dihydrobenzo[f]quinoline-4(1H)-carboxylate (11)
A suspension of 10 (113 mg, 0.314 mmol) and 20 (206 mg, 1.57 mmol) in CH2Cl2 (3.14 mL) was treated with HATU (179 mg, 0.471 mmol). i-Pr2NEt (82 μL, 0.471 mmol) was added at room temperature under Ar, after which the reaction mixture was stirred for 18 h. The reaction mixture was poured into H2O and extracted with EtOAc. The organic extract was washed with H2O and saturated aqueous NaCl, dried over Na2SO4, and concentrated. The residue was purified by PTLC (88% EtOAc/hexanes elution) to provide 11 as a pale yellow oil. The compound was further triturated with hexanes to give 11 (134 mg, 90%) as a pale yellow amorphous solid and as a mixture of rotamers (1:3): mp 72–73 °C; 1H NMR (acetone-d6, 500 MHz) δ 9.09 (br, 1H), 7.91 (d, J = 9.0 Hz, 0.25H), 7.87 (d, J = 9.0 Hz, 0.75H), 7.55 (br, 0.25H), 7.50 (t, J = 7.8 Hz, 0.75H), 7.36 (br, 0.25H), 7.29 (d, J = 1.5 Hz, 0.75H), 7.25 (d, J = 7.0 Hz, 1H), 4.62 (br, 1H), 4.26 (m, 2H), 4.01–3.95 (m, 1H), 3.57–3.48 (m, 2H), 3.40 (s, 3H), 3.37–3.34 (m, 1H), 3.08 (br, 1H), 2.92–2.86 (m, 1H), 1.81 (m, 1H), 1.62–1.57 (m, 1H), 1.52 (s, 9H), 1.39–1.27 (m, 3H), 1.15 (m, 1H); 13C NMR (acetone-d6, 150 MHz) δ 154.66, 154.60, 151.4, 138.2, 137.8, 134.5, 134.0, 126.9, 126.4, 124.6, 124.0, 120.4, 113.7, 113.3, 108.8, 108.3, 103.9, 101.9, 81.1, 65.2, 63.0, 62.8, 50.94, 50.89, 39.9, 34.3, 34.2, 28.5, 26.3, 25.8, 20.8; IR (film) νmax 2940, 1620, 1390, 1154 cm–1; [α]25D +15 (c 0.1, acetone); ESI-TOF HRMS m/z 473.2280 (M + H+, C25H32N2O7 requires 473.2282).
tert-Butyl (S)-9-Hydroxy-4-oxo-4,8,9,10-tetrahydro-7H-isobenzofuro[7,1-fg]quinoline-7-carboxylate (15)
In the conversion of 10 to 11, the lactone 15 could be isolated and characterized if the reaction was prematurely quenched. For 15: mp 190 °C; 1H NMR (acetone-d6, 500 MHz) δ 8.29 (d, J = 8.5 Hz, 1H), 8.11 (d, J = 7.5 Hz, 1H), 7.92 (dd, J = 8.5, 7.5 Hz, 1H), 7.52 (s, 1H), 4.36–4.34 (m, 2H), 3.93 (dd, J = 12.5, 2.5 Hz, 1H), 3.75 (dd, J = 12.5, 6.5 Hz, 1H), 3.48 (dd, J = 17.0, 5.5 Hz, 1H), 3.07 (dd, J = 17.0, 5.0 Hz, 1H), 1.54 (s, 9H); 13C NMR (acetone-d6, 150 MHz) δ 167.7, 154.9, 148.3, 140.0, 131.1, 130.2, 129.8, 127.9, 125.5, 121.9, 118.1, 106.8, 81.9, 64.7, 51.4, 33.1, 28.5 (3C); IR (film) νmax 3404, 2926, 1776, 1699, 1648, 1455, 1392, 1366, 1242, 1154 cm–1; ESI-TOF HRMS m/z 364.1161 (M + Na+, C19H19NO5 requires 364.1155).
tert-Butyl (9aS)-5-(Methyl((tetrahydro-2H-pyran-2-yl)oxy)carbamoyl)-4-oxo-9,9a-dihydro-1H-benzo[e]cyclopropa[c]indole-2(4H)-carboxylate (12)
A solution of 11 (66 mg, 0.14 mmol) in THF (14 mL) was treated with tributylphosphine (176 μL, 0.70 mmol), after which the reaction mixture was treated with ADDP (176 mg, 0.70 mmol). The reaction mixture was stirred for 1 h, quenched with the addition of H2O, and extracted with EtOAc. The organic layer was washed with H2O and saturated aqueous NaCl, dried over Na2SO4, and concentrated. The residue was purified by PTLC (88% EtOAc/hexanes elution) to provide 12 as a colorless oil. The compound was further triturated with hexanes to give 12 (48 mg, 75%) as a white amorphous solid and as a mixture of rotamers (1:1): mp 90–92 °C; 1H NMR (acetone-d6, 500 MHz) δ 7.56 (ddd, J = 7.5, 7.5, 3.5 Hz, 1H), 7.22 (t, J = 6.5 Hz, 1H), 7.14 (d, J = 7.5 Hz, 1H), 6.72 (d, J = 8.5 Hz, 1H), 4.53 (br, 0.5H), 4.49 (br, 0.5H), 4.09–4.02 (m, 2H), 3.89 (m, 1H), 3.51–3.47 (m, 1H), 3.40 (s, 1.5H), 3.39 (s, 1.5H), 3.11–3.08 (m, 1H), 1.72–1.59 (m, 3H), 1.55 (s, 9H), 1.42–1.41 (m, 2H), 1.33–1.21 (m, 3H); 13C NMR (acetone-d6, 150 MHz) δ 185.0, 184.7, 177.0, 176.5, 152.5, 142.0, 141.6, 138.0, 132.1, 125.4, 125.2, 123.22, 123.16, 108.6, 108.2, 105.2, 105.1, 102.0, 83.3, 63.14, 63.06, 53.98, 53.93, 38.58, 38.53, 32.5, 29.2, 28.40, 28.38, 25.96, 25.90, 23.4, 19.87, 19.84, 14.5; IR (film) νmax 3728, 3627, 1730, 1965, 1730, 1625, 1377, 1274 cm–1; [α]25D +89 (c 0.1, acetone); ESI-TOF HRMS m/z 455.2177 (M + H+, C25H30N2O6 requires 455.2177).
tert-Butyl (S)-1-(Chloromethyl)-5-hydroxy-6-(hydroxy(methyl)carbamoyl)-1,2-dihydro-3H-benzo[e]indole-3-carboxylate (13)
A sample of 12 (45 mg, 0.099 mmol) at −78 °C was treated with 4 N HCl in EtOAc (4.5 mL), and the solution was stirred at −78 °C for 2 h. The reaction mixture was diluted with EtOAc (13.5 mL) at the same temperature, after which H2O (9.0 mL) was added. The organic layer was separated, washed with H2O and saturated aqueous NaCl, dried over Na2SO4, and concentrated. The residue was purified by PTLC (50% EtOAc/hexanes elution) to provide 13 and 16. Compound 13 was further triturated with hexanes to give 13 (25 mg, 61%) as a pale yellow solid and as a mixture of rotamers (1:2): mp 115–116 °C; 1H NMR (CD3CN, 600 MHz) δ 8.23 (br, 0.33H), 8.07 (s, 0.67H), 7.76–7.67 (m, 2H), 7.44 (dd, J = 14.4, 7.2 Hz, 1H), 7.15 (d, J = 7.2 Hz, 1H), 7.16 (d, J = 7.0 Hz, 1H), 4.60–4.50 (br, 1H), 4.15–4.08 (m, 2H), 3.91–3.86 (m, 1H), 3.68–3.61 (m, 1H), 3.47 (br, 1H), 3.36 (s, 2H), 1.58 (s, 9H); 13C NMR (CD3CN, 150 MHz) δ 176.1, 154.7, 153.2, 142.9, 134.4, 131.4, 127.7, 127.3, 127.2, 124.4, 123.9, 105.0, 100.8, 81.6, 63.2, 53.6, 48.3, 48.2, 41.7, 37.9, 26.0, 25.6; IR (film) νmax 2934, 1701, 1622, 1406, 1370, 1334, 1249, 1142 cm–1; [α]25D +5 (c 0.08, acetone); ESI-TOF HRMS m/z 407.1367 (M + H+, C20H23ClN2O5 requires 407.1368).
Byproduct 16 was further triturated with hexanes to give 16 (6.8 mg, 19%) as a yellow solid: mp 185–186 °C; 1H NMR (acetone-d6, 600 MHz) δ 8.34 (d, J = 8.4 Hz, 1H), 8.19–8.05 (br, 1H), 8.03 (d, J = 6.6 Hz, 1H), 7.91 (dd, J = 8.4, 6.6 Hz, 1H), 4.34–4.27 (m, 2H), 4.23 (dd, J = 11.4, 3.6 Hz, 1H), 3.95 (dd, J = 11.4, 7.2 Hz, 1H), 1.60 (s, 9H); 13C NMR (acetone-d6, 150 MHz) δ 167.6, 152.9, 151.7, 145.9, 131.7, 129.3, 127.6, 126.7, 124.6, 122.6, 118.3, 99.0, 82.1, 54.1, 48.4, 41.4, 28.6 (3C); IR (film) νmax 1784, 1704, 1401, 1327, 1137 cm–1; [α]28D −41 (c 0.1, acetone); ESI-TOF HRMS m/z 360.0997 (M + H+, C19H18ClNO4 requires 360.0997).
tert-Butyl (S)-10-(Chloromethyl)-5-methyl-4-oxo-4,5,9,10-tetrahydro-8H-pyrrolo[3′,2′:5,6]naphtho[1,8-de][1,2]oxazine-8-carboxylate (14)
A stirred solution of (COCl)2 (6.2 μL, 0.074 mmol) in freshly distilled CH2Cl2 (1.0 mL) at −78 °C was treated with Me2SO (10.5 μL, 0.148 mmol) in 0.25 mL of freshly distilled CH2Cl2 dropwise. After 30 min, compound 13 (10.0 mg, 0.0246 mmol) in 2.0 mL of freshly distilled CH2Cl2 was added dropwise and the reaction mixture was stirred at −78 °C for 30 min, after which the reaction mixture was warmed to −15 °C and stirred for 2 h. The reaction was quenched with the addition of saturated aqueous NH4Cl, and the mixture was extracted with EtOAc. The organic layer was washed with saturated aqueous NaCl, dried over Na2SO4, and concentrated. The residue was purified by PTLC (50% EtOAc/hexanes elution) to provide 14 (4.75 mg, 50%) as a pale yellow solid: mp 147 °C; 1H NMR (acetone-d6, 600 MHz) δ 7.99 (d, J = 8.4 Hz, 1H), 7.82 (d, J = 7.2 Hz, 1H), 7.77 (br, 1H), 7.63 (t, J = 7.8 Hz, 1H), 4.21 (m, 2H), 4.14 (m, 1H), 4.00 (dd, J = 11.1, 3.6 Hz, 1H), 3.79 (dd, J = 8.4, 11.4 Hz, 1H), 3.56 (s, 3H), 1.58 (s, 9H); 13C NMR (acetone-d6, 150 MHz) δ 161.8, 153.7, 153.3, 144.8, 131.2, 130.0, 127.9, 123.9, 121.6, 118.5, 118.1, 99.2, 82.7, 54.7, 48.7, 45.5, 36.2, 29.5 (3C); IR (film) νmax 2978, 1702, 1404, 1141 cm–1; [α]23D −45 (c 1.0, THF); ESI-TOF HRMS m/z 389.1268 (M + H+, C20H21ClN2O4 requires 389.1263).
Benzyl ((Tetrahydro-2H-pyran-2-yl)oxy)carbamate (18)
A solution of O-(tetrahydro-2H-pyran-2-yl)hydroxylamine (2.0 g, 17.1 mmol) in CH2Cl2 (20 mL) was treated with i-Pr2NEt (3.28 mL, 18.8 mmol) and benzyl chloroformate (2.56 mL, 18.0 mmol) at 0 °C and stirred at room temperature overnight. The reaction mixture was poured into ice-cold H2O, extracted with CH2Cl2, washed with saturated aqueous NaCl, and dried over Na2SO4. The solvent was removed, and the residue was purified by flash chromatography (10–30% EtOAc/hexanes gradient elution) to give 18 (4.30 g, 100%) as a colorless oil: 1H NMR (CDCl3, 600 MHz) δ 7.64 (br, 1H), 7.37–7.31 (m, 5H), 7.79 (d, J = 8.5 Hz, 1H), 5.18 (dd, J = 16.2, 12.0 Hz, 2H), 4.93 (t, J = 3.0 Hz, 1H), 3.93 (ddd, J = 12.0, 9.6, 3.0 Hz, 1H), 3.61 (dddd, J = 11.4, 4.2, 4.2, 1.8 Hz, 1H), 1.80–1.76 (m, 3H), 1.64–1.54 (m, 3H); 13C NMR (CDCl3, 150 MHz) δ 157.0, 135.7, 128.7, 128.5, 128.4, 102.6, 67.6, 62.6, 28.2, 25.1, 18.8; IR (film) νmax 3265, 2945, 1723, 1454, 1242, 1205, 1108, 1036, 898, 874, 742 cm–1; ESI-TOF HRMS m/z 252.1231 (M + H+, C13H17NO4 requires 252.1230).
Benzyl Methyl((tetrahydro-2H-pyran-2-yl)oxy)carbamate (19)
A solution of 18 (4.30 g, 17.1 mmol) in DMF (50 mL) was treated with NaH (60% dispersion in mineral oil, 821 mg, 20.5 mmol) at 0 °C and stirred at the same temperature for 1 h, after which iodomethane (3.2 mL, 51.3 mmol) was added. The reaction mixture was stirred at room temperature overnight before being poured into ice-cold H2O. The mixture was extracted with EtOAc, washed with H2O and saturated aqueous NaCl, and dried over Na2SO4. The solvent was removed, and the residue was purified by flash chromatography (10–15% EtOAc/hexanes gradient elution) to give 19 (3.89 g, 86%) as a colorless oil: 1H NMR (CDCl3, 600 MHz) δ 7.37–7.31 (m, 5H), 5.19 (d, J = 12.0 Hz, 1H), 5.16 (d, J = 12.0 Hz, 1H), 5.02 (t, J = 3.0 Hz, 1H), 4.02 (ddd, J = 12.0, 9.0, 3.0 Hz, 1H), 3.60 (dddd, J = 11.4, 4.2, 4.2, 1.8 Hz, 1H), 3.28 (s, 3H), 1.79–1.72 (m, 3H), 1.64–1.54 (m, 3H); 13C NMR (CDCl3, 150 MHz) δ 157.8, 136.2, 128.6, 128.3, 128.1, 103.0, 67.9, 62.8, 38.7, 28.6, 25.3, 19.0; IR (film) νmax 2940, 2857, 1703, 1037 cm–1; ESI-TOF HRMS m/z 266.1390 (M + H+, C14H19NO4 requires 266.1387).
N-Methyl-O-(tetrahydro-2H-pyran-2-yl)hydroxylamine (20)
A solution of 19 (1.0 g, 3.77 mmol) in anhydrous Et2O (30 mL) was treated with 10% Pd/C (80 mg, 0.075 mmol), after which the atmosphere was exchanged with H2. The reaction mixture was stirred under H2 at room temperature for 2 h. The reaction mixture was diluted with Et2O, filtered through Celite, and concentrated in an ice-cold bath under reduced pressure to give 20 (530 mg, 87%) as a colorless oil: 1H NMR (CDCl3, 600 MHz) δ 5.68 (m, 1H), 4.80 (dd, J = 6.0, 3.0 Hz, 1H), 3.98–3.92 (m, 1H), 3.58–3.55 (m, 1H), 2.78 (s, 3H), 1.83–1.75 (m, 1H), 1.74–1.69 (m, 1H), 1.59–1.46 (m, 4H); 13C NMR (CDCl3, 150 MHz) δ 101.3, 63.3, 39.7, 29.4, 25.5, 20.4; IR (film) νmax 2940, 2857, 1073, 1037 cm–1; ESI-TOF HRMS m/z 132.1018 (M + H+, C6H13NO2 requires 132.1019).
Acknowledgments
We gratefully acknowledge the financial support from the National Institutes of Health (CA042056, DLB).
Supporting Information Available
Copies of the 1H and 13C NMR spectra and a figure of the HPLC chiral phase separation and establishment of the optical purity of 14. This material is available free of charge via the Internet at http://pubs.acs.org.
The authors declare no competing financial interests.
Funding Statement
National Institutes of Health, United States
Supplementary Material
References
- Ichimura M.; Ogawa T.; Takahashi K.; Kobayashi E.; Kawamoto I.; Yasuzawa T.; Takahashi I.; Nakano H. J. Antibiot. 1990, 43, 1037–1038. [DOI] [PubMed] [Google Scholar]
- Martin D. G.; Biles C.; Gerpheide S. A.; Hanka L. J.; Krueger W. C.; McGovren J. P.; Mizsak S. A.; Neil G. L.; Stewart J. C.; Visser J. J. Antibiot. 1981, 34, 1119–1125. [DOI] [PubMed] [Google Scholar]
- Takahashi I.; Takahashi K.; Ichimura M.; Morimoto M.; Asano K.; Kawamoto I.; Tomita F.; Nakano H. J. Antibiot. 1988, 41, 1915–1917. [DOI] [PubMed] [Google Scholar]
- Igarashi Y.; Futamata K.; Fujita T.; Sekine A.; Senda H.; Naoki H.; Furumai T. J. Antibiot. 2003, 56, 107–113. [DOI] [PubMed] [Google Scholar]
- For duocarmycin SA, see:; a Boger D. L.; Johnson D. S.; Yun W. J. Am. Chem. Soc. 1994, 116, 1635–1656. [Google Scholar]; For yatakemycin, see:; b Parrish J. P.; Kastrinsky D. B.; Wolkenberg S. E.; Igarashi Y.; Boger D. L. J. Am. Chem. Soc. 2003, 125, 10971–10976. [DOI] [PubMed] [Google Scholar]; For CC-1065, see; c Hurley L. H.; Lee C.-S.; McGovren J. P.; Warpehoski M. A.; Mitchell M. A.; Kelly R. C.; Aristoff P. A. Biochemistry 1988, 27, 3886–3892. [DOI] [PubMed] [Google Scholar]; d Boger D. L.; Johnson D. S.; Yun W.; Tarby C. M. Bioorg. Med. Chem. 1994, 2, 115–135. [DOI] [PubMed] [Google Scholar]; e Boger D. L.; Zarrinmayeh H.; Munk S. A.; Kitos P. A.; Suntornwat O. Proc. Natl. Acad. Sci. U. S. A. 1991, 88, 1431–1435. [DOI] [PMC free article] [PubMed] [Google Scholar]; For duocarmycin A, see:; f Boger D. L.; Ishizaki T.; Zarrinmayeh H.; Munk S. A.; Kitos P. A.; Suntornwat O. J. Am. Chem. Soc. 1990, 112, 8961–8971. [Google Scholar]; g Boger D. L.; Yun W.; Terashima S.; Fukuda Y.; Nakatani K.; Kitos P. A.; Jin Q. Bioorg. Med. Chem. Lett. 1992, 2, 759–765. [Google Scholar]
- Reviews:; a Boger D. L.; Johnson D. S. Angew. Chem., Int. Ed. Engl. 1996, 35, 1438–1474. [Google Scholar]; b Boger D. L. Acc. Chem. Res. 1995, 28, 20–29. [Google Scholar]; c Boger D. L.; Johnson D. S. Proc. Natl. Acad. Sci. U. S. A. 1995, 92, 3642–3649. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Boger D. L.; Garbaccio R. M. Acc. Chem. Res. 1999, 32, 1043–1052. [Google Scholar]; e Searcey M. Curr. Pharm. Des. 2002, 8, 1375–1389. [DOI] [PubMed] [Google Scholar]; f MacMillan K. S.; Boger D. L. J. Med. Chem. 2009, 52, 5771–5780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Review:Boger D. L.; Boyce C. W.; Garbaccio R. M.; Goldberg J. A. Chem. Rev. 1997, 97, 787–828. [DOI] [PubMed] [Google Scholar]
- Review:Boger D. L.; Garbaccio R. M. Bioorg. Med. Chem. 1997, 5, 263–276. [DOI] [PubMed] [Google Scholar]
- a Jin W.; Trzupek J. D.; Rayl T. J.; Broward M. A.; Vielhauer G. A.; Weir S. J.; Hwang I.; Boger D. L. J. Am. Chem. Soc. 2007, 129, 15391–15397. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Lajiness J. P.; Robertson W. M.; Dunwiddie I.; Broward M. A.; Vielhauer G. A.; Weir S. J.; Boger D. L. J. Med. Chem. 2010, 53, 7731–7738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe A. L.; Duncan K. K.; Parelkar N. K.; Brown D.; Vielhauer G. A.; Boger D. L. J. Med. Chem. 2013, 56, 4104–4115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reviews:; a Chen Y.; Hu L. Med. Res. Rev. 2009, 29, 29–64. [DOI] [PubMed] [Google Scholar]; b Ghosh N.; Sheldrake H. M.; Searcey M.; Pors K. Curr. Top. Med. Chem. 2009, 9, 1494–1524. [DOI] [PubMed] [Google Scholar]; c Tietze L. F.; Krewer B. Anti-Cancer Agents Med. Chem. 2009, 9, 304–325. [DOI] [PubMed] [Google Scholar]; d Wolkenberg S. E.; Boger D. L. Chem. Rev. 2002, 102, 2477–2495. [DOI] [PubMed] [Google Scholar]
- a Boger D. L.; Ishizaki T.; Kitos P. A.; Suntornwat O. J. Org. Chem. 1990, 55, 5823–5832. [Google Scholar]; b Boger D. L.; Ishizaki T. Tetrahedron Lett. 1990, 31, 793–796. [Google Scholar]; c Boger D. L.; Wysocki R. J.; Ishizaki T. J. Am. Chem. Soc. 1990, 112, 5230–5240. [Google Scholar]; d Boger D. L.; Munk S. A. J. Am. Chem. Soc. 1992, 114, 5487–5496. [Google Scholar]
- a Boger D. L.; McKie J. A.; Boyce C. W. Synlett 1997, 515–517. [Google Scholar]; b Kastrinsky D. B.; Boger D. L. J. Org. Chem. 2004, 69, 2284–2289. [DOI] [PubMed] [Google Scholar]; c Lajiness J. P.; Boger D. L. J. Org. Chem. 2011, 76, 583–587. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Ling L.; Xie Y.; Lown J. W. Heterocycl. Commun. 1997, 3, 405–408. [Google Scholar]; e Tietze L. F.; von Hof J. M.; Krewer B.; Muller M.; Major F.; Schuster H. J.; Schuberth I.; Alvers F. ChemMedChem. 2008, 3, 1946–1955. [DOI] [PubMed] [Google Scholar]
- Wolfe A. L.; Duncan K. K.; Parelkar N. K.; Weir S. J.; Vielhauer G. A.; Boger D. L. J. Med. Chem. 2012, 55, 5878–5886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tichenor M. S.; Trzupek J. D.; Kastrinsky D. B.; Shiga F.; Hwang I.; Boger D. L. J. Am. Chem. Soc. 2006, 128, 15683–15696. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.