Abstract
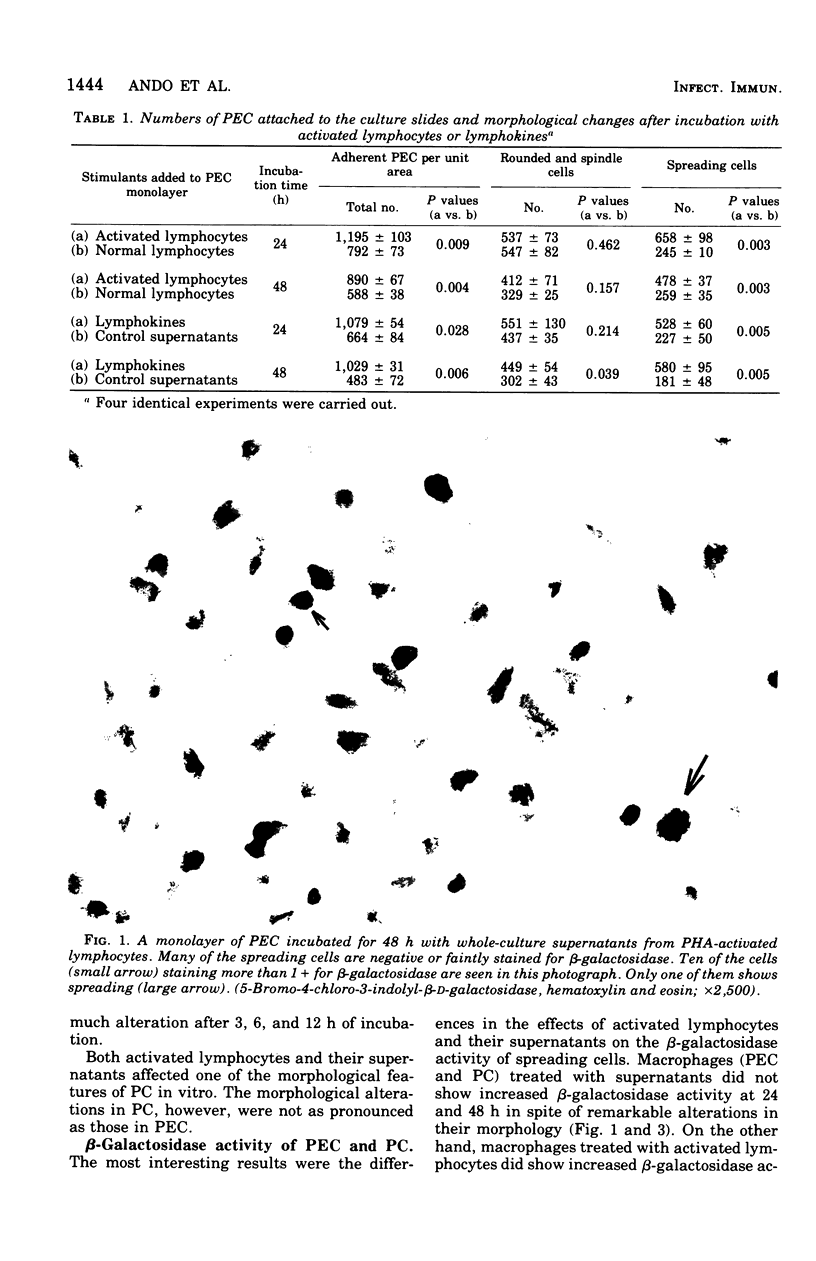
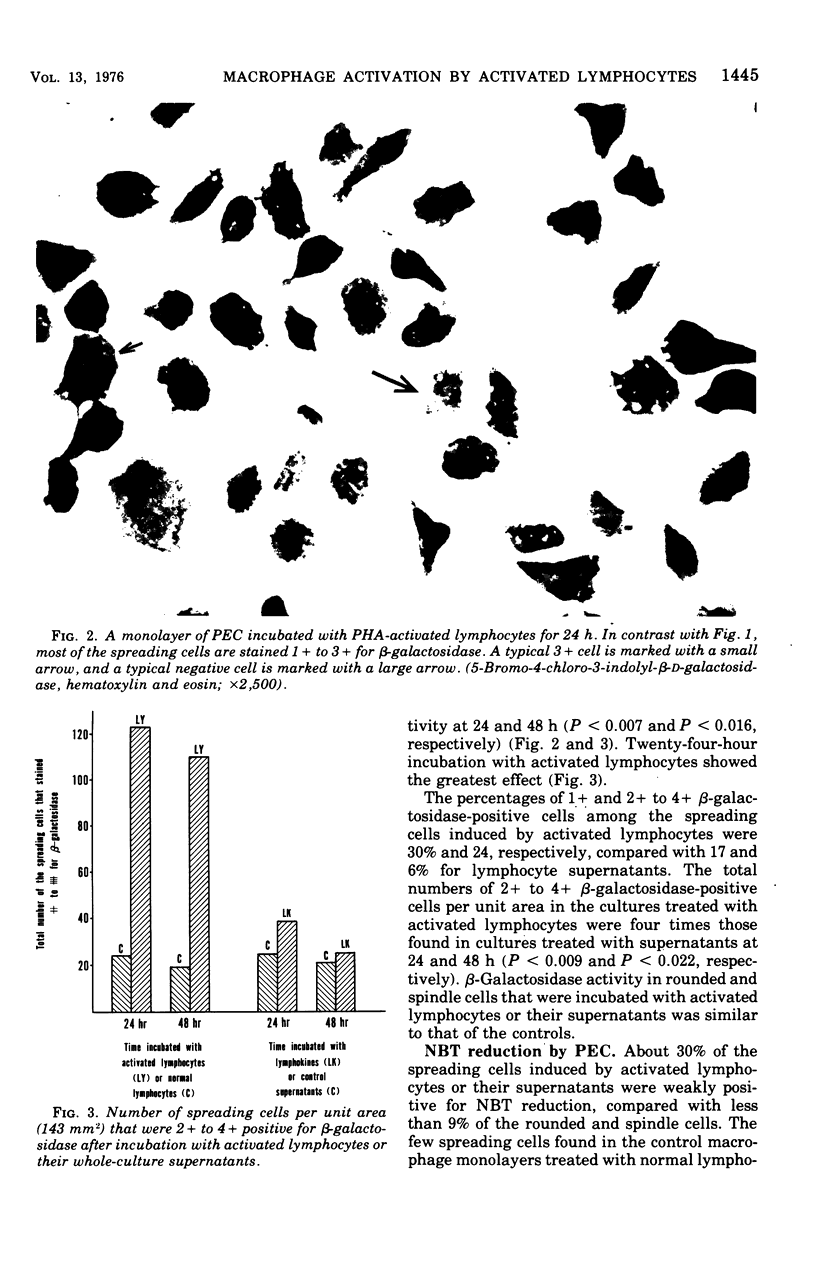
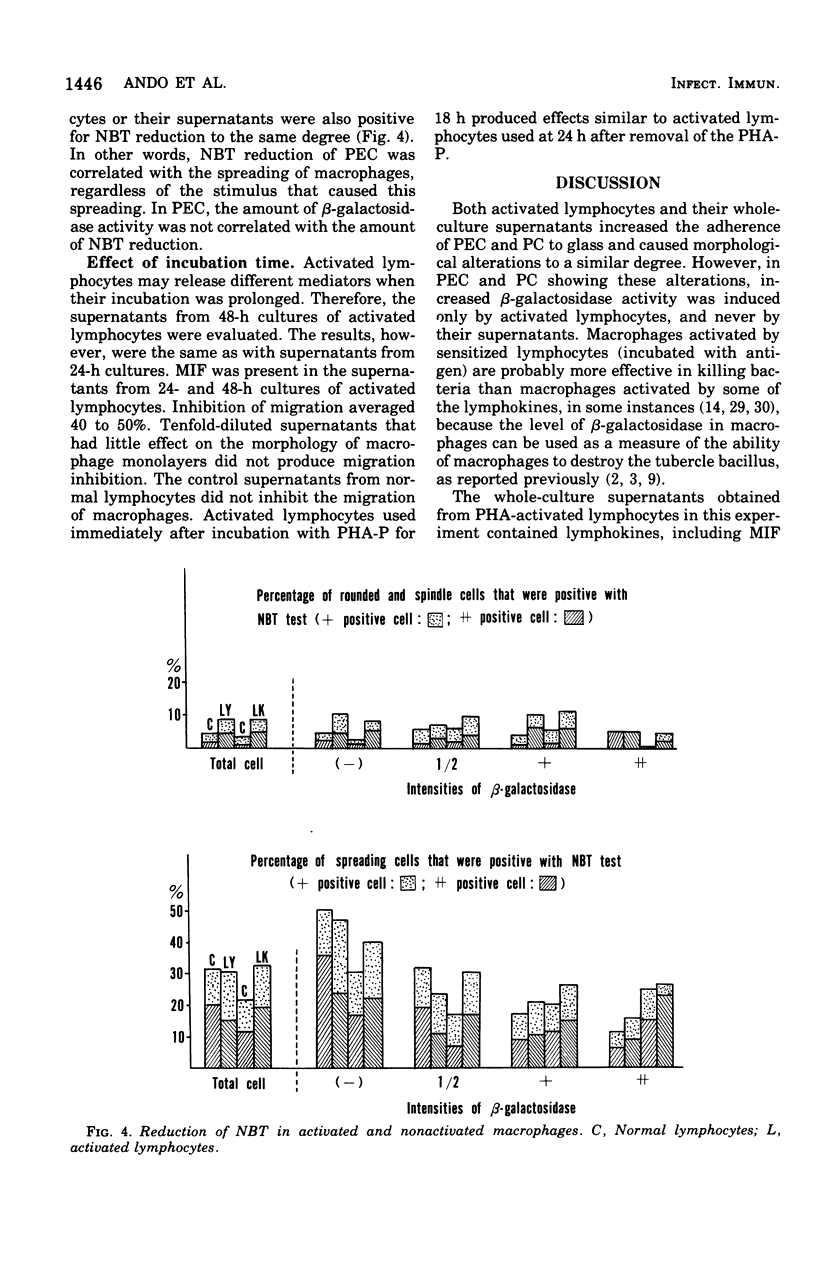
When rabbit peritoneal exudate cells were incubated for 24 and 48 h with phytohemagglutinin-activated lymphocytes or their culture supernatants, two times as many cells remained adherent to culture slides as in the controls. More spreading cells were found among the adherent cells in the stimulated cultures. Eighty percent of spreading cells that were induced by supernatants were negative or faintly positive for beta-galactosidase. On the other hand, half of the spreading cells induced by activated lymphocytes were positive (1+ to 4+) for beta-lymphocytes and their supernatants. Under similar conditions, unstimulated peritoneal cells showed less marked activation. These findings show that macrophages can appear morphologically activated and yet not be enzymatically activated by lymphokines. Possible mechanisms of direct interaction of activated lymphocytes and macrophages are discussed.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ando M., Dannenberg A. M., Jr Macrophage accumulation, division, maturation, and digestive and microbicidal capacities in tuberculous lesions. IV. Macrophage turnover, lysosomal enzymes, and division in healing lesions. Lab Invest. 1972 Nov;27(5):466–472. [PubMed] [Google Scholar]
- Ando M., Dannenberg A. M., Jr, Shima K. Macrophage accumulation, division, maturation and digestive and microbicidal capacities in tuberculous lesions. II. Rate at which mononuclear cells enter and divide in primary BCG lesions and those of reinfection. J Immunol. 1972 Jul;109(1):8–19. [PubMed] [Google Scholar]
- Ando M. Macrophage activation in tuberculin reactions of rabbits with primary BCG infection and reinfection. J Reticuloendothel Soc. 1973 Aug;14(2):132–145. [PubMed] [Google Scholar]
- Babior B. M., Kipnes R. S., Curnutte J. T. Biological defense mechanisms. The production by leukocytes of superoxide, a potential bactericidal agent. J Clin Invest. 1973 Mar;52(3):741–744. doi: 10.1172/JCI107236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baehner R. L., Nathan D. G., Karnovsky M. L. Correction of metabolic deficiencies in the leukocytes of patients with chronic granulomatous disease. J Clin Invest. 1970 May;49(5):865–870. doi: 10.1172/JCI106305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baehner R. L., Nathan D. G. Leukocyte oxidase: defective activity in chronic granulomatous disease. Science. 1967 Feb 17;155(3764):835–836. doi: 10.1126/science.155.3764.835. [DOI] [PubMed] [Google Scholar]
- Curnutte J. T., Whitten D. M., Babior B. M. Defective superoxide production by granulocytes from patients with chronic granulomatous disease. N Engl J Med. 1974 Mar 14;290(11):593–597. doi: 10.1056/NEJM197403142901104. [DOI] [PubMed] [Google Scholar]
- DAVID J. R., AL-ASKARI S., LAWRENCE H. S., THOMAS L. DELAYED HYPERSENSITIVITY IN VITRO. I. THE SPECIFICITY OF INHIBITION OF CELL MIGRATION BY ANTIGENS. J Immunol. 1964 Aug;93:264–273. [PubMed] [Google Scholar]
- Dannenberg A. M., Jr, Ando M., Shima K. Macrophage accumulation, division, maturation, and digestive and microbicidal capacities in tuberculous lesions. 3. The turnover of macrophages and its relation to their activation and antimicrobial immunity in primary BCG lesions and those of reinfection. J Immunol. 1972 Nov;109(5):1109–1121. [PubMed] [Google Scholar]
- Fowles R. E., Fajardo I. M., Leibowitch J. L., David J. R. The enhancement of macrophage bacteriostasis by products of activated lymphocytes. J Exp Med. 1973 Oct 1;138(4):952–964. doi: 10.1084/jem.138.4.952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godal T., Rees R. J., Lamvik J. O. Lymphocyte-mediated modification of blood-derived macrophage function in vitro; inhibition of growth of intracellular mycobacteria with lymphokines. Clin Exp Immunol. 1971 Apr;8(4):625–637. [PMC free article] [PubMed] [Google Scholar]
- Klebanoff S. J. Antimicrobial mechanisms in neutrophilic polymorphonuclear leukocytes. Semin Hematol. 1975 Apr;12(2):117–142. [PubMed] [Google Scholar]
- Lipsky P. E., Rosenthal A. S. Macrophage-lymphocyte interaction. I. Characteristics of the antigen-independent-binding of guinea pig thymocytes and lymphocytes to syngeneic macrophages. J Exp Med. 1973 Oct 1;138(4):900–924. doi: 10.1084/jem.138.4.900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandell G. L., Rubin W., Hook E. W. The effect of an NADH oxidase inhibitor (hydrocortisone) on polymorphonuclear leukocyte bactericidal activity. J Clin Invest. 1970 Jul;49(7):1381–1388. doi: 10.1172/JCI106355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooney J. J., Waksman B. H. Activation of normal rabbit macrophage monolayers by supernatants of antigen-stimulated lymphocytes. J Immunol. 1970 Nov;105(5):1138–1145. [PubMed] [Google Scholar]
- Nath I., Poulter L. W., Turk J. L. Effect of lymphocyte mediators on macrophages in vitro. A correlation of morphological and cytochemical changes. Clin Exp Immunol. 1973 Mar;13(3):455–466. [PMC free article] [PubMed] [Google Scholar]
- Nathan C. F., Karnovsky M. L., David J. R. Alterations of macrophage functions by mediators from lymphocytes. J Exp Med. 1971 Jun 1;133(6):1356–1376. doi: 10.1084/jem.133.6.1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan C. F., Remold H. G., David J. R. Characterization of a lymphocyte factor which alters macrophage functions. J Exp Med. 1973 Feb 1;137(2):275–290. doi: 10.1084/jem.137.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson R. J., Youmans G. P. Demonstration in tissue culture of lymphocyte-mediated immunity to tuberculosis. Infect Immun. 1970 Jun;1(6):600–603. doi: 10.1128/iai.1.6.600-603.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed P. W. Glutathione and the hexose monophosphate shunt in phagocytizing and hydrogen peroxide-treated rat leukocytes. J Biol Chem. 1969 May 10;244(9):2459–2464. [PubMed] [Google Scholar]
- SCHOENBERG M. D., MUMAW V. R., MOORE R. D., WEISBERGER A. S. CYTOPLASMIC INTERACTION BETWEEN MACROPHAGES AND LYMPHOCYTIC CELLS IN ANTIBODY SYNTHESIS. Science. 1964 Feb 28;143(3609):964–965. doi: 10.1126/science.143.3609.964. [DOI] [PubMed] [Google Scholar]
- Salvin S. B., Sell S., Nishio J. Activity in vitro of lymphocytes and macrophages in delayed hypersensitivity. J Immunol. 1971 Sep;107(3):655–662. [PubMed] [Google Scholar]
- Schmidt M. E., Douglas S. D., Rubin A. D. Human monocyte activation by supernatants from concanavalin A (con A) stimulated lymphocytes. Cell Immunol. 1973 Oct;9(1):45–59. doi: 10.1016/0008-8749(73)90166-4. [DOI] [PubMed] [Google Scholar]
- Shima K., Dannenberg A. M., Jr, Ando M., Chandrasekhar S., Seluzicki J. A., Fabrikant J. I. Macrophage accumulation, division, maturation, and digestive and microbicidal capacities in tuberculous lesions. I. Studies involving their incorporation of tritiated thymidine and their content of lysosomal enzymes and bacilli. Am J Pathol. 1972 Apr;67(1):159–180. [PMC free article] [PubMed] [Google Scholar]
- Simon H. B., Sheagren J. N. Enhancement of macrophage bactericidal capacity by antigenically stimulated immune lymphocytes. Cell Immunol. 1972 Jun;4(2):163–174. doi: 10.1016/0008-8749(72)90015-9. [DOI] [PubMed] [Google Scholar]
- Simon H. B., Sheagren J. N. Migration inhibitory factor and macrophage bactericidal function. Infect Immun. 1972 Aug;6(2):101–103. doi: 10.1128/iai.6.2.101-103.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]