Abstract
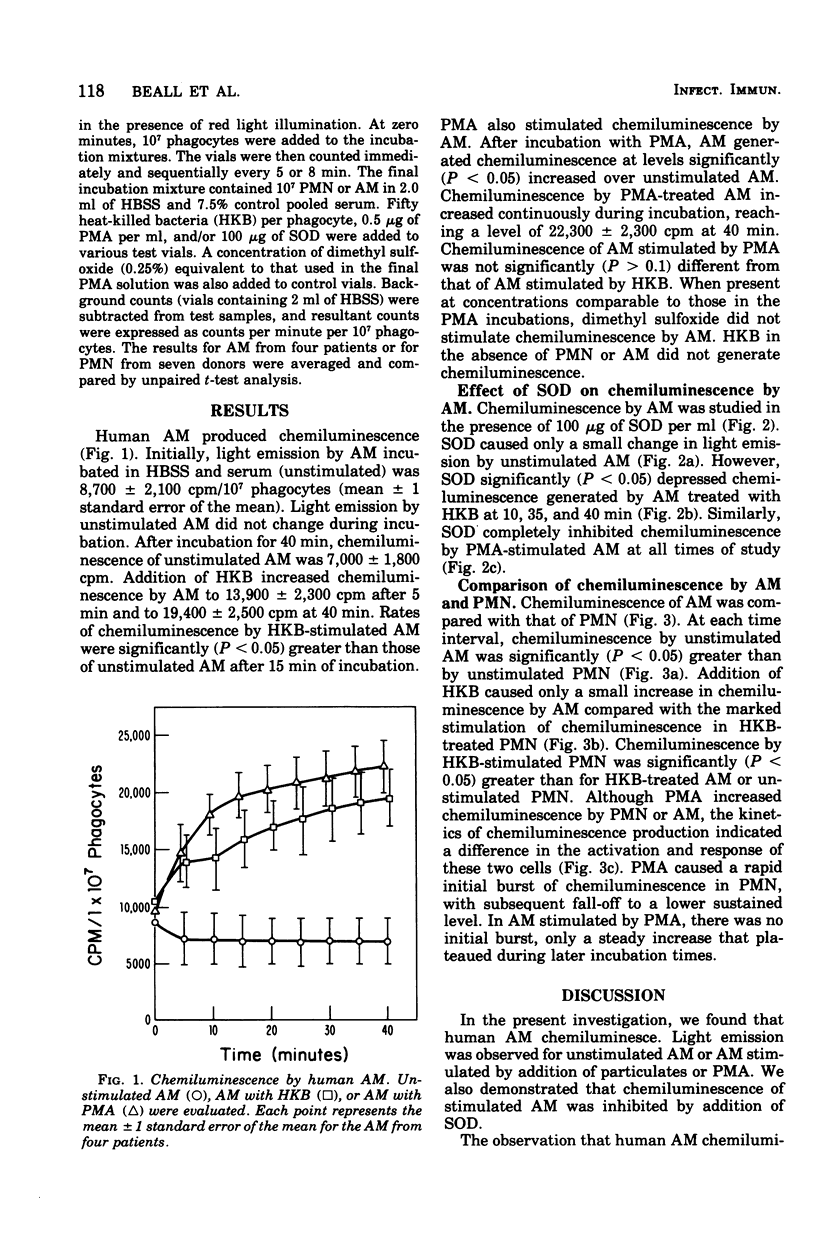
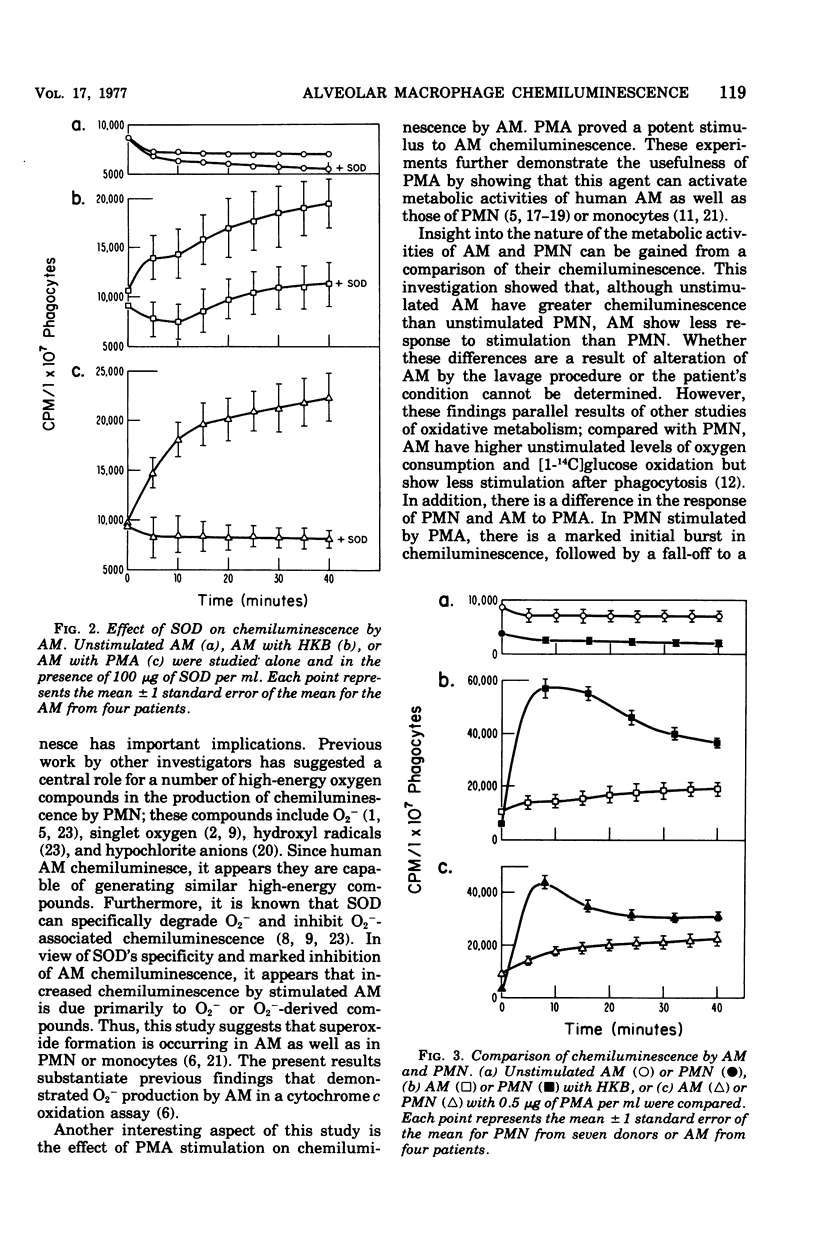
Chemiluminescence of human alveolar macrophages (AM) was evaluated in vitro. Unstimulated AM generated chemiluminescence that remained constant during incubation. Addition of heat-killed Staphylococcus aureus 502A (HKB) or a chemical agent, phorbol myristate acetate, produced high rates of chemiluminescence that were significantly (P less than 0.05) increased over unstimulated AM. Phorbol myristate acetate-and HKB-stimulated increases in AM chemiluminescence were completely blocked by the enzyme superoxide dismutase. In comparison with unstimulated polymorphonuclear leukocytes, unstimulated AM had significantly (P less than 0.005) greater levels of chemiluminescence. However, after stimulation by phorbol myristate acetate or HKB, AM showed less chemiluminescence than similarly treated polymorphonuclear leukocytes.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen R. C., Stjernholm R. L., Steele R. H. Evidence for the generation of an electronic excitation state(s) in human polymorphonuclear leukocytes and its participation in bactericidal activity. Biochem Biophys Res Commun. 1972 May 26;47(4):679–684. doi: 10.1016/0006-291x(72)90545-1. [DOI] [PubMed] [Google Scholar]
- Allen R. C., Yevich S. J., Orth R. W., Steele R. H. The superoxide anion and singlet molecular oxygen: their role in the microbicidal activity of the polymorphonuclear leukocyte. Biochem Biophys Res Commun. 1974 Oct 8;60(3):909–917. doi: 10.1016/0006-291x(74)90401-x. [DOI] [PubMed] [Google Scholar]
- Babior B. M., Curnutte J. T., Kipnes R. S. Biological defense mechanisms. Evidence for the participation of superoxide in bacterial killing by xanthine oxidase. J Lab Clin Med. 1975 Feb;85(2):235–244. [PubMed] [Google Scholar]
- Babior B. M., Kipnes R. S., Curnutte J. T. Biological defense mechanisms. The production by leukocytes of superoxide, a potential bactericidal agent. J Clin Invest. 1973 Mar;52(3):741–744. doi: 10.1172/JCI107236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeChatelet L. R., Shirley P. S., Johnston R. B., Jr Effect of phorbol myristate acetate on the oxidative metabolism of human polymorphonuclear leukocytes. Blood. 1976 Apr;47(4):545–554. [PubMed] [Google Scholar]
- Drath D. B., Karnovsky M. L. Superoxide production by phagocytic leukocytes. J Exp Med. 1975 Jan 1;141(1):257–262. doi: 10.1084/jem.141.1.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finley T. N., Swenson E. W., Curran W. S., Huber G. L., Ladman A. J. Bronchopulmonary lavage in normal subjects and patients with obstructive lung disease. Ann Intern Med. 1967 Apr;66(4):651–658. doi: 10.7326/0003-4819-66-4-651. [DOI] [PubMed] [Google Scholar]
- Fridovich I. Superoxide dismutases. Annu Rev Biochem. 1975;44:147–159. doi: 10.1146/annurev.bi.44.070175.001051. [DOI] [PubMed] [Google Scholar]
- Goda K., Chu J., Kimura T., Schaap A. P. Cytochrome c enhancement of singlet molecular oxygen production by the NADPH-dependent adrenodoxin reductase-adrenodoxin system: the role of singlet oxygen in damaging adrenal mitochondrial membranes. Biochem Biophys Res Commun. 1973 Jun 19;52(4):1300–1306. doi: 10.1016/0006-291x(73)90642-6. [DOI] [PubMed] [Google Scholar]
- Johnston R. B., Jr, Keele B. B., Jr, Misra H. P., Lehmeyer J. E., Webb L. S., Baehner R. L., RaJagopalan K. V. The role of superoxide anion generation in phagocytic bactericidal activity. Studies with normal and chronic granulomatous disease leukocytes. J Clin Invest. 1975 Jun;55(6):1357–1372. doi: 10.1172/JCI108055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston R. B., Jr, Lehmeyer J. E., Guthrie L. A. Generation of superoxide anion and chemiluminescence by human monocytes during phagocytosis and on contact with surface-bound immunoglobulin G. J Exp Med. 1976 Jun 1;143(6):1551–1556. doi: 10.1084/jem.143.6.1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KARNOVSKY M. L. Metabolic basis of phagocytic activity. Physiol Rev. 1962 Jan;42:143–168. doi: 10.1152/physrev.1962.42.1.143. [DOI] [PubMed] [Google Scholar]
- Klebanoff S. J. Antimicrobial mechanisms in neutrophilic polymorphonuclear leukocytes. Semin Hematol. 1975 Apr;12(2):117–142. [PubMed] [Google Scholar]
- Klebanoff S. J. Role of the superoxide anion in the myeloperoxidase-mediated antimicrobial system. J Biol Chem. 1974 Jun 25;249(12):3724–3728. [PubMed] [Google Scholar]
- Krinsky N. I. Singlet excited oxygen as a mediator of the antibacterial action of leukocytes. Science. 1974 Oct 25;186(4161):363–365. doi: 10.1126/science.186.4161.363. [DOI] [PubMed] [Google Scholar]
- Nelson R. D., Mills E. L., Simmons R. L., Quie P. G. Chemiluminescence response of phagocytizing human monocytes. Infect Immun. 1976 Jul;14(1):129–134. doi: 10.1128/iai.14.1.129-134.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repine J. E., White J. G., Clawson C. C., Holmes B. H. The influence of phorbol myristate acetate on the metabolism of neutrophils from carriers of sex-linked chronic granulomatous disease. J Lab Clin Med. 1975 Jan;85(1):82–86. [PubMed] [Google Scholar]
- Repine J. E., White J. G., Clawson C. C., Holmes B. M. Effects of phorbol myristate acetate on the metabolism and ultrastructure of neutrophils in chronic granulomatous disease. J Clin Invest. 1974 Jul;54(1):83–90. doi: 10.1172/JCI107752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repine J. E., White J. G., Clawson C. C., Holmes B. M. The influence of phorbol myristate acetate on oxygen consumption by polymorphonuclear leukocytes. J Lab Clin Med. 1974 Jun;83(6):911–920. [PubMed] [Google Scholar]
- Rosen H., Klebanoff S. J. Chemiluminescence and superoxide production by myeloperoxidase-deficient leukocytes. J Clin Invest. 1976 Jul;58(1):50–60. doi: 10.1172/JCI108458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagone A. L., Jr, King G. W., Metz E. N. A comparison of the metabolic response to phagocytosis in human granulocytes and monocytes. J Clin Invest. 1976 May;57(5):1352–1358. doi: 10.1172/JCI108403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley P. E., Williams S. G. Use of the liquid scintillation spectrometer for determining adenosine triphosphate by the luciferase enzyme. Anal Biochem. 1969 Jun;29(3):381–392. doi: 10.1016/0003-2697(69)90323-6. [DOI] [PubMed] [Google Scholar]
- Webb L. S., Keele B. B., Jr, Johnston R. B., Jr Inhibition of phagocytosis-associated chemiluminescence by superoxide dismutase. Infect Immun. 1974 Jun;9(6):1051–1056. doi: 10.1128/iai.9.6.1051-1056.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]


