Graphical abstract
Keywords: PPAR gamma, Nuclear receptor, Natural product, Nutrition, Diabetes
Abbreviations: 9-(S)-HODE, (9S,10E,12Z)-9-hydroxyoctadeca-10,12-dienoic acid; AF-2, activation function-2; CAP, c-Cbl-associated protein; Cdk5, cyclin-dependent kinase 5; DCM, dichloromethane; DIO, diet-induced obesity; DPP-4, dipeptidylpeptidase 4; EMA, European Medicines Agency; FDA, Food and Drug Administration; Glut4, glucose transporter type 4; HDL, high-density lipoprotein; HUVEC, human umbilical vein endothelial cells; LBD, ligand-binding domain; LDL, low-density lipoprotein; MAPK, mitogen-activated protein kinase; MeOH, methanol; NF-κB, nuclear factor-kappaB; PPAR, peroxisome proliferator-activated receptor; RXR, retinoid X receptor; PDB, protein data bank; PPRE, peroxisome proliferator response element; SPPARMs, selective PPARγ modulators; TCM, traditional Chinese medicine; TNF-α, tumor necrosis factor alpha
Chemical compounds studied in this article: Pioglitazone (PubChem CID: 4829), Magnolol (PubChem CID: 72300), Honokiol (PubChem CID: 72303), Falcarindiol (PubChem CID: 5281148), Resveratrol (PubChem CID: 445154), Amorfrutin 1 (PubChem CID: 10132170), Rosiglitazone (PubChem CID: 77999), Quercetin (PubChem CID: 5280343), (−)-Catechin (PubChem CID: 73160), Linolenic acid (PubChem CID: 5280934)
Abstract
Agonists of the nuclear receptor PPARγ are therapeutically used to combat hyperglycaemia associated with the metabolic syndrome and type 2 diabetes. In spite of being effective in normalization of blood glucose levels, the currently used PPARγ agonists from the thiazolidinedione type have serious side effects, making the discovery of novel ligands highly relevant.
Natural products have proven historically to be a promising pool of structures for drug discovery, and a significant research effort has recently been undertaken to explore the PPARγ-activating potential of a wide range of natural products originating from traditionally used medicinal plants or dietary sources. The majority of identified compounds are selective PPARγ modulators (SPPARMs), transactivating the expression of PPARγ-dependent reporter genes as partial agonists. Those natural PPARγ ligands have different binding modes to the receptor in comparison to the full thiazolidinedione agonists, and on some occasions activate in addition PPARα (e.g. genistein, biochanin A, sargaquinoic acid, sargahydroquinoic acid, resveratrol, amorphastilbol) or the PPARγ-dimer partner retinoid X receptor (RXR; e.g. the neolignans magnolol and honokiol). A number of in vivo studies suggest that some of the natural product activators of PPARγ (e.g. honokiol, amorfrutin 1, amorfrutin B, amorphastilbol) improve metabolic parameters in diabetic animal models, partly with reduced side effects in comparison to full thiazolidinedione agonists. The bioactivity pattern as well as the dietary use of several of the identified active compounds and plant extracts warrants future research regarding their therapeutic potential and the possibility to modulate PPARγ activation by dietary interventions or food supplements.
1. Significance of metabolic disorders
The metabolic syndrome is currently a major worldwide epidemic. It strongly associates with obesity, insulin resistance, type 2 diabetes, and cardiovascular diseases, which are major pathologies contributing to mortality and morbidity worldwide. At present the metabolic syndrome is already affecting more than a quarter of the world's adult population. Its prevalence is further growing in both adults and children due to a life style characterized by high calorie nutrition combined with low physical activity [1], [2].
The metabolic syndrome represents by definition a disorder related to imbalance of energy utilization and storage. Its features include abdominal obesity, hypertension, dyslipidemia (increased blood serum triglycerides; low high-density lipoprotein (HDL) and high low-density lipoprotein (LDL) cholesterol levels), insulin resistance with elevated fasting blood glucose, and glucose intolerance as well as establishment of pro-thrombotic and pro-inflammatory states [3]. People affected by the metabolic syndrome have a greater risk of developing cardiovascular diseases and type 2 diabetes. Moreover, recent research indicates that metabolic syndrome associated obesity causes chronic low-grade local tissue inflammation and increased susceptibility to other disease conditions such as fatty liver, sleep disturbances, cholesterol gallstones, polycystic ovary syndrome, asthma, and some types of cancer [3], [4].
The two main approaches in metabolic syndrome management are in the first place life style modifications that aim at restoring energy balance by reduced calorie intake and increased energy expenditure by physical activity, and on second place pharmaceutical interventions [1], [3]. Employed drugs target different relevant aspects of the metabolic syndrome such as body weight and fat distribution, insulin resistance, hypertension, dyslipidemia, hyperglycemia, or the established prothrombotic and proinflammatory state [3]. For the treatment of patients suffering from type 2 diabetes, aside from life-style alterations, insulin and insulin analogs were first applied [5]. Later a number of oral anti-hyperglycemic pharmaceuticals were developed and successfully used [6] including sulfonylureas (increasing insulin secretion) [7], biguanides (insulin sensitizers; e.g. metformin), alpha-glucosidase inhibitors (slowing the digestion of starch in the small intestine), meglitinides (increasing insulin secretion), dipeptidylpeptidase 4 (DPP-4) inhibitors (increasing insulin secretion) [6], as well as thiazolidinediones (agonists of PPARγ). Recent research strategies also explore targeting the nuclear factor-kappaB (NF-κB) pathway [8], mitogen-activated protein kinases (MAPK) signaling [9], fatty acid-binding proteins [10], as well as other targets involved in fatty acid metabolism [11], [12]. PPARγ, the molecular target of the thiazolidinediones, is particularly involved in the regulation of insulin sensitivity, inflammation, fatty acid storage, and glucose metabolism, and therefore represents an especially interesting pharmacological target which is able to simultaneously modulate several of the underlying pathologies of the metabolic syndrome [13], [14].
2. PPARγ and the metabolic regulation
PPARs belong to a subfamily of the nuclear receptor superfamily of ligand-inducible transcription factors [15]. To date, three PPAR isotypes encoded by separate genes have been identified, PPARα [16], PPARβ/δ, and PPARγ [17].
PPARs mainly control the expression of gene networks involved in adipogenesis, lipid metabolism, inflammation, and the maintenance of metabolic homeostasis. As they can be activated by dietary fatty acids and their metabolites, they act as lipid sensors that, upon activation, are able to markedly redirect metabolism [18], [19], [20]. The gene transcription process is identical in all three PPAR subtypes (Fig. 1): After ligand binding, PPARs form heterodimers with another ligand-activated nuclear receptor, the retinoid X receptor (RXR). The PPAR-RXR heterodimer binds to peroxisome proliferator response elements (PPREs) in the promoter region of the respective target genes. The transcription process is then initiated upon recruitment of different transcriptional cofactors [21], [22], [23], [24] (Fig. 1).
Fig. 1.
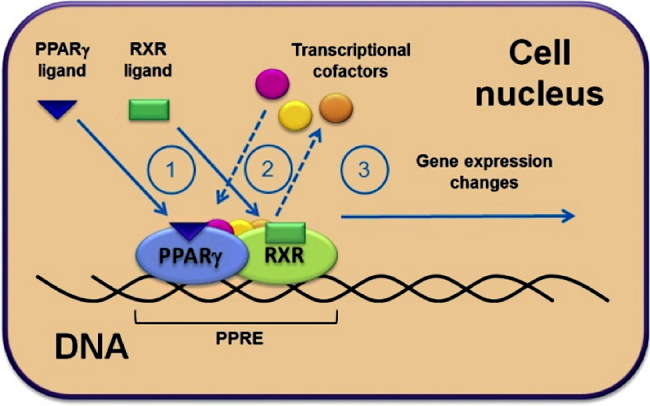
PPARγ transcriptional activation. (1) Binding of activating ligands to PPARγ and to its dimer partner RXR; (2) following the ligand binding there are conformational changes of the receptors, resulting in re-arrangement of the transcriptional complex and changes in the associated transcriptional cofactors; (3) resulting from this reorganization, the transcriptional complex is activated and initiates changes in the expression of the regulated PPARγ target genes.
The three PPAR isotypes possess a distinct tissue distribution and have different functions in the regulation of energy metabolism. PPARα is highly expressed in muscles, liver, heart, and kidney, and mainly regulates genes involved in the metabolism of lipids and lipoproteins [20], [25], [26], [27]. PPARβ/δ is abundantly expressed throughout the body but at low levels in the liver. It has emerged as an important regulator of lipid metabolism and energy balance primarily in adipose tissue, skeletal muscle, and the heart [25], [28], [29]. The PPARγ protein exists in two isoforms that are expressed from the same gene by utilizing distinct promoters and 5′exons. PPARγ2 differs from PPARγ1 by the presence of an additional stretch of 30 amino acid residues in the ligand-independent domain at the N-terminal end resulting in a higher transcriptional activity compared to PPARγ1 [30], [31], [32]. The two PPARγ isoforms also show a distinct expression pattern: PPARγ1 is abundantly expressed in adipose tissue, large intestine, and hematopoietic cells, and to a lower degree in kidney, liver, muscles, pancreas, and small intestine. PPARγ2 is restricted to white and brown adipose tissue under physiological conditions [25], [33], [34].
Endogenous ligands for PPARγ include fatty acids and prostanoids [19], [35] that act as weak agonists compared to the strong synthetic thiazolidinedione agonists [36], [37]. The question of whether PPARγ has some highly specific endogenous ligands or whether it operates as a rather promiscuous physiological lipid sensor activated in concert by a variety of fatty acids and eicosanoids is still not clearly resolved [38], [39], [40], [41], [42], [43].
In the human body, PPARγ is the master regulator of adipocyte differentiation, plays an important role in lipid metabolism and glucose homeostasis, modulates metabolism and inflammation in immune cells, as well as controls cell proliferation [44], [45], [46]. PPARγ is induced during the differentiation of preadipocytes into adipocytes [47], [48], [49]. The fact that PPARγ null mice are completely lacking adipose tissue clearly demonstrates that PPARγ is essential for adipocyte differentiation [50]. Furthermore, PPARγ directly activates many genes involved in adipocyte lipid storage [51], [52]. Adipose tissue is also the primary tissue responsible for the insulin-sensitizing effect of the thiazolidinedione-type PPARγ ligands. PPARγ controls the expression of numerous factors secreted from adipose tissue that influence insulin sensitivity positively (e.g. adiponectin, leptin) or negatively (e.g. resistin, tumor necrosis factor-α). In addition, PPARγ can directly modulate the expression of genes involved in glucose homeostasis, e.g. it upregulates glucose transporter type 4 (Glut4) and c-Cbl-associated protein (CAP) expression [53], [54]. PPARγ is also expressed in various immune system-related cell types, particularly in antigen-presenting cells such as macrophages and dendritic cells. In these cells, PPARγ does not only regulate genes related to lipid metabolism, but also immunity and inflammation related genes [55], [56], [57], [58]. Also the anti-atherosclerosis activity of PPARγ activating thiazolidinediones observed in animal models is thought to be generated primarily through modulation of PPARγ-regulated gene expression in macrophages [44], [59]. In addition to its metabolic and anti-inflammatory properties, PPARγ also modulates proliferation and apoptosis of many cancer cell types, and is expressed in many human tumors including lung, breast, colon, prostate, and bladder cancer. As natural and synthetic PPARγ activators have been found to inhibit cancer cell growth in vitro and in animal models, PPARγ might also be a target for new cancer therapies [44], [60], [61].
Aside from the availability of agonists and cofactors, the transcriptional activity of PPARγ is also regulated by its phosphorylation status, providing additional possibilities for fine-tuning [62], [63]. Phosphorylation of PPARγ at Ser273 by cyclin-dependent kinase 5 (Cdk5) was recently linked to obesity, and anti-diabetic PPARγ ligands (e.g. the thiazolidinedione rosiglitazone) were shown to inhibit the Cdk5-mediated phosphorylation of PPARγ in adipose tissue [62]. Moreover, several PPARγ ligands with poor agonistic activity but potent anti-diabetic effects in vivo revealed to be strong inhibitors of the PPARγ phosphorylation by Cdk5. The ligand's ability to suppress Ser273 phosphorylation correlated well with their anti-diabetic effectiveness but was independent of classical agonistic effects implied in some of the side-effects of PPARγ ligands currently used in clinics. Consequently, targeted inhibition of PPARγ Ser273 phosphorylation was suggested as a promising approach for development of a new generation of anti-diabetic agents [62].
While the application of PPARγ agonists is studied in many different disease conditions, the only approved use for PPARγ ligands so far is the application of thiazolidinediones (full PPARγ agonists) in type 2 diabetes. Thiazolidinediones first emerged as new class of drugs alleviating insulin resistance in patients with type 2 diabetes in the late 1990s [64], [65], [66]. The first approved drug of this class was troglitazone (CS-045), which became first available in March 1997 and was withdrawn from the US market in March 2000 [67]. Troglitazone activates preferentially PPARγ but is also a ligand of PPARα. As a drug counteracting type 2 diabetes, troglitazone increases insulin sensitivity and glucose tolerance in obese subjects [68], [69], [70], [71], [72], [73], [74], [75]. It was also demonstrated to inhibit the progression of early atherosclerotic lesions, to lower blood pressure, as well as to have favorable impact on other known cardiovascular risk factors [76], [77], [78]. In spite of its benefits in cardiovascular disease, troglitazone was removed from the market because it induced severe to fatal hepatotoxicity that outweighed its benefits for patients with diabetes [79], [80], [81], [82], [83], [84], [85].
Rosiglitazone (BRL-49653) and pioglitazone are both thiazolidinediones still in clinical use in many countries for glycemic control in the treatment of type 2 diabetes, although rosiglitazone-containing anti-diabetes medicines were taken off the market in the European Union following a European Medicines Agency (EMA) recommendation for suspension of the marketing authorizations (press release 23rd of September 2010: EMA/585784/2010). In the United States the use of rosiglitazone was restricted by the Food and Drug Administration (FDA) in September 2010 and in November 2013 the restrictions were removed again, although according to the officially released FDA Drug Safety Communication (from 25th of November 2013) “some scientific uncertainty about the cardiovascular safety of rosiglitazone medicines still remains”. Rosiglitazone has proven its effectiveness in reducing insulin resistance [86], [87], [88], [89], [90]. However, some meta-analyses indicated that among patients with impaired glucose tolerance or type 2 diabetes the use of rosiglitazone for at least 12 months was associated with a significantly increased risk of myocardial infarction and heart failure, as well as with an elevated risk of cardiovascular mortality [91], [92], [93], [94], [95]. Furthermore, some case reports rose concerns that the application of rosiglitazone might be associated with hepatocellular injury [96] and hepatic failure [97], side effects similar to those observed for troglitazone. Similar to rosiglitazone, treatment of type 2 diabetes patients with pioglitazone reduces insulin resistance significantly [98]. Compared to rosiglitazone, pioglitazone exerts beneficial effects on the plasma lipid profile, leading to a lower risk of acute myocardial infarction, stroke, or heart failure [99], [100], [101], [102], [103]. However, the clinical use of pioglitazone is also limited by the occurrence of several adverse events, including body-weight gain, fluid retention, and possibly bladder cancer [104], [105], [106].
3. PPARγ activation by natural products
The severe adverse effects of thiazolidinediones which led to their withdrawal from the market or restricted clinical application are suggested to be a result of full PPARγ activation, contrasting the weak agonistic effect of endogenous PPARγ ligands such as fatty acids and prostanoids [19], [107]. Therefore, great research efforts have recently been undertaken to explore the potential of selective PPARγ modulators (SPPARMs), compounds that improve glucose homeostasis but elicit reduced side effects due to partial PPARγ agonism based on selective receptor-cofactor interactions and target gene regulation [107], [108], [109]. An illustrative example for a recently identified SPPARM is N-acetylfarnesylcysteine, a compound with in vitro and in vivo effectiveness as both a full and partial agonist depending on the investigated PPARγ target gene [110]. A further research direction under consideration is to explore the therapeutic potential of dual- and pan-PPAR agonists activating simultaneously two or all three PPAR receptors, respectively [111], [112], [113], [114].
Medicinal plants have been used to treat various diseases for thousands of years, and since the 19th century many bioactive pure compounds isolated from these plants became very successful drugs [115]. Moreover, still today natural products are an important source for the discovery and development of new drugs [116]. Natural products possess a high chemical scaffold diversity and are evolutionary optimized to serve different biological functions, conferring them a high drug-likeness and making them an excellent source for identification of new drug leads [117], [118], [119]. The traditional use of plant preparations can often give strong hints for the pharmacological effects of their ingredients. A study examining 119 clinically used plant-derived drugs found that 74% of them were indeed used for disease indications related to the traditional use of the medicinal plants from which the substances were isolated [120]. Not surprisingly, significant research efforts were undertaken to explore the PPARγ activating potential of a wide range of natural products originating from medicinal plants. Summarized in Table 1 are some of the most interesting examples of investigated sources, their use in traditional medicine, and the identified PPARγ-activating constituents. Noteworthy, along with plants and mushrooms applied in traditional medicines, PPARγ-ligands were often identified in plants that are common food sources, including the tea plant (Camellia sinensis), soybeans (Glycine max), palm oil (Elaeis guineensis), ginger (Zingiber officinale), grapes and wine (Vitis vinifera), and a number of culinary herbs and spices (e.g. Origanum vulgare, Rosmarinus officinalis, Salvia officinalis, Thymus vulgaris) (Table 1). The presence of PPARγ ligands in food products warrants an exploration whether this nuclear receptor may be effectively activated by the intake of nutraceuticals (by consumption of functional foods or by dietary supplements). Although most of the agonists identified in food sources are weak PPARγ agonists per se, the effects of their metabolites deserve further research to better estimate their preventive potential. While research in this direction is largely missing, a previous study reported that some main metabolites of flavonoid constituents from red clover (Trifolium pratense) have an up to 100-fold higher PPARγ binding affinity than their precursors [121].
Table 1.
Species investigated as a source of PPARγ ligands, their traditional use, and identified activating natural products.
| Species name | Traditional use | Identified PPARγ activating natural products |
|---|---|---|
| Amorpha fruticosa L. (Fabaceae) | Traditionally used to treat hypertension, hematomas, and contusions in China, Japan, and Korea [201] | Amorfrutins (in the fruits) [187] |
| Astragalus membranaceus Moench (Fabaceae) | In TCM used to reinforce qi and strengthen the superficial resistance, and promote the discharge of pus and the growth of new tissue [202] | Formononetin (in ethanolic extracts) [138] |
| Bixa orellana L. (Bixaceae) | In traditional medicine of India different parts of the plant are used as diuretic, laxative, antibilious, antiemetic and astringent agents, as blood purifier, in jaundice, in dysentery, and externally as scar-preventive [203] | Bixin and norbixin (in annatto extracts) [204] |
| Camellia sinensis (L.) Kuntze (Theaceae) | Used worldwide for the preparation of tea; used in the traditional medicine of India as stimulant, diuretic, and astringent. In China it is used in the treatment of diarrhea and dysentery [203] | (−)-Catechin (in green tea) [205] |
| Cannabis sativa L. (Cannabaceae) | In traditional medicine of India used as hallucinogenic, hypnotic, sedative, analgesic, and anti-inflammatory agent [203] | Δ9-Tetrahydrocannabinol [170] |
| Chromolaena odorata (L.) R.M. King & H. Rob. (Asteraceae) | In traditional medicine of Thailand used for the treatment of wounds, rashes, diabetes, and as insect repellent [206] | (9S,13R)-12-Oxo-phytodienoic acid (in chloroform-soluble extract from the whole plant) [207] and odoratin (in DCM extract) [208] |
| Coix lacryma-jobi var. ma-yuen (Rom. Caill.) Stapf ex Hook. f. (Poaceae) | In TCM used to invigorate the spleen function and promote urination, alleviate arthritis, arrest diarrhea, remove heat and facilitate the drainage of pus [202] | Hydroxy unsaturated fatty acids (in acetone extract from the seeds) [209] |
| Commiphora mukul (Hook. ex Stocks) Engl. (Burseraceae) | The oleo-gum-resin is used in traditional medicine of India for reducing obesity, as well as in the treatment of rheumatoid arthritis, osteoarthritis and sciatica [203] | Commipheric acid (in guggulipid, the ethyl acetate extract of the gum of the tree) [210] |
| Cornus alternifolia L.f. (Cornaceae) | Used in TCM as tonic, analgesic, and diuretic [211], [212] | Kaempferol-3-O-β-glucopyranoside (in 90% methanol extract from dried leaves) [211] |
| Cymbopogon citratus (DC.) Stapf (Poaceae) | In traditional medicine of India the leaves are used as stimulant, sudorific, antiperiodic, and anticatarrhal; the essential oil is used as carminative, depressant, analgesic, antipyretic, antibacterial, and antifungal agent [203] | Citral (in lemongrass oil) [213] |
| Echinacea purpurea (L.) Moench (Asteraceae) | Used in indigenous medicine of the native American Indians: external application for wounds, burns, and insect bites, chewing of roots for toothache and throat infections; internal application for pain, cough, stomach cramps and snake bites [214] | Alkamides (in n-hexane extract of the flowers) [215] |
| Elaeis guineensis Jacq. (Arecaceae) | In traditional African medicine different parts of the plant are used as laxative and diuretic, as a poison antidote, as a cure for gonorrhea, menorrhagia, and bronchitis, to treat headaches and rheumatism, to promote healing of fresh wounds and treat skin infections [216] | Tocotrienols (in palm oil) [217] |
| Elephantopus scaber L. (Asteraceae) | Different parts of the plant are used in traditional medicine of India as astringent agent, cardiac tonic, diuretic, to treat ulcers and eczema, in rheumatism, to reduce fever, and to eliminate bladder stones [203] | Deoxyelephantopin [218] |
| Epimedium elatum C. Morren & Decne. (Berberidaceae) | Used in TCM to reinforce the kidney yang, strengthen the tendons and bones, and relieve rheumatic conditions [202] | Acylated flavonol glycosides (in ethanol extract from the whole plant) [219] |
| Euonymus alatus (Thunb.) Siebold (Celastraceae) | Used in TCM to promote blood stasis to promote menstruation, remove toxic materials, subside swelling, and kill insects or parasites [202] | Kaempferol and quercetin [134] |
| Glycine max (L.) Merr. (Fabaceae) | The edible beans of the plant are used worldwide as a food and plant-based protein source [203] | Genistein (in soya beans) [135] |
| Glycyrrhiza glabra L. (Fabaceae) | Used in TCM to reinforce the function of the spleen and replenish qi, remove heat and counteract toxicity, dispel phlegm and relieve cough, alleviate spasmodic pain, and moderate drug actions [202] | 5′-Formylglabridin, (2R,3R)-3,4′,7-trihydroxy-3′-prenylflavane, echinatin, (3R)-2′,3′,7-trihydroxy-4′- methoxyisoflavan, kanzonol X, kanzonol W, shinpterocarpin, licoflavanone A, glabrol, shinflavanone, gancaonin L, glabrone (in ethanol extract from the roots) [220] |
| Glycyrrhiza foetida Desf. (Fabaceae) | Used in the treatment of stomach and throat problems in traditional medicine of the Marrakech region in Morocco [221] | Amorfrutins (in the edible roots) [187] |
| Glycyrrhiza inflata Batalin (Fabaceae) | Used in TCM to reinforce the function of the spleen and replenish qi, remove heat and counteract toxicity, dispel phlegm and relieve cough, alleviate spasmodic pain, and moderate drug actions [202] | Licochalcone E (in roots) [222] |
| Glycyrrhiza uralensis Fisch. ex DC. (Fabaceae) | Used in TCM to reinforce the function of the spleen and replenish qi, remove heat and counteract toxicity, dispel phlegm and relieve cough, alleviate spasmodic pain, and moderate drug actions [202] | Flavonoids and 3-arylcoumarins (in ethanolic extract of the roots) [136] |
| Limnocitrus littoralis (Miq.) Swingle (Rutaceae) | In traditional Vietnamese medicine different parts of the plant have been used as an expectorant, antitussive product, for exudation, and the treatment of colds and fevers [223] | Meranzin (in ethyl alcohol/water (90/10, v/v) extract from the leaves) [224] |
| Lycium chinense Mill. (Solanaceae) | Used in TCM for the treatment of night-sweats, pneumonia, cough, hematemesis, inflammation, and diabetes mellitus [225] | Fatty acids (in root bark DCM extract) [128] |
| Magnolia officinalis Rehder & E.H. Wilson (Magnoliaceae) | Used in TCM to eliminate damp and phlegm, and relieve distension [202] | Magnolol [140], [193], [194] and honokiol [175], [190], [191], [192] |
| Melampyrum pratense L. (Orobanchaceae) | Used in traditional Austrian medicine for the treatment of gout and rheumatism [122], [129] | Lunularin and fatty acids (in aerial parts DCM and MeOH extracts) [129] |
| Momordica charantia L. (Cucurbitaceae) | In traditional medicine of India different parts of the plant are used to relieve diabetes, as stomachic, laxative, antibilious, emetic, and anthelmintic agent. Also used for the treatment of cough, respiratory diseases, skin diseases, wounds, ulcer, gout, and rheumatism [203] | Cucurbitane-type triterpene glycosides [226] |
| Notopterygium incisum C.T. Ting ex H.T. Chang (Apiaceae) | Used in TCM for the treatment of rheumatism, cold, and headache [227] | Polyacetylenes (in roots and rhizomes DCM extract) [228] |
| Origanum vulgare L. (Lamiaceae) | Used as a culinary herb worldwide; used in the traditional medicine of India as emmenagogue, antispasmodic, carminative, and expectorant [203] | Biochanin A (in dried leaves) [137] |
| Panax ginseng C.A. Mey. (Araliaceae) | Used in TCM to reinforce the vital energy, to remedy collapse and restore the normal pulse, benefit the spleen and lung, promote the production of body fluids, and anchor the mind [202] | Ginsenoside 20(S)-protopanaxatriol [229] and ginsenoside Rb1 (in ginseng roots) [230] |
| Pinellia ternata (Thunb.) Ten. ex Breitenb. (Araceae) | Used in TCM to remove damp and phlegm, relieve nausea and vomiting, and eliminate stuffiness in the chest and epigastrium [202] | Fatty acids (in different apolar extracts from the rhizomes) [130] |
| Pistacia lentiscus L. (var. Chia) (Anacardiaceae) | Uses of the resin in traditional medicine of India: as carminative, diuretic, stimulant, and astringent [203] | Oleanonic acid (in Chios mastic gum) [131] |
| Pseudolarix amabilis (J. Nelson) Rehder (published as Pseudolarix kaempferi Gordon) (Pinaceae) | Used in TCM as dermatologic antifungal remedy [231] | Pseudolaric acid B (in extracts of the root and trunk barks) [232] |
| Pueraria thomsonii Benth. (Fabaceae) | Used in TCM for the treatment of fever, acute dysentery, diarrhea, diabetes, and cardiovascular diseases [233] | Daidzein (in ethanolic extracts) [138] |
| Robinia pseudoacacia var. umbraculifer DC. (Fabaceae) | In traditional medicine of India different parts of Robinia pseudoacacia are used as laxative, antispasmodic, and diuretic [203] | Amorphastilbol (in seed extract) [234] |
| Rosmarinus officinalis L. (Lamiaceae) | Used as a culinary herb worldwide; in traditional medicine of India essential oil from flowers and leaves is used as anti-inflammatory agent, astringent, antiseptic, stomachic, carminative, and externally in circulatory disorders; flowering tops and leaves are used as carminative and diuretic [203] | Carnosic acid and carnosol (in ethanolic extract of rosemary) [235] |
| Salvia officinalis L. (Lamiaceae) | Used as a culinary herb worldwide; in traditional medicine of India different parts of the plant are used as astringent, anti-inflammatory, carminative, antispasmodic, antiseptic, hypoglycaemic, anti-asthmatic, cholagogue, emmenagogue, antisudoriferous, diaphoretic, and antipyretic agent, as well as for the treatment of sore throat, laryngitis, tonsillitis, and stomatitis [203] | Carnosic acid and carnosol (in ethanolic extract of sage) [235]; as well as 12-O-methyl carnosic acid and α-linolenic acid (in DCM extract of sage) [132] |
| Sambucus nigra L. (Adoxaceae) | In traditional medicine of India different parts of the plant are used as anti-inflammatory, anti-catarrhal, diuretic, and emetic agent, as well as for the treatment of common cold, influenza, nasal catarrh, and sinusitis [203] | α-Linolenic acid, linoleic acid, and naringenin (in MeOH extract of elderflowers) [133] |
| Saururus chinensis (Lour.) Baill. (Saururaceae) | In traditional Korean medicine aerial parts of the plant are used for the treatment of edema, jaundice, gonorrhea, and several inflammatory diseases [236] | Saurufuran A (in roots) [237] |
| Silybum marianum (L.) Gaertn. (Asteraceae) | Widely used worldwide as a supportive agent in the treatment of a variety of liver diseases; used in TCM to clear heat and relieve toxic material, to soothe the liver and to promote bile flow [202] | Isosilybin A (in silymarin, a phenolic mixture from the fruits of the plant) [238] |
| Terminalia bellerica Roxb. (Combretaceae) | The fruits are used in traditional medicine of India to treat anemia, asthma, cancer, diarrhea, hypertension, inflammation, and rheumatism [239] | Gallotannins (in the fruits) [240] |
| Thymus vulgaris L. (Lamiaceae) | Used as a culinary herb worldwide; used in traditional medicine of India as antiseptic, antibacterial, antifungal, antiviral, antispasmodic, mild sedative, and expectorant, for coughs and common cold [203] | Carvacrol (in thyme oil) [241] |
| Trifolium pratense L. (Fabaceae) | Used in traditional medicine of India as deobstruent, antispasmodic, expectorant, sedative, anti-inflammatory, and anti-dermatosis agent [203] | Isoflavones (in red clover extracts) [121] |
| Vitis vinifera L. (Vitaceae) | Widely used worldwide as food (grapes) and for beverage preparation (wine); used in traditional medicine of India in prescriptions for cough, respiratory tract catarrh, subacute cases of enlarged liver and spleen, as well as in alcohol-based tonics (Aasavs) [203] | Ellagic acid, epicatechin gallate, flavonoids (in grapes and wine) [242] |
| Wolfiporia extensa (Peck) Ginns (published as Poria cocos F.A. Wolf) (Polyporaceae) | In TCM this mushroom is used to cause urination, invigorate the spleen function, and calm the mind [202] | Dehydrotrametenolic acid (in dried sclerotia) [243] |
| Zingiber officinale Roscoe (Zingiberaceae) | Widely used as a spice worldwide; in TCM fresh rhizomes are used to dispel pathogenic factors from exterior and eliminate cold, arrest vomiting by warming the middle-energizer, remove phlegm and arrest cough; dried rhizomes are used to dispel cold from the spleen and the stomach, promote recovery from collapse, and warm the lung to expel retained morbid fluids [202] | 6-Shogaol (in ginger roots) [244] |
Although in some occasions the traditional use of the species presented in Table 1 might give hints for bioactivities linked to PPARγ activation, it is important to underline that the applications of traditional preparations often cover a broad range of symptoms that are unlikely to be related to PPARγ action (e.g. Echinacea purpurea is traditionally used for the treatment of wounds, burns, insect bites, toothache, throat infections, pain, cough, stomach cramps and snake bites; in this example the range of traditional uses is very likely linked to diverse bioactivities resulting from the interaction with different molecular targets).
While even many more plant extracts are reported to activate PPARγ [122], [123], [124], [125], [126], [127], Table 1 mainly summarizes studies that identified bioactive compounds present in the respective extracts. One reason for frequently omitting the identification of bioactive compounds might be the very high number of medicinal plant extracts inducing PPARγ activation in general. For example, a recent study examining the PPARγ transactivation potential of extracts from traditional Austrian medicinal plants identified that 40 out of 71 studied herbal drugs (56% hit rate) are able to induce PPARγ activation when tested at a concentration of 10 μg/mL [122]. This high number of active extracts makes it difficult to identify the bioactive compounds in each of them. In addition, the laborious phytochemical analysis is often not rewarded with the identification of interesting novel PPARγ ligands but with the re-isolation of some ubiquitous plant constituents activating the receptor such as fatty acids [128], [129], [130], [131], [132], [133] or flavonoids [121], [133], [134], [135], [136], [137], [138].
Besides testing of extracts and bio-guided approaches, virtual screening emerged as an effective strategy for the discovery of novel PPARγ ligands from natural sources. Rupp et al. used descriptor-based Gaussian process regression to search for PPARγ agonists based on a data set of 144 published PPARγ ligands [139]. A combination of prediction models and manual inspection of the hit list yielded 15 compounds, which were experimentally evaluated against PPARα and PPARγ activation. Eight compounds exhibited agonistic activity towards either of these receptors or both. The most active compound, a truxillic acid derivative, was a selective PPARγ agonist with an EC50 of 10 μM. Petersen et al. performed a pharmacophore-based virtual screening of a database containing over 57,000 traditional Chinese medicine constituents [131]. The ligand-based pharmacophore model consisted of one hydrogen bond acceptor and three hydrophobic features and was based on a set of 13 selective, partial PPARγ agonists. The virtual hit list contained 939 entries. Exemplarily, one virtual hit, present in Pistacia lentiscus, was experimentally investigated involving the testing of the Pistacia oleoresin extract and the bio-guided fractionation of the active extract. These efforts led to the discovery of oleanonic acid as a modestly active partial PPARγ agonist. Fakhrudin et al. discovered dieugenol, magnolol, and tetrahydrodieugenol as partial PPARγ agonists [140]. They used a structure-based pharmacophore model to screen natural compound databases. Among the highly ranked hits, several neolignans were isolated or synthesized and experimentally tested for their in vitro activity against PPARγ. Dieugenol, tetrahydrodieugenol, and magnolol with EC50 values in the low micromolar or submicromolar range also induced adipocyte differentiation in 3T3-L1 adipocytes. Lewis et al. used docking to select natural products for evaluation against PPARγ and in a mouse model for irritable bowel disease [141]. The top-ranked virtual hit from the docking, α-eleostearic acid, showed activity in the PPARγ binding assay, the cell-based reporter assay, and the in vivo mouse model for irritable bowel syndrome. Salam et al. screened a small in-house natural product library using a multi-step docking protocol [142]. They selected 29 hits from the 200 docked compounds for experimental analysis in a functional PPARγ activity assay. Six compounds, psi-baptigenin, hesperidin, apigenin, chrysin, biochanin A, and genistein, showed EC50s in the low micromolar range. Finally, Tanrikulu et al. used a structure-based pharmacophore model based on the common interactions of four PPARγ X-ray crystal structures in complex with different agonists [143]. They screened the Analyticon database, which contains natural products and their semi-synthetic derivatives. Their efforts led to the discovery of two α-santonin derivatives as PPARγ activators, while α-santonin itself was not active on the receptor. In summary, several 2D and 3D virtual screening approaches have successfully discovered structurally diverse natural product PPARγ activators, thereby indicating natural products as a rich source for novel PPARγ agonists.
A selection of natural products well characterized as PPARγ ligands is presented in Table 2. The PPARγ-agonistic effects of endogenous (e.g. fatty acids, prostanoids) [19], [26], [144], [145], [146], [147], [148], [149], [150], [151] and synthetic [13], [151], [152], [153] ligands of the receptor have been reviewed in numerous previous articles and therefore will not be discussed here. Natural products reported to activate or bind PPARγ with EC50 or respectively IC50 above 50 μM were considered as less relevant and were therefore omitted from Table 2. While numerous natural products were so far shown to interfere with PPARγ activity or expression (Table 1 and references [142], [154], [155], [156], [157], [158], [159], [160], [161], [162], [163], [164], [165], [166], [167], [168], [169], [170], [171], [172], [173]), the compounds depicted in Table 2 did not only show effectiveness in a cell model responsive to PPARγ activation (e.g. activation of PPARγ-dependent reporter gene expression), but also to directly bind to the receptor in an in vitro binding assay using purified PPARγ protein. While a binding assay with a purified receptor is one of the most direct approaches to confirm the potential of a compound to physically interact with PPARγ, application of a protein-based in vitro assay alone is not sufficient to assure that the respective compound can act also in intact cells (since the compound might not be able to reach PPARγ that is located inside the cell nucleus, due to various reasons such as inability to penetrate cellular membranes, extrusion from the cells mediated by membrane efflux transporters, metabolic transformation to products that do not bind PPARγ etc.). On the other side, the use of cellular models alone does not ensure that the studied compound is a direct receptor ligand, since PPARγ activation as observed in a luciferase reporter model might also be caused by indirect effects (e.g. increase in PPARγ protein expression, activation of the PPARγ dimer partner RXR). The 20 natural products covered in Table 2 include representatives of seven structural classes (flavonoids, neolignans, stilbenes, amorfrutins, polyacetylenes, sesquiterpene lactones, and diterpenequinone derivatives). This structural variety is consistent with the known ability of the PPARγ ligand-binding domain (LBD) to accommodate a diversity of chemical scaffolds due to the large size of the binding site cavity and its adaptability through the flexibility of side chains [43], [174]. With the exceptions of 6-hydroxydaidzein and (−)-catechin, all of the compounds reviewed in Table 2 revealed to be SPPARMs displaying partial agonistic effects towards PPARγ-dependent reporter gene expression. Genistein, biochanin A, sargaquinoic acid, sargahydroquinoic acid, resveratrol, and amorphastilbol were shown to be dual agonists able to activate also PPARα along with PPARγ (Table 2). Genistein also exerts estrogenic activity at low concentrations, leading to a concentration-dependent preferential activation of PPARγ or estrogen receptor, translating into opposite effects on osteogenesis and adipogenesis [135]. Six of the natural products, i.e. honokiol [175], magnolol [176], resveratrol [177], [178], [179], [180], [181], [182], [183], [184], [185], [186], amorfrutin 1 [187], amorfrutin B [188], and amorphastilbol [189], have been demonstrated to improve blood glucose levels and other relevant parameters in animal models of diabetes, on some occasions with reduced side effects in comparison to full thiazolidinedione PPARγ ligands (Table 2). In particular honokiol, amorfrutin 1, amorfrutin B, and amorphastilbol reduced weight gain in diabetic animal models. Furthermore, some of these compounds did not display adverse liver effects such as hepatomegaly (amorphastilbol) and hepatotoxicity (amorfrutin 1, amorfrutin B), and amorfrutin B also lacked adverse effects associated with osteoblastogenesis and fluid retention (Table 2). Among the studied natural products, amorfrutin 1 is the only one that was investigated so far for interference with PPARγ Ser273 phosphorylation and was found to suppress phosphorylation at this residue in the visceral white adipose tissue of diet-induced obesity (DIO) mice [187]. An interesting distinct mode of agonism is exerted by the neolignans honokiol and magnolol, which are dual agonists of PPARγ and its dimer activation partner RXR [140], [175], [190], [191], [192], [193], [194].
Table 2.
Natural products activating PPARγ.
| Bioactive compound | Notes |
|---|---|
| Flavonoids | |
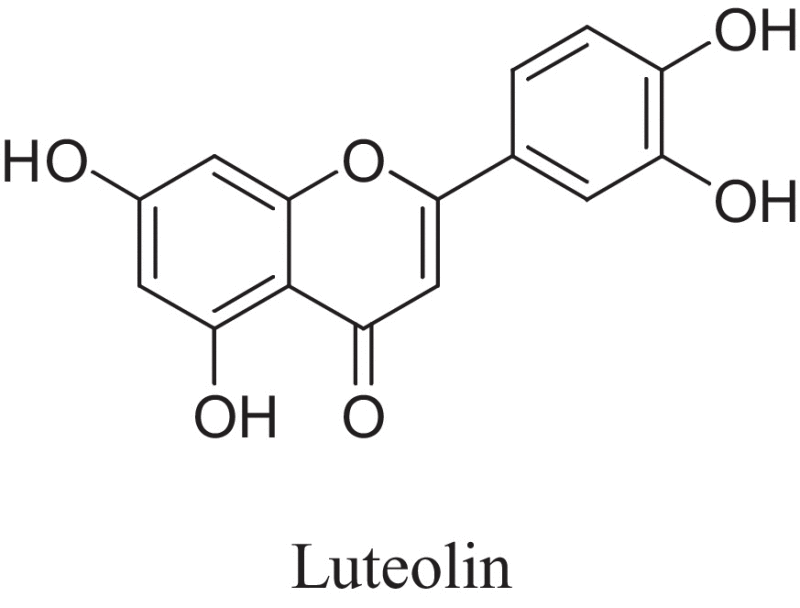 |
Binds to purified human PPARγ with IC50 = 3.9 [127] or 7.2 μM [137], activates chimeric Gal4-PPARγ-dependent reporter gene expression as partial agonist (with EC50 = 15.6 μM and maximal efficacy around 3-fold lower than rosiglitazone) [195], and antagonizes the effect of rosiglitazone (1 μM) upon co-treatment (with IC50 = 21.8 μM) [195], antagonizes the adipogenesis inducing action of rosiglitazone (1 μM) in 3T3-L1 cells upon co-treatment (at 5–20 μM) [195], regulates several PPARγ-dependent genes as a weak partial agonist/antagonist, but acts as a full PPARγ agonist on GLUT4 expression in 3T3-L1 cells (at 10–20 μM) [195], counteracts (at 1–5 μM) the IL-8 secretion in human corneal epithelial cells exposed to hypertonic stress or to the PPARγ antagonist GW9662 (at 1 μM) [195], was co-crystallized with the PPARγ LBD whereby luteolin and myristic acid simultaneously bind to the LBD (PDB: 3sz1) [195] |
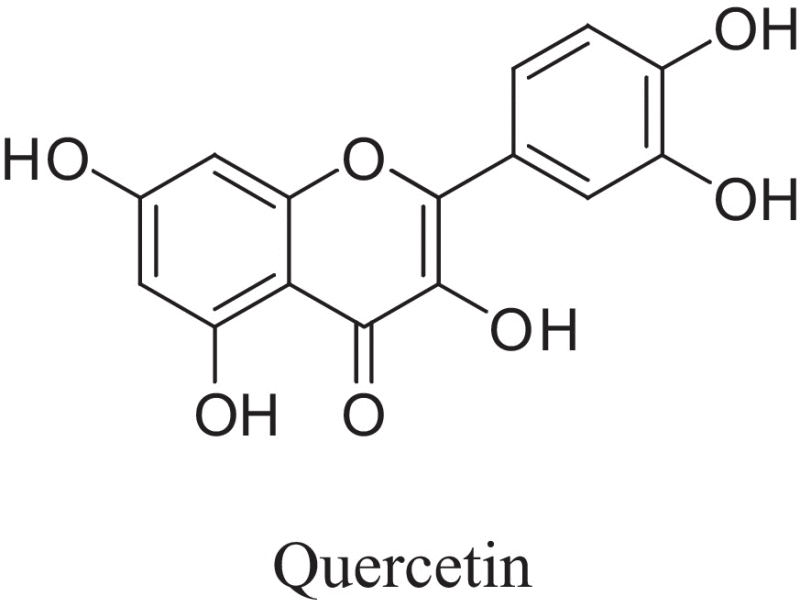 |
Binds to recombinant human PPARγ (IC50 reported to be 26.0 [134], 5.7 [242], or 2.8 μM [127]), activates PPARγ-dependent reporter gene expression as partial agonist when applied as a single treatment, and antagonizes the effect of rosiglitazone upon co-treatment (at 1–100 μM) [134], induces the insulin-dependent glucose uptake but not adipogenesis in 3T3-L1 cells (at 5–50 μM) [134], inhibits rosiglitazone-induced 3T3-L1 cell differentiation (at 5–50 μM) [134] |
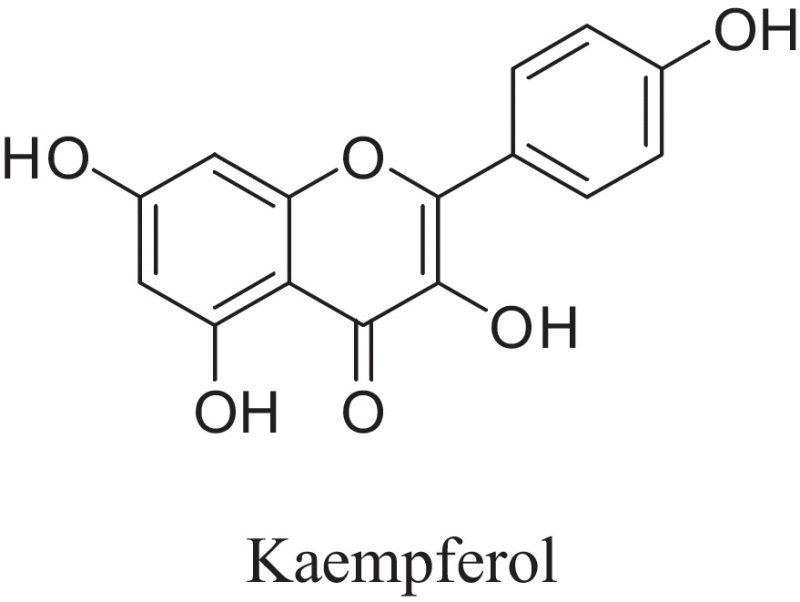 |
Binds to recombinant human PPARγ (IC50 = 23.1 [134], 30 [242] or 49.9 μM [245]), activates PPARγ-dependent reporter gene expression as partial agonist when applied as a single treatment, and antagonizes the effect of rosiglitazone upon co-treatment (at 1–100 μM) [134], induces the insulin-dependent glucose uptake but not adipogenesis in 3T3-L1 cells (at 5–50 μM) [134], inhibits rosiglitazone-induced 3T3-L1 cell differentiation (at 5–50 μM) [134] |
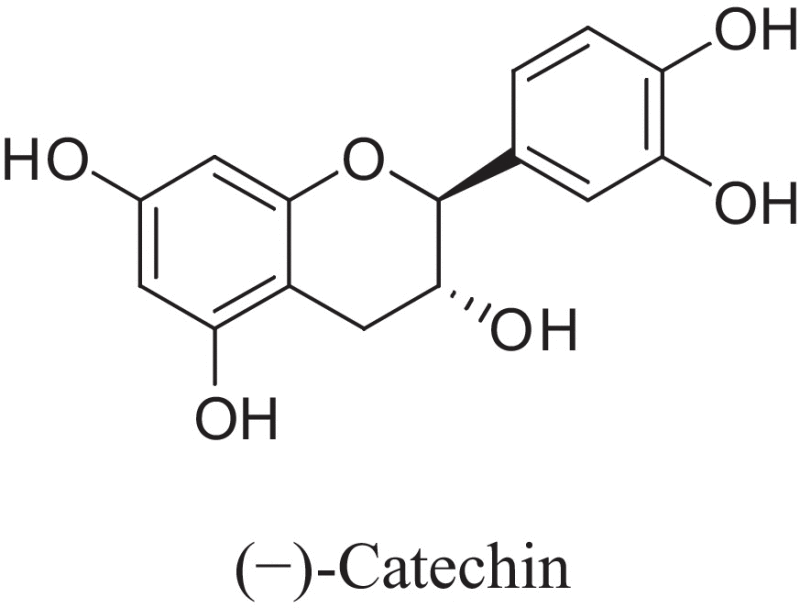 |
Binds to purified PPARγ-LBD with IC50 = 9.9 μM [205], activates PPARγ-dependent reporter gene expression as full agonist with EC50 of around 2 μM [205], modulates expression of PPARγ target genes, and promotes adipocyte differentiation of human bone marrow mesenchymal stem cells [205] |
 |
Binds to purified PPARγ (IC50 = 3.8 μM) and activates chimeric Gal4-PPARγ-dependent reporter gene expression as partial agonist (with EC50 = 3.8 μM and maximal efficacy around 3-fold lower than rosiglitazone) [127], [245] |
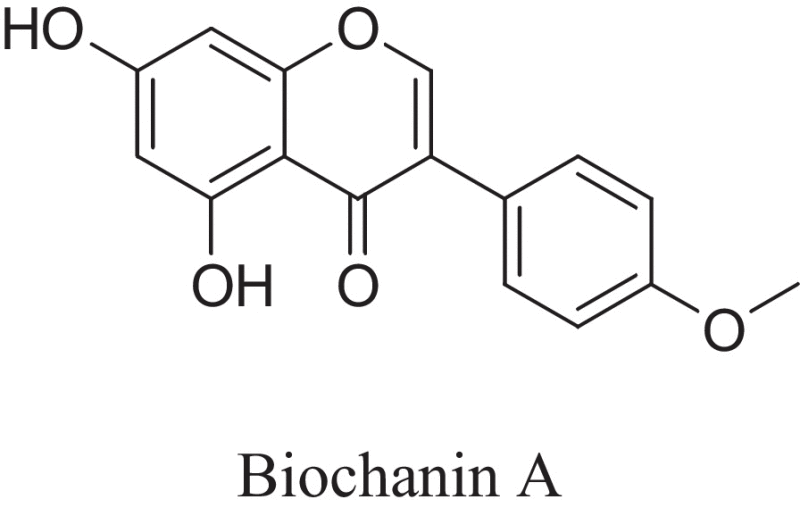 |
Binds to purified human PPARγ with IC50 = 19.6 [121] or 23.7 μM [137], activates chimeric Gal4-PPARγ-dependent reporter gene expression as partial agonist (with EC50 = 39.5 μM and maximal efficacy around 3-fold lower than pioglitazone) [121], induces adipogenesis in 3T3-L1 cells (at 1-5 μM) [138], activates PPARγ promoter activity in HUVEC transfected with PPRE-reporter plasmids and inhibits monocyte adhesion to TNF-α activated HUVEC in the presence of flow (at 1 μM) [246], activates also chimeric Gal4-PPARα-dependent reporter gene expression [138], [247] |
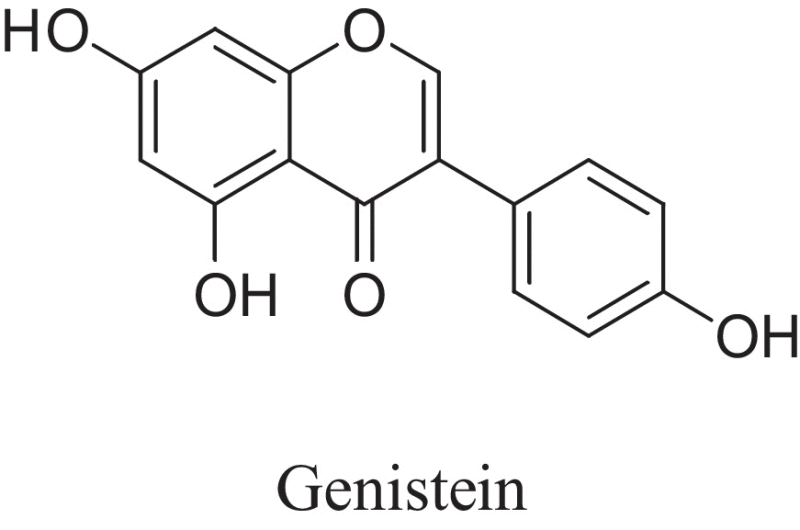 |
Binds to purified human PPARγ with Ki = 5.7 [135] or 22.5 μM [121], activates chimeric Gal4-PPARγ-dependent reporter gene expression as partial agonist (with EC50 = 18.7 μM and maximal efficacy around 4-fold lower than pioglitazone) [121], induces adipogenesis in 3T3-L1 cells (at 1-30 μM) [138], activates PPARγ promoter activity in HUVEC transfected with PPRE-reporter plasmids and inhibits monocyte adhesion to TNF-α activated HUVEC in the presence of flow (at 1 μM; the monocyte adhesion effect was abolished upon siRNA silencing of PPARγ) [246], activates also the transcriptional activity of PPARα [138], [247], [248], [249], was shown to act as an estrogen at low concentrations (≤1 μM) and as a ligand of PPARγ at high concentrations (>1 μM) leading to concentration-dependent opposite effects on osteogenesis and adipogenesis [135] |
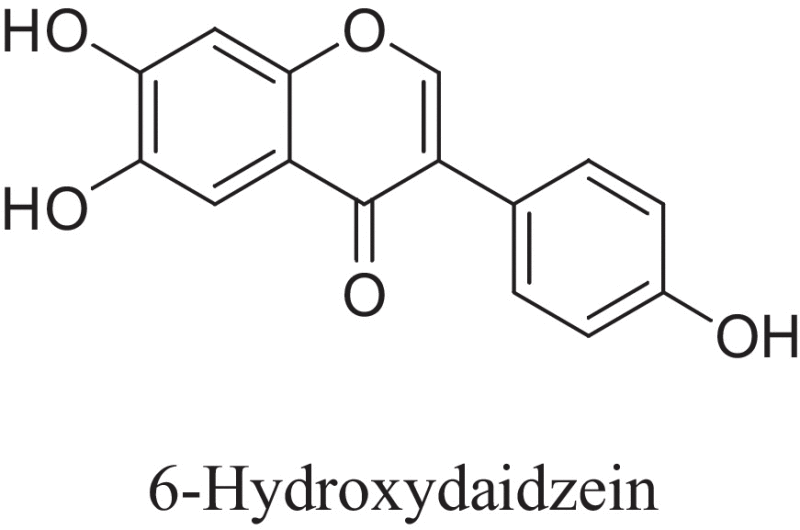 |
Binds to purified human PPARγ with IC50 = 3.3 μM [121], activates chimeric Gal4-PPARγ-dependent reporter gene expression as full agonist with EC50 = 48.6 μM [121] |
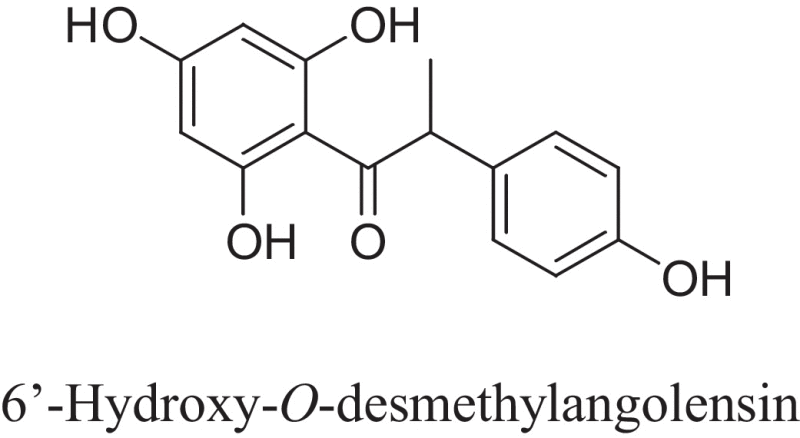 |
Binds to purified human PPARγ with IC50 = 16.7 μM [121], activates chimeric Gal4-PPARγ-dependent reporter gene expression as partial agonist with EC50 = 27.7 μM and maximal efficacy around 5-fold lower than rosiglitazone [121] |
| Neolignans | |
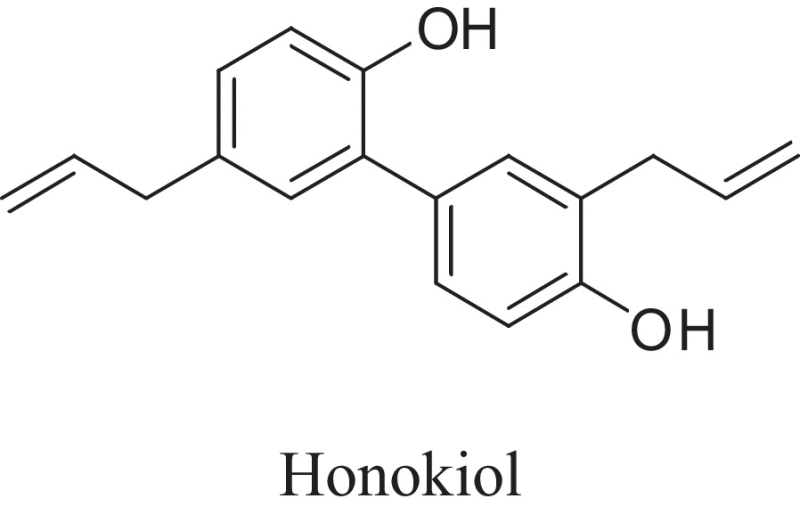 |
Dual agonist of PPARγ and RXR [175], [190], [191], [192], binds to purified human PPARγ (Ki = 22.9 μM) [175], activates PPARγ-dependent reporter gene expression as partial agonist (EC50 = 3.9 μM) [175], induces glucose uptake but not adipogenesis in 3T3-L1 cells (at 1–10 μM) [175], decreases blood glucose levels in diabetic KKAy mice with simultaneous suppression of weight gain [175] |
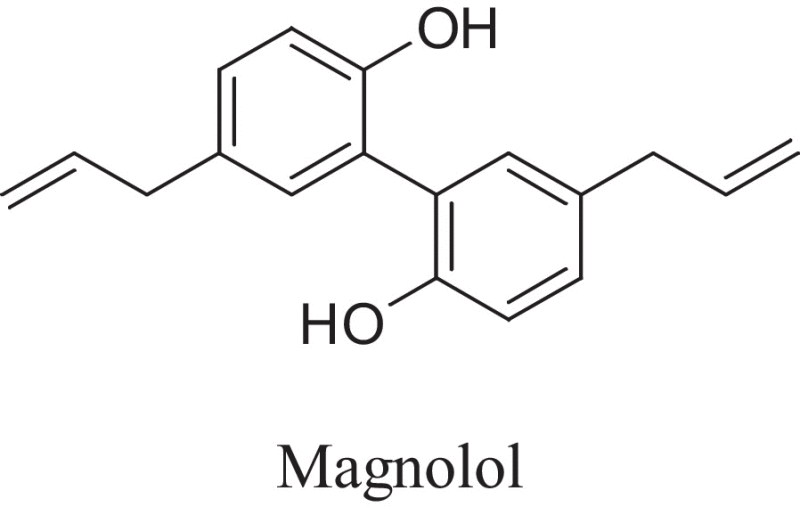 |
Dual agonist of PPARγ and RXRα [140], [193], [194], binds to purified human PPARγ (Ki = 2.0 μM) [140], activates PPARγ-dependent reporter gene expression as partial agonist (EC50 = 1.6 μM) [140], induces the recruitment of TRAP220/DRIP-2 coactivator peptide to purified PPARγ (with EC50 of around 0.5 μM and maximal efficacy around 3-fold lower than pioglitazone) [140], induces adipogenesis [140], [194] and glucose uptake [194] in 3T3-L1 cells (at 10 μM), decreases fasting blood glucose and plasma insulin levels and prevents or retards diabetic nephropathy in type 2 diabetic Goto-Kakizaki rats [176], was co-crystallized with the RXRα-LBD (PDB: 3r5 m) and the PPARγ-LBD (PDB: 3r5n) [193] |
| Stilbenes | |
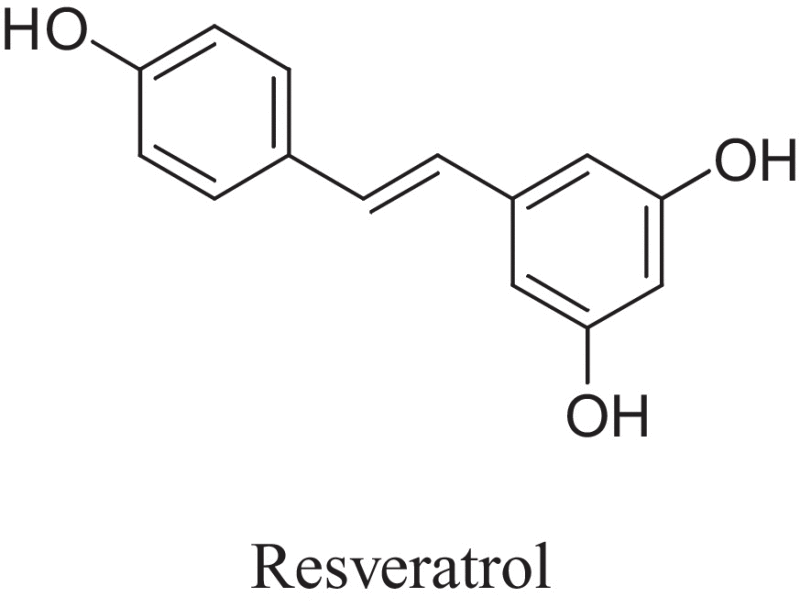 |
Binds to purified human PPARγ (Ki = 1.37 μM) [250], activates chimeric Gal4-PPARγ-dependent reporter gene expression as partial agonist (at 50–100 μM) [251], inhibits rosiglitazone-induced PPARγ luciferase reporter transactivation with IC50 = 27.4 μM [250], affects glucose and lipid metabolism as well as inflammation by interference with PPARγ in several in vitro and in vivo animal models [177], [178], [179], [180], [181], [182], [183], [184], [185] and improves insulin sensitivity in type 2 diabetic patients [186], is also a ligand of PPARα [250], [252], was co-crystallized with the PPARγ-LBD (PDB: 4jaz) [250] |
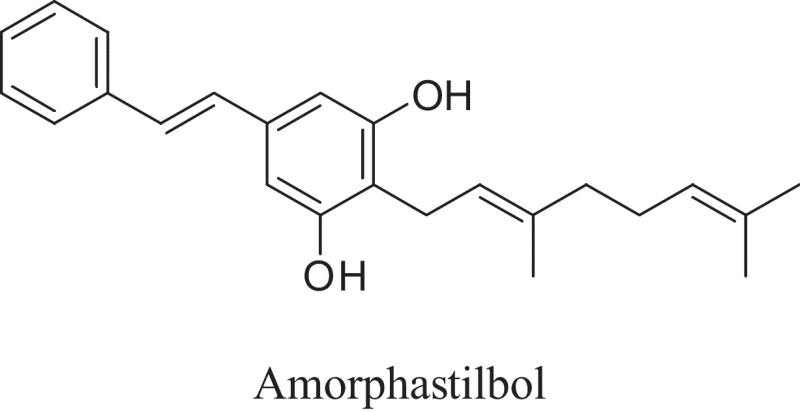 |
Binds to purified human PPARγ (IC50 = 0.85 μM) and activates human PPARγ-dependent luciferase reporter gene expression (EC50 = 5 μM; maximal fold activation of 83% as compared to the full agonist troglitazone) [234], binds and activates with a similar potency also PPARα [234], improves glucose and lipid impairment in db/db mice without significant side effects, such as weight gain or hepatomegaly [189] |
| Amorfrutins | |
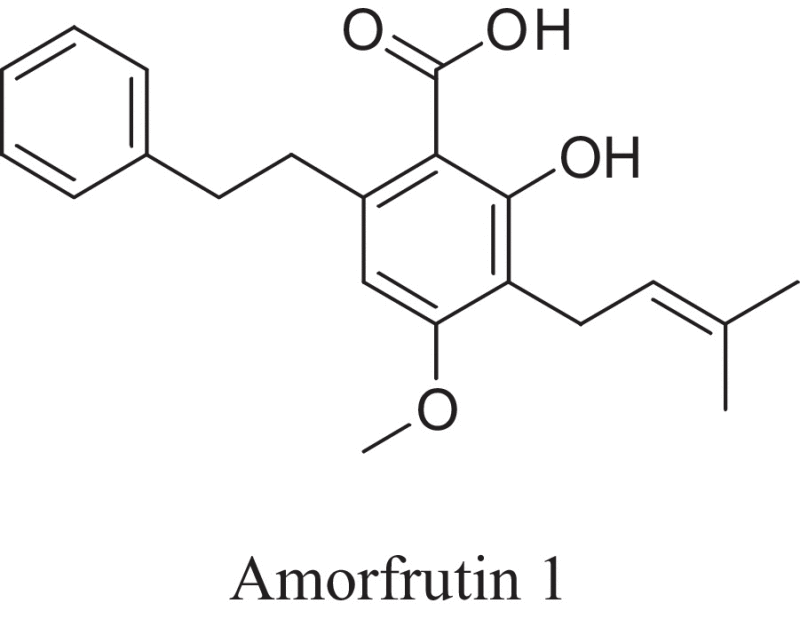 |
Binds to purified PPARγ (Ki = 0.24 μM) and activates chimeric Gal4-PPARγ-dependent reporter gene expression as partial agonist (with EC50 = 0.46 μM and maximal efficacy 61% lower than rosiglitazone) [187], selectively modulates PPARγ gene expression networks in human adipocytes with a different pattern in comparison to synthetic PPARγ agonists [187], improves insulin resistance and other metabolic and inflammatory parameters without concomitant increase of fat storage or other unwanted side effects such as hepatotoxicity in diet-induced obese and db/db mice [187], blocks PPARγ Ser273 phosphorylation in DIO mice [187], was co-crystallized with the PPARγ-LBD (PDB: 2yfe) [187] |
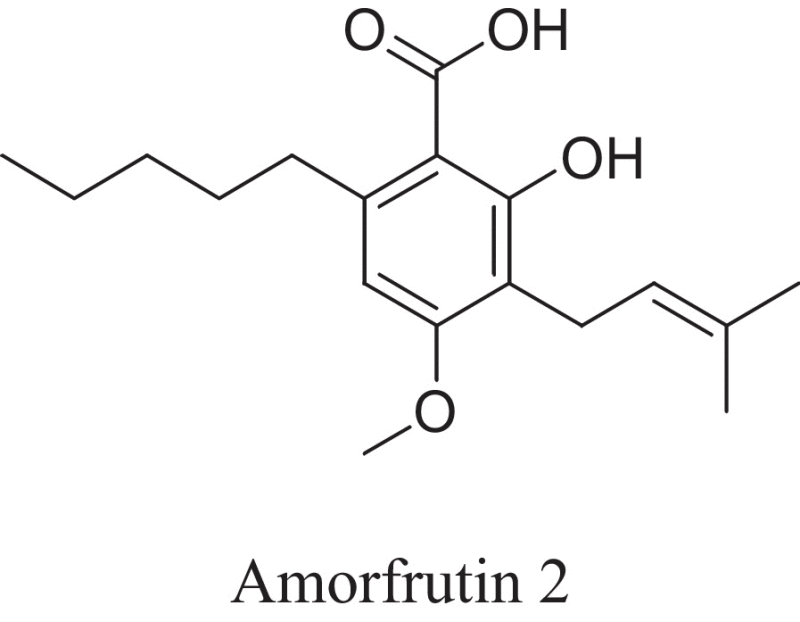 |
Binds to purified PPARγ (Ki = 0.29 μM), and activates chimeric Gal4-PPARγ-dependent reporter gene expression as partial agonist (with EC50 = 1.2 μM and maximal efficacy 70% lower than rosiglitazone) [187], selectively modulates PPARγ gene expression networks in human adipocytes with a different pattern in comparison to synthetic PPARγ agonists [187], was co-crystallized with the PPARγ-LBD (PDB: 4a4v) [197] |
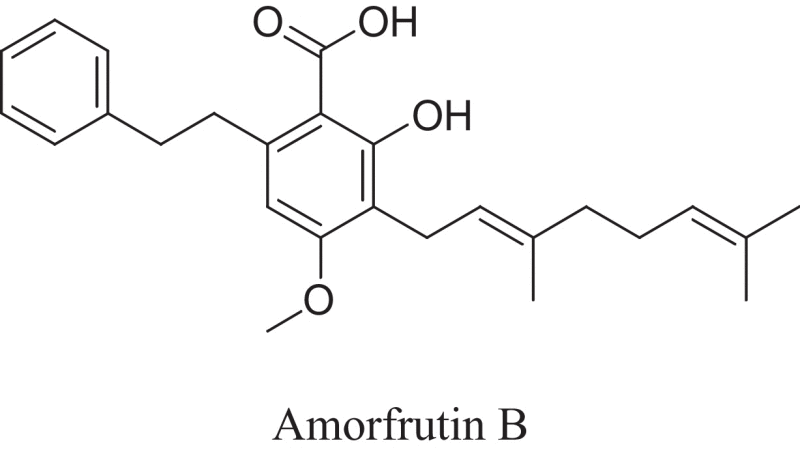 |
Binds to purified PPARγ (Ki = 0.019 μM) and activates chimeric Gal4-PPARγ-dependent reporter gene expression as partial agonist (with EC50 = 0.073 μM and maximal efficacy 4-fold lower than rosiglitazone) [188], induces partial recruitment of several PPARγ transcriptional coactivators [188], regulates gene expression in human adipocytes in a PPARγ-dependent manner [188], in insulin-resistant mice, it shows liver-protecting properties and improves insulin sensitivity, glucose tolerance, and blood lipid variables, without weight gain or adverse effects on osteoblastogenesis and fluid retention [188], was co-crystallized with the PPARγ-LBD (PDB: 4a4w) [197] |
| Polyacetylenes | |
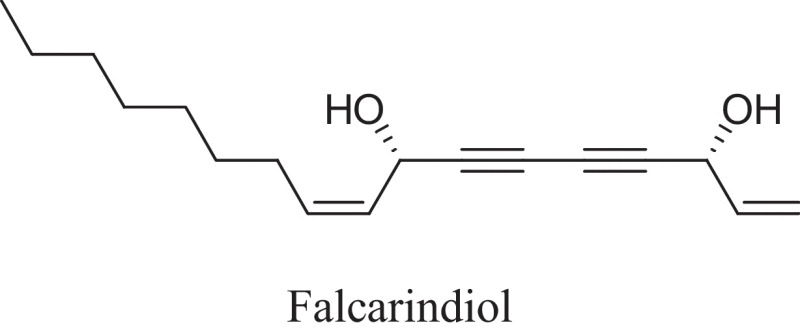 |
Binds to purified human PPARγ (Ki = 3.1 μM) [228], activates PPARγ-dependent reporter gene expression as partial agonist (at 1–30 μM), and antagonizes the effect of rosiglitazone upon co-treatment [228], induces adipogenesis and glucose uptake in 3T3-L1 adipocytes at 10 μM [228] |
| Sesquiterpene lactones | |
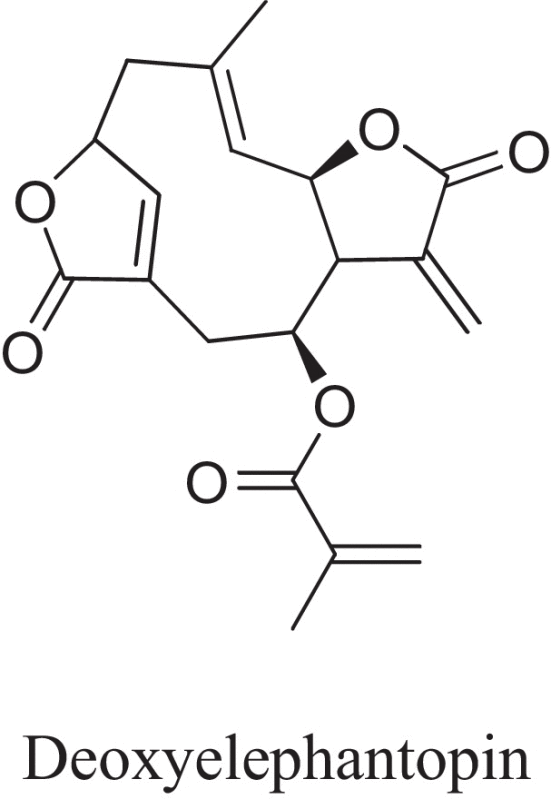 |
Binds to purified PPARγ-LBD (KD = 3.4 μM) but not to PPARα-LBD or PPARβ/δ-LBD [218], enhances the transcriptional activity of full-length PPARγ and Gal4-PPARγ-LBD chimera as a partial agonist (at 1–20 μM) [218], enhances the transcription activity of PPARγ upon co-treatment with non-saturating concentrations of rosiglitazone [218] |
| Diterpenequinone derivatives | |
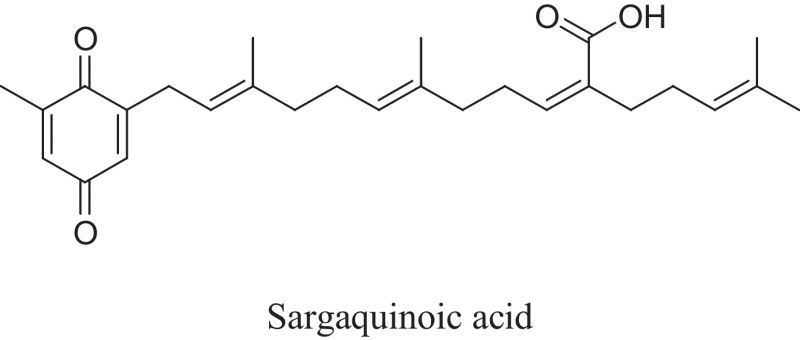 |
Binds to purified PPARγ (IC50 = 0.255 μM) [253], activates PPARγ-dependent reporter gene expression as a partial agonist (at 1–30 μM) [253], enhances adipocyte differentiation in 3T3-L1 cells by increasing the expression of genes critical for adipocyte phenotype (at 10 μM) [253], activates also PPARα (at 1–30 μM) [253] |
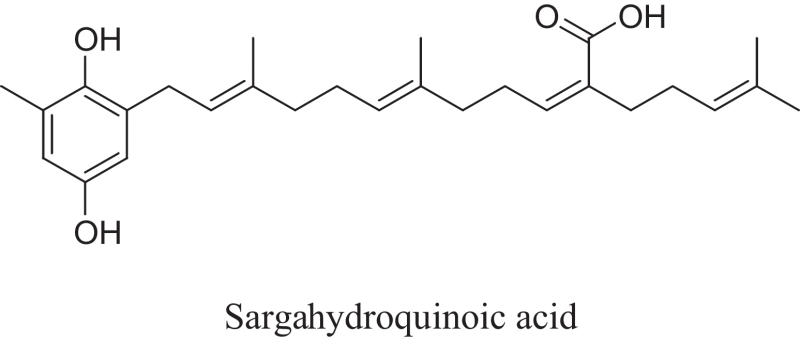 |
Binds to purified PPARγ (IC50 = 0.725 μM) [253], activates PPARγ-dependent reporter gene expression as a partial agonist (at 1–30 μM) [253], enhances adipocyte differentiation in 3T3-L1 cells by increasing the expression of genes critical for adipocyte phenotype (at 10 μM) [253], activates also PPARα (at 1–30 μM) [253] |
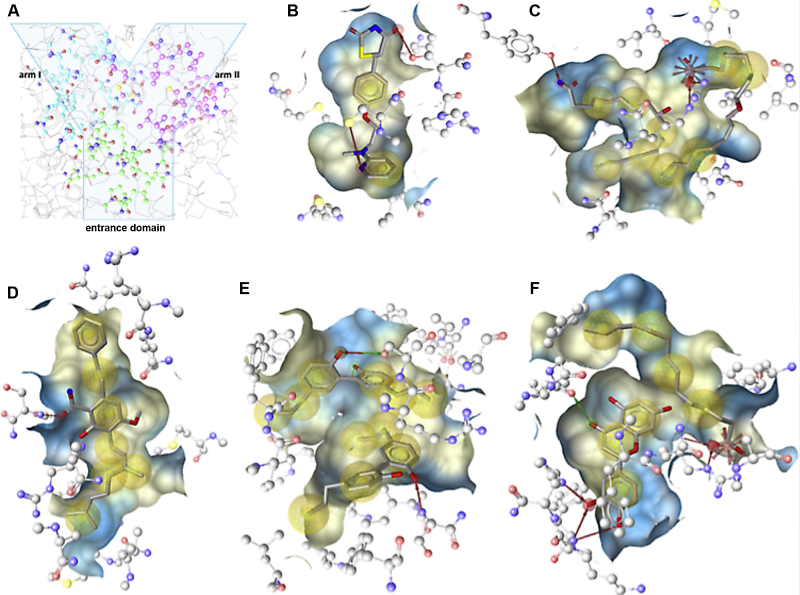
Structural details for the binding to PPARγ LBD are revealed by receptor-ligand crystal structures solved for several natural products (Table 2 and Fig. 2). The PPARγ protein comprises an N-terminal regulatory domain, a central DNA-binding domain, and a C-terminal LBD (amino acids 204-477) [43], [195]. The LBD consists of 13 α-helices and a small four-stranded β-sheet [196]. Helix H12 of the ligand-dependent activation domain (activation function-2, AF-2) is essential for ligand binding and PPAR function. H12 and the loop between H2′ and H3 are the most mobile parts of the LBD. Ligand binding leads to a more rigid conformation of the LBD, which causes recruitment of coactivators and consequently transcription of target genes [197]. The PPARγ LBD is a large Y-shaped cavity that is composed of an entrance domain and two pockets, arm I and arm II (Fig. 2A) [198]. The large size and the flexibility of the binding pocket allow PPARγ to interact with structurally distinct ligands. No ligand is known that completely fills this large cavity [43]. However, it enables in some instances the simultaneous binding of two or even three molecules, which interact with the binding pocket as well as with each other, resulting in a more stable binding conformation [199]. Moreover, different ligands bind different areas in the PPARγ LBD, representing different binding modes. Depicted in Fig. 2 are the binding modes of a selection of ligands co-crystallized with the PPARγ LBD: the full thiazolidinedione agonist rosiglitazone (protein data bank (PDB) [200] entry PDB: 4ema [199], Fig. 2B); (9S,10E,12Z)-9-hydroxyoctadeca-10,12-dienoic acid (9-(S)-HODE) as a representative endogenous ligand that binds as a homodimer (PDB: 2vsr [43], Fig. 2C); the natural product amorfrutin B (PDB: 4a4w [197], Fig. 2D); the neolignan magnolol that binds as a homodimer (PDB: 3r5n [193], Fig. 2E); and the flavonoid luteolin binding concomitantly with myristic acid (PDB: 3sz1 [195], Fig. 2F).
Fig. 2.
Binding modes of selected PPARγ ligands co-crystallized with PPARγ. (A) The Y-shaped PPARγ LBD composed of one entrance domain and two arms (arm I is substantially polar, arm II is mainly hydrophobic) [174]. Observed protein-ligand interactions are presented between the human PPARγ LBD and (B) the synthetic agonist rosiglitazone (PDB: 4ema), (C) the endogenous agonist 9-(S)-HODE binding as a homodimer (PDB: 2vsr), the natural ligands (D) amorfrutin B (PDB: 4a4w), (E) magnolol binding as homodimer (PDB: 3r5n), and (F) luteolin binding as a mixed dimer with myristic acid (PDB: 3sz1). The interactions were visualized by means of the software LigandScout [254] with the following color code: hydrogen bond acceptor (red arrow), hydrogen bond donor (green arrow), hydrophobic interaction (yellow sphere), and negative ionizable area (red star). The ligand binding pocket is depicted as surface; its colors are based on the lipo- and hydrophilicity. Contacts with active site water molecules are not shown.
In general, strong PPARγ agonists such as thiazolidinediones are known to bind to H12, whereas partial agonists stabilize the β-sheet and the H2′/H3 area. The full agonist rosiglitazone stabilizes H12 by building hydrogen bonds with Tyr473, which leads to coactivator recruitment [199]. Whereas just one molecule of the thiazolidinedione agonists such as rosiglitazone is binding to the LBD (PDB: 4ema [199], Fig. 2B), some endogenous ligands such as 9-(S)-HODE were demonstrated to activate the receptor as homodimers (PDB: 2vsr [43], Fig. 2C). The first 9-(S)-HODE molecule binds with its carboxy group via hydrogen bond to Tyr 473 of H12. This interaction is typical for carboxylate-containing ligands. The tail, which is located in an area also occupied by highly potent agonists, interacts via van der Waals contacts with Phe363 and other amino acids. The second molecule is located between H3 and the β-sheet, an area which is occupied also by synthetic partial agonists. Its carboxylate group forms a salt bridge with Arg288, an amino acid, which is not involved in the binding of thiazolidinediones [43].
The partial PPARγ agonists amorfrutin 1, 2, and B (PDB: 2yfe, PDB: 4a4 v, and PDB: 4a4w, respectively [187], [197]) are localized and oriented almost identically in the PPARγ LBD. They bind to and therefore stabilize the β-sheet as well as H3 of PPARγ by hydrogen bonds and van der Waals contacts. The reason for the high affinity of amorfrutins to PPARγ is the interaction of the carboxyl group to Ser342 of the β-sheet via hydrogen bonds. Also Arg288 of H3 is stabilized by amorfrutins. The replacement of Arg288 by threonins in PPARα and PPARβ/δ is likely the reason for the selective PPARγ activity of amorfrutins 1, 2, and B. However, there are also differences in their interactions with the LBD. Amorfrutin B shows significantly higher affinity than other reported amorfrutins, similar to that of rosiglitazone. This is caused by the long geranyl side chain, which forms additional hydrophobic interactions especially to Arg288 of H3 and to H4/5 [197].
According to the PDB: 3sz1, the PPARγ partial agonist luteolin binds to the PPARγ LBD simultaneously with the long-chain fatty acid myristic acid. The two molecules stabilize the β-sheet as well as the loop among H2′ and H3. Luteolin interacts via hydrogen bonds with Lys265 and His266 at the loop that links H2′ and H3 and builds hydrophobic contacts with various amino acids. Myristic acid occupies H3, H5, and H7 and interacts with Arg288 (H3) via a salt bridge. Luteolin and the carboxylate of myristic acid are connected via a water molecule through a hydrogen bond. This water molecule seems to be important for keeping luteolin in the LBD [195].
Similar to some endogenous ligands such as 9-(S)-HODE, two magnolol molecules were demonstrated to cooperatively occupy the PPARγ LBD. One magnolol molecule occupies AF-2, the other one the β-sheet. In AF-2, the hydroxyl group of magnolol makes a hydrogen bond with Ser289 in H3 and water-mediated hydrogen bonds with Tyr473. In the β-sheet, the hydroxyl group of the second magnolol forms a hydrogen bond with Ser342. Furthermore, there is also a water-mediated hydrogen bond in the β-sheet to magnolol. The magnolol structure is highly flexible due to the single bond connecting the two 5-ally-2-hydroxyphenyl moieties. It exhibits three different conformations when binding to PPARγ and RXRα, which bind two and one molecule of magnolol, respectively [193].
4. Concluding remarks
Natural products prove to be a rich source for the discovery of novel PPARγ ligands and many structurally diverse agonists of this receptor were recently identified from traditionally used medicinal plants or food sources. Interestingly, the majority of identified natural compounds are rather weak agonists of PPARγ, often activating the receptor as partial agonists, with activation pattern distinct from the full thiazolidinedione agonists and more similar to endogenous ligands with weaker activation potential such as fatty acids and prostanoids. Noteworthy, several PPARγ agonists were identified in plants used as culinary spices, beverages or food sources, opening the possibility to consider modulation of the activity of this nuclear receptor through dietary interventions. While most of the identified natural products only activate PPARγ as SPPARMs, some are dual agonists able to also activate PPARα (Table 2). An especially interesting activation pattern is observed for the neolignans magnolol and honokiol, which are ligands for both PPARγ and its dimer activation partner RXR. The neolignan honokiol and several other natural products have also demonstrated beneficial metabolic effects in diabetic animal models, with reduced side effects in comparison to full thiazolidinedione agonists. Many extracts from medicinal plants reported in the literature as PPARγ activators are so far not thoroughly investigated. The identification of their active constituents might provide further interesting ligands in the future.
In conclusion, a range of PPARγ activating natural products and plant extracts were recently described that bear a good potential to be further explored for therapeutic effectiveness as well as to be studied as potential dietary supplements to counteract the metabolic syndrome and type 2 diabetes.
Acknowledgments
This work was supported by the Austrian Science Fund (FWF): S10702/S10711, S10703, S10704, S10705, and S10706 (NFN ‘Drugs from Nature Targeting Inflammation’) and P25971-B23 (‘Improved cholesterol efflux by natural products’), as well as by the Tyrolean Science Fund (TWF).
References
- 1.Ford E.S., Li C., Zhao G. Prevalence and correlates of metabolic syndrome based on a harmonious definition among adults in the US. J Diabetes. 2010;2:180–193. doi: 10.1111/j.1753-0407.2010.00078.x. [DOI] [PubMed] [Google Scholar]
- 2.Friend A., Craig L., Turner S. The prevalence of metabolic syndrome in children: a systematic review of the literature. Metab Syndr Relat Disord. 2013;11:71–80. doi: 10.1089/met.2012.0122. [DOI] [PubMed] [Google Scholar]
- 3.Grundy S.M., Brewer H.B., Jr., Cleeman J.I., Smith S.C., Jr., Lenfant C. Definition of metabolic syndrome: report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Circulation. 2004;109:433–438. doi: 10.1161/01.CIR.0000111245.75752.C6. [DOI] [PubMed] [Google Scholar]
- 4.Ouchi N., Parker J.L., Lugus J.J., Walsh K. Adipokines in inflammation and metabolic disease. Nat Rev Immunol. 2011;11:85–97. doi: 10.1038/nri2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berenson D.F., Weiss A.R., Wan Z.L., Weiss M.A. Insulin analogs for the treatment of diabetes mellitus: therapeutic applications of protein engineering. Ann NY Acad Sci. 2011;1243:E40–E54. doi: 10.1111/j.1749-6632.2012.06468.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mizuno C.S., Chittiboyina A.G., Kurtz T.W., Pershadsingh H.A. Avery MA. Type 2 diabetes and oral antihyperglycemic drugs. Curr Med Chem. 2008;15:61–74. doi: 10.2174/092986708783330656. [DOI] [PubMed] [Google Scholar]
- 7.Seino S., Takahashi H., Takahashi T., Shibasaki T. Treating diabetes today: a matter of selectivity of sulphonylureas. Diabetes Obes Metab. 2012;14(Suppl 1):9–13. doi: 10.1111/j.1463-1326.2011.01507.x. [DOI] [PubMed] [Google Scholar]
- 8.Baker R.G., Hayden M.S., Ghosh S. NF-kappaB, inflammation, and metabolic disease. Cell Metab. 2011;13:11–22. doi: 10.1016/j.cmet.2010.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roth Flach R.J., Bennett A.M. Mitogen-activated protein kinase phosphatase-1 - a potential therapeutic target in metabolic disease. Expert Opin Ther Targets. 2010;14:1323–1332. doi: 10.1517/14728222.2010.528395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Furuhashi M., Hotamisligil G.S. Fatty acid-binding proteins: role in metabolic diseases and potential as drug targets. Nat Rev Drug Discovery. 2008;7:489–503. doi: 10.1038/nrd2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wakil S.J., Abu-Elheiga L.A. Fatty acid metabolism: target for metabolic syndrome. J Lipid Res. 2009;50(Suppl):S138–S143. doi: 10.1194/jlr.R800079-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cao Y. Adipose tissue angiogenesis as a therapeutic target for obesity and metabolic diseases. Nat Rev Drug Discovery. 2010;9:107–115. doi: 10.1038/nrd3055. [DOI] [PubMed] [Google Scholar]
- 13.Berger J.P., Akiyama T.E., Meinke P.T. PPARs: therapeutic targets for metabolic disease. Trends Pharmacol Sci. 2005;26:244–251. doi: 10.1016/j.tips.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 14.Monsalve F.A., Pyarasani R.D., Delgado-Lopez F., Moore-Carrasco R. Peroxisome proliferator-activated receptor targets for the treatment of metabolic diseases. Mediators Inflamm. 2013;2013:549627. doi: 10.1155/2013/549627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Laudet V., Hanni C., Coll J., Catzeflis F., Stehelin D. Evolution of the nuclear receptor gene superfamily. EMBO J. 1992;11:1003–1013. doi: 10.1002/j.1460-2075.1992.tb05139.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Issemann I., Green S. Activation of a member of the steroid hormone receptor superfamily by peroxisome proliferators. Nature. 1990;347:645–650. doi: 10.1038/347645a0. [DOI] [PubMed] [Google Scholar]
- 17.Dreyer C., Krey G., Keller H., Givel F., Helftenbein G., Wahli W. Control of the peroxisomal beta-oxidation pathway by a novel family of nuclear hormone receptors. Cell. 1992;68:879–887. doi: 10.1016/0092-8674(92)90031-7. [DOI] [PubMed] [Google Scholar]
- 18.Forman B.M., Chen J., Evans R.M. Hypolipidemic drugs, polyunsaturated fatty acids, and eicosanoids are ligands for peroxisome proliferator-activated receptors alpha and delta. Proc Natl Acad Sci USA. 1997;94:4312–4317. doi: 10.1073/pnas.94.9.4312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dussault I., Forman B.M. Prostaglandins and fatty acids regulate transcriptional signaling via the peroxisome proliferator activated receptor nuclear receptors. Prostaglandins Other Lipid Mediat. 2000;62:1–13. doi: 10.1016/s0090-6980(00)00071-x. [DOI] [PubMed] [Google Scholar]
- 20.Evans R.M., Barish G.D., Wang Y.X. PPARs and the complex journey to obesity. Nat Med. 2004;10:355–361. doi: 10.1038/nm1025. [DOI] [PubMed] [Google Scholar]
- 21.Gearing K.L., Gottlicher M., Teboul M., Widmark E., Gustafsson J.A. Interaction of the peroxisome-proliferator-activated receptor and retinoid X receptor. Proc Natl Acad Sci USA. 1993;90:1440–1444. doi: 10.1073/pnas.90.4.1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lemberger T., Desvergne B., Wahli W. Peroxisome proliferator-activated receptors: a nuclear receptor signaling pathway in lipid physiology. Ann Rev Cell Dev Biol. 1996;12:335–363. doi: 10.1146/annurev.cellbio.12.1.335. [DOI] [PubMed] [Google Scholar]
- 23.Yu S., Reddy J.K. Transcription coactivators for peroxisome proliferator-activated receptors. Biochim Biophys Acta. 2007;1771:936–951. doi: 10.1016/j.bbalip.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 24.Feige J.N., Auwerx J. Transcriptional coregulators in the control of energy homeostasis. Trends Cell Biol. 2007;17:292–301. doi: 10.1016/j.tcb.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 25.Auboeuf D., Rieusset J., Fajas L., Vallier P., Frering V., Riou J.P. Tissue distribution and quantification of the expression of mRNAs of peroxisome proliferator-activated receptors and liver X receptor-alpha in humans: no alteration in adipose tissue of obese and NIDDM patients. Diabetes. 1997;46:1319–1327. doi: 10.2337/diab.46.8.1319. [DOI] [PubMed] [Google Scholar]
- 26.Desvergne B., Wahli W. Peroxisome proliferator-activated receptors: nuclear control of metabolism. Endocr Rev. 1999;20:649–688. doi: 10.1210/edrv.20.5.0380. [DOI] [PubMed] [Google Scholar]
- 27.Fruchart J.C. Peroxisome proliferator-activated receptor-alpha (PPARalpha): at the crossroads of obesity, diabetes and cardiovascular disease. Atherosclerosis. 2009;205:1–8. doi: 10.1016/j.atherosclerosis.2009.03.008. [DOI] [PubMed] [Google Scholar]
- 28.Barish G.D., Narkar V.A., Evans R.M. PPAR delta: a dagger in the heart of the metabolic syndrome. J Clin Invest. 2006;116:590–597. doi: 10.1172/JCI27955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Seedorf U., Aberle J. Emerging roles of PPARdelta in metabolism. Biochim Biophys Acta. 2007;1771:1125–1131. doi: 10.1016/j.bbalip.2007.04.017. [DOI] [PubMed] [Google Scholar]
- 30.Zhu Y., Qi C., Korenberg J.R., Chen X.N., Noya D., Rao M.S. Structural organization of mouse peroxisome proliferator-activated receptor gamma (mPPAR gamma) gene: alternative promoter use and different splicing yield two mPPAR gamma isoforms. Proc Natl Acad Sci USA. 1995;92:7921–7925. doi: 10.1073/pnas.92.17.7921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fajas L., Auboeuf D., Raspe E., Schoonjans K., Lefebvre A.M., Saladin R. The organization, promoter analysis, and expression of the human PPARgamma gene. J Biol Chem. 1997;272:18779–18789. doi: 10.1074/jbc.272.30.18779. [DOI] [PubMed] [Google Scholar]
- 32.Werman A., Hollenberg A., Solanes G., Bjorbaek C., Vidal-Puig A.J., Flier J.S. Ligand-independent activation domain in the N terminus of peroxisome proliferator-activated receptor gamma (PPARgamma). Differential activity of PPARgamma1 and -2 isoforms and influence of insulin. J Biol Chem. 1997;272:20230–20235. doi: 10.1074/jbc.272.32.20230. [DOI] [PubMed] [Google Scholar]
- 33.Vidal-Puig A.J., Considine R.V., Jimenez-Linan M., Werman A., Pories W.J., Caro J.F. Peroxisome proliferator-activated receptor gene expression in human tissues. Effects of obesity, weight loss, and regulation by insulin and glucocorticoids. J Clin Invest. 1997;99:2416–2422. doi: 10.1172/JCI119424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Medina-Gomez G., Gray S.L., Yetukuri L., Shimomura K., Virtue S., Campbell M. PPAR gamma 2 prevents lipotoxicity by controlling adipose tissue expandability and peripheral lipid metabolism. PLoS Genet. 2007;3:e64. doi: 10.1371/journal.pgen.0030064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kliewer S.A., Sundseth S.S., Jones S.A., Brown P.J., Wisely G.B., Koble C.S. Fatty acids and eicosanoids regulate gene expression through direct interactions with peroxisome proliferator-activated receptors alpha and gamma. Proc Natl Acad Sci USA. 1997;94:4318–4323. doi: 10.1073/pnas.94.9.4318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lehmann J.M., Moore L.B., Smith-Oliver T.A., Wilkison W.O., Willson T.M., Kliewer S.A. An antidiabetic thiazolidinedione is a high affinity ligand for peroxisome proliferator-activated receptor gamma (PPAR gamma) J Bio Chem. 1995;270:12953–12956. doi: 10.1074/jbc.270.22.12953. [DOI] [PubMed] [Google Scholar]
- 37.Cho N., Momose Y. Peroxisome proliferator-activated receptor gamma agonists as insulin sensitizers: from the discovery to recent progress. Curr Top Med Chem. 2008;8:1483–1507. doi: 10.2174/156802608786413474. [DOI] [PubMed] [Google Scholar]
- 38.Tzameli I., Fang H., Ollero M., Shi H., Hamm J.K., Kievit P. Regulated production of a peroxisome proliferator-activated receptor-gamma ligand during an early phase of adipocyte differentiation in 3T3-L1 adipocytes. J Biol Chem. 2004;279:36093–36102. doi: 10.1074/jbc.M405346200. [DOI] [PubMed] [Google Scholar]
- 39.Lehrke M., Lazar M.A. The many faces of PPARgamma. Cell. 2005;123:993–999. doi: 10.1016/j.cell.2005.11.026. [DOI] [PubMed] [Google Scholar]
- 40.Subramani P.A., Reddy M.C., Narala V.R. The need for physiologically relevant peroxisome proliferator-activated receptor-gamma (PPAR-gamma) ligands. Endocr Metab Imm Disorders Drug Targets. 2013;13:175–183. doi: 10.2174/18715303113139990003. [DOI] [PubMed] [Google Scholar]
- 41.Goto T., Nagai H., Egawa K., Kim Y.I., Kato S., Taimatsu A. Farnesyl pyrophosphate regulates adipocyte functions as an endogenous PPARgamma agonist. Biochem J. 2011;438:111–119. doi: 10.1042/BJ20101939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schopfer F.J., Cole M.P., Groeger A.L., Chen C.S., Khoo N.K., Woodcock S.R. Covalent peroxisome proliferator-activated receptor gamma adduction by nitro-fatty acids: selective ligand activity and anti-diabetic signaling actions. J Biol Chem. 2010;285:12321–12333. doi: 10.1074/jbc.M109.091512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Itoh T., Fairall L., Amin K., Inaba Y., Szanto A., Balint B.L. Structural basis for the activation of PPARgamma by oxidized fatty acids. Nat Struct Mol Biol. 2008;15:924–931. doi: 10.1038/nsmb.1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tontonoz P., Spiegelman B.M. Fat and beyond: the diverse biology of PPARgamma. Annu Rev Biochem. 2008;77:289–312. doi: 10.1146/annurev.biochem.77.061307.091829. [DOI] [PubMed] [Google Scholar]
- 45.Na H.K., Surh Y.J. Peroxisome proliferator-activated receptor gamma (PPARgamma) ligands as bifunctional regulators of cell proliferation. Biochem Pharmacol. 2003;66:1381–1391. doi: 10.1016/s0006-2952(03)00488-x. [DOI] [PubMed] [Google Scholar]
- 46.Heikkinen S., Auwerx J., Argmann C.A. PPARgamma in human and mouse physiology. Biochim Biophys Acta. 2007;1771:999–1013. doi: 10.1016/j.bbalip.2007.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tontonoz P., Hu E., Graves R.A., Budavari A.I., Spiegelman B.M. mPPAR gamma 2: tissue-specific regulator of an adipocyte enhancer. Genes Dev. 1994;8:1224–1234. doi: 10.1101/gad.8.10.1224. [DOI] [PubMed] [Google Scholar]
- 48.Tontonoz P., Hu E., Spiegelman B.M. Stimulation of adipogenesis in fibroblasts by PPAR gamma 2, a lipid-activated transcription factor. Cell. 1994;79:1147–1156. doi: 10.1016/0092-8674(94)90006-x. [DOI] [PubMed] [Google Scholar]
- 49.Sears I.B., MacGinnitie M.A., Kovacs L.G., Graves R.A. Differentiation-dependent expression of the brown adipocyte uncoupling protein gene: regulation by peroxisome proliferator-activated receptor gamma. Mol Cell Biol. 1996;16:3410–3419. doi: 10.1128/mcb.16.7.3410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Barak Y., Nelson M.C., Ong E.S., Jones Y.Z., Ruiz-Lozano P., Chien K.R. PPAR gamma is required for placental, cardiac, and adipose tissue development. Mol Cell. 1999;4:585–595. doi: 10.1016/s1097-2765(00)80209-9. [DOI] [PubMed] [Google Scholar]
- 51.Auwerx J. PPARgamma, the ultimate thrifty gene. Diabetologia. 1999;42:1033–1049. doi: 10.1007/s001250051268. [DOI] [PubMed] [Google Scholar]
- 52.Christodoulides C., Vidal-Puig A. PPARs and adipocyte function. Mol Cell Endocrinol. 2010;318:61–68. doi: 10.1016/j.mce.2009.09.014. [DOI] [PubMed] [Google Scholar]
- 53.Picard F., Auwerx J. PPAR(gamma) and glucose homeostasis. Ann Rev Nutr. 2002;22:167–197. doi: 10.1146/annurev.nutr.22.010402.102808. [DOI] [PubMed] [Google Scholar]
- 54.Ahmadian M., Suh J.M., Hah N., Liddle C., Atkins A.R., Downes M. PPARgamma signaling and metabolism: the good, the bad and the future. Nat Med. 2013;19:557–566. doi: 10.1038/nm.3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Szatmari I., Rajnavolgyi E., Nagy L. PPARgamma, a lipid-activated transcription factor as a regulator of dendritic cell function. Ann NY Acad Sci. 2006;1088:207–218. doi: 10.1196/annals.1366.013. [DOI] [PubMed] [Google Scholar]
- 56.Szeles L., Torocsik D., Nagy L. PPARgamma in immunity and inflammation: cell types and diseases. Biochim Biophys Acta. 2007;1771:1014–1030. doi: 10.1016/j.bbalip.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 57.Huang W., Glass C.K. Nuclear receptors and inflammation control: molecular mechanisms and pathophysiological relevance. Arterioscl Throm Vas Biol. 2010;30:1542–1549. doi: 10.1161/ATVBAHA.109.191189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Glass C.K., Saijo K. Nuclear receptor transrepression pathways that regulate inflammation in macrophages and T cells. Nat Rev Immunol. 2010;10:365–376. doi: 10.1038/nri2748. [DOI] [PubMed] [Google Scholar]
- 59.Zhang L., Chawla A. Role of PPARgamma in macrophage biology and atherosclerosis. Trends Endocrinol Metabol: TEM. 2004;15:500–505. doi: 10.1016/j.tem.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 60.Han S., Roman J. Peroxisome proliferator-activated receptor gamma: a novel target for cancer therapeutics. Anticancer Drugs. 2007;18:237–244. doi: 10.1097/CAD.0b013e328011e67d. [DOI] [PubMed] [Google Scholar]
- 61.Reka A.K., Kurapati H., Narala V.R., Bommer G., Chen J., Standiford T.J. Peroxisome proliferator-activated receptor-gamma activation inhibits tumor metastasis by antagonizing Smad3-mediated epithelial-mesenchymal transition. Mol Cancer Ther. 2010;9:3221–3232. doi: 10.1158/1535-7163.MCT-10-0570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Choi J.H., Banks A.S., Estall J.L., Kajimura S., Bostrom P., Laznik D. Anti-diabetic drugs inhibit obesity-linked phosphorylation of PPARgamma by Cdk5. Nature. 2010;466:451–456. doi: 10.1038/nature09291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rangwala S.M., Rhoades B., Shapiro J.S., Rich A.S., Kim J.K., Shulman G.I. Genetic modulation of PPARgamma phosphorylation regulates insulin sensitivity. Dev Cell. 2003;5:657–663. doi: 10.1016/s1534-5807(03)00274-0. [DOI] [PubMed] [Google Scholar]
- 64.Fitzgerald M.L., Moore K.J., Freeman M.W. Nuclear hormone receptors and cholesterol trafficking: the orphans find a new home. J Mol Med (Berl) 2002;80:271–281. doi: 10.1007/s00109-001-0318-y. [DOI] [PubMed] [Google Scholar]
- 65.Fruchart J.C., Staels B., Duriez P. PPARS, metabolic disease and atherosclerosis. Pharmacol Res. 2001;44:345–352. doi: 10.1006/phrs.2001.0871. [DOI] [PubMed] [Google Scholar]
- 66.Lalloyer F., Staels B. Fibrates, glitazones, and peroxisome proliferator-activated receptors. Arterioscl Thromb Vas Biol. 2010;30:894–899. doi: 10.1161/ATVBAHA.108.179689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Martens F.M., Visseren F.L., Lemay J., de Koning E.J., Rabelink T.J. Metabolic and additional vascular effects of thiazolidinediones. Drugs. 2002;62:1463–1480. doi: 10.2165/00003495-200262100-00004. [DOI] [PubMed] [Google Scholar]
- 68.Nolan J.J., Ludvik B., Beerdsen P., Joyce M., Olefsky J. Improvement in glucose tolerance and insulin resistance in obese subjects treated with troglitazone. New England J Med. 1994;331:1188–1193. doi: 10.1056/NEJM199411033311803. [DOI] [PubMed] [Google Scholar]
- 69.Kumar S., Boulton A.J., Beck-Nielsen H., Berthezene F., Muggeo M., Persson B. Troglitazone, an insulin action enhancer, improves metabolic control in NIDDM patients. Troglitazone Study Group. Diabetologia. 1996;39:701–709. doi: 10.1007/BF00418542. [DOI] [PubMed] [Google Scholar]
- 70.Iwamoto Y., Kosaka K., Kuzuya T., Akanuma Y., Shigeta Y., Kaneko T. Effects of troglitazone: a new hypoglycemic agent in patients with NIDDM poorly controlled by diet therapy. Diabetes Care. 1996;19:151–156. doi: 10.2337/diacare.19.2.151. [DOI] [PubMed] [Google Scholar]
- 71.Cavaghan M.K., Ehrmann D.A., Byrne M.M., Polonsky K.S. Treatment with the oral antidiabetic agent troglitazone improves beta cell responses to glucose in subjects with impaired glucose tolerance. J Clin Invest. 1997;100:530–537. doi: 10.1172/JCI119562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Schwartz S., Raskin P., Fonseca V., Graveline J.F. Effect of troglitazone in insulin-treated patients with type II diabetes mellitus. Troglitazone and Exogenous Insulin Study Group. New England J Med. 1998;338:861–866. doi: 10.1056/NEJM199803263381302. [DOI] [PubMed] [Google Scholar]
- 73.Maggs D.G., Buchanan T.A., Burant C.F., Cline G., Gumbiner B., Hsueh W.A. Metabolic effects of troglitazone monotherapy in type 2 diabetes mellitus. A randomized, double-blind, placebo-controlled trial. Ann Internal Med. 1998;128:176–185. doi: 10.7326/0003-4819-128-3-199802010-00002. [DOI] [PubMed] [Google Scholar]
- 74.Tack C.J., Ong M.K., Lutterman J.A., Smits P. Insulin-induced vasodilatation and endothelial function in obesity/insulin resistance. Effects of troglitazone. Diabetologia. 1998;41:569–576. doi: 10.1007/s001250050948. [DOI] [PubMed] [Google Scholar]
- 75.Kumar S., Prange A., Schulze J., Lettis S., Barnett A.H. Troglitazone, an insulin action enhancer, improves glycaemic control and insulin sensitivity in elderly type 2 diabetic patients. Diabet Med. 1998;15:772–779. doi: 10.1002/(SICI)1096-9136(199809)15:9<772::AID-DIA677>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 76.Minamikawa J., Tanaka S., Yamauchi M., Inoue D., Koshiyama H. Potent inhibitory effect of troglitazone on carotid arterial wall thickness in type 2 diabetes. J Clin Endocrinol Metab. 1998;83:1818–1820. doi: 10.1210/jcem.83.5.4932. [DOI] [PubMed] [Google Scholar]
- 77.Ogihara T., Rakugi H., Ikegami H., Mikami H., Masuo K. Enhancement of insulin sensitivity by troglitazone lowers blood pressure in diabetic hypertensives. Am J Hypertension. 1995;8:316–320. doi: 10.1016/0895-7061(95)96214-5. [DOI] [PubMed] [Google Scholar]
- 78.Ghazzi M.N., Perez J.E., Antonucci T.K., Driscoll J.H., Huang S.M., Faja B.W. Cardiac and glycemic benefits of troglitazone treatment in NIDDM. The Troglitazone Study Group. Diabetes. 1997;46:433–439. doi: 10.2337/diab.46.3.433. [DOI] [PubMed] [Google Scholar]
- 79.Watkins P.B., Whitcomb R.W. Hepatic dysfunction associated with troglitazone. New England J Med. 1998;338:916–917. doi: 10.1056/NEJM199803263381314. [DOI] [PubMed] [Google Scholar]
- 80.Kohlroser J., Mathai J., Reichheld J., Banner B.F., Bonkovsky H.L. Hepatotoxicity due to troglitazone: report of two cases and review of adverse events reported to the United States Food and Drug Administration. Am J Gastroenterol. 2000;95:272–276. doi: 10.1111/j.1572-0241.2000.01707.x. [DOI] [PubMed] [Google Scholar]
- 81.Neuschwander-Tetri B.A., Isley W.L., Oki J.C., Ramrakhiani S., Quiason S.G., Phillips N.J. Troglitazone-induced hepatic failure leading to liver transplantation. A case report. Ann Internal Med. 1998;129:38–41. doi: 10.7326/0003-4819-129-1-199807010-00009. [DOI] [PubMed] [Google Scholar]
- 82.Gitlin N., Julie N.L., Spurr C.L., Lim K.N., Juarbe H.M. Two cases of severe clinical and histologic hepatotoxicity associated with troglitazone. Ann Internal Med. 1998;129:36–38. doi: 10.7326/0003-4819-129-1-199807010-00008. [DOI] [PubMed] [Google Scholar]
- 83.Shibuya A., Watanabe M., Fujita Y., Saigenji K., Kuwao S., Takahashi H. An autopsy case of troglitazone-induced fulminant hepatitis. Diabetes Care. 1998;21:2140–2143. doi: 10.2337/diacare.21.12.2140. [DOI] [PubMed] [Google Scholar]
- 84.Murphy E.J., Davern T.J., Shakil A.O., Shick L., Masharani U., Chow H. Troglitazone-induced fulminant hepatic failure. Acute Liver Failure Study Group. Dig Dis Sci. 2000;45:549–553. doi: 10.1023/a:1005405526283. [DOI] [PubMed] [Google Scholar]
- 85.Graham D.J., Green L., Senior J.R., Nourjah P. Troglitazone-induced liver failure: a case study. Am J Med. 2003;114:299–306. doi: 10.1016/s0002-9343(02)01529-2. [DOI] [PubMed] [Google Scholar]
- 86.Lebovitz H.E., Dole J.F., Patwardhan R., Rappaport E.B., Freed M.I. Rosiglitazone monotherapy is effective in patients with type 2 diabetes. J Clin Endocrinol Metabol. 2001;86:280–288. doi: 10.1210/jcem.86.1.7157. [DOI] [PubMed] [Google Scholar]
- 87.Raskin P., Rendell M., Riddle M.C., Dole J.F., Freed M.I., Rosenstock J. A randomized trial of rosiglitazone therapy in patients with inadequately controlled insulin-treated type 2 diabetes. Diabetes Care. 2001;24:1226–1232. doi: 10.2337/diacare.24.7.1226. [DOI] [PubMed] [Google Scholar]
- 88.Phillips L.S., Grunberger G., Miller E., Patwardhan R., Rappaport E.B., Salzman A. Once- and twice-daily dosing with rosiglitazone improves glycemic control in patients with type 2 diabetes. Diabetes Care. 2001;24:308–315. doi: 10.2337/diacare.24.2.308. [DOI] [PubMed] [Google Scholar]
- 89.Mayerson A.B., Hundal R.S., Dufour S., Lebon V., Befroy D., Cline G.W. The effects of rosiglitazone on insulin sensitivity, lipolysis, and hepatic and skeletal muscle triglyceride content in patients with type 2 diabetes. Diabetes. 2002;51:797–802. doi: 10.2337/diabetes.51.3.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Raji A., Seely E.W., Bekins S.A., Williams G.H., Simonson D.C. Rosiglitazone improves insulin sensitivity and lowers blood pressure in hypertensive patients. Diabetes Care. 2003;26:172–178. doi: 10.2337/diacare.26.1.172. [DOI] [PubMed] [Google Scholar]
- 91.Nissen S.E., Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. New England J Med. 2007;356:2457–2471. doi: 10.1056/NEJMoa072761. [DOI] [PubMed] [Google Scholar]
- 92.Singh S., Loke Y.K., Furberg C.D. Long-term risk of cardiovascular events with rosiglitazone: a meta-analysis. JAMA. 2007;298:1189–1195. doi: 10.1001/jama.298.10.1189. [DOI] [PubMed] [Google Scholar]
- 93.Home P.D., Pocock S.J., Beck-Nielsen H., Gomis R., Hanefeld M., Jones N.P. Rosiglitazone evaluated for cardiovascular outcomes--an interim analysis. New England J Med. 2007;357:28–38. doi: 10.1056/NEJMoa073394. [DOI] [PubMed] [Google Scholar]
- 94.Home P.D., Pocock S.J., Beck-Nielsen H., Curtis P.S., Gomis R., Hanefeld M. Rosiglitazone evaluated for cardiovascular outcomes in oral agent combination therapy for type 2 diabetes (RECORD): a multicentre, randomised, open-label trial. Lancet. 2009;373:2125–2135. doi: 10.1016/S0140-6736(09)60953-3. [DOI] [PubMed] [Google Scholar]
- 95.Nissen S.E., Wolski K. Rosiglitazone revisited: an updated meta-analysis of risk for myocardial infarction and cardiovascular mortality. Arch Internal Med. 2010;170:1191–1201. doi: 10.1001/archinternmed.2010.207. [DOI] [PubMed] [Google Scholar]
- 96.Al-Salman J., Arjomand H., Kemp D.G., Mittal M. Hepatocellular injury in a patient receiving rosiglitazone. A case report. Ann Int Med. 2000;132:121–124. doi: 10.7326/0003-4819-132-2-200001180-00006. [DOI] [PubMed] [Google Scholar]
- 97.Forman L.M., Simmons D.A., Diamond R.H. Hepatic failure in a patient taking rosiglitazone. Ann Internal Med. 2000;132:118–121. doi: 10.7326/0003-4819-132-2-200001180-00005. [DOI] [PubMed] [Google Scholar]
- 98.Fullert S., Schneider F., Haak E., Rau H., Badenhoop K., Lubben G. Effects of pioglitazone in nondiabetic patients with arterial hypertension: a double-blind, placebo-controlled study. J Clin Endocrinol Metab. 2002;87:5503–5506. doi: 10.1210/jc.2002-020963. [DOI] [PubMed] [Google Scholar]
- 99.Goldberg R.B., Kendall D.M., Deeg M.A., Buse J.B., Zagar A.J., Pinaire J.A. A comparison of lipid and glycemic effects of pioglitazone and rosiglitazone in patients with type 2 diabetes and dyslipidemia. Diabetes Care. 2005;28:1547–1554. doi: 10.2337/diacare.28.7.1547. [DOI] [PubMed] [Google Scholar]
- 100.Dormandy J.A., Charbonnel B., Eckland D.J., Erdmann E., Massi-Benedetti M., Moules I.K. Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive Study (PROspective pioglitAzone Clinical Trial In macroVascular Events): a randomised controlled trial. Lancet. 2005;366:1279–1289. doi: 10.1016/S0140-6736(05)67528-9. [DOI] [PubMed] [Google Scholar]
- 101.Nissen S.E., Nicholls S.J., Wolski K., Nesto R., Kupfer S., Perez A. Comparison of pioglitazone vs glimepiride on progression of coronary atherosclerosis in patients with type 2 diabetes: the PERISCOPE randomized controlled trial. JAMA. 2008;299:1561–1573. doi: 10.1001/jama.299.13.1561. [DOI] [PubMed] [Google Scholar]
- 102.Graham D.J., Ouellet-Hellstrom R., MaCurdy T.E., Ali F., Sholley C., Worrall C. Risk of acute myocardial infarction, stroke, heart failure, and death in elderly Medicare patients treated with rosiglitazone or pioglitazone. JAMA. 2010;304:411–418. doi: 10.1001/jama.2010.920. [DOI] [PubMed] [Google Scholar]
- 103.Aronoff S., Rosenblatt S., Braithwaite S., Egan J.W., Mathisen A.L., Schneider R.L. Pioglitazone hydrochloride monotherapy improves glycemic control in the treatment of patients with type 2 diabetes: a 6-month randomized placebo-controlled dose-response study, The Pioglitazone 001 Study Group. Diabetes Care. 2000;23:1605–1611. doi: 10.2337/diacare.23.11.1605. [DOI] [PubMed] [Google Scholar]
- 104.Cariou B., Charbonnel B., Staels B. Thiazolidinediones and PPARgamma agonists: time for a reassessment. Trends Endocrinol Metab: TEM. 2012;23:205–215. doi: 10.1016/j.tem.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 105.Bongartz T., Coras B., Vogt T., Scholmerich J., Muller-Ladner U. Treatment of active psoriatic arthritis with the PPARgamma ligand pioglitazone: an open-label pilot study. Rheumatology (Oxford) 2005;44:126–129. doi: 10.1093/rheumatology/keh423. [DOI] [PubMed] [Google Scholar]
- 106.Hirose H., Kawai T., Yamamoto Y., Taniyama M., Tomita M., Matsubara K. Effects of pioglitazone on metabolic parameters, body fat distribution, and serum adiponectin levels in Japanese male patients with type 2 diabetes. Metab: Clin Exp. 2002;51:314–317. doi: 10.1053/meta.2002.30506. [DOI] [PubMed] [Google Scholar]
- 107.Balakumar P., Kathuria S. Submaximal PPARgamma activation and endothelial dysfunction: new perspectives for the management of cardiovascular disorders. Brit J Pharmacol. 2012;166:1981–1992. doi: 10.1111/j.1476-5381.2012.01938.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Schupp M., Clemenz M., Gineste R., Witt H., Janke J., Helleboid S. Molecular characterization of new selective peroxisome proliferator-activated receptor gamma modulators with angiotensin receptor blocking activity. Diabetes. 2005;54:3442–3452. doi: 10.2337/diabetes.54.12.3442. [DOI] [PubMed] [Google Scholar]
- 109.Higgins L.S., Depaoli A.M. Selective peroxisome proliferator-activated receptor gamma (PPARgamma) modulation as a strategy for safer therapeutic PPARgamma activation. Am J Clin Nut. 2010;91:72S–267S. doi: 10.3945/ajcn.2009.28449E. [DOI] [PubMed] [Google Scholar]
- 110.Bhalla K., Hwang B.J., Choi J.H., Dewi R., Ou L., McLenithan J. N-Acetylfarnesylcysteine is a novel class of peroxisome proliferator-activated receptor gamma ligand with partial and full agonist activity in vitro and in vivo. J Biol Chem. 2011;286:41626–41635. doi: 10.1074/jbc.M111.257915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Heald M., Cawthorne M.A. Dual acting and pan-PPAR activators as potential anti-diabetic therapies. Handb Exp Pharmacol. 2011:35–51. doi: 10.1007/978-3-642-17214-4_2. [DOI] [PubMed] [Google Scholar]
- 112.Tenenbaum A., Motro M., Fisman E.Z. Dual and pan-peroxisome proliferator-activated receptors (PPAR) co-agonism: the bezafibrate lessons. Cardiovasc Diabetol. 2005;4:14. doi: 10.1186/1475-2840-4-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Tenenbaum A., Fisman E.Z. Balanced pan-PPAR activator bezafibrate in combination with statin: comprehensive lipids control and diabetes prevention? Cardiovasc Diabetol. 2012;11:140. doi: 10.1186/1475-2840-11-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Knouff C., Auwerx J. Peroxisome proliferator-activated receptor-gamma calls for activation in moderation: lessons from genetics and pharmacology. Endocr Rev. 2004;25:899–918. doi: 10.1210/er.2003-0036. [DOI] [PubMed] [Google Scholar]
- 115.Beutler J.A. Natural products as a foundation for drug discovery. Curr Protoc Pharmacol. 2009 doi: 10.1002/0471141755.ph0911s46. Chapter 9:Unit 9 11. [DOI] [PubMed] [Google Scholar]
- 116.Cragg G.M., Newman D.J. Natural products: a continuing source of novel drug leads. Biochim Biophys Acta. 2013;1830:3670–3695. doi: 10.1016/j.bbagen.2013.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Henrich C.J., Beutler J.A. Matching the power of high throughput screening to the chemical diversity of natural products. Nat Prod Rep. 2013;30:1284–1298. doi: 10.1039/c3np70052f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Clardy J., Walsh C. Lessons from natural molecules. Nature. 2004;432:829–837. doi: 10.1038/nature03194. [DOI] [PubMed] [Google Scholar]
- 119.Ertl P., Roggo S., Schuffenhauer A. Natural product-likeness score and its application for prioritization of compound libraries. J Chem Inf Model. 2008;48:68–74. doi: 10.1021/ci700286x. [DOI] [PubMed] [Google Scholar]
- 120.Farnsworth N.R., Akerele O., Bingel A.S., Soejarto D.D., Guo Z. Medicinal plants in therapy. Bull World Health Organ. 1985;63:965–981. [PMC free article] [PubMed] [Google Scholar]
- 121.Mueller M., Jungbauer A. Red clover extract: a putative source for simultaneous treatment of menopausal disorders and the metabolic syndrome. Menopause. 2008;15:1120–1131. doi: 10.1097/gme.0b013e31817062ce. [DOI] [PubMed] [Google Scholar]
- 122.Vogl S., Picker P., Mihaly-Bison J., Fakhrudin N., Atanasov A.G., Heiss E.H. Ethnopharmacological in vitro studies on Austria's folk medicine--an unexplored lore in vitro anti-inflammatory activities of 71 Austrian traditional herbal drugs. J Ethnopharmacol. 2013;149:750–771. doi: 10.1016/j.jep.2013.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Local Food-Nutraceuticals Consortium Understanding local Mediterranean diets: a multidisciplinary pharmacological and ethnobotanical approach. Pharmacol Res. 2005;52:353–366. doi: 10.1016/j.phrs.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 124.Christensen K.B., Minet A., Svenstrup H., Grevsen K., Zhang H., Schrader E. Identification of plant extracts with potential antidiabetic properties: effect on human peroxisome proliferator-activated receptor (PPAR), adipocyte differentiation and insulin-stimulated glucose uptake. Phytother Res. 2009;23:1316–1325. doi: 10.1002/ptr.2782. [DOI] [PubMed] [Google Scholar]
- 125.Yang M.H., Avula B., Smillie T., Khan I.A., Khan S.I. Screening of medicinal plants for PPARalpha and PPARgamma activation and evaluation of their effects on glucose uptake and 3T3-L1 adipogenesis. Planta Medica. 2013;79:1084–1095. doi: 10.1055/s-0033-1350620. [DOI] [PubMed] [Google Scholar]
- 126.Benhaddou-Andaloussi A., Martineau L.C., Vallerand D., Haddad Y., Afshar A., Settaf A. Multiple molecular targets underlie the antidiabetic effect of Nigella sativa seed extract in skeletal muscle, adipocyte and liver cells. Diabetes Obes Metab. 2010;12:148–157. doi: 10.1111/j.1463-1326.2009.01131.x. [DOI] [PubMed] [Google Scholar]
- 127.Mueller M., Jungbauer A. Culinary plants, herbs and spices–A rich source of PPARγ ligands. Food Chem. 2009;117:660–667. [Google Scholar]
- 128.Xie L.W., Atanasov A.G., Guo D.A., Malainer C., Zhang J.X., Zehl M. Activity-guided isolation of NF-kappaB inhibitors and PPARgamma agonists from the root bark of Lycium chinense Miller. J Ethnopharmacol. 2014;152:470–477. doi: 10.1016/j.jep.2014.01.029. [DOI] [PubMed] [Google Scholar]
- 129.Vogl S., Atanasov A.G., Binder M., Bulusu M., Zehl M., Fakhrudin N. The Herbal Drug Melampyrum pratense L. (Koch): Isolation and Identification of Its Bioactive Compounds Targeting Mediators of Inflammation. Evid Based Complement Alternat Med. 2013;2013:395316. doi: 10.1155/2013/395316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Rozema E., Atanasov A.G., Fakhrudin N., Singhuber J., Namduang U., Heiss E.H. Selected Extracts of Chinese Herbal Medicines: Their Effect on NF-kappaB, PPARalpha and PPARgamma and the Respective Bioactive Compounds. Evid Based Complement Alternat Med. 2012;2012:983023. doi: 10.1155/2012/983023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Petersen R.K., Christensen K.B., Assimopoulou A.N., Frette X., Papageorgiou V.P., Kristiansen K. Pharmacophore-driven identification of PPARgamma agonists from natural sources. J Comput-Aid Mol Des. 2011;25:107–116. doi: 10.1007/s10822-010-9398-5. [DOI] [PubMed] [Google Scholar]
- 132.Christensen K.B., Jorgensen M., Kotowska D., Petersen R.K., Kristiansen K., Christensen L.P. Activation of the nuclear receptor PPARgamma by metabolites isolated from sage (Salvia officinalis L.) J Ethnopharmacol. 2010;132:127–133. doi: 10.1016/j.jep.2010.07.054. [DOI] [PubMed] [Google Scholar]
- 133.Christensen K.B., Petersen R.K., Kristiansen K., Christensen L.P. Identification of bioactive compounds from flowers of black elder (Sambucus nigra L.) that activate the human peroxisome proliferator-activated receptor (PPAR) gamma. Phytother Res. 2010;24(Suppl 2):S129–S132. doi: 10.1002/ptr.3005. [DOI] [PubMed] [Google Scholar]
- 134.Fang X.K., Gao J., Zhu D.N. Kaempferol and quercetin isolated from Euonymus alatus improve glucose uptake of 3T3-L1 cells without adipogenesis activity. Life Sci. 2008;82:615–622. doi: 10.1016/j.lfs.2007.12.021. [DOI] [PubMed] [Google Scholar]
- 135.Dang Z.C., Audinot V., Papapoulos S.E., Boutin J.A., Lowik C.W. Peroxisome proliferator-activated receptor gamma (PPARgamma) as a molecular target for the soy phytoestrogen genistein. J Biol Chem. 2003;278:962–967. doi: 10.1074/jbc.M209483200. [DOI] [PubMed] [Google Scholar]
- 136.Kuroda M., Mimaki Y., Sashida Y., Mae T., Kishida H., Nishiyama T. Phenolics with PPAR-gamma ligand-binding activity obtained from licorice (Glycyrrhiza uralensis roots) and ameliorative effects of glycyrin on genetically diabetic KK-A(y) mice. Bioorg Med Chem Lett. 2003;13:4267–4272. doi: 10.1016/j.bmcl.2003.09.052. [DOI] [PubMed] [Google Scholar]
- 137.Mueller M., Lukas B., Novak J., Simoncini T., Genazzani A.R., Jungbauer A. Oregano: a source for peroxisome proliferator-activated receptor gamma antagonists. J Agr Food Chem. 2008;56:11621–11630. doi: 10.1021/jf802298w. [DOI] [PubMed] [Google Scholar]
- 138.Shen P., Liu M.H., Ng T.Y., Chan Y.H., Yong E.L. Differential effects of isoflavones, from Astragalus membranaceus and Pueraria thomsonii, on the activation of PPARalpha, PPARgamma, and adipocyte differentiation in vitro. J Nutr. 2006;136:899–905. doi: 10.1093/jn/136.4.899. [DOI] [PubMed] [Google Scholar]
- 139.Rupp M., Schroeter T., Steri R., Zettl H., Proschak E., Hansen K. From machine learning to natural product derivatives that selectively activate transcription factor PPARgamma. ChemMedChem. 2010;5:191–194. doi: 10.1002/cmdc.200900469. [DOI] [PubMed] [Google Scholar]
- 140.Fakhrudin N., Ladurner A., Atanasov A.G., Heiss E.H., Baumgartner L., Markt P. Computer-aided discovery, validation, and mechanistic characterization of novel neolignan activators of peroxisome proliferator-activated receptor gamma. Mol Pharmacol. 2010;77:559–566. doi: 10.1124/mol.109.062141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Lewis S.N., Brannan L., Guri A.J., Lu P., Hontecillas R., Bassaganya-Riera J. Dietary alpha-eleostearic acid ameliorates experimental inflammatory bowel disease in mice by activating peroxisome proliferator-activated receptor-gamma. PLoS One. 2011;6:e24031. doi: 10.1371/journal.pone.0024031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Salam N.K., Huang T.H., Kota B.P., Kim M.S., Li Y., Hibbs D.E. Novel PPAR-gamma agonists identified from a natural product library: a virtual screening, induced-fit docking and biological assay study. Chem Biol Drug Des. 2008;71:57–70. doi: 10.1111/j.1747-0285.2007.00606.x. [DOI] [PubMed] [Google Scholar]
- 143.Tanrikulu Y., Rau O., Schwarz O., Proschak E., Siems K., Muller-Kuhrt L. Structure-based pharmacophore screening for natural-product-derived PPARgamma agonists. Chembiochem. 2009;10:75–78. doi: 10.1002/cbic.200800520. [DOI] [PubMed] [Google Scholar]
- 144.Waku T., Shiraki T., Oyama T., Fujimoto Y., Maebara K., Kamiya N. Structural insight into PPARgamma activation through covalent modification with endogenous fatty acids. J Mol Biol. 2009;385:188–199. doi: 10.1016/j.jmb.2008.10.039. [DOI] [PubMed] [Google Scholar]
- 145.Kota B.P., Huang T.H., Roufogalis B.D. An overview on biological mechanisms of PPARs. Pharmacol Res. 2005;51:85–94. doi: 10.1016/j.phrs.2004.07.012. [DOI] [PubMed] [Google Scholar]
- 146.Marx N., Duez H., Fruchart J.C., Staels B. Peroxisome proliferator-activated receptors and atherogenesis: regulators of gene expression in vascular cells. Circ Res. 2004;94:1168–1178. doi: 10.1161/01.RES.0000127122.22685.0A. [DOI] [PubMed] [Google Scholar]
- 147.Willson T.M., Wahli W. Peroxisome proliferator-activated receptor agonists. Curr Opin Chem Biol. 1997;1:235–241. doi: 10.1016/s1367-5931(97)80015-4. [DOI] [PubMed] [Google Scholar]
- 148.Touyz R.M., Schiffrin E.L. Peroxisome proliferator-activated receptors in vascular biology-molecular mechanisms and clinical implications. Vasc Pharmacol. 2006;45:19–28. doi: 10.1016/j.vph.2005.11.014. [DOI] [PubMed] [Google Scholar]
- 149.Grygiel-Gorniak B. Peroxisome proliferator-activated receptors and their ligands: nutritional and clinical implications--a review. Nutr J. 2014;13:17. doi: 10.1186/1475-2891-13-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Michalik L., Auwerx J., Berger J.P., Chatterjee V.K., Glass C.K., Gonzalez F.J. International Union of Pharmacology. LXI. Peroxisome proliferator-activated receptors. Pharmacol Rev. 2006;58:726–741. doi: 10.1124/pr.58.4.5. [DOI] [PubMed] [Google Scholar]
- 151.Bensinger S.J., Tontonoz P. Integration of metabolism and inflammation by lipid-activated nuclear receptors. Nature. 2008;454:470–477. doi: 10.1038/nature07202. [DOI] [PubMed] [Google Scholar]
- 152.Doshi L.S., Brahma M.K., Bahirat U.A., Dixit A.V., Nemmani K.V. Discovery and development of selective PPAR gamma modulators as safe and effective antidiabetic agents. Exp Opin Invest Drugs. 2010;19:489–512. doi: 10.1517/13543781003640169. [DOI] [PubMed] [Google Scholar]
- 153.Shearer B.G., Billin A.N. The next generation of PPAR drugs: do we have the tools to find them. Biochim Biophys Acta. 2007;1771:1082–1093. doi: 10.1016/j.bbalip.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 154.Zhang D., Nie X., Pan H., Yu L., Yang X., Xu J. [Study on effect of total saponins from Semen Nigellae on inflammatory mediators and ERK/MAPK pathway in stimulated macrophage] Zhongguo Zhong Yao Za Zhi. 2010;35:2594–2598. [PubMed] [Google Scholar]
- 155.Lee Y.K., Lee W.S., Hwang J.T., Kwon D.Y., Surh Y.J., Park O.J. Curcumin exerts antidifferentiation effect through AMPKalpha-PPAR-gamma in 3T3-L1 adipocytes and antiproliferatory effect through AMPKalpha-COX-2 in cancer cells. J Agr Food Chem. 2009;57:305–310. doi: 10.1021/jf802737z. [DOI] [PubMed] [Google Scholar]
- 156.Saito T., Abe D., Sekiya K. Sakuranetin induces adipogenesis of 3T3-L1 cells through enhanced expression of PPARgamma2. Biochem Biophys Res Commun. 2008;372:835–839. doi: 10.1016/j.bbrc.2008.05.146. [DOI] [PubMed] [Google Scholar]
- 157.Huang T.H., Peng G., Kota B.P., Li G.Q., Yamahara J., Roufogalis B.D. Anti-diabetic action of Punica granatum flower extract: activation of PPAR-gamma and identification of an active component. Toxicol Appl Pharmacol. 2005;207:160–169. doi: 10.1016/j.taap.2004.12.009. [DOI] [PubMed] [Google Scholar]
- 158.Li Y.T., Li L., Chen J., Hu T.C., Huang J., Guo Y.W. 7-Chloroarctinone-b as a new selective PPARgamma antagonist potently blocks adipocyte differentiation. Acta Pharmacol Sin. 2009;30:1351–1358. doi: 10.1038/aps.2009.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Hwang J.T., Lee M.S., Kim H.J., Sung M.J., Kim H.Y., Kim M.S. Antiobesity effect of ginsenoside Rg3 involves the AMPK and PPAR-gamma signal pathways. Phytother Res. 2009;23:262–266. doi: 10.1002/ptr.2606. [DOI] [PubMed] [Google Scholar]
- 160.Lee J., Jung E., Kim S., Huh S., Kim Y., Byun S.Y. Isorhamnetin represses adipogenesis in 3T3-L1 cells. Obesity (Silver Spring) 2009;17:226–232. doi: 10.1038/oby.2008.472. [DOI] [PubMed] [Google Scholar]
- 161.Gong Z., Huang C., Sheng X., Zhang Y., Li Q., Wang M.W. The role of tanshinone IIA in the treatment of obesity through peroxisome proliferator-activated receptor gamma antagonism. Endocrinology. 2009;150:104–113. doi: 10.1210/en.2008-0322. [DOI] [PubMed] [Google Scholar]
- 162.Jacob A., Wu R., Zhou M., Wang P. Mechanism of the anti-inflammatory effect of Curcumin: PPAR-gamma Activation. PPAR Res. 2007;2007:89369. doi: 10.1155/2007/89369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Siddiqui A.M., Cui X., Wu R., Dong W., Zhou M., Hu M. The anti-inflammatory effect of curcumin in an experimental model of sepsis is mediated by up-regulation of peroxisome proliferator-activated receptor-gamma. Crit Care Med. 2006;34:1874–1882. doi: 10.1097/01.CCM.0000221921.71300.BF. [DOI] [PubMed] [Google Scholar]
- 164.Takahashi N., Kawada T., Goto T., Yamamoto T., Taimatsu A., Matsui N. Dual action of isoprenols from herbal medicines on both PPARgamma and PPARalpha in 3T3-L1 adipocytes and HepG2 hepatocytes. FEBS Lett. 2002;514:315–322. doi: 10.1016/s0014-5793(02)02390-6. [DOI] [PubMed] [Google Scholar]
- 165.Chung S.W., Kim M.K., Chung J.H., Kim D.H., Choi J.S., Anton S. Peroxisome proliferator-activated receptor activation by a short-term feeding of zingerone in aged rats. J Med Food. 2009;12:345–350. doi: 10.1089/jmf.2007.0660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Park J.Y., Kawada T., Han I.S., Kim B.S., Goto T., Takahashi N. Capsaicin inhibits the production of tumor necrosis factor alpha by LPS-stimulated murine macrophages, RAW 264.7: a PPARgamma ligand-like action as a novel mechanism. FEBS Lett. 2004;572:266–270. doi: 10.1016/j.febslet.2004.06.084. [DOI] [PubMed] [Google Scholar]
- 167.Zhang M., Deng C., Zheng J., Xia J., Sheng D. Curcumin inhibits trinitrobenzene sulphonic acid-induced colitis in rats by activation of peroxisome proliferator-activated receptor gamma. Int Immunopharmacol. 2006;6:1233–1242. doi: 10.1016/j.intimp.2006.02.013. [DOI] [PubMed] [Google Scholar]
- 168.Dong S.Z., Zhao S.P., Wu Z.H., Yang J., Xie X.Z., Yu B.L. Curcumin promotes cholesterol efflux from adipocytes related to PPARgamma-LXRalpha-ABCA1 passway. Mol Cell Biochem. 2011;358:281–285. doi: 10.1007/s11010-011-0978-z. [DOI] [PubMed] [Google Scholar]
- 169.Kuroyanagi K., Kang M.S., Goto T., Hirai S., Ohyama K., Kusudo T. Citrus auraptene acts as an agonist for PPARs and enhances adiponectin production and MCP-1 reduction in 3T3-L1 adipocytes. Biochem Biophys Res Commun. 2008;366:219–225. doi: 10.1016/j.bbrc.2007.11.119. [DOI] [PubMed] [Google Scholar]
- 170.O'Sullivan S.E., Tarling E.J., Bennett A.J., Kendall D.A., Randall M.D. Novel time-dependent vascular actions of Delta9-tetrahydrocannabinol mediated by peroxisome proliferator-activated receptor gamma. Biochem Biophys Res Commun. 2005;337:824–831. doi: 10.1016/j.bbrc.2005.09.121. [DOI] [PubMed] [Google Scholar]
- 171.Waki H., Park K.W., Mitro N., Pei L., Damoiseaux R., Wilpitz D.C. The small molecule harmine is an antidiabetic cell-type-specific regulator of PPARgamma expression. Cell Metab. 2007;5:357–370. doi: 10.1016/j.cmet.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 172.Lee I.K., Lee J.H., Yun B.S. Polychlorinated compounds with PPAR-gamma agonistic effect from the medicinal fungus Phellinus ribis. Bioorg Med Chem Lett. 2008;18:4566–4568. doi: 10.1016/j.bmcl.2008.07.034. [DOI] [PubMed] [Google Scholar]
- 173.Mora F.D., Jones D.K., Desai P.V., Patny A., Avery M.A., Feller D.R. Bioassay for the identification of natural product-based activators of peroxisome proliferator-activated receptor-gamma (PPARgamma): the marine sponge metabolite psammaplin A activates PPARgamma and induces apoptosis in human breast tumor cells. J Nat Prod. 2006;69:547–552. doi: 10.1021/np050397q. [DOI] [PubMed] [Google Scholar]
- 174.Zoete V., Grosdidier A., Michielin O. Peroxisome proliferator-activated receptor structures: ligand specificity, molecular switch and interactions with regulators. Biochim Biophys Acta. 2007;1771:915–925. doi: 10.1016/j.bbalip.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 175.Atanasov A.G., Wang J.N., Gu S.P., Bu J., Kramer M.P., Baumgartner L. Honokiol: a non-adipogenic PPARgamma agonist from nature. Biochim Biophys Acta. 2013;1830:4813–4819. doi: 10.1016/j.bbagen.2013.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Sohn E.J., Kim C.S., Kim Y.S., Jung D.H., Jang D.S., Lee Y.M. Effects of magnolol (5,5’-diallyl-2,2’-dihydroxybiphenyl) on diabetic nephropathy in type 2 diabetic Goto-Kakizaki rats. Life Sci. 2007;80:468–475. doi: 10.1016/j.lfs.2006.09.037. [DOI] [PubMed] [Google Scholar]
- 177.Beaudoin M.S., Snook L.A., Arkell A.M., Simpson J.A., Holloway G.P., Wright D.C. Resveratrol supplementation improves white adipose tissue function in a depot-specific manner in Zucker diabetic fatty rats. Am J Physiol Reg I. 2013;305:R542–R551. doi: 10.1152/ajpregu.00200.2013. [DOI] [PubMed] [Google Scholar]
- 178.Alberdi G., Rodriguez V.M., Miranda J., Macarulla M.T., Arias N., Andres-Lacueva C. Changes in white adipose tissue metabolism induced by resveratrol in rats. Nutr Metab. 2011;8:29. doi: 10.1186/1743-7075-8-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Chuang C.C., Martinez K., Xie G., Kennedy A., Bumrungpert A., Overman A. Quercetin is equally or more effective than resveratrol in attenuating tumor necrosis factor-{alpha}-mediated inflammation and insulin resistance in primary human adipocytes. Am J Clin Nutr. 2010;92:1511–1521. doi: 10.3945/ajcn.2010.29807. [DOI] [PubMed] [Google Scholar]
- 180.Floyd Z.E., Wang Z.Q., Kilroy G., Cefalu W.T. Modulation of peroxisome proliferator-activated receptor gamma stability and transcriptional activity in adipocytes by resveratrol. Metab: Clin Exp. 2008;57:S32–S38. doi: 10.1016/j.metabol.2008.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Dong W., Wang X., Bi S., Pan Z., Liu S., Yu H. Inhibitory effects of resveratrol on foam cell formation are mediated through monocyte chemotactic protein-1 and lipid metabolism-related proteins. Int J Mol Med. 2014;33:1161–1168. doi: 10.3892/ijmm.2014.1680. [DOI] [PubMed] [Google Scholar]
- 182.Voloshyna I., Hai O., Littlefield M.J., Carsons S., Reiss A.B. Resveratrol mediates anti-atherogenic effects on cholesterol flux in human macrophages and endothelium via PPARgamma and adenosine. Eur J Pharmacol. 2013;698:299–309. doi: 10.1016/j.ejphar.2012.08.024. [DOI] [PubMed] [Google Scholar]
- 183.Zhang Y., Luo Z., Ma L., Xu Q., Yang Q., Si L. Resveratrol prevents the impairment of advanced glycosylation end products (AGE) on macrophage lipid homeostasis by suppressing the receptor for AGE via peroxisome proliferator-activated receptor gamma activation. Int J Mol Med. 2010;25:729–734. doi: 10.3892/ijmm_00000398. [DOI] [PubMed] [Google Scholar]
- 184.Kennedy A., Overman A., Lapoint K., Hopkins R., West T., Chuang C.C. Conjugated linoleic acid-mediated inflammation and insulin resistance in human adipocytes are attenuated by resveratrol. J Lipid Res. 2009;50:225–232. doi: 10.1194/jlr.M800258-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Ge H., Zhang J.F., Guo B.S., He Q., Wang B.Y., He B. Resveratrol inhibits macrophage expression of EMMPRIN by activating PPARgamma. Vasc Pharmacol. 2007;46:114–121. doi: 10.1016/j.vph.2006.08.412. [DOI] [PubMed] [Google Scholar]
- 186.Brasnyo P., Molnar G.A., Mohas M., Marko L., Laczy B., Cseh J. Resveratrol improves insulin sensitivity, reduces oxidative stress and activates the Akt pathway in type 2 diabetic patients. Brit J Nutr. 2011;106:383–389. doi: 10.1017/S0007114511000316. [DOI] [PubMed] [Google Scholar]
- 187.Weidner C., de Groot J.C., Prasad A., Freiwald A., Quedenau C., Kliem M. Amorfrutins are potent antidiabetic dietary natural products. Proc Natl Acad Sci USA. 2012;109:7257–7262. doi: 10.1073/pnas.1116971109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Weidner C., Wowro S.J., Freiwald A., Kawamoto K., Witzke A., Kliem M. Amorfrutin B is an efficient natural peroxisome proliferator-activated receptor gamma (PPARgamma) agonist with potent glucose-lowering properties. Diabetologia. 2013;56:1802–1812. doi: 10.1007/s00125-013-2920-2. [DOI] [PubMed] [Google Scholar]
- 189.Lee W., Ham J., Kwon H.C., Kim Y.K., Kim S.N. Anti-diabetic effect of amorphastilbol through PPARalpha/gamma dual activation in db/db mice. Biochem Biophys Res Commun. 2013;432:73–79. doi: 10.1016/j.bbrc.2013.01.083. [DOI] [PubMed] [Google Scholar]
- 190.Kotani H., Tanabe H., Mizukami H., Makishima M., Inoue M. Identification of a naturally occurring rexinoid, honokiol, that activates the retinoid X receptor. J Nat Prod. 2010;73:1332–1336. doi: 10.1021/np100120c. [DOI] [PubMed] [Google Scholar]
- 191.Kotani H., Tanabe H., Mizukami H., Amagaya S., Inoue M. A naturally occurring rexinoid, honokiol, can serve as a regulator of various retinoid x receptor heterodimers. Biol Pharmaceut Bull. 2012;35:1–9. doi: 10.1248/bpb.35.1. [DOI] [PubMed] [Google Scholar]
- 192.Jung C.G., Horike H., Cha B.Y., Uhm K.O., Yamauchi R., Yamaguchi T. Honokiol increases ABCA1 expression level by activating retinoid X receptor beta. Biol Pharmaceut Bull. 2010;33:1105–1111. doi: 10.1248/bpb.33.1105. [DOI] [PubMed] [Google Scholar]
- 193.Zhang H., Xu X., Chen L., Chen J., Hu L., Jiang H. Molecular determinants of magnolol targeting both RXRalpha and PPARgamma. PLoS One. 2011;6:e28253. doi: 10.1371/journal.pone.0028253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Choi S.S., Cha B.Y., Lee Y.S., Yonezawa T., Teruya T., Nagai K. Magnolol enhances adipocyte differentiation and glucose uptake in 3T3-L1 cells. Life Sci. 2009;84:908–914. doi: 10.1016/j.lfs.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 195.Puhl A.C., Bernardes A., Silveira R.L., Yuan J., Campos J.L., Saidemberg D.M. Mode of peroxisome proliferator-activated receptor gamma activation by luteolin. Mol Pharmacol. 2012;81:788–799. doi: 10.1124/mol.111.076216. [DOI] [PubMed] [Google Scholar]
- 196.Nolte R.T., Wisely G.B., Westin S., Cobb J.E., Lambert M.H., Kurokawa R. Ligand binding and co-activator assembly of the peroxisome proliferator-activated receptor-gamma. Nature. 1998;395:137–143. doi: 10.1038/25931. [DOI] [PubMed] [Google Scholar]
- 197.de Groot J.C., Weidner C., Krausze J., Kawamoto K., Schroeder F.C., Sauer S. Structural characterization of amorfrutins bound to the peroxisome proliferator-activated receptor gamma. J Med Chem. 2013;56:1535–1543. doi: 10.1021/jm3013272. [DOI] [PubMed] [Google Scholar]
- 198.Guasch L., Sala E., Valls C., Blay M., Mulero M., Arola L. Structural insights for the design of new PPARgamma partial agonists with high binding affinity and low transactivation activity. J Comput-Aid Mol Des. 2011;25:717–728. doi: 10.1007/s10822-011-9446-9. [DOI] [PubMed] [Google Scholar]
- 199.Liberato M.V., Nascimento A.S., Ayers S.D., Lin J.Z., Cvoro A., Silveira R.L. Medium chain fatty acids are selective peroxisome proliferator activated receptor (PPAR) gamma activators and pan-PPAR partial agonists. PLoS One. 2012;7:e36297. doi: 10.1371/journal.pone.0036297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Berman H.M., Westbrook J., Feng Z., Gilliland G., Bhat T.N., Weissig H. The Protein Data Bank. Nucleic Acids Res. 2000;28:235–242. doi: 10.1093/nar/28.1.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Cho J.Y., Kim P.S., Park J., Yoo E.S., Baik K.U., Kim Y.K. Inhibitor of tumor necrosis factor-alpha production in lipopolysaccharide-stimulated RAW264.7 cells from Amorpha fruticosa. J Ethnopharmacol. 2000;70:127–133. doi: 10.1016/s0378-8741(99)00154-3. [DOI] [PubMed] [Google Scholar]
- 202.Gao Xuemin . Foreign Languages Press; 2006. Applied illustrated compendium of materia medica (Chinese-English edition) [Google Scholar]
- 203.Khare C.P. Springer; 2007. Indian Medicinal Plants: An Illustrated Dictionary. [Google Scholar]
- 204.Takahashi N., Goto T., Taimatsu A., Egawa K., Katoh S., Kusudo T. Bixin regulates mRNA expression involved in adipogenesis and enhances insulin sensitivity in 3T3-L1 adipocytes through PPARgamma activation. Biochem Biophys Res Commun. 2009;390:1372–1376. doi: 10.1016/j.bbrc.2009.10.162. [DOI] [PubMed] [Google Scholar]
- 205.Shin D.W., Kim S.N., Lee S.M., Lee W., Song M.J., Park S.M. (-)-Catechin promotes adipocyte differentiation in human bone marrow mesenchymal stem cells through PPAR gamma transactivation. Biochem Pharmacol. 2009;77:125–133. doi: 10.1016/j.bcp.2008.09.033. [DOI] [PubMed] [Google Scholar]
- 206.Inta A., Trisonthi P., Trisonthi C. Analysis of traditional knowledge in medicinal plants used by Yuan in Thailand. J Ethnopharmacol. 2013;149:344–351. doi: 10.1016/j.jep.2013.06.047. [DOI] [PubMed] [Google Scholar]
- 207.Dat N.T., Lee K., Hong Y.S., Kim Y.H., Minh C.V., Lee J.J. A peroxisome proliferator-activated receptor-gamma agonist and other constituents from Chromolaena odorata. Planta Medica. 2009;75:803–807. doi: 10.1055/s-0029-1185386. [DOI] [PubMed] [Google Scholar]
- 208.Zhang M.L., Irwin D., Li X.N., Sauriol F., Shi X.W., Wang Y.F. PPARgamma agonist from Chromolaena odorata. J Nat Prod. 2012;75:2076–2081. doi: 10.1021/np300386d. [DOI] [PubMed] [Google Scholar]
- 209.Yokoi H., Mizukami H., Nagatsu A., Ohno T., Tanabe H., Inoue M. Peroxisome proliferator-activated receptor gamma ligands isolated from adlay seed (Coix lacryma-jobi L. var. ma-yuen STAPF.) Biol Pharmaceut Bull. 2009;32:735–740. doi: 10.1248/bpb.32.735. [DOI] [PubMed] [Google Scholar]
- 210.Cornick C.L., Strongitharm B.H., Sassano G., Rawlins C., Mayes A.E., Joseph A.N. Identification of a novel agonist of peroxisome proliferator-activated receptors alpha and gamma that may contribute to the anti-diabetic activity of guggulipid in Lep(ob)/Lep(ob) mice. J Nutr Biochem. 2009;20:806–815. doi: 10.1016/j.jnutbio.2008.07.010. [DOI] [PubMed] [Google Scholar]
- 211.He Y.Q., Ma G.Y., Peng J.N., Ma Z.Y., Hamann M.T. Liver X receptor and peroxisome proliferator-activated receptor agonist from Cornus alternifolia. Biochim Biophys Acta. 2012;1820:1021–1026. doi: 10.1016/j.bbagen.2012.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Vareed S.K., Reddy M.K., Schutzki R.E., Nair M.G. Anthocyanins in Cornus alternifolia, Cornus controversa, Cornus kousa and Cornus florida fruits with health benefits. Life Sci. 2006;78:777–784. doi: 10.1016/j.lfs.2005.05.094. [DOI] [PubMed] [Google Scholar]
- 213.Katsukawa M., Nakata R., Takizawa Y., Hori K., Takahashi S., Inoue H. Citral, a component of lemongrass oil, activates PPARalpha and gamma and suppresses COX-2 expression. Biochim Biophys Acta. 2010;1801:1214–1220. doi: 10.1016/j.bbalip.2010.07.004. [DOI] [PubMed] [Google Scholar]
- 214.Hostettmann K. [History of a plant: the example of Echinacea] Forsch Komplementarmed Klass Naturheilkd. 2003;10(Suppl 1):9–12. doi: 10.1159/000071678. [DOI] [PubMed] [Google Scholar]
- 215.Christensen K.B., Petersen R.K., Petersen S., Kristiansen K., Christensen L.P. Activation of PPARgamma by metabolites from the flowers of purple coneflower (Echinacea purpurea) J Nat Prod. 2009;72:933–937. doi: 10.1021/np900003a. [DOI] [PubMed] [Google Scholar]
- 216.Sasidharan S., Nilawatyi R., Xavier R., Latha L.Y., Amala R. Wound healing potential of Elaeis guineensis Jacq leaves in an infected albino rat model. Molecules. 2010;15:3186–3199. doi: 10.3390/molecules15053186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Fang F., Kang Z., Wong C. Vitamin E tocotrienols improve insulin sensitivity through activating peroxisome proliferator-activated receptors. Mol Nutr Food Res. 2010;54:345–352. doi: 10.1002/mnfr.200900119. [DOI] [PubMed] [Google Scholar]
- 218.Zou G., Gao Z., Wang J., Zhang Y., Ding H., Huang J. Deoxyelephantopin inhibits cancer cell proliferation and functions as a selective partial agonist against PPARgamma. Biochem Pharmacol. 2008;75:1381–1392. doi: 10.1016/j.bcp.2007.11.021. [DOI] [PubMed] [Google Scholar]
- 219.Tantry M.A., Dar J.A., Idris A., Akbar S., Shawl A.S. Acylated flavonol glycosides from Epimedium elatum, a plant endemic to the Western Himalayas. Fitoterapia. 2012;83:665–670. doi: 10.1016/j.fitote.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 220.Kuroda M., Mimaki Y., Honda S., Tanaka H., Yokota S., Mae T. Phenolics from Glycyrrhiza glabra roots and their PPAR-gamma ligand-binding activity. Bioorg Med Chem. 2010;18:962–970. doi: 10.1016/j.bmc.2009.11.027. [DOI] [PubMed] [Google Scholar]
- 221.Ouarghidi A., Martin G.J., Powell B., Esser G., Abbad A. Botanical identification of medicinal roots collected and traded in Morocco and comparison to the existing literature. J Ethnobiol Ethnomed. 2013;9:59. doi: 10.1186/1746-4269-9-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Park H.G., Bak E.J., Woo G.H., Kim J.M., Quan Z., Yoon H.K. Licochalcone E has an antidiabetic effect. J Nutr Biochem. 2012;23:759–767. doi: 10.1016/j.jnutbio.2011.03.021. [DOI] [PubMed] [Google Scholar]
- 223.Sauvan N, Renimel I, Lamy C, Dupont D. Patent US 7527813 B2: Cosmetic composition containing an extract of Limnocitrus littoralis. Google Patents, 2009.
- 224.Do Q.T., Lamy C., Renimel I., Sauvan N., Andre P., Himbert F. Reverse pharmacognosy: identifying biological properties for plants by means of their molecule constituents: application to meranzin. Planta Medica. 2007;73:1235–1240. doi: 10.1055/s-2007-990216. [DOI] [PubMed] [Google Scholar]
- 225.Committee of National Pharmacopoeia . Chemical Industry Press; Beijing: 2010. China Pharmacopoeia (Part 1) [Google Scholar]
- 226.Nhiem N.X., Yen P.H., Ngan N.T., Quang T.H., Kiem P.V., Minh C.V. Inhibition of nuclear transcription factor-kappaB and activation of peroxisome proliferator-activated receptors in HepG2 cells by cucurbitane-type triterpene glycosides from Momordica charantia. J Med Food. 2012;15:369–377. doi: 10.1089/jmf.2011.1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Liu X., Jiang S., Xu K., Sun H., Zhou Y., Xu X. Quantitative analysis of chemical constituents in different commercial parts of Notopterygium incisum by HPLC-DAD-MS. J Ethnopharmacol. 2009;126:474–479. doi: 10.1016/j.jep.2009.09.011. [DOI] [PubMed] [Google Scholar]
- 228.Atanasov A.G., Blunder M., Fakhrudin N., Liu X., Noha S.M., Malainer C. Polyacetylenes from Notopterygium incisum--new selective partial agonists of peroxisome proliferator-activated receptor-gamma. PLoS One. 2013;8:e61755. doi: 10.1371/journal.pone.0061755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Han K.L., Jung M.H., Sohn J.H., Hwang J.K. Ginsenoside 20S-protopanaxatriol (PPT) activates peroxisome proliferator-activated receptor gamma (PPARgamma) in 3T3-L1 adipocytes. Biol Pharmaceut Bull. 2006;29:110–113. doi: 10.1248/bpb.29.110. [DOI] [PubMed] [Google Scholar]
- 230.Shang W., Yang Y., Jiang B., Jin H., Zhou L., Liu S. Ginsenoside Rb1 promotes adipogenesis in 3T3-L1 cells by enhancing PPARgamma2 and C/EBPalpha gene expression. Life Sci. 2007;80:618–625. doi: 10.1016/j.lfs.2006.10.021. [DOI] [PubMed] [Google Scholar]
- 231.Wong V.K., Chiu P., Chung S.S., Chow L.M., Zhao Y.Z., Yang B.B. Pseudolaric acid B, a novel microtubule-destabilizing agent that circumvents multidrug resistance phenotype and exhibits antitumor activity in vivo. Clin Cancer Res. 2005;11:6002–6011. doi: 10.1158/1078-0432.CCR-05-0209. [DOI] [PubMed] [Google Scholar]
- 232.Jaradat M.S., Noonan D.J., Wu B., Avery M.A., Feller D.R. Pseudolaric acid analogs as a new class of peroxisome proliferator-activated receptor agonists. Planta Medica. 2002;68:667–671. doi: 10.1055/s-2002-33785. [DOI] [PubMed] [Google Scholar]
- 233.Wong K.H., Li G.Q., Li K.M., Razmovski-Naumovski V., Chan K. Kudzu root: traditional uses and potential medicinal benefits in diabetes and cardiovascular diseases. J Ethnopharmacol. 2011;134:584–607. doi: 10.1016/j.jep.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 234.Kim T., Lee W., Jeong K.H., Song J.H., Park S.H., Choi P. Total synthesis and dual PPARalpha/gamma agonist effects of amorphastilbol and its synthetic derivatives. Bioorg Med Chem Lett. 2012;22:4122–4126. doi: 10.1016/j.bmcl.2012.04.062. [DOI] [PubMed] [Google Scholar]
- 235.Rau O., Wurglics M., Paulke A., Zitzkowski J., Meindl N., Bock A. Carnosic acid and carnosol, phenolic diterpene compounds of the labiate herbs rosemary and sage, are activators of the human peroxisome proliferator-activated receptor gamma. Planta Medica. 2006;72:881–887. doi: 10.1055/s-2006-946680. [DOI] [PubMed] [Google Scholar]
- 236.Yu M.H., Im H.G., Lee J.W., Bo M.H., Kim H.J., Kim S.K. Effects of ethanol extract from Saururus chinensis (Bour.) Baill on lipid and antioxidant metabolisms in rats fed a high-fat diet. Nat Prod Res. 2008;22:275–283. doi: 10.1080/14786410701590657. [DOI] [PubMed] [Google Scholar]
- 237.Hwang B.Y., Lee J.H., Nam J.B., Kim H.S., Hong Y.S., Lee J.J. Two new furanoditerpenes from Saururus chinenesis and their effects on the activation of peroxisome proliferator-activated receptor gamma. J Nat Prod. 2002;65:616–617. doi: 10.1021/np010440j. [DOI] [PubMed] [Google Scholar]
- 238.Pferschy-Wenzig E.M., Atanasov A.G., Malainer C., Noha S.M., Kunert O., Schuster D. Identification of isosilybin a from milk thistle seeds as an agonist of peroxisome proliferator-activated receptor gamma. J Nat Prod. 2014;77:842–847. doi: 10.1021/np400943b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Nampoothiri S.V., Binil Raj S.S., Prathapan A., Abhilash P.A., Arumughan C., Sundaresan A. In vitro antioxidant activities of the methanol extract and its different solvent fractions obtained from the fruit pericarp of Terminalia bellerica. Nat Prod Res. 2011;25:277–287. doi: 10.1080/14786419.2010.482053. [DOI] [PubMed] [Google Scholar]
- 240.Yang M.H., Vasquez Y., Ali Z., Khan I.A., Khan S.I. Constituents from Terminalia species increase PPARalpha and PPARgamma levels and stimulate glucose uptake without enhancing adipocyte differentiation. J Ethnopharmacol. 2013;149:490–498. doi: 10.1016/j.jep.2013.07.003. [DOI] [PubMed] [Google Scholar]
- 241.Hotta M., Nakata R., Katsukawa M., Hori K., Takahashi S., Inoue H. Carvacrol, a component of thyme oil, activates PPARalpha and gamma and suppresses COX-2 expression. J Lipid Res. 2010;51:132–139. doi: 10.1194/jlr.M900255-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Zoechling A., Liebner F., Jungbauer A. Red wine: a source of potent ligands for peroxisome proliferator-activated receptor gamma. Food Funct. 2011;2:28–38. doi: 10.1039/c0fo00086h. [DOI] [PubMed] [Google Scholar]
- 243.Sato M., Tai T., Nunoura Y., Yajima Y., Kawashima S., Tanaka K. Dehydrotrametenolic acid induces preadipocyte differentiation and sensitizes animal models of noninsulin-dependent diabetes mellitus to insulin. Biol Pharmaceut Bull. 2002;25:81–86. doi: 10.1248/bpb.25.81. [DOI] [PubMed] [Google Scholar]
- 244.Isa Y., Miyakawa Y., Yanagisawa M., Goto T., Kang M.S., Kawada T. 6-Shogaol and 6-gingerol, the pungent of ginger, inhibit TNF-alpha mediated downregulation of adiponectin expression via different mechanisms in 3T3-L1 adipocytes. Biochem Biophys Res Commun. 2008;373:429–434. doi: 10.1016/j.bbrc.2008.06.046. [DOI] [PubMed] [Google Scholar]
- 245.Jungbauer A., Medjakovic S. Anti-inflammatory properties of culinary herbs and spices that ameliorate the effects of metabolic syndrome. Maturitas. 2012;71:227–239. doi: 10.1016/j.maturitas.2011.12.009. [DOI] [PubMed] [Google Scholar]
- 246.Chacko B.K., Chandler R.T., D’Alessandro T.L., Mundhekar A., Khoo N.K., Botting N. Anti-inflammatory effects of isoflavones are dependent on flow and human endothelial cell PPARgamma. J Nutr. 2007;137:351–356. doi: 10.1093/jn/137.2.351. [DOI] [PubMed] [Google Scholar]
- 247.Mueller M., Hobiger S., Jungbauer A. Red clover extract: a source for substances that activate peroxisome proliferator-activated receptor alpha and ameliorate the cytokine secretion profile of lipopolysaccharide-stimulated macrophages. Menopause. 2010;17:379–387. doi: 10.1097/gme.0b013e3181c94617. [DOI] [PubMed] [Google Scholar]
- 248.Kim S., Shin H.J., Kim S.Y., Kim J.H., Lee Y.S., Kim D.H. Genistein enhances expression of genes involved in fatty acid catabolism through activation of PPARalpha. Mol Cell Endocrinol. 2004;220:51–58. doi: 10.1016/j.mce.2004.03.011. [DOI] [PubMed] [Google Scholar]
- 249.Mezei O., Banz W.J., Steger R.W., Peluso M.R., Winters T.A., Shay N. Soy isoflavones exert antidiabetic and hypolipidemic effects through the PPAR pathways in obese Zucker rats and murine RAW 264.7 cells. J Nutr. 2003;133:1238–1243. doi: 10.1093/jn/133.5.1238. [DOI] [PubMed] [Google Scholar]
- 250.Calleri E., Pochetti G., Dossou K.S., Laghezza A., Montanari R., Capelli D. Resveratrol and Its Metabolites Bind to PPARs. Chembiochem. 2014 doi: 10.1002/cbic.201300754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Ulrich S., Loitsch S.M., Rau O., von Knethen A., Brune B., Schubert-Zsilavecz M. Peroxisome proliferator-activated receptor gamma as a molecular target of resveratrol-induced modulation of polyamine metabolism. Cancer Res. 2006;66:7348–7354. doi: 10.1158/0008-5472.CAN-05-2777. [DOI] [PubMed] [Google Scholar]
- 252.Mueller M., Beck V., Jungbauer A. PPARalpha activation by culinary herbs and spices. Planta Medica. 2011;77:497–504. doi: 10.1055/s-0030-1250435. [DOI] [PubMed] [Google Scholar]
- 253.Kim S.N., Choi H.Y., Lee W., Park G.M., Shin W.S., Kim Y.K. Sargaquinoic acid and sargahydroquinoic acid from Sargassum yezoense stimulate adipocyte differentiation through PPARalpha/gamma activation in 3T3-L1 cells. FEBS Lett. 2008;582:3465–3472. doi: 10.1016/j.febslet.2008.09.011. [DOI] [PubMed] [Google Scholar]
- 254.Wolber G., Langer T. LigandScout: 3-D pharmacophores derived from protein-bound ligands and their use as virtual screening filters. J Chem Inf Model. 2005;45:160–169. doi: 10.1021/ci049885e. [DOI] [PubMed] [Google Scholar]