Abstract
Objective
Gestational diabetes mellitus (GDM) is more common in pregnancies complicated by obesity and both diseases increase the risk for fetal overgrowth and long-term adverse health consequences for the mother and child. Previous studies have linked low maternal serum adiponectin to GDM in normal and overweight women. We hypothesized that lower adiponectin, in particular the high-molecular-weight form, and insulin-like growth factor I (IGF-I) and its binding protein (IGFBP-1) are associated with GDM in pregnant obese Hispanic women.
Methods
72 obese, predominantly Hispanic (92%), women were recruited at 24–28 weeks of gestation. Adiposity was assessed, fasting serum samples were collected, and glucose, insulin, triglyceride, cholesterol levels, adipokines, and hormones associated with obesity and insulin resistance were measured. 30 women had been recently diagnosed with GDM.
Results
Gestational weeks, body mass index, triceps skinfold thickness, mid-arm circumference, serum leptin, IGF-I, tumor necrosis factor α, and interleukin-6 did not differ in the two groups. Obese women with GDM had significantly higher fasting glucose, A1C, triglycerides, very-low-density lipoprotein cholesterol and lower high-density lipoprotein cholesterol, adiponectin, and IGFBP-1 compared to obese women without GDM. Homeostasis model assessment of insulin resistance was positively correlated to IGF-I and negatively correlated to adiponectin.
Conclusions
Obese pregnant women with recently diagnosed GDM had a significantly exacerbated metabolic profile, low serum adiponectin and IGFBP-1 levels at 24–28 weeks of gestation, as compared to women with obesity alone. Because low adiponectin is well established to cause insulin resistance and decreased IGFBP-1 indicates increased IGF-I bioavailability, we propose that these changes are mechanistically linked to the development of GDM in obese Hispanic women.
Keywords: Pregnancy, Obesity, Adiponectin, Gestational Diabetes Mellitus
Key messages.
Obesity in pregnancy is highly prevalent but the mechanism underlying the development of gestational diabetes mellitus (GDM) in some but not all of these women is unclear.
At mid-pregnancy, obese mothers with GDM had significantly lower serum adiponectin and insulin-like growth factor I binding protein (IGFBP-1) compared with obese non-GDM mothers.
We speculate that low maternal adiponectin and IGFBP-1 is mechanistically linked to the development of GDM by promoting insulin resistance.
Introduction
Recent estimates indicate that 32% of women in reproductive age (20–39 years) are obese.1 The prevalence of obesity in pregnancy has become a significant obstetrical challenge due to the increased risk of pregnancy complications in these women including gestational hypertension, pre-eclampsia, and gestational diabetes mellitus (GDM) as well as operative and postoperative complications following cesarean delivery.2 The fetus can also be negatively affected by high maternal adiposity with increased risk for stillbirth, prematurity, shoulder dystocia, and fetal overgrowth.3
Obese pregnant women often enter pregnancy with mild insulin resistance, which is associated with hyperinsulinemia. The incidence of GDM in obese mothers is nearly double compared with mothers of normal body mass index (BMI, kg/m2).4 Advances in detection and treatment of GDM in recent years have not alleviated the unfavorable intrauterine environment for the developing fetus, and fetal overgrowth continues to be a major problem in these pregnancies.5 6 Large-for-gestational-age children have a higher long-term risk for metabolic and cardiovascular disease.7 Pregnancy complicated by GDM also increases the risk for the mother as well as the fetus to develop metabolic and cardiovascular diseases later in life.8
Mexican-American women of reproductive age (20–39) have a greater prevalence of overweight and obesity (69%) than non-Hispanic White women (51%).1 The frequency of GDM has been reported to be 13% in obese Hispanic women compared with 7% for obese Caucasian women.4 9 Therefore, babies of Hispanic women are at particular risk of exposure to the adverse intrauterine metabolic environment associated with maternal obesity and GDM, which may predispose them for obesity and diabetes. This may contribute to the very high rate of childhood and adolescent obesity (21% with BMI >95th centile) in Hispanic children and adolescents.10
Insulin resistance has been identified as a common underlying factor for poor pregnancy outcomes.11 The ability to diagnose abnormal insulin resistance in pregnancy is complicated by the natural decrease in insulin responsiveness that occurs in all pregnant women. Adiponectin has well-established insulin sensitizing properties and circulating levels of adiponectin decrease with increasing BMI, contributing to insulin resistance in obese non-pregnant participants. In pregnancy, low maternal adiponectin levels have been reported in women diagnosed with GDM.12–23 However, maternal BMI was not consistently accounted for in these reports.
Adiponectin is found in multimeric forms in the circulation commonly known as high-molecular-weight (HMW), middle-molecular-weight, and low-molecular-weight forms. Most metabolic effects of adiponectin can be attributed to HMW forms24 and the strongest predictor of insulin sensitivity in non-pregnant individuals is the HMW adiponectin/total adiponectin ratio. In pregnancy, the relationship between HMW adiponectin and insulin sensitivity appears to be similar with HMW adiponectin being positively correlated with insulin sensitivity and HMW forms reported to be reduced in GDM mothers.18 25 Naruse et al26 observed a decline in HMW adiponectin as gestation progresses. In contrast, Mazaki-Tovi et al17 reported no change with gestation in any multimeric form. Low maternal levels of HMW adiponectin have been reported in GDM pregnancies12–23 as well as in pregnancies complicated by small-for-gestational-age fetuses.27 Maternal HMW adiponectin is increased in pre-eclampsia.28 Interestingly, cord serum HMW adiponectin levels are higher than in maternal serum and are positively correlated to birth weight29–31 in most but not all32 studies.
Maternal circulating levels of insulin-like growth factor I (IGF-I) and its binding proteins (IGFBP) increase over gestation. This is likely due to the effects of placental growth hormone on hepatic IGF-I release.33 Maternal IGF-I correlates positively with birth weight and placental weight, and maternal IGF-I is significantly higher in GDM pregnancies compared with non-diabetic pregnancies.34 35 Maternal IGF-I levels are positively correlated with pre-pregnancy BMI, fasting insulin, and the homeostasis model assessment of insulin resistance (HOMA-IR) in individuals with gestational diabetes but not in control pregnancies suggesting that elevated IGF-I may contribute to insulin resistance in women with GDM.34 One study suggested that early pregnancy IGF-I plasma levels may be predictive of GDM.36 The predominant IGFBP-isoform 3 is degraded in pregnancy resulting in increased IGF-I availability.37 This data suggests that IGFBP-1 plays an important role in modulating IGF-I bioavailability during pregnancy. IGFBP-1 is negatively correlated with birth size in normal and diabetic pregnancies.38 39
Our study was designed to examine maternal metabolic parameters (glucose and lipids), hormones (insulin, IGF-I, IGFBP-1), and adipokine levels (adiponectin, leptin, interleukin (IL)-6, and tumor necrosis factor (TNF) α) in obese pregnant women. This study was conducted at 24–28 weeks of gestation in predominantly Hispanic women with pre-pregnancy BMI >30 with or without recent diagnosis of GDM. We hypothesized that lower adiponectin, in particular the HMW form, and IGFBP-1 and higher IGF-I are associated with the diagnosis of GDM in pregnancies complicated by maternal obesity.
Methods
Study subjects
All participants granted informed consent and approved use of their protected health information. Seventy-two pregnant women were included according to the inclusion criteria of high pre-pregnancy or early pregnancy (<12 weeks) BMI (30–45) with singleton pregnancies and between 18 and 40 years of age. Participants between 24 and 28 weeks of gestation were recruited from the University Hospital System prenatal clinics in South Central Texas. The exclusion criteria were concurrent inflammatory, vascular, or metabolic disease; current use of tobacco, street drugs, or medications known to affect inflammatory markers (including corticosteroids); excessive weight gain or loss prior to pregnancy (>20 lbs) including bariatric surgery in the past year; plans to leave the area; and inability to travel to study visit. Of the 72 women, 30 were diagnosed with GDM at or within 2 weeks of enrollment. GDM is carbohydrate intolerance of varying degrees of severity, with onset or first recognition during pregnancy. Diagnosis was confirmed when a 50 g carbohydrate load (1 h glucose challenge test) yielded a blood sugar 1 h later greater than 130 mg/dL and a subsequent 100 g carbohydrate load (3 h glucose tolerance test) resulted in a blood sugar greater than 95 (fasting), 180, 155, and 140 mg/dL, at 1, 2, and 3 h, respectively, with at least two abnormal values out of four.
In order to determine eligibility (pre-pregnant BMI >30), we calculated BMI (kg/m2) based on pre-pregnancy medical records or an estimate of pre-pregnancy BMI was calculated from self-reported weight when pre-pregnant medical records were not available. Participants visited the first outpatient research unit located at University of Texas Health Science Center, San Antonio (UTHSCSA) after an overnight fast (minimum 8 h). We completed anthropometric measurements, reviewed medical history, and obtained a fasting blood sample. After delivery, we reviewed the medical records for each participant and recorded information on pregnancy complications, medications, birth weight, and length.
Anthropometrics
In order to calculate total weight gain during pregnancy, we subtracted pre-pregnancy weight from the mother's weight at delivery. Maternal weight and height were also measured at 24–28 weeks of gestation, and regional adiposity was assessed by measurement of the tricep skinfold and the arm circumference using the protocol from the National Health and Nutrition Examination Survey.40 The tricep skinfold was measured using a Lange caliper and the mid-arm circumference using a non-stretchable tape measure. All measurements were done in triplicate, by the same investigator.
After birth, the gestational age at delivery, and weight, length, and head circumference of the neonate were recorded from the participant's medical record and ponderal index (PI) was calculated using the formula: PI=birth weight×100/(crown-heel length)3.
Maternal blood sampling
A maternal venous fasting blood sample was obtained and processed immediately for glucose, A1C, and lipid panel by standard clinical assays. Serum samples were aliquoted and stored at −80°C until batch analysis. Maternal serum hormone and cytokine levels (insulin, leptin, total adiponectin, IL-6, TNFα, and IGF-I) were determined by ELISA according to manufacturers’ instructions (R&D Systems). In a subset of serum samples, HMW adiponectin (ALPCO) and IGFBP-1 (Diagnostic System Lab) were determined by ELISA.
Statistical analysis
Student t test or a χ2 test were used to evaluate differences in continuous outcomes or categorical variables between the two groups. Pearson correlation coefficients were used to determine the relationships between maternal metabolic parameters. To understand the relationship between maternal metabolic factors, body size parameters, and insulin sensitivity, we combined data from all participants and computed Pearson correlation coefficients. We examined the relationship between pre-pregnancy BMI of the mother and metabolic parameters, similarly. In order to identify clusters of correlated factors, we computed the full correlation matrix between all key variables and this was permuted using hierarchical clustering with average linkage. When multiple factors were correlated with key outcomes, we applied linear regression to account for confounding effects. Data are expressed as mean±SEM. A cut-off of p<0.05 was considered statistically significant. Statistical analysis was performed using the R V.2.15 (Vienna, Austria) statistical package.
Results
Table 1 summarizes the demographics of the two study groups at 24–28 weeks of gestation. Obese participants without GDM were slightly younger (3 years) than the obese mothers with GDM. However, there was no relationship between maternal age and any of the measured metabolic parameters. There was also a difference in the genders of the neonates with 32% of obese mothers delivering female babies while 57% of GDM mothers had female babies. We found no significant differences between the two groups for other demographic parameters including Women Infants Child (WIC) benefit recipients and years of education completed. Pre-pregnancy BMI, gestational weight gain, birth weight, length, and PI of the neonates did not differ between the two groups. Maternal BMI, triceps skinfold thickness, and mid-arm circumference were similar in both groups indicating similar levels of adiposity (table 2).
Table 1.
Demographics of the study participants
| Variable | Obese | Obese with GDM | p Value |
|---|---|---|---|
| N | 42 | 30 | |
| Maternal age (years) | 28.1±0.83 | 30.87±0.85 | 0.02 |
| Pre-pregnancy BMI (kg/m2) | 34.38±0.58 | 34.07±0.82 | 0.76 |
| Gestational age at study (weeks) | 26.54±0.14 | 26.55±0.15 | 0.95 |
| Gestational weight gain (lbs) | 6.57±1.08 | 9.44±2.29 | 0.26 |
| Parity (% primiparous) | 14 | 10 | 0.86 |
| Race/ethnicity (% Hispanic) | 90 | 93 | 1 |
| Birth weight (g) | 3477.16±65.73 | 3521.81±86.08 | 0.68 |
| Birth length (cm) | 51.16±0.31 | 51.33±0.33 | 0.7 |
| Ponderal index (cm2) | 2.59±0.03 | 2.58±0.05 | 0.86 |
| Gender (% female) | 32 | 58 | 0.07 |
| WIC recipient (%) | 79 | 93 | 0.17 |
| Education (% HS or higher) | 57 | 50 | 0.7 |
BMI, body mass index; GDM, gestational diabetes mellitus; HS, high school; WIC, Women Infant Child.
Table 2.
Anthropomorphic measurements and biochemical analysis of plasma samples at 24–28 weeks of gestation
| Variable | Obese | Obese with GDM | p Value |
|---|---|---|---|
| N | 42 | 30 | |
| BMI (kg/m2) | 35.5±0.54 | 35.67±0.85 | 0.87 |
| Gestational age at study (week) | 26.54±0.14 | 26.55±0.15 | 0.95 |
| Triceps skinfold (mm) | 36.9±0.84 | 36.63±1.01 | 0.83 |
| Mid-arm circumference (cm) | 35.72±0.55 | 35.89±0.66 | 0.84 |
| Fasting glucose (mg/dL) | 79.31±0.9 | 89.93±2.95 | 1.5e-03 |
| Fasting insulin (µID/mL) | 12.97±0.94 | 16.41±1.83 | 0.1 |
| A1C (%) | 5.39±0.04 | 5.59±0.08 | 0.04 |
| Leptin (ng/mL) | 52.33±3.68 | 45.45±3.42 | 0.18 |
| TNFα (pg/mL) | 2.38±0.35 | 1.69±0.21 | 0.09 |
| IL-6 (pg/mL) | 1.85±0.28 | 1.47±0.19 | 0.27 |
BMI, body mass index; GDM, gestational diabetes mellitus; IL, interleukin; TNF, tumor necrosis factor.
Fasting glucose and A1C in obese GDM mothers was significantly higher compared to obese women without diabetes (table 2). Maternal adipokines (leptin, IL-6, or TNFα, see table 2) were not significantly different in the two groups. Insulin (+27%, p=0.07) appeared to be elevated in obese women with GDM but these differences did not reach statistical significance. HOMA-IR scores calculated according to Catalano and Kirwan41 were significantly higher in obese women recently diagnosed with GDM compared with obese women without GDM (figure 1).
Figure 1.
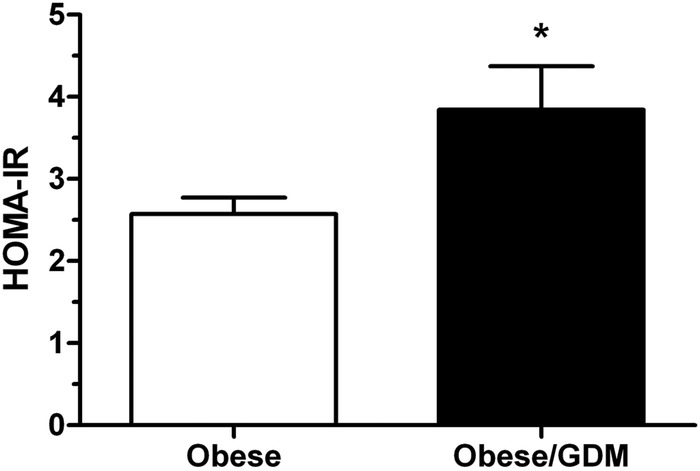
Homeostasis model assessment of insulin resistance (HOMA-IR) scores in obese women and obese women with gestational diabetes mellitus (GDM) at approximately 26 weeks of gestation. HOMA-IR scores were significantly increased in GDM women compared with their obese euglycemic counterparts. Values are expressed as mean+SEM; n=42 obese, n=30 obese/GDM. *p<0.05, Student t test.
Obese pregnant women with GDM demonstrated a significant dyslipidemia compared to obese mothers without diabetes. As shown in figure 2, total triglycerides and very-low-density lipoprotein cholesterol (VLDL-c) were elevated, high-density lipoprotein cholesterol (HDL-c) was reduced, whereas total cholesterol and LDL-c were not different in obese GDM mothers compared to mothers with obesity alone. The obese GDM mothers had markedly lower levels of total adiponectin compared to obese mothers without diabetes and this difference was predominantly due to a decrease in HMW adiponectin (figure 3). Total IGF-I levels were not significantly different in GDM obese women compared with obesity alone; however, IGFBP-1 was significantly lower in the GDM group (figure 4) suggesting increased IGF-I bioavailability.
Figure 2.

Obese gestational diabetes mellitus (GDM) women demonstrate significant dyslipidemia at 26 weeks of gestation compared to obese women without diabetes. Women with GDM had significantly higher triglycerides (TG), very-low-density lipoprotein cholesterol (VLDL-c), and lower high-density lipoprotein cholesterol (HDL-c) than non-diabetic pregnant women. Values are expressed as mean+SEM; n=42 obese (open bar), n=29 obese/GDM (closed bars). *p<0.05, analysis of variance.
Figure 3.
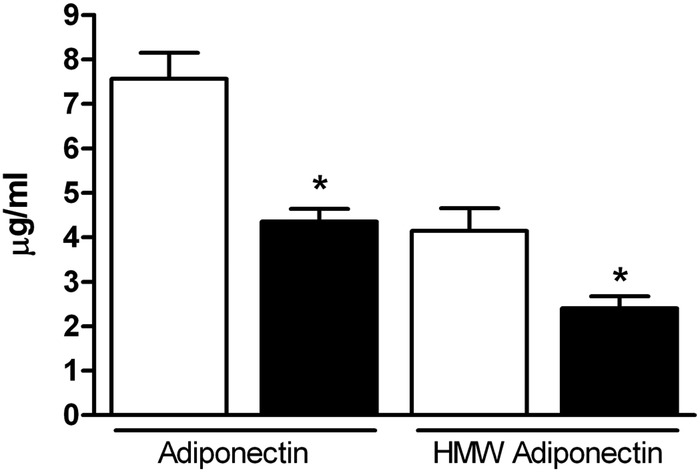
Serum adiponectin is significantly lower in obese gestational diabetes mellitus (GDM) women compared with obese women at 26 weeks of gestation. Total (n=42 obese, n=25 obese/GDM) and high-molecular-weight (HMW; n=18 obese, n=19 obese/GDM) adiponectin were significantly decreased in obese GDM women compared with non-diabetic obese women. Open bars, obese; closed bars, obese/GDM. Values are expressed as mean+SEM. *p<0.005, Student t test.
Figure 4.
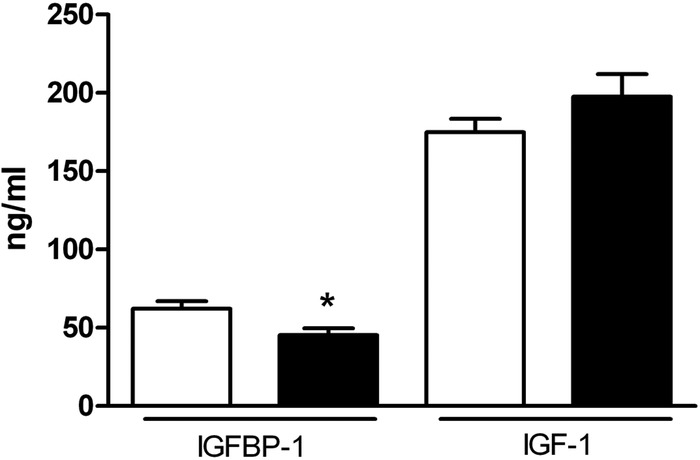
Decreased insulin-like growth factor I binding protein (IGFBP-1) in serum from obese women with gestational diabetes mellitus (GDM) at 26 weeks of gestation. IGFBP-1 was significantly lower in obese GDM women (n=14; closed bars) compared to obese women without GDM (n=20; open bars). Total IGF-I serum levels were not different between the two groups (n=42 obese, n=26 obese/GDM). Values are expressed as mean+SEM. *p<0.05, Student t test.
We found that HOMA-IR was negatively correlated to maternal adiponectin (figure 5A), positively correlated to IGF-I (figure 5B), and negatively correlated to IGFBP-1 (figure 5C) at 24–28 weeks of gestation. We observed the expected strong positive correlations between BMI (as well as other maternal adiposity indicators) and fasting glucose, insulin, leptin, and HOMA-IR (see online supplementary figure S1). Obesity in pregnancy has been suggested to be accompanied by proinflammatory activation.21 42 While we could not address this question directly because our study did not include lean mothers; there was no correlation between any body size measurements and circulating IL-6 and TNFα (see online supplementary figure S1). Furthermore, there was no relationship between IL-6 and TNFα and any insulin sensitivity measurements; fasting glucose, insulin, or HOMA-IR (see supplementary figure S1). We applied multiple linear regression to HOMA-IR to determine the simultaneous effects of adiponectin (effect −0.158, p<0.05) plus IGF-I (effect 0.015, p<0.001) and we found that both parameters are independently associated with HOMA-IR.
Figure 5.
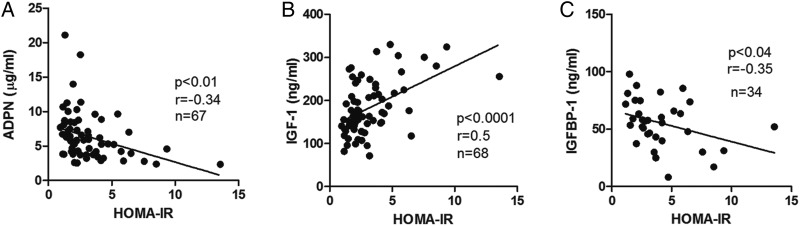
Homeostasis model assessment of insulin resistance (HOMA-IR) correlates strongly with serum adiponectin (ADPN), insulin-like growth factor I (IGF-I), and IGF-I binding protein (IGFBP-1). HOMA-IR values from obese and obese/gestational diabetes mellitus groups had (A) a significant inverse relationship between adiponectin (p<0.001), (B) a strong positive correlation with IGF-1 (p<0.0001), and (C) a significant inverse relationship between IGFBP-1 (p<0.042), Pearson.
Discussion
Our study evaluated pregnant women at the time in pregnancy when most women have an oral glucose tolerance test for diagnosis of GDM, 24–28 weeks. Our objective was to study women in which body size characteristics including weight, height, skinfold thickness, and mid-arm circumference were similar so that GDM was the relevant difference between the groups rather than degree of adiposity. Maternal age was different between the two groups but this did not influence the metabolic parameters measured and therefore do not account for the differences in adiponectin and triglycerides between the two groups. Selecting obese women based on BMI proved to be a good method for obtaining two groups of equivalent body size. The anthropomorphic measures and the finding of no significant difference in leptin levels indicate that we were successful in achieving our recruitment goal. Metabolically, obese women with recent diagnosis of GDM differed from those who were obese without diabetes by having a greater degree of insulin resistance (higher fasting glucose, A1C, and HOMA-IR scores), significantly lower adiponectin and IGFBP-1 as well as a significant dyslipidemia with higher triglycerides, VLDL-c, and lower HDL-c.
Owing to its insulin sensitizing action in peripheral tissues, the reduction in circulating adiponectin with increasing adiposity is considered to be an important component of the insulin resistance associated with weight gain.43 Adiponectin levels in pregnancy have been reported to be increased, not changed, or reduced over the course of gestation.17 26 A potential role for adiponectin as a factor in modulating insulin sensitivity in pregnancy has been previously addressed.18 25 One study indicated that early (11–13 weeks) low adiponectin levels may be predictive for later development of GDM.44 Obese pregnant women have more pronounced insulin resistance and glucose intolerance than pregnant women with normal BMI,11 however the underlying mechanisms remain elusive. The role of adipose tissue and circulating adipokines in regulating maternal glucose homeostasis has been recently recognized as an important component in the development of glucose intolerance and insulin resistance. Dyslipidemia and adipokine levels associated with GDM have been previously reported,45 but BMI and other body size parameters were not carefully controlled in many early studies. Therefore, identifying a definitive role of adipokines, such as adiponectin, in the insulin resistance of obese GDM mothers was difficult in those studies. We found significantly lower circulating adiponectin levels, in particular the HMW form, in those women recently diagnosed with GDM compared with euglycemic obese women matched for body size/adiposity. This is consistent with the possibility that adiponectin is a major contributor to the loss of insulin sensitivity in obese mothers who develop GDM. The nearly 50% reduction in total adiponectin was for the most part due to reduction in HMW form of adiponectin; however, the underlying mechanism for reduced HMW adiponectin release from adipocytes in some obese women but not others is currently unclear. Likewise, it is unknown whether the differences in adiponectin preceded pregnancy or developed during pregnancy. These are clear considerations for further investigation.
In addition to adiponectin, our data suggest that IGF-I and IGFBP-1 are determinants of maternal insulin sensitivity and altered IGF-I and IGFBP-1 levels may contribute to the development of GDM. IGF-I has been previously implicated as an important factor for metabolic health in pregnancy. Qui et al36 found that free IGF-I and IGFBP-1 measured at 13 weeks were inversely correlated with GDM risk. Likewise, lower amniotic fluid IGFBP-1 has been described in women prior to GDM diagnosis.46 Contrary to some studies, we found no difference in total IGF-I in obese women with GDM when compared with obese women.39 We did not measure free IGF-I, which we assume would have been significantly increased. There was a highly significant positive correlation between IGF-I and HOMA-IR when data from all participants were combined suggesting that IGF-I may be involved in regulating maternal insulin sensitivity in mid-pregnancy. IGFBP-1 was significantly reduced in the GDM group and was inversely related to HOMA-IR in all women studied. Given the role of IGFBP-1 in regulating IGF-I bioavailability, the reduced IGFBP-1 levels in women recently diagnosed with GDM may be an important contributor to the development of insulin resistance in obese pregnancies through increased IGF-I bioavailability.
Adipose tissue inflammation associated with obesity, leads to the release of proinflammatory cytokines to the circulation and obese pregnant women have been reported to have approximately a doubling of circulating proinflammatory cytokine levels.42 However, a recent systematic review concluded that the published studies to date do not support an increase in proinflammatory cytokines in pregnancies complicated by GDM.47 In the current study we did not find a difference in circulating IL-6 and TNFα between obese mothers with and without GDM. Because no lean mothers were included in this study, we cannot clearly determine whether the cytokines of obese Hispanic mothers were elevated, but GDM did not alter cytokine levels compared with obesity alone even though insulin resistance was increased. We found no relationship between these proinflammatory cytokines and insulin sensitivity (fasting glucose, insulin, or HOMA-IR) at 26 weeks of gestation. This is contrary to previously published studies at term that indicate that cytokines are a major contributor to insulin resistance. TNFα has been linked to insulin resistance by serine phosphorylation of insulin receptor substrate 1, which causes a reduction in the responsiveness to insulin.48 Maternal TNFα and IL-6 have also been strongly linked to fetal size.42 These discrepant findings may reflect ethnic differences in the inflammatory response to obesity and/or pregnancy. Nevertheless, our data indicate that elevated circulating levels of proinflammatory cytokines are unlikely to explain the high rate of GDM in Hispanic women.
The strength of this study is the use of two groups of women with similar degrees of adiposity allowing for distinction of factors specifically associated with diabetes. The limitations of the study are the small sample size and lack of a lean control group. The purpose of this study was to define metabolic parameters associated with maternal obesity that would lead to GDM. Metabolic changes associated with maternal obesity compared with lean or normal BMI women have been previously described.49 Therefore, we focused on delineating factors in obese women with GDM compared with BMI-matched mothers who remained euglycemic throughout pregnancy.
Obese women are more likely to develop GDM with a significantly greater prevalence in Hispanic mothers.9 Importantly, the majority of obese pregnant women do not develop GDM and there is a clear lack of early diagnostic tools to identify those obese women who will develop GDM prior to the occurrence of hyperglycemia after mid-gestation. GDM is associated with increased short-term and long-term risks for the mother as well as her developing fetus therefore early detection could aid in reducing the negative outcomes associated with this condition. Women who have been diagnosed with GDM have a high probability to develop type 2 diabetes in later life50 and the children of these pregnancies are also at greater risk to develop metabolic syndrome in the first 6–11 years of life.51 GDM may be a window of opportunity to identify mothers and babies at risk and to intervene in pregnancy to improve the in utero environment and contribute to reducing the negative outcomes. Our study suggests that in a subset of women the metabolic response to pregnancy results in dyslipidemia, reduced levels of HMW forms of adiponectin and decreased IGFBP-1 leading to increased IGF-I bioavailability. These factors likely contribute to the exacerbation of insulin resistance, leading to glucose intolerance, the hallmark of diabetes in pregnancy. A common mechanism linking low adiponectin and dyslipidemia may be altered adipose tissue function and we speculate that obese women who develop GDM in pregnancy have more pronounced adipose tissue dysfunction than those who do not. Two studies suggest that early pregnancy may provide a useful diagnostic window for women at risk because adiponectin levels measured in early gestation were significantly lower in those women who were later diagnosed with GDM compared with controls.23 52 However, the substantial variability in maternal adiponectin levels in early pregnancy may limit the usefulness of low adiponectin as a single predictive biomarker for later GDM. Adipose dysfunction in women who develop GDM may precede pregnancy because pre-pregnancy adiponectin levels have also been reported to be lower in women who were later diagnosed with GDM.53
Obese pregnant women with recently diagnosed GDM had a significantly exacerbated metabolic profile, low serum adiponectin and IGFBP-1 levels at 24–28 weeks of gestation, as compared to women with obesity alone. Because low adiponectin is well established to cause insulin resistance and decreased IGFBP-1 indicates increased IGF-I bioavailability, we propose that these changes are mechanistically linked to the development of GDM in obese Hispanic women. Several studies in non-pregnant individuals have demonstrated that adiponectin levels can be improved by moderate exercise, lowering caloric intake, increasing ω-3 fatty acid intake, and increasing dietary fiber.54 This is consistent with the possibility that a modification in fiber and fish intake and moderate exercise may improve outcomes in obese pregnant women with low adiponectin levels. Improving adiponectin levels in obese mothers may contribute to improved insulin sensitivity and perinatal outcomes. Future studies are needed to establish a causal link between adiponectin and/or IGFBP-1, and insulin sensitivity in pregnancy and to develop effective interventions.
Supplementary Material
Footnotes
Contributors: VIR, DAK, and TLP were involved in conception and design of the experiments carried out at University of Texas Health Science Center at San Antonio (UTHSCSA). VIR, EM, CLM, and TLP were involved in collection, analysis, and interpretation of data. JG was involved in statistical analysis. VIR and TLP were involved in drafting the article or revising it critically for important intellectual content. All authors approved the final version of the manuscript.
Funding: This study was generously supported by the National Institutes of Health, National Heart Lung Blood Institute (1R21HL093532), the National Center for Research Resources Clinical and Translational Science Award (CTSA) grant to UTHSCSA (8UL1TR000149) and the Mike Hogg Fund.
Competing interests: None.
Ethics approval: This study was approved by the UTHSCSA institutional review board (IRB).
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: No additional data are available.
References
- 1.Flegal KM, Carroll MD, Kit BK, et al. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999–2010. JAMA 2012;307:491–7 [DOI] [PubMed] [Google Scholar]
- 2.Mission JF, Marshall NE, Caughey AB. Obesity in pregnancy: a big problem and getting bigger. Obstet Gynecol Surv 2013;68:389–99 [DOI] [PubMed] [Google Scholar]
- 3.Galliano D, Bellver J. Female obesity: short- and long-term consequences on the offspring. Gynecol Endocrinol 2013;29:626–31. [DOI] [PubMed] [Google Scholar]
- 4.Baci Y, Ustuner I, Keskin HL, et al. Effect of maternal obesity and weight gain on gestational diabetes mellitus. Gynecol Endocrinol 2013;29:133–6 [DOI] [PubMed] [Google Scholar]
- 5.Black MH, Sacks DA, Xiang AH, et al. The relative contribution of prepregnancy overweight and obesity, gestational weight gain, and IADPSG-defined gestational diabetes mellitus to fetal overgrowth. Diabetes Care 2013;36:56–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Henriksen T. The macrosomic fetus: a challenge in current obstetrics. Acta Obstet Gynecol Scand 2008;87:134–45 [DOI] [PubMed] [Google Scholar]
- 7.Marshall NE, Spong CY. Obesity, pregnancy complications, and birth outcomes. Semin Reprod Med 2012;30:465–71 [DOI] [PubMed] [Google Scholar]
- 8.Yessoufou A, Moutairou K. Maternal diabetes in pregnancy: early and long-term outcomes on the offspring and the concept of ‘metabolic memory’. Exp Diabetes Res 2011;2011:218598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shah A, Stotland NE, Cheng YW, et al. The association between body mass index and gestational diabetes mellitus varies by race/ethnicity. Am J Perinatol 2011;28:515–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ogden CL, Carroll MD, Kit BK, et al. Prevalence of obesity and trends in body mass index among US children and adolescents, 1999–2010. JAMA 2012;307:483–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Catalano PM. Obesity, insulin resistance, and pregnancy outcome. Reproduction 2010;140:365–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Retnakaran R, Hanley AJ, Raif N, et al. Adiponectin and beta cell dysfunction in gestational diabetes: pathophysiological implications. Diabetologia 2005;48:993–1001 [DOI] [PubMed] [Google Scholar]
- 13.Kinalski M, Telejko B, Kuzmicki M, et al. Tumor necrosis factor alpha system and plasma adiponectin concentration in women with gestational diabetes. Horm Metab Res 2005;37:450–4 [DOI] [PubMed] [Google Scholar]
- 14.Altinova AE, Toruner F, Bozkurt N, et al. Circulating concentrations of adiponectin and tumor necrosis factor-alpha in gestational diabetes. Gynecol Endocrinol 2007;23:161–5 [DOI] [PubMed] [Google Scholar]
- 15.Georgiou HM, Lappas M, Georgiou GM, et al. Screening for biomarkers predictive of gestational diabetes mellitus. Acta Diabetol 2008;45:157–65 [DOI] [PubMed] [Google Scholar]
- 16.Lain KY, Daftary AR, Ness RB, et al. First trimester adipocytokine concentrations and risk of developing gestational diabetes later in pregnancy. Clin Endocrinol (Oxf) 2008;69:407–11 [DOI] [PubMed] [Google Scholar]
- 17.Mazaki-Tovi S, Romero R, Kusanovic JP, et al. Adiponectin multimers in maternal plasma. J Matern Fetal Neonatal Med 2008;21:796–815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mazaki-Tovi S, Romero R, Vaisbuch E, et al. Maternal serum adiponectin multimers in gestational diabetes. J Perinat Med 2009;37:637–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Soheilykhah S, Mohammadi M, Mojibian M, et al. Maternal serum adiponectin concentration in gestational diabetes. Gynecol Endocrinol 2009;25:593–6 [DOI] [PubMed] [Google Scholar]
- 20.Thyfault JP, Hedberg EM, Anchan RM, et al. Gestational diabetes is associated with depressed adiponectin levels. J Soc Gynecol Investig 2005;12:41–5 [DOI] [PubMed] [Google Scholar]
- 21.Lowe LP, Metzger BE, Lowe WL, Jret al. Inflammatory mediators and glucose in pregnancy: results from a subset of the Hyperglycemia and Adverse Pregnancy Outcome (HAPO) Study. J Clin Endocrinol Metab 2010;95:5427–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vitoratos N, Valsamakis G, Mastorakos G, et al. Pre-and early post-partum adiponectin and interleukin-1beta levels in women with and without gestational diabetes. Hormones 2008;7:230–6 [DOI] [PubMed] [Google Scholar]
- 23.Ferreira AF, Rezende JC, Vaikousi E, et al. Maternal serum visfatin at 11–13 weeks of gestation in gestational diabetes mellitus. Clin Chem 2011;57:609–13 [DOI] [PubMed] [Google Scholar]
- 24.Lara-Castro C, Fu Y, Chung BH, et al. Adiponectin and the metabolic syndrome: mechanisms mediating risk for metabolic and cardiovascular disease. Curr Opin Lipidol 2007;18:263–70 [DOI] [PubMed] [Google Scholar]
- 25.Retnakaran R, Connelly PW, Maguire G, et al. Decreased high-molecular-weight adiponectin in gestational diabetes: implications for the pathophysiology of type 2 diabetes. Diabet Med 2007;24:245–52 [DOI] [PubMed] [Google Scholar]
- 26.Naruse K, Noguchi T, Sado T, et al. Chemokine and free fatty acid levels in insulin-resistant state of successful pregnancy: a preliminary observation. Mediators Inflamm 2012;2012:432575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mazaki-Tovi S, Romero R, Vaisbuch E, et al. Maternal serum adiponectin multimers in patients with a small-for-gestational-age newborn. J Perinat Med 2009;37:623–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fasshauer M, Waldeyer T, Seeger J, et al. Circulating high-molecular-weight adiponectin is upregulated in preeclampsia and is related to insulin sensitivity and renal function. Eur J Endocrinol 2008;158:197–201 [DOI] [PubMed] [Google Scholar]
- 29.Inoue M, Itabashi K, Nakano Y, et al. High-molecular-weight adiponectin and leptin levels in cord blood are associated with anthropometric measurements at birth. Horm Res 2008;70:268–72 [DOI] [PubMed] [Google Scholar]
- 30.Basu S, Laffineuse L, Presley L, et al. In utero gender dimorphism of adiponectin reflects insulin sensitivity and adiposity of the fetus. Obesity (Silver Spring) 2009;17:1144–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ballesteros M, Simon I, Vendrell J, et al. Maternal and cord blood adiponectin multimeric forms in gestational diabetes mellitus: a prospective analysis. Diabetes Care 2011;34:2418–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ong GK, Hamilton JK, Sermer M, et al. Maternal serum adiponectin and infant birthweight: the role of adiponectin isoform distribution. Clin Endocrinol (Oxf) 2007;67:108–14 [DOI] [PubMed] [Google Scholar]
- 33.McIntyre HD, Zeck W, Russell A. Placental growth hormone, fetal growth and the IGF axis in normal and diabetic pregnancy. Curr Diabetes Rev 2009;5:185–9 [DOI] [PubMed] [Google Scholar]
- 34.Matuszek B, Lenart-Lipinska M, Burska A, et al. Increased serum insulin-like growth factor-1 levels in women with gestational diabetes. Adv Med Sci 2011;56:200–6 [DOI] [PubMed] [Google Scholar]
- 35.Luo ZC, Nuyt AM, Delvin E, et al. Maternal and fetal IGF-I and IGF-II levels, fetal growth, and gestational diabetes. J Clin Endocrinol Metab 2012;97:1720–8 [DOI] [PubMed] [Google Scholar]
- 36.Qiu C, Vadachkoria S, Meryman L, et al. Maternal plasma concentrations of IGF-1, IGFBP-1, and C-peptide in early pregnancy and subsequent risk of gestational diabetes mellitus. Am J Obstet Gynecol 2005;193:1691–7 [DOI] [PubMed] [Google Scholar]
- 37.Sakai K, Iwashita M, Takeda Y. Profiles of insulin-like growth factor binding proteins and the protease activity in the maternal circulation and its local regulation between placenta and decidua. Endocr J 1997;44:409–17 [DOI] [PubMed] [Google Scholar]
- 38.El-Masry SA, El-Ganzoury MM, El-Farrash RA, et al. Size at birth and insulin-like growth factor-I and its binding protein-1 among infants of diabetic mothers. J Matern Fetal Neonatal Med 2013;26:5–9 [DOI] [PubMed] [Google Scholar]
- 39.Olausson H, Lof M, Brismar K, et al. Maternal serum concentrations of insulin-like growth factor (IGF)-I and IGF binding protein-1 before and during pregnancy in relation to maternal body weight and composition and infant birth weight. Br J Nutr 2010;104:842–8 [DOI] [PubMed] [Google Scholar]
- 40. Committee on Nutritional Anthropometry, Food and Nutrition Board, National Research Council. Recommendations concerning body measurements for the characterization of nutritional status. In Brozek J, ed. Body Measurements. Detroit: Wayne University Press, 1956. [Google Scholar]
- 41.Catalano PM, Kirwan JP. Clinical utility and appraoches for estimating insulin sensitivity in pregnancy. Semin Perinatol 2002;26:181–89 [DOI] [PubMed] [Google Scholar]
- 42.Kirwan JP, Hauguel-de Mouzon S, Lepercq J, et al. TNF-alpha is a predictor of insulin resistance in human pregnancy. Diabetes 2002;51:2207–13 [DOI] [PubMed] [Google Scholar]
- 43.Kadowaki T, Yamauchi T, Kubota N, et al. Adiponectin and adiponectin receptors in insulin resistance, diabetes, and the metabolic syndrome. J Clin Invest 2006;116:1784–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nanda S, Savvidou M, Syngelaki A, et al. Prediction of gestational diabetes mellitus by maternal factors and biomarkers at 11 to 13 weeks. Prenat Diagn 2011;31:135–41 [DOI] [PubMed] [Google Scholar]
- 45.Herrera E, Ortega-Senovilla H. Disturbances in lipid metabolism in diabetic pregnancy—are these the cause of the problem? Best Pract Res Clin Endocrinol Metab 2010;24:515–25 [DOI] [PubMed] [Google Scholar]
- 46.Tisi DK, Burns DH, Luskey GW, et al. Fetal exposure to altered amniotic fluid glucose, insulin, and insulin-like growth factor-binding protein 1 occurs before screening for gestational diabetes mellitus. Diabetes Care 2011;34:139–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gomes CP, Torloni MR, Gueuvoghlanian-Silva BY, et al. Cytokine levels in gestational diabetes mellitus: a systematic review of the literature. Am J Reprod Immunol 2013;69:545–57 [DOI] [PubMed] [Google Scholar]
- 48.Capurso C, Capurso A. From excess adiposity to insulin resistance: the role of free fatty acids. Vascul Pharmacol 2012;57:91–7 [DOI] [PubMed] [Google Scholar]
- 49.Jansson N, Nilsfelt A, Gellerstedt M, et al. Maternal hormones linking maternal body mass index and dietary intake to birth weight. Am J Clin Nutr 2008;87:1743–9 [DOI] [PubMed] [Google Scholar]
- 50.Retnakaran R. Glucose tolerance status in pregnancy: a window to the future risk of diabetes and cardiovascular disease in young women. Curr Diabetes Rev 2009;5:239–44 [DOI] [PubMed] [Google Scholar]
- 51.Boney CM, Verma A, Tucker R, et al. Metabolic syndrome in childhood: association with birth weight, maternal obesity, and gestational diabetes. Pediatrics 2005;115:e290–6 [DOI] [PubMed] [Google Scholar]
- 52.Lacroix M, Battista MC, Doyon M, et al. Lower adiponectin levels at first trimester of pregnancy are associated with increased insulin resistance and higher risk of developing gestational diabetes mellitus. Diabetes Care 2013;36:1577–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hedderson MM, Darbinian J, Havel PJ, et al. Low prepregnancy adiponectin concentrations are associated with a marked increase in risk for development of gestational diabetes mellitus. Diabetes Care 2013;36:3930–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Silva FM, de Almeida JC, Feoli AM. Effect of diet on adiponectin levels in blood. Nutr Rev 2011;69:599–612 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


