Abstract
Purpose of review
In this era of modern combination antiretroviral therapy (cART) HIV-associated neurocognitive disorders (HAND) continues to be a debilitating condition affecting a large portion of the infected population. In this review we highlight recent discoveries that help to define the interplay between HIV life cycle, the innate immune system, and cellular autophagy in the context of the CNS.
Recent findings
Investigators have recently elucidated themes in HAND, which place it in a unique framework. Cells of macrophage lineage and probably astrocytes play a role in disseminating virus through the CNS. Each of these cell types responds to a diverse population of constantly evolving virus existing in an inflammatory environment. This occurs though the failure of both host antiviral mechanisms, such as autophagy, and innate immunological signaling pathways to control viral replication.
Summary
The newest findings detailed in this review help define why HIV CNS disease is a difficult target for therapeutics and create hope that these new mechanisms may be exploited to attenuate viral replication and eliminate disease.
Keywords: HIV, innate immunity, autophagy, central nervous system
Introduction
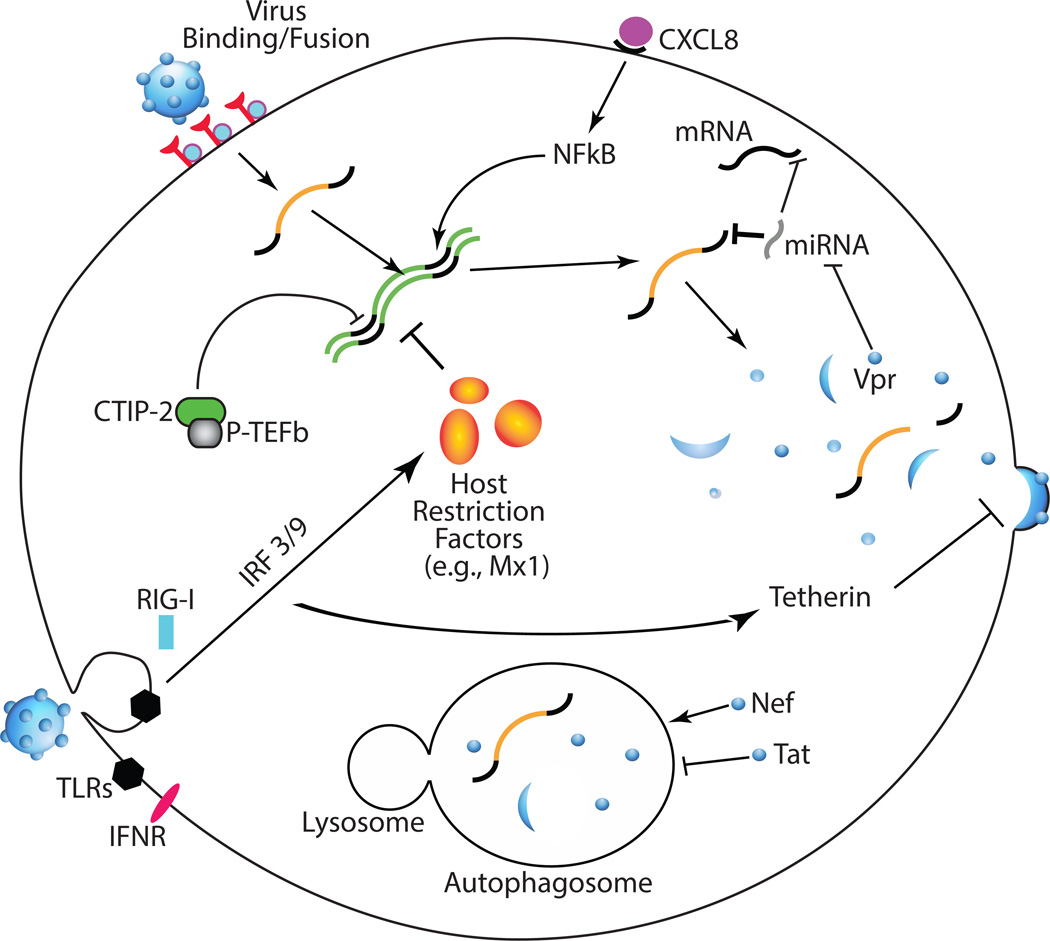
The most deadly consequence of HIV infection, acquired immunodeficiency syndrome (AIDS), can be successfully combated by combination antiretroviral therapy (cART). However, HIV enters the CNS very early after infection (1) and continues to cause HIV-associated neurocognitive disorders (HAND) in approximately 50% of HIV-infected individuals in the post-cART era (2, 3). cART-treated HIV-infected individuals are living longer and there is growing evidence that the aging HIV-infected population may suffer from earlier onset of neurocognitive deficits (4). In the periphery, HIV primarily replicates in CD4+ T cells. However, cells of the macrophage lineage are the primary virus producers in the CNS. Thus, viral life cycle and immune responses are governed by unique mechanisms in brain. In this review we highlight the most recent advances in understanding viral life cycle, innate immune responses, and autophagy, one aspect of the innate immune response, in the CNS (Fig 1). These concepts are of critical importance for both eradication strategies and developing therapeutic targets to treat HAND.
Fig 1.
Recent discoveries in HIV life cycle, innate immunity, and autophagy in the CNS. Virus binding and fusion with macrophages is based on divergent Env proteins’ ability to engage cell surface receptors despite a low CD4 density. Once in the cell, viral replication is influenced by a number of host and viral factors. CTIP-2 can form an inhibitory complex with P-TEFb, shutting down viral transcription and inducing latency. CXCL8 production by multiple cells in the brain enhances viral replication via NFkB signaling. miRNAs, inhibitors of mRNA translation, can affect nearly any stage of viral life cycle by targeting viral mRNAs or by targeting mRNAs of inhibitors or enhancers of viral replication. Furthermore, HIV Vpr can affect global miRNA production by degrading Dicer. PRRs (TLRs, RIG-I, IFNR) respond to interstitial and cytoplasmic viral epitopes and intracellular cytokines inducing expression of signaling molecules, which produce restriction factors such as Mx1 and tetherin. The viral protein Nef can subvert these innate host responses by increasing host clearance of viral components though autophagy. Inversely Tat can further increase replication by inhibiting the autophagosome.
HIV life cycle in the CNS
During the course of the thirty-year epidemic, much has been learned about HIV infection of neuroglia and viral replication in the CNS. However, many recent discoveries have important implications as the field renews hope for solving the problem of eradication and searches for treatments for HAND.
Viral entry into cells of macrophage lineage
In the CNS, cells of macrophage lineage are the primary virus producers whereas astrocytes likely support only restricted virus replication (5). Having a complete understanding of viral entry into these cells and evolution of viral tropism during different stages of disease has important treatment consequences and may affect both peripheral and CNS disease progression.
Viruses were historically categorized based on two somewhat related sets of criteria: 1.) the ability to infect T cells (T-Tropic) or macrophages (M-tropic) or 2.) usage of the coreceptor CCR5 (R5) or CXCR4 (X4) for binding and fusion with target cells. Defining true macrophage tropism has been historically difficult due to the high level of inter- and intra-donor macrophage variation, in addition to the fact that viral tropism/coreceptor usage are not always mutually exclusive (6). Two recent studies used the recently developed Affinofile cell line (7) to titrate levels of CD4 and CCR5 and quantitatively evaluate CD4 and CCR5 requirements of both classically defined and patient-derived T-tropic (isolated from blood or lymph node) and M-tropic (isolated from brain or CSF) Env proteins. They found that M-tropic Env proteins are not CD4 independent, but rather are capable of infecting cells with very low CD4 surface densities (8, 9). Furthermore, the conformation of brain-derived, M-tropic Env proteins had increased CD4 binding site exposure and alterations in the physical interactions with CCR5 compared to lymph node-derived, T-tropic isolates (9).
Viral replication cells of macrophage lineage
Once inside the host cell, many factors can modify viral replication. Proinflammatory conditions enhance viral replication in most cell types. CXCL8 (IL-8) is produced by most cell types in the brain, enhances HIV replication in macrophages and T cells (10), and is elevated in the CSF of patients with HIV-associated dementia compared with neurocognitively normal HIV-infected patients (11). Mamik and Ghorpade extended these studies and found that CXCL8 increased formation of 2-LTR circles, a marker of nuclear import of viral DNA or enhanced infectivity, in macrophages and primary microglia and that enhanced replication was dependent on NF-κB (12).
Micro RNAs (miRNAs) have also gained recent attention as modulators of HIV replication. miRNAs are small ~22 nucleotide RNAs that inhibit translation of target mRNAs, usually via binding to the 3’ UTR (13). miRNAs can regulate the HIV life cycle by directly binding to and inhibiting translation of viral mRNA or by inhibiting translation of proteins involved in any stage the HIV life cycle. The number of publications implicating miRNAs in HIV regulation has exploded over the past several years (14–17). This review will highlight only the most recent publications that have relevance to viral replication in cells of the macrophage lineage. Using simian immunodeficiency virus (SIV) as a model, Sisk and colleagues recently identified 4 host miRNAs (miRs-29a, -29b, -9 and -146a) that directly inhibit virus production and are produced in macrophages upon viral infection, likely via stimulation of interferon β (IFNβ) or tissue necrosis factor α (TNFα) (18). These miRNAs were also upregulated in human macrophages in response to IFNβ or TNFα and 2 of the miRNAs (miRs-29a and -29b) directly target HIV-1 transcripts (17, 18). These findings support the growing body of literature highlighting the importance of miRNAs in not just the viral life cycle, but also in the context of the immune response (19, 20). Rather than directly binding to viral mRNA, Ma and colleagues recently showed that miRNA-1236 represses translation of Vpr binding protein (VprBP), thereby inhibiting HIV infection of monocytes, implicating miRNA-1236 as an endogenous inhibitor of viral replication (21). Differential expression of miRNA-1236 in monocytes (high) vs macrophages (low) appears to partly explain the differential permissivity of these cells to viral replication (21). Virus itself also usurps miRNA mechanisms for its own benefit. Casey Klockow recently demonstrated that HIV enhances macrophage infection by targeting the miRNA processing protein Dicer for proteosomal degradation via Vpr (22). Overall, miRNA regulation of HIV replication is a rapidly growing field of investigation with many questions left to answer at all stages of viral replication, particularly in the CNS.
Viral latency in macrophages and microglia
In the current atmosphere of renewed hope for eradication, understanding latency in macrophages and microglia is of utmost importance. While multiple mechanisms of latency have been identified (23), recent studies have further defined the role of COUP-TF interacting protein 2 (CTIP-2). CTIP-2 was previously shown to inhibit LTR transcription via histone deacetylase (HDAC) and histone methyltransferase (HMT) recruitment (24). The same group has now found that CTIP-2 is part of a large, inactive P-TEFb complex on the HIV LTR in a microglial cell line and is recruited by HMGA1 (25, 26). Furthermore, the P-TEFb-related transcriptional silencing mechanism is independent of CTIP-2 recruitment of HDAC and HMT (25).
Infection of astrocytes
In contrast to macrophages, microglia, and T cells, much less is known about HIV entry and replication in astrocytes, which do not express CD4. Gray and colleagues recently identified intracellular vesicles containing the tetraspanin-family protein CD81 as compartments containing HIV in astrocytes (27). Knocking down this protein decreased cell-associated virus, but the virus still associated with residual CD81-containing vesicles, further supporting CD81-vesicles as important compartments for the viral life cycle in astrocytes.
Viral latency in astrocytes
Once infected, astrocytes only support very limited or restricted viral replication, although they may be capable of producing infectious virus in proinflammatory conditions (5). Because astrocytes are the most numerous cell type in the brain, fully understanding mechanisms of viral replication and latency in these cells is critical. Narasipura and colleagues used primary human progenitor-derived astrocytes and astrocytic cell lines to demonstrate that class I HDACs and HMTs, likely SU(VAR)3–9 in particular, are important in epigenetic silencing of HIV proviral DNA in astrocytes (28). SAHA, the class I HDAC inhibitor used for these experiments, is currently used in latency reversing eradication strategies (29, 30). While the view of astrocytes as viral reservoirs is contentious, reactivation of HIV in these cells may have yet unforeseen consequences.
Innate immunity in the context of HIV Associated Neurological Disease
The innate and adaptive immune systems play integral roles in minimizing host damage and clearing infection. Our understanding of HIV-induced dysfunction of the immune system is still evolving. For example, recent studies have revealed that pyroptosis, cell death specifically induced by inflammasome activity and mediated by caspase 1, is responsible for much of T cell death during infection. Monroe et al (31) recently showed that loss of bystander, unactivated, CD4+ T cells is largely due to pyroptosis and accompanies IL-1β secretion. This was demonstrated with both in vitro and in vivo data and pyroptosis may be able to be attenuated therapeutically. Doitsh et al (32) identified an inducible protein which binds both dsDNA and ssDNA and induces CD4+ T cell pyroptosis. Further, knockdown of this protein reduced CD4+ T cell depletion. While no specific role for pyroptosis in the development of HAND has been identified, it is a potential mechanism for cellular loss in the CNS.
A better understanding of the role of host immunity, particularly innate immunity, in HAND could mitigate both direct cytopathological effects and hyper-activation of adaptive immunity. In the last decade immunologists have discovered and defined several mechanisms by which cells of the innate immune system respond to challenge; discoveries that drive our appreciation of innate immunity well beyond the convention of generic interferon (IFN) response.. Of particular interest are pattern recognition receptors (PRRs), which respond to specific pathogen associated molecular patterns (PAMPs), and have made investigators take note of the complex responses the immune system can mount without adaptive immunity and memory. PRRs trigger a variety of signal transduction events that result in the expression of genes that have activity against pathogens (TNF, INFα/β, chemokines, interleukins, etc). PRRs such as Toll like receptors (TLRs), retinoic acid-inducible gene 1 (RIG-I), and RIG-I like receptors (RLRs), have been implicated in contributing to aberrant inflammation in the CNS through recognition of tissue damage molecular signals and aberrant responses to self (33–37) (Fig 1).
Specific components of the innate immune system have been associated with HAND. For example, CXCL10 is induced by several PRRs as well as HIV proteins. CXCL10 also recruits both T cells and monocytes/macrophages to the brain exacerbating inflammation (34, 36). Further, innate immune stimulation in HAND is attenuated by a number of evasive strategies. Host factors in brain tissue that restrict viral replication such as interferon regulatory factor 3 (IRF3) and RIG-I are targeted by HIV proteins for sequestration and degradation (34, 36, 37) (Fig 1).
Recent developments in the literature have elaborated on and reaffirmed some of these previously described immunological processes. In the setting of acute SIV infection in macaques, IFNα transcripts were substantially lower in the brain vs the periphery and changes specifically involved IFNα subtype 6 (38). Polyak et al (39) used human fetal microglia to reveal that stimulation with IFNα and HIV induced tetherin expression, a host restriction factor, which restricts viral budding. Additionally, virus isolated from the brain of a HAND patient induced significantly less tetherin and Mx1 than virus isolated from toxoplasmosis encephalitis CSF. This indicates that neurovirulent HIV strains may evade tetherin and Mx1 via an undefined mechanism. These data were recapitulated using two feline immunodeficiency virus (FIV) strains, divergent in their Env proteins; after ten days post infection tetherin and Mx1 were elevated in both. Further, analysis of an in vivo FIV model of HIV neurovirulence demonstrated that while one strain induced TNFα and CD40, the other had a significant increase in tetherin and Mx1. These changes in tetherin/Mx1 were associated with IFN-related genes IRF3/9 and not IFNα, TNFα, or IL1-β. These studies support previous evidence that the brain is immunologically discrete, neurological inflammation may be dependent on viral strain, and its complexities present unique challenges as therapeutic targets (34, 36, 37, 40) (Fig 1).
Recent studies have elucidated some functions of multi nucleated giant cells (MNGCs), which are a common hallmark of HIV infection. Tarassishin et al (41) recently demonstrated that MNGCs in the brain that were highly associated with IRF3 induction. Another group, Hornik et al (42) demonstrated in a microglia cell line that multinucleation could be triggered by TLR ligands, IFNβ, and proteins associated with Parkinson’s disease alpha-synuclein) and Alzheimer’s disease ((amyloid-beta). This multinucleation occurred through abscission failure, not fusion or endocytosis. Additionally, these MNGCs had increased phagocytic activity. These studies reinforce the idea that HIV neurological disease has mechanisms in common with other neurodegenerative disorders.
Specific microenvironments in the brain contain unique cells that exacerbate cytopathy in the brain. In a brain endothelial cell (EC) line it was shown by Li et al (43) that TLR ligands can induce IFNβ/λ expression in a dose-dependent manner. Application of supernatants from polyI:C activated ECs to infected macrophages resulted in reduction of viral replication in a dose dependent manner. This was accompanied by an increase in the IFN associated genes ISG56, Mx1, and OAS-1. Treatment of neutralizing antibody to IFNβ and IL10-β receptor inhibited these effects. Geffin et al (44) used neural epithelial cell (NEP) derived astrocyte/neuron co-cultures to further investigate gene expression. Treatment with virus containing medium up regulated 20 genes having to do with antigen presentation and immunity; including tetherin. The gene Apolipoprotein E (ApoE), which is linked with other neurocognitive diseases, was also investigated. Between NEP cultures with either E3/E3 or E3/E4 genotypes, E3/E4 cultures had significantly reduced expression patterns in eight of the previously identified 20 genes. These data demonstrate that cells other than classical inflammatory cells may play a critical role in inflammatory homeostasis in the brain. Further, host factors like genetics may dictate dysregulation in these cell types of the brain (Fig 1).
Autophagy and HIV infection of the CNS
Autophagy is an important cellular mechanism that provides quality control of proteins and eliminates defective older intracellular organelles (45). This is accomplished by the creation of autophagosomes, which consist of double membrane bodies that form within the cytoplasm and engulf cytoplasmic constituents such as sub-cellular organelles and microbial pathogens, including viruses, and target them for degradation by fusion with a lysosome (Fig 1). This process constitutes an important innate immune defense mechanism for host cells.
HIV appropriates autophagy for replication
As an intracellular parasite, HIV is dependent upon its ability to subvert host cell machinery for replication and dissemination, and to circumvent cellular processes that prevent its growth. Autophagy is one such intracellular process. HIV proteins can both promote and delay autophagy to facilitate different stages of viral replication in cells of monocyte/macrophage lineage. This suggests that these processes are also important in viral replication in the brain, where productive replication occurs almost exclusively in cells of macrophage lineage, which might modulate the pathogenesis of HAND. In fact, post mortem examination of brains from individuals with HIVE have more markers of autophagy than brains of HIV-infected individuals without encephalitis or uninfected individuals (46, 47), suggesting a dysregulation of autophagy in HIVE (48).
HIV replication in primary macrophages and monocytes is inhibited when autophagy-associated genes are silenced by RNAi (49–52), suggesting that HIV induces autophagy but blocks the late proteolytic stage. HIV assembly is thought to occur on endocytic membranes that intersect with recycling endosomes in macrophages (53, 54), and Gag proteins colocalize with autophagosome membrane proteins suggesting that autophagy might be involved in Gag processing (55). The HIV accessory protein Nef has been shown to induce autophagosome formation and enhance HIV replication (56). Additionally, during permissive infection, HIV prevents the fusion of autophagosomes with lysosomes (57), further demonstrating how HIV may subvert autophagosomes to benefit the viral life cycle.
Autophagy as a host innate defense mechanism
On the other hand, there is much evidence supporting autophagy as an important player in the host’s innate defense against HIV pathogenesis. In a recent study, PBMCs from HIV-infected long term non-progressors and elite controllers were shown to have significantly higher numbers of autophagic vesicles and higher levels of autophagic markers than normal progressors. In macrophages, virions were detectable in cells with moderate but not high levels of accumulated autophagosomes (52). This suggests that autophagic vesicles limit viral pathogenesis in HIV-1 infection by targeting viral components for degradation (58).
Deficits in autophagy have been identified in a number of aging-associated diseases including Alzheimer’s disease (59, 60) and Parkinson’s disease (61, 62). This association with aging is important, given that the aging population represents one of the fastest-growing populations of HIV-infected individuals (63).
The association between aging and autophagy dysfunction was supported by a recent study, in which differences in HIV viral load (VL), CNS immune activation and expression of autophagy-related proteins were compared between HIV-infected individuals with encephalitis that were older than or younger than 50 years of age. Young HIVE patients displayed an increase in beclin-1, cathepsin-D and light chain (LC)3, whereas these autophagy markers were reduced in aged HIVE cases compared to age-matched HIV+ donors (46). Similar alterations in autophagy markers were observed in aged gp120 transgenic (tg) mice (46).
Autophagy as a therapeutic target for HAND
Since autophagy works at the host cellular level to induce degradation of HIV, there has been substantial interest in identifying therapeutics that can manipulate this cellular process in combination with antiretroviral drugs to treat and potentially eradicate HIV. In addition, since autophagy is a naturally occurring host clearance mechanism, it is unlikely that viral resistance would develop.
Rapamycin, a specific mTOR inhibitor and inducer of autophagy, inhibits HIV replication through both the downregulation of CCR5 (64) and the induction of autophagy (55). It also inhibits HIV infection in human peripheral blood leukocyte-reconstituted SCID mice and synergizes with a number of inhibitors of HIV entry (65). However, rapamycin has immunosuppressive and other adverse effects that limit its potential usefulness in the treatment of HIV.
Vitamin D has well known antimicrobial effects, and low levels of 25-hydroxycholecalciferol (25D3) and/or the active metabolite 1a,25-dehydroxycholecalciferol (1,25D3) are associated with increased risk of or severity of infection with HIV (64, 66), thought to occur as a result of upregulation of autophagy (51, 67). Vitamin D has also been implicated in the autophagic response to PAMPs by PRRs as TLR7/8 stimulation activates a variety of antiviral effector functions including the induction of autophagy (50, 68).
A recent study described production of a cell-permeable HIV Tat-Beclin-1 fusion protein that binds to Nef and markedly inhibits HIV replication in primary human macrophages through the induction of autophagy (69). This peptide, which also restricts replication of a number of other viruses that cause encephalitis, holds therapeutic promise.
Conclusions
Surprisingly, after 30 years of intensely studying the HIV life cycle, we still are discovering new facets of this process, particularly in cells of macrophage lineage, which emphasize the complexity of this virus’s adaptations to host cell defenses. These new data emphasize the importance of innate defense mechanisms and basic homeostatic mechanisms for control of virus in the CNS, where adaptive immune mechanisms are inadequate. Manipulation of the process of autophagy is attractive as a potential therapeutic target, but this complex mechanism requires further investigation.
Key Points.
Macrophage tropic viruses require lower cell densities of CD4.
CTIP-2 promotes HIV latency in macrophages by multiple mechanisms.
HIV initiates inflammatory cascades in both infected and bystander cells; the interplay between the two is what dictates neurological disease.
Host factors such as age and genetics are important determinants of susceptibility to HAND.
The increased severity of HIV CNS disease in aging patients is likely at least partly associated with age-related declines in cellular autophagy.
Acknowledgements
The authors thank Julia Drewes for helpful comments.
M. Christine Zink has received funding from the National Institutes of Health (grants R01 MH087233, R01 MH085554, P01 MH070306, P30 MH075673, P40 OD013117, and T32 OD011089).
Footnotes
Conflicts of Interest
The authors declare no conflicts of interest.
References
- 1.Davis LE, Hjelle BL, Miller VE, Palmer DL, Llewellyn AL, Merlin TL, et al. Early viral brain invasion in iatrogenic human immunodeficiency virus infection. Neurology. 1992;42(9):1736–1739. doi: 10.1212/wnl.42.9.1736. Epub 1992/09/01. [DOI] [PubMed] [Google Scholar]
- 2.Ances BM, Ellis RJ. Dementia and neurocognitive disorders due to HIV-1 infection. Seminars in neurology. 2007;27(1):86–92. doi: 10.1055/s-2006-956759. Epub 2007/01/18. [DOI] [PubMed] [Google Scholar]
- 3.Heaton RK, Clifford DB, Franklin DR, Jr, Woods SP, Ake C, Vaida F, et al. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology. 2010;75(23):2087–2096. doi: 10.1212/WNL.0b013e318200d727. Epub 2010/12/08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pathai S, Bajillan H, Landay AL, High KP. Is HIV a Model of Accelerated or Accentuated Aging? The journals of gerontology Series A, Biological sciences and medical sciences. 2014;69(7):833–842. doi: 10.1093/gerona/glt168. Epub 2013/10/26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Churchill M, Nath A. Where does HIV hide? A focus on the central nervous system. Current opinion in HIV and AIDS. 2013;8(3):165–169. doi: 10.1097/COH.0b013e32835fc601. Epub 2013/02/23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arrildt KT, Joseph SB, Swanstrom R. The HIV-1 env protein: a coat of many colors. Current HIV/AIDS reports. 2012;9(1):52–63. doi: 10.1007/s11904-011-0107-3. Epub 2012/01/13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Johnston SH, Lobritz MA, Nguyen S, Lassen K, Delair S, Posta F, et al. A quantitative affinity-profiling system that reveals distinct CD4/CCR5 usage patterns among human immunodeficiency virus type 1 and simian immunodeficiency virus strains. Journal of virology. 2009;83(21):11016–11026. doi: 10.1128/JVI.01242-09. Epub 2009/08/21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Joseph SB, Arrildt KT, Swanstrom AE, Schnell G, Lee B, Hoxie JA, et al. Quantification of entry phenotypes of macrophage-tropic HIV-1 across a wide range of CD4 densities. Journal of virology. 2014;88(4):1858–1869. doi: 10.1128/JVI.02477-13. Epub 2013/12/07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Salimi H, Roche M, Webb N, Gray LR, Chikere K, Sterjovski J, et al. Macrophage-tropic HIV-1 variants from brain demonstrate alterations in the way gp120 engages both CD4 and CCR5. Journal of leukocyte biology. 2013;93(1):113–126. doi: 10.1189/jlb.0612308. Epub 2012/10/19. Important advancements in understanding mechanisms of M-tropic vs T-tropic env proteins. Demonstrated that M-tropic viruses have increased CD4 binding site exposure and alterations physical interactions with CCR5
- 10.Lane BR, Lore K, Bock PJ, Andersson J, Coffey MJ, Strieter RM, et al. Interleukin-8 stimulates human immunodeficiency virus type 1 replication and is a potential new target for antiretroviral therapy. Journal of virology. 2001;75(17):8195–8202. doi: 10.1128/JVI.75.17.8195-8202.2001. Epub 2001/08/03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zheng JC, Huang Y, Tang K, Cui M, Niemann D, Lopez A, et al. HIV-1-infected and/or immune-activated macrophages regulate astrocyte CXCL8 production through IL-1beta and TNF-alpha: involvement of mitogen-activated protein kinases and protein kinase R. Journal of neuroimmunology. 2008;200(1–2):100–110. doi: 10.1016/j.jneuroim.2008.06.015. Epub 2008/07/26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mamik MK, Ghorpade A. Chemokine CXCL8 promotes HIV-1 replication in human monocyte-derived macrophages and primary microglia via nuclear factor-kappaB pathway. PloS one. 2014;9(3):e92145. doi: 10.1371/journal.pone.0092145. Epub 2014/03/26. Shows CXCL8 signaling, mediated by NFkB, is capable of facilitating HIV replication.
- 13.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116(2):281–297. doi: 10.1016/s0092-8674(04)00045-5. Epub 2004/01/28. [DOI] [PubMed] [Google Scholar]
- 14.Fowler L, Saksena NK. Micro-RNA: new players in HIV-pathogenesis, diagnosis, prognosis and antiviral therapy. AIDS reviews. 2013;15(1):3–14. Epub 2013/03/02. [PubMed] [Google Scholar]
- 15.Harwig A, Das AT, Berkhout B. Retroviral microRNAs. Current opinion in virology. 2014;7C:47–54. doi: 10.1016/j.coviro.2014.03.013. Epub 2014/04/29. [DOI] [PubMed] [Google Scholar]
- 16.Swaminathan G, Navas-Martin S, Martin-Garcia J. MicroRNAs and HIV-1 infection: antiviral activities and beyond. Journal of molecular biology. 2014;426(6):1178–1197. doi: 10.1016/j.jmb.2013.12.017. Epub 2013/12/29. [DOI] [PubMed] [Google Scholar]
- 17.Swaminathan S, Murray DD, Kelleher AD. miRNAs and HIV: unforeseen determinants of host-pathogen interaction. Immunological reviews. 2013;254(1):265–280. doi: 10.1111/imr.12077. Epub 2013/06/19. [DOI] [PubMed] [Google Scholar]
- 18. Sisk JM, Witwer KW, Tarwater PM, Clements JE. SIV replication is directly downregulated by four antiviral miRNAs. Retrovirology. 2013;10:95. doi: 10.1186/1742-4690-10-95. Epub 2013/08/31. Identified four host miRNAs which inhibit viral replication in macrophages.
- 19.Rebane A, Akdis CA. MicroRNAs: Essential players in the regulation of inflammation. The Journal of allergy and clinical immunology. 2013;132(1):15–26. doi: 10.1016/j.jaci.2013.04.011. Epub 2013/06/04. [DOI] [PubMed] [Google Scholar]
- 20.Sedger LM. microRNA control of interferons and interferon induced anti-viral activity. Molecular immunology. 2013;56(4):781–793. doi: 10.1016/j.molimm.2013.07.009. Epub 2013/08/22. [DOI] [PubMed] [Google Scholar]
- 21.Ma L, Shen CJ, Cohen EA, Xiong SD, Wang JH. miRNA-1236 Inhibits HIV-1 Infection of Monocytes by Repressing Translation of Cellular Factor VprBP. PloS one. 2014;9(6):e99535. doi: 10.1371/journal.pone.0099535. Epub 2014/06/17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Casey Klockow L, Sharifi HJ, Wen X, Flagg M, Furuya AK, Nekorchuk M, et al. The HIV-1 protein Vpr targets the endoribonuclease Dicer for proteasomal degradation to boost macrophage infection. Virology. 2013;444(1–2):191–202. doi: 10.1016/j.virol.2013.06.010. Epub 2013/07/16. Found HIV Vpr can have a broad effect on miRNA processing by targeting Dicer for degradation.
- 23.Kumar A, Abbas W, Herbein G. HIV-1 latency in monocytes/macrophages. Viruses. 2014;6(4):1837–1860. doi: 10.3390/v6041837. Epub 2014/04/25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marban C, Suzanne S, Dequiedt F, de Walque S, Redel L, Van Lint C, et al. Recruitment of chromatin-modifying enzymes by CTIP2 promotes HIV-1 transcriptional silencing. The EMBO journal. 2007;26(2):412–423. doi: 10.1038/sj.emboj.7601516. Epub 2007/01/25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cherrier T, Le Douce V, Eilebrecht S, Riclet R, Marban C, Dequiedt F, et al. CTIP2 is a negative regulator of P-TEFb. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(31):12655–12660. doi: 10.1073/pnas.1220136110. Discovered a new role for CTIP-2 in HIV latency in microglial cells. CTIP-2 is part of a large, inactive P-TEFb complex on the HIV LTR in a microglial cell line, which is independent of its recruitment of HDACs and HMT.
- 26.Eilebrecht S, Le Douce V, Riclet R, Targat B, Hallay H, Van Driessche B, et al. HMGA1 recruits CTIP2-repressed P-TEFb to the HIV-1 and cellular target promoters. Nucleic acids research. 2014;42(8):4962–4971. doi: 10.1093/nar/gku168. Epub 2014/03/14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gray LR, Turville SG, Hitchen TL, Cheng WJ, Ellett AM. HIV-1 Entry and Trans-Infection of Astrocytes Involves CD81 Vesicles (vol 9, e90620, 2014) PloS one. 2014;9(3) doi: 10.1371/journal.pone.0090620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Narasipura SD, Kim S, Al-Harthi L. Epigenetic Regulation of HIV-1 Latency in Astrocytes. Journal of virology. 2014;88(5):3031–3038. doi: 10.1128/JVI.03333-13. Discovered that HDACs and HMTs contribute to silencing HIV transcription in astrocytes, which has important implications for eradication strategies involving latency reversing agents.
- 29.Sgarbanti M, Battistini A. Therapeutics for HIV-1 reactivation from latency. Current opinion in virology. 2013;3(4):394–401. doi: 10.1016/j.coviro.2013.06.001. Epub 2013/07/03. [DOI] [PubMed] [Google Scholar]
- 30.Shan L, Siliciano RF. From reactivation of latent HIV-1 to elimination of the latent reservoir: the presence of multiple barriers to viral eradication. BioEssays : news and reviews in molecular, cellular and developmental biology. 2013;35(6):544–552. doi: 10.1002/bies.201200170. Epub 2013/04/25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Monroe K, Yang Z, Johnson J, Geng X, Doitsh G, Krogan N, et al. IFI16 DNA Sensor Is Required for Death of Lymphoid CD4 T Cells Abortively Infected with HIV. Science. 2013;343(6169):428432. doi: 10.1126/science.1243640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Doitsh G, Galloway N, Geng X, Yang Z, Monroe K, Zepeda O, et al. Cell death by pyroptosis drives CD4 T-cell depletion in HIV-1 infection. Nature. 2014;505(7484):509–514. doi: 10.1038/nature12940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zaritsky LA, Dery A, Leong WY, Gama L, Clements JE. Tissue-specific interferon alpha subtype response to SIV infection in brain, spleen, and lung. Journal of interferon & cytokine research : the official journal of the International Society for Interferon and Cytokine Research. 2013;33(1):24–33. doi: 10.1089/jir.2012.0018. This is the first report of IFN alpha specificity in the brain.
- 34. Polyak MJ, Vivithanaporn P, Maingat FG, Walsh JG, Branton W, Cohen EA, et al. Differential type 1 interferon-regulated gene expression in the brain during AIDS: interactions with viral diversity and neurovirulence. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2013;27(7):2829–2844. doi: 10.1096/fj.13-227868. This research highlights not only the importance of tetherin (among other genes) as a restriction factor but the dynamic between host responses and diverse viral population.
- 35.Mogensen TH, Melchjorsen J, Larsen CS, Paludan SR. Innate immune recognition and activation during HIV infection. Retrovirology. 2010;7:54. doi: 10.1186/1742-4690-7-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tarassishin L, Bauman A, Suh HS, Lee SC. Anti-viral and anti-inflammatory mechanisms of the innate immune transcription factor interferon regulatory factor 3: relevance to human CNS diseases. Journal of neuroimmune pharmacology : the official journal of the Society on NeuroImmune Pharmacology. 2013;8(1):132–144. doi: 10.1007/s11481-012-9360-5. These authors highlight the importance of innate immunity signal transduction through direct links with hallmarks of HIV disease.
- 37.Yao H, Bethel-Brown C, Li CZ, Buch SJ. HIV neuropathogenesis: a tight rope walk of innate immunity. Journal of neuroimmune pharmacology : the official journal of the Society on NeuroImmune Pharmacology. 2010;5(4):489–495. doi: 10.1007/s11481-010-9211-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Li J, Wang Y, Wang X, Ye L, Zhou Y, Persidsky Y, et al. Immune activation of human brain microvascular endothelial cells inhibits HIV replication in macrophages. Blood. 2013;121(15):2934–2942. doi: 10.1182/blood-2012-08-450353. Researchers here show how specific cell types in brain structures influence replication in other cells.
- 39. Geffin R, Martinez R, Perez R, Issac B, McCarthy M. Apolipoprotein E-dependent differences in innate immune responses of maturing human neuroepithelial progenitor cells exposed to HIV-1. Journal of neuroimmune pharmacology : the official journal of the Society on NeuroImmune Pharmacology. 2013;8(4):1010–1026. doi: 10.1007/s11481-013-9478-0. This paper shows a host genotype which may increase susceptibility to HAND in patients.
- 40.Dube M, Roy BB, Guiot-Guillain P, Binette J, Mercier J, Chiasson A, et al. Antagonism of tetherin restriction of HIV-1 release by Vpu involves binding and sequestration of the restriction factor in a perinuclear compartment. PLoS pathogens. 2010;6(4):e1000856. doi: 10.1371/journal.ppat.1000856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tarassishin L, Bauman A, Suh HS, Lee SC. Anti-viral and anti-inflammatory mechanisms of the innate immune transcription factor interferon regulatory factor 3: relevance to human CNS diseases. Journal of neuroimmune pharmacology : the official journal of the Society on NeuroImmune Pharmacology. 2013;8(1):132–144. doi: 10.1007/s11481-012-9360-5. [DOI] [PubMed] [Google Scholar]
- 42.Hornik TC, Neniskyte U, Brown GC. Inflammation induces multinucleation of Microglia via PKC inhibition of cytokinesis, generating highly phagocytic multinucleated giant cells. Journal of neurochemistry. 2014;128(5):650–661. doi: 10.1111/jnc.12477. [DOI] [PubMed] [Google Scholar]
- 43.Li J, Wang Y, Wang X, Ye L, Zhou Y, Persidsky Y, et al. Immune activation of human brain microvascular endothelial cells inhibits HIV replication in macrophages. Blood. 2013;121(15):2934–2942. doi: 10.1182/blood-2012-08-450353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Geffin R, Martinez R, Perez R, Issac B, McCarthy M. Apolipoprotein E-dependent differences in innate immune responses of maturing human neuroepithelial progenitor cells exposed to HIV-1. Journal of neuroimmune pharmacology : the official journal of the Society on NeuroImmune Pharmacology. 2013;8(4):1010–1026. doi: 10.1007/s11481-013-9478-0. [DOI] [PubMed] [Google Scholar]
- 45.Cuervo AM. Autophagy: many paths to the same end. Molecular and cellular biochemistry. 2004;263(1–2):55–72. doi: 10.1023/B:MCBI.0000041848.57020.57. Epub 2004/11/05. [DOI] [PubMed] [Google Scholar]
- 46. Fields J, Dumaop W, Rockenstein E, Mante M, Spencer B, Grant I, et al. Age-dependent molecular alterations in the autophagy pathway in HIVE patients and in a gp120 tg mouse model: reversal with beclin-1 gene transfer. Journal of neurovirology. 2013;19(1):89–101. doi: 10.1007/s13365-012-0145-7. Epub 2013/01/24. This paper shows a link between the reduction of autophagy markers in the brains of individuals with HIVE and then validates the link with HIV with a gp120 transgenic mouse model
- 47.Zhou D, Masliah E, Spector SA. Autophagy is increased in postmortem brains of persons with HIV-1-associated encephalitis. The Journal of infectious diseases. 2011;203(11):1647–1657. doi: 10.1093/infdis/jir163. Epub 2011/05/20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Spector SA, Zhou D. Autophagy: an overlooked mechanism of HIV-1 pathogenesis and neuroAIDS? Autophagy. 2008;4(5):704–706. doi: 10.4161/auto.6105. Epub 2008/04/22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Campbell GR, Spector SA. Hormonally active vitamin D3 (1alpha,25-dihydroxycholecalciferol) triggers autophagy in human macrophages that inhibits HIV-1 infection. The Journal of biological chemistry. 2011;286(21):18890–18902. doi: 10.1074/jbc.M110.206110. Epub 2011/04/02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Campbell GR, Spector SA. Toll-like receptor 8 ligands activate a vitamin D mediated autophagic response that inhibits human immunodeficiency virus type 1. PLoS pathogens. 2012;8(11):e1003017. doi: 10.1371/journal.ppat.1003017. Epub 2012/11/21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Campbell GR, Spector SA. Vitamin D inhibits human immunodeficiency virus type 1 and Mycobacterium tuberculosis infection in macrophages through the induction of autophagy. PLoS pathogens. 2012;8(5):e1002689. doi: 10.1371/journal.ppat.1002689. Epub 2012/05/17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Espert L, Varbanov M, Robert-Hebmann V, Sagnier S, Robbins I, Sanchez F, et al. Differential role of autophagy in CD4 T cells and macrophages during X4 and R5 HIV-1 infection. PloS one. 2009;4(6):e5787. doi: 10.1371/journal.pone.0005787. Epub 2009/06/06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Deneka M, Pelchen-Matthews A, Byland R, Ruiz-Mateos E, Marsh M. In macrophages, HIV-1 assembles into an intracellular plasma membrane domain containing the tetraspanins CD81, CD9, and CD53. The Journal of cell biology. 2007;177(2):329–341. doi: 10.1083/jcb.200609050. Epub 2007/04/18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pelchen-Matthews A, Kramer B, Marsh M. Infectious HIV-1 assembles in late endosomes in primary macrophages. The Journal of cell biology. 2003;162(3):443–455. doi: 10.1083/jcb.200304008. Epub 2003/07/30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kyei GB, Dinkins C, Davis AS, Roberts E, Singh SB, Dong C, et al. Autophagy pathway intersects with HIV-1 biosynthesis and regulates viral yields in macrophages. The Journal of cell biology. 2009;186(2):255–268. doi: 10.1083/jcb.200903070. Epub 2009/07/29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gregoire IP, Richetta C, Meyniel-Schicklin L, Borel S, Pradezynski F, Diaz O, et al. IRGM is a common target of RNA viruses that subvert the autophagy network. PLoS pathogens. 2011;7(12):e1002422. doi: 10.1371/journal.ppat.1002422. Epub 2011/12/17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Campbell GR, Spector SA. Inhibition of human immunodeficiency virus type-1 through autophagy. Current opinion in microbiology. 2013;16(3):349–354. doi: 10.1016/j.mib.2013.05.006. Epub 2013/06/12. Authors describe a mechanism though which HIV hijacks the autophagosome to promote replication.
- 58. Nardacci R, Amendola A, Ciccosanti F, Corazzari M, Esposito V, Vlassi C, et al. Autophagy plays an important role in the containment of HIV-1 in nonprogressor-infected patients. Autophagy. 2014;10(7) doi: 10.4161/auto.28678. Epub 2014/05/13. Research here reaffirms autophagy as a host mecahnism capable of supression replication and HIV pathogenesis.
- 59.Nixon RA, Wegiel J, Kumar A, Yu WH, Peterhoff C, Cataldo A, et al. Extensive involvement of autophagy in Alzheimer disease: an immuno-electron microscopy study. Journal of neuropathology and experimental neurology. 2005;64(2):113–122. doi: 10.1093/jnen/64.2.113. Epub 2005/03/09. [DOI] [PubMed] [Google Scholar]
- 60.Pickford F, Masliah E, Britschgi M, Lucin K, Narasimhan R, Jaeger PA, et al. The autophagy-related protein beclin 1 shows reduced expression in early Alzheimer disease and regulates amyloid beta accumulation in mice. The Journal of clinical investigation. 2008;118(6):2190–2199. doi: 10.1172/JCI33585. Epub 2008/05/24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Crews L, Spencer B, Desplats P, Patrick C, Paulino A, Rockenstein E, et al. Selective molecular alterations in the autophagy pathway in patients with Lewy body disease and in models of alpha-synucleinopathy. PloS one. 2010;5(2):e9313. doi: 10.1371/journal.pone.0009313. Epub 2010/02/23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cuervo AM, Stefanis L, Fredenburg R, Lansbury PT, Sulzer D. Impaired degradation of mutant alpha-synuclein by chaperone-mediated autophagy. Science. 2004;305(5688):1292–1295. doi: 10.1126/science.1101738. Epub 2004/08/31. [DOI] [PubMed] [Google Scholar]
- 63.Scott JC, Woods SP, Carey CL, Weber E, Bondi MW, Grant I. Neurocognitive consequences of HIV infection in older adults: an evaluation of the "cortical" hypothesis. AIDS and behavior. 2011;15(6):1187–1196. doi: 10.1007/s10461-010-9815-8. Epub 2010/09/25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lake JE, Adams JS. Vitamin D in HIV-Infected Patients. Current HIV/AIDS reports. 2011;8(3):133–141. doi: 10.1007/s11904-011-0082-8. Epub 2011/06/08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nicoletti F, Lapenta C, Donati S, Spada M, Ranazzi A, Cacopardo B, et al. Inhibition of human immunodeficiency virus (HIV-1) infection in human peripheral blood leucocytes-SCID reconstituted mice by rapamycin. Clinical and experimental immunology. 2009;155(1):28–34. doi: 10.1111/j.1365-2249.2008.03780.x. Epub 2008/12/17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Viard JP, Souberbielle JC, Kirk O, Reekie J, Knysz B, Losso M, et al. Vitamin D and clinical disease progression in HIV infection: results from the EuroSIDA study. AIDS. 2011;25(10):1305–1315. doi: 10.1097/QAD.0b013e328347f6f7. Epub 2011/04/28. [DOI] [PubMed] [Google Scholar]
- 67.Campbell GR, Spector SA. Autophagy induction by vitamin D inhibits both Mycobacterium tuberculosis and human immunodeficiency virus type 1. Autophagy. 2012;8(10):1523–1525. doi: 10.4161/auto.21154. Epub 2012/08/16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Delgado MA, Elmaoued RA, Davis AS, Kyei G, Deretic V. Toll-like receptors control autophagy. The EMBO journal. 2008;27(7):1110–1121. doi: 10.1038/emboj.2008.31. Epub 2008/03/14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Shoji-Kawata S, Sumpter R, Leveno M, Campbell GR, Zou Z, Kinch L, et al. Identification of a candidate therapeutic autophagy-inducing peptide. Nature. 2013;494(7436):201–6. doi: 10.1038/nature11866. Epub 2013/02/01. Research discussed here has trememdous clinical implications for a therapeutic which may be capable of inhibiting not only HIV replication but other viruses which may induce encephalitis.