Abstract
Background
Approximately 90% of patients who die of Prostate Cancer (PCa) have bone metastases, which promote a spectrum of osteoblastic, osteolytic or mixed bone responses. Numerous secreted proteins have been reported to promote osteoblastic or osteolytic bone responses. We determined whether previously identified and/or novel proteins were associated with the osteoblastic or osteolytic response in clinical specimens of PCa bone metastases.
Methods
Gene expression was analyzed on 14 PCa metastases from 11 patients by microarray profiling and qRT-PCR, and protein expression was analyzed on 33 PCa metastases from 30 patients by immunohistochemistry on highly osteoblastic and highly osteolytic bone specimens.
Results
Transcript and protein levels of BMP-2, BMP-7, DKK-1, ET-1 and Sclerostin were not significantly different between osteoblastic and osteolytic metastases. However, levels of OPG, PGK1 and Substance P proteins were increased in osteoblastic samples. In addition, Emu1, MMP-12 and sFRP-1 were proteins identified with a novel role of being associated with either the osteoblastic or osteolytic bone response.
Conclusions
This is the first detailed analysis of bone remodeling proteins in human specimens of PCa bone metastases. Three proteins not previously shown to be involved may have a role in the PCa bone response. Furthermore, our data suggests that the relative expression of numerous, rather than a single, bone remodeling proteins determine the bone response in PCa bone metastases.
Keywords: prostate cancer, osteoblastic, osteolytic, bone, metastasis
Introduction
Approximately 90% of patients that die of prostate cancer (PCa) have bone metastases [1–5]. Tumor cells disseminate to the bone, which may progress to clinically relevant metastases that promote bone formation and/or resorption. This alteration in bone remodeling is thought to be due to aberrant cell-cell interactions between the metastatic tumor cells and the bone microenvironment. The complexity of the bone response in PCa is underscored by the variety of soluble factors, signaling pathways and transcriptional regulators involved. The abnormal bone response is further promoted by the osteomimic potential of the tumor cells signaling in a paracrine fashion within the bone environment, and an autocrine signaling cascade of the bone cells themselves [4, 6–8]. These interactions between the PCa cells and bone cells often yield a predominantly osteoblastic response. However, the formation of osteoblastic bone is also often associated with an osteolytic component, leading to a mixed, woven bone response in the same patient at different metastatic sites [2, 9, 10]. The alterations of normal bone equilibrium by metastatic PCa leads to the destruction of bone marrow, spinal cord compression, severe bone pain, cachexia and even death [6, 9]. Therefore, the identification of soluble factors responsible for the new bone formation in response to PCa is critical to increase our understanding of the biology of bone metastases and to successfully treat the morbidity associated with PCa.
While chemotherapeutic strategies show promise, there is no effective treatment for castration resistant PCa that substantially prolongs survival. A number of therapies have been developed to treat the osteolytic response with bisphosphonates (BP), and more recently, the RANKL inhibitor Denosumab [4, 6, 11]. In contrast, the mechanisms promoting the osteoblastic response are still unclear. A number of soluble proteins have been described that promote bone formation including, but not limited to, the BMP’s, ET-1, NELL-1, OPG, and PGK1 [4, 6, 12–17]. While each of these proteins can promote bone formation, it is not known if any of these proteins are directly responsible for the osteoblastic response in PCa patients.
Previous work focusing on PCa bone metastases has been restricted to animal models and in vitro investigations due to the lack of patient tissues to interrogate factors of interest. Herein, we describe the use of clinical human specimens of bone metastases (n=33) with distinct osteoblastic or osteolytic responses. Our objective was to provide a detailed expression analysis of recognized bone remodeling proteins in both highly osteoblastic and highly osteolytic PCa bone metastases, in addition to identifying novel PCa bone remodeling-associated proteins. The transcript and protein levels of ten recognized bone-remodeling proteins were assessed in both osteoblastic and osteolytic PCa metastases – bone morphogenetic protein 2 (BMP-2) [13, 15], bone morphogenetic protein 7 (BMP-7) [15], dickkopf-related protein 1 (DKK-1) [18, 19], receptor tyrosine-protein kinase erbB-3 (ErbB3) [20, 21], endothelin-1 (ET-1) [17, 22, 23], NEL-like protein 1 (NELL-1), tumor necrosis factor receptor superfamily 11B (OPG) [13, 14, 24, 25], phosphoglycerate kinase 1 (PGK1) [16], sclerostin [26, 27], Substance P [28, 29] and. In addition, we identified a putative osteoblastic factor EMI domain-containing protein 1 (Emu1), and two putative osteolytic factors, matrix metalloproteinase-12 (MMP-12) and secreted frizzled-related protein 1 (sFRP-1) in PCa bone metastases.
Materials and Methods
Clinical Data
Human PCa metastasis were obtained as part of the University of Washington Medical Center Prostate Cancer Donor Rapid Autopsy Program, which is approved by the University of Washington Institutional Review Board [1]. Thirty-three bone samples from rapid autopsies of 30 patients who died with a diagnosis of metastatic castration resistant prostate cancer were processed. One patient did not have complete clinical data available. For all patients with clinical data the mean age at autopsy was 70.9 years. All had received androgen deprivation therapy with mean treatment duration of 5.3 years. Twenty-five patients (86%) received BP therapy. Zoledronate was the most common BP medication; among those treated with BP the median treatment duration was 15.9 months (n=25).
Tissue Acquisition and Processing
From 30 patients, 33 metastatic cores were isolated at autopsy and divided into two portions – one flash frozen in liquid nitrogen used for RNA isolation and one decalcified in formic acid, fixed in 10% neutral buffered formalin and embedded in paraffin used for immunohistochemistry (IHC). From a selected subset of 11 patients, seven bone metastases were identified as highly osteoblastic and seven as highly osteolytic (Figure 1A). The corresponding frozen tissue was used for RNA isolation.
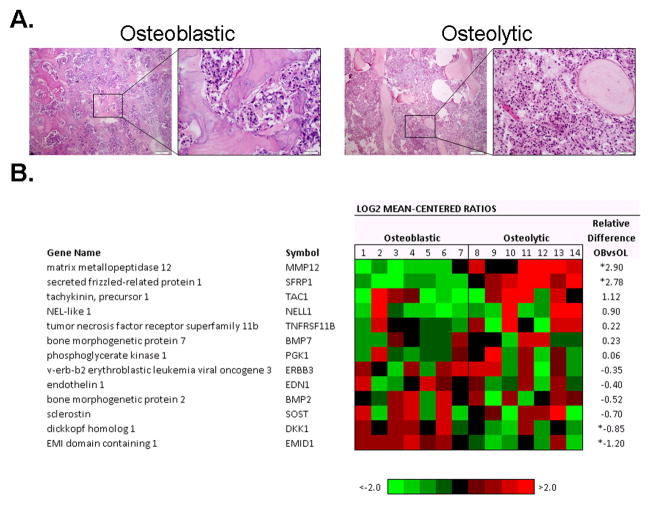
Figure 1. Histology and Gene Expression of PCa Bone Metastasis.
A. Representative H&E staining of an osteoblastic and osteolytic sample. Scale bar is equal to 50 μm. B. Agilent whole human genome microarrays were used to profile seven highly osteoblastic and seven highly osteolytic bone metastases. Mean-centered ratios of known and novel bone remodeling genes are colored according to scale. *Probes returning significance in signal strength (p<0.05) using paired t-test.
Due to the extreme difficulty in sectioning osteoblastic bone, laser capture microdissection (LCM) was not used to isolate RNA. However, macroscopic assessment of paraffin embedded tissues confirmed specimens to be at least 90% tumor, verified by IHC analysis.
RNA amplification and microarray hybridization
Agilent 44K whole human genome expression oligonucleotide microarrays (Agilent Technologies, Inc., Santa Clara, CA) were used to profile frozen bone cores. To provide a reference standard RNA for use on two-color oligonucleotide microarrays, we pooled equal amounts of total RNA isolated from four human prostate tumor cell lines LNCaP, DU145, PC3, and CWR22 (American Type Culture Collection, Manassas, VA) growing at log phase in dye-free RPMI-1640 medium supplemented with 10% fetal bovine serum (FBS) (Invitrogen, Carlsbad, CA). Two micrograms reference total RNA was amplified two rounds while 500 ng total RNA isolated from either osteoblastic or osteolytic bone metastatic cores was amplified one round using the Ambion MessageAmp aRNA Kit (Ambion Inc, Austin, TX), incorporating amino-allyl UTP into amplified antisense RNA. A total of 825 ng of amplified amino-allyl aRNA from each experimental sample was labeled with Cy3 fluorescent dye (reference amino-allyl aRNA was labeled with Cy5) and hybridized to custom Agilent 44K whole human genome expression oligonucleotide microarray slides (Agilent Technologies, Inc., Santa Clara, CA) following the manufacturer’s suggested protocols. Fluorescence array images were collected using the Agilent microarray scanner G2565BA. Agilent Feature Extraction software was used to grid, extract, and normalize data. Spots of poor quality or average intensity levels <300 were removed from further analysis. Statistical Analysis of Microarray (SAM) program (http://www-stat.stanford.edu/~tibs/SAM/) was used to analyze expression differences between the osteoblastic and osteolytic groups. Unpaired, t-tests were calculated for all probes passing filters and controlled for multiple testing by estimation of q-values using the false discovery rate (FDR) method [30]. Microarray data are deposited in the Gene Expression Omnibus database under the accession number GSE41619.
Quantitative reverse transcription-polymerase chain reaction (qRT-PCR)
Primers (Integrated DNA Technologies, Coralville, IA) were designed for β-actin (ACTB), BMP2, BMP7, DKK1, EDN1, EMID1, ERBB3 [20], MMP12, NELL1, PGK1, sFRP1, SOST and Substance P (TAC1), TNFRSF11B (Supplemental Table 1) to span intron-exon boundaries using Primer3 software (http://frodo.wi.mit.edu). One microgram of amplified RNA from each patient sample was reverse transcribed into cDNA using the Advantage RT-for-PCR kit according to manufacturer’s protocol (BD Biosciences, Palo Alto, CA). Reactions contained 10 μl of Absolute QPCR SYBR Green Mix (Thermo Scientific, Wilmington, DE), 2μl of cDNA template (1:10 dilution of reverse transcribed RNA), 0.4μl each of forward and reverse primer (200nM) and 7.2μl H2O. qRT-PCR was performed on a Rotor-Gene RG-3000 (Corbett Research, Sydney, Australia) using the following parameters: 95°C for 15 min followed by 40 cycles of denaturing at 95°C for 15 sec and annealing/extension at T(m)/72°C for 30 sec each. qRT-PCR quality was accessed using a four-fold dilution standard curve of LNCaP cDNA with a R2 value >0.99. Amplicon product was confirmed by melt curve analysis and gel electrophoresis. Using cycle threshold values, average expression levels were normalized against β-actin. Fold changes and significance using paired t-tests were determined between the osteoblastic and osteolytic samples.
Immunohistochemistry
Formalin-fixed paraffin-embedded tissue sections (5μm) were deparaffinized and rehydrated. Antigen retrieval was performed with 10 mM citrate buffer (pH 6.0) in a pressure cooker (20 psi for 5 min). Endogenous peroxide and avidin/biotin were blocked for 15 min respectively with corresponding reagents (Vector Laboratories Inc.). Sections were blocked with 5% normal goat-horse-chicken serum at room temperature for 1h, and incubated with primary antibody (Table 1) at 4°C overnight. Slides were incubated with corresponding biotinylated secondary antibody (1:150) (Vector Laboratories Inc.) for 30 min., followed by ABC reagent (Vector Laboratories Inc.) and stable DAB (Invitrogen Corp.). All sections were counterstained with hematoxylin and mounted with Cytoseal XYL (Richard Allan Scientific). Mouse, rabbit or goat IgG was used at the same concentration as the primary antibody for negative controls.
Table 1.
Antibodies used for IHC analysis of human PCa bone metastases.
| Protein | Antibody | Company | Clone/Lot | IHC Working Condition |
|---|---|---|---|---|
| Bone morphogenetic protein 2 | BMP-2 | LifeSpan BioSciences | LS-B785/20546 | 1:100 |
| Bone morphogenetic protein 7 | BMP-7 | Abcam | Ab56023 | 1:250 |
| Dickkopf-related protein 1 | Dkk-1 | SIGMA | HPA75704 | 1:30 |
| EMI domain-containing protein 1 | Emu1 | SIGMA | HPA000592 | 1:100 |
| Endothelin-1 | ET-1 | Thermo Scientific | TR.ET.48.5 | 1:250 |
| Macrophage metalloelastase | MMP-12 | Abcam | Ab52897 | 1:150 |
| Tumor necrosis factor receptor superfamily member 11B | OPG | IMGENEX | IMG-103A | 1:500 |
| Phosphoglycerate kinase 1 | PGK-1 | Abcam | Ab38007 | 1:25 |
| Sclerostin | Sclerostin | Abcam | Ab63097 | 1:250 |
| Secreted frizzled-related protein 1 | sFRP-1 | Abcam | Ab4193 | 1:800 |
| Protachykinin-1 | Substance P | Millipore | AB1566 | 1:200 |
Immunohistochemical assessment
Immunostaining was assessed using a quasi continuous score system, created by multiplying each intensity level (“0” for no brown color, “1” for faint and fine brown chromogen deposition, and “2” for clear and coarse granular chromogen clumps) with the corresponding percentage of cells expressing the particular intensity, and then summing all values to final score for each sample (scores ranging from 0–200) [31]. Cytoplasms and nuclei immunoreactivities were evaluated separately. The distribution of the tissue scores were grouped as “none” (score range: 0), “weak” (score range: 0~100), “moderate” (score range: 100~150) and “intense” (score range: 150~200). Samples with missing or damaged sections were excluded from analysis.
Statistical analysis
Significance of differences for the qRT-PCR and IHC analyses were calculated using a students t-test, with p values ≤ 0.05 indicating statistical significance.
Results
Gene expression analysis of bone remodeling genes
To assess transcript levels in bone metastases, mRNA was isolated from seven highly osteoblastic and seven highly osteolytic frozen tissues samples from 11 patients with castration resistant metastatic PCa. Our analysis of previously described genes associated with bone formation (BMP2, BMP7, EDN1, ERBB3, NELL1, PGK1, TAC1 and TNFRSF11B) and wnt signaling pathway inhibitors that restrict bone formation (DKK1 and SOST) were analyzed using gene expression arrays (Figure 1B). Of these ten genes, only DKK1 had significantly differential expression; exhibiting higher expression in osteoblastic samples than in the osteolytic samples (p=0.016). Additionally, three novel genes (EMID1, MMP12 and SFRP1) were identified in the PCa bone metastases, each exhibiting differential expression between the osteoblastic and osteolytic samples. EMID1 (p=0.002) had higher expression in osteoblastic samples, whereas MMP12 (p<0.001) and SFRP1 (p<0.001) had higher expression in osteolytic samples. The gene expression array results were confirmed by qRT-PCR using the same sample set, and protein expression was further analyzed by IHC.
Transcript and protein expression of bone forming factors
The qRT-PCR results for the eight previously described bone-promoting genes (BMP2, BMP7, EDN1, ERBB3, NELL1, PGK1, TAC1 and TNFRSF11B) were consistent with the gene expression array analysis. None of the aforementioned genes displayed any significant transcript differences (p<0.05) between the osteoblastic and osteolytic PCa bone samples (Figure 2A and Supplemental Figure 1). EMID1 had significantly higher expression in osteoblastic samples when compared to osteolytic samples (p=0.014) (Figure 2A).
Figure 2. Validation of gene expression arrays by qRT-PCR.
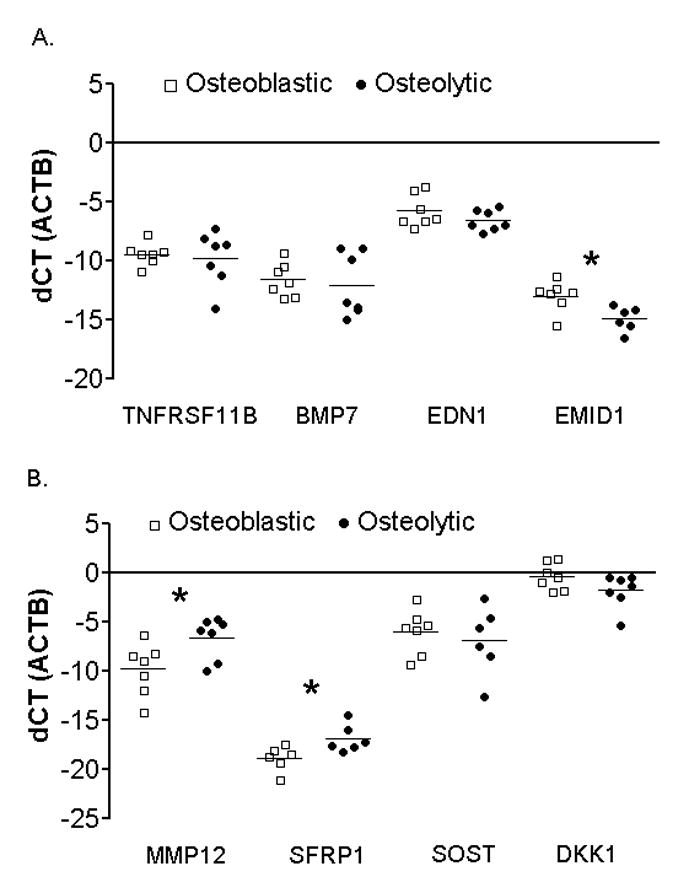
A. qRT-PCR of proposed osteoblastic-associated factors TNFRSF11B, BMP7, EDN1, and EMID1 in osteoblastic (white squares) and osteolytic (black circles) PCa metastases. B. qRT-PCR of proposed osteolytic-associated factors MMP12, SFRP1, SOST, and DKK1 in osteoblastic (white squares) and osteolytic (black circles) PCa metastases. All samples are normalized against β-actin. * indicates significant difference with a p-value of p < 0.05.
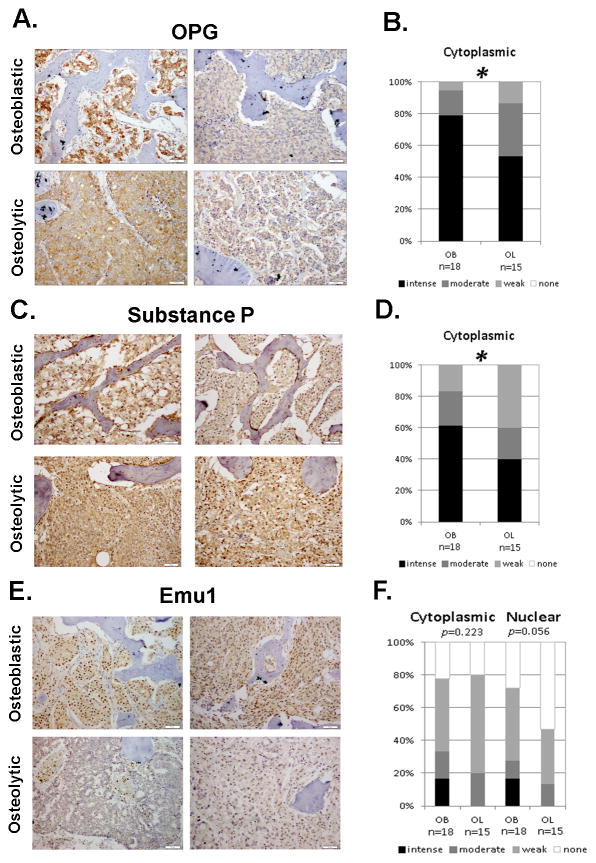
To further confirm the association of the selected genes with the osteoblastic bone response we calculated differential protein expression in human metastatic PCa metastasis from 27 patients. Three of the proposed bone forming proteins (BMP-2, BMP-7 and ET-1) displayed no significant difference in protein expression between osteoblastic and osteolytic samples (Supplemental Figure 2 and 3). This finding was consistent with our gene expression analysis, suggesting a uniform expression of the two BMP’s and ET-1 in the PCa bone metastases. Although present in all samples, cytoplasmic OPG, Substance P and PGK1 were significantly higher in osteoblastic samples than in osteolytic samples at the protein level (p=0.026, p=0.045 and p=0.009, respectively) (Figure 3A–D, Supplemental Figure 2 and 3). Cytoplasmic Emu1, while displaying higher expression in osteoblastic samples, was not significantly different in comparison to the osteolytic samples (p=0.223). Nuclear Emu1 trended towards significance in osteoblastic samples (p=0.056) when compared to osteolytic samples (Figure 3E, F). ErbB3 and NELL-1 were not analyzed because either no suitable commercial antibody was available or the staining was determined to be of insufficient quality to assess after repeated attempts.
Figure 3. Protein expression in osteoblastic (OB) and osteolytic (OL) PCa Bone Metastases.
A, C and E. IHC staining of two known (OPG and Substance P) and one novel (Emu1) osteoblastic-associated factor in PCa bone metastases. Scale bar is equal to 50 μm. B, D and F. Statistical analysis reveals significance in OPG and Substance P staining and approaching significance in Emu1 nuclear staining between the osteoblastic and osteolytic bone metastases. Staining intensity ranges from none (white) to intense (black). * p<0.05
Transcript and protein expression of bone degrading factors
By qRT-PCR, SOST expression was not significantly different between osteoblastic and osteolytic bone metastases (p=0.163) (Figure 2B). Although differentially expressed on the gene expression array, DKK1 did not show differential gene expression by qRT-PCR (p=0.112). The two genes that were significantly higher in osteolytic samples than in osteoblastic samples by gene expression arrays, and validated by qRT-PCR, were SFRP1 (p=0.024) and MMP12 (p=0.027) (Figure 2B).
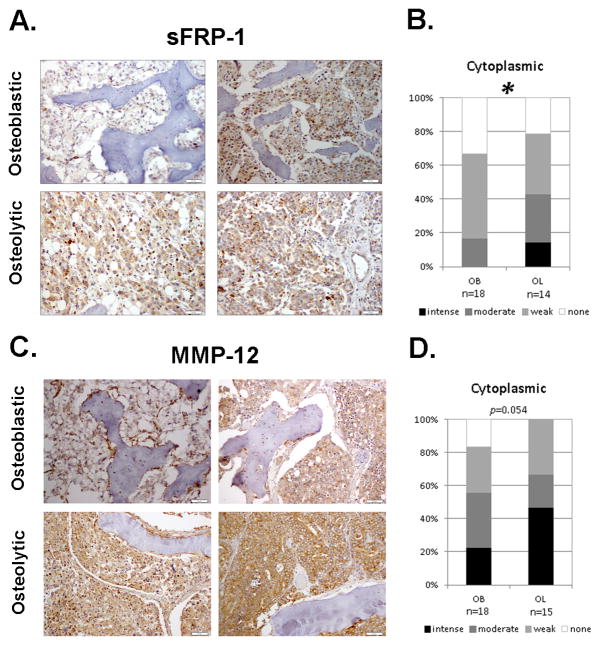
By IHC the previously identified osteoblastic inhibitor proteins, Sclerostin and DKK-1, did not show any significant differences in cytoplasmic staining for both (p=0.163; p=0.458, respectively), and nuclear staining for DKK-1 (p=0.476) (Supplemental Figure 2 and Supplemental Figure 3). Interestingly, cytoplasmic sFRP-1 was significantly higher in osteolytic bone metastases (p=0.044) (Figure 4A, B). Additionally, cytoplasmic expression of MMP-12 was higher in metastatic PCa osteolytic bone reactions, trending towards significance (p=0.054) (Figure 4C, D).
Figure 4. Protein expression in osteoblastic (OB) and osteolytic (OL) PCa Bone Metastases.
A,C. IHC staining of novel (sFRP-1, MMP-12) osteolytic factors in PCa bone metastases. Scale bar is equal to 50 μm. B,D. Statistical analysis reveals significance in sFRP-1 and approaching significance in MMP-12 cytoplasmic staining between the osteoblastic and osteolytic bone metastases. Staining intensity ranges from none (white) to intense (black). *p<0.05
Discussion
Despite the osteoblastic bone response being a significant problem for patients with advanced stage prostate cancer, the mechanism of osteoblastic stimulation by PCa cells and the factor, or factors, responsible for new bone formation have not yet been identified. In order to identify such proteins that will have clinical relevance, human samples of bone metastases are needed. Multiple studies have been performed to identify factors responsible for stimulation of the osteoblastic reaction by PCa tumor cells using in vitro and in vivo experimental models. However, the use of clinical samples has been hindered by a relative lack of relevant samples as well as by technical difficulties with extracting RNA and sectioning frozen bone tissue from the highly osteoblastic samples.
Our study provided interesting findings that show a substantial discrepancy between our results generated using clinical samples compared to those from in vitro and in vivo assessments. Furthermore, we provided novel information about the association of three proteins that have not yet been investigated in connection with osteoblastic or osteolytic PCa bone metastases.
The BMP’s are known to stimulate differentiation towards the osteoblastic lineage. Additionally, studies have shown expression of BMP-2/4, -6 and -7 in PCa bone metastases [32, 33]. Similarly, ET-1 has been associated with bone growth in prostate cancer, stimulating osteoblastic activity and negatively regulating osteoclastic activity [34, 35]. Surprisingly, BMP-2, BMP-7 and ET-1 were not differentially expressed in osteoblastic compared to osteolytic PCa bone metastases. Conversely, Sclerostin was elevated in PCa bone metastases, shown to inhibit bone formation and promote osteoclast formation [36]. Likewise, in vitro studies have shown that blocking DKK-1 expression in osteolytic PCa cells stimulated osteoblast differentiation and mineralization [37]. However, both cytoplasmic Sclerostin and DKK-1 were present in all osteoblastic and osteolytic samples. So while Sclerostin and DKK-1 promote bone degradation, they are not sufficient to activate a predominantly osteolytic response. Additionally, several proteins such as the BMP’s and ET-1 have been portrayed as being involved in the vicious cycle paradigm. This theory describes tumor burden regulation within the bone due to crosstalk between tumor cells and the bone microenvironment, particularly osteoblasts [38–40]. Our data does not support or oppose this theory.
Studies have shown that OPG and PGK1 promote bone growth by inhibiting osteoclastogenesis in intratibial and subcutaneous injected mice or in PCa cell lines, respectively [14, 16]. PGK1 has also been shown to induce osteoblastic differentiation [16]. Additionally, Substance P has been shown to have a dose-related osteogenic stimulating effect, promoting the osteoblastic phenotype in bone [29]. Our data support these studies, as OPG, PGK1 and Substance P displayed an increased protein expression in the osteoblastic samples in comparison to the osteolytic samples. However, both osteoblastic and osteolytic metastatic samples analyzed expressed OPG, Substance P and PGK1. Although there was increased protein expression, there was no significant difference at the transcript level between the osteoblastic and osteolytic samples. This discrepancy could be attributed to protein stabilization or other factors influencing protein stability and turnover. The OPG/RANK/RANKL pathway is known to regulate osteoclast proliferation and activity [41]. We have previously shown that OPG and RANK are expressed in PCa bone metastases [42]. We observed no RANKL expression in the tumor cells of PCa bone metastases. RANKL expression was however observed in rare spindle-like cells on the surface of new bone and in stromal cells. Therefore, given the scattered presence of RANKL expressing cells in the stroma we could not accurately assess the expression of RANKL in these cells relative to the expression of OPG in the tumor cells populating he osteoblastic and osteolytic PCa bone metastases. Nevertheless, osteoclast numbers have been shown to be significantly higher in high versus low bone volume PCa bone metastases [43]. This is consistent with the idea that OPG is expressed at higher levels in osteoblastic prostate cancer bone metastases and that there are fewer osteoclast like cells present in osteoblastic metastases.
Interestingly, the secreted protein Emu1 had elevated levels of nuclear staining in osteoblastic bone metastases. It has been shown to be prevalent in the epithelium during embryonic development. The glycosylated protein Emu1 maintains a cysteine-rich domain that is frequently involved in cellular signaling, forming homo- or heteromers on extracellular surfaces. Proteoglycans often signal to ligands associated with the extracellular matrix such as the BMPs and Wnts; therefore, it can be hypothesized that Emu1 aids in cell adhesion events [44]. Although this hypothesis is relevant in the expression of Emu1 in PCa bone metastases, it does not explain its increased presence in the osteoblastic bone.
sFRP1 displayed significant differential expression in osteolytic PCa bone metastases than in osteoblastic metastases. The extensively studied bone degrading protein sFRP1 has been identified as an inhibitor of the Wnt signaling cascade, inhibiting osteoblast viability [45]. Recent gene expression profiles have identified SFRP1 as having significant expression in advanced PCa patients in comparison to BPH and pT2, pT3/4 PCa stages, indicating its importance in advanced stage disease [46]. Our results suggest sFRP1 has a role in promoting the osteolytic response in PCa bone metastases.
MMP-12 also displayed increased expression in the osteolytic PCa bone metastases compared to the osteoblastic metastases. The proteolytic enzymes, MMP’s, are involved in a complex, collaborative role in cancer progression by the degradation of the extracellular matrix, regulating tumor-bone cell interactions and enhancing angiogenesis [47–49]. For example, in vitro studies have shown MMP-12 can activate MMP-2, which may then activate MMP-13 [47]. MMP-13 is expressed in breast cancer lesions, and has been shown to be involved in osteoclastogenesis and osteoclast activity [49]. Previous studies also identified MMP-12 as being expressed on PC3 PCa cells [50]. MMP-12 has now been identified to not only be expressed in clinical PCa samples, but present in osteolytic PCa bone metastases. It could be hypothesized that similar to MMP-13, MMP-12 could be involved in osteoclastogenesis, osteoclast differentiation and activity.
This study shows that PCa bone metastases express a variety of proteins that promote bone formation and bone breakdown. While some of these factors displayed significant differences in expression between osteoblastic and osteolytic metastases, no one factor stood out as being highly expressed in osteoblastic or osteolytic metastases. This leads to the conclusion that the proteins described are not the sole driving force for either the osteoblastic or osteolytic bone responses in PCa metastases in the bone. This suggests that a possible explanation for the heterogeneous, but predominantly osteoblastic bone response in PCa is that the process is multi-factorial. We propose two hypotheses as to why PCa bone metastases are predominantly osteoblastic. First, a single osteoblastic factor that is highly expressed in PCa cells provides the predominant driving force behind the osteoblastic response in PCa. Consequently, every other protein expressed has a secondary role. Alternatively, the process is multifactoral with PCa expressing a disproportionate number of pro-osteoblastic factors when compared to other solid tumor types that metastasize to the bone. The bone response while predominantly osteoblastic would depend on the relative levels and presence or absence of the factors in the PCa tumor in question. The heterogeneous nature of metastatic PCa would favor this hypothesis [1].
Supplementary Material
qRT-PCR of the known osteoblastic factors – NELL1, TAC1, PGK1, ERBB3 and BMP2 - in osteoblastic (white squares) and osteolytic (black circles) PCa metastases. Samples are normalized against β-actin.
Osteoblastic A. BMP-7, B. PGK1, C. ET-1, D. BMP-2 and osteolytic E. Sclerostin, F. DKK-1 associated factors involved in bone response using IHC. Scale bar is equal to 50 μm.
IHC protein expression of osteoblastic A. BMP-7, B. PGK-1, C. ET-1, D. BMP-2 and osteolytic E. Sclerostin, F. Dkk-1 associated proteins. Staining intensity ranges from intense (black) to none (white). Analysis reveals no statistical differential expression between osteoblastic samples and osteolytic samples (p<0.05).
Primer sequences used for qRT-PCR.
Acknowledgments
We would like to thank Jennifer Noteboom, Lisha Brown and Belinda Nghiem for assistance with this study. This material is the result of work supported by resources from the VA Puget Sound Health Care System, Seattle, Washington (RLV is a Research Career Scientist, PHL is a Staff Physician), by the Pacific Northwest Prostate Cancer SPORE (P50CA97186), the PO1 NIH grant (PO1CA085859), the Richard M. LUCAS Foundation, and by a generous donation from Jim and Catherine Allchin. CM is a recipient of a Career Development Award from the Pacific Northwest Prostate Cancer SPORE (P50CA097186).
References
- 1.Roudier MP, True LD, Higano CS, Vesselle H, Ellis W, Lange P, Vessella RL. Phenotypic heterogeneity of end-stage prostate carcinoma metastatic to bone. Hum Pathol. 2003;34:646–653. doi: 10.1016/s0046-8177(03)00190-4. [DOI] [PubMed] [Google Scholar]
- 2.Roudier MP, Morrissey C, True LD, Higano CS, Vessella RL, Ott SM. Histopathological assessment of prostate cancer bone osteoblastic metastases. J Urol. 2008;180:1154–1160. doi: 10.1016/j.juro.2008.04.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bubendorf L, Schopfer A, Wagner U, Sauter G, Moch H, Willi N, Gasser TC, Mihatsch MJ. Metastatic patterns of prostate cancer: an autopsy study of 1,589 patients. Hum Pathol. 2000;31:578–583. doi: 10.1053/hp.2000.6698. [DOI] [PubMed] [Google Scholar]
- 4.Sturge J, Caley MP, Waxman J. Bone metastasis in prostate cancer: emerging therapeutic strategies. Nat Rev Clin Oncol. 2011;8:357–368. doi: 10.1038/nrclinonc.2011.67. [DOI] [PubMed] [Google Scholar]
- 5.Lange PH, Vessella RL. Mechanisms, hypotheses and questions regarding prostate cancer micrometastases to bone. Cancer Metastasis Rev. 1998;17:331–336. doi: 10.1023/a:1006106209527. [DOI] [PubMed] [Google Scholar]
- 6.Roato I, D’Amelio P, Gorassini E, Grimaldi A, Bonello L, Fiori C, Delsedime L, Tizzani A, De LA, Isaia G, Ferracini R. Osteoclasts are active in bone forming metastases of prostate cancer patients. PLoS One. 2008;3:e3627. doi: 10.1371/journal.pone.0003627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rucci N, Teti A. Osteomimicry: how tumor cells try to deceive the bone. Front Biosci (Schol Ed) 2010;2:907–915. doi: 10.2741/s110. [DOI] [PubMed] [Google Scholar]
- 8.Koeneman KS, Yeung F, Chung LW. Osteomimetic properties of prostate cancer cells: a hypothesis supporting the predilection of prostate cancer metastasis and growth in the bone environment. Prostate. 1999;39:246–261. doi: 10.1002/(sici)1097-0045(19990601)39:4<246::aid-pros5>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 9.Olson KB, Pienta KJ. Pain management in patients with advanced prostate cancer. Oncology (Williston Park) 1999;13:1537–1549. [PubMed] [Google Scholar]
- 10.Morrissey C, Roudier MP, Dowell A, True LD, Ketchanji M, Welty C, Corey E, Lange PH, Higano CS, Vessella RL. Effects of androgen deprivation therapy and bisphosphonate treatment on bone in patients with metastatic castration resistant prostate cancer: results from the University of Washington rapid autopsy series. J Bone Miner Res. 2012 doi: 10.1002/jbmr.1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yee AJ, Raje NS. Denosumab, a RANK ligand inhibitor, for the management of bone loss in cancer patients. Clin Interv Aging. 2012;7:331–338. doi: 10.2147/CIA.S14566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fizazi K, Carducci M, Smith M, Damiao R, Brown J, Karsh L, Milecki P, Shore N, Rader M, Wang H, Jiang Q, Tadros S, Dansey R, Goessl C. Denosumab versus zoledronic acid for treatment of bone metastases in men with castration-resistant prostate cancer: a randomised, double-blind study. Lancet. 2011;377:813–822. doi: 10.1016/S0140-6736(10)62344-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aghaloo T, Cowan CM, Zhang X, Freymiller E, Soo C, Wu B, Ting K, Zhang Z. The effect of NELL1 and bone morphogenetic protein-2 on calvarial bone regeneration. J Oral Maxillofac Surg. 2010;68:300–308. doi: 10.1016/j.joms.2009.03.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang J, Dai J, Qi Y, Lin DL, Smith P, Strayhorn C, Mizokami A, Fu Z, Westman J, Keller ET. Osteoprotegerin inhibits prostate cancer-induced osteoclastogenesis and prevents prostate tumor growth in the bone. J Clin Invest. 2001;107:1235–1244. doi: 10.1172/JCI11685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Feeley BT, Gamradt SC, Hsu WK, Liu N, Krenek L, Robbins P, Huard J, Lieberman JR. Influence of BMPs on the formation of osteoblastic lesions in metastatic prostate cancer. J Bone Miner Res. 2005;20:2189–2199. doi: 10.1359/JBMR.050802. [DOI] [PubMed] [Google Scholar]
- 16.Jung Y, Shiozawa Y, Wang J, Wang J, Wang Z, Pedersen EA, Lee CH, Hall CL, Hogg PJ, Krebsbach PH, Keller ET, Taichman RS. Expression of PGK1 by prostate cancer cells induces bone formation. Mol Cancer Res. 2009;7:1595–1604. doi: 10.1158/1541-7786.MCR-09-0072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yin JJ, Mohammad KS, Kakonen SM, Harris S, Wu-Wong JR, Wessale JL, Padley RJ, Garrett IR, Chirgwin JM, Guise TA. A causal role for endothelin-1 in the pathogenesis of osteoblastic bone metastases. Proc Natl Acad Sci U S A. 2003;100:10954–10959. doi: 10.1073/pnas.1830978100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thudi NK, Martin CK, Murahari S, Shu ST, Lanigan LG, Werbeck JL, Keller ET, McCauley LK, Pinzone JJ, Rosol TJ. Dickkopf-1 (DKK-1) stimulated prostate cancer growth and metastasis and inhibited bone formation in osteoblastic bone metastases. Prostate. 2011;71:615–625. doi: 10.1002/pros.21277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hall CL, Kang S, MacDougald OA, Keller ET. Role of Wnts in prostate cancer bone metastases. J Cell Biochem. 2006;97:661–672. doi: 10.1002/jcb.20735. [DOI] [PubMed] [Google Scholar]
- 20.Lin SH, Cheng CJ, Lee YC, Ye X, Tsai WW, Kim J, Pasqualini R, Arap W, Navone NM, Tu SM, Hu M, Yu-Lee LY, Logothetis CJ. A 45-kDa ErbB3 secreted by prostate cancer cells promotes bone formation. Oncogene. 2008;27:5195–5203. doi: 10.1038/onc.2008.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen N, Ye XC, Chu K, Navone NM, Sage EH, Yu-Lee LY, Logothetis CJ, Lin SH. A secreted isoform of ErbB3 promotes osteonectin expression in bone and enhances the invasiveness of prostate cancer cells. Cancer Res. 2007;67:6544–6548. doi: 10.1158/0008-5472.CAN-07-1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nelson JB, Hedican SP, George DJ, Reddi AH, Piantadosi S, Eisenberger MA, Simons JW. Identification of endothelin-1 in the pathophysiology of metastatic adenocarcinoma of the prostate. Nat Med. 1995;1:944–949. doi: 10.1038/nm0995-944. [DOI] [PubMed] [Google Scholar]
- 23.Nelson J, Bagnato A, Battistini B, Nisen P. The endothelin axis: emerging role in cancer. Nat Rev Cancer. 2003;3:110–116. doi: 10.1038/nrc990. [DOI] [PubMed] [Google Scholar]
- 24.Chen G, Sircar K, Aprikian A, Potti A, Goltzman D, Rabbani SA. Expression of RANKL/RANK/OPG in primary and metastatic human prostate cancer as markers of disease stage and functional regulation. Cancer. 2006;107:289–298. doi: 10.1002/cncr.21978. [DOI] [PubMed] [Google Scholar]
- 25.Zhang X, Zara J, Siu RK, Ting K, Soo C. The role of NELL-1, a growth factor associated with craniosynostosis, in promoting bone regeneration. J Dent Res. 2010;89:865–878. doi: 10.1177/0022034510376401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gkotzamanidou M, Dimopoulos MA, Kastritis E, Christoulas D, Moulopoulos LA, Terpos E. Sclerostin: a possible target for the management of cancer-induced bone disease. Expert Opin Ther Targets. 2012;16:761–769. doi: 10.1517/14728222.2012.697154. [DOI] [PubMed] [Google Scholar]
- 27.Wijenayaka AR, Kogawa M, Lim HP, Bonewald LF, Findlay DM, Atkins GJ. Sclerostin stimulates osteocyte support of osteoclast activity by a RANKL-dependent pathway. PLoS One. 2011;6:e25900. doi: 10.1371/journal.pone.0025900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goto T, Nakao K, Gunjigake KK, Kido MA, Kobayashi S, Tanaka T. Substance P stimulates late-stage rat osteoblastic bone formation through neurokinin-1 receptors. Neuropeptides. 2007;41:25–31. doi: 10.1016/j.npep.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 29.Shih C, Bernard GW. Neurogenic substance P stimulates osteogenesis in vitro. Peptides. 1997;18:323–326. doi: 10.1016/s0196-9781(96)00280-x. [DOI] [PubMed] [Google Scholar]
- 30.Tusher VG, Tibshirani R, Chu G. Significance analysis of microarrays applied to the ionizing radiation response. Proc Natl Acad Sci U S A. 2001;98:5116–5121. doi: 10.1073/pnas.091062498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang X, Morrissey C, Sun S, Ketchandji M, Nelson PS, True LD, Vakar-Lopez F, Vessella RL, Plymate SR. Androgen receptor variants occur frequently in castration resistant prostate cancer metastases. PLoS One. 2011;6:e27970. doi: 10.1371/journal.pone.0027970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Spanjol J, Djordjevic G, Markic D, Klaric M, Fuckar D, Bobinac D. Role of bone morphogenetic proteins in human prostate cancer pathogenesis and development of bone metastases: immunohistochemical study. Coll Antropol. 2010;34 (Suppl 2):119–125. [PubMed] [Google Scholar]
- 33.Morrissey C, Brown LG, Pitts TE, Vessella RL, Corey E. Bone morphogenetic protein 7 is expressed in prostate cancer metastases and its effects on prostate tumor cells depend on cell phenotype and the tumor microenvironment. Neoplasia. 2010;12:192–205. doi: 10.1593/neo.91836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bagnato A, Loizidou M, Pflug BR, Curwen J, Growcott J. Role of the endothelin axis and its antagonists in the treatment of cancer. Br J Pharmacol. 2011;163:220–233. doi: 10.1111/j.1476-5381.2011.01217.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chiao JW, Moonga BS, Yang YM, Kancherla R, Mittelman A, Wu-Wong JR, Ahmed T. Endothelin-1 from prostate cancer cells is enhanced by bone contact which blocks osteoclastic bone resorption. Br J Cancer. 2000;83:360–365. doi: 10.1054/bjoc.2000.1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yavropoulou MP, van Lierop AH, Hamdy NA, Rizzoli R, Papapoulos SE. Serum sclerostin levels in Paget’s disease and prostate cancer with bone metastases with a wide range of bone turnover. Bone. 2012;51:153–157. doi: 10.1016/j.bone.2012.04.016. [DOI] [PubMed] [Google Scholar]
- 37.Hall CL, Daignault SD, Shah RB, Pienta KJ, Keller ET. Dickkopf-1 expression increases early in prostate cancer development and decreases during progression from primary tumor to metastasis. Prostate. 2008;68:1396–1404. doi: 10.1002/pros.20805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Casimiro S, Guise TA, Chirgwin J. The critical role of the bone microenvironment in cancer metastases. Mol Cell Endocrinol. 2009;310:71–81. doi: 10.1016/j.mce.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 39.Ibrahim T, Flamini E, Mercatali L, Sacanna E, Serra P, Amadori D. Pathogenesis of osteoblastic bone metastases from prostate cancer. Cancer. 2010;116:1406–1418. doi: 10.1002/cncr.24896. [DOI] [PubMed] [Google Scholar]
- 40.Itoh T, Ito Y, Ohtsuki Y, Ando M, Tsukamasa Y, Yamada N, Naoe T, Akao Y. Microvesicles released from hormone-refractory prostate cancer cells facilitate mouse pre-osteoblast differentiation. J Mol Histol. 2012 doi: 10.1007/s10735-012-9415-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wittrant Y, Theoleyre S, Chipoy C, Padrines M, Blanchard F, Heymann D, Redini F. RANKL/RANK/OPG: new therapeutic targets in bone tumours and associated osteolysis. Biochim Biophys Acta. 2004;1704:49–57. doi: 10.1016/j.bbcan.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 42.Roudier M, Morrissey C, Huang L, Rohrbach K, Keller E, True L, Higano C, Vessella R, Dougall W. Immunohistochemical detection in post-mortem specimens of RANK/RANKL/OPG in prostate cancer bone and soft tissue metastases. American Society of Clinical Oncology Prostate Cancer Symposium; 2007. [Google Scholar]
- 43.Morrissey C, Roudier MP, Dowell A, True LD, Ketchanji M, Welty C, Corey E, Lange PH, Higano CS, Vessella RL. Effects of androgen deprivation therapy and bisphosphonate treatment on bone in patients with metastatic castration resistant prostate cancer: results from the University of Washington rapid autopsy series. J Bone Miner Res. 2012 doi: 10.1002/jbmr.1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Leimeister C, Steidl C, Schumacher N, Erhard S, Gessler M. Developmental expression and biochemical characterization of Emu family members. Dev Biol. 2002;249:204–218. doi: 10.1006/dbio.2002.0764. [DOI] [PubMed] [Google Scholar]
- 45.Thiele S, Rauner M, Goettsch C, Rachner TD, Benad P, Fuessel S, Erdmann K, Hamann C, Baretton GB, Wirth MP, Jakob F, Hofbauer LC. Expression profile of WNT molecules in prostate cancer and its regulation by aminobisphosphonates. J Cell Biochem. 2011;112:1593–1600. doi: 10.1002/jcb.23070. [DOI] [PubMed] [Google Scholar]
- 46.Monroe DG, Gee-Lawrence ME, Oursler MJ, Westendorf JJ. Update on Wnt signaling in bone cell biology and bone disease. Gene. 2012;492:1–18. doi: 10.1016/j.gene.2011.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kerkela E, Bohling T, Herva R, Uria JA, Saarialho-Kere U. Human macrophage metalloelastase (MMP-12) expression is induced in chondrocytes during fetal development and malignant transformation. Bone. 2001;29:487–493. doi: 10.1016/s8756-3282(01)00595-6. [DOI] [PubMed] [Google Scholar]
- 48.Kerkela E, la-Aho R, Jeskanen L, Rechardt O, Grenman R, Shapiro SD, Kahari VM, Saarialho-Kere U. Expression of human macrophage metalloelastase (MMP-12) by tumor cells in skin cancer. J Invest Dermatol. 2000;114:1113–1119. doi: 10.1046/j.1523-1747.2000.00993.x. [DOI] [PubMed] [Google Scholar]
- 49.Pivetta E, Scapolan M, Pecolo M, Wassermann B, bu-Rumeileh I, Balestreri L, Borsatti E, Tripodo C, Colombatti A, Spessotto P. MMP-13 stimulates osteoclast differentiation and activation in tumour breast bone metastases. Breast Cancer Res. 2011;13:R105. doi: 10.1186/bcr3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nabha SM, dos Santos EB, Yamamoto HA, Belizi A, Dong Z, Meng H, Saliganan A, Sabbota A, Bonfil RD, Cher ML. Bone marrow stromal cells enhance prostate cancer cell invasion through type I collagen in an MMP-12 dependent manner. Int J Cancer. 2008;122:2482–2490. doi: 10.1002/ijc.23431. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
qRT-PCR of the known osteoblastic factors – NELL1, TAC1, PGK1, ERBB3 and BMP2 - in osteoblastic (white squares) and osteolytic (black circles) PCa metastases. Samples are normalized against β-actin.
Osteoblastic A. BMP-7, B. PGK1, C. ET-1, D. BMP-2 and osteolytic E. Sclerostin, F. DKK-1 associated factors involved in bone response using IHC. Scale bar is equal to 50 μm.
IHC protein expression of osteoblastic A. BMP-7, B. PGK-1, C. ET-1, D. BMP-2 and osteolytic E. Sclerostin, F. Dkk-1 associated proteins. Staining intensity ranges from intense (black) to none (white). Analysis reveals no statistical differential expression between osteoblastic samples and osteolytic samples (p<0.05).
Primer sequences used for qRT-PCR.