Abstract
During prion infections, the cellular glycosylphosphatidylinositol-anchored glycoprotein PrP is converted into a conformational isoform. This abnormal conformer is thought to recruit and convert the normal cellular PrP into a likeness of itself and is proposed to be the infectious agent. We investigated the distribution of the PrP protein on the surface of Rov cells, an epithelial cell line highly permissive to prion multiplication, and we found that PrP is primarily expressed on the apical side. We further show that prion transmission to Rov cells is much more efficient if infectivity contacts the apical side, indicating that the apical and basolateral sides of Rov cells are not equally competent for prion infection and adding prions to the list of the conventional infectious agents (viruses and bacteria) that infect epithelial cells in a polarized manner. These data raise the possibility that apically expressed PrP may be involved in this polarized process of infection. This would add further support for a crucial role of PrP at the cell surface in prion infection of target cells.
The host-encoded PrP protein is essential for prion multiplication. Inhibiting its expression renders susceptible tissues or cells incapable of replicating prions (10, 33). PrP is a glycoprotein of unknown function anchored to the external leaflet of the plasma membrane through a glycosylphosphatidylinositol (GPI) anchor (38). Like other membrane proteins, PrP is synthesized and translocated into the rough endoplasmic reticulum and then transits through the Golgi before being delivered to the plasma membrane (reviewed in references 13 and 14). In contrast to other GPI proteins, however, PrP does not remain at the cell surface for long periods. It is rapidly endocytosed and constitutively cycles between endocytic compartments and the plasma membrane (21, 28, 35, 40). PrP undergoes numerous posttranslational modifications, including cleavage of the signal peptide, addition of the GPI anchor, formation of a single intrachain disulfide bond, N-glycosylation on two possible glycosylation sites, and also posttranslational cleavages during endocytosis and recycling of the protein (13, 14).
During prion pathogenesis, multiplication of the infectious agent occurs concomitantly with the conversion of the PrP protein into a detergent-insoluble, protease-resistant isoform called PrPsc (reviewed in reference 30). The most widely accepted hypothesis is that PrPsc, or a precursor of it, is the infective agent (29); introduced into the organism, the abnormal conformer is thought to recruit and convert the cellular PrP into a likeness of itself. However, the molecular details of PrP conversion, including possible cofactor requirements, are unclear (8). At the cellular level, too, a number of uncertainties remain. Analysis by immunotechniques indicates that accumulated abnormal PrP is widely distributed in cultured infected cells (13, 14); however, the initial interaction of exogenous abnormal PrP with a target cell is poorly characterized, and the subcellular site(s) at which the first conversion events occur has yet to be identified. In addition, very few cultured cell lines are able to replicate prions (31, 37), indicating that prion multiplication requires a number of unidentified cellular and/or molecular factors, the conjunction of which may be restricted to a few cell lines, most of them with neuronal features.
It was recently shown that efficient multiplication of prions can occur in nonneuronal cultured cells (44). Rov cells are derived from rabbit kidney (RK13) epithelial cells by transfection of a Tet-regulatable ovine PrP gene. These epithelial cells can be infected with low doses of brain homogenate, and there is evidence that Rov is a relevant model to study the influence of natural PrP polymorphisms on sheep scrapie infection (32). Using cells grown on porous filters, we show here that the efficiency of prion transmission to Rov cells strongly depends on the cellular side to which exogenous sheep prions are presented. Rov cells were much more easily infected if exogenous prions contacted their apical sides, indicating that the apical and basolateral sides of Rov cells are not equally susceptible to prion infection. The PrP protein, the presence of which is shown here to be restricted to the Rov cell apical surface, may be involved in this polarized process of infection.
MATERIALS AND METHODS
Cell culture.
Rov cells (44) were maintained at 37°C in 6% CO2 in α minimal essential medium containing 10% fetal calf serum, 100 U of penicillin/ml, and 10 μg of streptomycin/ml and were split at a 1:4 dilution weekly. To induce the expression of the transfected ovine PrP protein, the cultures were treated with 1 μg of doxycycline (Dox)/ml. To obtain polarized monolayers of Rov cells, we used transwell inserts (Transwell-clear; 12-mm diameter; 0.4 or 3 μm pore size; Corning-Costar, Corning, N.Y.) placed in each well of a 12-well tissue culture plate (Costar). Cells (105) were seeded on the porous filter and were allowed to reach confluence (∼4 × 105 cells per filter). The transmembrane resistance (TER) was measured daily with a Millicell-ERS meter (Millipore). The TER (100 Ω · cm2) was stable 7 days after seeding, and the Rov monolayers were used after 10 days.
MovS cells (the MovS6 clone) are mouse Schwann-like cells established from mice transgenic for the ovine PrP and constitutively expressing this protein (1). These cells were cultured in a mixture of Dulbecco's modified Eagle's medium and F12 medium (3/4 and 1/4, respectively) containing 10% fetal calf serum, 100 U of penicillin/ml, and 10 μg of streptomycin/ml and were split at a 1:10 dilution weekly.
Flotation of ovine PrP in sucrose gradient.
Detergent and flotation procedures were performed as described previously (7). Dox-induced Rov cells were grown to confluence in 25-cm2 tissue culture dishes and then placed on ice. The monolayers were rinsed three times with phosphate-buffered saline (PBS) and lysed in 1 ml of TNE/TX100 buffer (20 mM Tris-HCl, pH 7.4, 150 mM NaCl, 1 mM EDTA, 1% Triton X-100). The lysates were collected with a rubber scraper and homogenized by being passed 10 times through a 25-gauge needle and were left on ice for 30 min. The lysate was then brought to 40% sucrose by using 80% sucrose in TNE and placed in an ultracentrifuge tube. A discontinuous (40, 35, and 5%) sucrose gradient was layered over the lysate, and the flotation gradient was ultracentrifuged at 180,000 × g for 18 h at 4°C in a Beckman SW41 rotor. Twelve fractions (1 ml each) were harvested from the top of the gradient and sonicated. Each fraction was dot blotted for GM1 ganglioside detection with peroxidase-conjugated cholera toxin and also analyzed for PrP by immunoblotting after methanol precipitation.
Preparation of cell inocula and infection of cell cultures.
Extracts from prion-infected Rov cultures were prepared by scraping cells into PBS, pelleting them by centrifugation, and resuspending the cell pellets in a sterile 5% glucose solution. The cellular membranes were destroyed by four freezing-thawing cycles. Infection of Rov and MovS cells was carried out on porous filters (24-mm diameter; pore size, 3 μm). In the case of basolateral infection, the transwell insert was turned over (so that the bottom side faced up), and cells were seeded on it. The cells were allowed to attach for 3 h, after which the filter was turned back to the upright position in six-well culture plates (see Fig. 3A).
FIG. 3.
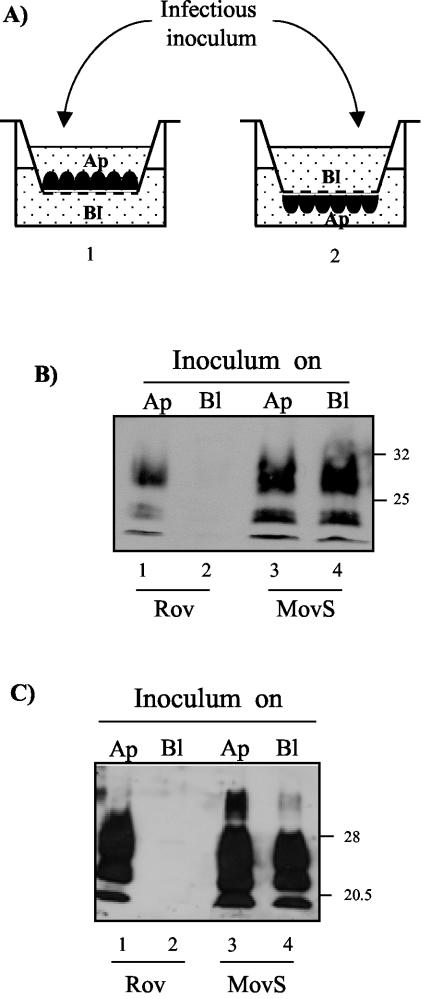
Prion infection of Rov cells occurs preferentially through the apical membrane domain. (A) Experimental setup. (1) Cells were seeded on porous filters to present their apical sides (Ap) to the infectious inoculum. (2) The cells were seeded onto inverted inserts and allowed to attach for a few hours. The filters were then placed upright in the culture plates, thus orienting the basolateral sides (Bl) of the monolayers upward. The inoculum was placed in the upper chamber and allowed to sediment during basolateral infection. (B) Infectious inoculum was loaded for 2 days either on the basolateral (lanes 2 and 4) or on the apical (lanes 1 and 3) domains of Rov or MovS cells. The inoculum was removed, and the cells were rinsed and lysed on the filter 1 week later. The cellular lysates were PK digested, and PrPres from a representative experiment is shown after being immunoblotted with ICSM18 MAb. Incubation of the infectious inoculum on the apical sides of Rov cells grown in the absence of Dox indicates that most of the PrPres signal in lane 1 is neoformation of PrPres (not shown). The positions of molecular mass marker proteins are indicated (in kilodaltons). (C) Infectious inoculum was loaded for 2 days either on the basolateral (lanes 2 and 4) or on the apical (lanes 1 and 3)domains of Rov or MovS cells. The inoculum was removed, and the cells were then trypsinized and passaged twice in plastic dishes. The resulting cultures were extracted with detergents, the cellular lysates were digested with PK, and PrPres of a representative experiment is shown after being immunoblotted with ICSM18 MAb. The lower level of PrPres in MovS cells exposed to prions through the porous filter (compare lane 4 to lane 3) may be due to some adsorption of prions on the filter. When the infectious inoculum was incubated on the apical sides of Rov cells expressing no PrP (i.e., grown in the absence of Dox), no PrPres was detected (not shown), as previously reported (44). The positions of molecular mass marker proteins are indicated (in kilodaltons).
Rov and MovS cells were exposed for 2 days to infectious cellular extract that had been sonicated for 2 min and diluted in culture medium. The infectious inoculum was removed, and the cells were incubated on the filters for 5 more days before being lysed. Alternatively, rinsed cells were scraped and subcultured twice in new tissue culture flasks before being analyzed for PrPres.
Immunoprecipitation of cell surface PrP.
Dox-induced Rov cells grown on porous cell filters (pore size, 0.4 μm) were placed on ice. The anti-PrP monoclonal antibody (MAb) 4F2 (ascites fluid, 1/50) (16) was added for 1 h in the upper compartment against the apical side of the monolayer or in the lower compartment against the basolateral side. The monolayers were then extensively washed in PBS buffer, lysed with Triton-DOC lysis buffer (50 mM Tris · HCl, pH 7.4-0.5% Triton X-100-0.5% sodium deoxycholate), and the cellular extracts were cleared (2,000 rpm; 1 min). The antibody-PrP complexes were adsorbed with 30 μl of protein A-Sepharose beads (Invitrogen), incubated on a wheel at 4°C for 1 h, and washed three times with lysis buffer. Cell surface PrP was also immunoprecipitated with ICSM18 MAbs (5). In that case, protein G-agarose (Roche Molecular Biochemicals) was used. The immunoprecipitated polypeptides were analyzed by Western blotting.
Immunofluorescence microscopy.
Dox-induced Rov cells were grown on porous filters (pore size, 0.4 μm). Polarized cells were then washed once with ice-cold PBS-CM (1 mM MgCl2, 0.1 mM CaCl2), fixed with 4% paraformaldehyde-4% sucrose for 10 min, and permeabilized for 3 min with 0.2% Triton X-100 in PBS. The cells were then stained with the following antibodies. PrPc was detected by using either 4F2 ascites fluid (1/7,500) or ICSM18 MAbs (0.75 μg/ml). Filamentous actin was detected with rhodamine-coupled phalloidin (Molecular Probes). ZO-1 expression was detected by using a rat anti-ZO-1 MAb (39). Bound MAbs were revealed with Texas red- or fluorescein isothiocyanate-coupled goat anti-mouse secondary antibody at room temperature for 30 min. After being washed in PBS, specimens were mounted in anti-fade medium (Vectashield; Vector Laboratories). Dual-immunofluorescence confocal images were acquired by using a confocal laser scanning microscope (CLSM 310; Carl Zeiss) equipped with a Plan-Apochromat 63× oil immersion objective (1.4 numerical aperture). Stacks of confocal images were acquired at 0.4-μm intervals. Images were processed using Zeiss software for overlays of red and green channels.
Isolation of abnormal PrP and Western blot analysis.
Infected cells were lysed for 10 min at 4°C in Triton-DOC lysis buffer (see above). The lysates were clarified (2,000 rpm; 1 min), and the equivalent of 500 μg of protein was digested with proteinase K (PK) for 2 h at 37°C (2 μg of PK for 500 μg of protein) in the presence of 0.02% bromophenol blue. Pefabloc (4 mM) was added at the end of the incubation, and blue pellets of aggregated PK-resistant PrP were collected by centrifugation at 13,000 rpm in a Microfuge for 20 min at room temperature and were boiled for 10 min in Laemmli denaturing sample buffer. All samples were electrophoresed on sodium dodecyl sulfate-12% polyacrylamide gels before transfer to nitrocellulose filters. The anti-PrP MAb was 4F2 or ICSM18, as appropriate. Bound MAbs were revealed with horseradish peroxidase-conjugated goat anti-rabbit antibodies. The reactivity was detected using the Western blotting detection reagent ECL kit (Amersham) and visualized with Hyperfilm/ECL (Amersham).
RESULTS AND DISCUSSION
The PrP protein, as a GPI-anchored protein, is directed to specialized membrane domains, which due to their particular lipid and protein composition, resist solubilization in nonionic detergents (e.g., Triton X-100). These detergent-resistant microdomains (DRM) are thought to select and concentrate molecules in a particular environment (reviewed in references 6 and 36), and indeed, PrP association with DRM (27, 43) is important for the conversion of PrP into the abnormal isoform, at least in neuroblastoma cells infected with a murine strain of prions (15, 22, 26, 41). In order to examine whether ovine PrP expressed in epithelial Rov cells was associated with DRM, PrP-expressing (Dox-treated) Rov cultures were extracted with cold Triton X-100, and detergent-insoluble complexes were separated on sucrose density gradients. Immunoblotting analysis of the different fractions showed that a substantial amount of PrP colocalized with the raft-resident sphingoglycolipid GM1 (Fig. 1A and C, fraction 4). When Rov cells were extracted at 37°C, the PrP in the fraction was fully solubilized (Fig. 1B), showing that in epithelial Rov cells, as already shown in neuronal cells (20, 27, 43), a fraction of the PrP was associated with DRM.
FIG. 1.
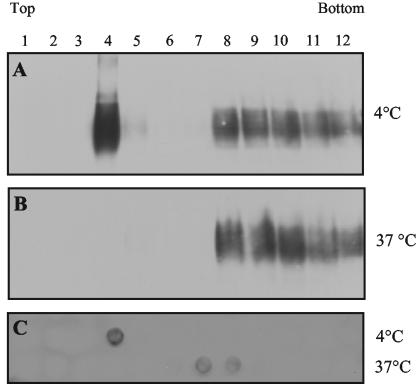
Ovine PrP expressed in Rov cells is present in DRM. (A and B) To determine if PrP was associated with DRM, Dox-treated Rov cells were extracted in Triton X-100 at 4 (A) or 37°C (B), and cell lysates were subjected to a standard flotation assay in sucrose gradient. Twelve fractions were collected and analyzed by immunoblotting for the presence of PrP (4F2 MAbs). (C) Aliquots of the fractions in panels A and B were also dot blotted onto nitrocellulose filters to detect the DRM-associated GM1 ganglioside.
A feature of epithelial cells is the targeting of specific proteins (e.g., GPI-anchored proteins) and lipids to distinct functional domains of the plasma membrane. This results in a polarized organization of the cell surface (25), with apical and basolateral domains separated from each other by tight junctions. To study PrP distribution in epithelial Rov cells, Dox-treated Rov cultures were grown to confluence on porous filters. The electrical resistance across the epithelial monolayer (TER) was measured daily, and stable TER (100 Ω · cm2) was considered an indication that Rov cells had formed a tight, polarized monolayer. Filter-grown Rov cells were fixed, permeabilized, and processed for indirect immunofluorescence with anti-PrP MAbs. Up to 50% of the Rov cells expressed detectable levels of ovine PrP, as previously shown (44), and analysis of the labeling by confocal microscopy revealed that PrP was mainly expressed at the apical surface of Rov cells (Fig. 2A). The steady-state localization of PrP at the apical and basolateral sides was further quantified by cell surface immunoprecipitation. Filter-grown Rov cells were cooled on ice, and anti-PrP MAbs were added to either the apical or the basolateral compartment. The monolayers were then washed and lysed, and PrP bound to MAbs was collected with protein A-Sepharose beads before being analyzed by immunoblotting. The results obtained with 4F2 antibodies, which do not recognize NH2-terminally truncated PrP (Fig. 2B), or with ICSM18 MAbs (not shown) show that steady-state total PrP was present almost exclusively on the apical side of Rov cells. This localization was not unexpected, as GPI-anchored proteins are generally sorted to the apical side of polarized cultured epithelial cells (18, 19). However, our finding is in sharp contrast to the basolateral localization of a transfected PrP observed in MDCK and FRT epithelial cell lines (34). The localization of PrP in Rov cells also differs from that in Caco-2 cells and human enterocytes, where it was observed in the cell-cell upper lateral junctional domains (24). This illustrates that different types of epithelial cells may target the same protein differently and confirms that a GPI anchor is not always sufficient to dictate apical sorting of a given protein (4, 47). As the mechanisms responsible for the polarized sorting of at least some proteins are similar in epithelial cells and neurons (11), distribution of PrP on the cell surface may also vary between different types of neurons.
FIG. 2.
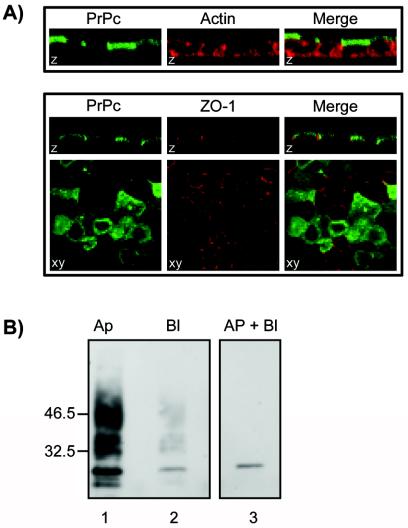
PrP protein is mainly present at the apical sides of Rov cells. (A) Confocal microscopic examination of PrP expression in polarized Rov cells. The apical distribution of PrPc (green) in filter-grown Rov cells was determined by immunostaining, together with the filamentous actin (red) or the tight-junctional marker ZO-1 (red). Images of polarized cells are either z sections through confocal images taken at sequential focal planes or xy views. (B) Immunoprecipitation of cell surface PrP. The apical (Ap) (lane 1) or basolateral (Bl) (lane 2) sides of filter-grown, Dox-stimulated (lanes 1 and 2) or unstimulated (lane 3) Rov cells were incubated at 4°C with 4F2 anti-PrP MAb. Antibody-bound PrP was then immunoprecipitated and analyzed by Western blotting with ICSM18. More than 90% of the steady-state PrP was apically localized. The positions of molecular mass marker proteins are indicated (in kilodaltons).
Transmission of prions to PrP-expressing Rov cells is achieved by incubating the monolayers for a few days in the presence of the infectious inoculum (usually brain homogenate from a scrapie-affected animal) diluted in Dox-containing growth medium (44). Inoculated cells can either be left unsettled and analyzed 1 week later or trypsinized and subcultured every week. The efficiency of prion infection is deduced from the levels of aggregated PK-resistant PrP (PrPres) in Rov cell lysates, as assessed by immunoblotting. To test if transmission of prions to Rov cells was a polarized event, infectious inoculum was put in contact with either the apical or the basolateral surface of filter-grown Rov cells, after which the inoculum was removed by successive washings. Transmission of prion infection to filter-grown cells was assessed in two ways. Monolayers were left on the filter for 5 more days before being analyzed for the presence of accumulated PrPres (Fig. 3B). Alternatively, filter-grown inoculated cells were scraped and passaged in new plastic flasks, and the efficiency of prion transmission was deduced from the levels of PrPres produced in the corresponding cultures after two passages (Fig. 3C). Since preliminary experiments indicated that 0.4-μm-pore-size filters prevented infectious insoluble material in brain homogenate from efficiently crossing the filter and accessing the basolateral cell surface, we grew the cells on 3.0-μm-pore-size filters. In addition, cells were seeded on the lower side of the filter, allowing prions in the insoluble material to more readily reach the basolateral surfaces of the cells (Fig. 3A), and cell extracts from prion-infected Rov cultures were used as the inoculum rather than crude brain homogenate. Rov cell susceptibility to apically and basolaterally applied prions was compared to that of a nonpolarized cell line: the recently isolated MovS neuroglial cell line, also permissive to sheep prions (1). We found that MovS cells were readily infected irrespective of the cellular side to which the infectious inoculum was presented (Fig. 3B and C), showing that prion infection can be successfully transmitted to cells through 3-μm-pore-size filters. In striking contrast, efficient transmission to Rov cells was observed only when sheep prions were put in contact with their apical sides (Fig. 3B and C). This result demonstrates that the interaction of prions with permissive cells does not necessarily lead to successful infection.
It is tempting to speculate that efficient transmission of apically presented prions is related to the presence of PrP at the apical surfaces of epithelial Rov cells. Consistent with the proposed importance of cell surface PrP, its release from the cell surface by phosphatidylinositol-specific phospholipase C or treatments with anti-PrP MAb were shown to prevent prion transmission to N2a cells (12). Failure so far to transmit prions to epithelial MDCK and FRT cells (S. Lehmann, personal communication) is consistent with apical sorting of PrP as one component of the permissiveness of Rov cells to prions. It would also be very interesting to grow MDCK and FRT cells on plastic coated with infectious prions, thus exposing their PrP-expressing basolateral sides to prions. However, a possible role of another polarization-dependent factor cannot be excluded at this point. Depending on where conversion of PrPc into PrPsc occurs in the cell, two broad scenarios, not mutually exclusive, could be proposed to explain why the apical and basolateral surfaces of Rov cells have such dramatically different competences to prion infection. Studies of prion-infected cells have shown that abnormal PrP seems to be widely distributed. It has been visualized in the late endocytic lysosomal compartment (2, 23, 42) and can also be detected at the plasma membrane (17, 43). However, as PrP permanently cycles between endosomes and the plasma membrane (21, 28, 35, 40), some of these sites may merely represent subcellular sites of accumulation rather than sites where PrP conversion actually occurs. The plasma membrane is a plausible site for conversion to take place when uninfected target cells are exposed to prions. Cell-free reaction conversion was observed in isolated membrane vesicles containing both the normal and abnormal isoforms of PrP (3). Also, N2a neuroblastoma cells can be successfully infected by contact with physical supports, such as stainless steel wires, onto which prion infectivity was bound (46). Assuming irreversible binding to the wires, this study suggests that initiation of infection is very likely to involve the plasma membranes of the target cells. Accordingly, exogenous PrPsc in contact with the apical sides of Rov cells could interact with cellular PrP, either directly or after incorporation in the apical plasma membrane, initiating conversion events. In contrast, exogenous prions would inefficiently infect Rov cells through the basolateral membrane, as low levels of PrP are expressed on that side.
PrP conversion could also occur intracellularly (9), and conversion occurring in cell compartments with distinct PrP glycoforms has been proposed as a possible explanation for strain-specific glycosylation patterns of abnormal PrP (45). In that case, exogenous PrPsc should be internalized before cellular PrP could be converted in intracellular compartments. Uptake of PrPsc through the basolateral cell surface might be much less efficient than uptake through the apical surface. Alternatively, PrPsc taken up by the Rov cell basolateral surface may not be targeted to the relevant intracellular site(s) where conversion could take place. That efficient uptake and/or proper intracellular targeting of exogenous PrPsc might require the presence of PrP is an appealing possibility that needs further investigation.
Acknowledgments
We thank P. Adenot (Unité de Biologie du Développement et de la Reproduction, INRA, Jouy-en-Josas, France) for use of his confocal facilities; J. Grassi (Service de Pharmacologie et Immunologie, Commissariat à l'Energie Atomique, Saclay, France) and S. Hawke (Imperial College, London, United Kingdom) for 4F2 and ICSM18 MAbs, respectively; and W. Fifield, V. Beringue, and M. Moudjou for reading the manuscript.
S.P. was supported by a fellowship from INRA and the Ile de France region.
REFERENCES
- 1.Archer, F., C. Bachelin, O. Andreoletti, N. Besnard, G. Perrot, C. Langevin, A. Le Dur, D. Vilette, A. Baron-Van Evercooren, J. L. Vilotte, and H. Laude. 2004. Cultured peripheral neuroglial cells are highly permissive to sheep prion infection. J. Virol. 78:482-490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arnold, J. E., C. Tipler, L. Laszlo, J. Hope, M. Landon, and R. J. Mayer. 1995. The abnormal isoform of the prion protein accumulates in late-endosome-like organelles in scrapie-infected mouse brain. J. Pathol. 176:403-411. [DOI] [PubMed] [Google Scholar]
- 3.Baron, G. S., K. Wehrly, D. W. Dorward, B. Chesebro, and B. Caughey. 2002. Conversion of raft associated prion protein to the protease-resistant state requires insertion of PrP-res (PrP(Sc)) into contiguous membranes. EMBO J. 21:1031-1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Benting, J. H., A. G. Rietveld, and K. Simons. 1999. N-Glycans mediate the apical sorting of a GPI-anchored, raft-associated protein in Madin-Darby canine kidney cells. J. Cell Biol. 146:313-320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beringue, V., G. Mallinson, M. Kaisar, M. Tayebi, Z. Sattar, G. Jackson, D. Anstee, J. Collinge, and S. Hawke. 2003. Regional heterogeneity of cellular prion protein isoforms in the mouse brain. Brain 126:2065-2073. [DOI] [PubMed] [Google Scholar]
- 6.Brown, D. A., and E. London. 1998. Functions of lipid rafts in biological membranes. Annu. Rev. Cell Dev. Biol. 14:111-136. [DOI] [PubMed] [Google Scholar]
- 7.Brown, D. A., and J. K. Rose. 1992. Sorting of GPI-anchored proteins to glycolipid-enriched membrane subdomains during transport to the apical cell surface. Cell 68:533-544. [DOI] [PubMed] [Google Scholar]
- 8.Caughey, B., G. J. Raymond, M. A. Callahan, C. Wong, G. S. Baron, and L. W. Xiong. 2001. Interactions and conversions of prion protein isoforms. Adv. Protein Chem. 57:139-169. [DOI] [PubMed] [Google Scholar]
- 9.Caughey, B., G. J. Raymond, D. Ernst, and R. E. Race. 1991. N-terminal truncation of the scrapie-associated form of PrP by lysosomal protease(s): implications regarding the site of conversion of PrP to the protease-resistant state. J. Virol. 65:6597-6603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Daude, N., M. Marella, and J. Chabry. 2003. Specific inhibition of pathological prion protein accumulation by small interfering RNAs. J. Cell Sci. 116:2775-2779. [DOI] [PubMed] [Google Scholar]
- 11.Dotti, C. G., and K. Simons. 1990. Polarized sorting of viral glycoproteins to the axon and dendrites of hippocampal neurons in culture. Cell 62:63-72. [DOI] [PubMed] [Google Scholar]
- 12.Enari, M., E. Flechsig, and C. Weissmann. 2001. Scrapie prion protein accumulation by scrapie-infected neuroblastoma cells abrogated by exposure to a prion protein antibody. Proc. Natl. Acad. Sci. USA 98:9295-9299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harris, D. A. 1999. Cellular biology of prion diseases. Clin. Microbiol. Rev. 12:429-444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harris, D. A. 2003. Trafficking, turnover and membrane topology of PrP. Br. Med. Bull. 66:71-85. [DOI] [PubMed] [Google Scholar]
- 15.Kaneko, K., M. Vey, M. Scott, S. Pilkuhn, F. E. Cohen, and S. B. Prusiner. 1997. COOH-terminal sequence of the cellular prion protein directs subcellular trafficking and controls conversion into the scrapie isoform. Proc. Natl. Acad. Sci. USA 94:2333-2338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krasemann, S., M. Groschup, G. Hunsmann, and W. Bodemer. 1996. Induction of antibodies against human prion proteins (PrP) by DNA-mediated immunization of PrP0/0 mice. J. Immunol. Methods 199:109-118. [DOI] [PubMed] [Google Scholar]
- 17.Lehmann, S., and D. A. Harris. 1996. Mutant and infectious prion proteins display common biochemical properties in cultured cells. J. Biol. Chem. 271:1633-1637. [DOI] [PubMed] [Google Scholar]
- 18.Lisanti, M. P., A. Le Bivic, A. R. Saltiel, and E. Rodriguez-Boulan. 1990. Preferred apical distribution of glycosyl-phosphatidylinositol (GPI) anchored proteins: a highly conserved feature of the polarized epithelial cell phenotype. J. Membr. Biol. 113:155-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lisanti, M. P., M. Sargiacomo, L. Graeve, A. R. Saltiel, and E. Rodriguez-Boulan. 1988. Polarized apical distribution of glycosyl-phosphatidylinositol-anchored proteins in a renal epithelial cell line. Proc. Natl. Acad. Sci. USA 85:9557-9561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Madore, N., K. L. Smith, C. H. Graham, A. Jen, K. Brady, S. Hall, and R. Morris. 1999. Functionally different GPI proteins are organized in different domains on the neuronal surface. EMBO J. 18:6917-6926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Magalhaes, A. C., J. A. Silva, K. S. Lee, V. R. Martins, V. F. Prado, S. S. Ferguson, M. V. Gomez, R. R. Brentani, and M. A. Prado. 2002. Endocytic intermediates involved with the intracellular trafficking of a fluorescent cellular prion protein. J. Biol. Chem. 277:33311-33318. [DOI] [PubMed] [Google Scholar]
- 22.Mange, A., N. Nishida, O. Milhavet, H. E. McMahon, D. Casanova, and S. Lehmann. 2000. Amphotericin B inhibits the generation of the scrapie isoform of the prion protein in infected cultures. J. Virol. 74:3135-3140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McKinley, M. P., A. Taraboulos, L. Kenaga, D. Serban, A. Stieber, S. J. DeArmond, S. B. Prusiner, and N. Gonatas. 1991. Ultrastructural localization of scrapie prion proteins in cytoplasmic vesicles of infected cultured cells. Lab. Investig. 65:622-630. [PubMed] [Google Scholar]
- 24.Morel, E., S. Fouquet, D. Chateau, L. Yvernault, Y. Frobert, M. Pincon-Raymond, J. Chambaz, T. Pillot, and M. Rousset. 2004. The cellular prion protein PrPc is expressed in human enterocytes in cell-cell junctional domains. J. Biol. Chem. 279:1499-1505. [DOI] [PubMed] [Google Scholar]
- 25.Mostov, K., T. Su, and M. ter Beest. 2003. Polarized epithelial membrane traffic: conservation and plasticity. Nat. Cell Biol. 5:287-293. [DOI] [PubMed] [Google Scholar]
- 26.Naslavsky, N., H. Shmeeda, G. Friedlander, A. Yanai, A. H. Futerman, Y. Barenholz, and A. Taraboulos. 1999. Sphingolipid depletion increases formation of the scrapie prion protein in neuroblastoma cells infected with prions. J. Biol. Chem. 274:20763-20771. [DOI] [PubMed] [Google Scholar]
- 27.Naslavsky, N., R. Stein, A. Yanai, G. Friedlander, and A. Taraboulos. 1997. Characterization of detergent-insoluble complexes containing the cellular prion protein and its scrapie isoform. J. Biol. Chem. 272:6324-6331. [DOI] [PubMed] [Google Scholar]
- 28.Peters, P. J., A. Mironov, Jr., D. Peretz, E. van Donselaar, E. Leclerc, S. Erpel, S. J. DeArmond, D. R. Burton, R. A. Williamson, M. Vey, and S. B. Prusiner. 2003. Trafficking of prion proteins through a caveolae-mediated endosomal pathway. J. Cell Biol. 162:703-717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Prusiner, S. B. 1982. Novel proteinaceous infectious particles cause scrapie. Science 216:136-144. [DOI] [PubMed] [Google Scholar]
- 30.Prusiner, S. B. 1998. Prions. Proc. Natl. Acad. Sci. USA 95:13363-13383. [DOI] [PMC free article] [PubMed]
- 31.Race, R. 1991. The scrapie agent in vitro. Curr. Top. Microbiol. Immunol. 172:181-193. [DOI] [PubMed] [Google Scholar]
- 32.Sabuncu, E., S. Petit, A. Le Dur, T. Lan Lai, J. L. Vilotte, H. Laude, and D. Vilette. 2003. PrP polymorphisms tightly control sheep prion replication in cultured cells. J. Virol. 77:2696-2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sailer, A., H. Bueler, M. Fischer, A. Aguzzi, and C. Weissmann. 1994. No propagation of prions in mice devoid of PrP. Cell 77:967-968. [DOI] [PubMed] [Google Scholar]
- 34.Sarnataro, D., S. Paladino, V. Campana, J. Grassi, L. Nitsch, and C. Zurzolo. 2002. PrPC is sorted to the basolateral membrane of epithelial cells independently of its association with rafts. Traffic 3:810-821. [DOI] [PubMed] [Google Scholar]
- 35.Shyng, S. L., M. T. Huber, and D. A. Harris. 1993. A prion protein cycles between the cell surface and an endocytic compartment in cultured neuroblastoma cells. J. Biol. Chem. 268:15922-15928. [PubMed] [Google Scholar]
- 36.Simons, K., and E. Ikonen. 1997. Functional rafts in cell membranes. Nature 387:569-572. [DOI] [PubMed] [Google Scholar]
- 37.Solassol, J., C. Crozet, and S. Lehmann. 2003. Prion propagation in cultured cells. Br. Med. Bull. 66:87-97. [DOI] [PubMed] [Google Scholar]
- 38.Stahl, N., D. R. Borchelt, K. Hsiao, and S. B. Prusiner. 1987. Scrapie prion protein contains a phosphatidylinositol glycolipid. Cell 51:229-240. [DOI] [PubMed] [Google Scholar]
- 39.Stevenson, B. R., J. M. Anderson, D. A. Goodenough, and M. S. Mooseker. 1988. Tight junction structure and ZO-1 content are identical in two strains of Madin-Darby canine kidney cells which differ in transepithelial resistance. J. Cell Biol. 107:2401-2408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sunyach, C., A. Jen, J. Deng, K. T. Fitzgerald, Y. Frobert, J. Grassi, M. W. McCaffrey, and R. Morris. 2003. The mechanism of internalization of glycosylphosphatidylinositol-anchored prion protein. EMBO J. 22:3591-3601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Taraboulos, A., M. Scott, A. Semenov, D. Avrahami, L. Laszlo, S. B. Prusiner, and D. Avraham. 1995. Cholesterol depletion and modification of COOH-terminal targeting sequence of the prion protein inhibit formation of the scrapie isoform. J. Cell Biol. 129:121-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Taraboulos, A., D. Serban, and S. B. Prusiner. 1990. Scrapie prion proteins accumulate in the cytoplasm of persistently infected cultured cells. J. Cell Biol. 110:2117-2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vey, M., S. Pilkuhn, H. Wille, R. Nixon, S. J. DeArmond, E. J. Smart, R. G. Anderson, A. Taraboulos, and S. B. Prusiner. 1996. Subcellular colocalization of the cellular and scrapie prion proteins in caveolae-like membranous domains. Proc. Natl. Acad. Sci. USA 93:14945-14949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vilette, D., O. Andreoletti, F. Archer, M. F. Madelaine, J. L. Vilotte, S. Lehmann, and H. Laude. 2001. Ex vivo propagation of infectious sheep scrapie agent in heterologous epithelial cells expressing ovine prion protein. Proc. Natl. Acad. Sci. USA 98:4055-4059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vorberg, I., and S. A. Priola. 2002. Molecular basis of scrapie strain glycoform variation. J. Biol. Chem. 277:36775-36781. [DOI] [PubMed] [Google Scholar]
- 46.Weissmann, C., M. Enari, P. C. Klohn, D. Rossi, and E. Flechsig. 2002. Transmission of prions. J. Infect. Dis. 186(Suppl. 2):S157-S165. [DOI] [PubMed] [Google Scholar]
- 47.Zurzolo, C., M. P. Lisanti, I. W. Caras, L. Nitsch, and E. Rodriguez-Boulan. 1993. Glycosylphosphatidylinositol-anchored proteins are preferentially targeted to the basolateral surface in Fischer rat thyroid epithelial cells. J. Cell Biol. 121:1031-1039. [DOI] [PMC free article] [PubMed] [Google Scholar]


