Abstract
Tsetse flies are viviparous insects that nurture a single intrauterine progeny per gonotrophic cycle. The developing larva is nourished by the lipid-rich, milk-like secretions from a modified female accessory gland (milk gland). An essential feature of the lactation process involves lipid mobilization for incorporation into the milk. In this study, we examined roles for juvenile hormone (JH) and insulin/IGF-like (IIS) signaling pathways during tsetse pregnancy. In particular, we examined the roles for these pathways in regulating lipid homeostasis during transitions between non-lactating (dry) and lactating periods. The dry period occurs over the course of oogenesis and embryogenesis, while the lactation period spans intrauterine larvigenesis. Genes involved in the JH and IIS pathways were upregulated during dry periods, correlating with lipid accumulation between bouts of lactation. RNAi suppression of Forkhead Box Sub Group O (FOXO) expression impaired lipolysis during tsetse lactation and reduced fecundity. Similar reduction of the JH receptor Methoprene tolerant (Met), but not its paralog germ cell expressed (gce), reduced lipid accumulation during dry periods, indicating functional divergence between Met and gce during tsetse reproduction. Reduced lipid levels following Met knockdown led to impaired fecundity due to inadequate fat reserves at the initiation of milk production. Both the application of the JH analog (JHA) methoprene and injection of insulin into lactating females increased stored lipids by suppressing lipolysis and reduced transcripts of lactation-specific genes, leading to elevated rates of larval abortion. To our knowledge, this study is the first to address the molecular physiology of JH and IIS in a viviparous insect, and specifically to provide a role for JH signaling through Met in the regulation of lipid metabolism.
Keywords: Insulin, juvenile hormone, lipid metabolism, lactation, reproduction, tsetse, Diptera
1. Introduction
Tsetse flies (Glossina spp.) are the sole insect vectors responsible for cyclical transmission of African trypanosomes, parasites responsible for Human African Trypanosomiasis (HAT)/sleeping sickness and for African Animal Trypanosomiasis (AAT)/Nagana. While most insects are oviparous and produce multiple eggs during each gonotrophic cycle, tsetse flies represent an intriguing instance of viviparous reproduction in insects (Meier et al., 1999) and produce only 8–12 progeny per lifetime (Tobe and Langley, 1978). Females have reduced ovarian capacity, generating a single oocyte in the left or right ovary during each gonotrophic cycle (Tobe and Langley, 1978). In addition, the reproductive tract expands to accommodate embryonic and larval development, which proceeds through growth to the third instar. At parturition (birth), the larval mass is almost equivalent to that of the mother (Denlinger and Ma, 1974; Attardo et al., 2012). Shortly after parturition, the third instar larva burrows into the ground and pupariates.
To accommodate viviparity, the tsetse female accessory gland has been expanded into a specialized milk secreting organ (milk gland) that provides the nutritional components necessary for larval and pupal growth and development (Tobe and Langley, 1978; Denlinger and Ma, 1974; Denlinger and Ma, 1975). During the four to six day larvigenesis period, the milk gland secretes 20–30 mg of milk, which consists of various carbohydrates, lipids, and proteins (Cmelik et al., 1969). The protein constituents of the milk include a lipocalin (milk gland protein, MGP1; Attard et al., 2006), several novel, tsetse-specific proteins called milk gland proteins (MGP2 and MGP3, Yang et al., 2010; Transferrin, Guz et al. 2007; Acid sphingomyelinase 1, aSMase1, Benoit et al. 2012) and Peptidoglycan Recognition Protein-LB (Wang et al., 2012). The lipids present in the milk originate directly from the digestion products of the blood meal and from lipolysis of stored lipids in the fat body (Attardo et al., 2012; Langley et al., 1981; Pimley et al., 1981; 1982). The rapid incorporation of lipids into the milk reduces total maternal lipid content by nearly 50% during lactation. Recovery of maternal lipid reserves occurs in the dry periods during oogenesis and embryogenesis (Attardo et al., 2012; Langley et al., 1981; Pimley et al., 1981; 1982). Previously we showed that the massive lipid breakdown required for milk production is coordinated by the Brummer lipase (Bmm, insect adipose triglyceride lipase) and adipokinetic hormone (AKH) signaling pathways (Attardo et al., 2012). Interference with these two lipolytic pathways hinders utilization of lipid reserves, which in turn extends the pregnancy cycle and decreases fecundity, indicating that regulation of stored lipid levels is a critical facet of tsetse pregnancy (Attardo et al., 2012). Currently, factors that control the lipid content in tsetse mothers, specifically hormones that regulate the drastic physiological changes associated with lactation and dry periods, are unknown.
One candidate regulatory mechanism for modulating lipid content during tsetse fly pregnancy is the insulin/insulin-like signaling (IIS) pathway. In Drosophila melanogaster, this pathway mediates nutrient storage/metabolism and is critical for reproduction, growth and development (Teleman, 2010). D. melanogaster has eight insulin-like peptides (ILP1-8) that share amino acid sequence identity with mammalian insulin (Brogiolo et al., 2001; Garelli et al. 2012; Colombani et al., 2012). Distinct temporal and spatial expression profiles are characteristics of each of the Drosophila ILPs according to the physiological state of the fly (Teleman, 2010; Brogiolo et al., 2001; Ikeya et al., 2002; Broughton et al., 2005). Upon ligand binding, constituent genes in the IIS pathway direct the uptake of hemolymph carbohydrates and lipids by the fat body and other storage locations, but the specific physiological response depends on the type or combination of types of ILPs that bind the insulin receptor (Teleman, 2010; Brogiolo et al. 2001; Belgacem et al., 2006; Zhang et al., 2009; Grönke et al., 2010). In D. melanogaster, knockdown of genes in the IIS pathway yields insulin insensitivity, resulting in high circulating carbohydrates, altered whole body lipid levels, behavioral changes and reduced fecundity (Teleman, 2010 and references within). In addition, insulin insensitivity in D. melanogaster promotes longevity, increases survival during periods of starvation, and promotes oxidative stress tolerance [24–26] (Kapahi et al., 2004; Tatar et al., 2001; Clancy et al., 2001). Recent studies show that insulin signaling acts through a Ser/Thr kinase (SIK3) and a class II deacetylase, histone deacetylase 4 (HDAC4), to control lipolysis (Wang et al., 2011). During periods of starvation, SIK3 remains inactive, resulting in the dephosphorylation and subsequent nuclear translocation of HDAC4, which in turn promotes a Forkhead Box Subgroup O (FOXO)-dependent increase in the expression of bmm (Wang et al, 2011). In addition, the Acyl Co-A synthetase (ACS) Pudgy has been identified as a key regulator of lipid levels in Drosophila is under positive control by FOXO and is repressed by insulin (Xu et al., 2012). Until now, no studies have investigated the role(s) for the IIS pathway in tsetse reproductive physiology.
The juvenile hormone (JH) signaling pathway, which directs numerous aspects of insect growth/development, metamorphosis (Goodman and Cusson, 2012) and reproduction (Wyatt and Davey, 1996; Bownes, 2004), interacts with the IIS pathway in several insect species (Parthasarathy and Palli, 2011; Sheng et al., 2011; Richard et al., 2005; Tu et al., 2005; Riddiford, 2012). However, the molecular basis of JH/IIS interaction remains poorly characterized. Recent studies show that the JH signaling may intersect with the IIS pathway through the Chico (an adaptor protein that interacts with the insulin-like peptide receptor) and/or FOXO (the downstream transcriptional regulator of the insulin pathway) proteins or may even directly alter the expression of specific ILPs (Parthasarathy and Palli, 2011; Sheng et al., 2011). Genetic suppression of chico or FOXO reduces or increases JH biosynthesis, respectively (Tu et al., 2005; Suren-Castillo et al., 2012). Unlike the IIS pathway, JH signaling is unique to insects. Elucidation of the complete JH pathway and its role in insect physiology has been a fundamental problem to insect biology for decades (Riddiford, 2008). Recent advances in our understanding of JH signaling implicate the Methoprene tolerant (Met) protein as a bona fide JH receptor in beetles (Konopova and Jindra, 2007; Charles et al., 2011) and in lower Diptera, including mosquitoes (Zhu et al., 2010; Li et al., 2011; Wang et al., 2007). In contrast, higher Diptera (Brachycera) including Drosophila possesses two genes, Met and its paralog germ cell expressed (gce),that have an intimate and stoichiometric relationship with JH signal transduction (Baumann et al., 2010). Met was first identified in a mutagenesis screen for D. melanogaster resistance to the toxic and morphogenetic effects incurred by exposure to the JH analog (JHA) insecticide methoprene (Wilson and Fabian, 1986). The Met27 null allele confers high-level resistance to methoprene and other JHAs, but not to other classes of insecticides (Baumann et al., 2010; Wilson and Ashok, 1998; Riddiford et al., 2010). Met27 mutants display severe reproductive phenotypes (Wilson and Ashok, 1998) but are otherwise viable due to the presence of the functionally redundant paralog gce (Abdou et al., 2011). Met/gce double mutants fail to complete the pupal-adult transition, displaying similar morphogenetic defects observed following genetic ablation of the corpus allatum (CA; the primary JH biosynthetic organ) (Liu et al., 2009). It has been recently demonstrated that this phenotype results from reduced expression of the JH response gene Kr-h1 and the subsequent precocious expression of the early 20E response gene Broad (Abdou et al., 2011), which acts to drive pupal development in holometabolous insects (Zhou and Riddiford, 2008; Suzuki et al., 2008). Early studies on JH/JHA application (Denlinger, 1975; Langley et al., 1988; Langley et al., 1990) or removal of JH producing organs in tsetse (Ejezie and Davey, 1976) demonstrated reproductive impairment, implicating JH as critical for tsetse reproduction. However, little is known about the molecular genetics underlying JH signaling in tsetse, in particular with respect to tsetse's viviparous reproductive biology.
In this study, we examined the roles of JH and IIS pathways during tsetse fly reproduction, paying particular attention to the regulation of maternal lipid accumulation and decline during pregnancy. Our results suggest both JH (mediated primarily by Met) and IIS pathways play a critical role in regulating lipolysis/lipogenesis during the lactation and dry periods of tsetse pregnancy. We propose that both of these pathways promote lipid accumulation during the dry periods separating bouts of lactation; perturbation of either pathway leads to rapid lipolysis associated with the lactation period. This study is the first to address the molecular mechanisms of the JH and IIS pathways in a viviparous insect.
2. Materials and Methods
2.1. Flies
The Glossina morsitans morsitans colony originated from populations in Zimbabwe. Flies were maintained at 24°C and 50–60% RH and received defibrinated bovine blood through an artificial feeding system three times per week [83]. Female flies were mated 3d after emergence and stored in groups of 15 flies. Female flies were collected for analysis according to developmental markers based on the progeny developmental state which were established to analyze pregnancy according to previous studies (Attardo et al., 2006; Benoit et al., 2012).
2.2. Quantitative PCR analysis
Expression profiles for the following genes were obtained via quantitative reverse transcription PCR (RT-PCR): FOXO (JX291116), chico (JX291117), InR (JX291127), Met (JX291118), Kr-h1 (JX291126), gce (JX291119), E75 (JX291125), JHAMT (JX291120), bmm (AEV59505), midway (AD19427), tg lipase (JX291121), mogat (JX291122), mgp (CAY49913.1), HDAC4 (JX291128), pudgy (JX291124) and asmase1 (AFJ68090.1). Transcript expression was monitored using the CFX96 real-time PCR detection system (Bio-Rad, Hercules, CA) using gene specific primers (Supplemental Table 1). The data were obtained in triplicate and were normalized to tsetse tubulin (tub, DQ377071.1) expression and analyzed with software version 3.1 (Bio-Rad).
2.3. Lipid content analysis
Analyses of total lipid contents were conducted using a standard vanillin assay developed for mosquitoes (Van Handel, 1985) and modified for tsetse flies (Attardo et al., 2012). Flies were dried at 0% RH and 60°C until their mass was constant and then homogenized in 2 ml of chloroform:methanol (2:1). The supernatant was removed from the 5 ml glass tube and the solvent was evaporated at 90°C. The lipids were heated in 0.4 ml of concentrated sulfuric acid at 90°C for 12 minutes. Acid/lipid mixture (40 μl) was then added to 4 ml vanillin reagent. Samples were measured spectrophotometrically at 525 nm, and total lipid content was calculated against a canola oil lipid standard.
2.4. RNA interference of juvenile hormone and insulin signaling genes
Short interfering RNA (siRNA) constructs consisting of two Duplex sequences (Table S2) were purchased commercially (IDT, Coralville, IA) to target Met, gce, JHAMT, and FOXO. siRNA for bmm was generated according to previous studies on tsetse fly lipid metabolism studies. Control siRNA molecules were designed as complementary to green fluorescent protein (GFP) mRNA (IDT, Cordville, IA). The siRNA concentration was determined spectrophotmetrically, adjusted to 1–1.5 μg/μl in PBS, and individual flies were injected with 1.5μl siRNA. For determination of the knockdown phenotypes, flies were injected either 1–2d after adult emergence with siRNAs targeting genes expressed early in pregnancy (Met, gce and JHAMT) or 7–9d after adult emergence to target genes expressed late in pregnancy (bmm and FOXO). Previous studies have shown that siRNA injection into the mother has no discernible effect on larval transcript levels (Attardo et al., 2012; Benoit et al., 2012). Three to five days after siRNA injection, expression levels of target and downstream genes were determined by qPCR (as described) and normalized to tub after removal of the larva. Total lipid content in female flies was measured following removal of the developing intrauterine larva 5d after injection. Fecundity was measured for 40d (the first two gonotrophic cycles). For flies that deposited larvae, the duration of the first gonotrophic cycle was determined as the time between adult emergence and parturition.
2.5. Methoprene application and insulin injection
Methoprene (juvenile hormone analog, JHA) application studies were conducted according to those previously developed for tsetse flies (Langley et al., 1988; Denlinger, 1975). Mated female flies, 12–14d after adult emergence, were given a topical application of either 10 μg (low dose) or 25 μg (high dose) of methoprene dissolved in 2μl of acetone. This time point was chosen to test for potential reversal of lactation-associated lipolysis, which is highest at this time based on our previous studies (Attardo et al. 2012). Following methoprene application, the flies were stored in groups of five and transcript levels were measured by qPCR (as described) after 12–16h and following removal of the larva. Total lipid content in female flies was measured following removal of the intrauterine larva 72–76h after methoprene exposure. Fecundity and duration of the gonotrophic cycle were determined as previously described over 40d.
Insulin injection experiments were conducted based on those developed for D. melanogaster (Belgacem et al., 2006), but scaled up to accommodate the larger size of tsetse flies. Flies received two injections of 1μl bovine insulin (Sigma) at a concentration of 0.05mg/μl 12d and 14d after adult emergence. Transcript levels were measured 6h after the first insulin injection. Total lipid content in females was measured following removal of the developing intrauterine larva 72h after the second injection. Fecundity and duration of the gonotrophic cycle were determined as previously described.
For combined methoprene and insulin treatments, flies were treated with 25μg of methoprene at 12d and injected with insulin 12h later. Gene transcript levels were measured 6–8h after insulin treatment and 18–20h after methoprene application. Total lipid content in female flies was measured following removal of the developing intrauterine larva 72h after the insulin injection. Fecundity and duration of the gonotrophic cycle were determined as previously described.
3. Results
3.1. Lipid metabolism and insulin signaling during tsetse pregnancy
During the viviparous reproductive process, large quantities of milk secretions are transferred from the mother to her intrauterine progeny in each gonotrophic cycle. We analyzed the lipid levels and the expression of genes involved in lipid metabolism and insulin signaling during the 1st and 2nd gonotrophic cycles in pregnant females following removal of their intrauterine larva. During the course of pregnancy there was a substantial fluctuation in total body lipid content, which increased by about 3-fold during the dry periods and peaked toward the end of embryogenesis (Figure 1a). Lipid levels then declined over the course of larval development, during which time lactation occurs. This alternating pattern of lipogenesis and lipolysis was then repeated in subsequent gonotrophic cycles (Figure 1a; Figure S1a). Notably, this drastic, temporal fluctuation in lipid levels correlated with the expression of genes involved in lipid accumulation (midway and monoacylglycerol O-acyltransferase, mogat) and breakdown (bmm, tg lipase (triacylglycerol lipase) and pudgy) as shown in Figure 1b and Figure S1b, c. In contrast, lactation-specific genes including mgp and asmase1 increased during lactation and declined during dry periods (Figure 1c; Figure S1d). These results indicate that lipids accumulate during the dry periods between lactation-associated lipolysis and milk protein synthesis.
Fig. 1.

Lipid and transcript levels through two tsetse gonotrophic cycles. A. Total lipid levels in female flies throughout pregnancy. Each point represents the mean ± SE of three flies. B. Fold change of transcripts for lipid metabolism genes (bmm, tg lipase, pdgy, mdy and mogat). C. Fold change of transcripts for pregnancy-specific genes (mgp, asmase1). D. Fold change of transcripts for IIS genes (chico, FOXO, InR, and HDAC4). E. Fold change of transcripts for JH signaling genes (JHE, JHAMT, Met, Kr-h1, gce, and E75). Transcript levels were determined by qPCR using the iCycler iQ real-time PCR detection system (Bio-Rad, Hercules). The data were normalized to tub transcript levels and each point represents the mean ± SE of three to five flies. Fold changes represent a summary of transcript levels in Figure S2. Right ovary, uterus, and left ovary denote the presence of a developing oocyte, embryo, or larva in these locations.
In addition to lipid metabolism and accumulation during larvigenesis, we measured transcript levels of genes involved in the IIS pathway. Although not significant, an inverse trend for chico and FOXO expression was observed, with maximal FOXO and minimal chico transcript levels detected during lactation (Figure 1d; Figure S1e). InR showed no significant change during pregnancy (Figure 1d; Figure S1f). In contrast, transcript levels for the tsetse ortholog of DmHDAC4 varied substantially throughout pregnancy, reaching maximal levels during the transition between dry and lactating periods (Figure 1d; Figure S1f). These results, in combination with the observed temporal fluctuation in lipid levels, suggest an increase in IIS-induced lipid accumulation during the dry period.
3.2. JH signaling gene identification and expression during tsetse pregnancy
Based on homology searches using D. melanogaster sequences as queries, we identified tsetse orthologs for several genes involved in JH pathways. Like D. melanogaster, the G. morsitans genome has distinct Met and gce orthologs (Figure S2), indicating that Met arose from a gce-like ancestor (Baumann et al. 2011) sometime before the origin of the dipteran infraorder Muscomorpha. Glossina is thus the second brachyceran genus identified with distinct Met/gce orthologs.
The JH titer during tsetse pregnancy is unknown. To make an indirect determination of the JH titer and to identify potential regulatory mechanisms for JH signaling during pregnancy, we measured the expression profiles for several key JH signaling genes including Met, gce, E75, Kr-h1, JHE and JHAMT. JHAMT catalyzes the penultimate step in JH III biosynthesis, converting JH acid to JH III, and its expression correlates with increased levels of JH (Daimon et al. 2012). Conversely, JHE clears circulating JH from the hemolymph at developmentally appropriate times, such as at the onset of metamorphosis in holometabolous insects. Kr-h1 induction requires the presence of JH with severe reduction as a results of allatectomy (removal of the corpus allatum, the JH biosynthetic organ; Konopova et al., 2011). Kr-h1 induction by JH is hampered by dsRNA targeting Met, comfirming that Kr-H1 is responsive to JH with Met as the receptor (Charles et al. 2011). Lastly, Met27/gce2.5k mutants fail to express Kr-h1(Abdou et al. 2011). Therefore, JH titer can be estimated by extrapolating from the combined expression of JHAMT, JHE, and Kr-h1expression during pregnancy.
JHAMT/JHE expression suggests that an increase in maternal JH titer during dry periods is followed by a steep decline that immediately precedes lactation (Figure 1e; Figure S1g). As expected, expression of the JH response gene Kr-h1 increases during dry periods and declines during lactation (Figure S1h). In addition, Met expression closely mirrors the temporal profile of Kr-h1, suggesting that Met regulates Kr-h1 expression (Figure 1e; Figure S1h), consistent with the regulatory hierarchy observed in other insects (e.g. [Zhu et al., 2010]). In contrast, gce expression shows an oscillatory pattern with a slight peak towards the end of lactation (Figure1e; Figure S1i). This pattern is mirrored by E75 expression (Figure 1e; Figure S1i), corroborating recent results in D. melanogaster S2 cells showing that gce rather than Met is critical for JH-mediated induction of E75A (Dubrovsky et al., 2011). Overall, we show that expression profiles for several JH signaling genes correlate with those of genes involved in IIS pathway and with temporal patterns of lipid accumulation in dry flies.
3.3. Methoprene application and insulin injection results
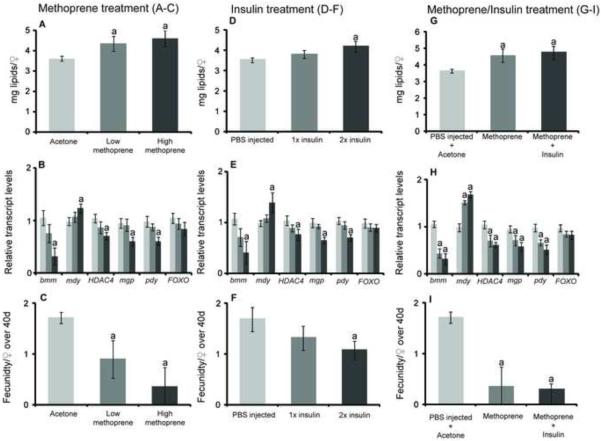
We next examined the effects of methoprene application on the expression of key genes in the JH and IIS pathways. We exposed pregnant females to two different concentrations (10 and 25 μg) of methoprene. As expected, methorpene application caused Kr-h1 induction (Fig. S3). Methoprene application resulted in a dose-dependent increase in stored lipid content, with an observed increase of 32–36% following the high (25 μg) JHA treatment (Figure 2a). Methoprene treatment significantly elevated transcript levels of midway, a gene involved in triacylglycerol (TAG) biosynthesis, but downregulated the expression of bmm and pudgy, two genes involved in TAG catabolism (Figure 2b). In particular, bmm expression was sensitive even to low doses of methoprene. There was no significant change in FOXO transcript levels in response to methoprene treatment at either dosage. However, expression of the lactation-specific gene mgp1 was significantly reduced following treatment with a high dose of methoprene (Fig 2b). In addition, the gonotrophic cycle of flies exposed to a high dose of methoprene was extended by about 5–6 days, concomitant with a 30–40% reduction in fecundity over the 1st and 2nd gonotrophic cycles (Figure 2c; Figure S4). Insulin injection produced similar effects, including increased expression of Kr-h1, elevated lipid levels, increased expression of the lipogenic gene midway, decreased expression of lipolytic genes including bmm and pudgy, reduced fecundity and extended larval gestation (Figure 2d–f; Figure S4,S4). Thus, insulin injection, like methoprene application, induces a heterochronic shift from lipid breakdown to accumulation, consequently extending larval gestation time and reducing fecundity.
Fig. 2.
Methoprene and insulin treatments increase lipid levels, alter lipid metabolism gene expression, and reduce fecundity. A, D and G. Total lipid levels in female flies. Each point represents the mean ± SE of eight flies. B, E and H. Relative transcript levels of bmm, mdy, HDAC4, mgp, pdy, FOXO, and Kr-h1. Each point represents the mean ± SE of three flies. C, F, I. Number of pupae per female over 40d, mean ± SE of three groups of 30 flies. Significant differences (indicated by “a” at P < 0.01) were determined by ANOVA followed by Tukey's test compared to controls.
Simultaneous treatment with insulin and methoprene did not significantly alter the transcription of lipolytic/lipogenic genes or lipid levels beyond treatment with insulin or methoprene alone (Figure 2g, h). The additional effects on fecundity and chronological extension of the gonotrophic cycle did not statistically differ in the combined treatment versus the individual treatments of methoprene or insulin alone (Figure 2i; Figure S4). This result suggests that insulin injection can only minimally enhance the transcriptional or physiological effects incurred by high dosage of methoprene. Given that in D. melanogaster, Kr-h1 expression is upregulated via JHA application to a saturable level [59], we asked what effect insulin injection, in combination with methoprene, might have on Kr-h1 levels in tsetse. As anticipated, methoprene application led to a substantial increase in Kr-h1 transcripts in tsetse (Fig 2e), while a combined treatment with both methoprene and insulin failed to appreciably amplify Kr-h1 expression beyond levels obtained with methoprene alone (Figure 2h). These results suggest that Kr-h1 regulation by Met/JH occurs downstream of a potential intersection between IIS and JH pathways.
3.4. Knockdown by siRNA injection: transcriptional consequences
Our data show that similar temporal expression patterns for E75 and gce during tsetse pregnancy (Fig 1i). Accordingly, there was a significant decrease in E75 transcripts in flies treated with siRNA constructs targeting gce (Fig 3a). In contrast, siMet treatment had no effect on E75 expression, whereas Kr-h1 transcript levels were reduced substantially (Fig 3b). Importantly, knockdown of Met, but not gce, resulted in upregulation of bmm and HDAC4 concomitant downregulation of midway expression (Figure3a, b). In addition, lipid levels were significantly reduced upon knockdown of Met, but not gce (Figure 3d). Fecundity was reduced and the length of pregnancy was extended under Met knockdown and to a lesser extent by gce knockdown (Figure 3e, f). None of the transcriptional or physiological consequences of Met knockdown were amplified when Met and gce were simultaneously suppressed (Figure 3c–f). These results suggest that Met and Gce are functionally divergent in respect to lipid metabolism associated with tsetse reproduction, but both may contribute to fecundity.
Fig. 3.
Effect of Met and gce knockdown on tsetse lipid metabolism and fecundity. A. Relative transcript levels of Met, gce, Kr-h1, E75, bmm, mdy, and HDAC4 following injection of short interfering (si) RNA targeting GCE. Each point represents the mean ± SE of four to five flies. B. siMet injection and expression analysis of downstream target genes under same conditions as Figure 3a. C. siMet/siGCE injection and analysis as in Figure 3a. D. Total lipid levels in female flies after injection in siGFP, siMet, siGCE, or siMet/siGCE (both). Each point represents the mean ± SE of eight flies. E. Number of pupae deposited per female over 40d after injection in siGFP, siMet, siGCE, or siMet/siGCE (both), mean ± SE of three groups of 30 flies. F. Duration of the first gonotrophic cycle after injection in siGFP, siMet, siGCE, or siMet/siGCE (both), mean ± SE of three groups of 15 flies. Significant differences (indicated by “a” at P < 0.01) were determined by ANOVA followed by Tukey's test compared to controls. siGFP, siRNA targeting green fluorescent protein.
To examine the reproductive and transcriptional effects of JH titer, we knocked down the expression of JHAMT, a critical enzyme for JH biosynthesis (Fig. 4). Suppression of JHAMT resulted in concomitant reduction of Kr-h1, E75 and midway while Met and gce expression were unaffected and bmm and HDAC4 expression increased (Figure 4a). These results indicate that partial blockage of JH synthesis through suppression of JHAMT expression phenocopies Met deficiency with respect to Kr-h1, bmm, HDAC4 and midway expression, and gce deficiency with respect to E75 expression. Both fecundity and lipid content were reduced under JHAMT suppression to similar levels seen following Met/gce knockdown (Fig 4b, c). The first gonotrophic cycle of pregnancy was equally extended following either JHAMT or Met/gce knockdown (Figure 3f). Thus, reduction of JHAMT incurred similar transcriptional and physiological consequences as Met/gce reduction during tsetse pregnancy.
Fig. 4.
Effect of JHAMT knockdown on tsetse lipid metabolism and fecundity. A. Relative transcript levels of JHAMT, Met, gce, Kr-h1, E75, bmm, mdy, and HDAC4 following injection of siJHAMT and siGFP. B. Total lipid levels in female flies after injection siGFP, siJHAMT, or siMet/siGCE (both). Each point represents the mean ± SE of eight flies. C. Number of pupae per female over 40d after injection siGFP, siJHAMT, or siMet/siGCE (Both), mean ± SE of three groups of 30 flies. D. Duration of the first gonotrophic cycle after injection siGFP, siJHAMT, or siMet/siGCE (both), mean ± SE of three groups of 15 flies. Significant differences (indicated by “a” at P < 0.01) were determined by ANOVA followed by Tukey's test compared to controls. siGFP, short interfering RNA targeting green fluorescent protein.
Next, we knocked down the expression of FOXO, since several recent studies have indicated that FOXO may act as a molecular bridge between IIS and JH pathways (Sheng et al., 2011; Suren-Castillo et al., 2010; Sim and Denlinger, 2008). Suppression of FOXO significantly increased JHAMT expression, while reducing bmm expression (Figure 5a). Therefore, FOXO reduction increases JH biosynthesis through upregulation of JHAMT, as predicted by Suren-Castillo et al (2012). Since the promoter region of bmm was reported to harbor several FOXO response elements in Drosophila (Wang et al., 2011), reduction of bmm expression following FOXO reduction is not unexpected. Lipid levels were elevated in mothers receiving siFOXO treatment (Figure 5b), consistent with the role of FOXO as a negative regulator of the IIS pathway and a positive regulator of bmm gene expression in the absence of insulin stimulation (Wang et al. 2011). In D. melanogaster, bmm mutants display an obese phenotype (Grönke et al., 2005; Grönke et al., 2007). This is in stark contrast to the results we obtained following Met/gce knockdown in tsetse, where lipid levels were significantly depressed in dry flies, consistent with our finding that Met reduction promotes increased bmm expression (Fig 5a). These results support previous work showing that JH counteracts FOXO activity (Sheng et al., 2011). Similar negative consequences to fecundity and on the duration of pregnancy were noted after FOXO and Met/gce knockdown (Fig 5c,d). Although these results appear contradictory, this similarity results from the timing of each treatment; FOXO was reduced during lactation and Met/gce transcripts were reduced early in the dry period. Thus, similar negative phenotypes are observed due to prevention of lipid accumulation in dry flies by inhibiting JH signal transduction (Met/gce knockdown), or by the prevention of lipid metabolism along with increased JH biosynthesis in lactating flies (FOXO knockdown); both processes are critical for tsetse fecundity.
Fig. 5.
Effects of knockdown of FOXO on juvenile hormone signaling and lipid metabolism. A. Relative transcript levels of Met, gce, bmm, mdy, Kr-h1, E75, JHAMT, and FOXO following injection of siFOXO. B. Total lipid levels in female flies after siGFP, siFOXO, or siMet/siGCE treatments, respectively. Each point represents the mean ± SE of eight flies. C. Number of pupae deposited per female over 40d after siGFP, siFOXO, or siMet/siGCE treatments, respectively, mean ± SE of three groups of 30 flies. D. Duration of the first gonotrophic cycle after siGFP, siFOXO, or siMet/siGCE treatments, respectively, mean ± SE of three groups of 15 flies. Significant differences (indicated by “a” at P < 0.01) were determined by ANOVA followed by Tukey's test compared to controls. siGFP, short interfering green fluorescent protein.
3.5. Knockdown of bmm followed by methoprene application
Our methoprene application experiments with pregnant females showed increased lipid levels, which we reasoned could result from reduced expression of insulin-suppressed bmm. To test this hypothesis, we injected mothers with siBmm prior to methoprene application. Injection with siBmm resulted in a substantial reduction of bmm expression to similar levels as seen under methoprene treatment (JHA; Figure 6a). However, suppression of bmm followed by methoprene application led to a greater reduction in fecundity than that observed with either methoprene treatment or bmm knockdown alone (Figure 6b). This effect was not a general feature of siRNA injection, since siGFP treatment followed by methoprene application failed to reduce fecundity beyond methoprene treatment alone (Figure 6b). Flies receiving methoprene in combination with bmm knockdown showed increased lipid levels compared to untreated or siGFP-injected controls (Figure 6c). Lipid levels were not significantly increased in flies receiving a combination of methoprene application and siBmm injection compared to flies receiving methoprene or siBmm injection alone (Figure 6c). These results suggest that the observed methoprene-induced lipid reduction likely results from 1) a positive interaction with insulin signaling or 2) another mechanism through which methoprene suppresses the expression of bmm and other genes involved in lipolysis.
Fig. 6.

Effect of methoprene application on bmm levels, fecundity, and lipid levels following knockdown of bmm. A. Relative bmm levels after treatment. Each point represents the mean ± SE of three flies. B. Number of pupae deposited per female over 40d, mean ± SE of three groups of 30 flies. C. Total lipid levels in female flies. Each point represents the mean ± SE of eight flies. Significant differences (indicated by “a” at P < 0.01) were determined by ANOVA followed by Tukey's test compared to controls and siGFP-injected flies. Control, PBS-injected; siGFP, short interfering RNA targeting green fluorescent protein; JHA, juvenile hormone analog (methoprene). siGFP +JHA, siGFP injection followed by JHA. siBmm + JHA, siGFP injection followed by JHA.
4. Discussion
In adult insects, JH primarily functions as a gonadotropic hormone whose action manifests in a species-specific manner. For example, in A. aegypti JH directs previtellogenic oocyte development and primes the fat body for competence to respond to 20E-directed oocyte maturation (Clements, 1992). Conversely, in D. melanogaster JH is necessary both for the synthesis and endocytotic uptake of vitellogenins into the developing oocyte (Soller et al., 1999). It has been recognized for decades that JHA application to tsetse can induce larval abortion (Denlinger, 1975) or production of nonviable offspring (Langley et al., 1988; Langley et al., 1990). In G. austeni, JH was proposed as critical for tsetse reproduction based on experiments in which surgical allatectomy inhibited the production of viable offspring, a phenotype which was rescued in subsequent gonotrophic cycles by provision of exogenous JH III (Ejezie and Davey, 1976). In addition, insulin/IGF-like signaling (IIS) is necessary for growth, reproduction, and nutrient homeostasis in D. melanogaster and other insects (Teleman, 2010; Wu and Brown, 2006). Similarly, it is known that JH treatment of D. melanogaster adults leads to increased lipid content (Butterwood and Bodenste, 1969), but the mechanisms responsible for this phenotype are unknown. Until now, no studies have investigated the role of IIS in tsetse reproductive physiology. In this study, we show that like Drosophila spp., tsetse JH signal is transduced through functionally distinct Met and gce orthologs. Importantly, suppression of Met, but not its paralog gce, results in misexpression of genes in the IIS pathway, consequently leading to misexpression of genes involved in lipid homeostasis. This is the first study reporting on the molecular mechanisms of JH and IIS as mediators of the critical aspects of lipid metabolism underlying viviparous reproduction in tsetse flies. In relation to general insect physiology, these are to our knowledge the first results indicating that Met promotes lipid accumulation through synergism with IIS under conditions of high JH titer.
Tsetse has distinct Met and gce orthologs
Our in silico analysis of the G. morsitans genome identified distinct Met and gce orthologs, which were previously only known from 12 members of the genus Drosophila. The genomes of several mosquito species (Wang et al., 2007) and the red flour beetle T. castaneum (Konopova and Jindra, 2007) each carry a single, gce-like ancestor from which Met originated via gene duplication, apparently during dipteran evolution (Baumann et al., 2010). While the genome of the silk moth, Bombyx mori, carries two Met-like genes, Met1 and Met2, Met1 protein is particular to Lepidoptera while Met2 appears to be a gce ancestor (Kayukawa et al., 2012). Therefore, the identification of both Met and gce in tsetse indicates that these genes predate the origin of the lineage leading to the dipteran infraorder Muscomorpha.
JH signaling changes associated with tsetse pregnancy
During tsetse pregnancy, suppression of Met, but not gce, resulted in concomitant reduction in expression of the JH-response gene Kr-h1. In both A. aegypti (Zhu et al., 2010) and T. castaneum (Zhang et al., 2011) Kr-h1 expression is mediated via the single Met-like (gce) ortholog. Similarly, in B. mori, the gce-like Met2 is required along with BmSRC for proper BmKr-h1 expression (Kayukawa et al., 2012). In D. melanogaster, RNAi mediated silencing of Met (and to a much lesser extent gce) results in decreased Kr-h1 transcript levels, whereas in the Met/gce double null mutant, Kr-h1 expression is blocked entirely. During tsetse pregnancy, our tandem suppression of Met and gce minimally enhanced the level of Kr-h1 blockage beyond that seen upon Met suppression alone (Fig 3c). This difference could either be due to our inability to fully suppress both Met and gce by RNA interference or to dissimilarity in the gene regulatory hierarchies during D. melanogaster development versus those acting during tsetse reproduction. Concurrent reduction of both Met and gce during tsetse pregnancy did not result in appreciable enhancement of any transcriptional or physiological consequence incurred by Met reduction alone. Functional partitioning of Met and Gce across reproduction and development has been demonstrated in D. melanogaster. While Met and Gce share redundant function during preadult development (Abdou et al., 2011), ectopically expressed gce cannot rescue the reproductive consequences of Met deficiency, namely a severe reduction in oogenesis (Baumann et al., 2010). This argues for divergent roles for Met and Gce during tsetse reproduction.
We additionally show that Gce but not Met, regulates E75 expression during tsetse pregnancy. Three E75 isoforms, E75A, B, and C, are transcribed from different promoters in D. melanogaster. Since individual E75 isoforms in G. morsitans have not yet been defined, we designed our primers using a pair wise alignment consisting of the core D. melanogaster E75 sequence and the tsetse ortholog predicted from assembled transcripts mapped against genomic scaffolds. Therefore, our results represent changes in regulation of whichever E75 variants are expressed at the indicated time points during pregnancy. Recent studies show that intracellular expression of JHE in D. melanogaster S2 cells inhibits E75A transcription, indicating that JH must enter the cell, is necessary for E75A induction, and that JH III-mediated transcription of E75A is blocked by suppression of gce or gce and Met expression, but not by Met suppression alone (Dubrovsky et al., 2011). While our data suggest E75 regulation through Gce as a conserved mechanism in higher Diptera, the importance of this regulatory mechanism during tsetse pregnancy remains unexplored.
Interestingly, tandem suppression of Met and gce in tsetse mothers resulted in elevated rates of adult mortality compared to siGFP-treated controls (50% mortality in Met/gce double knockdown vs. > 5% in the siGFP group). Roles for JH, Met and/or Gce in adult viability have not been described, but this result is not unprecedented. In a previous study, we drove expression of a gce RNAi construct from either a tubulin or actin promoter in D. melanogaster with a Met27 null genetic background. RNAi constructs expressed under the control of the tubulin promoter induced lethality during pupal development; gce knockdown driven by the slightly weaker actin promoter (Barry et al., 2008) allowed a subset of flies to survive to adulthood (Baumann et al., 2010). However, all adults that eclosed in this experiment died within two days of eclosion. The mechanism of mortality was not investigated, but induction of adult lethality through Met/gce suppression is a phenomenon that clearly warrants further study.
IIS and lipid accumulation during tsetse pregnancy
Our results indicate that IIS is critical for lipid homeostasis during pregnancy in tsetse flies. Previous studies in D. melanogaster have shown that manipulation of the IIS pathway via targeted gene knockdown results in insulin insensitivity and subsequent changes in lipid content (Teleman, 2010; Bohni et al., 1999; Werz et al., 2009; Shingleton et al., 2005). In addition to its role in maintaining lipid homeostasis, IIS is critical for growth, development and reproduction (Teleman, 2010; GrÄnke et al., 2010; Bohni et al., 1999). In tsetse, we show that genes in the IIS pathway exhibit an oscillatory expression pattern that is synchronized to alternating dry and lactation periods. Observed changes in the transcript abundance of insulin signaling genes correlated both with differences in total maternal lipid content and with the transcript abundance of genes involved in lipid metabolism, suggesting that IIS acts as a key regulator of lipid levels during tsetse pregnancy.
Predominantly, gene knockdown experiments, rather than gene overexpression or insulin injection, have been used to analyze the IIS pathway in insects (Teleman, 2010; Wu and Brown, 2006; Baker and Thummel, 2007). Experiments performed using D. melanogaster Kc cells demonstrated that insulin treatment increased glucose production via the pentose phosphate pathway, but no alteration of lipid levels was observed (Ceddia et al., 2003). Overexpression of InR leads to increased body weight in D. melanogaster (Brogiolo et al., 2001), and activation of the IIS pathway in the D. melanogaster fat body promotes triglyceride accumulation (DiAngelo and Birnbaum, 2009). Lactating tsetse flies receiving insulin injection showed reduced transcripts of genes associated with lipolysis and an increase in those associated with lipogenesis, a phenotype that is similar to the physiological state in dry flies. The insulin-induced decrease in lipolysis likely reduces fats available for incorporation into the milk, leading to reduced fecundity.
It is important to note that the physiological state during tsetse lactation, in which metabolism of storage lipids counteracts lipid shortages associated with lactation (Attardo et al., 2012), is analogous to starvation in D. melanogaster. Under starvation, knockout of FOXO in D. melanogaster leads to decreased levels of bmm (Wang et al., 2011), similar to what we observed following siFOXO treatment in tsetse during lactation. This phenotype of decreased lipolysis and increased lipogenesis is similar to that seen in flies receiving insulin injection, consistent with the role of FOXO as a negative regulator of the IIS pathway. Thus, it is likely that insulin-suppressed lipolysis through bmm is governed by a conserved mechanism in tsetse and Drosophila. Furthermore, bmm suppression may result from reduced HDAC4 expression, since HDAC4 functions as a modulator of FOXO activity, which in turn regulates bmm transcription (Wang et al., 2011). Additional studies are necessary to confirm the effects of FOXO deacetylation by HDAC4 and the subsequent consequences on bmm levels in tsetse. Reduced expression of Pudgy following insulin treatment suggests that this ACS is likely a critical component for promoting FOXO-mediated lipolysis during tsetse lactation. Taken together, our results suggest that the IIS pathway mediates changes in lipid metabolism during cyclical transitions between dry and lactating periods of tsetse pregnancy, whereby positive regulation of the IIS pathway promotes a shift toward lipid accumulation in the fat body, restricting lipid content available for incorporation into the milk.
Potential interaction of IIS and JH pathways
The IIS pathway is critical for various aspects of growth and development, reproduction (Sheng et al., 2011), diapause (Sim and Denlinger, 2008; 2009), and in the modulation of lifespan (Tatar et al., 2001). JH is a similarly pleiotropic hormone and JH/IIS interaction in diverse insect species has recently received considerable attention. For example, in C. pipiens, dsRNA suppression of CpILP-1 results in a physiological state mimicking diapause, which can be rescued by topical application of JH III in a dose-dependent manner (Sim and Denlinger, 2008). A similar state of arrest, obtained via suppression of InR expression, is rescued via provision of exogenous JH (Sim and Denlinger, 2008), suggesting that JH and upstream positive regulators of IIS pathway share some critical intersection to yield similar physiological outcomes. In contrast, in the imaginal discs of final instar Manduca sexta, IIS pathway counteracts JH signal by derepressing the induction of Broad, a gene in Holometabola whose expression is diagnostic for pupal commitment (Koyama et al., 2008). Therefore it appears that the underlying mechanisms of JH/IIS interaction may be species-, stage-, and tissue-specific. Here, we demonstrate that JH and IIS both act to increase lipid levels, likely through increased lipogenesis and decreased lipolysis through suppressed bmm expression. Similar to insulin treatment, we noted a 20–30% decline in HDAC4, 30–40% decline in bmm and 30–35% decline in pudgy transcripts following treatment with methoprene, indicating a JH-induced reduction in lipolysis likely occurs through cross talk with IIS. JH/IIS interaction in tsetse is further supported by the fact that knockdown of genes involved in JH synthesis reception, JHAMT and Met, leads to increased lipolysis, the opposite physiological effect as observed following methoprene treatment. Lastly, this interaction is corroborated by the observation that bmm reduction prior to methoprene treatment yields similar results to methoprene treatment alone, indicating that the lipid accumulation observed following methoprene application likely occurs through the suppression of bmm. Thus, lipid homeostasis in tsetse mothers is likely coordinated through the combined activities of IIS and JH pathways. Importantly, it appears that this interaction is mediated through JH-Met, not JH-Gce, supporting the notion that the paralogous proteins Met and Gce are functionally divergent in higher dipteran reproduction. Met is proposed as a component of a complex molecular bridge that coordinates crosstalk between JH and 20E signaling pathways (Wilson et al., 2006; Li et al., 2007; Bitra and Palli, 2009). Perhaps this regulatory machinery also interacts directly with IIS and additional, unidentified factors to suppress lipolysis through IIS in a complex tissue- and stage-specific manner. Here, we implicate Met as a critical link between IIS and JH signaling in coordinating lipid metabolism during tsetse pregnancy.
Conclusions
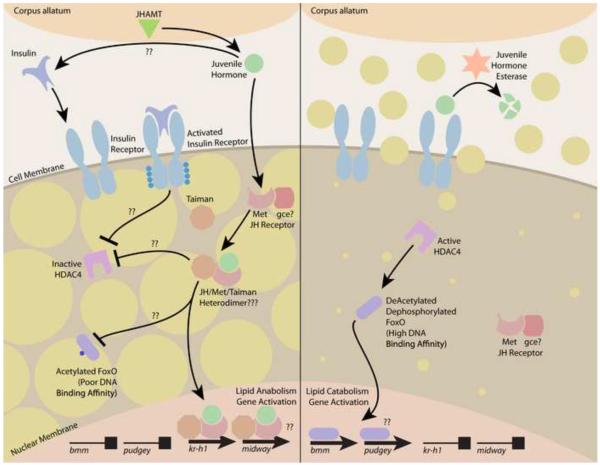
We have demonstrated that both JH and IIS promote lipid accumulation during dry periods between bouts of lactation in tsetse. Based on our results, we have developed a model for these interacting endocrine pathways during tsetse pregnancy (Figure 7). In this model, positive effectors of the insulin signaling pathway, which we propose operates in a manner similar to D. melanogaster, stimulate an increase in lipid content in dry flies. Elevated JH titer acts in unison with the IIS pathway to promote an increase in lipid content, likely through interaction with IIS. When insulin and JH titers are depressed during lactation, lipolytic genes are upregulated, resulting in the mobilization of lipids necessary for incorporation into tsetse milk. Overall, this study demonstrates that changes in lipid metabolism, associated with lactation during tsetse intrauterine progeny development, are under JH/IIS control.
Fig. 7.
Model for the role of JH/IIS signaling in mediating lipid level changes associated with tsetse pregnancy. Left, lipid accumulation during dry periods is promoted by elevation of insulin and JH titer. Right, lipid breakdown during lactation during periods results from reduced insulin and JH titer. JHAMT catalyzes the conversion of JH acid to JH III, increasing JH titer during dry periods. JH signal is transduced through the JH receptor Met; the JH and IIS pathways share a critical intersection to promote lipid accumulation in the mother (lipid content indicated by yellow circles). A decrease in JH and insulin levels during the lactation phase promotes a rapid breakdown of lipids for their incorporation into tsetse milk through the insulin-controlled genes Bmm and Pdgy.
Supplementary Material
Highlights.
Tsetse flies possess two juvenile hormone receptors, Methoprene-tolerant and germ cell expressed, similar to Drosophila.
Juvenile hormone and insulin promote lipid accumulation in tsetse flies.
Juvenile hormone and insulin leads to increased fat storage during non-lactating periods.
Our results indicate the insulin and juvenile hormone are key to nutritional mobilization during tsetse lactation.
Acknowledgements
We thank Oleg Kruglov and Yineng Wu for their technical expertise. We additionally extend thanks to Dr. Lynn Riddiford for valuable comments on this manuscript. This work was supported by the National Institutes of Health (AI081774 to SA, F32AI093023 to JB and RF01228833 to TGW) and Ambrose Monell Foundation (http://www.monellvetlesen.org/) to SA.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abdou M,A, He QY, Wen D, Zyaan O, Wang J, et al. Drosophila Met and Gce are partially redundant in transducing juvenile hormone action. Insect Biochem. Mol. Biol. 2011;41:938–945. doi: 10.1016/j.ibmb.2011.09.003. [DOI] [PubMed] [Google Scholar]
- Attardo GM, Benoit JB, Michalkova V, Yang G, Roller L, et al. Analysis of lipolysis underlying lactation in the tsetse fly, Glossina morsitans. Insect Biochem. Mol. Biol. 2012;41:360–370. doi: 10.1016/j.ibmb.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attardo GM, Guz N, Strickler-Dinglasan P, Aksoy S. Molecular aspects of viviparous reproductive biology of the tsetse fly (Glossina morsitans morsitans): Regulation of yolk and milk gland protein synthesis. J. Insect Physiol. 2006;52:1128–1136. doi: 10.1016/j.jinsphys.2006.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker KD, Thummel CS. Diabetic larvae and obese flies-emerging studies of metabolism in Drosophila. Cell Met. 2007;6:257–266. doi: 10.1016/j.cmet.2007.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry J, Wang SL, Wilson TG. Overexpression of Methoprene-tolerant, a Drosophila melanogaster gene that is critical for juvenile hormone action and insecticide resistance. Insect Biochem. Mol. Biol. 2008;38:346–353. doi: 10.1016/j.ibmb.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann A, Wilson TG. Molecular evolution of juvenile hormone signaling. In: Friedberg F, editor. Gene duplication Rijeka. InTech; Croatia: 2011. [Google Scholar]
- Baumann A, Barry J, Wang SL, Fujiwara Y, Wilson TG. Paralogous genes involved in juvenile hormone action in Drosophila melanogaster. Genetics. 2010a;185:1327–1336. doi: 10.1534/genetics.110.116962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann A, Fujiwara Y, Wilson TG. Evolutionary divergence of the paralogs Methoprene tolerant (Met) and germ cell expressed (gce) within the genus Drosophila. J. Insect Physiol. 2010b;56:1445–1455. doi: 10.1016/j.jinsphys.2010.05.001. [DOI] [PubMed] [Google Scholar]
- Belgacem YH, Martin JR. Disruption of insulin pathways alters trehalose level and abolishes sexual dimorphism in locomotor activity in Drosophila. J. Neurobiol. 2006;66:19–32. doi: 10.1002/neu.20193. [DOI] [PubMed] [Google Scholar]
- Benoit JB, Attardo GM, Michalkova V, Takac P, Bohova J, et al. Sphingomyelinase activity in mother's milk is essential for juvenile development: a case from lactating tsetse flies. Biol. Reprod. 2012;87:1–10. doi: 10.1095/biolreprod.112.100008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benoit JB, Yang G, Krause TB, Patrick KR, Aksoy S, et al. Lipophorin acts as a shuttle of lipids to the milk gland during tsetse fly pregnancy. J. Insect Physiol. 2011;57:1553–1561. doi: 10.1016/j.jinsphys.2011.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitra K, Palli SR. Interaction of proteins involved in ecdysone and juvenile hormone signal transduction. Arch. Insect Biochem. Physiol. 2009;70:90–105. doi: 10.1002/arch.20281. [DOI] [PubMed] [Google Scholar]
- Bohni R, Riesgo-Escovar J, Oldham S, Brogiolo W, Stocker H, et al. Autonomous control of cell and organ size by CHICO, a Drosophila homolog of vertebrate IRS1-4. Cell. 1999;97:865–875. doi: 10.1016/s0092-8674(00)80799-0. [DOI] [PubMed] [Google Scholar]
- Bownes M. The regulation of yolk protein gene expression and vitellogenesis in higher Diptera. In: Raikhel AS, editor. Reproductive Biology of Invertebrates, Part B: Progress in Vitellogenesis. Science Publishers; Enfield, USA/Plymonth, UK: 2004. pp. 95–128. [Google Scholar]
- Butterwood FM, Bodenste D. Adipose tissue of Drosophila melanogaster 4. effect of corpus allatum and synthetic juvenile hormone on tissue of adult male. Gen. Comp. Endocrinol. 1969;13:68–75. doi: 10.1016/0016-6480(69)90222-6. [DOI] [PubMed] [Google Scholar]
- Brogiolo W, Stocker H, Ikeya T, Rintelen F, Fernandez R, et al. An evolutionarily conserved function of the Drosophila insulin receptor and insulin-like peptides in growth control. Current Biol. 2001;11:213–221. doi: 10.1016/s0960-9822(01)00068-9. [DOI] [PubMed] [Google Scholar]
- Broughton SJ, Piper MDW, Ikeya T, Bass TM, Jacobson J, et al. Longer lifespan, altered metabolism, and stress resistance in Drosophila from ablation of cells making insulin-like ligands. Proc. Natl Acad. Sci. USA. 2005;102:3105–3110. doi: 10.1073/pnas.0405775102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceddia RB, Bikopoulos GJ, Hilliker AJ, Sweeney G. Insulin stimulates glucose metabolism via the pentose phosphate pathway in Drosophila Kc cells. FEBS Lett. 2003;555:307–310. doi: 10.1016/s0014-5793(03)01261-4. [DOI] [PubMed] [Google Scholar]
- Charles JP, Iwema T, Epa VC, Takaki K, Rynes J, et al. Ligand-binding properties of a juvenile hormone receptor, Methoprene-tolerant. Proc. Natl Acad. Sci USA. 2011;108:21128–21133. doi: 10.1073/pnas.1116123109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clancy DJ, Gems D, Harshman LG, Oldham S, Stocker H, et al. Extension of lifespan by loss of CHICO, a Drosophila insulin receptor substrate protein. Science. 2001;292:104–106. doi: 10.1126/science.1057991. [DOI] [PubMed] [Google Scholar]
- Clements AN. In: The Biology of Mosquitoes. Clements AN, editor. Vol. 1. Chapman & Hall; London: 1992. [Google Scholar]
- Cmelik SHW, Bursell E, Slack E. Composition of the gut contents of thrid-instar tsetse larvae (Glossina morsitans Westwood) Comp. Biochem. Physiol. 1969;29:447–453. [Google Scholar]
- Colombani J, Andersen DS, Leopold P. Secreted peptide Dilp8 coordinates Drosophila tissue growth with developmental timing. Science. 2012;336:582–585. doi: 10.1126/science.1216689. [DOI] [PubMed] [Google Scholar]
- Daimon T, Kozaki T, Niwa R, Kobayashi I, Furuta K, et al. Precocious metamorphosis in the juvenile-hormone-deficient mutant of the silkworm, Bombyx mori. PLoS Genet. 2012;8:e1002486. doi: 10.1371/journal.pgen.1002486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denlinger DL, Ma W-C. Dynamics of the pregnancy cycle in the tsetse Glossina morsitans. J. Insect Physiol. 1974;20:1015–1026. doi: 10.1016/0022-1910(74)90143-7. [DOI] [PubMed] [Google Scholar]
- Denlinger DL, Ma WC. Maternal nutritive secretions as possible channels for vertical transmission of microorganisms in insects: the tsetse fly example. Ann. NY Acad. Sci. 1975;266:162–165. doi: 10.1111/j.1749-6632.1975.tb35097.x. [DOI] [PubMed] [Google Scholar]
- Denlinger DL. Insect hormones as tsetse abortifacients. Nature. 1975;253:347–348. doi: 10.1038/253347a0. [DOI] [PubMed] [Google Scholar]
- DiAngelo JR, Birnbaum MJ. Regulation of fat cell mass by insulin in Drosophila melanogaster. Mol. Cell. Biol. 2009;29:6341–6352. doi: 10.1128/MCB.00675-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubrovsky EB, Dubrovskaya VA, Bernardo T, Otte V, DiFilippo R, et al. The Drosophila FTZ-F1 nuclear receptor mediates juvenile hormone activation of E75A gene expression through an intracellular pathway. J. Biol. Chem. 2011;286:33689–33700. doi: 10.1074/jbc.M111.273458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ejezie GC, Davey KG. Some effects of allatectomy in female tsetse, Glossina austeni. J. Insect Physiol. 1976;22:1743–1749. doi: 10.1016/0022-1910(76)90068-8. [DOI] [PubMed] [Google Scholar]
- Garelli A, Gontijo AM, Miguela V, Caparros E, Dominguez M. Imaginal discs secrete insulin-like peptide 8 to mediate plasticity of growth and maturation. Science. 2012;336:579–582. doi: 10.1126/science.1216735. [DOI] [PubMed] [Google Scholar]
- Goodman WG, Cusson M. In: The juvenile hormones. Gilbert LI, editor. Insect Endocrinology Amsterdam Elsevier; 2012. pp. 310–365. [Google Scholar]
- Grönke S, Clarke DF, Broughton S, Andrews TD, Partridge L. Molecular evolution and functional characterization of Drosophila insulin-like peptides. PLoS Genetics. 2010;6:e1000857. doi: 10.1371/journal.pgen.1000857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grönke S, Mildner A, Fellert S, Tennagels N, Petry S, et al. Brummer lipase is an evolutionary conserved fat storage regulator in Drosophila. Cell Met. 2005;1:323–330. doi: 10.1016/j.cmet.2005.04.003. [DOI] [PubMed] [Google Scholar]
- Grönke S, Muller G, Hirsch J, Fellert S, Andreou A, et al. Dual lipolytic control of body fat storage and mobilization in Drosophila. PLoS Biol. 2007;5:e137. doi: 10.1371/journal.pbio.0050137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guz N, Attardo GM, Wu Y, Aksoy S. Molecular aspects of transferrin expression in the tsetse fly (Glossina morsitans morsitans) J. Insect Physiol. 2007;53:715–723. doi: 10.1016/j.jinsphys.2007.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeya T, Galic M, Belawat P, Nairz K, Hafen E. Nutrient-dependent expression of insulin-like peptides from neuroendocrine cells in the CNS contributes to growth regulation in Drosophila. Current Biol. 2002;12:1293–1300. doi: 10.1016/s0960-9822(02)01043-6. [DOI] [PubMed] [Google Scholar]
- Kapahi P, Zid BM, Harper T, Koslover D, Sapin V, et al. Regulation of lifespan in Drosophila by modulation of genes in the TOR signaling pathway. Current Biol. 2004;14:885–890. doi: 10.1016/j.cub.2004.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayukawa T, Minakuchi C, Namiki T, Togawa T, Yoshiyama M, et al. Transcriptional regulation of juvenile hormone-mediated induction of Kruppel homolog 1, a repressor of insect metamorphosis. Proc. Natl Acad. Sci. USA. 2012;109:11729–11734. doi: 10.1073/pnas.1204951109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konopova B, Jindra M. Juvenile hormone resistance gene Methoprene-tolerant controls entry into metamorphosis in the beetle Tribolium castaneum. Proc. Natl Acad. Sci. USA. 2007;104:10488–10493. doi: 10.1073/pnas.0703719104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konopova B, Smykal V, Jindra M. Common and distinct role for juvenile hormone signaling genes in metamorphosis of holometabolous and hemimetabolous isnects. PLoS One. 2011;6:e28728. doi: 10.1371/journal.pone.0028728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyama T, Syropyatova MO, Riddiford LM. Insulin/IGF signaling regulates the change in commitment in imaginal discs and primordia by overriding the effect of juvenile hormone. Dev. Biol. 2008;324:258–265. doi: 10.1016/j.ydbio.2008.09.017. [DOI] [PubMed] [Google Scholar]
- Langley PA, Bursell E, Kabayo J, Pimley RW, Trewen MA, et al. Hemolymph lipid transport from fat-body to uterine gland in pregnant females of Glossina morsitans. Insect Biochem. 1981;11:225–231. [Google Scholar]
- Langley PA, Felton T, Oouchi H. Juvenile hormone mimics as effective sterilants for the tsetse fly Glossina morsitans morsitans. Med. Vet. Entomol. 1988;2:29–35. doi: 10.1111/j.1365-2915.1988.tb00046.x. [DOI] [PubMed] [Google Scholar]
- Langley PA, Felton T, Stafford K, Oouchi H. Formulation of pyriproxyfen, a juvenile hormone mimic, for tsetse control. Med. Vet. Entomol. 1990;4:127–133. doi: 10.1111/j.1365-2915.1990.tb00269.x. [DOI] [PubMed] [Google Scholar]
- Li M, Mead EA, Zhu J. Heterodimer of two bHLH-PAS proteins mediates juvenile hormone-induced gene expression. Proc. Natl Acad. Sci. USA. 2011;108:638–643. doi: 10.1073/pnas.1013914108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Zhang Z, Robinson GE, Palli SR. Identification and characterization of a juvenile hormone response element and its binding proteins. J. Biol. Chem. 2007;282:37605–37617. doi: 10.1074/jbc.M704595200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Sheng ZT, Liu HH, Wen D, He QY, et al. Juvenile hormone counteracts the bHLH-PAS transcription factors MET and GCE to prevent caspase-dependent programmed cell death in Drosophila. Development. 2009;136:2015–2025. doi: 10.1242/dev.033712. [DOI] [PubMed] [Google Scholar]
- Meier R, Kotrba M, Ferrar P. Ovoviviparity and viviparity in the Diptera. Biol. Rev. Cambridge Phil. Soc. 1999;74:199–258. [Google Scholar]
- Minakuchi C, Zhou X, Riddiford LM. Kruppel homolog 1 (Kr-h1) mediates juvenile hormone action during metamorphosis of Drosophila melanogaster. Mech. Devel. 2008;125:91–105. doi: 10.1016/j.mod.2007.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moloo SK. An artificial feeding technique for Glossina. Parasitology. 1971;63:507–512. doi: 10.1017/s0031182000080021. [DOI] [PubMed] [Google Scholar]
- Parthasarathy R, Palli SR. Molecular analysis of nutritional and hormonal regulation of female reproduction in the red flour beetle, Tribolium castaneum. Insect Biochem. Mol. Biol. 2011;41:294–305. doi: 10.1016/j.ibmb.2011.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pimley RW, Langley PA. Hormone stimulated lipolysis and proline synthesis in the fat body of the adult tsetse fly, Glossina morsitans. J. Insect Physiol. 1982;28:781–789. [Google Scholar]
- Pimley RW, Langley PA. Hormonal control of lipid synthesis in the fat body of the adult female tsetse fly, Glossina morsitans. J. Insect Physiol. 1981;27:839–847. [Google Scholar]
- Richard DS, Rybczynski R, Wilson TG, Wang Y, Wayne ML, et al. Insulin signaling is necessary for vitellogenesis in Drosophila melanogaster independent of the roles of juvenile hormone and ecdysteroids: female sterility of the chico: insulin signaling mutation is autonomous to the ovary. J. Insect Physio.l. 2005;51:455–464. doi: 10.1016/j.jinsphys.2004.12.013. [DOI] [PubMed] [Google Scholar]
- Riddiford LM. How does juvenile hormone control insect metamorphosis and reproduction? Gen. Comp. Endocrinol. 2012 doi: 10.1016/j.ygcen.2012.06.001. In press. [DOI] [PubMed] [Google Scholar]
- Riddiford LM, Truman JW, Mirth CK, Shen YC. A role for juvenile hormone in the prepupal development of Drosophila melanogaster. Development. 2010;137:1117–1126. doi: 10.1242/dev.037218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riddiford LM. Juvenile hormone action: a 2007 perspective. J. Insect Physiol. 2008;54:895–901. doi: 10.1016/j.jinsphys.2008.01.014. [DOI] [PubMed] [Google Scholar]
- Sheng ZT, Xu JJ, Bai H, Zhu F, Palli SR. Juvenile hormone regulates vitellogenin gene expression through insulin-like peptide signaling pathway in the red flour beetle, Tribolium castaneum. J. Biol. Chem. 2011;286:41924–41936. doi: 10.1074/jbc.M111.269845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shingleton AW, Das J, Vinicius L, Stern DL. The temporal requirements for insulin signaling during development in Drosophila. PLoS Biol. 2005;3:e289. doi: 10.1371/journal.pbio.0030289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sim C, Denlinger DL. Insulin signaling and FOXO regulate the overwintering diapause of the mosquito Culex pipiens. Proc. Natl Acad. Sci. USA. 2008;105:6777–6781. doi: 10.1073/pnas.0802067105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sim C, Denlinger DL. A shut-down in expression of an insulin-like peptide, ILP-1, halts ovarian maturation during the overwintering diapause of the mosquito Culex pipiens. Insect Mol. Biol. 2009;18:325–332. doi: 10.1111/j.1365-2583.2009.00872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soller M, Bownes M, Kubli E. Control of oocyte maturation in sexually mature Drosophila females. Dev. Biol. 1999;208:337–351. doi: 10.1006/dbio.1999.9210. [DOI] [PubMed] [Google Scholar]
- Suren-Castillo S, Abrisqueta M, Maestro JL. FoxO inhibits juvenile hormone biosynthesis and vitellogenin production in the German cockroach. Insect Biochem. Mol. Biol. 2012;42:491–498. doi: 10.1016/j.ibmb.2012.03.006. [DOI] [PubMed] [Google Scholar]
- Suzuki Y, Truman JW, Riddiford LM. The role of Broad in the development of Tribolium castaneum: implications for the evolution of the holometabolous insect pupa. Development. 2008;135:569–577. doi: 10.1242/dev.015263. [DOI] [PubMed] [Google Scholar]
- Tatar M, Kopelman A, Epstein D, Tu MP, Yin CM, et al. Mutations in the Drosophila insulin receptor homologue extend lifespan and impair neuroendocrine function. Diabetes. 2001;50:A79–A79. [Google Scholar]
- Teleman AA. Molecular mechanisms of metabolic regulation by insulin in Drosophila. Biochem. J. 2010;425:13–26. doi: 10.1042/BJ20091181. [DOI] [PubMed] [Google Scholar]
- Tobe SS, Langley PA. Reproductive Physiology of Glossina. Ann. Rev. Entomol. 1978;23:283–307. doi: 10.1146/annurev.en.23.010178.001435. [DOI] [PubMed] [Google Scholar]
- Truman JW, Hiruma K, Allee JP, MacWhinnie SGB, Champlin DT, et al. Juvenile hormone is required to couple imaginal disc formation with nutrition in insects. Science. 2006;312:1385–1388. doi: 10.1126/science.1123652. [DOI] [PubMed] [Google Scholar]
- Tu MP, Yin CM, Tatar M. Mutations in insulin signaling pathway alter juvenile hormone synthesis in Drosophila melanogaster. Gen. Comp. Endocrinol. 2005;142:347–356. doi: 10.1016/j.ygcen.2005.02.009. [DOI] [PubMed] [Google Scholar]
- Van Handel E. Rapid determination of total lipids in mosquitoes. J. Am. Mosquito. Cont. Assoc. 1985;1:302–304. [PubMed] [Google Scholar]
- Wang J, Aksoy S. PGRP-LB is a maternally transmitted immune milk protein that influences symbiosis and parasitism in tsetse's offspring. Proc. Natl Acad. Sci. USA. 2012;109:10552–7. doi: 10.1073/pnas.1116431109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang SL, Baumann A, Wilson TG. Drosophila melanogaster Methoprene-tolerant (Met) gene homologs from three mosquito species: Members of PAS transcriptional factor family. J. Insect Physiol. 2007;53:246–253. doi: 10.1016/j.jinsphys.2006.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, Moya N, Niessen S, Hoover H, Mihaylova MM, et al. A hormone-dependent module regulating energy balance. Cell. 2011;145:596–606. doi: 10.1016/j.cell.2011.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werz C, Kohler K, Hafen E, Stocker H. The Drosophila SH2B family adaptor Lnk acts in parallel to chico in the insulin signaling pathway. PLoS Genetics. 2009;5:e1000596. doi: 10.1371/journal.pgen.1000596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson TG, Fabian J. A Drosophila melanogaster mutant resistant to a chemical analog of juvenile hormone. Dev. Biol. 1986;118:190–201. doi: 10.1016/0012-1606(86)90087-4. [DOI] [PubMed] [Google Scholar]
- Wilson TG, Ashok M. Insecticide resistance resulting from an absence of target-site gene product. Proc. Natl Acad. Sci. USA. 1998;95:14040–14044. doi: 10.1073/pnas.95.24.14040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson TG, Yerushalmi Y, Donnell DM, Restifo LL. Interaction between hormonal signaling pathways in Drosophila melanogaster as revealed by genetic interaction between methoprene-tolerant and broad-complex. Genetics. 2006;172:253–264. doi: 10.1534/genetics.105.046631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Q, Brown MR. Signaling and function of insulin-like peptides in insects. Annu. Rev. Entomol. 2006;51:1–24. doi: 10.1146/annurev.ento.51.110104.151011. [DOI] [PubMed] [Google Scholar]
- Wyatt GR, Davey KG. Cellular and molecular actions of juvenile hormone. II. Roles of juvenile hormones in adult insects. Adv. Insect Physiol. 1996;26:1–155. [Google Scholar]
- Xu XJ, Gopalacharyulu P, Seppanen-Laakso T, Ruskeepaa AL, Aye CC, et al. Insulin signaling regulates fatty acid catabolism at the level of CoA activation. PLoS Genetics. 2012:8. doi: 10.1371/journal.pgen.1002478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang G, Attardo GM, Lohs C, Aksoy S. Molecular characterization of two novel milk proteins in the tsetse fly (Glossina morsitans morsitans) Insect Mol. Biol. 2010;19:253–262. doi: 10.1111/j.1365-2583.2009.00987.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang ZL, Xu JJ, Sheng ZT, Sui YP, Palli SR. Steroid receptor co-activator is required for juvenile hormone signal transduction through a bHLH-PAS transcription factor, Methoprene-tolerant. J. Biol. Chem. 2011;286:8437–8447. doi: 10.1074/jbc.M110.191684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Liu JN, Li CR, Momen B, Kohanski RA, et al. Deletion of Drosophila insulin-like peptides causes growth defects and metabolic abnormalities. Proc. Natl Acad. Sci. USA. 2009;106:19617–19622. doi: 10.1073/pnas.0905083106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Riddiford LM. Broad specifies pupal development and mediates the 'status quo' action of juvenile hormone on the pupal-adult transformation in Drosophila and Manduca. Development. 2002;129:2259–2269. doi: 10.1242/dev.129.9.2259. [DOI] [PubMed] [Google Scholar]
- Zhu JS, Busche JM, Zhang X. Identification of juvenile hormone target genes in the adult female mosquitoes. Insect Biochem. Mol. Biol. 2010;40:23–29. doi: 10.1016/j.ibmb.2009.12.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.