Abstract
The structure of the essential oil secretory tissues of Prostanthera ovalifolia R.Br was investigated using bright‐ and dark‐field optical microscopy, and scanning and transmission electron microscopy. The leaves of P. ovalifolia have glandular trichomes of the peltate type common to many Lamiaceae species. The trichomes consist of a basal cell embedded in the epidermis, a stalk cell with heavily cutinized walls and a 16‐celled secretory head, but they differ from those of many previously reported Lamiaceae species in their morphological form defined by the elevated cuticle. The sub‐cuticular space contains a mixture of lipid and aqueous phases. Secretory cells have dense cytoplasm with many leucoplasts present. Volatile terpenoids are eliminated from the cytoplasm into the sub‐cuticular space, the site of essential oil accumulation, via granulocrine secretion.
Key words: Trichomes, Lamiaceae, secretion, essential oil, volatile terpenoids, Prostanthera ovalifolia, optical microscopy, electron microscopy
INTRODUCTION
The Lamiaceae comprise many commercially important essential oil‐accumulating species. The structure and function of glandular trichomes occurring in plants of the Lamiaceae is well documented (Werkeret al., 1985; Fahn, 1988, 2000; Werker, 1993; Ascensaoet al., 1995) and they are recognized as the site of essential oil biosynthesis, secretion and accumulation (Croteau, 1986; Gershenzonet al., 1989). Some variability exists between Lamiaceae genera as to the types of trichomes (peltate, capitate, non‐glandular and different versions and combinations of these) that can occur in a given species (Venkatachalamet al., 1984).
Reports indicate that, within the family, different species can have both peltate and capitate trichomes, peltate or capitate only or, more rarely, neither. In a recent study of Lamiaceae secretory structures, Corsi and Bottega (1999) identified four different types of capitate trichomes on the leaves of Salvia officinalis, in addition to peltate trichomes. Each type had a different spatial arrangement and function, secreting different combinations, or proportions, of lipophilic and hydrophilic material.
Many species endemic in Australia are known to synthesize and accumulate significant quantities of volatile terpenoids. The range of known essential oil plants, their essential oil compositions and the properties of and potential uses for the oil have been reviewed periodically (Lassak and Southwell, 1977; Southwell, 1987; Southwell and Brophy, 2000). The Myrtaceae family is the most extensively studied, with the genera Eucalyptus (Bolandet al., 1991) and Melaleuca (Riedl, 1997) containing species of considerable economic significance. Essential oil secretory tissues have been studied in some endemic Australian Myrtaceae including Eucalyptus species (Carr and Carr, 1970), Angophora species (Ladiges, 1984) and Melaleuca alternifolia (Listet al., 1995).
The native Lamiaceae have received relatively less attention in the field of essential oil‐related research. The genus Prostanthera, belonging to the family Lamiaceae, consists of around 100 species that are endemic to Australia (Conn, 1992). They are collectively known as ‘mint bushes’ and are generally reported to have aromatic foliage (Lassak and McCarthy, 1983). Volatile extracts of relatively few species have been analysed, and there are no publications dealing with secretory structures in the genus, apart from the general observation that oil glands exist on the leaves.
The species P. ovalifolia (purple mint bush) is endemic to the coastal regions of New South Wales and southern Queensland. A comprehensive description of the species’ growth and morphology is available (Conn, 1992). It secretes and accumulates in the leaves an essential oil particularly rich in 1,8‐cineole, and containing significant proportions of p‐cymene and the sesquiterpene ether cis‐dihydroagarofuran (Southwell and Tucker, 1993).
This paper describes the use of several imaging techniques including bright‐ and dark‐field optical microscopy, and scanning (SEM) and transmission (TEM) electron microscopy, to investigate structural and functional aspects of the essential oil secretory structures of P. ovalifolia. It is usually preferable to study a specimen in its natural state if possible, and this report demonstrates the application of environmental SEM to non‐invasive imaging of plant secretory structures.
MATERIALS AND METHODS
Bright‐field microscopy
Fresh leaves of Prostanthera ovalifolia R.Br were collected from plants grown in the native annex of the Royal Botanic Gardens, Mt Annan, NSW. Leaves at various stages of maturity were selected, ranging from the first fully expanded leaves from flush growth to immature leaves of approx. 500 mm in length. The external morphology of intact leaves, and fresh sectioned tissue, with relevance to secretory structures, was examined by light microscopy.
We used an Olympus SZH zoom stereomicroscope equipped with a DF‐Plan 1·0× objective, with the aperture adjusted to the minimum setting, and an Olympus BH‐2 transmitted light microscope with 10× and 40× PlanApo objectives. For photography, both instruments were fitted with a 2·5× projection eyepiece and an Olympus C35AD‐4 camera, operated by an Olympus PM‐10AD5 automatic photomicrographic system attachment. Images were recorded on 100 ASA print film.
Fluorescence microscopy
Hand‐sections were cut from leaves and mounted in water on glass slides with cover slips, then examined by fluorescence microscopy. An Olympus BHC light microscope equipped with a BH2‐RFL epifluorescence attachment was used. The fluorescence attachment contained two filter assemblies designed to provide specific wavelengths of light from the blue and ultra‐violet regions of the spectrum. The blue light irradiation assembly consisted of an excitation filter BP‐490, dichroic mirror DM‐500 and barrier filter O‐515, with an additional excitation filter EY‐455 and two additional barrier filters B‐460 and G‐520. This combination of filters produced bright lines at 405 and 435 nm and continuous spectrum regions near 490 nm. The ultra‐violet irradiation assembly consisted of excitation filter UG‐1, dichroic mirror DM‐400 and barrier filters L‐420 and L‐435, resulting in excitation wavelengths of 334 and 365 nm.
The light source was an Olympus HBO‐100 high pressure mercury lamp, mounted directly onto the fluorescence attachment. The objectives used were Olympus D‐PlanApo‐10× UV, and D‐Plan 40× UV‐RiO oil immersion (both fluorescence‐free). The microscope was also fitted with 10× eyepieces and a trinocular observation tube incorporating an Olympus C35AD‐4 camera and 2·5× projection eyepiece. The sections were examined and photographed using ultra‐violet and blue light excitation. Images were recorded on 400 ASA print film, and resulted from autofluorescence of the particular tissues or structures; no stains or fluorochromes of any description were used.
Scanning electron microscopy
Freeze fracture.
Sections of tissue approx. 10 mm2 were cut from the leaves. These were frozen and fractured in liquid nitrogen, then placed in vented 10 ml polypropylene tubes that had been pre‐cooled in liquid nitrogen. The tubes containing the frozen tissue were transferred to a freeze dryer and dried over a period of 3 d at –50 °C. The dried sections were mounted on SEM stubs with silver paint and sputter coated with gold. The sections were examined and photographed with a Jeol JSM‐T330 scanning electron microscope with the accelerating voltage at 15·0 kV.
Environmental SEM.
Entire fresh leaves were mounted on SEM stubs and imaged in the microscope while still biologically functional. Examination and photography of the plant material was performed with an Electroscan environmental SEM equipped with a Peltier cold stage and an environmental secondary electron detector. Operating conditions were stage temperature 7·5 °C, chamber pressure 800 Pa and accelerating voltage 10·0 kV.
Transmission electron microscopy.
Sections of tissue approx. 1 mm2 were cut from leaves and fixed in a double aldehyde solution (2·0 % w/w formaldehyde, 2·5 % w/w glutaraldehyde, 0·05 m sucrose and 2·0 mm CaCl2 in 0·10 m phosphate buffer, pH 6·8) in separate glass vials, initially under a low vacuum at room temperature until the tissue was infiltrated with fixative, and then for a further 12 h at 4·0 °C. After fixation the tissue was washed three times with phosphate buffer, 15 min per wash. Sections were post‐fixed in 2·5 % w/w OsO4 for 2 h, followed by a further three × 15 min washes in buffer and a final wash for 15 min in distilled water. Dehydration was in a water/ethanol series consisting of 5, 10, 20, 30, 40, 50, 60, 70, 80 and 90 % w/w ethanol for 1 h each, followed by three changes of anhydrous ethanol for 1 h each.
The dehydrated sections were infiltrated with 10, 20, 40, 60 and 80 % w/w Spurr’s resin in anhydrous ethanol for 6 h each, followed by five changes of pure resin for 6 h each. The sections were embedded in flat moulds with fresh resin and polymerized at 70 °C for 24 h. The resin blocks were sectioned with a Reichert Ultracut‐E ultramicrotome fitted with a diamond knife. Semi‐thin sections of 2 µm were cut first, stained with Toluidine Blue O, examined with a light microscope and photographed. Ultra‐thin sections of 60– 75 nm were cut and collected on formvar‐coated copper slot grids. The mounted ultra‐thin sections were stained, first with 5·0 % w/w uranyl acetate in a 1 : 1 solution of methanol and water for 3 min followed by three × 1 min washes with distilled water, then with Reynolds lead citrate for 2 min followed by another three × 1 min washes with distilled water. After drying, the sections were examined and photographed using a Jeol 1010 transmission electron microscope with the accelerating voltage at 80 kV.
RESULTS AND DISCUSSION
The glandular trichomes of Prostanthera ovalifolia were up to 120 µm in diameter, and their distribution was apparently random at approx. 25 trichomes mm–2 over adaxial and abaxial leaf surfaces and the calyx. A leaf, and the arrangement and relative size and density of the trichomes, is shown in Fig. 1 (bright‐field images). The trichomes were all of the peltate variety; no capitate trichomes were noted and non‐glandular hairs were few and sparsely distributed. The peltate glandular trichomes (PGTs) consisted of one, or occasionally two, basal cells embedded in the epidermis, a single stalk cell connecting the basal cell with the secretory head, which incorporated 16 secretory cells, and an elevated cuticle enclosing the accumulated secreted material.
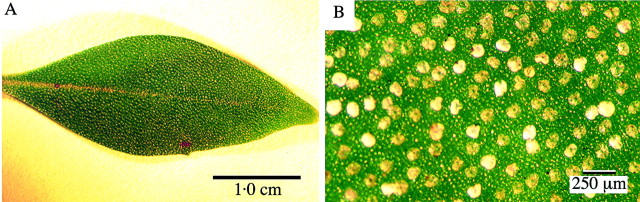
Fig. 1. A, Bright‐field image of a Prostanthera ovalifolia leaf. The presence of external oil glands is evident even at low magnification. B, Bright‐field image showing size and distribution of peltate glandular trichomes on adaxial leaf surface.
The trichomes of P. ovalifolia appeared to follow a similar ontogenic pattern to that described for other Lamiaceae species (Ascensaoet al., 1995), originating from a single protodermal cell that could be distinguished from neighbouring cells by its darker‐staining content (Fig. 2A). Trichomes at various stages of development were observed on very young leaves; however, the proportion of mature to immature trichomes increased with leaf maturity, with immature trichomes being exceedingly rare on fully mature leaves. Immature trichomes were smaller than 120 µm, with the stalk cell and head protruding above the leaf epidermis as shown in Fig. 2B, while mature trichomes were assumed to be those sunken below the normal level of the epidermis as shown in Fig. 3.
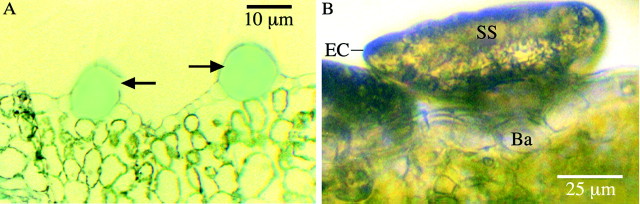
Fig. 2. A, Transmitted light micrograph of leaf section showing enlarged epidermal cells (arrows) of the type from which trichomes will develop. These cells stain more darkly with Toluidine Blue than normal epidermal cells. B, Immature glandular trichome. Secreted material has accumulated in the sub‐cuticular space (SS), but the stalk cell is still above the level of the epidermis. EC, Elevated cuticle; Ba, basal cell.
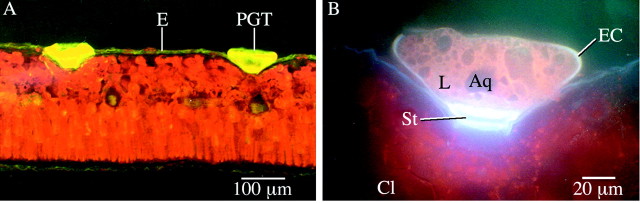
Fig. 3. A, Section of leaf imaged by epifluorescence microscopy with blue light excitation showing peltate glandular trichomes (PGT) embedded in epidermis (E). The trichomes and their contents contribute to the yellow/green autofluorescence; the red autofluorescence is emitted by chlorophyll. B, Autofluorescence of glandular trichome imaged with UV excitation. Note the characteristic shape of the trichome as defined by the elevated cuticle (EC), the presence of lipid (L) and aqueous (Aq) secretions accumulated in the sub‐cuticular space, the relatively intense blue/white autofluorescence of the stalk cell (St) wall and the apparent absence of intact secretory cells. Cl, Red autofluorescence of chlorophyll.
The PGTs differed from many previously recorded examples in the Lamiaceae in the occasional presence of two basal cells, and in the overall shape of the trichome, largely defined by the elevated cuticle. The stylized representation of a lamiaceous PGT (Fahn, 1988) suggests a rounded, bulbous appearance. In contrast, in P. ovalifolia the elevated cuticle was broader and flattened, and had a distinctly segmented appearance. These features are clearly illustrated in the fluorescence microscopy images (Fig. 3), the freeze fractured SEM images (Fig. 4) and the environmental SEM images (Fig. 5). There were always 16 sectors, which coincidently corresponded with the number of secretory cells. Segmented peltate trichomes have previously been noted in some Plectranthus (Lamiaceae) species (Ascensaoet al., 1998).
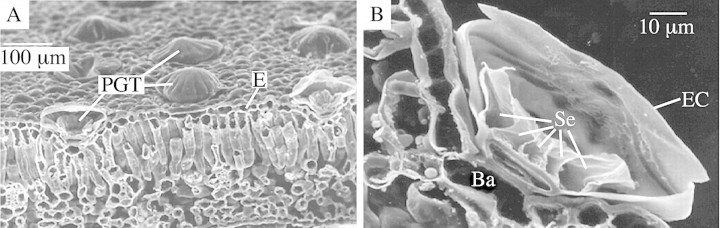
Fig. 4. SEM micrographs of freeze‐fractured leaf sections. A, Glandular trichomes (PGT) on leaf surface and in section at the fractured surface. E, Epidermis. B, Section through a glandular trichome showing internal structure. Se, secretory cells; Ba, basal cell; EC, elevated cuticle.
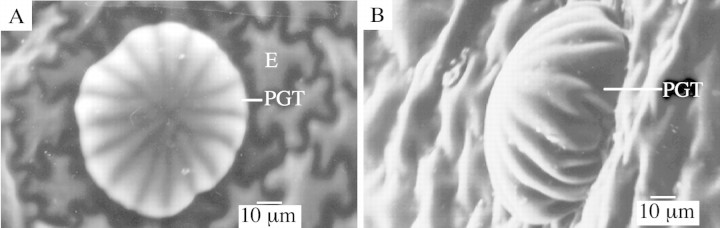
Fig. 5. Environmental SEM micrographs of fresh leaf showing a glandular trichome (PGT). The elevated cuticle is segmented, the number of sectors always 16. E, Epidermal cells. The stalk and basal cells, not visible, are embedded below the normal level of the epidermis.
The basal cells were not readily distinguishable from other epidermal cells, with the possible exception of being slightly larger in some instances. The stalk cell was relatively short, in the order of 5–10 µm from the points of attachment to basal and secretory cells. Stalk cells in Lamiaceae have been reported to possess heavily cutinized cell walls, and this feature has the dual function of adding structural support to the trichome and enabling the stalk cell to act as a barrier to intercellular movement of secretions. Partitioning of terpenes in well‐defined compartments is a general feature of essential oil‐accumulating plants (Fahn, 1988).
A stalk cell of a P. ovalifolia PGT is illustrated in Fig. 3B. The image is of autofluorescence emitted by a PGT as a result of UV light excitation. The stalk cell wall fluoresced an intense blue‐white. Fluorescence of this nature, clearly different from that emitted by other tissues, is evidence of the specialized function of the cell. In addition to chlorophyll, which can be observed fluorescing in Fig. 3, cutin and suberin are compounds in which autofluorescence is particularly marked (Rost, 1992). As suggested above, cutinization of cell walls in trichomes can be related to aspects of structural support and to compartmentation of secreted material. This type of fluorescence has been noted in previous studies of secretory idioblasts in Cymbopogon citratus (Poaceae) (Lewinsohnet al., 1998), and was attributed to the presence of cutin in the idioblast cell wall.
The secreted material visible in this image appeared to consist of a mixture of both lipid and aqueous phases. The darker areas in the sub‐cuticular space presumably contain mostly water, which does not fluoresce, while most of the fluorescent light would be emitted by terpenoid compounds in the lipid phase, such as p‐cymene, and which contain an aromatic ring (Devet al., 1982), possibly with a contribution from other sources such as flavones (Bosabalidiset al., 1998).
There are two possible explanations for the presence of the aqueous phase in the sub‐cuticular space. It could be attributed to the degradation of secretory cells subsequent to maturity, the contents of which have become part of the secreted material in the sub‐cuticular space. A contribution to the aqueous phase from other types of secreted materials is also possible, as trichomes in some Lamiaceae are known to secrete compounds such as carbohydrates in solution, in addition to volatile terpenes (Amelunxenet al., 1969; Werker, 1993).
The 16 cells in the secretory head of the trichomes were arranged in a circle. If viewed from directly below the trichome, as in Fig. 6A, each cell represents a sector of the circle, with an arc of approx. 22·5°. Prior to secretion commencing in new trichomes, the cuticle was in contact with the secretory cells. Although not observed directly, it is proposed that when secretion commences, very early in the development of a trichome, the cuticle detaches from the secretory cells, forming the sub‐cuticular space in which the secreted material accumulates, as described previously (Werker, 1993). In fully mature trichomes the space had reached its maximum dimensions and the secretory cells often appeared to be lysing (Figs 6B, 7A) or absent (Fig. 2B).
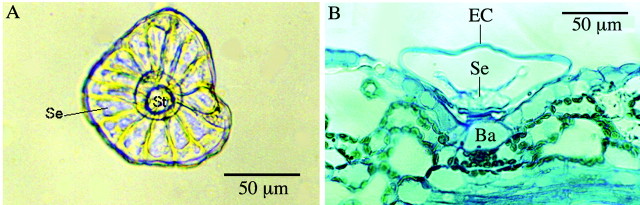
Fig. 6. A, Transmitted light micrograph of an immature glandular trichome that has been detached from a leaf to show the arrangement of secretory cells (Se) in an intact trichome from below. There are always 16 secretory cells, and viewed from this angle they appear to be arranged like sectors of a circle, with the stalk cell (St) at the centre. B, Embedded section through a mature glandular trichome in which the secretory cells (Se) appear to have partially degraded. EC, Elevated cuticle; Ba, basal cell.
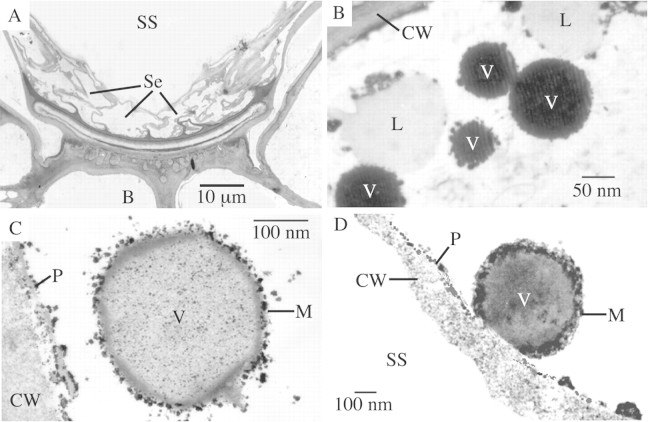
Figure 7B is a section through a secretory cell acquired by TEM, showing leucoplasts and oil droplets in the cell cytoplasm. Compared with normal mesophyll cells, trichome secretory cells have denser cytoplasm containing many leucoplasts. These plastids are a noted feature of secretory cells (Cheniclet and Carde, 1985) and are implicated as the site of mono‐ and sesquiterpenoid biosynthesis. The plastids observed in the secretory cells of P. ovalifolia were often adjacent to oil droplets, as in Fig. 7B, and were of the type devoid of internal structure, denoting primarily monoterpene synthesis (Fahn, 1988). In the secretory cell cytoplasm of P. ovalifolia trichomes, oil droplets were observed in membrane‐bound vesicles (Fig. 7C) that fused with the plasmalemma (Fig. 7D). On this evidence, exocytosis was favoured as the probable mechanism for elimination of the essential oil from the cell.
Fig. 7. Transmission electron micrographs. A, Section through glandular trichome showing basal cell (B), sub‐cuticular space (SS) and degrading secretory cells (Se). B, Section through active secretory cell showing leucoplasts (L), which are implicated in essential oil biosynthesis, and vesicles (V) containing osmium‐staining material (lipid). C, Membrane‐bound vesicle (V) containing lipid material, in secretory cell cytoplasm. D, Membrane‐bound oil vesicle (V) fused with secretory cell plasmalemma (P), suggesting essential oil is secreted from the cell by exocytosis.
The genus Prostanthera contains some of Australia’s best examples of lamiaceous essential oil plants. The Australian flora as a whole offers many more opportunities for the study of essential oil secretory structures. Many species synthesize and accumulate volatile terpenoids, some of which show commercial potential. There is also the incentive to improve upon existing records of secretory structures through the adaptation of potentially non‐invasive methods such as environmental SEM and confocal microscopy, which have not previously been used for this purpose.
ACKNOWLEDGEMENTS
The author thanks V. Sarafis of the Centre for Microscopy and Microanalysis (University of Queensland) for facilitating access to electron microscopes, and J. Nailon and R. Gould of CMM (UQ) for technical assistance and advice on electron microscopy and sample preparation.
Supplementary Material
Received: 13 June 2001; Returned for revision: 3 October 2001; Accepted: 13 November 2001.
References
- AmelunxenF, Wahlig T, Arbeiter T.1969. Über den Nachweis des ätherischen Öls in isolierten Drüsenhaaren und Drüsenschuppen von Mentha piperita L. Zeitschrift für Pflanzenphysiologie 61: 68–72. [Google Scholar]
- AscensaoL, Marques N, Pais MS.1995. Glandular trichomes on vegetative and reproductive organs of Leonotis leonurus (Lamiaceae). Annals of Botany 75: 619–626. [Google Scholar]
- AscensaoL, Figueiredo AC, Barroso JG, Pedro LG, Schripsema J, Deans SG, Scheffer JCJ.1998. Plectranthus madagascariensis – morphology of the glandular trichomes, essential oil composition, and its biological activity. International Journal of Plant Sciences 159: 31–38. [Google Scholar]
- BolandDJ, Brophy JJ, House APN.1991. Eucalyptus leaf oils: use, chemistry, distillation and marketing. Melbourne: Inkata Press. [Google Scholar]
- BosabalidisA, Gabrieli C, Niopas I.1998. Flavone aglycones in glandular hairs of Oreganum × intercedens Phytochemistry 49: 1549–1553. [DOI] [PubMed] [Google Scholar]
- CarrDJ, Carr SGM.1970. Oil glands and ducts in Eucalyptus L’ Hérit. II. Development and structure of oil glands in the embryo. Australian Journal of Botany 18: 191–212. [Google Scholar]
- ChenicletC, Carde JP.1985. Presence of leucoplasts in secretory cells and of monoterpenes in the essential oil: a correlative study. Israel Journal of Botany 34: 219–238. [Google Scholar]
- ConnBJ.1992. Flora of New South Wales, vol 3. Kensington NSW: NSW University Press. [Google Scholar]
- CorsiG, Bottega S.1999. Glandular hairs of Salvia officinalis: New data on morphology, localisation and histochemistry in relation to function. Annals of Botany 84: 657–664. [Google Scholar]
- CroteauR.1986. Biochemistry of monoterpenes and sesquiterpenes of the essential oils. In: Craker LE, Simon JE, eds. Herbs, spices and medicinal plants: Recent advances in botany, horticulture and pharmacology, vol 1. Phoenix: Oryx Press. [Google Scholar]
- DevS, Narula A, Yadav J.1982. CRC handbook of terpenoids – monoterpenoids. Boca Raton, Florida: CRC Press. [Google Scholar]
- FahnA.1988. Secretory tissues in vascular plants. New Phytologist 108: 229–257. [DOI] [PubMed] [Google Scholar]
- FahnA.2000. Structure and function of secretory cells. In: Hallahan DL, Gray JC, Callow JA, eds. Advances in botanical research incorporating advances in plant pathology, volume 31: plant trichomes. London: Academic Press. [Google Scholar]
- GershenzonJ, Maffei M, Croteau R.1989. Biochemical and histochemical localisation of monoterpene biosynthesis in the glandular trichomes of spearmint (Mentha spicata). Plant Physiology 89: 1351–1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LadigesP.1984. A comparative study of trichomes in Angophora Cav. and Eucalyptus L’Herit – a question of homology. Australian Journal of Botany 32: 561–574. [Google Scholar]
- LassakEV, McCarthy T.1983. Australian medicinal plants. Hawthorn, Victoria: Methuen Australia Pty Ltd. [Google Scholar]
- LassakEV, Southwell IA.1977. Essential oil isolates from the Australian flora. In: Flavour and Fragrance Additives Sydney: Museum of Applied Arts and Sciences. pp 126–132. [Google Scholar]
- LewinsohnE, Dudai N, Tadmor Y, Katzir I, Ravid U, Putievsky E, Joel DM.1998. Histochemical localisation of citral accumulation in lemongrass leaves (Cymbopogon citratus, Poaceae). Annals of Botany 81: 35–39. [Google Scholar]
- ListS, Brown PH, Walsh KB.1995. Functional anatomy of the oil glands of Melaleuca alternifolia (Myrtaceae). Australian Journal of Botany 43: 629–641. [Google Scholar]
- RiedlRW.1997. Practical methods for using tea tree oil. Agro Food Industry Hi‐Tech 8: 34–36. [Google Scholar]
- RostFWD.1992. Fluorescence microscopy. Cambridge, New York: Cambridge University Press. [Google Scholar]
- SouthwellIA.1987. Essential oil isolated from the Australian flora. Part 2. Flavour and Fragrance Journal 2: 21–27. [Google Scholar]
- SouthwellIA, Brophy JJ.2000. Essential oil isolates from the Australian flora. Part 3. Journal of Essential Oil Research 12: 267–278. [Google Scholar]
- SouthwellIA, Tucker DJ.1993. Cis‐dihydroagarofuran from Prostanthera sp aff. ovalifolia Phytochemistry 33: 857–862. [Google Scholar]
- VenkatachalamKV, Kjonaas R, Croteau R.1984. Development and essential oil content of secretory glands of sage (Salvia officinalis). Plant Physiology 76: 148–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WerkerE.1993. Function of essential oil‐secreting glandular hairs in aromatic plants of the Lamiaceae – a review. Flavour and Fragrance Journal 8: 249–255. [Google Scholar]
- WerkerE, Ravid U, Putievsky E.1985. Structure of glandular hairs and identification of the main components of their secreted material in some species of the Labiatae. Israel Journal of Botany 34: 31–45. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.