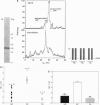
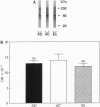
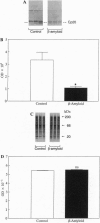
Abstract
The two proteins most consistently identified in the brains of patients with Alzheimer disease (AD) have been beta-amyloid and tau, whose roles in the physiology or pathophysiology of brain cells are not fully understood. To identify other protein(s) involved in AD that have been implicated in physiological contexts, we undertook to analyze a specific memory-associated protein, Cp20, in fibroblasts from AD and control donors. Cp20, a GTP-binding protein that is a member of the ADP-ribosylation factor family, was significantly decreased in fibroblasts from AD patients. Normal control fibroblasts exposed to 10 nM beta-amyloid, the same concentration that induced AD-like K+ changes in control fibroblasts, showed a similar decrease in Cp20. Since it has been previously demonstrated that Cp20 is a potent regulator of K+ channels, these findings suggest that changes in this memory-associated protein may explain previously observed differences in AD K+ channels and suggest a pathophysiologic involvement linked to soluble beta-amyloid metabolism that could contribute to the characteristic memory loss of AD.
Full text
PDF

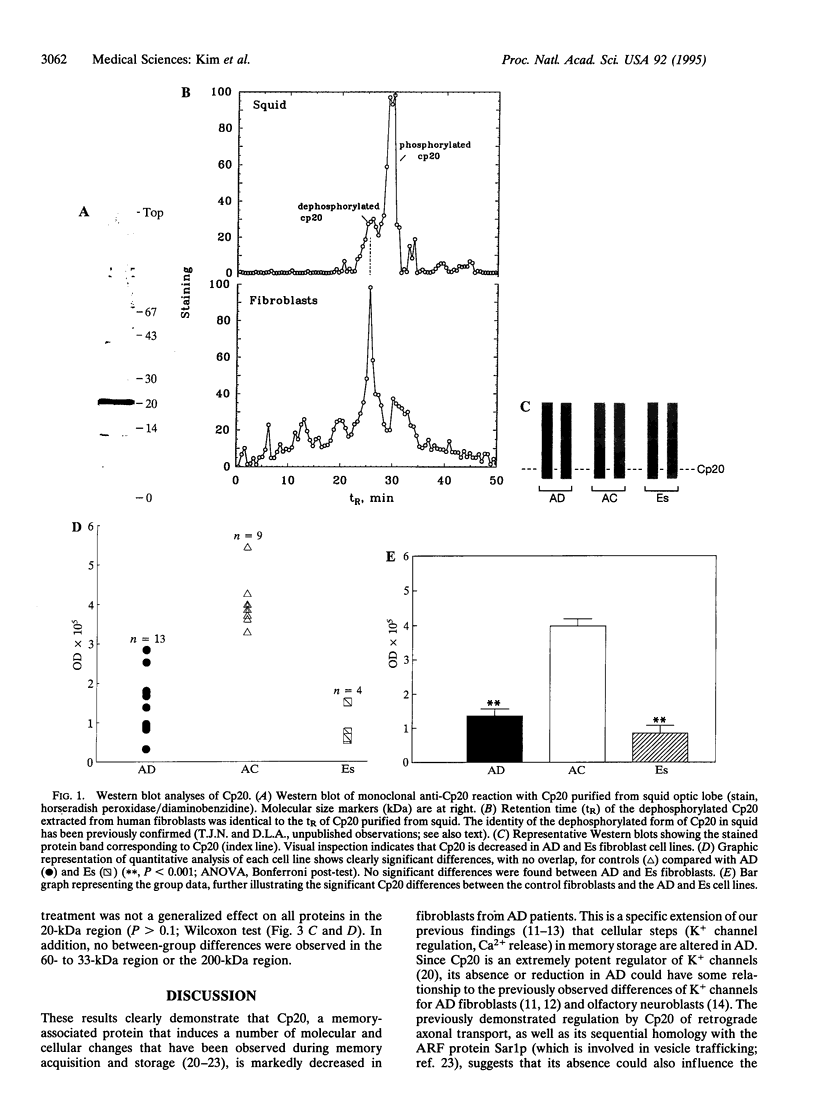

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alkon D. L., Ikeno H., Dworkin J., McPhie D. L., Olds J. L., Lederhendler I., Matzel L., Schreurs B. G., Kuzirian A., Collin C. Contraction of neuronal branching volume: an anatomic correlate of Pavlovian conditioning. Proc Natl Acad Sci U S A. 1990 Feb;87(4):1611–1614. doi: 10.1073/pnas.87.4.1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alkon D. L., Naito S., Kubota M., Chen C., Bank B., Smallwood J., Gallant P., Rasmussen H. Regulation of Hermissenda K+ channels by cytoplasmic and membrane-associated C-kinase. J Neurochem. 1988 Sep;51(3):903–917. doi: 10.1111/j.1471-4159.1988.tb01827.x. [DOI] [PubMed] [Google Scholar]
- Arispe N., Pollard H. B., Rojas E. Giant multilevel cation channels formed by Alzheimer disease amyloid beta-protein [A beta P-(1-40)] in bilayer membranes. Proc Natl Acad Sci U S A. 1993 Nov 15;90(22):10573–10577. doi: 10.1073/pnas.90.22.10573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bomsel M., Mostov K. Role of heterotrimeric G proteins in membrane traffic. Mol Biol Cell. 1992 Dec;3(12):1317–1328. doi: 10.1091/mbc.3.12.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busciglio J., Gabuzda D. H., Matsudaira P., Yankner B. A. Generation of beta-amyloid in the secretory pathway in neuronal and nonneuronal cells. Proc Natl Acad Sci U S A. 1993 Mar 1;90(5):2092–2096. doi: 10.1073/pnas.90.5.2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush A. I., Beyreuther K., Masters C. L. Beta A4 amyloid protein and its precursor in Alzheimer's disease. Pharmacol Ther. 1992;56(1):97–117. doi: 10.1016/0163-7258(92)90039-3. [DOI] [PubMed] [Google Scholar]
- Collin C., Papageorge A. G., Lowy D. R., Alkon D. L. Early enhancement of calcium currents by H-ras oncoproteins injected into Hermissenda neurons. Science. 1990 Dec 21;250(4988):1743–1745. doi: 10.1126/science.2176747. [DOI] [PubMed] [Google Scholar]
- Cotton P. Constellation of risks and processes seen in search for Alzheimer's clues. JAMA. 1994 Jan 12;271(2):89–91. [PubMed] [Google Scholar]
- Crutcher K. A., Anderton B. H., Barger S. W., Ohm T. G., Snow A. D. Cellular and molecular pathology in Alzheimer's disease. Hippocampus. 1993;3(Spec No):271–287. [PubMed] [Google Scholar]
- Estus S., Golde T. E., Kunishita T., Blades D., Lowery D., Eisen M., Usiak M., Qu X. M., Tabira T., Greenberg B. D. Potentially amyloidogenic, carboxyl-terminal derivatives of the amyloid protein precursor. Science. 1992 Feb 7;255(5045):726–728. doi: 10.1126/science.1738846. [DOI] [PubMed] [Google Scholar]
- Etcheberrigaray R., Ito E., Kim C. S., Alkon D. L. Soluble beta-amyloid induction of Alzheimer's phenotype for human fibroblast K+ channels. Science. 1994 Apr 8;264(5156):276–279. doi: 10.1126/science.8146663. [DOI] [PubMed] [Google Scholar]
- Etcheberrigaray R., Ito E., Oka K., Tofel-Grehl B., Gibson G. E., Alkon D. L. Potassium channel dysfunction in fibroblasts identifies patients with Alzheimer disease. Proc Natl Acad Sci U S A. 1993 Sep 1;90(17):8209–8213. doi: 10.1073/pnas.90.17.8209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gajdusek D. C., Beyreuther K., Brown P., Cork L. C., Cunningham D. D., Frangione B., Gibbs C. J., Jr, Goldfarb L. G., Goldgaber D., Hsiao K. K. Regulation and genetic control of brain amyloid. FESN Study Group. Brain Res Brain Res Rev. 1991 Jan-Apr;16(1):83–114. doi: 10.1016/0165-0173(91)90021-y. [DOI] [PubMed] [Google Scholar]
- Golde T. E., Estus S., Younkin L. H., Selkoe D. J., Younkin S. G. Processing of the amyloid protein precursor to potentially amyloidogenic derivatives. Science. 1992 Feb 7;255(5045):728–730. doi: 10.1126/science.1738847. [DOI] [PubMed] [Google Scholar]
- Govoni S., Bergamaschi S., Racchi M., Battaini F., Binetti G., Bianchetti A., Trabucchi M. Cytosol protein kinase C downregulation in fibroblasts from Alzheimer's disease patients. Neurology. 1993 Dec;43(12):2581–2586. doi: 10.1212/wnl.43.12.2581. [DOI] [PubMed] [Google Scholar]
- Haass C., Schlossmacher M. G., Hung A. Y., Vigo-Pelfrey C., Mellon A., Ostaszewski B. L., Lieberburg I., Koo E. H., Schenk D., Teplow D. B. Amyloid beta-peptide is produced by cultured cells during normal metabolism. Nature. 1992 Sep 24;359(6393):322–325. doi: 10.1038/359322a0. [DOI] [PubMed] [Google Scholar]
- Ito E., Oka K., Etcheberrigaray R., Nelson T. J., McPhie D. L., Tofel-Grehl B., Gibson G. E., Alkon D. L. Internal Ca2+ mobilization is altered in fibroblasts from patients with Alzheimer disease. Proc Natl Acad Sci U S A. 1994 Jan 18;91(2):534–538. doi: 10.1073/pnas.91.2.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosik K. S. The molecular and cellular biology of tau. Brain Pathol. 1993 Jan;3(1):39–43. doi: 10.1111/j.1750-3639.1993.tb00724.x. [DOI] [PubMed] [Google Scholar]
- Lane R. D., Federman D., Flora J. L., Beck B. L. Computer-assisted determination of protein concentrations from dye-binding and bicinchoninic acid protein assays performed in microtiter plates. J Immunol Methods. 1986 Sep 27;92(2):261–270. doi: 10.1016/0022-1759(86)90174-2. [DOI] [PubMed] [Google Scholar]
- Langstrom N. S., Anderson J. P., Lindroos H. G., Winblad B., Wallace W. C. Alzheimer's disease-associated reduction of polysomal mRNA translation. Brain Res Mol Brain Res. 1989 Jun;5(4):259–269. doi: 10.1016/0169-328x(89)90060-0. [DOI] [PubMed] [Google Scholar]
- Moshiach S., Nelson T. J., Sanchez-Andres J. V., Sakakibara M., Alkon D. L. G-protein effects on retrograde axonal transport. Brain Res. 1993 Mar 12;605(2):298–304. doi: 10.1016/0006-8993(93)91754-g. [DOI] [PubMed] [Google Scholar]
- Neary J. T., Crow T., Alkon D. L. Change in a specific phosphoprotein band following associative learning in Hermissenda. Nature. 1981 Oct 22;293(5834):658–660. doi: 10.1038/293658a0. [DOI] [PubMed] [Google Scholar]
- Nelson T. J., Alkon D. L. Prolonged RNA changes in the Hermissenda eye induced by classical conditioning. Proc Natl Acad Sci U S A. 1988 Oct;85(20):7800–7804. doi: 10.1073/pnas.85.20.7800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson T. J., Alkon D. L. Specific high molecular weight mRNAs induced by associative learning in Hermissenda. Proc Natl Acad Sci U S A. 1990 Jan;87(1):269–273. doi: 10.1073/pnas.87.1.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson T. J., Collin C., Alkon D. L. Isolation of a G protein that is modified by learning and reduces potassium currents in Hermissenda. Science. 1990 Mar 23;247(4949 Pt 1):1479–1483. doi: 10.1126/science.247.4949.1479. [DOI] [PubMed] [Google Scholar]
- Nelson T. J., Sanchez-Andres J. V., Schreurs B. G., Alkon D. L. Classical conditioning-induced changes in low-molecular-weight GTP-binding proteins in rabbit hippocampus. J Neurochem. 1991 Dec;57(6):2065–2069. doi: 10.1111/j.1471-4159.1991.tb06423.x. [DOI] [PubMed] [Google Scholar]
- Nelson T. J., Yoshioka T., Toyoshima S., Han Y. F., Alkon D. L. Characterization of a GTP-binding protein implicated in both memory storage and interorganelle vesicle transport. Proc Natl Acad Sci U S A. 1994 Sep 27;91(20):9287–9291. doi: 10.1073/pnas.91.20.9287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimoto I., Okamoto T., Matsuura Y., Takahashi S., Okamoto T., Murayama Y., Ogata E. Alzheimer amyloid protein precursor complexes with brain GTP-binding protein G(o) Nature. 1993 Mar 4;362(6415):75–79. doi: 10.1038/362075a0. [DOI] [PubMed] [Google Scholar]
- Olds J. L., Anderson M. L., McPhie D. L., Staten L. D., Alkon D. L. Imaging of memory-specific changes in the distribution of protein kinase C in the hippocampus. Science. 1989 Aug 25;245(4920):866–869. doi: 10.1126/science.2772638. [DOI] [PubMed] [Google Scholar]
- Selkoe D. J. Physiological production of the beta-amyloid protein and the mechanism of Alzheimer's disease. Trends Neurosci. 1993 Oct;16(10):403–409. doi: 10.1016/0166-2236(93)90008-a. [DOI] [PubMed] [Google Scholar]
- Selkoe D. J. The molecular pathology of Alzheimer's disease. Neuron. 1991 Apr;6(4):487–498. doi: 10.1016/0896-6273(91)90052-2. [DOI] [PubMed] [Google Scholar]
- Wolozin B., Sunderland T., Zheng B. B., Resau J., Dufy B., Barker J., Swerdlow R., Coon H. Continuous culture of neuronal cells from adult human olfactory epithelium. J Mol Neurosci. 1992;3(3):137–146. doi: 10.1007/BF02919405. [DOI] [PubMed] [Google Scholar]