Abstract
Global climate change is expected to shift regional rainfall patterns, influencing species distributions where they depend on water availability. Comparative studies have demonstrated that C4 grasses inhabit drier habitats than C3 relatives, but that both C3 and C4 photosynthesis are susceptible to drought. However, C4 plants may show advantages in hydraulic performance in dry environments. We investigated the effects of seasonal variation in water availability on leaf physiology, using a common garden experiment in the Eastern Cape of South Africa to compare 12 locally occurring grass species from C4 and C3 sister lineages. Photosynthesis was always higher in the C4 than C3 grasses across every month, but the difference was not statistically significant during the wettest months. Surprisingly, stomatal conductance was typically lower in the C3 than C4 grasses, with the peak monthly average for C3 species being similar to that of C4 leaves. In water-limited, rain-fed plots, the photosynthesis of C4 leaves was between 2.0 and 7.4 μmol m−2 s−1 higher, stomatal conductance almost double, and transpiration 60% higher than for C3 plants. Although C4 average instantaneous water-use efficiencies were higher (2.4–8.1 mmol mol−1) than C3 averages (0.7–6.8 mmol mol−1), differences were not as great as we expected and were statistically significant only as drought became established. Photosynthesis declined earlier during drought among C3 than C4 species, coincident with decreases in stomatal conductance and transpiration. Eventual decreases in photosynthesis among C4 plants were linked with declining midday leaf water potentials. However, during the same phase of drought, C3 species showed significant decreases in hydrodynamic gradients that suggested hydraulic failure. Thus, our results indicate that stomatal and hydraulic behaviour during drought enhances the differences in photosynthesis between C4 and C3 species. We suggest that these drought responses are important for understanding the advantages of C4 photosynthesis under field conditions.
Keywords: C3 photosynthesis, C4 photosynthesis, drought, gas exchange, PACMAD, Poaceae, stomatal conductance, water potential
Introduction
C4 photosynthesis is a fascinating example of a complex phenotype that has evolved repeatedly (Sage et al., 2011), and influences a suite of ecophysiological traits that determine plant performance in natural settings (Long, 1999). Today, C4 grasses are vital as agricultural crops (e.g., maize and sugarcane) and dominate the ground cover over large areas of Africa, Australia, South Asia and the Americas (Edwards et al., 2010). The role of climate in determining the relative performance of C4 and C3 species from both monocot and eudicot lineages is, therefore, a key question in studies of global change (Epstein et al., 1997; Murphy & Bowman, 2006; Arnone et al., 2011; Morgan et al., 2011).
The principal physiological innovation common to C4 lineages is the development of a biochemical CO2 pump that operates as an extension of the dark reactions of photosynthesis (Hatch & Osmond, 1976). The C4 pump elevates CO2 concentrations in photosynthesising chloroplasts, virtually eliminating O2 competition for the active site of Rubisco and therefore photorespiration, while in C3 plants, photorespiration limits net CO2 assimilation (A) at higher temperatures and low partial pressures of CO2 (Osmond et al., 1982). The efficient delivery of CO2 to Rubisco in C4 plants improves photosynthetic efficiency at high temperatures, but bears an energetic cost that limits the maximum efficiency of photosynthesis in C4 species at low temperatures (Ehleringer & Pearcy, 1983). The initial CO2 assimilation step in C4 plants, which is catalyzed by PEP-carboxylase in combination with carbonic anhydrase, also has a higher affinity for its substrate than that of C3 plants. This generates the CO2 concentrating effect of the C4 pump and, in combination with the increased assimilation rates driven by the pump, means that C4 leaves are able to maintain higher A at lower internal CO2 concentrations (Collatz et al., 1992). The rates of supply of CO2 to the intercellular spaces of leaves and the loss of water through transpiration (E) are intrinsically linked, and water use can be limited by reducing stomatal conductance (gs; Raschke, 1975). C4 photosynthesis therefore has important consequences for leaf water-use efficiency, i.e. net CO2 assimilation per unit of water loss, a key observation noted in the earliest studies of C4 ecophysiology (Black et al., 1969; Bjorkman, 1971).
Despite the important consequences of C4 physiology for water use, until recently the primary ecophysiological explanation for C4 grass species distributions was considered to be growing season temperature (Teeri & Stowe, 1976). For C4 eudicots, however, adaptation to arid environments has long been accepted as important in shaping species distributions (Ehleringer & Monson, 1993; Ehleringer et al., 1997). For the grass family (Poaceae), within which the majority of C4 species and 30% or more of C4 evolutionary origins occur (Sage, 2009; Sage et al., 2011), habitat water availability and plant hydraulics are receiving renewed attention for their role determining the evolutionary success and distribution of C4 species (Edwards & Still, 2008; Osborne & Freckleton, 2009; Edwards & Smith, 2010; Osborne & Sack, 2012; Pau et al., 2012; Taylor et al., 2012; Griffiths et al., 2013). Recent use of molecular phylogenies has provided new insights into how evolutionary processes have shaped species distributions with respect to climate. Occupation of cooler habitats by C3 species is now known to be associated with a preference for cooler climates in two (Edwards & Smith, 2010; Visser et al., 2014) of the nine monophyletic subfamilies of Poaceae (Grass Phylogeny Working Group II, 2012): Pooideae (Vigeland et al., 2013) and Danthonioideae (Humphreys & Linder, 2013), species of which are all C3 (Edwards & Smith, 2010). In contrast, comparisons between C3 and C4 grasses within the PACMAD clade are most appropriate to studies of the adaptive advantages of C4 photosynthesis among grasses (Edwards et al., 2007; Edwards & Still, 2008). The PACMAD clade includes the monophyletic subfamilies Panicoideae, Arundinoideae, Chloridoideae, Micrairoideae, Aristidoideae and Danthonioideae, excludes the Pooideae, and encompasses the evolutionary origins of all contemporary C4 grass species (Christin et al., 2009; Grass Phylogeny Working Group II, 2012). Within the PACMAD clade, the evolution of C4 photosynthesis has resulted in preferences for drier habitats by C4 lineages (Edwards & Still, 2008; Osborne & Freckleton, 2009; Edwards & Smith, 2010; Pau et al., 2012), and divergence in water-use traits between C3 and C4 grasses (Taylor et al., 2012; Griffiths et al., 2013).
Paradoxically, as evidence has mounted to support the importance of drier habitats to the evolutionary success of C4 photosynthesis in grasses, it has become clear that photosynthesis in these species may be more susceptible to failure under declining leaf water status (reviewed by Ghannoum, 2009; Driever & Kromdijk, 2013). Following restriction of watering in pot-based experiments, gS of C3 grasses declines to a greater degree and C3 water-use efficiency can increase to match that of C4 plants (Ripley et al., 2010; Taylor et al., 2011). However, there is evidence that C3 grasses commonly operate at more negative leaf water potentials (Ψ) than C4 species (Ripley et al., 2010; Taylor et al., 2010, 2011). As a consequence of these observations, it has been proposed that differences in plant hydraulics may have played an important role in allowing C4 grasses to colonize and adapt to dry and open habitats (Osborne & Sack, 2012): decreased responsiveness of Ψ, gs and E to water availability may result in photosynthesis among C4 grasses showing greater resistance to the effects of drought.
To date, observations of susceptibility to drought among C4 species have been made primarily in pot-based studies, which have several potential limitations (Poorter et al., 2012). There is, therefore, only limited evidence that can be used to compare the impacts of drought on the leaf physiology of closely related C3 and C4 species under natural growing conditions (Ripley et al., 2007; Frole, 2008; Ibrahim et al., 2008). Crucially, all of these studies have focused on comparisons within the Panicoideae subfamily, and there is no evidence addressing contrasts across other key PACMAD lineages. We therefore established an outdoor common garden experiment using twelve C3 and C4 grass species, sampled from four closely related PACMAD lineages. All of the species used in the experiment are found within 60 km of the study site, in a region of the Eastern Cape of South Africa where climate, according to the Koppen–Geiger classification, is warm temperate, fully humid, with warm summers (Peel et al., 2007). Our goal was to compare physiological responses of C3 and C4 grasses to an experimental manipulation of water availability, testing whether responses of leaf gas exchange and water potential previously observed under more controlled conditions are important under natural climatic conditions.
Based on our previous experiments (Ripley et al., 2007, 2010; Taylor et al., 2010, 2011), we hypothesized that C4 grasses would show higher A, lower gs, and higher water-use efficiency when well watered. During periods of progressive drought, we expected that gs in C3 grasses would decrease to a greater extent and that differences in leaf water-use efficiency might also diminish between C3 and C4 grasses (Frole, 2008; Ripley et al., 2010; Taylor et al., 2011). We further hypothesized that limitation of photosynthesis observed during drought in C3 species would be principally driven by decreased gs, but in C4 species would instead be associated with decreased midday leaf water potential (Ψmidday; Ghannoum et al., 2003; Ripley et al., 2007). We also predicted that C4 grasses would show less negative Ψmidday and maintain smaller hydrodynamic gradients from soil to leaf (ΔΨ = Ψpredawn – Ψmidday) when well watered, differences that we expected to be reduced under drought (Taylor et al., 2010, 2011). Finally, we aimed to test whether differences in leaf Ψ were associated with greater plant hydraulic conductance in C4 grasses (Kplant = E/−ΔΨ; Osborne & Sack, 2012).
Materials and methods
Experimental design and plant species
Twelve grass species of open habitats were drawn from four lineages found in the regional species pool of the Eastern Cape of South Africa (Gibbs Russell et al., 1990), based on a random sample of three species per lineage (Table1). The two C4 groups were the genus Aristida and the tribe Andropogoneae, which share a biochemical subtype (NADP-me) but have independent origins of their C4 syndrome (Christin et al., 2009; Grass Phylogeny Working Group II, 2012). The C3 subfamily Danthonioideae and C3 species from the tribe Paniceae were used in comparison; both are important components of grassland ecosystems in southern Africa.
Table 1.
Details of species used, collection locations and commonly inhabited biome types
| Clade (photosynthetic type) | Species | Collection location(S:E; deg,min,sec) | Altitude(m) | Biome description (Gibbs Russell et al., 1990) | Number of plants surviving, by treatment, in May 2009 | |
|---|---|---|---|---|---|---|
| Watered | Rain-fed | |||||
| Panicoideae, Paniceae (C3) | Alloteropsis semialata ssp. eckloniana | 33,19,44.54: 26,28,44.21 | 726 | Savanna, Grassland | 7 | 8 |
| Panicum aequinerve | 33,19,38.09: 26,31,14.44 | 660 | Grassland, Forest | 8 | 8 | |
| Panicum ecklonii | 33,19,46.38: 26,28,35.82 | 714 | Grassland | 2 | 4 | |
| Panicoideae, Andropogoneae (C4) | Heteropogon contortus | 33,19,11.87: 26,30,29.61 | 631 | Savanna, Grassland, Fynbos, Nama-Karoo | 8 | 7 |
| Hyparrhenia hirta | 39,19,06.94: 26,30,37.57 | 618 | Savanna, Grassland, Fynbos, Nama-Karoo | 8 | 8 | |
| Themeda triandra | 33,17,05.23: 26,29,21.15 | 639 | Savanna, Grassland, Fynbos, Nama-Karoo | 7 | 8 | |
| Danthonioideae (C3) | Karoochloa curva | 33,14,54.27: 26,21,26.78 | 492 | Grassland, Fynbos, Nama-Karoo | 5 | 7 |
| Merxmuellera disticha | 33,14,54.27: 26,21,26.77 | 492 | Grassland, Fynbos, Nama-Karoo, Afro-Montane | 8 | 8 | |
| Pentaschistis curvifolia | 33,19,46.38: 26,28,35.83 | 714 | Fynbos | 6 | 6 | |
| Aristidoideae (C4) | Aristida congesta ssp. barbicollis | 33,13,09.22: 26,37,40.04 | 487 | Savanna, Grassland | 7 | 7 |
| Aristida diffusa ssp. burkei | 33,14,54.27: 26,21,26.76 | 492 | Savanna, Grassland, Nama-Karoo | 8 | 8 | |
| Aristida junciformis ssp. junciformis | 33,33,44.46: 26,53,39.36 | 80 | Savanna, Grassland, Fynbos | 8 | 8 | |
Plants were collected from field locations (Table1) between January 2007 and January 2008 and established in the outdoor common garden. The common garden had a blocked design, in which individual plots were separated by 2 m of short lawn, and paired 2 × 2 m plots within each of eight blocks were either watered or allowed to receive natural rainfall. Plants were regularly spaced and species locations were randomized within each plot but matched between watered and natural-rainfall plots in each block. All plots were watered on a regular basis until October 2008. After this time, only the plots in the watered treatment received additional water; approximately 28 l (equivalent to approximately 7 mm rainfall) was added to each plot every 2–3 days during the growing season. Following rainfall greater than 10 mm in 48 h, watering was halted for 2 weeks. Plots were weeded and the surrounding lawn mown on a regular basis.
Weather
Air temperature, humidity, wind-speed and direction, precipitation and photosynthetic photon flux density (PPFD) were recorded using a weather station. This comprised a datalogger (DL2e Delta T, Cambridge, UK); two relative humidity and temperature sensors (RHT2 nl, Delta T) positioned at 0.5 and 2 m; an anemometer (AN4, Delta T) positioned at 2 m; a rain gauge (RG2, Delta T) and a quantum sensor (QS2, Delta T).
Estimation of reference crop evapotranspiration
To assess the effects of our watering treatment, R Language and Environment version 3.0.1 (R Core Team, 2013) was used to calculate reference crop evapotranspiration (ET0, mm day−1), which was compared with rainfall and watering inputs. Daily mean values (mean of maximum and minimum) from weather station measurements were used in combination with the Penman–Monteith equation, following Allen et al., (1998; Data S1). The method assumes an extensive surface of growing, green grass, completely shading the ground and not short of water. Water shortage was observed at our site and bare soil was maintained between plants, thus the calculated ET0 is an approximate guide of true evapotranspiration.
Leaf water potential
To assess plant water deficits, Ψmidday and Ψpredawn were measured and ΔΨ was estimated as the difference between them, assuming Ψpredawn was equilibrated with Ψsoil. Measurements of Ψmidday were paired with measurements of gas exchange (below). For measurement, leaves were enclosed in polythene and immediately excised using a razor blade. The balancing pressure was determined using a Scholander-type pressure bomb. Ψpredawn of leaves selected using similar criteria to those used for Ψmidday was determined before sunrise within 48 h. If rainfall occurred between the collection of midday and predawn measurements, Ψpredawn measurements were either discarded or repeated the following day to better represent prevailing daytime conditions.
Leaf gas exchange
Gas exchange measurements were made during the final 2 weeks of each month during the growing season. Measurements were made under all but wet and extremely overcast conditions to obtain representative snapshots of seasonal gas exchange. During each day on which leaf gas exchange was measured, measurements were taken for one block between 09:30 h and 15:00 h. The first treatment to be measured was rotated each day, and the order of sampling between species was determined by their randomized positions within each plot.
A portable open gas exchange system (LI-6400; LI-COR, Inc., Lincoln, NE, USA) was used for gas exchange measurements, equipped with a CO2 mixer (LI-6400-01) and 30 mm × 20 mm chamber/red-blue LED light source (LI-6400-02B). The CO2 mole fraction of air entering the chamber was maintained at 400 μmol mol−1. Light levels were matched to a PPFD sensor (LI-190); attached via a 1.5 m extension lead and mounted prior to measurements in each plot, in an unshaded, north-facing position, at 45 ° from vertical and roughly 30 cm above the soil surface. Air temperature in the chamber was not controlled, but the equipment was shaded to prevent excessive heating and to allow the chamber temperature to track that of the air. Leaf temperature was estimated using an energy balance calculation. Incoming air was not scrubbed of water vapour.
As the leaves of most species were narrow (1 to 3 mm wide), multiple leaves were usually inserted into the chamber, with a minimum of 100 mm2 total projected leaf area used for all measurements. Leaves selected for gas exchange were the youngest fully emerged leaves on their tillers, with flowering tillers being avoided wherever possible and sections of canopy where leaf blades were exposed to full sun being preferred. Leaf area was calculated based on the known dimensions of the chamber and the combined widths of the inserted leaves at either edge of the chamber, measured using a ruler. Low fluxes were encountered regularly, especially during dry periods, forcing the use of flow rates down to 100 μmol s−1 to obtain resolvable differences in CO2 (ΔCO2 > 10 μmol mol−1) and H2O (ΔH2O > 1 mmol mol−1) between the reference air-stream and the chamber. The chamber was tested for leaks by exhaling around the seals immediately after inserting leaves. Measurements were taken as soon as the predicted intercellular CO2 concentration (ci) stabilized. If ci failed to stabilize within 3 min, if ΔCO2 < 10 μmol mol−1, or if leaves being measured were thick/rolled, the chamber was re-tested for leaks and, if necessary, the seal on the chamber was re-adjusted before re-commencing measurements. In all cases where ΔCO2 was < 10 μmol mol−1, reference and chamber gas analyzers were matched prior to measurement.
For the first set of measurements in November 2008, rolled leaves were routinely unrolled to take measurements. Paired measurements, taken with leaves first rolled and then unrolled, indicated that by unrolling leaves, values for ci were elevated to an unusual degree due to increases in estimated gs (data not shown). Thus, from December 2008 onwards, tightly rolled leaves were not unrolled during gas exchange measurements.
Estimation of leaf transpiration
To assess water use at the leaf level, a model implemented in R Language and Environment version 3.0.1 (R Core Team, 2013) was used to estimate E for individual leaves from each species in the study. The Penman–Monteith equation (Penman, 1948; Monteith, 1965) was combined with an iterative approach to modelling of leaf energy balance for a horizontal leaf suspended over a lawn (Jones, 1992; Data S2). The model was parameterized using leaf widths based on published values for each species (Data S2), mean values for climate variables (Data S3) and gs (Data S4) from each measurement period during the growing season.
Statistical analysis
Statistical analyses were carried out using the R Language and Environment, version 3.0.1 (R Core Team, 2013). To determine the effects of the watering treatments, a Wilcoxon signed rank test was used to test for differences in weekly ET0 − (watering+rainfall) values.
Linear mixed effect models of seasonal changes in physiological traits were fitted using maximum likelihood, and tested for significance using tools in the lme4 package (Bates et al., 2013). The data used in models were species mean values calculated for each month × treatment combination. Prior to analysis, mean values based on ≤ 2 replicates were eliminated from the dataset and, to improve balance in the dataset, species means that were unpaired across treatments in any given month were also removed. The full datasets used for analysis are plotted in Data S5. Average values for C3 and C4 groups in both treatments during each monthly sampling interval were predicted as fixed effects. Clade was modelled as a random effect dependent on the month of sampling. Model validation was carried out by inspection of residuals and, except for the model of instantaneous water-use efficiency (A/E), log-transformation was used to improve homoscedasticity of data. Bootstrapped 95% confidence intervals for fixed effect predictions were generated using 1000 simulations of each model.
Results
Weather and effect of watering
Maximum temperatures were observed in January (mean of daily maxima during January, 28 °C), whereas rainfall and relative humidity were greatest in February (total rainfall, 139 mm; relative humidity, mean of daily minima February 74%; Fig.1a). Relative humidity was lowest during early November (mean of daily minima November 1st–15th 53%) and late March–early April (mean of daily minima March 15th–April 15th 47%; Fig.1b).
Figure 1.
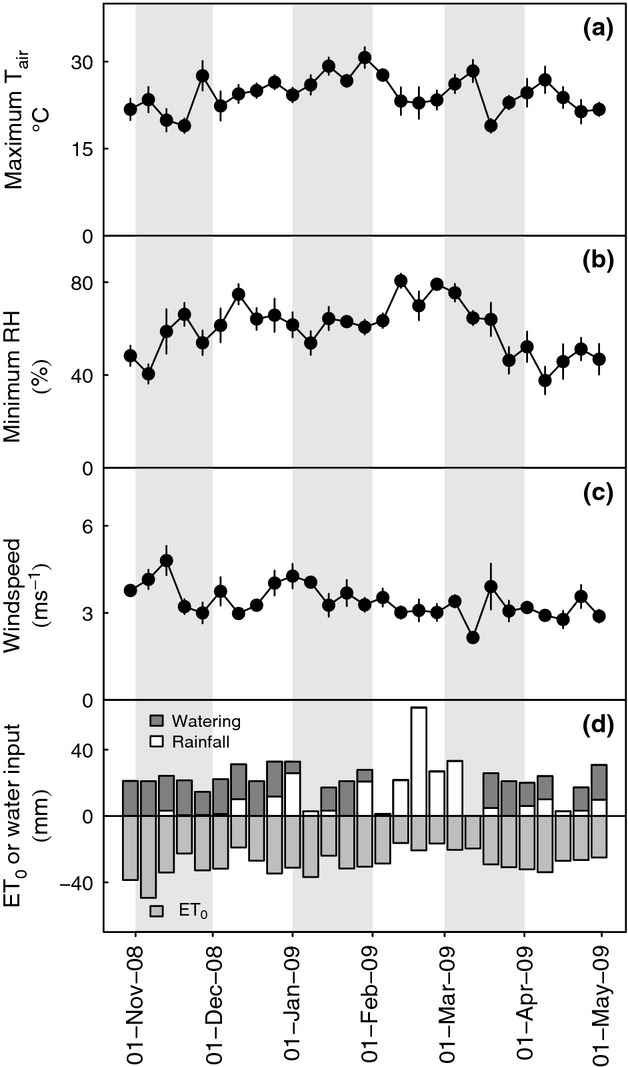
Climate conditions relevant for midday photosynthesis in the common garden experiment carried out in Grahamstown, Eastern Cape of South Africa, during November 2008–April 2009. Weekly values (mean ± SEM) for: (a) daily maximum temperature; (b) daily minimum relative humidity; (c) daily mean windspeed. Weekly totals (d) for water added to the supplementary water treatment, rainfall, and reference crop evapotranspiration calculated using micrometerological data (ET0, shown as negative values). Months in the experiment are highlighted by grey-filled areas.
Supplementary watering significantly reduced the cumulative water deficit, indicated by rainfall deficit, ET0−(watering + rainfall), on a week-by-week basis (Wilcoxon signed rank test, P < 0.001; windspeeds used to calculate ET0 are shown in Fig.1c). The total accumulated water deficit in the rain-fed plots was estimated to have been more than 3 times that in the plots receiving supplementary water (differences in water input are shown in Fig.1d). Rainfall peaked during the week ending February 19th (Fig.1d). In the 16 weeks prior to the peak of rainfall, total deficits in the watered treatments were 155 mm, compared with 386 mm in the rain-fed treatments. Peak rainfall in February was followed by a further period in which rainfall was low: rainfall deficits were 120 mm in the rain-fed plots and 15 mm in the watered plots over the final 10 weeks of the experiment. We note, however, that as an approximation of soil water balance, rainfall deficit calculated in this manner does not account for soil hydrology and depends on the method used to estimate ET0.
Plant survival
A number of plants died during the 2008–2009 growing season (Table1). The small sample size meant that there was no clear evidence that mortality for any species differed between the watered and rain-fed plots (Table1). Compared with nine deaths in the rain-fed plots, 14 plants died in the watered plots, but six of the dead plants in watered plots were of a single species, P. ecklonii. This was one of three species for which more than two of the 16 planted individuals died; P. ecklonii (ten dead), K. curva (four dead), and P. curvifolia (four dead), are all C3 plants. Overall, therefore, 19 C3 plants died, compared with four C4 plants.
Leaf water potentials
Leaf water potentials in rain-fed plots were significantly more negative than in watered plots. Watering caused significant increases in average Ψpredawn, of 0.26–1.07 MPa, for both C3 and C4 photosynthetic types during January and April (Fig.2a,b). In December and March, watering led to significant improvements in average Ψpredawn for C3 grasses (increase by 0.18–0.29 MPa), but smaller improvements for C4 grasses (0–0.05 MPa) were not statistically significant (Fig.2a,b).
Figure 2.
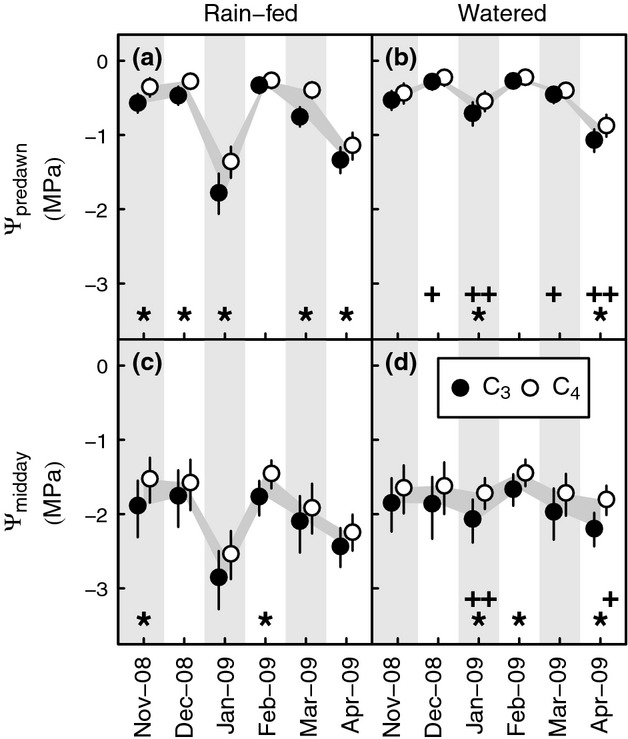
Seasonal contrasts in leaf water potential between C3 and C4 PACMAD grasses in a common garden experiment at Grahamstown, Eastern Cape of South Africa, between November 2008–April 2009. (a,b) Predawn water potential, Ψpredawn; (c,d) midday water potential, Ψmidday. Points represent pooled means and 95% confidence intervals for 4–6 species in plots that were rain-fed (a,c) or given supplemental water (b,d). Values are back-transformed from the log-transformed scale used for statistical analysis. Differences between photosynthetic types and months in the experiment are highlighted by grey-filled areas. Photosynthetic type comparisons for which confidence intervals indicate significance at the P < 0.05 level are highlighted by * within each pane. Significant differences within photosynthetic types that resulted from watering are indicated by + below the relevant means in (b) and (d).
Mean Ψ values were always more negative for the C3 than C4 groups (Fig.2). Except during the wettest month, February, differences in Ψpredawn between the photosynthetic types in rain-fed plots (0.19–0.42 MPa) were statistically significant (Fig.2a). These differences were eliminated in the watered plots, except when drought was most acute during January (0.16 MPa difference) and April (0.19 MPa difference, Fig.2b).
Significant differences in Ψmidday (Fig.2c,d) did not entirely mirror the pattern of drought response shown by Ψpredawn. Significant positive effects of watering on Ψmidday were observed for C4 grasses in January (0.82 MPa) and April (0.43 MPa; Fig.2d). However, watering significantly increased Ψmidday among C3 plants only in January (0.79 MPa), not in April (0.24 MPa; Fig.2d); a contrast with Ψpredawn which was affected consistently across the photosynthetic types at the two timepoints. During the wetter month of February, differences between the average Ψmidday of C3 and C4 grass leaves were 0.22–0.31 MPa and were significant in both rain-fed and watered plots (Fig.2c,d): these differences were particularly notable, given the lack of differences in Ψpredawn in February (Fig.2a,b). In addition, Ψmidday differed significantly between the photosynthetic types in the rain-fed plots during November. In the watered plots during January and April (Fig.2c,d), significant differences in Ψmidday between the photosynthetic types were coincident with significant effects of watering on Ψmidday of one or both types (Fig.2c,d).
Gas exchange
In each month, average A was always higher among C4 grasses (range of means 5.1–14.7 μmol m−2 s−1) than among C3 grasses (range of means 0.6–11.5 μmol m−2 s−1). In the rain-fed treatment, differences between the photosynthetic types during the drought periods, December–January and March–April, ranged between 3.1 and 8.2 μmol m−2 s−1, and confidence limits indicated that they were statistically significant (Fig.3a). In the wettest month, February, differences between the photosynthetic types were smaller in the rain-fed plots and were not significant (1.7 μmol m−2 s−1). This was also true at the start of the growing season in November (2.4 μmol m−2 s−1; Fig.3a). During this first month of the experiment water deficits in the rain-fed plots may still have been establishing, as watering ceased during October.
Figure 3.
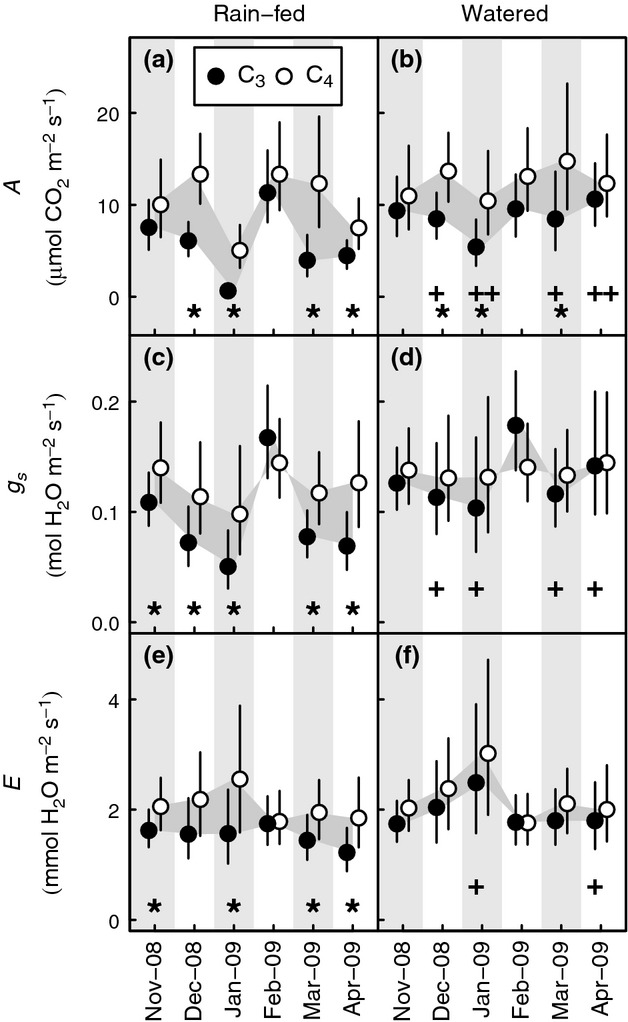
Seasonal contrasts in leaf gas exchange between C3 and C4 PACMAD grasses in a common garden experiment at Grahamstown, Eastern Cape of South Africa, between November 2008 and April 2009. (a,b) Net CO2 assimilation, A; (c,d) stomatal conductance to H2O, gs; (e,f) transpiration, E. Experimental plots were rain-fed (a,c,e) or given supplemental water (b,d,f). Points represent pooled means and lines 95% confidence intervals for 4–6 species, values are back-transformed from the log-transformed scale used for statistical analysis. Differences between photosynthetic types and months in the experiment are highlighted by the grey-filled areas. Photosynthetic type comparisons for which confidence intervals indicate significance at the P < 0.05 level are highlighted by * within each pane. Significant differences within photosynthetic types that resulted from watering are indicated by + below the relevant means in (b), (d) and (f).
In the watered plots, there were also significant differences in A between the two photosynthetic types during December, January and March (Fig.3b), which ranged from 4.9 to 6.2 μmol m−2 s−1. Both photosynthetic types showed significant increases in A (4.8–6.0 μmol m−2 s−1) in response to watering during the most severe drought periods (January and April, Fig.3b), but only the C3 group increased A significantly in response to watering under less severe drought during December (increase of 2.3 μmol m−2 s−1) and March (increase of 4.5 μmol m−2 s−1; Fig.3b). Watering had no significant effect on A in the wettest month, February, or in November, at the start of the growing season (Fig.3b).
Unexpectedly, in the natural-rainfall treatment, average gs among C3 species was often significantly lower, by 0.031–0.056 mol m−2 s−1, than among C4 species (Fig.3c); the exception was the wettest month, February, during which average gs among C3 species was slightly, but not significantly greater than among C4 species (0.022 mol m−2 s−1, Fig.3c). Differences in average gs between the photosynthetic types in the watered treatment were never significant, but showed a similar pattern to those in the rain-fed plots (Fig.3d); the average gs of C4 species was greater by 0.001–0.028 mol m−2 s−1, except during February when the C3 value was 0.038 mol m−2 s−1 greater than for C4 species. Similar values for gs between the photosynthetic types in the watered plots were a result of significant increases in mean gs among C3 species in December, January, March and April, relative to rain-fed plots, of 0.039–0.075 mol m−2 s−1 (Fig.3d); watering did not significantly influence the mean gs among C4 species (Fig.3d). These results contrasted with our expectation that well-watered C3 plants would show significantly higher gs than their C4 relatives.
As expected, patterns in modelled E were broadly consistent with the patterns seen for gs (Fig.3e,f). Average values of E were 0.04–0.64 mmol m−2 s−1 higher for C4 species when compared with C3 species in rain-fed plots, and significantly so in November, January, March and April (Fig.3e). Watering eliminated these differences in February, and the maximum difference between photosynthetic types in the watered plots was 0.54 mmol m−2 s−1 (Fig.3f). The smaller difference in E between C3 and C4 species in the watered plots resulted from watering-induced increases of 2–59% in E among C3 species. Differences in average E in the rain-fed plots were also eliminated during the wettest month, February (Fig.3e). However, in contrast with patterns in gs, where watering had a significant influence on values in four of six months, watering had a significant effect on E among C3 species only in the driest months, January and April (Fig.3d,f).
To summarize, although watering always eliminated the differences in gs and E between photosynthetic types under rain-fed conditions (Fig.3d,f), the differences in A between the photosynthetic types persisted in both watered and rain-fed treatments during drought (Fig.3b). Thus, C4 grasses held a photosynthetic advantage over their C3 relatives when operating at similar E and gs. Furthermore, while decreased photosynthesis among C3 species was associated with significant declines in gs and Ψpredawn due to drought, the same was not true for their C4 counterparts. Among C4 species, gs and E were not significantly affected by water supply, and decreases in A coincided instead with significant decreases in Ψpredawn.
Water-use efficiency
Although leaf-level water-use efficiency tended to be higher, on average, among C4 grasses, differences between the photosynthetic types during each monthly sampling interval were rarely significant (Fig.4). At the beginning (November) and end (April) of the growing season, differences in intrinsic water-use efficiency (A/gs) between the photosynthetic types in rain-fed plots were small (6–7 mmol mol−1) and were not significant (Fig.4a). Throughout the remainder of the growing season, average A/gs of C4 leaves in rain-fed plots was 64–125 mmol mol−1, greater than the values for C3 leaves (26–91 mmol mol−1) and significantly so during periods of intermediate drought stress in December and March (Fig.4a). This A/gs advantage to C4 grasses in rain-fed plots was therefore at its maximum when A, gs and Ψpredawn were water limited (i.e. significantly affected by the watering treatment) among C3 but not C4 grasses (Fig.3). When drought was most severe during January, however, although the difference in A/gs between the photosynthetic types in the rain-fed plots was large (38 mmol mol−1) it was not significant. In contrast, during the wettest month, February, differences of a similar size to that seen in January were significant in both watered (33 mmol mol−1) and rain-fed (37 mmol mol−1) plots (Fig.4a). To summarize, advantages to the C4 grasses in A/gs were largest in well-watered soil and during mild drought, but were lost under severe drought and were also small at the beginning and end of the growing season (Fig.4a,b).
Figure 4.
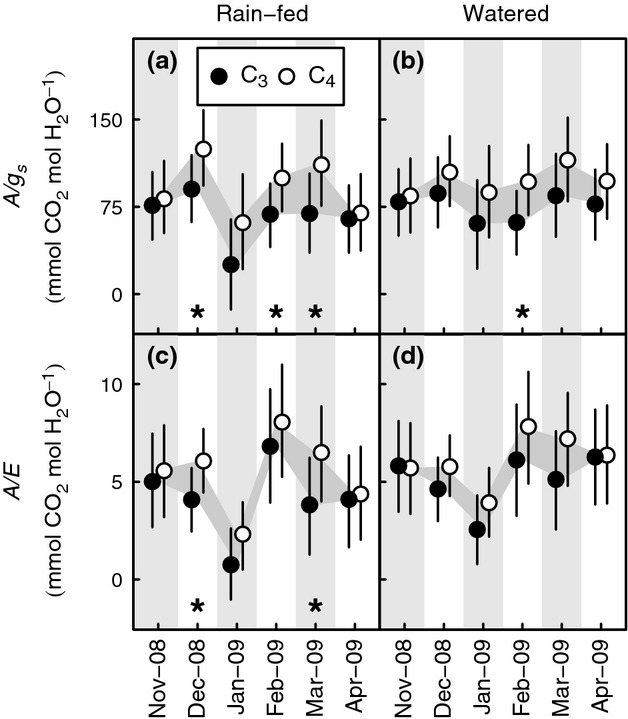
Seasonal contrasts in leaf-level water-use efficiency between C3 and C4 PACMAD grasses in a common garden experiment at Grahamstown, Eastern Cape of South Africa, between November 2008 and April 2009. (a,b) Intrinsic water-use efficiency, A/gs; (c and d) instantaneous water-use efficiency, A/E. Points represent pooled means and 95% confidence intervals for 4–6 species in plots that were rain-fed (a,c) or given supplemental water (b,d). Values are back-transformed from the log-transformed scale used for statistical analysis (a,b), analysis was carried out on untransformed data (c,d). Differences between photosynthetic types and months in the experiment are highlighted by the grey-filled areas. Photosynthetic type comparisons for which confidence intervals indicate significance at the P < 0.05 level are highlighted by * within each pane. There were no significant differences due to watering.
Variation over the growing season and across treatments meant that average instantaneous water-use efficiency (A/E) ranged from 0.72 to 6.89 mmol mol−1 for C3 and 2.29 to 7.99 mmol mol−1 for C4 plants, and was 1.10–2.61 mmol mol−1 greater among C4 grasses from December to March. Differences in A/E were similar to A/gs in that they usually favoured C4 grasses and that in the rain-fed treatment they were smallest in November and April (0.14–0.5 mmol mol−1). Indeed, C3 grasses showed very similar A/E to their C4 relatives in the watered plots (−0.1–0.06 mmol mol−1 difference; Fig.4c,d) in November and April. However, statistically significant advantages to C4 grasses were observed for A/E in the rain-fed plots in December and March (Fig.4c), consistent with significant differences in A/gs. We were surprised to find that, in contrast with A/gs, having accounted for the effects of leaf energy budget by calculating A/E, differences in leaf instantaneous water-use efficiency between the photosynthetic types were not significant in either treatment during February (Fig.4c,d), the wettest month in the study.
Plant hydraulics
The size of ΔΨ reflects the hydraulic balance between water loss from the leaves and supply from the roots and soil, with more negative values for individual plants indicating greater strain. Average values by photosynthetic type ranged between −0.73 and −1.59 MPa. In watered plots, although differences in ΔΨ between C3 and C4 species were never significant (Fig.5b), average values for C4 species were consistently smaller (−0.91 to −1.38 MPa) than those for C3 species (−1.10 to −1.59 MPa; Fig.5a,b). In rain-fed plots, average values for C3 species were similar to or smaller than those for C4 plants during drought in December (C3 −1.27 MPa, C4 −1.29 MPa), March (C3 −1.30 MPa, C4 −1.51 MPa) and April (C3 −1.04 MPa, C4 −1.08 MPa), differences that were not statistically significant (Fig.5a). The only significant differences in ΔΨ were observed under the most severe drought in January, when the C3 ΔΨ (−0.73 MPa) was significantly smaller than both C3 grasses in the watered plots (−1.39 MPa) and C4 grasses in the rain-fed plots (−1.14 MPa; Fig.5a). This significantly smaller value of average ΔΨ among C3 grasses was observed during a period of acute leaf water deficit (Fig.2). Therefore, the nonsignificant changes in a similar direction, which were observed in December, March and April, might also be interpreted as indicative of reduced or more variable hydraulic performance among C3 species.
Figure 5.
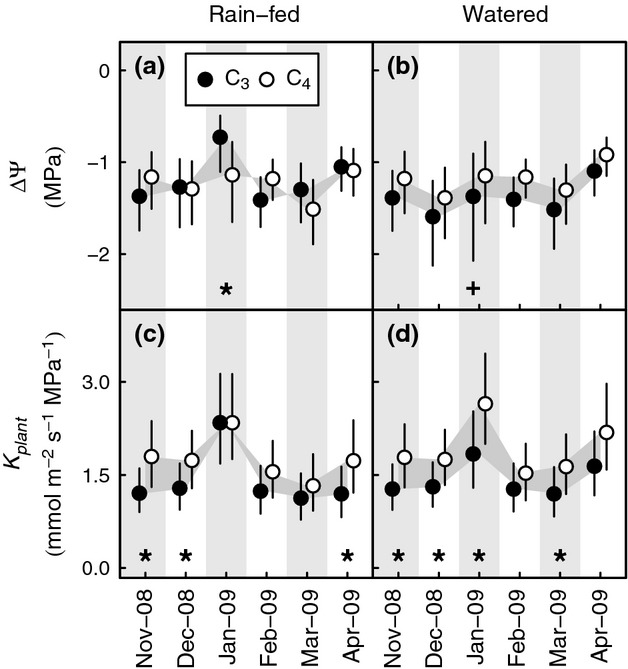
Seasonal contrasts in plant hydraulics between C3 and C4 PACMAD grasses in a common garden experiment at Grahamstown, Eastern Cape of South Africa, between November 2008 and April 2009. (a,b) Hydrodynamic gradient, ΔΨ = Ψmidday − Ψpredawn; (c,d) hydraulic conductance, Kplant = E/−ΔΨ. Points represent pooled means and 95% confidence intervals for 4–6 species exposed to natural rainfall (a,c) or given supplemental water (b,d). Values are back-transformed from the log-transformed scale used for statistical analysis. Differences between photosynthetic types and months in the experiment are highlighted by the grey-filled areas. Photosynthetic type comparisons for which confidence intervals indicate significance at the P < 0.05 level are highlighted by * within each pane. The significant difference within the C3 photosynthetic type that resulted from watering is indicated by + below the relevant mean in (b); there were no significant differences due to watering in (d).
A measure of whole-plant leaf-specific hydraulic conductance (Kplant, mmol m−2 s−1 MPa−1) is provided by the flux of water due to E (mmol m−2 s−1) normalized by ΔΨ (MPa). Kplant was almost always greater among C4 species (1.32–2.69 mmol m−2 s−1 MPa−1) than C3 species (1.12–2.35 mmol m−2 s−1 MPa−1; Fig.5c,d), a difference of 0.2–0.88 mmol m−2 s−1 MPa−1. The exception to the general rule that Kplant was greater among C4 species was in the rain-fed plots during January (Fig.5c), when the difference was almost zero (Fig.5a), coincident with the significant decline in average ΔΨ for C3 species. Differences in Kplant between C3 and C4 species were significant in November, December and April in the rain-fed plots (Fig.5c) and in November, December, January and March in the watered plots (Fig.5d), but watering had no significant effects on Kplant (Fig.5d).
Discussion
Our results demonstrate that plant water relations play a key role in maintaining the physiological advantages of C4 over C3 PACMAD grasses under field conditions. We found that A, E and gs among C3 grasses declined significantly in response to drought, in concert with reductions in ΔΨ. In contrast, C4 grasses maintained ΔΨ, E and gs throughout the growing season, and A was limited by water supply only under the most extreme drought conditions when both Ψpredawn and Ψmidday decreased. The findings that gs is more sensitive to drought among C3 grasses and that A is more obviously associated with Ψmidday than with gs in C4 grasses are consistent with evidence from previous experiments (Ghannoum et al., 2003; Ripley et al., 2010; Taylor et al., 2011). That C4 gas exchange was relatively independent of water supply and that gs among C3 species was commonly lower than among C4 relatives are novel findings that highlight the importance of both taking a field-based approach and monitoring performance throughout a growing season. While our measurements of ΔΨ and estimates of Kplant provide some support for the hypothesis that Kplant is often higher among C4 grasses, these differences were not clear-cut, and their physiological basis remains unclear. Therefore, important questions remain about the causes of C4 resistance to drought.
We found that gs among C4 plants was independent of our watering treatment, but A decreased in conjunction with Ψmidday under more severe drought. Among C3 species, decreases in photosynthesis were paired with decreases in Ψpredawn and gs, but among C4 species, decreases in Ψpredawn occurred later and gs never decreased significantly. Although they represent average responses and summarize the performance of species with sometimes distinct behaviours, these results are consistent with previous demonstrations that drought sensitivity of C4 photosynthesis in grasses depends on metabolic rather than stomatal limitations (Ghannoum et al., 2003; Ripley et al., 2007, 2010). Ultimately, photosynthesis in both C3 and C4 grasses was limited by drought in our experiment, but our results suggest that the cause differed and show that significant effects on C4 photosynthesis occurred later. Consistent with our expectations, the greatest photosynthetic advantages for C4 grasses occurred during the development of drought in the warmest parts of the growing season.
We have previously shown that drought can narrow the gap in water-use efficiency between C3 and C4 grasses (Ripley et al., 2010; Taylor et al., 2011), which is large in well-watered, controlled conditions (e.g., Taylor et al., 2010 found that C4 A/gs was double that of C3 grasses). Here we show that, under natural conditions, with a relatively diverse group of PACMAD species, the intrinsic water-use efficiency advantage to C4 species was much smaller when well watered (ca. 40% greater than C3). It was often difficult to distinguish C3 and C4 species based on differences in A/gs, which, outside of the wettest periods in the experiment were significant only during periods of intermediate drought. Assuming that water deficits were generally greater in the field environment than controlled growth conditions, this result is consistent with our previous finding that drought reduces the difference in intrinsic water-use efficiency between C3 and C4 grasses (Taylor et al., 2011), but implies that advantages may be regained as water availability continues to decline. The response of A to gs is expected to saturate more quickly in C3 than C4 grasses (Osborne & Sack, 2012), and C3 species under well-watered conditions often operate above the point where increasing gs results in diminishing returns for A (this study, data not shown). When faced with a need to reduce gs, the leaves of well-watered C3 plants initially face a relatively small penalty in A, and A/gs increases, but C4 leaves retain a clear photosynthetic advantage at low gs (Osborne & Sack, 2012). Importantly, we found that, when we accounted for leaf energy balance, the C4 advantage in A/gs translated into significant differences in A/E only during periods of intermediate drought.
We were surprised to find that gs was similar across the two photosynthetic types, even in watered plots. In previous comparisons of well-watered grasses from a diverse array of habitats, gs was significantly higher among C3 grasses, though the full range of gs observed across C3 and C4 species overlapped substantially (Taylor et al., 2010, 2011). However, we have previously observed similar gs between C3 and C4 grasses in a subset of the species studied here (Frole, 2008; Ripley et al., 2010). We have also previously demonstrated that habitat water availability is important in determining stomatal trait differences among C3 and C4 grasses (Taylor et al., 2012), and it has been repeatedly shown that C3 and C4 lineages sort into distinct hydrological niches (Edwards & Still, 2008; Osborne & Freckleton, 2009; Edwards & Smith, 2010; Pau et al., 2012; Visser et al., 2014). It is possible that differential sensitivity to vapour pressure deficit between C3 and C4 leaves (Bunce, 1983; El-Sharkawy et al., 1985) contributed to the smaller difference in gs values observed in these experiments. However, we suggest that similar gs was observed among the species in this study because they were sampled from a restricted suite of habitats within a seasonally dry climate region. It follows that the smaller differences we observed in A and A/gs may also depend on these factors. This interpretation reinforces the importance of plant water relations in structuring species assemblages, and leads to the prediction that differences in gas exchange traits between C3 and C4 grasses are likely to be more extreme among species from diverse habitats (Taylor et al., 2010, 2011, 2012).
The clear advantage for C4 grasses in midday gas exchange, particularly A, during the growing season implies a disadvantage to C3 grasses that might ultimately influence their local persistence. Of the deaths observed during the 2008–2009 growing season, the majority were among C3 plants, but they were not clearly associated with the watering treatment, a reminder that other factors may ultimately determine the local habitat preferences of these grasses (Visser et al., 2011). Seasonal differences in performance are one possibility: differences in leaf survival during winter have been demonstrated for the C3 and C4 subspecies of A. semialata when grown close to our field site (Ibrahim et al., 2008; Osborne et al., 2008), and a recent phylogenetic study investigating the grass flora of Hawaii demonstrated that the niche of C3 PACMAD species is associated with winter precipitation (Pau et al., 2012). Seasonal differences in productivity are also important in mixed C3/C4 grasslands (Ode et al., 1980; Still et al., 2003) and will no doubt be influenced by shifting patterns of precipitation and seasonality under global change. We found that performance of C3 and C4 grasses was most similar at the beginning and end of the growing season. A key question remaining to be tested, therefore, is whether performance and growth of our C3 species in the late autumn, winter and early spring offset the physiological advantages of their C4 relatives during the summer.
When the soil was wetter, leaf Ψ was less negative and ΔΨ smaller among the C4 than C3 species, consistent with our expectations (Taylor et al., 2010, 2011). During drought, we observed that ΔΨ decreased among C3 grasses and was maintained among C4 grasses. Because declines in ΔΨ were paired with decreasing E among C3 species, whereas E among C4 grasses increased with evaporative demand, we interpret the pattern of decreases in ΔΨ among C3 grasses as indicating greater vulnerability of their hydraulic systems to failure under drought. Smaller ΔΨ was associated with more negative Ψpredawn, not less negative Ψmidday. It is plausible that night-time rehydration under severe drought was insufficient to bring Ψpredawn into equilibrium with soil Ψ in at least some of the C3 species (predawn disequilibrium; Donovan et al., 2001, 2003). Alternatively, increases of Kplant, if genuine, may act to maintain E, hydration and physiological function in the face of increased evaporative demand (Jones, 1992). Changes in Kplant can be regulated by several physiological processes, including changes in tissue conductance due to aquaporin activity (Kaldenhoff et al., 2008); changes in water requirements of growing tissues (Boyer, 1985); or changes in mass allocation such that water supply via roots is enhanced relative to water demand from leaf area (Maseda & Fernández, 2006). Differences in hydraulic traits will have contributed to the overall differences in Kplant in this experiment, and one realistic possibility is that less negative Ψ and smaller ΔΨ in C4 grasses in this experiment was a result of better root system access to available soil water; we have previously observed that the C4 lineages in this experiment have higher root mass ratios than the C3 lineages (Taylor et al., 2010). However, it is important to note that, although Kplant tended to be lower among C3 species, it was not always so: although suggestive, our evidence is not sufficient to claim that a clear difference in Kplant between C3 and C4 species was the principal driver for C4 performance advantages. Nonetheless, our results highlight a need to address mechanistic questions about the integration of hydraulic and photosynthetic performance among grasses, ideally in field experiments and especially under drought.
The characterization of ecophysiological traits associated with C4 photosynthesis is vital for understanding the natural diversity and ecological success of C4 species, and the differential impacts of global change on C3 and C4 species. The comparisons reported here, using four lineages of PACMAD grasses sampled from the same regional species pool and grown under natural climatic conditions, are a crucial complement to previous experiments that were pot-based, carried out in controlled environments, or completed using a less diverse panel of species. Our experimental manipulation of water availability influenced contrasts in leaf physiology during a growing season and we found that C4 photosynthetic advantages were maintained when a diverse panel of grass species were exposed to natural water shortages. Under mild drought, the C4 advantage in A was increased, as C3 leaves faced stomatal limitation of photosynthesis associated with earlier decreases in Ψ. We show that, under native climatic conditions in a location where both C3 and C4 PACMAD grasses are naturally abundant, water availability plays a crucial role in determining the magnitude of differences in physiological performance associated with photosynthetic type. Importantly, our experimental evidence supports a need to rigorously examine the proposition that advantages of C4 photosynthesis in dry environments are significantly modified by dynamic responses of the stomata and hydraulic system to drought (Osborne & Sack, 2012). Understanding the interplay between C4 photosynthesis and hydraulics will be crucial as we aim to better understand the response of plant communities to global change, including the question of why the distribution of C4 PACMAD grasses is so strongly linked with water availability (Edwards & Smith, 2010; Pau et al., 2012).
Acknowledgments
Research was funded by the NERC grant NE/DO13062/1 awarded to CPO and FIW. Matthew Gilbert and Kristen Frole assisted with initial set-up of the experiments. Our gardeners Joe, Xhanti and Wiseman were invaluable. SHT thanks Laura Premack for a critical reading of the manuscript.
Supporting Information
Additional Supporting Information may be found in the online version of this article
Equations used for estimation of reference crop evapotranspiration (ET0).
Model for estimation of leaf-level transpiration (E).
Mean values for microclimate used in modelling of leaf-level transpiration (E).
Tabulated values for physiological traits determined in the field, by species, month of measurement and treatment.
Plotted species means used for analysis.
References
- Allen RG, Pereira LS, Raes D, Smith M. Crop evapotranspiration–guidelines for computing crop water requirements–FAO irrigation and drainage paper. 1998;56:1–15. [Google Scholar]
- Arnone JA, III, Jasoni RL, Lucchesi AJ, et al. A climatically extreme year has large impacts on C4 species in tallgrass prairie ecosystems but only minor effects on species richness and other plant functional groups. Journal of Ecology. 2011;99:678–688. [Google Scholar]
- Bates D, Maechler M, Bolker B. lme4: Linear mixed-effects models using S4 classes. R package version 0.999999-2. 2013. http://cran.r-project.org/package=lme4.
- Bjorkman O. Comparative photosynthetic CO2 exchange in higher plants. In: Hatch MD, Osmond CB, Slatyer RO, editors. Photosynthesis and Photorespiration. Canberra: Australian Academy of Science; 1971. pp. 18–32. [Google Scholar]
- Black CC, Chen TM, Brown RH. Biochemical basis for plant competition. Weed Science. 1969;17:338–34. [Google Scholar]
- Boyer JS. Water transport. Annual Review of Plant Physiology. 1985;36:473–516. [Google Scholar]
- Bunce JA. Differential sensitivity to humidity of daily photosynthesis in the field in C3 and C4 species. Oecologia. 1983;57:262–265. doi: 10.1007/BF00379588. [DOI] [PubMed] [Google Scholar]
- Christin P-A, Salamin N, Kellogg EA, Vicentini A, Besnard G. Integrating phylogeny into studies of C4 variation in the grasses. Plant Physiology. 2009;149:82–87. doi: 10.1104/pp.108.128553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collatz GJ, Ribas-Carbó M, Berry JA. Coupled photosynthesis-stomatal conductance model for leaves of C4 plants. Australian Journal of Plant Physiology. 1992;19:519–538. [Google Scholar]
- Donovan LA, Linton MJ, Richards JH. Predawn plant water potential does not necessarily equilibrate with soil water potential under well-watered conditions. Oecologia. 2001;129:328–335. doi: 10.1007/s004420100738. [DOI] [PubMed] [Google Scholar]
- Donovan LA, Richards JH, Linton MJ. Magnitude and mechanisms of disequilibrium between predawn plant and soil water potentials. Ecology. 2003;84:463–470. [Google Scholar]
- Driever SM, Kromdijk J. Will C3 crops enhanced with the C4 CO2-concentrating mechanism live up to their full potential (yield)? Journal of Experimental Botany. 2013;64:3925–3935. doi: 10.1093/jxb/ert103. [DOI] [PubMed] [Google Scholar]
- Edwards EJ, Smith SA. Phylogenetic analyses reveal the shady history of C4 grasses. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:2532–2537. doi: 10.1073/pnas.0909672107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards EJ, Still CJ. Climate, phylogeny and the ecological distribution of C4 grasses. Ecology Letters. 2008;11:266–276. doi: 10.1111/j.1461-0248.2007.01144.x. [DOI] [PubMed] [Google Scholar]
- Edwards EJ, Still CJ, Donoghue MJ. The relevance of phylogeny to studies of global change. Trends in Ecology & Evolution. 2007;22:243–249. doi: 10.1016/j.tree.2007.02.002. [DOI] [PubMed] [Google Scholar]
- Edwards EJ, Osborne CP, Strömberg CAE, Smith SA, Consortium CG. The origins of C4 grasslands: integrating evolutionary and ecosystem science. Science. 2010;328:587–591. doi: 10.1126/science.1177216. [DOI] [PubMed] [Google Scholar]
- Ehleringer JR, Monson RK. Evolutionary and ecological aspects of photosynthetic pathway variation. Annual Review of Ecology and Systematics. 1993;24:411–439. [Google Scholar]
- Ehleringer JR, Pearcy RW. Variation in quantum yield for CO2 uptake among C3 and C4 plants. Plant Physiology. 1983;73:555–559. doi: 10.1104/pp.73.3.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehleringer JR, Cerling TE, Helliker BR. C4 photosynthesis, atmospheric CO2, and climate. Oecologia. 1997;112:285–299. doi: 10.1007/s004420050311. [DOI] [PubMed] [Google Scholar]
- El-Sharkawy MA, Cock JH, Del Pilar HernandezA. Stomatal response to air humidity and its relation to stomatal density in a wide range of warm climate species. Photosynthesis Research. 1985;7:137–149. doi: 10.1007/BF00037004. [DOI] [PubMed] [Google Scholar]
- Epstein HE, Lauenroth WK, Burke IC, Coffin DP. Productivity patterns of C3 and C4 functional types in the U.S. great plains. Ecology. 1997;78:722–731. [Google Scholar]
- Frole KM. Drought Responses of C3 and C4 (NADP-ME) Panicoid Grasses. Grahamstown: Rhodes University; 2008. Unpublished MSc thesis, 114 pp. [Google Scholar]
- Ghannoum O. C4 photosynthesis and water stress. Annals of Botany. 2009;103:635–644. doi: 10.1093/aob/mcn093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghannoum O, Conroy JP, Driscoll SP, Paul MJ, Foyer CH, Lawlor DW. Nonstomatal limitations are responsible for drought-induced photosynthetic inhibition in four C4 grasses. New Phytologist. 2003;159:599–608. doi: 10.1046/j.1469-8137.2003.00835.x. [DOI] [PubMed] [Google Scholar]
- Gibbs Russell GE, Watson L, Koekmoer L, Smook L, Barker NP, Anderson HM, Dallwitz MJ. Grasses of Southern Africa. Pretoria: National Botanical Gardens; 1990. [Google Scholar]
- Grass Phylogeny Working Group II. New grass phylogeny resolves deep evolutionary relationships and discovers C4 origins. New Phytologist. 2012;193:304–312. doi: 10.1111/j.1469-8137.2011.03972.x. [DOI] [PubMed] [Google Scholar]
- Griffiths H, Weller G, Toy LFM, Dennis RJ. You're so vein: bundle sheath physiology, phylogeny and evolution in C3 and C4 plants. Plant, Cell & Environment. 2013;36:249–261. doi: 10.1111/j.1365-3040.2012.02585.x. [DOI] [PubMed] [Google Scholar]
- Hatch MD, Osmond CB. Compartmentation and transport in C4 photosynthesis. In: Stocking CR, Heber U, editors. Transport in Plants III. Berlin: Heidelberg, Springer Verlag; 1976. pp. 144–184. [Google Scholar]
- Humphreys AM, Linder HP. Evidence for recent evolution of cold tolerance in grasses suggests current distribution is not limited by (low) temperature. New Phytologist. 2013;198:1261–1273. doi: 10.1111/nph.12244. [DOI] [PubMed] [Google Scholar]
- Ibrahim DG, Gilbert ME, Ripley BS, Osborne CP. Seasonal differences in photosynthesis between the C3 and C4 subspecies of Alloteropsis semialata are offset by frost and drought. Plant, Cell & Environment. 2008;31:1038–1050. doi: 10.1111/j.1365-3040.2008.01815.x. [DOI] [PubMed] [Google Scholar]
- Jones HG. Plants and Microclimate: A Quantitative Approach to Environmental Plant Physiology. United Kingdom, Cambridge: Cambridge University Press; 1992. [Google Scholar]
- Kaldenhoff R, Ribas-Carbo M, Sans JF, Lovisolo C, Heckwolf M, Uehlein N. Aquaporins and plant water balance. Plant, Cell & Environment. 2008;31:658–666. doi: 10.1111/j.1365-3040.2008.01792.x. [DOI] [PubMed] [Google Scholar]
- Long SP. Environmental responses. In: Sage RF, Monson RK, editors. C4 Plant Biology. San Diego, CA, USA: Academic Press; 1999. pp. 215–249. [Google Scholar]
- Maseda PH, Fernández RJ. Stay wet or else: three ways in which plants can adjust hydraulically to their environment. Journal of Experimental Botany. 2006;57:3963–3977. doi: 10.1093/jxb/erl127. [DOI] [PubMed] [Google Scholar]
- Monteith JL. Evaporation and environment. Symposia of the Society for Experimental Biology. 1965;19:205–234. [PubMed] [Google Scholar]
- Morgan JA, LeCain DR, Pendall E, et al. C4 grasses prosper as carbon dioxide eliminates desiccation in warmed semi-arid grassland. Nature. 2011;476:202–206. doi: 10.1038/nature10274. [DOI] [PubMed] [Google Scholar]
- Murphy BP, Bowman DMJS. Seasonal water availability predicts the relative abundance of C3 and C4 grasses in Australia. Global Ecology and Biogeography. 2006;16:160–169. [Google Scholar]
- Ode DJ, Tieszen LL, Lerman JC. The seasonal contribution of C3 and C4 plant species to primary production in a mixed prairie. Ecology. 1980;61:1304–1311. [Google Scholar]
- Osborne CP, Freckleton RP. Ecological selection pressures for C4 photosynthesis in the grasses. Proceedings of the Royal Society B: Biological Sciences. 2009;276:1753–1760. doi: 10.1098/rspb.2008.1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborne CP, Sack L. Evolution of C4 plants: a new hypothesis for an interaction of CO2 and water relations mediated by plant hydraulics. Philosophical Transactions of the Royal Society B: Biological Sciences. 2012;367:583–600. doi: 10.1098/rstb.2011.0261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborne CP, Wythe EJ, Ibrahim DG, Gilbert ME, Ripley BS. Low temperature effects on leaf physiology and survivorship in the C3 and C4 subspecies of Alloteropsis semialata. Journal of Experimental Botany. 2008;59:1743–1754. doi: 10.1093/jxb/ern062. [DOI] [PubMed] [Google Scholar]
- Osmond CB, Winter K, Ziegler H. Functional significance of different pathways of CO2 fixation in photosynthesis. In: Lange OL, Nobel P, Osmond CB, Ziegler H, editors. Encyclopedia of Plant Physiology. Berlin: Springer Verlag; 1982. pp. 479–547. [Google Scholar]
- Pau S, Edwards EJ, Still CJ. Improving our understanding of environmental controls on the distribution of C3 and C4 grasses. Global Change Biology. 2012;19:184–196. doi: 10.1111/gcb.12037. [DOI] [PubMed] [Google Scholar]
- Peel MC, Finlayson BL, McMahon TA. Updated world map of the Köppen-Geiger climate classification. Hydrology and Earth System Sciences. 2007;11:1633–1644. [Google Scholar]
- Penman HL. Natural evaporation from open water, bare soil and grass. Proceedings of the Royal Society A. 1948;193:120–145. doi: 10.1098/rspa.1948.0037. [DOI] [PubMed] [Google Scholar]
- Poorter H, Fiorani F, Stitt M, et al. The art of growing plants for experimental purposes: a practical guide for the plant biologist. Functional Plant Biology. 2012;39:821–838. doi: 10.1071/FP12028. [DOI] [PubMed] [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2013. [Google Scholar]
- Raschke K. Stomatal action. Annual Review of Plant Physiology. 1975;26:309–340. [Google Scholar]
- Ripley BS, Gilbert ME, Ibrahim DG, Osborne CP. Drought constraints on C4 photosynthesis: stomatal and metabolic limitations in C3 and C4 subspecies of Alloteropsis semialata. Journal of Experimental Botany. 2007;58:1351–1363. doi: 10.1093/jxb/erl302. [DOI] [PubMed] [Google Scholar]
- Ripley BS, Frole K, Gilbert M. Differences in drought sensitivities and photosynthetic limitations between co-occurring C3 and C4 (NADP-ME) Panicoid grasses. Annals of Botany. 2010;105:493–503. doi: 10.1093/aob/mcp307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sage RF. The evolution of C4 photosynthesis. New Phytologist. 2009;161:341–370. doi: 10.1111/j.1469-8137.2004.00974.x. [DOI] [PubMed] [Google Scholar]
- Sage RF, Christin P-A, Edwards EJ. The C4 plant lineages of planet Earth. Journal of Experimental Botany. 2011;62:3155–3169. doi: 10.1093/jxb/err048. [DOI] [PubMed] [Google Scholar]
- Still CJ, Berry JA, Ribas-Carbo M, Helliker BR. The contribution of C3 and C4 plants to the carbon cycle of a tallgrass prairie: an isotopic approach. Oecologia. 2003;136:347–359. doi: 10.1007/s00442-003-1274-8. [DOI] [PubMed] [Google Scholar]
- Taylor SH, Hulme SP, Rees M, Ripley BS, Woodward FI, Osborne CP. Ecophysiological traits in C3 and C4 grasses: a phylogenetically controlled screening experiment. New Phytologist. 2010;185:780–791. doi: 10.1111/j.1469-8137.2009.03102.x. [DOI] [PubMed] [Google Scholar]
- Taylor SH, Ripley BS, Woodward FI, Osborne CP. Drought limitation of photosynthesis differs between C3 and C4 grass species in a comparative experiment. Plant Cell & Environment. 2011;34:65–75. doi: 10.1111/j.1365-3040.2010.02226.x. [DOI] [PubMed] [Google Scholar]
- Taylor SH, Franks PJ, Hulme SP, et al. Photosynthetic pathway and ecological adaptation explain stomatal trait diversity amongst grasses. New Phytologist. 2012;193:387–396. doi: 10.1111/j.1469-8137.2011.03935.x. [DOI] [PubMed] [Google Scholar]
- Teeri JA, Stowe LG. Climatic patterns and the distribution of C4 grasses in North America. Ecology. 1976;23:1–12. doi: 10.1007/BF00351210. [DOI] [PubMed] [Google Scholar]
- Vigeland MD, Spannagl M, Asp T, et al. Evidence for adaptive evolution of low-temperature stress response genes in a Pooideae grass ancestor. New Phytologist. 2013;199:1060–1068. doi: 10.1111/nph.12337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visser V, Woodward FI, Freckleton RP, Osborne CP. Environmental factors determining the phylogenetic structure of C4 grass communities. Journal of Biogeography. 2011;39:232–246. [Google Scholar]
- Visser V, Clayton WD, Simpson DA, Freckleton RP, Osborne CP. Mechanisms driving an unusual latitudinal diversity gradient for grasses. Global Ecology and Biogeography. 2014;23:61–75. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Equations used for estimation of reference crop evapotranspiration (ET0).
Model for estimation of leaf-level transpiration (E).
Mean values for microclimate used in modelling of leaf-level transpiration (E).
Tabulated values for physiological traits determined in the field, by species, month of measurement and treatment.
Plotted species means used for analysis.


