Abstract
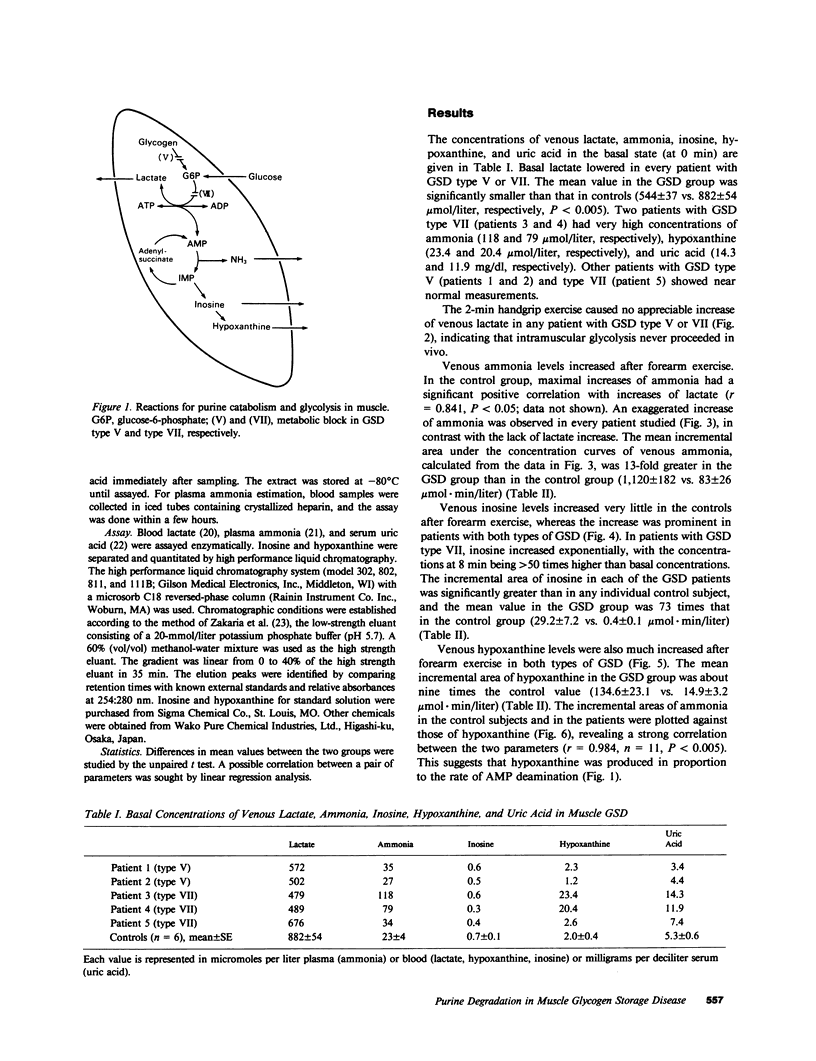
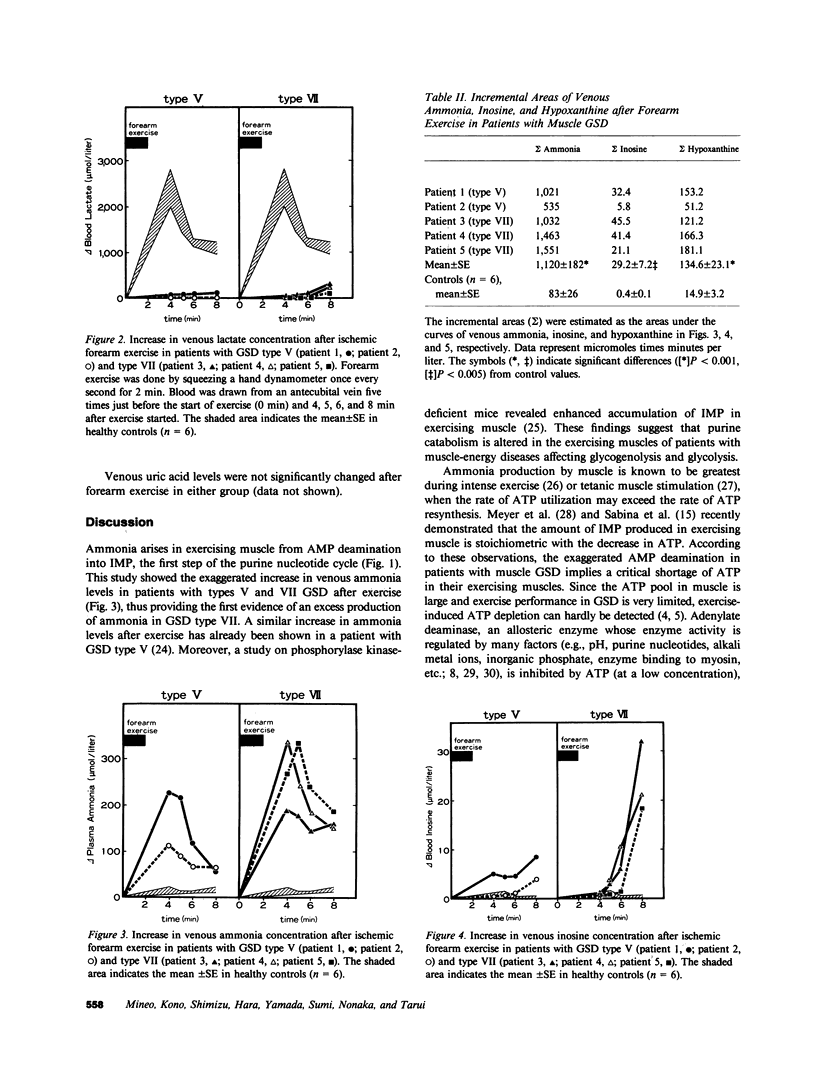
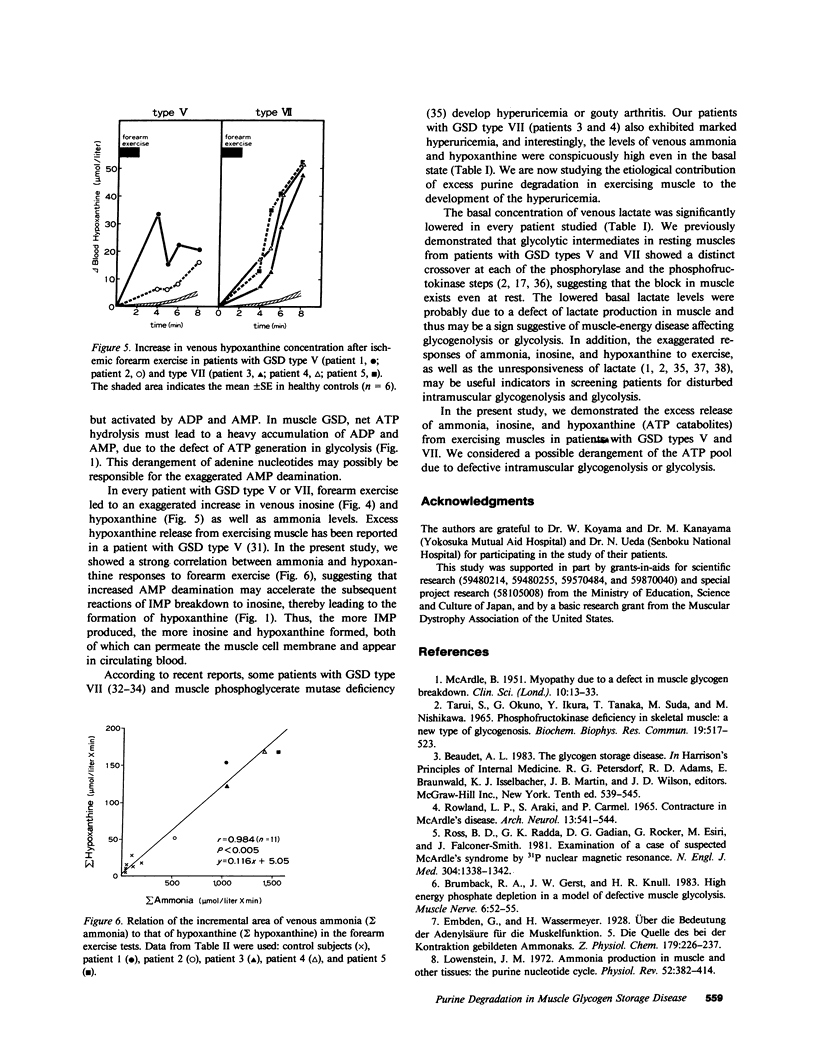
To investigate purine catabolism in exercising muscles of patients with muscle glycogen storage disease, we performed ischemic forearm exercise tests and quantitated metabolites appearing in cubital venous blood. Two patients with glycogen storage disease type V and three with glycogen storage disease type VII participated in this study. Basal lactate concentrations lowered in every patient with glycogen storage disease type V or type VII. Two patients with glycogen storage disease type VII, who had markedly elevated concentrations of serum uric acid (14.3 and 11.9 mg/dl, respectively), showed high basal concentrations of ammonia (118 and 79 mumol/liter, respectively; 23 +/- 4 mumol/liter in healthy controls) and of hypoxanthine (23.4 and 20.4 mumol/liter, respectively; 2.0 +/- 0.4 mumol/liter in healthy controls). Other patients showed near normal measurements of these metabolites. After forearm exercise, ammonia, inosine, and hypoxanthine levels increased greatly in every patient studied, in contrast with the lack of increase in lactate levels. The incremental area under the concentration curves for venous ammonia was 13-fold greater in the glycogen storage disease group than in controls (1,120 +/- 182 vs. 83 +/- 26 mumol X min/liter). The incremental areas of inosine and hypoxanthine were also greater in the glycogen storage disease group (29.2 +/- 7.2 vs. 0.4 +/- 0.1 and 134.6 +/- 23.1 vs. 14.9 +/- 3.2 mumol X min/liter, respectively). The incremental areas of ammonia in controls and in glycogen storage disease patients strongly correlated with those of hypoxanthine (r = 0.984, n = 11, P less than 0.005). These findings indicated that excess purine degradation occurred in the exercising muscles of patients with glycogen storage disease types V and VII, and suggested that the ATP pool in the exercising muscles may be deranged because of defective glycogenolysis or glycolysis.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agamanolis D. P., Askari A. D., Di Mauro S., Hays A., Kumar K., Lipton M., Raynor A. Muscle phosphofructokinase deficiency: two cases with unusual polysaccharide accumulation and immunologically active enzyme protein. Muscle Nerve. 1980 Nov-Dec;3(6):456–467. doi: 10.1002/mus.880030602. [DOI] [PubMed] [Google Scholar]
- Aragón J. J., Tornheim K., Goodman M. N., Lowenstein J. M. Replenishment of citric acid cycle intermediates by the purine nucleotide cycle in rat skeletal muscle. Curr Top Cell Regul. 1981;18:131–149. doi: 10.1016/b978-0-12-152818-8.50014-1. [DOI] [PubMed] [Google Scholar]
- Aragón J. J., Tornheim K., Lowenstein J. M. On a possible role of IMP in the regulation of phosphorylase activity in skeletal muscle. FEBS Lett. 1980 Aug 25;117 (Suppl):K56–K64. doi: 10.1016/0014-5793(80)80570-9. [DOI] [PubMed] [Google Scholar]
- Brooke M. H., Patterson V. H., Kaiser K. K. Hypoxanthine and Mcardle disease: a clue to metabolic stress in the working forearm. Muscle Nerve. 1983 Mar-Apr;6(3):204–206. doi: 10.1002/mus.880060307. [DOI] [PubMed] [Google Scholar]
- Brumback R. A., Gerst J. W., Knull H. R. High energy phosphate depletion in a model of defective muscle glycolysis. Muscle Nerve. 1983 Jan;6(1):52–55. doi: 10.1002/mus.880060109. [DOI] [PubMed] [Google Scholar]
- DiMauro S., Dalakas M., Miranda A. F. Phosphoglycerate kinase deficiency: another cause of recurrent myoglobinuria. Ann Neurol. 1983 Jan;13(1):11–19. doi: 10.1002/ana.410130104. [DOI] [PubMed] [Google Scholar]
- DiMauro S., Miranda A. F., Khan S., Gitlin K., Friedman R. Human muscle phosphoglycerate mutase deficiency: newly discovered metabolic myopathy. Science. 1981 Jun 12;212(4500):1277–1279. doi: 10.1126/science.6262916. [DOI] [PubMed] [Google Scholar]
- Hochachka P. W., Mommsen T. P. Protons and anaerobiosis. Science. 1983 Mar 25;219(4591):1391–1397. doi: 10.1126/science.6298937. [DOI] [PubMed] [Google Scholar]
- Kanno T., Sudo K., Takeuchi I., Kanda S., Honda N., Nishimura Y., Oyama K. Hereditary deficiency of lactate dehydrogenase M-subunit. Clin Chim Acta. 1980 Dec 8;108(2):267–276. doi: 10.1016/0009-8981(80)90013-3. [DOI] [PubMed] [Google Scholar]
- Kono N., Mineo I., Sumi S., Shimizu T., Kang J., Nonaka K., Tarui S. Metabolic basis of improved exercise tolerance: muscle phosphorylase deficiency after glucagon administration. Neurology. 1984 Nov;34(11):1471–1476. doi: 10.1212/wnl.34.11.1471. [DOI] [PubMed] [Google Scholar]
- Meyer R. A., Dudley G. A., Terjung R. L. Ammonia and IMP in different skeletal muscle fibers after exercise in rats. J Appl Physiol Respir Environ Exerc Physiol. 1980 Dec;49(6):1037–1041. doi: 10.1152/jappl.1980.49.6.1037. [DOI] [PubMed] [Google Scholar]
- Mineo I., Kono N., Shimizu T., Sumi S., Nonaka K., Tarui S. A comparative study on glucagon effect between McArdle disease and Tarui disease. Muscle Nerve. 1984 Sep;7(7):552–559. doi: 10.1002/mus.880070706. [DOI] [PubMed] [Google Scholar]
- Patterson V. H., Kaiser K. K., Brooke M. H. Exercising muscle does not produce hypoxanthine in adenylate deaminase deficiency. Neurology. 1983 Jun;33(6):784–786. doi: 10.1212/wnl.33.6.784. [DOI] [PubMed] [Google Scholar]
- Rahim Z. H., Perrett D., Lutaya G., Griffiths J. R. Metabolic adaptation in phosphorylase kinase deficiency. Changes in metabolite concentrations during tetanic stimulation of mouse leg muscles. Biochem J. 1980 Jan 15;186(1):331–341. doi: 10.1042/bj1860331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross B. D., Radda G. K., Gadian D. G., Rocker G., Esiri M., Falconer-Smith J. Examination of a case of suspected McArdle's syndrome by 31P nuclear magnetic resonance. N Engl J Med. 1981 May 28;304(22):1338–1342. doi: 10.1056/NEJM198105283042206. [DOI] [PubMed] [Google Scholar]
- Rowland L. P., Araki S., Carmel P. Contracture in McArdle's disease. Stability of adenosine triphosphate during contracture in phosphorylase-deficient human muscle. Arch Neurol. 1965 Nov;13(5):541–544. doi: 10.1001/archneur.1965.00470050089010. [DOI] [PubMed] [Google Scholar]
- Rumpf K. W., Wagner H., Kaiser H., Meinck H. M., Goebel H. H., Scheler F. Increased ammonia production during forearm ischemic work test in McArdle's disease. Klin Wochenschr. 1981 Dec 1;59(23):1319–1320. doi: 10.1007/BF01711182. [DOI] [PubMed] [Google Scholar]
- Sabina R. L., Swain J. L., Olanow C. W., Bradley W. G., Fishbein W. N., DiMauro S., Holmes E. W. Myoadenylate deaminase deficiency. Functional and metabolic abnormalities associated with disruption of the purine nucleotide cycle. J Clin Invest. 1984 Mar;73(3):720–730. doi: 10.1172/JCI111265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiraki H., Miyamoto S., Matsuda Y., Momose E., Nakagawa H. Possible correlation between binding of muscle type AMP deaminase to myofibrils and ammoniagenesis in rat skeletal muscle on electrical stimulation. Biochem Biophys Res Commun. 1981 Jun 16;100(3):1099–1103. doi: 10.1016/0006-291x(81)91936-7. [DOI] [PubMed] [Google Scholar]
- Sutton J. R., Toews C. J., Ward G. R., Fox I. H. Purine metabolism during strenuous muscular exercise in man. Metabolism. 1980 Mar;29(3):254–260. doi: 10.1016/0026-0495(80)90067-0. [DOI] [PubMed] [Google Scholar]
- Swain J. L., Hines J. J., Sabina R. L., Harbury O. L., Holmes E. W. Disruption of the purine nucleotide cycle by inhibition of adenylosuccinate lyase produces skeletal muscle dysfunction. J Clin Invest. 1984 Oct;74(4):1422–1427. doi: 10.1172/JCI111553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TARUI S., OKUNO G., IKURA Y., TANAKA T., SUDA M., NISHIKAWA M. PHOSPHOFRUCTOKINASE DEFICIENCY IN SKELETAL MUSCLE. A NEW TYPE OF GLYCOGENOSIS. Biochem Biophys Res Commun. 1965 May 3;19:517–523. doi: 10.1016/0006-291x(65)90156-7. [DOI] [PubMed] [Google Scholar]
- Tornheim K., Lowenstein J. M. The purine nucleotide cycle. 3. Oscillations in metabolite concentrations during the operation of the cycle in muscle extracts. J Biol Chem. 1973 Apr 25;248(8):2670–2677. [PubMed] [Google Scholar]
- Vora S., Davidson M., Seaman C., Miranda A. F., Noble N. A., Tanaka K. R., Frenkel E. P., Dimauro S. Heterogeneity of the molecular lesions in inherited phosphofructokinase deficiency. J Clin Invest. 1983 Dec;72(6):1995–2006. doi: 10.1172/JCI111164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler T. J., Lowenstein J. M. Adenylate deaminase from rat muscle. Regulation by purine nucleotides and orthophosphate in the presence of 150 mM KCl. J Biol Chem. 1979 Sep 25;254(18):8994–8999. [PubMed] [Google Scholar]
- Wilkerson J. E., Batterton D. L., Horvath S. M. Exercise-induced changes in blood ammonia levels in humans. Eur J Appl Physiol Occup Physiol. 1977 Dec 22;37(4):255–263. doi: 10.1007/BF00430955. [DOI] [PubMed] [Google Scholar]
- Zakaria M., Brown P. R., Farnes M. P., Barker B. E. HPLC analysis of aromatic amino acids, nucleosides, and bases in plasma of acute lymphocytic leukemia on chemotherapy. Clin Chim Acta. 1982 Nov 24;126(1):69–80. doi: 10.1016/0009-8981(82)90362-x. [DOI] [PubMed] [Google Scholar]
- Zenella A., Mariani M., Meola G., Fagnani F., Sirchia G. Phosphofructokinase (PFK) deficiency due to a catalytically inactive mutant M-type subunit. Am J Hematol. 1982 May;12(3):215–225. doi: 10.1002/ajh.2830120303. [DOI] [PubMed] [Google Scholar]
- van Anken H. C., Schiphorst M. E. A kinetic determination of ammonia in plasma. Clin Chim Acta. 1974 Oct 30;56(2):151–157. doi: 10.1016/0009-8981(74)90223-x. [DOI] [PubMed] [Google Scholar]


