Abstract
Background
HNA-3a specific antibodies can cause severe, sometimes fatal, transfusion related acute lung injury (TRALI) when present in transfused blood. The HNA3-a/b antigens are determined by an R154Q polymorphism in the first of five extracellular loops of the 10-membrane spanning choline transporter-like protein 2 (CTL2) expressed on neutrophils, lymphocytes and other tissues. About 50% of HNA-3a antibodies (Type 1) can be detected using CTL2 Loop 1 peptides containing R154; the remaining 50% (Type 2) fail to recognize this target. Understanding the basis for this difference could guide efforts to develop practical assays to screen blood donors for HNA-3 antibodies.
Study design and methods
Reactions of HNA-3a antibodies against recombinant versions of human, mouse, and human/mouse (chimeric) CTL2 were characterized using flow cytometry and various solid phase assays.
Results
Findings made show that, for binding to CTL2, Type 2 HNA-3a antibodies require non-polymorphic amino acid residues in the third, and possibly the second, extracellular loops of CTL2 to be in a configuration comparable to that found naturally in the cell membrane. In contrast, Type 1 antibodies require only peptides from the first extracellular loop that contain R154 for recognition.
Conclusion
Although Type 1 HNA-3a antibodies can readily be detected in solid phase assays that use a CTL2 peptide containing R154 as a target, development of a practical test to screen blood donors for Type 2 antibodies will pose a serious technical challenge because of the complex nature of the epitope(s) recognized by this antibody sub-group.
Keywords: TRALI, HNA-3a, CTL2, alloantibody
INTRODUCTION
Transfusion of antibodies specific for Class I and II HLA antigens and human neutrophil antigens (HNA) can cause transfusion-related acute lung injury (TRALI)1–4, currently the major cause of transfusion-associated mortality in the United States5,6. New approaches to donor selection7,8 have reduced the incidence of TRALI but are not feasible in all situations and are not totally effective. Consequently, TRALI remains an important cause of morbidity and mortality in transfusion recipients. Antibodies specific for the antigen HNA-3a cause particularly severe and often fatal TRALI3,9,10 for reasons that are only partially understood11–13. However, a method suitable for large-scale screening of blood donors to identify those who have HNA-3a antibodies is not currently available.
The HNA-3a/b antigens are determined by an arginine to glutamine (R154Q) amino acid polymorphism in the first extracellular loop of choline transporter-like protein 2 (CTL2)14,15, a 68–72 kDa membrane protein of uncertain function predicted to consist of 10 transmembrane domains and 5 extracellular loops16–18. HNA-3a/b was widely thought to be neutrophil-specific for many years, but later was found to be present in B-lymphocytes, T-lymphocytes, platelets and other tissues14,18–20. Blood cells express isoform 2 of two known isoforms resulting from alternative splicing of exon 120. Identification of the molecular basis for the HNA-3a/b antigens suggested the possibility that synthetic peptides comprising amino sequences from the first extracellular loop of CTL2 and containing R154 or Q154 could be used as targets to screen blood donors for anti-HNA-3a and anti-HNA-3b antibodies, thereby excluding these potentially dangerous antibodies from the blood supply. However, we21 and others22,23 found that first loop CTL2 peptides containing R154 are recognized by only about half of the HNA-3a antibodies identified in donors whose blood products caused TRALI. Subsequent reports showed that 20 of 2024, 31 of 3123 and 14 of 1425 HNA-3a antibodies recognized recombinant full length CTL2 (R154) expressed in HEK293 cells. These findings indicate that there are at least two distinct types of anti-HNA-3a antibodies, one that recognizes peptides from the first extracellular loop that contain R154 (Type 1) and another (Type 2) that fails to recognize peptide but reacts with both native and recombinant forms of full length CTL2 (R154) expressed on cell membranes.
Here, we describe studies to define the specificity of Type 2 HNA-3a antibodies. Findings made provide evidence that reactions of this antibody subset with CTL2 require both extracellular Loop 1 and the adjacent extracellular Loops 2 and 3 to be contiguous and in their native, membrane-associated conformations. These characteristics imply that novel strategies will be required to produce a natural or recombinant target protein capable of detecting both Type 1 and Type 2 HNA-3a antibodies in blood donors.
METHODS
Reagents
Unless otherwise stated, reagents were purchased from Sigma-Aldrich (St. Louis, MO). Fetal Bovine serum (FBS) was purchased from Atlanta Biologicals (Atlanta, GA); DMEM+ media, G418 and gentamycin from Mediatech Inc. (Herndon, VA); alkaline-phosphatase-labeled (AP) goat anti-human IgG (H+L), AP-goat anti-human IgG (Fc), APC goat (Fab’)2 anti-human IgG (H+L) from Jackson ImmunoResearch (West Grove, PA) and 9E10 (anti-myc, ATCC) was purified from ascites with protein G (GE Healthcare Piscataway, NJ). Human CTL2 isoform 2 cDNA18 was a gift from Dr T. Carey (University of Michigan, Ann Arbor, MI) and encodes the shorter isoform of 704 amino acids in which arginine determining the HNA-3a antigen is at position 152. In this report, we refer to this amino acid residue as “R154” to be consistent with the previous reports of Curtis14 and Greinacher15, which are based on the longer CTL2 isoform 1 containing 706 amino acids.
Antibodies
Twenty HNA-3a antibodies from donors whose blood was implicated in a TRALI reaction were previously used to show that 10 (here designated “Type 1”) recognized a 36-mer peptide from the first extracellular CTL2 loop (residues D131-K166) containing R154 whereas 10 others (designated “Type 2”) did not21. For studies described here, we used 7 members of this antibody panel that were available in quantities sufficient for this work. Two of these (1 and 5) were “Type 1” and five (2–4, 6, 13) were “Type 2.” Code numbers assigned to these antibodies correspond to those used in previous publications in which reactions of the antibody panel against CTL2 peptides21 and full-length recombinant CTL224 were described. All patients studied satisfied the standard diagnostic criteria for TRALI6.
Construction, expression and purification of srCTL2 56–197
cDNA corresponding to the signal peptide of glycoprotein IIIa fused in frame with CTL2 residues 56 through 197 was generated with overlapping PCR methodology26. After digestion with BamHI and Xba I, the PCR product was inserted into pcDNA3.1/myc-his B (Invitrogen Life Technologies, Grand Island, NY). Site-directed mutagenesis was then used to change arginine to glutamine at residue 154 with QuikChange II XL Site-Directed Mutagenesis Kit (Agilent Technologies, Inc., Santa Clara, CA). Plasmids were transfected into HEK293 cells with transfection reagent Fugene HD (Roche, Atlanta, GA). Stable cell lines were maintained and supplemented with 10% fetal bovine serum, G418 (50 ug/ml) and Gentamicin (25 ug/ml). srCTL2 56–197 was isolated from culture supernatant by affinity chromatography using MoAb 9E10 and elution with Gentle Elute (Thermo Scientific, Rockford, IL).
Recombinant mouse CTL2
CTL2 isoform 2 (704AA) was PCR amplified from cDNA isolated from the cultured murine cell line EL4 (ATCC, derived from the C57BL6/N strain) and was ligated into the pcDNA3.1/CT-GFP-TOPO vector. The construct was validated by sequencing the entire insert. HEK293 cells were transfected with this vector using TransIT-293 following the manufacturer’s protocol (Mirus Bio, Madison WI), followed by selection with G418 (50ug/ml). Cells were sorted to achieve expression approximately equal to that of lines expressing human CTL2 based on GFP fluorescence as previously described24. Expression of mouse CTL2-GFP fusion protein was confirmed by gel electrophoresis (8% SDS PAGE), and detection of GFP fluorescence (Typhoon laser scanner, GE Healthcare).
Human/mouse chimeric CTL2 constructs
Chimeric human/mouse recombinant CTL2 constructs containing R at position 154 and tagged with GFP at their C-termini were generated by overlapping PCR methodology27. Constructs encoding human CTL2 Loop1 with the remainder mouse (H1M) or mouse CTL2 Loop1 with the remainder human (M1H) were produced and expressed in HEK293 cells sorted for high expression as described above. In extracellular Loop 2, human and mouse CTL2 differ by only one amino acid (F303L). A construct (H2M) with human amino acid sequence in extracellular Loops 1 and 2 was generated from the H1M construct by site directed mutagenesis (QuickChange XL, Agilent Technologies, Santa Clara, CA) following the manufacture’s protocol. An H3M construct was generated by overlapping PCR as for the H1M construct. All constructs were validated by direct sequencing. Cell lines were sorted for high expression by GFP fluorescence and protein expression was confirmed by gel electrophoresis.
Antibody detection by flow cytometry
Studies were performed as previously described24. Briefly, stably transfected HEK293 cells were lifted with trypsin/EDTA and washed with buffer [1% BSA (bovine serum albumin) in PBS]. Cells (100,000) and 10ul of patient serum were combined in a final volume of 50ul and allowed to incubate at room temperature for 1h. The cells were washed with 150ul buffer, suspended in 50ul buffer containing APC labeled goat F(ab’)2 anti-human IgG (H+L) diluted at 1:100 and incubated for 45 minutes. The cells were again washed, suspended in 250ul buffer and analyzed by flow cytometry (LSRII, Becton Dickinson). During analysis cells were gated for equal expression of GFP. Cells expressing CTL2 R154 (HNA-3a positive) and CTL2 Q154 (HNA-3a negative) were used as controls.
Modified Antigen Capture ELlSA (MACE)
MACE was carried out as described previously28. In brief, HEK293 cell lines stably expressing recombinant human CTL2-GFP (R154 or Q154) were sensitized with human HNA-3a antibodies (diluted 1:5), washed and lysed with 1.0% Triton X-100. CTL2-GFP was captured in a microtiter plate with anti-myc MoAb 9E10. Bound human antibody was detected with AP-labeled goat anti-human IgG (H+L).
Indirect Antibody Capture ELISA
HEK293 cells, expressing recombinant proteins, were harvested with 0.05% Trypsin/0.53 mM EDTA in Hank’s balanced salt solution and washed twice with 1% bovine serum albumin tris buffered saline solution (BSA/TBS). The cells were then suspended in BSA/TBS at 5×104 cells/uL, incubated with 40 ul of anti-HNA-3a serum for one hour at room temperature, washed three times with BSA/TBS and incubated with 1:5000 AP-labeled goat anti-human IgG (Fc-specific) F(ab’)2 for 45 minutes in buffer. Cells were washed three times with BSA/TBS and lysed with 150 uL of 1.0%Triton X-100/0.1% Tween-20/1:100 protease inhibitor cocktail for 30 minutes on ice while vortexing every five minutes. Lysates were spun down at 18,000×g for 20 minutes at 4 degrees. IgG in sample supernatants was then captured in triplicate with bovine anti-human IgG (H+L) plated at 10ug/mL in the wells of a microtiter plate. After washing ×3 with TBS/0.1% Tween-20, AP-labeled goat anti-human IgG immobilized with the captured human IgG was detected with pNPP (Zymed/Invitrogen Life Technologies) in diethanolamine substrate buffer.
Human research approval
Studies involving human subjects were approved by the Institutional Review Board of the BloodCenter of Wisconsin.
RESULTS
A full-length Loop 1 construct is insufficient for detection of Type 2 HNA-3a antibodies
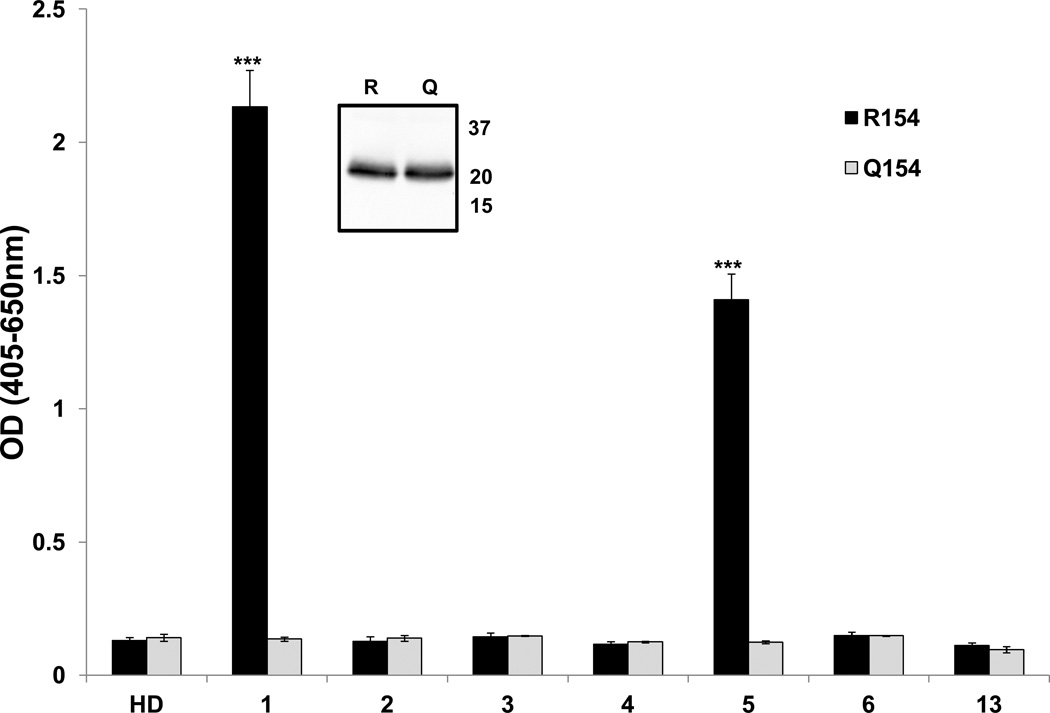
As noted, peptides containing R154 from the first extracellular (EC) loop of the HNA-3a allele of CTL2 are recognized by Type 1, but not Type 2 HNA-3a antibodies. To determine whether a longer segment of the first EC loop might be recognized by both types of antibody, a recombinant construct comprising almost the entire loop (amino acids 56–197) was tested against the antibody panel. As shown in Figure 1, this target was recognized only by the Type 1 antibodies 1 and 5. Similar findings were described recently by Wozniak et al23.
Figure 1. A soluble recombinant (sr) construct comprising extracellular Loop1 of CTL2 is recognized only by Type 1 HNA-3a antibodies.
Sr Loop 1 constructs containing R154 (black bars) or Q154 (gray bars) were immobilized in microtiter plates and their reactions with HNA-3a antibodies were characterized by ELISA. Only Type 1 antibodies reacted with the R154 construct. No antibodies recognized the Q154 construct. Studies were done in triplicate. Error bars represent ± 1.0 SD; *** = p<0.001 relative to the reaction with the Q154 construct. HD=Healthy donor serum. A Western blot performed with anti-myc (mAb 9E10) showed that equal quantities of the two constructs were tested (insert).
The epitope recognized by Type 2 HNA-3a antibodies is disrupted by detergent solubilization
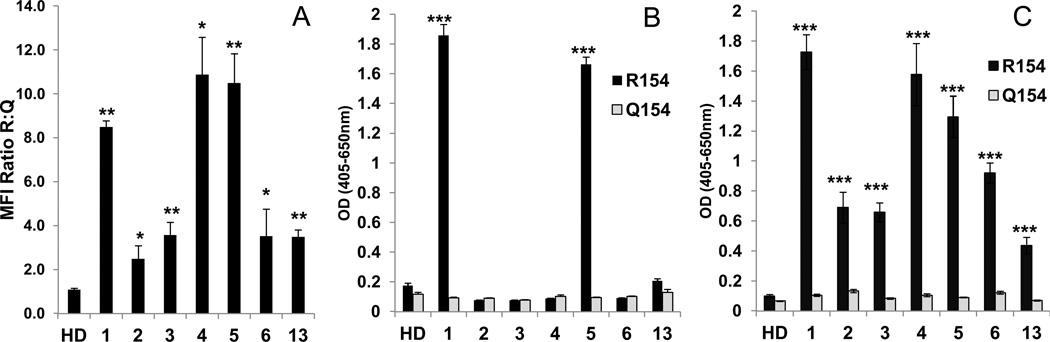
When tested against full-length CTL2 expressed in HEK293 cells, all seven members of the antibody panel recognized CTL2 (R154) but not CTL2 (Q154) using flow cytometry for detection of cell-bound antibodies (Figure 2A). When the cells were sensitized with antibody, washed and solubilized and CTL2 was captured with anti-myc in microtiter tray wells, only Type 1 antibodies (1 and 5) could be detected bound to the captured protein (Figure 2B). The cells were then sensitized with the HNA-3a antibodies, washed, and cell bound antibody was marked with alkaline-phosphatase (AP)-labeled goat anti-human IgG. The cells were then lysed with detergent and centrifuged and human IgG present in the supernatants was captured with bovine anti-human IgG. The AP tag, captured with the human IgG, was detected by ELISA. As shown in Figure 2C, all seven HNA-3a antibodies were identified in lysates prepared from cells expressing CTL2 (R154). The findings indicate that both Type 1 and Type 2 HNA-3a antibodies recognize full-length recombinant CTL2 (R154) expressed on the cell surface and survive serial washes in buffer. However, detergent solubilization perturbs CTL2 structure in such a way that Type 2 HNA-3a antibodies (but not Type 1) are disengaged from their target.
Figure 2. Detergent solubilization of recombinant CTL2 disrupts the epitope recognized by Type 2 antibodies.
A) HEK293 cells expressing full-length CTL2 (R154) were recognized by both Type 1 (1, 5) and Type 2 (2–4, 6, 13) HNA-3a antibodies in flow cytometry; values on the ordinate indicate median fluorescence intensity (MFI) relative to the signal obtained with cells expressing CTL2 Q154 (HPA-3a-negative). B) HEK293 cells expressing CTL2 were sensitized with the HNA-3a antibody panel and lysed with detergent. CTL2 was then captured with anti-myc monoclonal 9E10 and CTL2-bound IgG was detected with ELISA. Only Type 1 antibodies (1, 5) were detected with CTL2 (R154) (black bars). No antibodies recognized CTL2 (Q154) (gray bars). C) Same as Figure 3B except that cell bound antibody was marked with alkaline phosphatase (AP)-labeled goat anti-human IgG (H + L) before lysis. Released IgG was then captured with bovine anti-human IgG (Fc specific) and AP was measured by ELISA. All seven HNA-3a antibodies were detected (black bars); no antibodies were detected when cells expressing CTL2 (Q154) were used (gray bars). Studies were done in triplicate. Error bars represent ± 1.0 SD. *** = p<0.001, ** = p<0.01, * = p<0.05 relative to reaction with the Q154 construct. HD=Healthy donor serum.
Type 1, but not Type 2 HNA-3a antibodies recognize full-length mouse CTL2
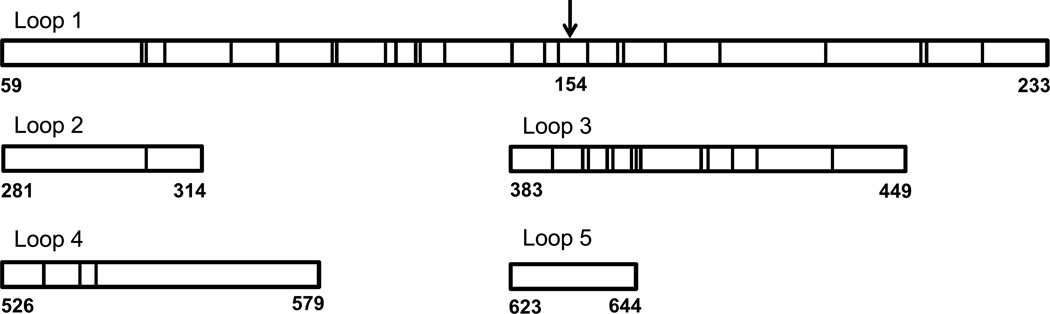
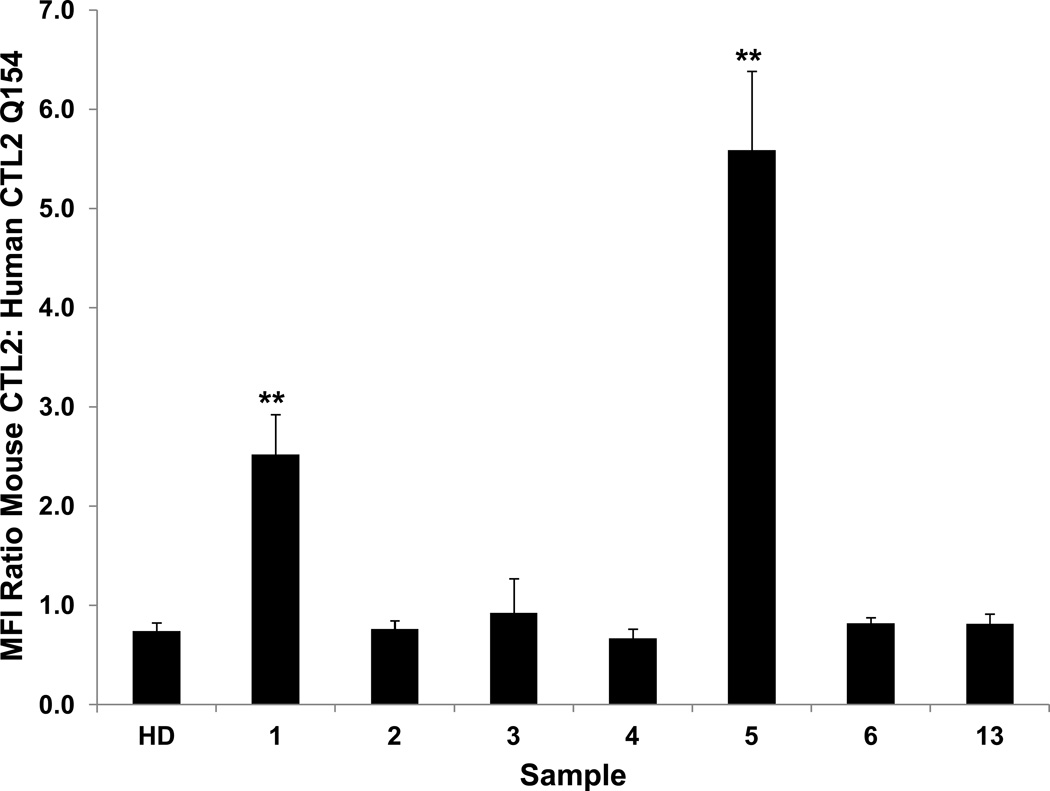
Full-length human and mouse CTL2 (reference mRNA sequences NM_001199186.1 and NM_001145056.1, respectively) are 91% identical and 94% homologous (http://imed.med.ucm.es/Tools/sias.html). The five predicted extracellular domains are 88% identical (Figure 3). Both human and mouse CTL2 possess arginine 154, the amino acid critical for recognition of human CTL2 by anti-HNA-3a. When full-length mouse CTL2 (mCTL2) was expressed in HEK293 cells and tested against the HNA-3a antibody panel (Figure 4), it was found that the Type 1 HNA-3a antibodies 1 and 5 reacted with the full-length mouse protein but the five Type 2 antibodies did not. The findings indicate that R154 in mouse CTL2 is sufficient to detect Type 1 but not Type 2 HNA-3a antibodies.
Figure 3. Comparison of the extracellular loops of human and mouse CTL2.
R154, present in both species, is indicated by the arrow. Vertical lines indicate sites at which human and mouse CTL2 sequence differs. In extracellular loops 2 and 3, human and mouse CTL2 differ by one and 13 residues, respectively. Beginning and ending amino acid residues are numbered at the ends of each loop.
Figure 4. Recombinant mouse CTL2 (mCTL2) is recognized only by Type 1 HNA-3a antibodies.
HEK293 cells expressing mCLT2 were sensitized with the HNA-3a antibody panel and washed. Bound human antibody was detected by flow cytometry. Values on the ordinate indicate ratio of median fluorescence intensity (MFI) obtained with cells expressing mouse CTL2 to that obtained with cells expressing human CTL2 (Q154) (not shown). Mouse CTL2 was recognized only by the Type 1 HNA-3a antibodies 1 and 5. Studies were done in triplicate. Error bars represent ± 1.0 SD, ** = p<0.01 relative to reaction with human CTL2 Q154. HD=Healthy donor serum.
Incorporation of human CTL2 extracellular (EC) Loops 2–5 into mCTL2 reconstitutes the epitope recognized by most Type 2 HNA-3a antibodies
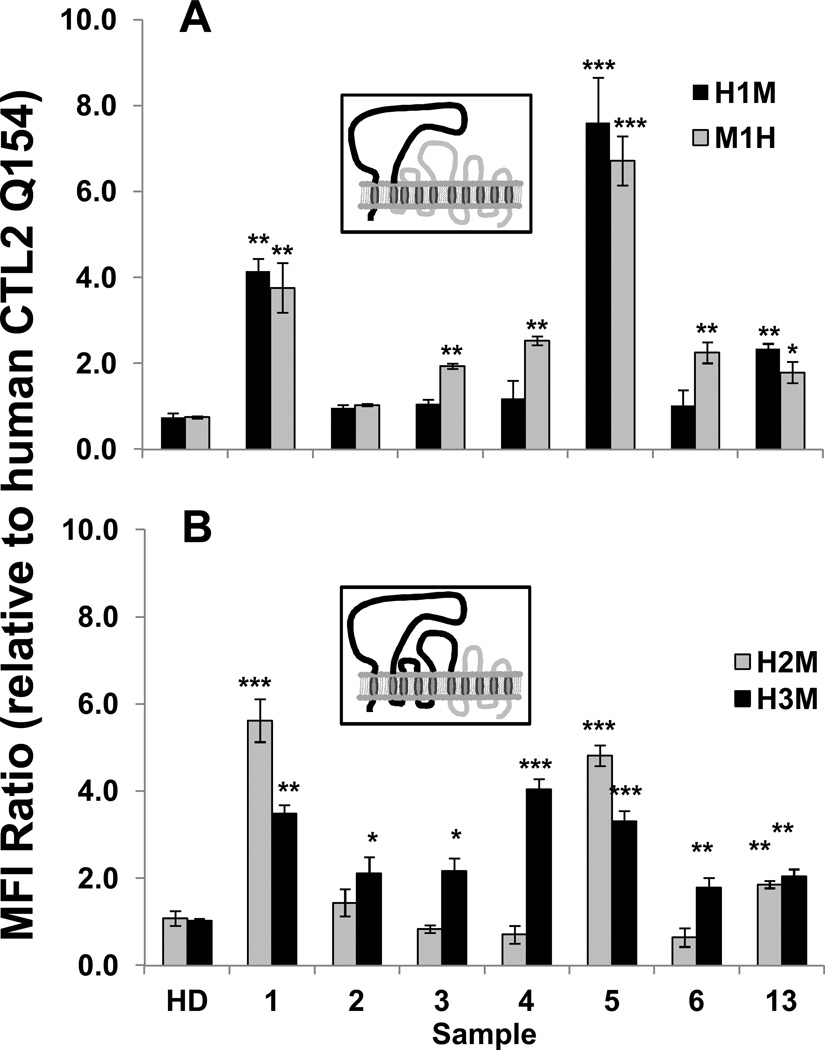
Recombinant CTL2 constructs (R154) consisting of human sequence through the second transmembrane domain (Loop 1), remainder mouse (H1M) and the reciprocal construct (M1H) were expressed in HEK293 cells and tested with the antibody panel. As shown in Figure 5A, the Type 1 antibodies 1 and 5 reacted strongly with H1M and Type 2 antibody 13 reacted weakly but other Type 2 antibodies were non-reactive. In contrast, the M1H chimera was recognized by each member of the panel except for the Type 2 antibody 2.
Figure 5. HNA-3a antibody binding to chimeric (human/mouse) CTL2 (R154).
A) A construct containing human extracellular Loop 1, remainder mouse (H1M black bars, depicted in the insert) was recognized only by Type 1 antibodies 1 and 5 and (weakly) by Type 2 antibody 13. The reciprocal construct (M1H, gray bars) reacted with all antibodies except Type 2 antibody 2; B) A construct containing human extracellular Loops 1 and 2, remainder mouse (H2M, gray bars) reacted only with Type 1 antibodies 1 and 5 and (weakly) with Type 2 antibody 13. However, construct H3M (black bars, depicted in the insert) comprising human Loops 1–3, remainder mouse, was recognized by all seven members of the antibody panel. Values on the ordinate indicate ratio of the median fluorescence intensity (MFI) signal obtained with chimeric protein to that obtained with cells expressing human CTL2 (Q154) (not shown). Studies were done in triplicate. Error bars represent ± 1.0 SD, *** = p<0.001, ** = p<0.01 relative to reaction with human CTL2 (Q154). HD=Healthy donor serum.
Human sequence in EC Loops 1–3 of CTL2 is sufficient for recognition by all members of the HNA-3 antibody panel
Recombinant CTL2 constructs comprising of human EC Loops 1 and 2, remainder mouse (H2M) and human EC Loops 1–3, remainder mouse (H3M) were similarly expressed in HEK293 cells and tested with the antibody panel. Mouse and human CTL2 differ by only one amino acid in Loop 2 and by 13 amino acids in Loop 3 (Figure 3). As shown in Figure 5B, H2M was recognized only by Type 1 antibodies 1 and 5 and less well by the Type 2 antibody 13. However, all seven members of the antibody panel reacted with H3M
DISCUSSION
The HNA-3a/b antigens were originally identified by J van Rood and co-workers in 1964 as a diallelic system designated “5b/5a” expressed on neutrophils and possibly other tissues29, but identification of the HNA-3a/b carrier protein as CTL2 and recognition that an R154Q amino acid polymorphism is essential for HNA-3a/b antigen expression was achieved only recently14,15. Recognition of the molecular basis for HNA-3a/b raised the hope that fragments of CTL2 derived from the first extracellular loop where R154Q is located could be used in solid phase assays to identify HNA-3-specific antibodies in blood donors. However, studies by several groups showed that only about half of the HNA-3a antibodies identified in persons whose blood was implicated in a TRALI reaction can be detected by this approach21–23. Studies described here were conducted to define binding requirements for HNA-3a antibodies (here designated “Type 2”) that fail to recognize peptides derived from the first extracellular (EC) loop of CTL2.
Findings made show that a peptide comprising the entire first extracellular loop of the HNA-3a version of CTL2 is insufficient to permit detection of Type 2 antibodies (Figure 1), a finding also made recently by Wozniak et al23. EC Loop 1 is predicted to be N-glycosylated at several asparagine residues16,17. Wozniak et al showed that a soluble recombinant Loop 1 construct expressed in HEK293 cells does possess glycan residues23, arguing against the possibility that glycans are required for Type 2 antibody binding. Our finding that binding of Type 2, but not Type 1 HNA-3a antibodies is disrupted by detergent solubilization of CTL2 suggests that Type 2 antibodies may require the target protein to be a configuration comparable to the one assumed naturally in the cell membrane and raises the possibility that CTL2 structures outside of Loop 1 could be required for binding.
Mouse CTL2, is closely homologous to human CTL2 and possesses the R154 residue critical for human HNA-3a antibody binding (Figure 3). Studies with HEK293 cells expressing mouse CTL2, showed that the mouse protein was recognized only by Type 1 members of the antibody panel. This made it possible to use chimeric (human/mouse) CTL2 constructs to define the minimum amount of human sequence required for Type 2 antibody binding. Findings showed that a construct containing human Loop 1, remainder mouse (H1M) reacted strongly with Type 1 antibodies and weakly with the Type 2 antibody 13, but was not recognized by Type 2 antibodies 2–4 or 6. However, the reciprocal construct containing mouse Loop 1, remainder human (M1H) reacted with all members of the panel except Type 2 antibody number 2. A second set of chimeric proteins containing human Loops 1 and 2 (H2M) and Loops 1–3 (H3M) was then expressed and tested against the antibody panel. Reactions of the H2M construct were similar to those of H1M. However, the H3M construct reacted with all seven antibodies.
Together, these findings argue that, for effective binding of Type 2 HNA-3 antibodies to CTL2 (R154), two requirements must to be met: 1) the protein must be in a conformation comparable to the one assumed naturally in the cell membrane and 2) human amino acid sequence must be present in Loop 3 (and possibly Loop 2). This is in distinct contrast to Type 1 antibodies, which require only Loop 1 for binding and even recognize shorter peptides containing R15421–23. All HNA-3a antibodies may, of course, not fit neatly into these two categories. Even studies with our limited antibody panel suggest that the Type 2 antibodies 2 and 13 differ slightly from antibodies 3, 4 and 6 in that antibody 2 failed to recognize the M1H construct and may therefore be sensitive to one of several amino acids at which the human and mouse sequences differ in Loop 1 close to R154. Similarly, antibody 13 was atypical in reacting weakly with the H1M and H2M chimeric proteins. Possibly, antibody 13 recognizes amino acids residues in Loops 2 and/or 3 that are shared between human and mouse but requires all-human sequence in Loop 1. It will not be surprising if further studies of HNA-3a antibody fine specificity reveal additional heterogeneity.
In EC Loops 2 and 3, sequences of human and mouse CTL2 differ at one and 13 amino acid residues, respectively (Figure 3). The simplest explanation for the behavior of Type 2 antibodies is that, in addition to recognizing R154 in Loop 1, they require direct contact with non-polymorphic amino acid residues in Loop 3 (and possibly Loop 2) of human CTL2 for tight binding. An alternative possibility is that CTL2 Loops 2 and 3 stabilize Loop 1 in a conformation optimal for Type 2 antibody recognition. That seems unlikely, however, in view of the failure of Type 2 antibodies to recognize mouse CTL2, the H1M chimera, and the H2M chimera despite the fact that Loops 2 and 3 of human and mouse CTL2 are exactly the same length and are closely homologous in amino acid composition. The suggestion that an alloantibody specific for an epitope on an extracellular loop of a protein with multiple transmembrane domains may require amino acid residues on an adjacent loop for effective binding is not unprecedented. For example, studies of the 12-membrane-spanning RhD protein have provided evidence that some Rh-specific alloantibodies may require amino acid residues on up to four extracellular loops for recognition of this target30–32.
Regardless of the molecular basis for differing serologic behaviors of Type 1 and Type 2 antibodies, findings described here indicate that detection of antibodies with Type 2 serologic behavior, comprising about half of all HNA-3a antibodies, will require a target consisting of at least the first three extracellular loops of CTL2 and perhaps even the full-length protein, in a configuration comparable to its native state in the cell membrane. Achievement of this objective is likely to present a serious technical challenge.
Acknowledgments
The authors are grateful to Hope Campbell of the Blood Research Institute’s Flow Cytometry core lab for her assistance with cell sorting.
Supported by grants HL-106286 and HL-13629 from the National Heart Lung and Blood Institute.
Footnotes
Conflict of interest disclosure: the authors report no conflicts of interest
Author contributions: DWB, JAP and RHA designed research, interpreted data and wrote the manuscript. DWB and AJK performed experiments and analyzed data. BRC provided vital patient material.
References
- 1.Muschter S, Berthold T, Greinacher A. Developments in the definition and clinical impact of human neutrophil antigens. Curr Opin Hematol. 2011;18:452–460. doi: 10.1097/MOH.0b013e32834babdd. [DOI] [PubMed] [Google Scholar]
- 2.Popovsky MA. Transfusion-related acute lung injury. Transfusion. 1995;35:180–181. [PubMed] [Google Scholar]
- 3.Reil A, Keller-Stanislawski B, Gunay S, Bux J. Specificities of leucocyte alloantibodies in transfusion-related acute lung injury and results of leucocyte antibody screening of blood donors. Vox Sang. 2008;95:313–317. doi: 10.1111/j.1423-0410.2008.01092.x. [DOI] [PubMed] [Google Scholar]
- 4.Kopko PM, Paglieroni TG, Popovsky MA, et al. TRALI: correlation of antigen-antibody and monocyte activation in donor-recipient pairs. Transfusion. 2003;43:177–184. doi: 10.1046/j.1537-2995.2003.00307.x. [DOI] [PubMed] [Google Scholar]
- 5.Silliman CC, Fung YL, Ball JB, Khan SY. Transfusion-related acute lung injury (TRALI): current concepts and misconceptions. Blood Rev. 2009;23:245–255. doi: 10.1016/j.blre.2009.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Toy P, Gajic O, Bacchetti P, et al. Transfusion-related acute lung injury: incidence and risk factors. Blood. 2012;119:1757–1767. doi: 10.1182/blood-2011-08-370932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eder AF, Dy BA, Perez JM, et al. The residual risk of transfusion-related acute lung injury at the American Red Cross (2008–2011): limitations of a predominantly male-donor plasma mitigation strategy. Transfusion. 2013;53:1442–1449. doi: 10.1111/j.1537-2995.2012.03935.x. [DOI] [PubMed] [Google Scholar]
- 8.Kanai R, Iijima T, Hashimoto S, et al. Impact of immunoreactive substances contained in apheresis platelet concentrate on postoperative respiratory function in surgical patients receiving platelet transfusion: a prospective cohort study. Transfus Med. 2013 doi: 10.1111/tme.12056. [DOI] [PubMed] [Google Scholar]
- 9.Kopko PM, Marshall CS, MacKenzie MR, et al. Transfusion-related acute lung injury: report of a clinical look-back investigation. JAMA. 2002;287:1968–1971. doi: 10.1001/jama.287.15.1968. [DOI] [PubMed] [Google Scholar]
- 10.Davoren A, Curtis BR, Shulman IA, et al. TRALI due to granulocyte-agglutinating human neutrophil antigen-3a (5b) alloantibodies in donor plasma: a report of 2 fatalities. Transfusion. 2003;43:641–645. doi: 10.1046/j.1537-2995.2003.00374.x. [DOI] [PubMed] [Google Scholar]
- 11.Thomas GM, Carbo C, Curtis BR, et al. Extracellular DNA traps are associated with the pathogenesis of TRALI in humans and mice. Blood. 2012;119:6335–6343. doi: 10.1182/blood-2012-01-405183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schubert N, Berthold T, Muschter S, et al. Human neutrophil antigen-3a antibodies induce neutrophil aggregation in a plasma-free medium. Blood Transfus. 2013:1–7. doi: 10.2450/2013.0294-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bayat B, Tjahjono Y, Sydykov A, et al. Anti-human neutrophil antigen-3a induced transfusion-related acute lung injury in mice by direct disturbance of lung endothelial cells. Arterioscler Thromb Vasc Biol. 2013;33:2538–2548. doi: 10.1161/ATVBAHA.113.301206. [DOI] [PubMed] [Google Scholar]
- 14.Curtis BR, Cox NJ, Sullivan MJ, et al. The neutrophil alloantigen HNA-3a (5b) is located on choline transporter-like protein 2 and appears to be encoded by an R>Q154 amino acid substitution. Blood. 2010;115:2073–2076. doi: 10.1182/blood-2009-11-248336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Greinacher A, Wesche J, Hammer E, et al. Characterization of the human neutrophil alloantigen-3a. Nat Med. 2010;16:45–48. doi: 10.1038/nm.2070. [DOI] [PubMed] [Google Scholar]
- 16.O'Regan S, Traiffort E, Ruat M, et al. An electric lobe suppressor for a yeast choline transport mutation belongs to a new family of transporter-like proteins. Proc Natl Acad Sci U S A. 2000;97:1835–1840. doi: 10.1073/pnas.030339697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nair TS, Kozma KE, Hoefling NL, et al. Identification and characterization of choline transporter-like protein 2, an inner ear glycoprotein of 68 and 72 kDa that is the target of antibody-induced hearing loss. J Neurosci. 2004;24:1772–1779. doi: 10.1523/JNEUROSCI.5063-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kommareddi PK, Nair TS, Thang LV, et al. Isoforms, expression, glycosylation, and tissue distribution of CTL2/SLC44A2. Protein J. 2010;29:417–426. doi: 10.1007/s10930-010-9268-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Traiffort E, Ruat M, O'Regan S, Meunier FM. Molecular characterization of the family of choline transporter-like proteins and their splice variants. J Neurochem. 2005;92:1116–1125. doi: 10.1111/j.1471-4159.2004.02962.x. [DOI] [PubMed] [Google Scholar]
- 20.Flesch BK, Wesche J, Berthold T, et al. Expression of the CTL2 transcript variants in human peripheral blood cells and human tissues. Transfusion. 2013 doi: 10.1111/trf.12160. [DOI] [PubMed] [Google Scholar]
- 21.Curtis BR, Sullivan MJ, Holyst MT. HNA-3a-specific antibodies recognize choline transporter-like protein-2 peptides containing arginine, but not glutamine at Position 154. Transfusion. 2011 doi: 10.1111/j.1537-2995.2011.03145.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Berthold T, Wesche J, Kuhnert K, et al. Epitope mapping of antibodies directed against the human neutrophil alloantigen 3a. Transfusion. 2011;51:2160–2167. doi: 10.1111/j.1537-2995.2011.03115.x. [DOI] [PubMed] [Google Scholar]
- 23.Wozniak MJ, Bowring C, Lucas G, Ridgwell K. Detection of HNA-3a and-3b antibodies using transfected cell lines and recombinant proteins. Transfusion. 2012;52:1458–1467. doi: 10.1111/j.1537-2995.2011.03490.x. [DOI] [PubMed] [Google Scholar]
- 24.Kanack AJ, Peterson JA, Sullivan MJ, et al. Full-length recombinant choline transporter-like protein 2 containing arginine 154 reconstitutes the epitope recognized by HNA-3a antibodies. Transfusion. 2012;52:1112–1116. doi: 10.1111/j.1537-2995.2011.03411.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bayat B, Tjahjono Y, Werth S, et al. Implication of transfected cell lines for the detection of alloantibodies against human neutrophil antigen-3. Transfusion. 2012;52:613–621. doi: 10.1111/j.1537-2995.2011.03303.x. [DOI] [PubMed] [Google Scholar]
- 26.Peterson JA, Nelson TN, Kanack AJ, Aster RH. Fine specificity of drug-dependent antibodies reactive with a restricted domain of platelet GPIIIA. Blood. 2008;111:1234–1239. doi: 10.1182/blood-2007-09-112680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bougie DW, Birenbaum J, Rasmussen M, et al. Quinine-dependent, platelet-reactive monoclonals mimic antibodies found in patients with quinine-induced immune thrombocytopenia. Blood. 2009;113:1105–1111. doi: 10.1182/blood-2008-09-177279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peterson JA, Pechauer SM, Gitter ML, et al. New platelet glycoprotein polymorphisms causing maternal immunization and neonatal alloimmune thrombocytopenia. Transfusion. 2012;52:1117–1124. doi: 10.1111/j.1537-2995.2011.03428.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Van Leeuwen A, Eernisse JG, Van Rood JJ. A NEW LEUCOCYTE GROUP WITH TWO ALLELES: LEUCOCYTE GROUP FIVE. Vox Sang. 1964;9:431–446. doi: 10.1111/j.1423-0410.1964.tb03311.x. [DOI] [PubMed] [Google Scholar]
- 30.Avent ND, Madgett TE, Lee ZE, et al. Molecular biology of Rh proteins and relevance to molecular medicine. Expert Rev Mol Med. 2006;8:1–20. doi: 10.1017/S1462399406010969. [DOI] [PubMed] [Google Scholar]
- 31.Chang TY, Siegel DL. Genetic and immunological properties of phage-displayed human anti-Rh(D) antibodies: implications for Rh(D) epitope topology. Blood. 1998;91:3066–3078. [PubMed] [Google Scholar]
- 32.Liu W, Avent ND, Jones JW, et al. Molecular configuration of Rh D epitopes as defined by site-directed mutagenesis and expression of mutant Rh constructs in K562 erythroleukemia cells. Blood. 1999;94:3986–3996. [PubMed] [Google Scholar]