Abstract
• Background and aims Much recent study of plant trichomes has focused on various aspects of glandular secreting trichomes (GSTs) and differentiation of simple trichomes. This Botanical Briefing will highlight:
‐ research on various aspects of, and manipulation of glandular secreting trichomes;
‐ molecular aspects of the differentiation and development of simple trichomes of arabidopsis and cotton;
‐ how methods for manipulation of model systems used in the above work can be applied to expand our understanding of less studied surface structures of plants.
• Scope The Briefing will cover:
‐ established and suggested roles of simple and glandular secreting trichomes;
‐ recent results regarding solute and ion movement in trichomes;
‐ methods for isolating trichomes;
‐ recent studies of trichome differentiation and development;
‐ attempts to modify metabolism in secreting trichomes;
‐ efforts to exploit trichomes for commercial and agronomic purposes.
Keywords: Plant secretion, simple trichomes, glandular trichomes, secreted phytochemicals, arabidopsis, tobacco, gene library, pest resistance, molecular farming, trichome differentiation, trichome functions
INTRODUCTION
Considerable recent research has focused on glandular secreting trichomes (GSTs) to understand and exploit their ability to secrete phytochemicals that might improve resistance to insects, microbes and herbivores, modify gland metabolism towards improving properties of exudates (e.g. flavour and aroma in herbs) and allow commercial production of useful compounds (molecular farming). Plant sources are increasingly being exploited for new drug development, and there is increased interest in validating traditional medicines and herbal remedies (Verpoorte, 2000). Many of the phytochemical principals involved are surface secretions.
Simple non‐glandular trichomes (e.g. those of Arabidopsis thaliana and cotton) currently represent useful systems for studying cellular differentiation and development at the molecular level. This Botanical Briefing will highlight recent research in these areas and suggest how methods and approaches now being applied to these major goals have potential for expanding our understanding of other functions of trichomes and other surface protuberances, for which there is, as yet, little direct evidence. Except for inclusion of a few notes, the cellular basis of GST secretion will not be considered here (see Fahn, 2000; Werker, 2000). No attempt will be made to review the vast literature on trichome and trichome exudate interactions with particular organisms [but see Thomson and Healey (1984), Kronestedt‐Robards and Robards (1991) and Wagner (1991) for the former, and Kelsey et al. (1984), Bennett and Wallsgrove (1994), Duke (1999), Berenbaum (1995) and Kessler and Baldwin (2002) for the latter].
TRICHOMES AND OTHER SURFACE APPENDAGES OF PLANTS: GENERAL ASPECTS
Trichomes were among the first anatomical features of plants to be recognized by early microscopists, and they have played a key role in plant taxonomy (see Behnke, 1984). Trichomes have been defined as epidermal protuberances that have distinguishing height/width ratios (see Behnke, 1984), although this criterion is not wholly suitable for classification of the many types of plant surface appendages. For the purposes of this Botanical Briefing, trichomes are defined as two general types of relatively high height/width, stalked protuberances: ‘simple’ or non‐glandular (presumably non‐secreting, but see below) and ‘glandular‐secreting’ (GST) types (Fig. 1A). The latter often produce copious secretions to the plant surface, outside the cuticle, or stored within glands. Morphology of both of these trichome types, as found on different plants, varies greatly. Specialized salt glands, lacticifers, nectaries, surface resin ducts and digestive glands (Uphof, 1962; Dell and McComb, 1978; Fahn, 2000; Kronestedt‐Robards and Robards, 1991; Werker, 2000) are other secretory structures that will, for the most part, not be discussed here. These are perhaps less well characterized than GSTs. Stalked hydathodes (Fig. 1B), sometimes referred to as trichomes, or trichome hydathodes, differ somewhat from simple trichomes and GSTs in morphology and, as with all hydathodes, appear to have a more direct connection to the vascular system than do simple trichomes or GSTs. Recent evidence for this comes from studies in arabidopsis where intense production of auxin in developing leaf hydathodes was correlated with differentiation of midveins and secondary vascular bundles (Aloni et al., 2003).
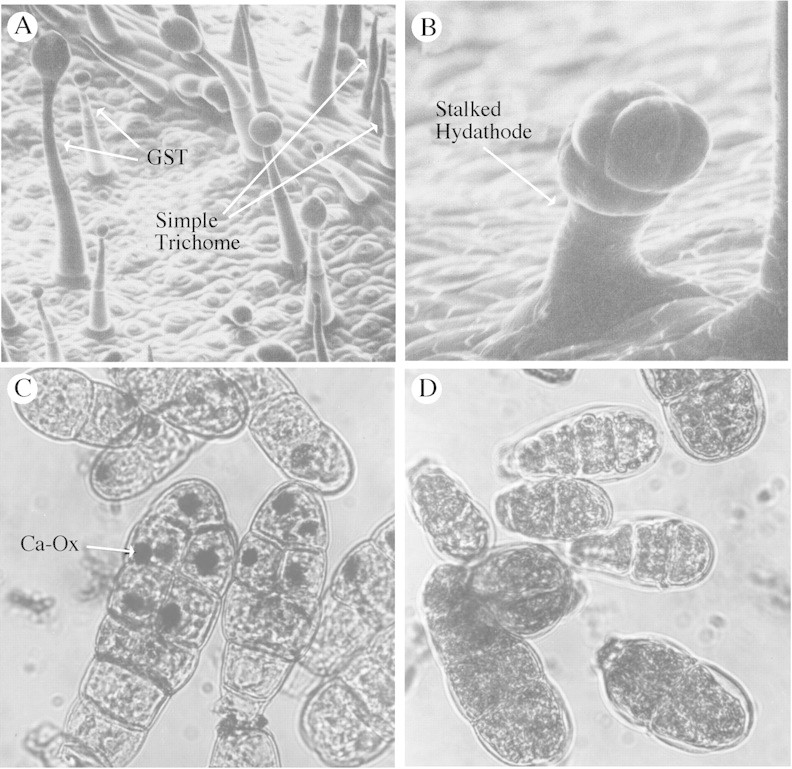
Fig. 1. A, Nicotiana tabacum leaf surface showing exudate laden GSTs and simple trichomes ×110. B, N. tabacum hydathode ×700. C, N. tabacum GST glands with exudate removed, pretreated with 5 % HOAc then silver nitrate–rubeanic acid to stain calcium oxalate crystals ×560. D, N. tabacum GST glands, treated as in C, but first pretreated with 0·25 m HCl to disolve calcium oxalate ×560. C and D by Vogeli‐Lange and Wagner, unpublished.
Simple trichomes are present on aerial surfaces of most angiosperms and on some gymnosperms and bryophytes (Uphof, 1962; Johnson, 1975). In angiosperms, trichomes may occur on leaves, petals, stems, petioles, peduncles and seed coats, depending on the species. GSTs are found on perhaps 30 % of vascular plants (Dell and McComb, 1978; Fahn, 2000). The amount of exudate produced by GSTs may reach 30 % of mature leaf dry weight, as found in certain Australian desert plants (Dell and McComb, 1978). In Nicotiana tabacum cultivars, trichome exudate varies from approx. 0·3 to >17 % of leaf dry weight, and amounts are influenced by environmental conditions (Severson et al., 1985).
ROLES OF SIMPLE TRICHOMES AND GSTs
Simple trichomes serve the plant, and humans, in many ways. The morphological and mechanical features (density, size, shape, surface texture, hair orientation) of trichomes can influence many aspects of plant physiology and ecology (Table 1). Simple trichomes (and GSTs) can form at various stages of organ development, and some senesce (then are or are not shed) before the organ reaches maturity, while others remain until plant senescence. Dead trichomes may continue to function in water absorption, seed dispersal and abrasion protection (Werker, 2000). Hairs may serve to protect buds of some plants until defence phytochemicals are produced (Johnson, 1975). Thus, although simple trichomes are not known to accumulate pest‐deterrent phytochemicals, their importance to plants and humans should not be underestimated. Regarding secretion potential, little direct evidence exists that they are indeed incapable of low‐level secretion. This aspect might now be investigated using Northern analysis and in situ hybridization to determine if trichome‐specific genes for GST biosynthesis are expressed in simple trichomes (Wang et al., 2002). The importance of simple trichomes to humans is well illustrated by cotton. Simple trichomes of cotton ovules (cotton fibres, which aid seed dispersal) are exploited more by humans than any single medicinal phytochemical or spice principal.
Table 1.
Apparent functions of simple trichomes, GSTs and hydathode trichomes
| Apparent functions | Trichome type* | Reference |
| Reduced insect movement | ST and GST | Levin, 1973; Johnson, 1975; Kessler, 2002; Kennedy, 2003 |
| Temperature regulation | ST and GST | Johnson, 1975; Dell and McComb, 1978; Ehleringer, 1984 |
| Increased light reflectance (including UV) | ST and GST | Ehleringer, 1984 |
| Decreased water loss through reflection | ST and GST | Ehlringer, 1984 |
| Reduced mechanical abrasion | ST and GST | Uphof, 1962; Johnson, 1975 |
| Protection of phylloplane organisms | ST and GST | Beattie and Lindow, 1999 |
| Reduced leaf wetness | ST and GST | Brewer and Smith, 1997 |
| Reduced photosynthesis thru reflectance | ST and GST | Ehleringer, 1984 |
| Seed dispersal and establishment | ST and GST | Uphof, 1962; Werker, 2000 |
| Epidermal Ca++ homeostasis | ST and GST | DeSilva et al., 2001 |
| Pollen collection and dispersal | ST and GST | Uphof, 1962; Werker, 2000 |
| Provide insect and herbivore deterrence | GST | Johnson, 1975; Kelsey et al., 1984;Wollenweber, 1984;Bennett and Wallsgrove, 1994; Berenbaum, 1995 |
| Insect immobilization | GST | Kennedy, 2003 |
| Fungal and bacterial toxicity/nutrition | GST | Johnson, 1975; Behnke, 1984 |
| Pollinator attraction | GST | Werker, 2000 |
| Ion and pollutant metal secretion | GST | Salt et al., 1995 Choi et al., 2001; Kupper et al., 2000 |
| Water retention and decreased desiccation in seeds | GST | Werker, 2000 |
| Allelopathy | GST | Macias et al., 1999; Werker, 2000 |
| Assist in climbing, and seed establishment | ST | Uphof, 1962; Werker, 2000 |
| Absorb water and nutrients | ST | Uphof, 1962; Sakai et al., 1980; Werker, 2000 |
| Guide pollinators | ST | Johnson, 1975 |
| Water and ion secretion | HT | Johnson, 1975; Aloni et al., 2003 |
* ST, simple trichomes; GST, glandular secreting trichomes; HT, hydathode trichomes.
GSTs, like simple trichomes, can influence plant functions by virtue of their physical properties (size, density). However, in addition, GSTs can affect host disease and pest resistance based on phytochemicals they secrete (Table 1). As an example of a multifunctional nature, GSTs of tobaccos that can produce up to 17 % of leaf dry weight as exudate (under normal growth conditions), can accumulate large calcium oxalate crystals in their glands (Fig. 1C and D) as well as providing the physiological consequences of a substantial protuberance.
SOLUTE AND ION MOVEMENT IN TRICHOMES
Although simple trichomes and GSTs (also specialized salt glands and nectaries) are generally not directly connected to the vascular system, they are the outermost aerial structures of many tissues. Recent evidence from fluorescent dye and dextran microinjection studies (Waigmann and Zambryski, 2000) suggests that solute movement through the stalk of GSTs is facilitated, presumably to supply adequate precursor carbon for massive exudate synthesis in the gland and to facilitate ion secretion. Stalk cells of chickpea GSTs are shown to have an extensive ‘vacuolar–tubular continuum’ to facilitate transport from the base to the gland. Trichome stalk cell plasmodesmata of Nicotiana clevelandii have an open desmotubule lumen (in contrast to mesophyll cells) suggesting an enhanced passageway for solute transport (Lazzaro and Thomson, 1996). In nectaries, large numbers of plasmodesmata appear to facilitate high solute flux to the tip. Glands of some GSTs contain chloroplasts while others do not (Wagner, 1991). Though isolated, photosynthetically competent gland cells of tobacco GSTs can convert bicarbonate to complex secreted phytochemicals: it is likely in situ that these glands also depend primarily on carbon supplied from the epidermis for massive exudate production (Wagner, 1991).
It is well known that glands of GSTs accumulate within, and secrete to, the gland surface elements including Na and Cl (salt glands), Ca, Cd, Zn, Mn, Ni, Pb, S, Si and others (Uphof, 1962; Salt et al., 1995; Kupper et al., 2000; Choi et al., 2001). Salts containing these elements may form crystals on the GST gland surface. The mechanism that releases primin from stinging nettle GSTs depends on the brittleness of the GST tip, which is due to the presence of silica in its cell wall. Crystals containing Ca and Cd are found on the outer surfaces of both long glandular and short (hydathode) trichomes of tobacco after exposure to high Cd (Choi et al., 2001). Enrichment in expression of plant metallothionein‐like genes has been shown in arabidopsis and Vicia trichomes (see Gutierrez‐Alcala et al., 2000). Sulphur metabolism is at the basis of Cd and Zn chelation in plants, being the element for coordination of these ions in glutathione, phytochelatins and metallothioneins. Glutathione levels are higher in arabidopsis trichomes than in epidermal cells, and several enzymes related to glutathione synthesis are enriched in trichomes (Gutierrez‐Alcala et al., 2000). These observations are consistent with the role of GSTs, as well as hydathodes, in removal of excess ions from plants. Trichomes may regulate apoplastic calcium by removing it to the surface, thereby preventing high Ca accumulation near stomata and assisting in stomatal homeostasis (DeSilva et al., 2001). Similar to ions, secreted secondary compounds are positioned to be leached to the soil, in some cases for the purpose of contributing to allelopathy (Macias et al., 1999). Thus there are many recognized roles of simple trichomes and GSTs. In some cases, the evidence for these functions is largely descriptive and correlative, yet logical and compelling. As will be described below, modern experimental approaches can help to substantiate these proposed functions.
ASPECTS OF GST EXUDATE CHEMISTRY AND DISPOSITION
Terpenoids and phenylpropanoids (and allied phenolics) are often secreted by GSTs, in a species‐ and cultivar‐specific fashion. Apparently, alkaloids, the third major class of plant secondary compounds, are not common in GST exudates. Nicotine, sometimes cited as a GST product, is formed in roots and translocated en masse to leaves and GSTs (Laue et al., 2000): the possibility that tobacco GST glands may form some nicotine has apparently not been tested directly. A recent report suggests that the precursor to the highly valued antineoplastic alkaloid camptothecin is accumulated in trichomes of Camptotheca acuminata (Li et al., 2002), but the single‐cell glands said to produce this compound are more like resin ducts than trichomes. Terpenoids (monoterpenes, sesquiterpenes, diterpenes and triterpenes) are clearly the most common and structurally diverse compound group found in GST exudates (Kelsey et al., 1984). Certain plants produce copious amounts of mixed‐terpenoids, such as the terpenophenolic cannabinoids of Cannabis (Mahlberg and Kim, 1992). Flavonoids occur as aglycones in trichome exudates of a number of species, sometimes as crystalline deposits (Wollenweber, 1984). The occurrence of flavonoids as aglycones is not surprising since they are lipophyllic and would be highly soluble in mixed exudates containing terpenoids. In contrast, flavonoid glycosides, as accumulated in many plants, occur in vacuoles and chromoplasts. Examples of trichome‐secreted phenolics include primin (Primula sp.), chlorogenic acid and rutin (Solanum sp.).
The accumulation of toxic compounds at the surface allows their direct contact with insects, pathogens and herbivores. Thus trichome exudates are ideally positioned to provide a first line of defence against attacking organisms, perhaps providing time for activation of induced defences. This same argument can be made for secretions of surface resin ducts, lacticifers and certain other surface‐secreting structures.
Many phytochemicals found in GST exudates participate in host defence, but plants also have inducible defences. Many plants have evolved the capability of using oligosaccharides released from plant cell walls during fungal attack to elicit defence responses. Recently a novel labdadiene‐diol diterpenoid from tobacco was shown to act as an endogenous signal that activated several wound‐inducible protein kinases, and enhanced resistance to tobacco mosaic virus infection (Seo et al., 2003). It is intriguing to question if this labdadiene‐diol is a metabolic product of the closely related labdene‐diol that is common in tobacco trichome exudates, or if the plant recruits labdane biosynthesis to form this labadiene‐diol signalling molecule.
Exudate compounds of GSTs can apparently escape cuticular containment via pores (striae) and flow out onto the epidermal plane in depressions between cells where, for example, fungal spores may collect. As shown in Fig. 2A, exudate can migrate down from the GST gland like molten wax on a candle. At the base of the trichome this exudate flows out along depressions between cells (Fig. 2B). Incidentally, amphipathic sugar esters of tobacco GSTs are known to aid in solubilization of lipophylic diterpenoids. This solubilization/wetting role is perhaps further evidenced by the behaviour of Rhodamine B stained tobacco exudate (Lin and Wagner, 1994). When it makes contact with aphids placed on a stained leaf, Rhodamine B–sugar ester conjugate rapidly coats the insect, and is found in highest concentration (yellow fluorescence) at the joints where it can penetrate to the insect interior (Fig. 2D). Subtle functions like the solubilization role just described may be important, but little recognized and appreciated.
Fig. 2. A, Nicotiana tabacum GSTs after treatment with rhodamine B to stain sucrose esters of exudates. B, Top view of rhodamine B‐treated leaf showing migration of stained exudate to the base of trichomes, then out onto the epidermal surface. C, Aphid walking on a rhodamine B‐treated leaf, note exudate on the legs. D, Fluorescence micrograph of aphid after it had walked on a rhodamine B‐treated leaf for 1 min. Yellow fluorescence denotes highest stain accumulation. Control aphids show no autofluorescence.
Methods for isolating GSTs, exudates, glands with stalks, and glands
Being surface protuberances, simple trichomes, GSTs, and their exudates are more easily accessed by mechanical means than cells or components of cells embedded in tissues within the plant. This accessibility has led to the development of several methods for removing exudates, trichomes with stalks, or glands alone. These methods include touching individual exudate droplets atop trichomes with a micro‐capillary containing a solvent that readily dissolves exudate. Although tedious, this method has the advantage of making contact only around glands, and therefore yields potentially pure exudate. A related, but less precise, method is to remove glands with exudate using, for example, forceps. The most efficient method to remove exudates is to wash surfaces with an organic solvent. This approach can quickly provide complete removal of large quantities of exudate, allows for efficient estimation of the exudate amount on a surface area basis, but can also access non‐trichome cuticular components. Rapid rinsing with methylene chloride or acetonitrile does not appear to leach components from within the leaf, while chloroform, hexane and other non‐polar solvents may, as does steam distillation.
Trichomes, both simple and GST types, may be removed by brushing surfaces, shaking in aqueous solution with an abrasive, or freezing tissue then brushing (see McCaskill et al., 1992; Wang et al., 2001). In order to demonstrate directly that exudate phytochemicals are produced in glands and for enzyme isolation, GSTs have been prepared in two ways. The stickiness of tobacco trichome exudate has been exploited to prepare photosynthetically competent GST glands (Fig. 1C). Incubation of such preparations with NaH14CO3 in the light resulted in synthesis of radio‐labelled diterpenoids and sugar esters (Wagner, 1991). The second general procedure for preparing biosynthetically competent secretory cells of GSTs involves shaking tissues of peppermint or basil plants with glass beads at 4 °C in a complex medium (see McCaskill et al., 1992; Gang et al., 2001). While glands isolated in this manner were permeable to low molecular weight, water‐soluble molecules, they retained the metabolic machinery for synthesis of exudate phytochemicals. Therefore, general methods are now available that allow for characterizing metabolism and gene expression in glands. Some new methods for isolating secreting structures from plants will be discussed below.
Recent studies of trichome differentiation and development
Current understanding of trichome development is largely based on studies of mutants of Arabidopsis thaliana, which have revealed the importance of over 40 genes that regulate trichome development (Schwab et al., 2000). Trichomes are the first cells to differentiate from the epidermis in developing leaf primordia: protodermal cells destined to become trichomes cease to divide, while surrounding epidermal cells continue normal division (Schnittger and Hulskamp, 2002). In arabidopsis, protodermal cells undergo an average of four DNA replication cycles (endoreduplication) without completing mitosis or undergoing cytokinesis. Recent studies have revealed that the first endoreduplication cycle probably occurs before protodermal cell outgrowth is visible, the second is concomitant with the first branching event, the third with the second branching event, and the last occurs during cell expansion. ZW1, the first trichome branching gene cloned from arabidopsis, encodes a kinesin‐like Ca/calmodulin‐regulated microtubule motor protein (see Schnittger and Hulskamp, 2002). Recent studies using drugs that disrupt cytoskeletal elements have shown that polarized trichome growth is dependent on microtubules, and coordination and maintenance of normal trichome growth in the later stages of morphogenesis requires actin microfiliments (Szymanski, 2001).
Studies of trichome development have also revealed much about development of root hairs. Trichome and root hair development in arabidopsis are known to involve common genes and gene products. A mutation in the TRANSPARENT TESTA GLABRA (TTG) gene results in loss of leaf trichomes, but increased production of root hairs. A mutation in the GLABRA2 gene produces malformed trichomes on leaves, but ectopic hairs on roots (Kellogg, 2001). Another example may be AGL16, a recently discovered MADS‐box gene that is expressed in trichomes, guard cells and root epidermal cells (Alvarez‐Buylla et al., 2000). MADS‐box genes encode transcription factors that are involved in plant development. Several other recent reports link transcription factors and cofactors to trichome mutations (Schnittger and Hulskamp, 2002). To summarize, our understanding of molecular determinants in trichome (and root hair) development is advancing rapidly, mainly because of the ease in monitoring trichomes and the many advantages of arabidopsis (mutants, full genome sequence). But arabidopsis produces only simple trichomes lacking glands, and presumably trichomes that are incapable of secretion. Little is known about the molecular determinants of GST gland differentiation. Partial EST (expressed sequence tag) libraries from peppermint and sweet basil (see below) have apparently not yet been mined for the presence of trichome development regulatory genes. It would be interesting to know if homologs to trichome development genes of arabidopsis can impact trichome, and particularly gland, development when overexpressed in a GST‐bearing plant. It is possible that overexpression or knockdown of homologues of arabidopsis genes that regulate trichome number or morphology may be useful for investigating the importance of trichome density and morphology in, for example, trichome/insect, trichome/UV interactions. The GL3‐homologue R gene of maize causes trichome formation when overexpressed in arabidopsis (Schellmann et al., 2002), so such manipulations may be feasible. A major advantage of trichomes for such study is that they are not essential for plant growth and development.
Recent efforts to modify trichome metabolism
Commercially important products produced by simple trichomes and GSTs include cotton fibres, medicinals (e.g. the sesquiterpene antidepressant and antiviral, hypericin, and the sesquiterpene lactone antimalarial, artemisinin), spice principals (e.g. the monoterpenes of mints) and cosmeceuticals (e.g. sclareol). Other commercial products are produced by other secretory structures (e.g. turpentine components of conifer oleoresins and latex of lacticifers). Since the commercial value of cotton fibres or trichomes is dependent on fibre length (Kim and Triplett, 2001), mechanisms underlying cotton fibre elongation (turgor‐driven expansion and cellulose synthesis, in particular) and trichome differentiation are currently key targets for improving production of this crop. Cotton fibre gene libraries have been created to identify transcription factors regulating these mechanisms (Jaradat and Allen, 1999) and the mechanisms of turgor generation underlying fibre expansion have been studied by monitoring expression of turgor‐related genes during fibre elongation (Smart et al., 1998).
Genome mining and directed gene knockdown are two approaches being used to investigate and modify GSTs. Gene mining of peppermint and basil trichome EST libraries have provided several cDNAs of genes directly and indirectly (e.g. lipid transfer proteins) involved in trichome exudate synthesis. The Croteau and Pichersky laboratories prepared randomly selected, partial cDNA libraries of peppermint and basil gland genes, respectively, beginning with mRNAs prepared from isolated secretory glands or cells. About 13–25 % of genes in these partial libraries were found to encode genes related to GST exudates from these plants (Lange et al., 2000; Gang et al., 2001). Similarly, an EST library was prepared for GSTs of Artemisia annua, and up to 6 % of ESTs were related to isoprenoid biosynthesis (C. M. Bertea and H. J. Bouwmeester, pers. comm.). EST mining will undoubtedly provide many targets for knockdown and overexpression to generate GST exudates having modified chemical composition. The next challenges will be to optimize production of desirable components, and test mixtures for useful properties (e.g. pest resistance, pharmacological activity, useful organoleptic properties).
GSTs may also be modified by targeting specific genes for knockdown using post‐transcriptional gene silencing (particularly RNAi). Direct suppression of an endogenous enzyme can result in precursor accumulation and altered GST exudate chemistry. An example is the knockdown of the gene for the terminal enzyme (a P450) that catalyses conversion of cembratriene‐ol (CBTol) to cembratriene‐diol (CBTdiol) in tobacco GST glands. cDNAs were prepared from isolated GSTs, and leaf tissue with glands removed. These were subtracted to yield about 24 unique, partial cDNA sequences, one of which was identified as a gene for a cytochrome P450. Antisense and co‐suppression using this cDNA resulted in a few plants having increased CBTol, correspondingly reduced CBTdiol, and greatly increased aphid resistance in the glasshouse (Wang et al., 2001) and in a field trial (Wang et al., 2003). Subsequently, RNAi proved highly efficient for knockdown. About 45 % of plants had a range of CBTol hydroxylase suppression. A gland‐specific promoter that should be useful for directed manipulation of, at least, tobacco trichomes was isolated using the CBTol hydroxylase gene (Wang et al., 2002). RNAi was also used to suppress an apparent CBTol synthase gene. Sixty‐four per cent of plants had reduced CBTol and CBTdiol, identifying this gene as the CBTol synthase that converts geranylgeranyl pyrophosphate to CBTol. Thus, RNAi has proved to be a very useful tool for selective knockdown of two tobacco trichome genes that encode very different enzymes. Gene knockdown may also be useful for enhancing metabolic pathways that produce very low levels of useful compounds. Knockdown of the CBTol synthase gene has observed to result in substantially increased cis‐abienol, normally a minor product (unpublished). In addition, suppressed plants produce several minor diterpenoids that be may present in very low amounts, or absent, from wild‐type exudates (unpublished). Therefore, the knockdown approach may be useful for amplifying minor, existing and, perhaps even serendipitously produced, novel compounds that may be tested for useful properties.
Overexpression and antisense knockdown have also been used to alter the quantity and quality of monoterpenes produced by peppermint GSTs (Mahmoud and Croteau, 2001). Over‐expression of a cDNA for a key enzyme in the methyl‐erythritol‐4‐phosphate pathway that supplies isopentenyl pyrophosphate for monoterpene biosynthesis resulted in about a 50 % increase in monoterpene production. Oil composition did not change appreciably. Antisense knockdown using a (+)‐menthofuran synthase cDNA resulted in a 50 % reduction of (+)‐methofuran, an undesirable component of peppermint oil. Interestingly, the precursor of (+)‐menthofuran, (+)‐pulegone, also decreased, but (–)‐menthol, one end‐product, increased substantially, so total oil yield did not change appreciably. Some changes observed were predictable while others were not (perhaps due to subtle aspects of pathway regulation), but significant modification of GST products was observed.
The examples discussed above demonstrate the potential of gene overexpression and knockdown for altering both the composition and quantity of GST exudate. Clearly, the ability to monitor trichomes easily on intact living tissue, the relative ease of isolating trichomes and trichome exudate, and the non‐essential nature of trichomes has allowed the application of tools of molecular genetics, genomics and biochemistry to advance our understanding of these fascinating structures. It might be thought that similar studies of surface resin ducts, for example, would be more difficult or impossible because these structures are embedded in the epidermal plane. However, EST libraries have been prepared from guard cell protoplasts (Kwak et al., 1997) and crystal idioblasts have been isolated from Pistia stratiotes (Kostman et al., 2001). Thus, while it may not be feasible to isolate secreting structures embedded in the epidermal plane or elsewhere using mechanical means, these may be accessible via protoplast technology. Finally, laser‐capture micro‐dissection has been used to isolate resin blisters (resin ducts) from grand fir (Abies grandis; M. Xu and R. J. Peters, pers. comm.). Theoretically, this method could be applied to isolation of any surface or subsurface structure or cell type. Methods for preparing high quality RNA from plants have improved greatly in recent years, so once such structures are isolated, they may, like trichomes, yield EST libraries for applying the genomics approach.
Exploitation of trichomes for biotechnology
The high production capacity of GSTs from certain plants and the external accumulation of exudates enhance the potential utility of GSTs as ‘green factories’ for producing commercially useful compounds (Wagner and Wang, 2001). Natural GST products are often phytotoxic but do not affect the parent plant because they are segregated outside the plant body. So, commercially useful phytotoxins may be efficiently produced by genetically engineered GSTs. Also, a major cost of any molecular farming strategy arises during purification of the product. Because GST exudates can be recovered by washing aerial surfaces with an appropriate solvent, the ease and cost of recovery and purification will most likely be greatly reduced. Washed plants can be used subsequently for ‘value added’ purposes (Wagner and Wang, 2001).
A possible limitation of GSTs for commercial production is the exposure of accumulating products to the atmosphere and sunlight, which may cause undesirable chemical modifications. However, this problem may be avoided by growing plants in a controlled environment. GSTs generally produce a particular class and sub‐class of secondary products; e.g. tobacco GSTs mainly produce diterpenoids and, further, mostly cembranoid and labdenoid diterpenoid types. Such a system, in which isoprenoid metabolism is accentuated, would presumably not be efficient in producing flavonoids, for example. However, the recent demonstration that cyanogenic glycoside biosynthesis can be engineered into a non‐cyanogenic plant forces us to remain open‐minded about the flexibility of plant metabolism. Tattersall et al. (2001) transformed arabidopsis with three genes that encode biosynthetic enzymes for the cyanogenic glycoside dhurrin. Transgenic plants produced low‐level, but sufficient dhurrin to provide resistance to the flea beetle, Phyllotreta nemorum, demonstrating that an endogenous, primary metabolite precursor (in this case tyrosine) can be tapped by an introduced metabolic pathway. High‐level production would probably be more difficult to achieve. For high‐level production of a desired/novel, non‐endogenous product it is likely that accumulation will be limited by the host plant’s capacity to accept the product, and the robustness of the precursor supply. Therefore, for high‐level production of novel compounds, it may be necessary to choose a ‘factory’ plant based on chemical similarities (e.g. solubility characteristics, similar precursor and cofactor requirements) between endogenous exudate molecules and the target product. High‐level production of a novel compound will probably also require suppression of endogenous exudate biosynthesis at a point in a metabolic pathway close to the common precursor. If endogenous competing pathways produce essential metabolites, the problem becomes more difficult, and it largely remains to be seen if exogenous enzymes can ‘spatially fit’ into metabolons (apparently common in plant secondary product pathways). Perhaps the successful engineering by Tattersall et al. (2001) of an entire exogenous metabolic pathway circumvented some of these potential problems, particularly impacts of regulation. From this perspective, discounting considerations of product yield, it might, in some cases, be less complicated to introduce new pathways into host plants than to infiltrate existing pathways. Despite some possible disadvantages of using trichomes for molecular farming, there are many advantages that should allow their exploitation for production of pharmaceuticals, nutriceuticals and cosmeceuticals.
Finally, it is hoped that distinctions between GSTs, simple trichomes (as defined here) and other secreting, surface structures (e.g. surface resin ducts) are diminished rather than accentuated by this review, as an attempt has been made here to present all such structures as highly functional entities (see Fig. 3). There are clear differences in, for example, the focus of metabolism among different surface secreting systems, but most epidermal secretion structures have the common purpose of secretion to the plant surface. Future studies will undoubtedly discover more similarities between secreting structures, in processes leading to secretory cell development, mechanisms of carbon transfer to secretory cells and lipophylic product transfer during secretion. Gene mining and other tools of modern biology will rapidly expand our understanding of the functions of trichomes and other secretory structures. Clearly manipulation of trichomes to enhance natural‐product‐based host resistance, to modify endogenous organoleptic properties and to facilitate ‘molecular farming’ is well underway.
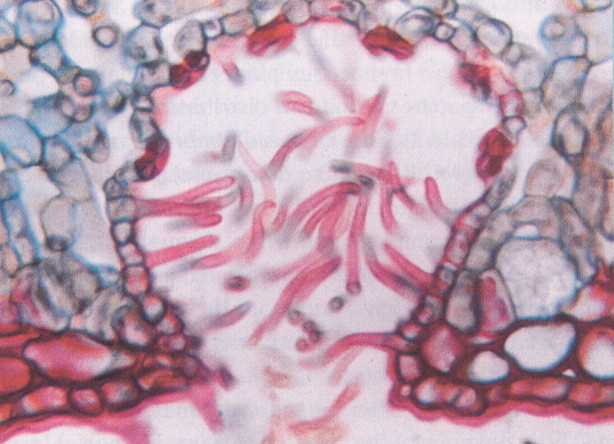
Fig. 3. Leaf pit on the abaxial leaf surface of oleander. The inner surface of the pit is lined with stomata and the opening with simple trichomes that are thought to reduce water loss in this highly drought‐resistant plant. Photographs by Jack M. Bostrack/Visuals Unlimited. Reproduced with permission of H. W. Freeman and Co.
Supplementary Material
Received: 3 June 2003; Returned for revision: 29 July 2003; Accepted: 2 October 2003
References
- AloniR, Schwalm K, Langhans M, Ullrich CI.2003. Gradual shifts in sites of free‐auxin production during leaf‐primordium development and their role in vascular differentiation and leaf morphogenesis in Arabidopsis Planta 216: 841–853. [DOI] [PubMed] [Google Scholar]
- Alvarez‐BuyllaER, Liljegren SJ, Pelaz S, Gold SE, Burgeff C, Ditta GS, Vergara‐Silva F, Yanofsky MF.2000. MADS‐box gene evolution beyond flowers: expression in pollen, endosperm, guard cells, roots and trichomes. Plant Journal 24: 457–466. [DOI] [PubMed] [Google Scholar]
- BeattieGA, Lindow SE.1999. Bacterial colonization of leaves: a spectrum of strategies. Phytopathology 89: 353–359. [DOI] [PubMed] [Google Scholar]
- BehnkeHD.1984. Plant trichomes‐structure and ultrastructure: general terminology, taxonomic applications, and aspects of trichome‐bacterial interaction in leaf tips of Dioscorea In: Rodriguez E, Healey PL, Mehta I, eds. Biology and chemistry of plant trichomes New York: Plenum Press, 1–21. [Google Scholar]
- BennettNR, Wallsgrove, RM.1994. Transley Review No. 72. Secondary metabolites in plant defence mechanisms. New Phytologist 127: 617–633. [DOI] [PubMed] [Google Scholar]
- BerenbaumMR.1995. The chemistry of defense: theory and practice. In: Eisner T, Meinwald J, eds. Chemical ecology: the chemistry of biotic interaction Washington DC, National Academy Press, 1–15. [Google Scholar]
- BrewerCA, Smith WK.1997. Patterns of leaf surface wetness for mountain and subalpine plants. Plant Cell and Environment 20: 1–11. [Google Scholar]
- ChoiYE, Harada E, Wada M, Tsuboi H, Morita Y, Kusano T, Sano H.2001. Detoxification of cadmium in tobacco plants: formation and active excretion of crystals containing cadmium and calcium through trichomes. Planta 213: 45–50. [DOI] [PubMed] [Google Scholar]
- DellB, McComb AJ.1978. Plant Resins‐their formation, secretion and possible functions. Advances in Botanical Research 6: 227–316. [Google Scholar]
- DeSilvaDLR, Mansfield TA, McAinsh MR.2001. Changes in stomatal behaviour in the calcicole Leontodon hispidus due to the disruption by ozone of the regulation of apoplastic Ca 2+ by trichomes. Planta 214: 158–162. [DOI] [PubMed] [Google Scholar]
- DukeSO, Rimando AM, Duke MV, Paul RN, Ferreira JFS, Smeda RJ.1999. Sequestration of phytotoxins by plants: implication for biosynthetic production. In: Cutler HG, Cutler SJ. eds. Biologically active natural products: agrochemical, Vol. 10 Boca Raton, FL: CRC Press, 127–136. [Google Scholar]
- EhleringerJ.1984. Ecology and ecophysiology of leaf pubescence in North American desert plants. In: Rodriguez E, Healey PL, Mehta I, eds. Biology and chemistry of plant trichomes New York: Plenum Press, 113–132. [Google Scholar]
- FahnA.2000. Structure and function of secretory cells. Advances in Botanical Research 31: 37–75. [Google Scholar]
- GangDR, Wang J, Dudareva N, Nam KH, Simon JE, Lewinsohn E, Pichersky E.2001. An investigation of the storage and biosynthesis of phenylpropenes in sweet basil. Plant Physiology 125: 539–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez‐AlcalaG, Gotor C, Meyer JA, Fricker M, Vega MJ, Romero CL.2000. Glutathione biosynthesis in Arabidopsis trichome cells. Proceedings of the National Academy of Science 97: 11108–1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JaradatTT, Allen RD.1999. Isolation of a novel cDNA encoding an auxin‐induced basic helix–loop–helix transcription factor (Accession no. AF 165924) from cotton (Gossypium hirsutum L.). Plant Physiology 121: 685–686.10517861 [Google Scholar]
- JohnsonHB.1975. Plant pubescence: an ecological perspective. Botanical Review 41: 233–253. [Google Scholar]
- KelloggEA.2001. Root hairs, trichomes and the evolution of duplicate genes. Trends in Plant Science 6: 550–552. [DOI] [PubMed] [Google Scholar]
- KelseyRG, Reynolds GW, Rodriguez E.1984. The chemistry of biologically active constituents secreted and stored in plant glandular trichomes. In: Rodriguez E, Healey PL, Mehta I, eds. Biology and chemistry of plant trichomes New York: Plenum Press, 187–240. [Google Scholar]
- KennedyGG.2003. Tomato, pest, parasitoids, and predators: tritrophic interactions involving the genus Lycopersicon Annual Review of Entomology 48: 51–72. [DOI] [PubMed] [Google Scholar]
- KesslerA, Baldwin IT.2002. Plant responses to insect herbivory:the emerging molecular analysis. Annual Review of Plant Biology 53: 299–328. [DOI] [PubMed] [Google Scholar]
- KimHJ, Triplett BA.2001. Cotton fiber growth in plants and in vitro Models for plant cell elongation and cell wall biogenesis. Plant Physiology 127: 1361–1366. [PMC free article] [PubMed] [Google Scholar]
- Kronestedt‐RobardsE, Robards AW.1991. Exocytosis in glands cells. Society for Experimental Biology, Seminar Series 45: 199–232. [Google Scholar]
- KostmanTA, Tarlyn NM, Loewus FA, Franceschi VR.2001. Biosynthesis of L‐Ascorbic acid and conversion of carbons 1 and 2 of L‐ascorbic acid to oxalic acid occurs within individual calcium oxalate crystal idioblasts. Plant Physiology 125: 634–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KupperH, Lombi E, Zhao FJ, McGrath SP.2000. Cellular compartmentation of cadmium and zinc in relation to other elements in the hyperaccumulator Arabidopsis halleri Planta 212: 75–84. [DOI] [PubMed] [Google Scholar]
- KwakJM, Kim SA, Hong SW, Nam HG.1997. Evaluation of 515 expressed sequence tags obtained from guard cells of Brassica campestris Planta 202: 9–17. [DOI] [PubMed] [Google Scholar]
- LangeBM, Wildung MR, Stauber EJ, Sanchez C, Pouchnik D, Croteau R.2000. Probing essential oil biosynthesis and secretion by functional evaluation of expressed sequence tags from mint glandular trichomes. Proceedings of the National Academy of Science 97: 2934–2939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaueG, Preston CA, Baldwin IT.2000. Fast track to the trichome: induction of N‐acyl nornicotines precedes nicotine induction in Nicotiana repanda Planta 210: 510–514. [DOI] [PubMed] [Google Scholar]
- LazzaroMD, Thomson WW.1996. The vaculolar‐tubular continuum in living trichomes of chickpea (Cicer arietinum) provides a rapid means of solute delivery from base to tip. Protoplasma 193: 181–190. [Google Scholar]
- LevinDA.1973. The role of trichomes in plant defence. Quarterly Review of Biology 48: 3–15. [Google Scholar]
- LiS, Yi Y, Wang Y, Zhang Z, Beasley RS.2002. Camptothecin accumulation and variations in Camptotheca Planta Medica 68: 1010–1016. [DOI] [PubMed] [Google Scholar]
- LinY, Wagner GJ.1994. Rapid and simple method for estimation of sugar esters. Journal of Agriculture and Food Chemistry 42: 1709–1712. [Google Scholar]
- McCaskillD, Gershenzon J, Croteau R.1992. Morphology and monoterpene biosynthetic capabilities of secretory cell clusters isolated from glandular trichomes of peppermint (Mentha piperita L.). Planta 187: 445–454. [DOI] [PubMed] [Google Scholar]
- MaciasFA, Molinillo JMG, Galindo JCG, Varela RM, Torres A, Simonet AM,1999. Terpenoids with potential use as natural herbicide templates. In: Cutler HG, Cutler SJ, eds. Biologically active natural products: agrochemicals, Vol. 2 Boca Raton, FL: CRC Press, 15–29. [Google Scholar]
- MahlbergPG, Kim ES.1992. Secretory vesicle formation in glandular trichomes of Cannabis sativa (Cannabaceae). American Journal of Botany 79: 166–173. [PubMed] [Google Scholar]
- MahmoudSS, Croteau RB.2001. Metabolic engineering of essential oil yield and composition in mint by altering expression of deoxyxylulose phosphate reductoisomerase and menthofuran synthase. Proceeding of the National Academy of Science 98: 8915–8920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SakaiWS, Sanford WG.1980. Ultrastructure of the water‐absorbing trichomes of Pineapple (Ananas comosus, Bromeliaceae). Annals of Botany 46: 7–11. [Google Scholar]
- SaltDE, Prince RC, Pickering IJ, Raskin I.1995. Mechanisms of cadmium mobility and accumulation in Indian mustard. Plant Physiology 109: 1427–1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SchellmannS, Schnittger A, Kirik V, Wada T, Okada K, Beermann A, Thumfahrt J, Jurgens G, Hulskamp M.2002. TRIPTYCHON and CAPRICE mediate lateral inhibition during trichome and root hair patterning in Arabidopsis. EMBO Journal 21: 5036–5046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SchnittgerA, Hulskamp M.2002. Trichome morphogenesis: a cell‐cyle perspective. Philosophical Transactions of the Royal Society of London B 357: 823–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SchwabB, Folkers U, Ilgenfritz H, Hulskamp M.2000. Trichome morphogenesis in Arabidopsis Philosophical Transactions of the Royal Society of London B 355: 879–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SeoS, Seto H, Koshino H, Yoshida S, Ohashi Y.2003. A diterpene as an endogenous signal for the activation of defense responses to infection with Tobacco mosaic virus and wounding in tobacco. Plant Cell 15: 863–873. [PMC free article] [PubMed] [Google Scholar]
- SeversonRF, Johnson AW, Jackson DM.1985. Cuticular constituents of tobacco: factors affecting their production and their role in insect and disease resistance and smoke quality. Recent Advances in Tobacco Science 11: 105–173. [Google Scholar]
- SmartLB, Vojdani F, Maeshima M, Wilkins, TA.1998. Genes involved in osmoregulation during turgor‐driven cell expansion of developing cotton fibers are differentially regulated. Plant Physiology 116: 1539–1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SzymanskiDB.2001.Arabidopsis trichome morphogenesis: a genetic approach to studying cytoskeletal function. Journal of Plant Growth Regulation 20: 131–140. [Google Scholar]
- TattersallDB, Bak S, Jones PR, Olsen CE, Nielsen JK, Hansen ML, Hoj PB, Moller BL.2001. Resistance to an herbivore through engineered cyanogenic glucoside synthesis. Science 293: 1826–1828. [DOI] [PubMed] [Google Scholar]
- ThomsonWW, Healey PL.1984. Cellular basis of trichome secretion. In: Rodriguez E, Healey PL, Mehta I, eds. Biology and chemistry of plant trichomes New York: Plenum Press, 95–112. [Google Scholar]
- UphofJCT.1962. Plant hairs. Encyclopedia of Plant Anatomy IV, 5: 1–206. [Google Scholar]
- VerpoorteR.2000. Pharmacognosy in the new millennium: leadfinding and biotechnology. Journal of Pharmacy and Pharmacology 52: 253–262. [DOI] [PubMed] [Google Scholar]
- WagnerGJ.1991. Secreting glandular trichomes: more than just hairs. Plant Physiology 96: 675–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WagnerGJ, Wang E.2001. Exploiting the ooze: engineering surface secretion systems of plants. Secretion systems of plants may be molecular farming and pest/disease resistance factor factories of the future. Agricultural Biotechnology Net 3: 1–3. [Google Scholar]
- WangE, Wagner GJ.2003. Elucidation of the functions of genes central to diterpene metabolism in tobacco trichomes using post transcriptional gene silencing. Planta 216: 686–691. [DOI] [PubMed] [Google Scholar]
- WangE, Gan S, Wagner GJ.2002. Isolation and characterization of the CYP71D16 trichome‐specific promoter from Nicotiana tabacum L. Journal of Experimental Botany 53: 1891–1897. [DOI] [PubMed] [Google Scholar]
- WangE, Hall JT, Wagner GJ.2003. Transgenic Nicotiana tabacum L. with enhanced trichome exudate cembratrieneols has reduced aphid infestation in the field. Molecular Breeding (in press). [Google Scholar]
- WangE, Wang R, DeParasis J, Loughrin JH, Gan S, Wagner GJ.2001. Suppression of a P450 hydroxylase gene in plant trichome glands enhances natural‐product‐based aphid resistance. Nature Biotechnology 19: 371–374. [DOI] [PubMed] [Google Scholar]
- WaigmannE, Zambryski P.2000. Trichome plasmodesmata: a model system for cell‐to‐cell movement. Advances in Botanical Research 31: 261–283. [Google Scholar]
- WerkerE.2000. Trichome diversity and development. Advances in Botanical Research 31: 1–35. [Google Scholar]
- WollenweberE.1984. The systematic implication of flavonoids secretedby plants. In: Rodriguez E, Healey PL, Mehta I, eds. Biology and chemistry of plant trichomes New York: Plenum Press, 53–69. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.