Abstract
Myriapods (e.g., centipedes and millipedes) display a simple homonomous body plan relative to other arthropods. All members of the class are terrestrial, but they attained terrestriality independently of insects. Myriapoda is the only arthropod class not represented by a sequenced genome. We present an analysis of the genome of the centipede Strigamia maritima. It retains a compact genome that has undergone less gene loss and shuffling than previously sequenced arthropods, and many orthologues of genes conserved from the bilaterian ancestor that have been lost in insects. Our analysis locates many genes in conserved macro-synteny contexts, and many small-scale examples of gene clustering. We describe several examples where S. maritima shows different solutions from insects to similar problems. The insect olfactory receptor gene family is absent from S. maritima, and olfaction in air is likely effected by expansion of other receptor gene families. For some genes S. maritima has evolved paralogues to generate coding sequence diversity, where insects use alternate splicing. This is most striking for the Dscam gene, which in Drosophila generates more than 100,000 alternate splice forms, but in S. maritima is encoded by over 100 paralogues. We see an intriguing linkage between the absence of any known photosensory proteins in a blind organism and the additional absence of canonical circadian clock genes. The phylogenetic position of myriapods allows us to identify where in arthropod phylogeny several particular molecular mechanisms and traits emerged. For example, we conclude that juvenile hormone signalling evolved with the emergence of the exoskeleton in the arthropods and that RR-1 containing cuticle proteins evolved in the lineage leading to Mandibulata. We also identify when various gene expansions and losses occurred. The genome of S. maritima offers us a unique glimpse into the ancestral arthropod genome, while also displaying many adaptations to its specific life history.
Author Summary
Arthropods are the most abundant animals on earth. Among them, insects clearly dominate on land, whereas crustaceans hold the title for the most diverse invertebrates in the oceans. Much is known about the biology of these groups, not least because of genomic studies of the fruit fly Drosophila, the water flea Daphnia, and other species used in research. Here we report the first genome sequence from a species belonging to a lineage that has previously received very little attention—the myriapods. Myriapods were among the first arthropods to invade the land over 400 million years ago, and survive today as the herbivorous millipedes and venomous centipedes, one of which—Strigamia maritima—we have sequenced here. We find that the genome of this centipede retains more characteristics of the presumed arthropod ancestor than other sequenced insect genomes. The genome provides access to many aspects of myriapod biology that have not been studied before, suggesting, for example, that they have diversified receptors for smell that are quite different from those used by insects. In addition, it shows specific consequences of the largely subterranean life of this particular species, which seems to have lost the genes for all known light-sensing molecules, even though it still avoids light.
Introduction
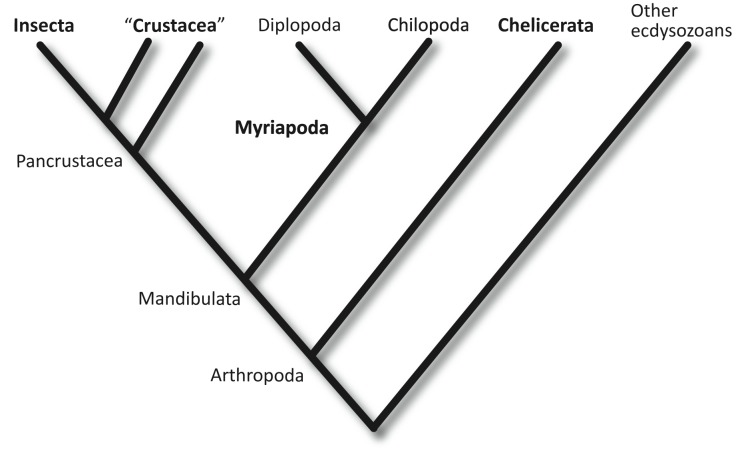
Arthropods are the most species-rich animal phylum on Earth. Of the four extant classes of arthropods (Insecta, Crustacea, Myriapoda, and Chelicerata) (Figure 1), only the Myriapoda (centipedes, millipedes, and their relatives) are currently not represented by any sequenced genome [1],[2]. This absence is particularly unfortunate, as myriapods have recently been recognised as the living sister group to the clade that encompasses all insects and crustaceans [3]–[6]. Hence, the Myriapoda are particularly well placed to provide an outgroup for comparison, to determine ancestral character states and the polarity of evolutionary change within insects and crustaceans, which together represent the most diverse animal clade on Earth.
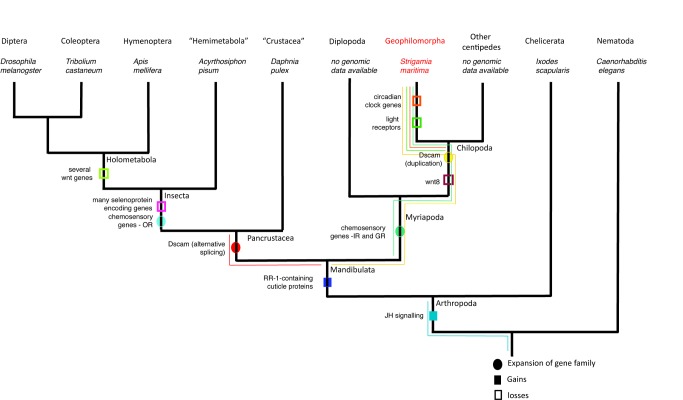
Figure 1. The phylogenetic position of the centipedes (Chilopoda), with respect to other arthropods, according to the currently best-supported phylogeny.
(See text for details). The four traditionally accepted arthropod classes are marked in bold.
Although Drosophila melanogaster is the best studied arthropod, it lacks many genes present in the ancestral bilaterian gene set, and chromosome rearrangements have disrupted all obvious evidence of synteny with other phyla [7]. Thus it is not fully representative of other arthropods. More comprehensive sampling of arthropod genomes will establish their basic structure, and determine when unique genomic characteristics of different taxa, such as the holometabolous insects, appear.
Phylogenetic Position of the Myriapods
Myriapods are today represented by two major lineages—the herbivorous millipedes (Diplopoda) and the carnivorous centipedes (Chilopoda), together with two minor clades, the Symphyla, which look superficially like small white centipedes, and the minute Pauropoda [8]. All are characterised by a multi-segmented trunk of rather similar (homonomous) segments, with no differentiation into thorax or abdomen. All recent studies, molecular and morphological, support the monophyly of myriapods [3]–[5],[8]–[10] suggesting that they share a single common ancestor.
Myriapods, insects, and crustaceans have traditionally been identified as a clade of mandibulate arthropods, characterised by head appendages that include antennae and biting jaws [11]. Some molecular datasets have challenged this idea, suggesting instead that the myriapods are a sister group to the chelicerates [12],[13]. The most comprehensive phylogenomic datasets thus far reject this, and strongly support the phylogeny that proposes that the chelicerates are the most basal of the four major extant arthropod clades, and the mandibulates represent a true monophyletic group [3],[5],[10],[14]–[17].
Within the mandibulates, myriapods were believed until recently to share a common origin with insects as terrestrial arthropods. This view, based on a number of shared characters including uniramous limbs, air breathing through tracheae, the lack of a second pair of antennae, and excretion using Malpighian tubules, was widely supported by morphologically based phylogenies [9],[18]. However, molecular phylogenies robustly reject the sister group relationship between insects and myriapods, placing the origin of myriapods basal to the diversification of crustaceans [5], and identifying insects as a derived clade within the Crustacea [19]–[21]. As crustaceans are overwhelmingly a marine group today, and were so ancestrally, this implies that myriapods and insects represent independent invasions of the land (with the chelicerates representing an additional, unrelated invasion). Their shared characteristics are striking convergences, not synapomorphies.
S. maritima as a Model Myriapod
We chose S. maritima as the species to sequence partly for pragmatic reasons: geophilomorph centipedes, such as S. maritima, have relatively small genome sizes, certainly compared to other centipedes [22]. More importantly, it is a species that has attracted interest for ecological and developmental studies [23]–[25], especially the process of segment patterning [26]–[32]. S. maritima is a common centipede of north western Europe, found along the coastline from France to the middle of Norway. It is a specialist of shingle beaches and rocky shores, occurring around the high tide mark, and feeding on the abundant crustaceans and insect larvae associated with the strand line. It is by far the most abundant centipede in these habitats around the British Isles, sometimes occurring at densities of thousands per square metre in suitable locations [25]. Eggs can be harvested from these abundant populations in large numbers with relatively little effort during the summer breeding season [27]. They can be reared in the lab from egg lay to at least the first free-living stage, adolescens I [24],[33].
Some aspects of S. maritima biology are not common to all centipedes. Notable among these is epimorphic development, wherein the embryos hatch from the egg with the final adult number of leg-bearing segments. Epimorphic development is found in two centipede orders: geophilomorphs (including S. maritima) and scolopendromorphs. In contrast, more basal clades display anamorphic development and add segments post-embryonically [34]. These anamorphic clades have relatively few leg-bearing segments, generally 15, while geophilomorphs have many more, up to nearly 200 in some species [6]. These unique characteristics probably arose at least 300 million years ago, as the earliest fossils of the much larger scolopendromorph centipedes date to the Upper Carboniferous [35]. These share the same mode of development as the geophilomorphs, and are their likely sister group. Geophilomorphs are also adapted to a subsurface life style, the whole order having lost all trace of eyes [36],[37], though apparently not photosensitivity [38].
We have sequenced the genome of S. maritima as a representative of the phylogenetically important myriapods. In contrast to the intensively sampled holometabolous insects, our analysis of this myriapod genome finds conservative gene sets and conserved synteny, shedding light on general genomic features of the arthropods.
Results and Discussion
Genome Assembly, Gene Densities, and Polymorphism
Genomic DNA from multiple individuals of a wild Scottish population of S. maritima was sequenced and assembled into a draft genome sequence spanning 176.2 Mb. This assembled sequence omits many repeat sequences including heterochromatin, which probably accounts for the difference between the assembly length and the total genome size estimate of 290 Mb. An analysis of repetitive elements within the assembly is presented in Text S1.
The assembly incorporates 14,992 automatically generated gene models, 1,095 of which have been additionally manually annotated. We re-sequenced four individuals comprising three females and one male. The frequency of identified polymorphism, with SNP density of 4.5 variants/kb, is comparable with the five variants per kb in the Drosophila genetic reference panel [39]. It is hard to say how typical this is for soil dwelling arthropods, as very little population data are available for such species.
Phylome Analysis and Phylogenomics
To understand general patterns of gene evolution in S. maritima we reconstructed the evolutionary histories of all of its genes, i.e., the phylome. The resulting gene phylogenies, available through phylomeDB [40], were analysed to establish orthology and paralogy relationships with other arthropod genomes [41], transfer functional knowledge from annotated orthologues, and to detect and date gene duplication events [42]. Some 32% of S. maritima genes can be traced back to duplications specific to this myriapod lineage since its divergence from other arthropod groups included in the analysis. Functions enriched among these genes include those related to, among other processes, catabolism of peptidoglycans, sodium transport, glutamate receptor, and sensory perception of taste. Related to this latter function, two of the largest gene expansions specific to the S. maritima lineage detected in our analysis are the gustatory receptor (GR) and ionotropic receptor (IR) families encoding putative membrane-associated gustatory and/or olfactory receptors (see Text S1, and Chemosensory section below).
Sex Chromosomes
No obviously differentiated sex chromosomes are apparent in the diploid S. maritima karyotype, which comprises one long pair of metacentric chromosomes, together with seven pairs of much shorter telocentric chromosomes (P. Woznicki, unpublished data; J. Green et al., unpublished). Read-depth data from the genome assembly show that a proportion of the genome is underrepresented compared to the bulk of the data. One obvious reason for underrepresentation would be sequences derived from sex chromosomes. To confirm this, the coverage of individual scaffolds from the assembly was examined in sequence obtained from single individuals. A distinct fraction of underrepresented scaffolds is present in DNA derived from a male, but absent in female sequence (Figure 2), implying an XY sex determination mechanism. Quantitative PCR from three scaffolds in the underrepresented fraction confirmed that they are present at approximately twice the copy number in females as in males, identifying them as X chromosome derived (J. Green et al., unpublished). Other scaffolds of this fraction contain male specific sequences, and therefore presumably derive from a Y chromosome (J. Green et al., unpublished) [31]. Combined with the karyotype data, this finding suggests that S. maritima possesses a weakly differentiated pair of X and Y chromosomes.
Figure 2. Plot showing that DNA from a male individual contains a distinct fraction of scaffolds that is underrepresented (black arrow), and presumably derives from heterogametic sex chromosomes.
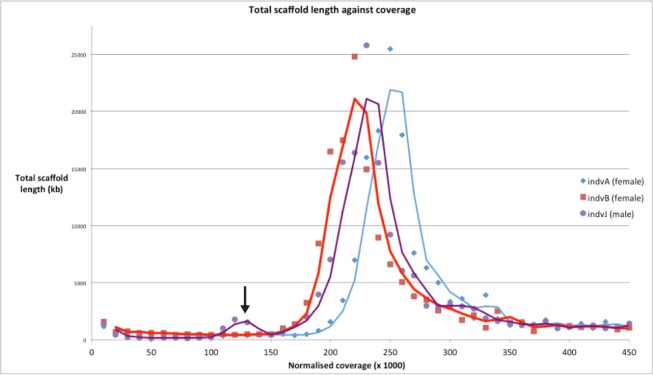
No such fraction is present in the sequenced DNA of two individual females. The data underlying this plot is presented in File S4.
Mitochondrial Genome
From the whole genome assembly, S. maritima scaffold scf7180001247661 was found to contain a complete copy of the mitochondrial coding regions, flanked by a TY1/Copia-like retrotransposon, which all together spanned approximately 20 kb. This is unusually large for a metazoan mitochondrial genome and, as mis-assembly was suspected, PCR was used to clone the DNA between the genes at either end of the scaffold. This enabled us to close the circle of the mitochondrial genome, correct frameshifts, and confirm an unusual gene arrangement, resulting in a final circular assembly of 14,983 bp (Table S11). The gene arrangement in the S. maritima mitochondrial genome is striking (Figure S6). It diverges dramatically from the basic arthropod genome arrangement and differs from all other known centipede mitochondrial gene arrangements [43]. Although small sections of the S. maritima gene order are conserved with respect to the arthropod ground pattern found in Limulus polyphemus and the lithobiomorph centipede Lithobius forficatus (e.g., trnaF-nad5-H-nad4-nad4L on the minus strand), other sections are completely rearranged to an extent unusual in arthropods, and metazoans (ACR and MJT, unpublished). This confounds attempts to use S. maritima mitochondrial gene order in phylogenetic reconstructions.
Conserved Synteny with Other Phyla
With the exception of some conserved local gene clusters, the location of genes on the chromosomes of Drosophila and other Diptera retains no obvious trace of the ancestral bilaterian gene linkage. Other holometabolous insects such as Bombyx mori and Tribolium castaneum do show significant conservation of large-scale gene linkage with other phyla, for example, in the chordate Branchiostoma floridae (amphioxus) and the cnidarian Nematostella vectensis [44],[45]. The last common ancestor of these two lineages pre-dated the ancestor of all bilaterian animals, and yet the genomes of these species retain detectable conserved synteny: orthologous genes are found together on the same chromosomes, or chromosome fragments, far more often than would be expected by chance.
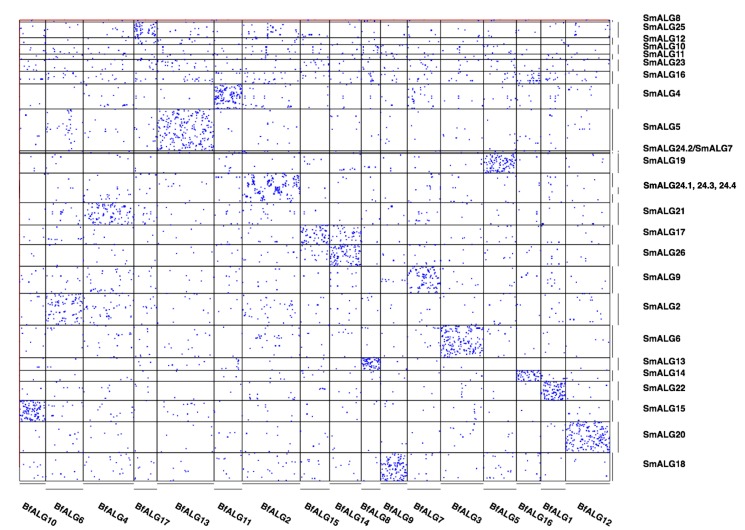
We find the S. maritima genome also retains significant traces of the large-scale genome organisation that was present in the bilaterian ancestor. Although the assignment of scaffolds to chromosomes is not determined in S. maritima, there are sufficient gene linkage data within scaffolds to reveal clear retained synteny between amphioxus and S. maritima (Figure 3), at a higher level than any of the Insecta or Pancrustacea we have examined.
Figure 3. Conserved macro synteny signal between S. maritima and the chordate lancelet B. floridae clustered into ancestral linkage groups.
Each dot represents a pair of genes, one in B. floridae, one in S. maritima, assigned to the same gene family by our orthology analysis. The ancestral linkage group identifiers refer to groups of scaffolds from the S. maritima (SmALG) or B. floridae (BfALG) assemblies, as detailed in File S2. The identification of ALGs is described in the SI. Note that two S. maritima scaffolds were divided across ALGs, and so appear multiple times in File S2.
Of the 62 scaffolds with at least 20 genes from ancestral bilaterian orthology groups, 37 show enrichment of shared orthologues with one or (in the case of a single scaffold) two chordate ancestral linkage groups (ALGs) at a significance threshold of p<0.0001 (after Bonferroni correction for 1,116 pairwise ALG-scaffold comparisons). Of these scaffolds' genes that have predicted human orthologues, 57% are found in a conserved macro-synteny context. At a more relaxed significance threshold (p<0.01), 71% of these scaffolds have a significant association with at least one chordate ALG, and 17 of the 18 chordate ALGs hit at least one of these scaffolds.
Stronger synteny is also detected for the genome of the nematode Caenorhabditis elegans with S. maritima than with insects or other Metazoa. The C. elegans genome is highly rearranged, and shows low synteny with higher insects, or with chordates [7],[46],[47]. As members of the Ecdysozoa, nematodes last shared a common ancestor with the arthropods more recently than with chordates. This shared ancestry allows traces of conserved genome organisation to be detected with slowly rearranging arthropod genomes, even when it is only weakly apparent with chordates.
By implication, the last common ancestor of the arthropods retained significant synteny with the last common ancestor of bilaterians as well as the last common ancestors of other phyla, such as the Chordata. This conserved synteny is more complete with this S. maritima genome sequence, due to the relative scrambling of the genomes of those other arthropods that have been sequenced previously.
Homeobox Gene Clusters: Hox, ParaHox, SuperHox, and Mega-homeobox
The clustering of genes in a genome is often of functional significance (e.g., reflecting co-regulation), as well as providing important insights into the origins of particular gene families when clusters are composed of genes from the same class or family. Gene clusters can also be a useful proxy for the degree of genome rearrangement. The homeobox gene super-class is one type of gene for which clustering has been extensively explored. S. maritima has 113 homeobox-containing genes, which is slightly more than seen in other sequenced arthropods such as D. melanogaster, T. castaneum, and Apis mellifera. This is due to some lineage-specific duplications in S. maritima as well as the retention of some homeobox families that have been lost in other arthropods, including Vax, Dmbx, and Hmbox (see Text S1).
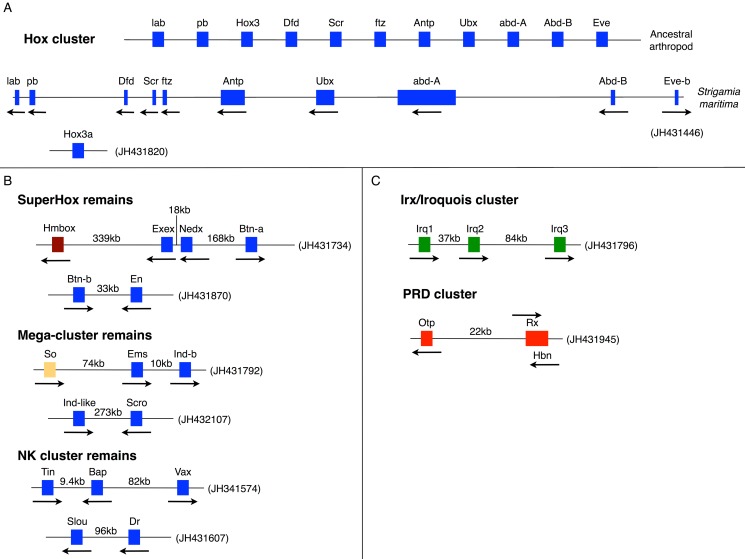
The homeobox-containing genes of the Hox gene cluster are renowned for their role in patterning the anterior-posterior axis of animal embryos. S. maritima has an intact, well-ordered Hox cluster containing one orthologue of each of the ten expected arthropod Hox genes, except for Hox3. There are two potential Hox3 genes elsewhere in the S. maritima genome [48], but the true orthology of these genes remains slightly ambiguous; it remains possible that they are the first example of ecdysozoan Xlox ParaHox genes (see Text S1). The Hox cluster spans 457 kb (labial to eve), a span similar to assembled Hox clusters in a range of other invertebrate groups (crustacean, mollusc, echinoderm, cephalochordate). This suggests that the contrasting very large (and frequently broken) Hox clusters of Drosophilids and some other insects are a derived characteristic. However, the spectrum of alternatively spliced and polyadenylated transcripts encoded by the Hox genes of S. maritima is comparable with what is known from D. melanogaster (details in Text S1). Exceptionally among protostomes, the S. maritima Hox cluster retains tight linkage to one orthologue of evx/evenskipped, as it does in some chordates and cnidarians.
Further instances of homeobox gene clustering and linkage, and reconstructions of ancestral states, are summarized in Figure 4 and Table 1 (and see Text S1). The Hox gene cluster is hypothesized to have evolved within the context of a Mega-homeobox cluster that existed before the origin of the bilaterians and consisted of an array of many ANTP-class genes [49]–[51]. By the time of the last common ancestor of bilaterians the Hox cluster existed within the context of a SuperHox cluster, containing the Hox genes themselves and at least eight further ANTP-class genes [52]. The conservative nature of the S. maritima genome has left several fragments from the Mega-homeobox and SuperHox clusters still intact (Figure 4; Table 1). Furthermore, homeobox linkages in S. maritima raise the possibility that further genes could have been members of the Mega-homeobox and SuperHox clusters, including the ANTP-class gene Vax, as well as the SINE-class gene sine oculis and the HNF-class gene Hmbox (see Text S1 for further details).
Figure 4. Homeobox gene clusters.
(A) The Hox gene cluster of S. maritima compared to the cluster that can be deduced for the ancestral arthropod. S. maritima provides the first instance of an arthropod Hox cluster with tight linkage of an Even-skipped (Eve) gene (see text). Hox3 is the only gene missing from the S. maritima Hox cluster, but may be present elsewhere in the genome on a separate scaffold (see main text and Text S1 for details). The S. maritima cluster is drawn approximately to scale and spans 457 kb from the start codon of labial (lab) to the start codon of Eve-b. Arrows denote the transcriptional orientation. (B) Remains of clustering and linkage of ANTP class genes in S. maritima. The blue boxes are genes belonging to the ANTP class. The brown box is a gene belonging to the HNF class. The orange box is a gene belonging to the SINE class. The intergenic distances are indicated in kb. (C) Clusters of non-ANTP class homeobox genes in S. maritima. The green boxes are genes belonging to the TALE class. The red boxes are genes belonging to the PRD class. The intergenic distances are indicated in kb, except in the case of Rx-Hbn as these genes are overlapping but with opposite transcriptional orientations. All scaffold numbers are indicated in brackets.
Table 1. Instances of homeobox gene clustering and linkage.
| Gene Cluster | Details | Conclusion or Hypothesis |
| Hox Cluster | Intact well ordered, but lacking Hox3 (Figure 4A). Two potential Hox3 genes elsewhere in the genome, but these could also be Xlox homologues | Has Xlox really been lost from all lineages of the ecdysozoan super phylum? |
| NK - Vax linkage | Centipede has gene pair remnants from the ancestral NK cluster slouch and drop, and tinman and bagpipe (now with Vax linkage, which also seen in mollusc) (Figure 4B) | Vax linkage likely ancestral, Vax a new member of the ancestral ANTP class mega-homeobox cluster. |
| IRX/Iroquois | Cluster of three Irx genes(Figure 4C) | Independent expansion from Drosophila by duplication of mirror. |
| Orthopedia, Rax, and Homeobrain | Cluster present in S. maritima (Figure 4C) | An ancestral cluster also found in insects, cnidarians, and molluscs. |
| SuperHox cluster remains | Linkage of BtnN and En on Scaffold JH431870. Linkage of Exex-Nedx-BtnA on scaffold JH431734 (Figure 4B) with Hmbox. | Remnants of the Super-Hox cluster? |
| ParaHox - NK linkage (Mega-cluster remains) | Tight linkage of Ems (NK gene) with IndB (ParaHox gene), and Ind-like (ParaHox like) with scro (NK gene) (Figure 4B) | Possible remnant of ParaHox and NK clusters from ancestral Mega-Clustera |
| SINE-ANTP class linkage | linkage of sine oculis & Ems | Also seen in humans and zebrafish - thus linkage of SINE and ANTP genes in bilaterian ancestor |
Chemosensory Gene Families (Gustatory Receptors, Ionotropic Receptors, Odorant Binding Proteins, Chemosensory Proteins)
The chemosensory system of arthropods is best known in insects. During the evolutionary transition from water to terrestrial environments, insects evolved a new set of genes to detect airborne molecules (odorants) [53]–[55]. The independent colonization of land by insects and myriapods raises two interesting questions: (1) what are the genes involved in chemosensation in non-insect arthropods, and (2) what genes are responsible for the detection of airborne molecules in other terrestrial arthropods? We searched the S. maritima genome for homologues of the insect chemosensory genes, included in six gene families, three ligand binding protein families: odorant binding proteins (OBPs) [56],[57], chemosensory proteins (CSPs) [58],[59], and CheA/B [60],[61]; and three membrane receptor families: GRs [62],[63], odorant receptors (ORs) [64],[65], and IRs [66],[67].
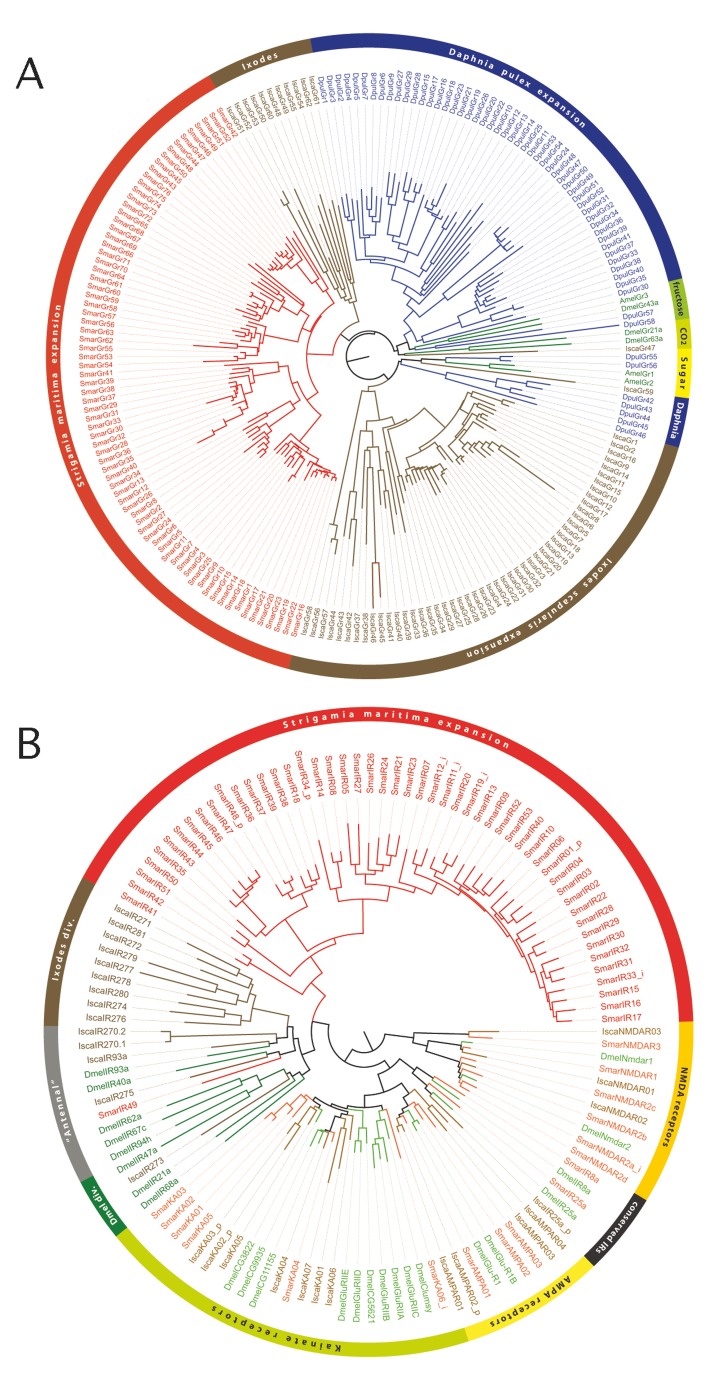
Of the ligand binding proteins, we found only two genes belonging to the CSP family, but no representatives of the OBP or CheA/B families. Among the membrane receptor families, we identified a number of genes of both the GR and IR families, but no OR genes. The GR family in S. maritima is represented by 77 genes, 17 of which seem to be pseudogenes, with similar numbers of genes and pseudogenes being fairly typical features of this gene family in other arthropods. A phylogenetic tree revealed that none of the S. maritima GR genes have 1∶1 orthology to other arthropod GRs. Instead, all S. maritima GRs cluster in a single clade, with six major subclades, representing separate expansions of the GR repertoire in the centipede lineage (Figure 5A and see Text S1). The IR family is known to be ancient [67], but S. maritima has a relative expansion of this family. The search for IRs led to the annotation of 69 genes, 15 of which belong to the IGluR subfamily, which is not involved in chemosensation, but is highly conserved among arthropods and animals in general. Among the remaining 54 IRs, three are orthologues of conserved IR genes that have been shown to have an olfactory function in D. melanogaster. However, 51 of the S. maritima IRs do not have orthologues either in D. melanogaster or in Ixodes scapularis, clustering together in a single clade (the expansion clade in Figure 5B). This finding suggests that most S. maritima IRs, as observed with GRs, have duplicated from a common ancestral gene exclusive to the centipede lineage.
Figure 5. Expansion of chemosensory receptor families.
(A) Phylogenetic relationships among S. maritima (Smar), I. scapularis (Isca), D. pulex (Dpul), and a few insect GRs that encode for sugar, fructose, and carbon dioxide receptors (Dmel, D. melanogaster, and Amel, A. mellifera). (B) Phylogenetic relationships among S. maritima, I. scapularis, and a few D. melanogaster IRs and IgluR genes (the suffix at the end of the protein names indicates: i, incomplete and p, pseudogene).
The absence of the insect OR family agrees with the prediction of Robertson and colleagues [54] that this lineage of the insect chemoreceptor superfamily evolved with terrestriality in insects, and it is also missing from the water flea Daphnia pulex [53]. The same appears to be true for the OBPs. We therefore infer that, as centipedes adapted to terrestriality independently from the hexapods, they utilized a novel combination of expanded GR and IR protein families for olfaction, in addition to their more ancestral roles in gustation.
Light Receptors and Circadian Clock Genes
S. maritima, like all species of the order Geophilomorpha, is blind [37]. Nevertheless, it avoids open spaces and negative phototaxis has been demonstrated in other species of Geophilomorpha [38],[68]. We searched the S. maritima genome for light receptor genes. Interestingly, we have found no opsin genes, no homologue of gustatory receptor 28b (GR28b), which is involved in larval light avoidance behaviour in Drosophila [69], and no cryptochromes. Thus, none of the known arthropod light receptors are present. Furthermore, there are no photolyases, which would repair UV light induced DNA damage. As a consequence, the critical avoidance of open spaces by S. maritima must either be mediated by other sensory instances than light perception, or S. maritima possesses yet unknown light receptor molecules.
The absence of light receptors, particularly cryptochromes, also raises the issue of the entrainment and composition of a potential S. maritima circadian clock. Strikingly, we could not identify any components of the major regulatory feedback loop of the canonical arthropod circadian clock (including period, cycle, b-mal/clock, timeless, cryptochromes 1 and 2, jetlag [70]). The only circadian clock genes found (timeout, vrille, pdp1, clockwork orange) are generally known to be involved in other physiological processes as well [71]–[73]. The extensive secondary gene loss of both light receptors and circadian clock genes raises questions about the actual existence of a circadian clock in S. maritima. One could hypothesize that a circadian clock may not be required in S. maritima's subsurface habitat, although other periodicities, such as tide cycles, might be important. If S. maritima does have a circadian clock then it must be operating via a mechanism distinct from the canonical arthropod system.
Other blind or subterranean animals do maintain a circadian rhythm, despite complete loss of vision and connection with the surface (e.g., Spalax) [74]–[76]. In other cases (e.g., blind cave crayfish [77]), despite the loss of vision, opsin proteins remain functional, and are hypothesized to have a role in circadian cycles. However, both these examples represent species that have become blind and subterranean relatively recently. To confirm that the loss of these genes is not general for all centipedes, we performed BLASTP analyses searching for the set of light sensing and circadian clock genes that are missing from S. maritima in RNAseq data from the house centipede Scutigera coleoptrata (NCBI SRA accession SRR1158078), a species with well-developed eyes. We find homologs to period, cycle, b-mal/clock, jetlag, cryptochrome1, cryptochrome 2, (6-4)-photolyase, and nina-e (rhodopsin 1), suggesting that both light sensing and circadian clock systems were present in ancestor of myriapods. Although we have no direct information about photoreceptors or circadian genes in other geophilomorph species, the fact that all geophilomorphs are blind suggests that the loss of the related genes is very ancient, and may date back to the origin of the clade.
Putative Cuticular Proteins
A defining characteristic of arthropods is an exoskeleton with chitin and cuticular proteins as the primary components. Although several families of cuticular proteins have been recognized, the CPR family (Cuticular Proteins with the Rebers and Riddiford consensus) is by far the largest in every arthropod for which a complete genome is available, with 32 to >150 members [78]. Proteins in the CPR family have a consensus region in arthropods of about 28 amino acids, first recognized by Rebers and Riddiford [79], which was subsequently extended to ∼64 amino acid residues and shown to be necessary and sufficient for binding to chitin [80]. No clear instances of the Rebers and Riddiford (RR) consensus have been identified outside the arthropods. We identified 38 members of the CPR family in S. maritima. There are two main forms of the consensus, designated RR-1 and RR-2, with the former primarily associated with flexible cuticle, the latter with rigid cuticle. Interestingly, while chelicerates studied to date have no members of the RR-1 subfamily (as classified at CutProtFam-Pred, http://aias.biol.uoa.gr/CutProtFam-Pred/home.php), seven of the S. maritima CPR proteins clearly belong to this class. This would be consistent with the origin of the RR1-coding genes being in the mandibulate ancestor after this lineage had diverged from the chelicerate lineage. Further data are needed to verify that the identified proteins are indeed important constituents of the cuticle.
Neuro-endocrine Hormone Signalling
Cell-to-cell communication in arthropods occurs via a variety of neurotransmitters and neuro-endocrine hormones, including biogenic amines, neuropeptides, protein hormones, juvenile hormone (JH), and ecdysone. These signalling molecules and their receptors steer central processes such as growth, metamorphosis, feeding, reproduction, and behaviour. Most receptors for biogenic amines, neuropeptides, and protein hormones are G protein-coupled receptors (GPCRs) [81]. Intracellularly, the G proteins initiate second messenger cascades [82]. JH and ecdysone, however, are lipophilic and can diffuse through the cell membrane to bind with nuclear receptors [83],[84]. In addition, ecdysone can also activate a specific GPCR, and initiate a second messenger cascade [85]. There is extensive cross-talk between these extracellular signal molecules.
S. maritima possesses 19 biogenic amine receptors, a number similar to the 18–22 biogenic amine receptors that have been identified in other arthropods (Table S19). In S. maritima, there are four octopamine GPCRs, one octopamine/tyramine, one tyramine, four dopamine and three serotonin GPCRs, three GPCRs for acetylcholine, one GPCR for adenosine, and two orphan biogenic amine receptors. Although this distribution resembles very much that of Drosophila and other arthropods, there are some interesting differences with Drosophila, which expresses two additional β-adrenergic-like octopamine receptors compared to S. maritima, while S. maritima expresses two putative β-adrenergic-like octopamine receptors (Sm-OctBetaRHK and Sm-D1/OctBeta), which are expressed in a number of insect and tick species, but not in Drosophila (Table S20) [86]. The true functional identities of all the putative S. maritima biogenic amine GPCRs awaits their cloning, functional expression, and pharmacological characterization in cell lines.
In addition, 36 neuropeptide and protein hormone precursor genes are present in this centipede. Each neuropeptide precursor contains one or more (up to seven) immature neuropeptide sequences (Figure S20). Interestingly, the centipede contains two CCHamide-1, two eclosion hormone, and two FMRFamide genes, whereas these genes are only present as single copies in the genomes of most other arthropods [87]. In concert with the presence of 36 neuropeptide genes, we found 33 genes for neuropeptide receptors (31 GPCRs and two guanylcyclase receptors) (see Table S21). As observed for the neuropeptide genes, a number of the neuropeptide receptor genes, which are only found as single copies in most other arthropods, have also been duplicated. S. maritima has two inotocin GPCR genes, two SIFamide, two corazonin, two eclosion hormone guanylcyclase receptor genes, two eclosion triggering hormone GPCR genes, three sulfakinin GPCR genes, and three LGR-4 (Leu-rich-repeats-containing-GPCR-4) genes. The latter receptors are orphans (GPCRs without an identified ligand) and only present as single-copy genes in most other arthropods [88]. Several of these duplicated GPCR genes are located in close vicinity to each other in the genome (Figure S21, suggesting recent duplication events. Furthermore, duplications of both the eclosion hormone and its receptor genes and the duplication of the ecdysis triggering hormone receptor genes suggest that the process of ecdysis (moulting) has undergone some sort of modification, perhaps requiring more complex control in the lineage leading to centipedes.
We summarize in Table S22 the neuropeptide/protein hormone signalling systems that are present or absent in selected arthropod genome sequences. Each arthropod species, including S. maritima, has its own characteristic pattern, or “barcode,” of present/absent neuropeptide signalling systems. However, the relationship between the specific neuropeptide “barcode” and physiology remains to be elucidated.
Insect JH is important for growth, moulting, and reproduction in arthropods [84]. This hormone is a terpenoid (unsaturated hydrocarbon) that is synthesized from acetyl-CoA by several enzymatic steps (Figure S22). In several insects the production of JH is stimulated by the neuropeptide allatotropin, while it is inhibited by either allotostatin-A, -B, or -C [89],[90]. We found that S. maritima has orthologues of many of the biosynthetic enzymes needed for JH biosynthesis in insects (Table S23). Also, the JH binding proteins are encoded in the centipede genome as well as JH degradation enzymes (Table S24). This implies that the complete JH system is present in this centipede. Similarly, neuropeptides that could stimulate or inhibit the synthesis and release of JH, such as allatotropin and the allatostatins -A, -B, and -C, are also present in S. maritima (Figure S22, suggesting that the overall functioning of the JH system in centipedes might be very similar to that of insects) (Table S23). To date, the existence of JH signalling systems has been demonstrated in insects, crustaceans, and recently in spider mites [89],[91],[92]. Its occurrence in S. maritima and spider mites (Chelicerata) indicates that JH signalling has deep evolutionary roots and we suggest that it might have evolved together with the emergence of the exoskeleton in arthropods.
Developmental Signalling Systems
Certain signalling systems, including transforming growth factor (TGF)-beta, Wnt, and fibroblast growth factor (FGF), are used throughout development across the animal kingdom. Various lineage-specific modifications of these systems have occurred, particularly within the arthropods. With regards to TGF-beta signalling we found single orthologues of all members of the Activin family, except Alp (Activin-like protein) (see Figure S23; Text S1). In the BMP-family, the S. maritima genome contains two divergent BMP sequences, as well as a clear orthologue of glass-bottom boat (gbb) and two decapentaplegic (dpp) orthologues. In addition, the S. maritima sequences confirm the ancestral presence of an anti-dorsalizing morphogenetic protein (ADMP) and a BMP9/10 orthologue in arthropods, which are both absent from Drosophila [93]. Most interestingly, the S. maritima genome includes the antagonistic BMP ligand BMP3 (previously suggested to be present only in deuterostomes [94]), a potential gremlin/neuroblastoma suppressor of tumorigenicity, and two nearly identical bambi genes (absent from Drosophila), and the BMP inhibitor noggin (present in vertebrates but lost in most holometabolous insects). The multiple BMP-agonists and -antagonists indicate that considerable changes have occurred in the TGF-beta signalling system during arthropod evolution, particularly in the Holometabola.
Reconstructions of Wnt gene evolutionary history suggest that the ancestral bilaterian possessed at least 13 distinct Wnt gene subfamilies [95],[96]. This initial number has been secondarily reduced in many taxa. This trend of secondary gene loss is readily apparent within the arthropods, with holometabolous insects such as D. melanogaster retaining only seven Wnt subfamilies [97],[98]. In contrast, the Wnt signalling complement in S. maritima comprises 11 of the 13 Wnt-ligand subfamilies (Figure S24). Phylogenetic investigation has identified these genes as wnt1, wnt2, wnt4, wnt5, wnt6, wnt7, wnt9, wnt10, wnt11, wnt16, and wntA. wnt3 and wnt8 are missing from the S. maritima genome. While the absence of wnt3 is common to protostomes, wnt8 or wnt8-like sequences occur in other protostome genomes, including insects, spiders, and another myriapod, Glomeris marginata [97]. The Wnt genes are known to display a degree of linkage and clustering in many arthropods. Some conservation of this is also found in S. maritima, with wnt1, wnt6, and wnt10 adjacent to each other on the same scaffold, possibly representing part of an ancient clustering (Table S25) [99].
The primary receptors for Wnt ligands in the canonical Wnt signalling pathway are the trans-membrane receptors of the Frizzled family. Five of these have been identified: Frizzled1, Frizzled4, Frizzled5/8, Frizzled7, and Frizzled10. As is the case for the wnt genes themselves, this is a larger number than is found in most arthropods. Other Fz-related genes are also present: smoothened, involved in Hedgehog signalling, and secreted frizzled related protein, which has inhibitory roles in Wnt signalling in other taxa. Putative non-canonical Wnt receptors are also encoded, including two subfamilies of receptor tyrosine kinase-like orphan receptor (ror). In addition to ror2, there is a lineage-specific duplication of ror1, making a total of three ror genes, as opposed to only one in D. melanogaster. Another Wnt agonist, the R-spondin orthologue was also found. As part of the Wnt-binding complex we found one arrow-LRP5/6-like Wnt-coreceptor gene in the genome: lrp6. Other LRP-molecules with potential Wnt-binding activity also exist: LRP1, LRP2, and LRP4. Because of the absence of an intracellular signalling domain these could potentially function as Wnt-inhibitors. Together, the large number of ligand and receptor genes point towards both the conservation of an ancestral Wnt signalling system and to a certain degree of unusual complexity in of this system in S. maritima.
Concerning the FGF pathway, we identified two closely related FGF receptors. These two S. maritima receptors are likely to stem from a duplication in the myriapod lineage that was independent from that which generated the two Drosophila FGFRs, Heartless and Breathless (Figure S25). The number of FGF ligands found in the genomes of insects such as D. melanogaster (three fgf genes) or T. castaneum (four fgf genes) is small when compared to 22 fgf genes found in the genomes of vertebrates. In the S. maritima genome, we identified three fgf-genes (Figure S26). One of them potentially represents an fgf 18/8/24 orthologue to which the fgf8-like genes of Tribolium and of Drosophila (pyramus and thisbe) are associated. The second S. maritima fgf groups with the fgf1 genes, while the third groups with the fgf 16/9/20 clade (the first known arthropod member of this clade). Low support values for this grouping raise the possibility that it might actually be an orthologue of insect branchless genes. Other FGF-pathway genes present in S. maritima include stumps (Downstream-of-FGF-signalling [DOF]) and sprouty related.
Protein Kinases
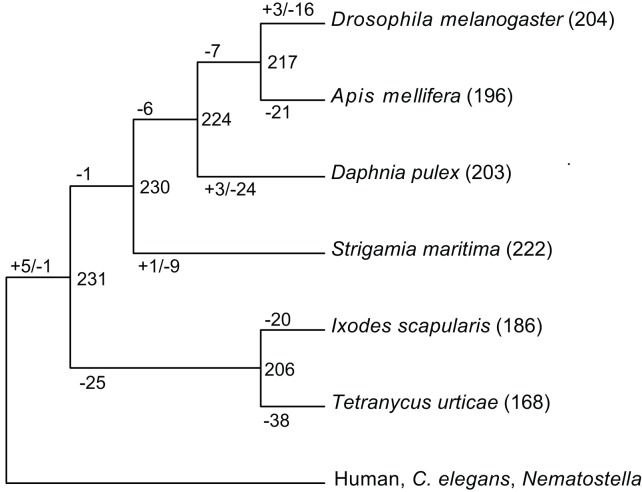
Kinases make up about 2% of all proteins in most eukaryotes, while they phosphorylate over 30% of all proteins and regulate virtually all biological functions. We identified 393 protein kinases in the S. maritima genome, representing 2.6% of the proteome. We classified these into conserved families and subfamilies, compared the kinome to those of 26 other arthropods and inferred the evolutionary history of all kinases across the arthropods (Figure 6). We predict that an early arthropod had at least 231 distinct kinases and see considerable loss of ancestral kinases in most extant species. S. maritima has the smallest number of losses among the arthropods, with only ten kinases lost relative to the arthropod ancestor. In contrast, the two chelicerates T. urticae and I. scapularis have lost 63 and 45 kinases, respectively, and D. melanogaster lost 30, giving S. maritima the richest repertoire of conserved kinases of any arthropod examined. All but one of the losses in S. maritima have been lost in other arthropods, suggesting that these genes may be partially redundant or particularly prone to loss. The one unique loss is NinaC, which in Drosophila is required for vision, likely associated with other vision related gene loss described above. As in many other species, we also see some novelties and expansions of existing families: the SRPK kinase family, involved in splicing and RNA regulation, has expanded to 36 members, and the nuclear VRK family is expanded to 16. A novel family of receptor guanylate cyclases (nine genes) and three clusters of unique protein-kinase-like (PKL) kinases, containing 28 genes in total, are also seen, though their functions are not known.
Figure 6. Ancestral protein kinases are extensively lost during arthropod evolution.
S. maritima is an exception and retains the largest number of ancestral kinases. Numbers of kinase subfamilies in selected species are shown in parentheses after species names. The gains, losses, and inferred content of common ancestors are listed on internal branches. Kinases found in at least two species from human, C. elegans and Nematostella vectenesis were used as an outgroup.
Developmental Transcription Factors
DNA binding proteins with the capacity to regulate the expression of other genes are central players in the control of development and many other processes. Since one of the original interests in S. maritima was for its developmental characteristics, we carried out a survey of developmentally relevant transcription factors, with an emphasis on transcription factors suspected to be involved in processes of axial specification, segmentation, mesoderm formation, and brain development. We identified orthologues of ∼80 transcription factors of the Zinc finger and helix-loop-helix families in addition to the 113 homeobox-containing transcription factors already discussed (see Text S1). In no case did we fail to find at least one orthologue of the gene families expected from our knowledge of Drosophila, though individual duplications and losses among gene families were not uncommon. Among the set of pair-rule segmentation genes, for example, S. maritima has multiple homologues of paired, even-skipped, odd-skipped, odd-paired, and hairy-like genes, but only a single orthologue of sloppy-paired and runt-like genes, whereas Drosophila has multiple runt and sloppy-paired genes but only single orthologues of even-skipped and odd-paired. Where both lineages have multiple copies, (paired, hairy, odd-skipped), sequence alone rarely defines one-to-one orthologous relationships, and the evolutionary history remains unclear [29]. Other notable duplications include caudal (three genes) and brachyury (two genes). In a number of cases, transcription factors known to play a role in vertebrate development, but apparently missing from Drosophila and other insects, are retained in S. maritima. Examples include the homeobox genes Dmbx and Vax noted above, and the FoxJ1, FoxJ2, and FoxL1 subfamilies of forkhead/Fox factors.
One of the developmental transcription factors provides an example where insects use isoforms to generate alternative proteins that are encoded by paralogous genes in S. maritima. Two centipede orthologues of the developmental transcription factor cap‘n’collar encode isoforms that differ at their N-terminal end. The longer protein, encoded by the gene cnc1, contains sequence motifs that align to Drosophila cnc isoform C (Figure S27, which is broadly expressed throughout embryonic development) [100]. S. maritima cnc1 is similarly expressed ubiquitously, whereas the other orthologue, cnc2, shows a segment specific pattern of expression similar to that of the shorter Drosophila cnc isoform B (VSH and MA, unpublished) [100].
Immune System
Arthropods can mount an innate immune response against pathogenic bacteria, fungi, viruses, and metazoan parasites. The nature of the responses to these invaders, such as phagocytosis, encapsulation, melanisation, or the synthesis of antimicrobial peptides, is often similar across arthropods [101]. Furthermore, key aspects of innate immunity are conserved between insects and mammals, which suggests an ancient origin of these defences. Previous studies have revealed extensive conservation of key pathways and gene families across the insects and crustaceans [102]. Beyond the Pancrustacea the extent of immunity gene conservation is unclear. Therefore, we searched the S. maritima genome for homologues of immunity genes characterised in other arthropods.
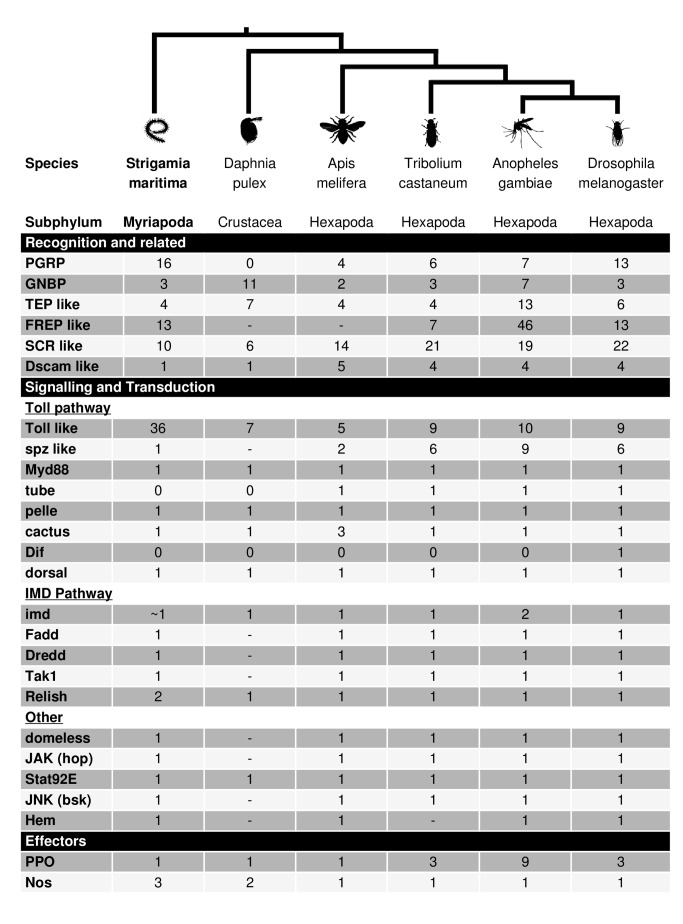
We found conservation of most immunity gene families between insects and S. maritima (Table S30), suggesting that the immune gene complement known from Drosophila was largely present in the most recent common ancestor of the myriapods and pancrustaceans. The humoral immune response of insects recognises infection using proteins that bind to conserved molecular patterns on pathogens [103]. Sequence homologues for the major recognition protein families found in Drosophila, peptidoglycan recognition proteins (PGRPs), and gram-negative bacteria-binding proteins (GNBPs), were found with the expected protein domains. These proteins then activate signalling pathways [103], and all four major insect immune signalling pathways (Toll, IMD, JAK/STAT, and JNK) are present in S. maritima, with 1∶1 sequence homologues of most pathway members. The cellular immune response of insects relies on receptors and opsonins including thioester-containing proteins (TEPs), fibrinogen related proteins (FREPs), and scavenger receptors [103],[104], and these are also present in S. maritima, often with protein domains in the same arrangement as Drosophila. We also find sequence homologues for effector gene classes including nitric oxide synthases (NOS) and prophenoloxidase (PPO). However, we failed to identify any antimicrobial peptide homologues, possibly as these genes are often short and highly divergent between species. In insects, it is common to find that certain immune gene families have undergone expansions in certain lineages [105]. Again, this is mirrored in S. maritima, where we found lineage-specific expansions of the PGRP and Toll-like receptor genes (TLRs) (Figure 7). Overall, the presence of the main families of immunity genes suggests that there is also functional conservation of the immune response.
Figure 7. Presence and absence of immunity genes in different arthropods.
Counts of immune genes are shown for S. maritima, D. pulex [131], A. mellifera [86], T. castaneum, Anopheles gambiae, and D. melanogaster [132]. ∼, identity of the gene is uncertain; -, not investigated.
The innate immune system is thought to rely on a small number of immune receptors that bind to conserved molecules associated with pathogens. This view was challenged by the discovery in Drosophila that the gene Dscam (Down syndrome cell adhesion molecule), which has the potential to generate over 150,000 different protein isoforms by alternative splicing, functions as an immune receptor in addition to its roles in nervous system development [106]. Dscam family members are membrane receptors composed of several immunoglobulin (Ig) and fibronectin domains (FNIII). In pancrustaceans one member of the Dscam family has a large number of internal exon duplications and a sophisticated mechanism of mutually exclusive alternative splicing, which enables a single Dscam locus to somatically generate thousands of isoforms, which differ in half of two Ig domains (Ig2 and Ig3) and in another complete Ig domain (Ig7). This creates a high diversity of adhesion properties, useful for immune responses.
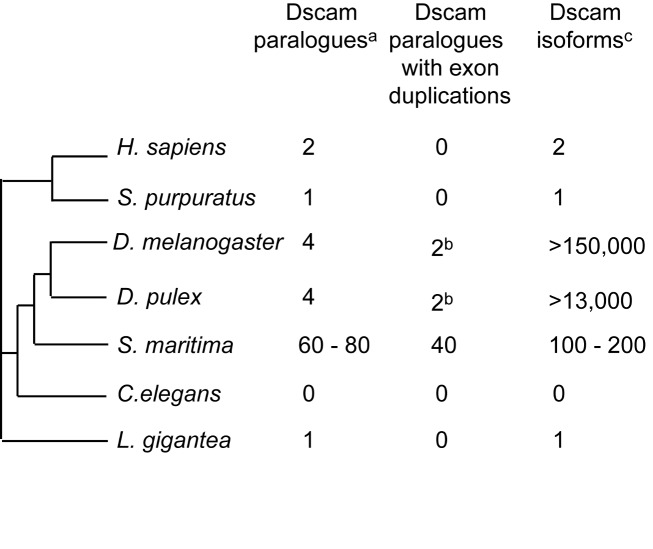
We found that S. maritima has evolved a different strategy to generate a diversity of Dscam isoforms [107]. The genome contains 60 to 80 canonical Dscam paralogues and over 20 other Dscam related incomplete or non-canonical genes (Figure 8). In 40 Dscam genes, the exon coding for Ig7 is duplicated two to five times (but not the exons coding for Ig2 and Ig3, which are duplicated in pancrustaceans). Our analysis of transcripts suggests that many of those duplicated exons might be alternatively spliced in a mutually exclusive fashion, supporting the hypothesis that the mechanism of mutually exclusive alternative splicing of Dscam probably evolved in the common ancestor of both pancrustaceans and myriapods. According to our phylogenetic analysis, which included 12 paralogues, the S. maritima Dscams share a common origin and arose by duplication in the centipede lineage [107]. In the chelicerate I. scapularis, Dscam has also been duplicated extensively, both by whole-gene and by domain duplications [107]. These Dscam homologues however do not have a canonical domain composition and whether or not alternative splicing is also present in chelicerates remains unknown. The independent evolution of Dscam diversification in different arthropod groups (one locus with dozens of exon duplications in pancrustaceans versus many gene duplications coupled with a few exon duplications in S. maritima (Figure 8) suggests that the functional diversity in adhesion properties was important in the early evolution of arthropods. Whether all of these genes function in the immune system or nervous system development remains to be determined.
Figure 8. Dscam diversity caused either by gene and/or exon duplication in different Metazoa.
aOnly canonical Dscam paralogues were considered. bIn D. melanogaster and D. pulex the paralogue Dscam-L2 has two Ig7 alternative coding exons. cPotential number of Dscam isoforms, circulating in one individual, produced by mutually exclusive alternative splicing of duplicated exons.
The short-interfering RNA (siRNA) pathway is the primary defence of insects against RNA viruses, while the piRNA pathway silences transposable elements in the germ line and micro RNAs (miRNAs) function in gene regulation [108]. These RNAi pathways appear to be intact in S. maritima, as we found homologues of key genes, including Ago1 and Dicer-1 in the miRNA pathway, Ago2 and Dcr2 in the siRNA pathway, and Ago3 and piwi in the piRNA pathway (Table S30). We found two paralogues of Ago2 and three paralogues of piwi, suggesting that RNAi may be more complex than in D. melanogaster. In other arthropods, expansion of the piwi family has been linked to neo- or subfunctionalization of germ line and soma roles, and so it remains to be seen whether this is also the case for S. maritima.
Selenoproteins
Selenoproteins are peculiar proteins including a selenocysteine (Sec) residue, a very reactive amino acid typically found in the catalytic site of redox proteins, which is inserted through the recoding of a UGA codon [109]. While vertebrates possess 24–38 selenoproteins [110], insects have very few (D. melanogaster has three) or none at all. Several events of complete selenoproteome loss have been observed in insects [111]. These were ascribed to the fundamental differences in the insect antioxidant systems, which would favour selenoprotein loss or their conversion to standard proteins (cysteine homologues). The analysis of a myriapod selenoproteome is then crucial for a phylogenetic mapping of such differences.
The S. maritima genome was found to be surprisingly rich in selenoproteins: we have identified 20 predicted proteins (Table S26). Downstream of the coding sequence of each selenoprotein gene, we detected a selenocysteine insertion sequence (SECIS) element, the stem-loop structure necessary to target the Sec recoding machinery during selenoprotein translation. The full set of factors necessary for selenocysteine insertion and production was also found: tRNA-Sec, SecS, SBP2, eEFsec, pstk, secp43, SPS2. The centipede selenoproteome is rather similar to that predicted for the ancestral vertebrate (see [110]). This supports the idea that selenoprotein losses are specific to insects and can be ascribed to changes in that lineage, supporting the idea that a massive selenoproteome reduction occurred specifically in insects. A notable difference with vertebrates was found for the protein methionine sulfoxide reductase A (MsrA). This enzyme catalyzes the reduction of methionine-L-oxide to methionine, repairing proteins that were inactivated by oxidation. A selenoenzyme from this family has been previously characterized in the green alga Chlamydomonas, and selenocysteine containing forms were also observed in some non-insect arthropods [112]. In contrast, only cysteine homologues are present in vertebrate and insect genomes. We found a Sec-containing MsrA in the centipede genome, as well as in arthropods D. pulex, I. scapularis, and also in the chordate B. floridae. This, along with phylogenetic reconstruction analysis, supports the idea that the selenoprotein MsrA was present in their last common ancestor, and was later converted to a cysteine homologue independently in insects and vertebrates.
The two major antioxidant selenoprotein families in vertebrates, glutathione peroxidases (GPx), and thioredoxin reductases (TrxR), were also found with selenocysteine in the centipede genome. In contrast, all holometabolous insects possess only cysteine forms, and consistently, important differences were noted in these and other enzymes in the glutathione and thioredoxin system (see [113] for an overview). Thus, on the basis of gene content, we expect the antioxidant systems of S. maritima to be more similar to vertebrates and other animals than to holometabolous insects like D. melanogaster.
DNA Methylation
Invertebrate DNA methylation occurs predominantly on gene bodies (exons and introns), via addition of a methyl group to a cytosine residue in a CpG context [114]–[116]. The exact function of gene body methylation is currently unknown, though it is correlated with active transcription in a wide range of species [116], and has been implicated in alternative splicing [117],[118] and regulation of chromatin organization [118]. Methylated cytosines are susceptible to deamination, to form a uracil, which is recognized as a thymine. Thus, over evolutionary time, highly methylated genes (in germ-line cells) will have comparatively low CpG content. The “observed CpG/expected CpG” (CpG(o/e)) ratio is an indicator of C-methylation: plots of CpG(o/e) for a gene set produce a bimodal distribution where a proportion of the genes have an evolutionary history of methylation [119]. In contrast, species without methylation systems, such as D. melanogaster, yield a unimodal distribution [119].
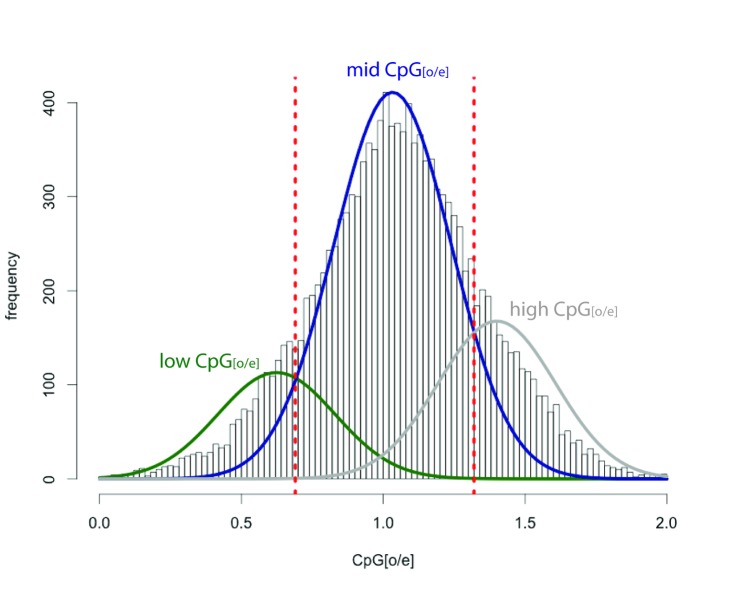
The S. maritima gene body CpG(o/e) plot has a trimodal distribution, with the majority of genes having a ratio close to 1 (Figure 9; Text S1). Underlying this major peak are two smaller peaks, one “low” and one “high” centred around ratios of 0.62 and 1.48, respectively. This “high” peak, that contains genes with higher than expected CpG content, is unusual and is not seen in this analysis of other arthropods [91],[119]–[121]. Applying the same analysis to 1,000 bp windows across the entire genome (including both coding and non-coding regions) reveals a similar peak of high CpG content (Figure S29). This implies that the peak of “high” CpG content seen in gene bodies is due to unusually high CpG content in some regions of the genome rather than a specific feature of those coding regions. The “low” peak, however, indicates that 9.5% of genes have been methylated in the germ-line over evolutionary time. The number of genes contained within the “low” peak in S. maritima is smaller than observed in insect species with methylation, which can be as high as 40% in exceptional species such as the pea aphid and the honeybee [119],[120], where the mechanism is likely involved in polyphenism and caste differences respectively. However, the number of genes methylated is less in non-social hymenopteran such as Nasonia vitripennis, in beetles, and in mites [91],[121],[122]. Consistent with the low-levels of germ-line methylation detected, the genome contains a single orthologue of the de novo DNA methylation enzyme Dnmt3 and four orthologues of the maintenance DNA methyltransferases Dnmt1(a–d). Two of the Dnmt1 orthologues have lost amino acids that are required for methyltransferase activity, but these genes are represented in the transcriptome data, and are thus unlikely to be pseudogenes. One Dnmt1 gene shows sex-specific splicing, with a shorter transcript producing a truncated protein seen in female-derived transcription libraries. We also find a single orthologue of Tet1, a putative DNA demethylation enzyme [123],[124]. Taken together these data indicate that S. maritima has an active DNA methylation system, and that over evolutionary time a small number of genes have been methylated in the germ-line, resulting in a lower than expected CpG dinucleotide content.
Figure 9. Frequency histogram of CpG(o/e) observed in S. maritima gene bodies.
The y-axis depicts the number of genes with the specific CpG(o/e) values given on the x-axis. The distribution of CpG(o/e) in S. maritima is a trimodal distribution, with a low-CpG(o/e) peak consistent with the presence of historical DNA methylation in S. maritima and the presence of a high CpG(o/e) peak. The data underlying this plot are available in File S4.
Non-Protein-Coding RNAs in the S. maritima Genome
We annotated over 900 homologues of known non-coding RNAs in the S. maritima genome, including over 600 predicted tRNAs (plus an additional 300 tRNA pseudogenes), 71 copies of 5S rRNA and 12 5.8S rRNAs, 88 copies of RNA components of the major spliceosome, and three out of the four RNA components of the minor U12 spliceosome, and 54 microRNA genes. As is common for whole genome assemblies, we did not identify intact copies of the 18S or 28S rRNAs. Further details of our methodology are provided in Text S1.
The predicted tRNA gene set includes all anticodons necessary to code for the 21 amino acids, including four potential SeC tRNAs. We identify a massive expansion of the tRNA-Ala-GGC family, with 322 sequences classified as functional tRNAs by tRNAscan-SE and an additional 172 classified as pseudogenes. These appear scattered throughout the scaffolds of the genome assembly. It is highly likely that the majority of these genes are pseudogenes, and the expansion may represent co-option of the tRNA into a transposable element.
Three S. maritima microRNA genes have been reported previously, and are available in the miRBase database (version 18) [125]. Two of these, mir-282 and mir-965, have homologues in crustaceans and insects. The third, mir-3930, is specific to myriapods [15]. In addition, we found 52 homologues of known microRNAs (Figure S34). These include 28 homologues of the 34 ancient microRNA families found throughout the Bilateria [126]. Four of these families were previously reported to be lost at various stages during animal evolution and, consistent with this, we failed to identify them in the S. maritima genome. Surprisingly, we also could not identify the S. maritima homologue of mir-125, a member of the ancient mir-100/let-7/mir-125 cluster, which is found in almost all bilaterians and has a well-established function in the regulation of development of many species [127]–[129]. Mir-100 and let-7 are well-conserved and localized within a 1 kb region on the same scaffold in S. maritima. Whilst we cannot rule out the possibility that the missing mir-125 is an artefact of the draft-quality genome assembly, the size of the scaffold strongly suggests that it is not present in the mir-100/let-7 cluster. We also identified 17 homologues of microRNAs common to ecdysozoans, and nine microRNAs known only from arthropods. Among the former, there are five homologues of mir-2 localized in close proximity to each other and downstream of mir-71. This clustering is conserved across protostomes, and it has previously been shown that the mir-2 family underwent various expansions during evolution [130]. Finally, we discovered a homologue of mir-2788, which was previously only known from insects, suggesting that this microRNA had an earlier origin.
Conclusions
The sequencing of the centipede genome extends significantly the diversity of available arthropod genomes, and provides novel information pertinent to a range of evolutionary questions. Myriapods show a simple body organization that has remained relatively unchanged in comparison to their ancestors from the Silurian or even earlier [6], leading to an expectation of general conservatism. The myriapods are descendants of an independent terrestrialisation event from the hexapods and chelicerates, opening the opportunity for studying convergent evolution in these taxa. Naturally, S. maritima itself has its own evolutionary history, including both lineage specific features of the geophilomorphs and adaptations to their subterranean environment, allowing us to identify specific genomic signatures of ecological adaptations. Finally, the phylogenetic position of the myriapods within the arthropods has been the subject of intense debate for several years, and the availability of genomic data for a myriapod should contribute to the future resolution of this debate.
The morphological conservatism of centipedes is mirrored in many conservative aspects of the S. maritima genome. From the analyses of the various gene families outlined above it becomes clear that the S. maritima genome has undergone much less gene loss and rearrangement than the genomes of other sequenced arthropods, in particular those of the holometabolous insects such as D. melanogaster. This prototypical nature of the S. maritima genome is illustrated by the conservation of synteny relative to the arthropod and bilaterian ancestors, and the conservation of some ancient gene linkages and clustering, as seen for numerous homeobox genes. As such, the S. maritima genome can serve as a guide to the ancestral state of the arthropod genomes, or as a reference in the reconstruction of evolutionary events in the history of arthropod genomes.
The independent terrestrialisation of the myriapods and insects is evidenced by the use of different evolutionary solutions to similar problems. Figure 10 summarizes some of the gene gains and losses observed. We see this most clearly in the independent expansions of gustatory receptor proteins in myriapods and insects and the differential expansions of ionotropic and odorant receptors to deal with terrestrial chemosensation in the two lineages. Similarly, though probably not for the same reasons, we see a divergent solution for the generation of Dscam diversity in the immune response through the use of paralogues instead of the insect strategy of alternative splicing. The chelicerates also attained terrestriality independently. However, our understanding of chelicerate genomes still lags behind our understanding of insect, and now myriapod, genomes. Thus, extending this comparison to chelicerates, intriguing as it may be, will have to await future analysis of their genomes.
Figure 10. Arthropod phylogenetic tree (with nematode outgroup) showing selected events of gene loss, gene gain, and gene family expansions.
Main taxa are listed on the tips, with representative species for which there is a fully sequenced genome listed below. Major nodes are also named. Data from the genome of S. maritima allow us to map when in arthropod evolution these events occurred, even when these events did not occur on the centipede lineage. A plausible node for the occurrence of each event is marked and colour-coded, with the possible range marked with a thin line of the same colour. The events, listed from left to right are: (1) Dscam alternative splicing as a strategy for increasing immune diversity is known from D. melanogaster, as well as the crustacean D. pulex, and thus probably evolved in the lineage leading to pancrustacea, after the split from centipedes. (2) Several wnt genes have been lost in holometabolous insects, leaving only seven of the 13 ancestral families. This loss occurred gradually over arthropod evolution, but reached its peak at the base of the Holometabola. (3) Selenoproteins are rare in insects. The presence of a large number of selenoproteins in S. maritima as well as in other non-insect arthropods suggests that the loss of many selenoproteins occurred at the base of the Insecta. (4) Expansion of chemosensory gene families occurred independently in different arthropod lineages as they underwent terrestrialisation. The OR family is expanded in insects only. (5) Chemosensory genes of the GR and IR genes have undergone a lineage specific expansion in the genome of S. maritima. As these are probably also linked with terrestrialisation we suggest that this expansion happened at the base of the Chilopoda, but it could have also occurred later in the lineage leading to S. maritima. (6) Cuticular proteins of the RR-1 family are numerous in the S. maritima genome. They are found in other arthropods, but not in chelicerates nor in any non-arthropod species. This suggests that the RR-1 subfamily evolved at the base of the Mandibulata. (7) The genome of S. maritima has a large complement of wnt genes, but is missing wnt8. Since this gene is found in the Diplopod G. marginata (a species without a fully sequenced genome), the loss most likely occurred at the base of the Chilopoda. (8) Unlike the situation in D. melanogaster, immune diversity in the S. maritima genome is achieved through multiple copies of the Dscam gene. This expansion of the family could have happened at any time after the split between Myriapoda and Pancrustacea. (9) Both circadian rhythm genes and many light receptors are missing in S. maritima. These losses are most likely due to the subterranean life style of geophilomorph centipedes and are probably specific to this group. However, we cannot rule out the possibility that they were lost somewhere in the lineage leading to myriapods. (10) The existence of JH signalling in S. maritima as well as in all other arthropods studied to date strengthens the idea that this signalling system evolved with the exoskeleton of arthropods, though its origins could be even more ancient and date back to the origin of moulting at the base of the Ecdysozoa.
Lineage specific features of the S. maritima genome include the apparent loss of all known photoreceptors and a loss of the canonical circadian clock system based around period and its associated gene network. The characterization of whether S. maritima does have a circadian clock, and if it does how this is controlled, awaits further work, as does the pinpointing of when in their evolutionary history these systems were lost. The absence of the microRNA miR-125 is another surprising evolutionary loss. The extensive rearrangement of the mitochondrial genome is striking in comparison with the general conservatism seen in other known arthropod mitochondrial genomes, and especially in contrast with the conservative nature of S. maritima's nuclear genome.
Materials and Methods
The S. maritima raw sequence, and assembled genome sequence data are available at the NCBI under bioproject PRJNA20501 (http://www.ncbi.nlm.nih.gov/bioproject/PRJNA20501) Assembly ID GCA_000239455.1. The genome was sequenced using 454 sequencing technology, assembled using the celera assembler, annotated using a combination of the Maker 2.0 pipeline, and custom perl scripts followed by manual annotation of selected genes. Text S1 includes detailed methods for these steps, and additionally for the individuals sequenced, library construction and sequencing protocols used, repeat analysis, RNA sequencing, phylome db analysis, specific protocols for manual annotation of gene families, CpG analysis, and phylome and synteny re-construction.
Supporting Information
Frequency histogram showing the distribution of gene lengths in the S. maritima genome. Gene length data used in this plot are available in File S4.
(PDF)
Multi-gene phylogeny for the 18 species included in the phylogenomics analysis. 1,491 widespread single-copy sets of orthologue sequences in at least 15 out of the 18 species were concatenated into a single alignment of 842,150 columns. Then, a maximum-likelihood tree was inferred using LG as evolutionary model by using PhyML.
(PDF)
Multi-gene phylogeny for 12 species included in the phylogenomics analysis plus five additional Chelicerata species. 1,491 widespread single-copy sets of orthologue sequences were concatenated into a single alignment of 829,729 positions. Then, a maximum-likelihood tree was inferred using LG as the evolutionary model by using PhyML.
(PDF)
Alternative topological placements of S. maritima relative to the main arthropod groups considered in the study: Chelicerata and Pancrustacea. Internal organization of each group was initially collapsed and, therefore, optimized during maximum-likelihood reconstruction.
(PDF)
Clusters of genes specifically expanded in the centipede lineage. On the plot, only clusters grouping five or more protein-coding genes were considered. The data underlying this plot are available in File S4.
(PDF)
Mitochondrial gene organisation. Shaded regions represent differences from the ground pattern. Gene translocations in Myriapoda have been noted in Scutigerella causeyae (Myriapoda: Symphyla) [49]. The previous example of the small conserved region trnaF-nad5-H-nad4-nad4L on the minus strand between Limulus, Lithobius, and Strigamia is not a conserved feature in all Chilopoda, because Scutigera colepotrata have an interruption between nad5 and H-nad4 with elements on the minus and plus strands accompanied by a translocation of nad4L to a position immediately preceding nad5.
(PDF)
Classification of all S. maritima (Sma) homeodomains (excluding Pax2/5/8/sv) via phylogenetic analysis using T. castaneum (Tca) and B. floridae (Bfl) homeodomains. This phylogenetic analysis was constructed using neighbour-joining with a JTT distance matrix and 1,000 bootstrap replicates. Gene classes are indicated by colours. The genes coloured in grey are those genes that cannot be assigned to known classes. Further classification was performed using additional domains outside the homeodomain and by performing additional phylogenetic analysis for particular gene classes using maximum-likelihood and bayesian approaches. Pax2/5/8/sv is excluded due to the gene possessing only a partial homeobox.
(PDF)
Phylogenetic analysis of ANTP class homeodomains of S. maritima (Sma) using T. castaneum (Tca) and B. floridae (Bfl) for comparison. These phylogenetic analyses were constructed using neighbour-joining with a JTT distance matrix, 1,000 bootstrap replicates (support values in black). Nodes with support equal to or above 500 in the maximum-likelihood (LG+G) analysis are in blue and nodes with posterior probabilities equal to or above 0.5 (LG+G) in the Bayesian analysis are in red.
(PDF)
Phylogenetic analysis of PRD class homeodomains of S. maritima (Sma) using T. castaneum (Tca) and B. floridae (Bfl) for comparison. These phylogenetic analyses were constructed using neighbour-joining with a JTT distance matrix, 1,000 bootstrap replicates (support values in black). Nodes with support equal to or above 500 in the maximum-likelihood (LG+G) analysis are in blue and nodes with posterior probabilities equal to or above 0.5 (LG+G) in the Bayesian analysis are in red.
(PDF)
Phylogenetic analysis of HNF class homeodomains of S. maritima (Sma) using B. floridae (Bfl), human ( Homo sapiens , Hsa), and sea anemone ( N. vectensis , Nve) for comparison. These phylogenetic analyses were constructed using neighbour-joining with a JTT distance matrix, 1,000 bootstrap replicates (support values in black). Nodes with support equal to or above 500 in the maximum-likelihood (LG+G) analysis are in blue and nodes with posterior probabilities equal to or above 0.5 (LG+G) in the Bayesian analysis are in red.
(PDF)
Phylogenetic analysis of Xlox/Hox3 genes of S. maritima (Sma) using a selection of Hox1, Hox2, Hox3, Hox4, and Xlox sequences. This analysis was based upon the whole coding sequence of the genes, and was constructed using neighbour-joining with a JTT distance matrix and 1,000 bootstrap replicates. The blue support value (of 333) is the node that reveals the affinity between the Xlox/Hox3 genes of S. maritima and Xlox sequences. Ame, A. mellifera; Bfl, B. floridae; Cte, Capitella teleta; Dme, D. melanogaster; Lgi, Lottia gigantea; and Tca, T. castaneum.
(PDF)
Multiple alignment of relevant residues of the Hox1, Hox2, Hox3, Hox4, and Xlox sequences of different lineages compared to S. maritima Hox3a and Hox3b sequences. Three paired class genes are included as an outgroup. The grading of purple colouring of the amino acids shows the identity level of these sequences. The red rectangles in the multiple alignment delimit the core of the hexapeptide motif and the homeodomain. This is the alignment used to construct the phylogenetic tree in Figure S13. Ame, A. mellifera; Bfl, B. floridae; Cte, Capitella teleta; Dme, D. melanogaster; Lgi, Lottia gigantea; and Tca, T. castaneum.
(PDF)
Phylogenetic analysis of S. maritima Xlox/Hox3 homeodomain and hexapeptide motifs using a selection of Hox1, Hox2, Hox3, Hox4, and Xlox sequences. This analysis used a section of the coding sequence including the hexapeptide and some flanking residues plus the homeodomain (alignment in Figure S12). Three paired class genes are included as an outgroup. This phylogeny was constructed using neighbour-joining with the JTT distance matrix and 1,000 bootstrap replicates. Maximum likelihood support values are shown in blue and Bayesian posterior probabilities in red. Ame, A. mellifera; Bfl, B. floridae; Cte, Capitella teleta; Dme, D. melanogaster; Lgi, Lottia gigantean; Tca, T. castaneum.
(PDF)
Fisher's exact test to distinguish whether S. maritima scaffold 48457 has significant synteny conservation with ParaHox or Hox chromosomes of humans. No significant Hox or ParaHox association is found.
(PDF)
Phylogenetic analysis of TALE class homeodomains of S. maritima (Sma) using T. castaneum (Tca) and B. floridae (Bfl), including the Iroquois/Irx genes. These phylogenetic analyses were constructed using neighbour-joining with a JTT distance matrix, 1,000 bootstrap replicates (support values in black). Nodes with support equal to or above 500 in the maximum-likelihood (LG+G) analysis are in blue and nodes with posterior probabilities equal to or above 0.5 (LG+G) in the Bayesian analysis are in red.
(PDF)
RNA processing in the Hox cluster of S. maritima . The transcriptome of S. maritima (Sm) eggs (blue), females (green), and males (red) was mapped to the Hox gene cluster (top panel; see Figure 4 in the main text) and transcript models were inferred for each gene within the cluster (shaded area) taking into account the presence of ORF and polyadenylation signals (PAS) to support the existence of RNA processing events. We note the occurrence of more than one mRNA isoform of six S. maritima Hox genes (i.e., Antp, Ubx, abd-A, lab, Dfd, pb). In all these six cases alternative polyadenylation (APA) generates mRNAs bearing distinct 3′ UTRs (alternative UTR sizes at the bottom). Alternative splicing (AS) with concomitant alternative promoter use (APU) events concern two S. maritima Hox genes Dfd and ftz (see alternative ORF sizes at the bottom). We also see that some genes such as S. maritima Ubx display high heterogeneity in 3′UTR sequences within the embryonic transcriptome (“eggs” data) suggesting the possibility that S. maritima Ubx APA might be developmentally controlled and/or display a tissue-specific pattern (see inset for further details on symbols).
(PDF)
RNA processing in the S. maritima and D. melanogaster Hox clusters. (A) The incidence of alternatively processed mRNAs is comparable between S. maritima and D. melanogaster, in that over 75% of the S. maritima Hox genes undergo RNA processing of one type or another. Similarly, seven out of the eight Drosophila Hox genes produce different mRNA isoforms (FlyBase, http://flybase.org/). (B) Three D. melanogaster Hox genes undergo AS (blue) and five produce different transcripts via APA (red, FlyBase http://flybase.org/). In addition five fruit fly Hox genes form different RNA species by APU (green). (C) Classification of all alternatively processed mRNA events in the S. maritima Hox cluster based on the same categorisation as in (B). Note that patterns of AS and APA affecting S. maritima and D. melanogaster Hox genes are relatively comparable; in contrast, APU seems more prevalent in the Drosophila (five out of eight genes) than in the centipede (two out of nine genes) Hox genes.
(PDF)
Phylogenetic tree of the S. maritima , D. pulex , I. scapularis , and representative insect GRs, part one. This is a corrected distance tree and was rooted at the midpoint in the absence of a clear outgroup, an approach that clearly indicates the distinctiveness of the centipede GRs. It is a more detailed version of Figure 5A. The S. maritima, D. pulex, I. scapularis, and representative insect gene/protein names are highlighted in red, blue, brown, and green, respectively, as are the branches leading to them to emphasize gene lineages. Bootstrap support levels in percentage of 10,000 replications of neighbour-joining with uncorrected distance is shown above major branches. Comments on major gene lineages are on the right. Suffixes after the gene/protein names are: PSE, pseudogene; FIX, sequence fixed with raw reads; JOI, gene model joined across scaffolds. Note than in Figure 5A for space reasons the IsGr47 and 59 proteins are included in the carbon dioxide and sugar receptor groupings, respectively; however, there is no bootstrap support for these branches, and no such functional assignment is claimed. Similarly, it is unlikely that the DpGr57/58 proteins are fructose receptors.
(PDF)
Phylogenetic tree of the S. maritima , D. pulex , I. scapularis , and representative insect GRs, part two. This is a corrected distance tree and was rooted at the midpoint in the absence of a clear outgroup, an approach that clearly indicates the distinctiveness of the centipede GRs. It is a more detailed version of Figure 5A. The S. maritima, D. pulex, I. scapularis, and representative insect gene/protein names are highlighted in red, blue, brown, and green, respectively, as are the branches leading to them to emphasize gene lineages. Bootstrap support levels in percentage of 10,000 replications of neighbour-joining with uncorrected distance is shown above major branches. Comments on major gene lineages are on the right. Suffixes after the gene/protein names are: PSE, pseudogene; FIX, sequence fixed with raw reads; JOI, gene model joined across scaffolds. Note than in Figure 5A for space reasons the IsGr47 and 59 proteins are included in the carbon dioxide and sugar receptor groupings, respectively; however, there is no bootstrap support for these branches, and no such functional assignment is claimed. Similarly, it is unlikely that the DpGr57/58 proteins are fructose receptors.
(PDF)
Neuropeptide precursor sequences identified in the S. maritima genome. The putative signal peptides (predicted by SignalP) are underlined, the putative active neuropeptides or protein hormones (based on similarity to neuropeptides or protein hormones identified in other invertebrates) are marked in yellow. Green indicates putative basic cleavage sites flanking the putative neuropeptides. Glycines used for amidation are shown in blue, cysteines proposed to form cysteine bridges are shown in red. Dots indicate missing N- or C-termini.
(DOCX)
Examples of tandem duplications of neuropeptide receptor genes. Structure of the two inotocin receptor genes found head-to-head on opposite strands of scaffold JH431865 (A). Structure of the two SIFamide receptor genes found tail-to-head on the same strand of scaffold JH432116 (B).
(PDF)
Schematic diagram showing sesquiterpenoids/juvenoids synthesis (upper) and degradation (lower) pathways in arthropods. Molecules/hormones in synthesis are shown in bold, enzymes are shown in italics, and species/clades are shown in bold italics.
(PDF)
Phylogenetic analysis of the TGFβ ligands in arthropods. See Text S1 for details. Abbreviations: Ag, Anopheles gambiae; Am, A. mellifera; Ap, Acyrthosiphon pisum; Ca, Clogmia albipunctata; Dm, Drosophila melanogaster; Dp, D. pulex; Is, I. scapularis; Lg, Lottia gigantea Ma, Megaselia abdita; Nv, Nasonia vitripennis; Ph, Pediculus humanus; Tc, T. castaneum;.
(EPS)
Range of Wnt genes present in S. maritima . Wnt genes present and number of Wnt subfamilies absent in S. maritima in comparison with other arthropods and three non-arthropod outgroups.
(TIF)
Phylogeny of FGFR genes indicating that FGFR genes duplicated independently in S. maritima and D. melanogaster . See text for details. Alignment was performed using Clustal-Omega (http://www.ebi.ac.uk/Tools/services/web). The evolutionary history was inferred using the neighbour-joining method with bootstrapping to determine node support values (10,000 replicates). The evolutionary distances were computed using the Poisson correction method. Evolutionary analyses were conducted in MEGA5.
(EPS)
Phylogeny including the three FGF genes of S. maritima . See text for details. Alignment was performed using Clustal-Omega (http://www.ebi.ac.uk/Tools/services/web). The evolutionary history was inferred using the neighbour-joining method with bootstrapping to determine node support values (10,000 replicates). The evolutionary distances were computed using the Poisson correction method. Evolutionary analyses were conducted in MEGA5.
(EPS)
Cap ‘n’ collar (cnc) genes. (A) The two genes are located on different scaffolds. Cnc1 is a long transcript consisting of 11 exons. Cnc2 is shorter (eight exons), the three exons at the 3′ end of the gene that encode the C-terminal region of the protein including the conserved domain (B) show a similar structure. (B) S. maritima Cnc protein structure. Both proteins contain the bZip domain in a similar position at the C-terminus. Cnc1 encodes a long protein (925 amino acids). Bits of the N-terminal region (blue lines) align with D. melanogaster Cnc isoform C and T. castaneum Cnc variant A. (C) Cnc protein sequence alignment, only showing the aligning bits in the N-terminal region. Blue lines show short stretches of sequence that form a consensus motif. These motifs are not present in the proteins encoded by Sm-cnc2, Dm-cnc isoforms A and B, and T. castaneum cnc variant B.
(JPG)
Frequency histograms of observed versus expected dinucleotide content in S. maritima gene bodies. (A–P) The y-axis depicts the number of genes with the specific dinucleotide[o/e] values given on the x-axis. The distribution of all dinucleotide pairs, with the exception of CpG, is best described as a unimodal distribution. The distribution of CpG dinucleotides is best described as a trimodal distribution, with “high” and “low” CpG[o/e] classes. The data underlying this figure are available in File S5.
(TIF)
Frequency histogram of CpG[o/e] observed in 1,000 bp windows of the S. maritima genome. The y-axis depicts the number of genes with the specific CpG[o/e] values given on the x-axis. The distribution of CpG[o/e] in S. maritima genome is a bimodal distribution, with a high CpG[o/e] peak observed similar to that observed in the gene bodies (Figure 9). The data underlying this figure are available in File S6.
(TIF)
Contrasting patterns of DNA methylation, as measured by over- and underrepresentation of CpG dinucleotides in coding regions (CpG(o/e)), within arthropod species. In all graphs the y-axis depicts the number of genes with the specific CpG(o/e) values given on the x-axis. (A) D. melanogaster coding regions show a unimodal peak reflective of the lack of DNA methylation in this species. (B) A. mellifera shows a bimodal peak consisting of genes with a lower than expected CpG(o/e) (green distribution) and a higher than expected CpG(o/e) (blue distribution). The presence of a bimodal distribution in this species is consistent with depletion of CpG dinculeotides in the coding regions of genes over evolutionary time as a result of DNA methylation. (C) A single unimodal peak is also observed for Tetranychus urticae, a species that has very low levels of DNA methylation. (D) The S. maritima distribution is best explained as a mixture of three distinct distributions that we have deemed “low” (green distribution), “medium” (blue distribution), and “high” (grey distribution). The genes within the low distribution likely contain genes that are historically methylated, whilst the “high” distribution can be explained by regions of the genome that are comparatively CpG-rich (as determined by the analysis of the S. maritima genome, Figure S29). The data underlying this figure are available in File S7.
(PDF)
Chromosomal organisation of histone gene clusters in S. maritima . In insects such as Drosophila [115] and the pea aphid [109] histone encoding genes are present in quintet clusters, each cluster containing one gene from each of the five classes of histone. Only one such cluster could be identified in S. maritima (A). The other four clusters identified in the S. maritima genome (B–D) all consist of two to three copies of a histone encoding gene of a single class. It is possible that these have arisen as a result of recent gene duplication.
(EPS)
S. maritima vasa DEAD-box helicase germline gene phylogeny. Maximum likelihood tree of vasa/PL10 family genes. One gene is a likely vasa orthologue (SMAR015390), one groups with the PL10 family (SMAR005518), and the majority group in an apparently distinct DEAD-box-containing clade. Bootstrap values for 2,000 replicates are shown at each node. Accession numbers for protein sequences are as follows: Apis Belle (XP_391829.3), Apis Vasa (NP_001035345.1), Danio PL10 (NP_571016.2), Danio Vasa (AAI29276.1), Drosophila Belle (NP_536783.1), Drosophila Vasa (NP_723899.1), Gryllus Vasa (BAG65665.1), Mus Mvh (NP_001139357.1), Mus PL10 (NP_149068.1), Nasonia Belle (XP_001605842.1), Nasonia Vasa (XP_001603956.2), Nematostella PL10 (XP_001627306.1), Nematostella Vasa 1 (XP_001628238.1), Nematostella Vasa 2 (XP_001639051.1), Oncopeltus Vasa (AGJ83330.1), Parhyale Vasa (ABX76969.1), Tribolium Belle (NP_001153721.1), Tribolium Vasa (NP_001034520.2), Xenopus PL10 (NP_001080283.1), Xenopus VLG1 (NP_001081728.1).
(EPS)
Phylogenomic inventory of meiotic genes in arthropods. Red genes are specific to meiosis in model species in which functional data are available. “+” and “−” indicate the presence and absence of orthologues, respectively. Numbers indicate copy number of duplicated genes.
(PDF)
Patterns of microRNA gain and loss across the animal kingdom with the inclusion of S. maritima . The number of microRNAs that were gained or lost at each node are shown in green and red, respectively, and names are listed below each taxon. MicroRNAs that are found in the S.maritima genome are in bold, and families for which more than one homologue is found are marked with an asterisk. The tree depicts the Mandibulata hypothesis rather than the Myriochelata, as in [124].
(EPS)
Detailed overview for the repetitive elements in S. maritima . For each group the number of elements (putative families), the number of their fragments or copies in the genome, the cumulative length, the proportion of the assembly, and some features are shown. This includes elements containing nested inserts of other elements (n), elements that appear to be complete (i.e., all typical structural and coding parts present, even if containing stop codons or frameshifts), elements with a RT or Tase domain detected (n), elements that potentially could be active as they contain an intact ORF with all the typical domains even though they could lack other structural features like terminal repeats, and elements that contain an intact ORF for the RT domain or parts of the Tase domain and could thus be partly active. The elements that could not be categorized or contained features of protein coding regions are shown at the bottom, whereby they probably do not belong to the transposable elements.
(XLSX)
Set of species used in the comparative genomics analyses related to the S. maritima genome. Columns include, in this order, scientific names, the species code according to UNIPROT, the number of the longest unique transcript used in the analyses, the data source, and the date in which data were retrieved.
(DOCX)
Orthologues detected between a given species and S. maritima . First column indicates how many trees have been used to detect such orthologues. Columns “uniq” refers to the number of orthologues detected for each pair of species after removing redundancy. In one-to-many and many-to-many orthology relationships it is possible to count a given protein more than once. Regarding the ratios values, “all” column refers to the orthology ratio computed using all orthologue pairs meanwhile “uniq” refers to the ratio computed using “uniq” columns.
(DOCX)
Orthology ratios for a given species related to S. maritima . This table is similar to Table S3, but in this case orthology relationships with ten or more proteins for any of the species are discarded in order to avoid biases introduced by species-specific gene family expansions.
(DOCX)
Newly added Chelicerata species used to increase the taxon sampling for the species phylogeny. First column indicates the scientific species name, the second one indicates which strategy has been used to identify single copy protein-coding genes. Third column shows how many single-copy genes have been identified in each species from the initial set of 1,491 used to reconstruct the species phylogeny. Last two columns show the data source and the date on which data were retrieved.
(DOCX)
Results after applying the different statistical tests implemented in CONSEL for the alternative placement of S. maritima relative to Pancrustacea and Chelicerata groups of species (as shown in Figure S4) in the context of the 18 species used for the phylogenomics analyses. The “item” column relates to Figure S4 as follows: (1) topology arrangement corresponding to Figure S4 left-hand panel, in which S. maritima was grouped with Chelicerata species. (2) Topology arrangement corresponding to Figure S4 central panel, in which S. maritima branches off before the split of Pancrustacea and Chelicerata. (3) Topology arrangement corresponding to Figure S4 right-hand panel, in which S. maritima was grouped with Pancrustacea species.
(DOCX)
Results after applying the different statistical tests implemented in CONSEL for the alternative placement of S. maritima relative to the two arthropod groups, Pancrustacea and Chelicerata (as shown in Figure S4), with the inclusion of extra chelicerates. Taxon sampling for the Chelicerata was increased after including sequences from five additional species. In order to reduce any potential bias introduced by distant and/or fast-evolving out-groups, six out-group species from the initial set were removed. The “item” column relates to Figure S4 as follows: (1) topology arrangement corresponding to Figure S4 left-hand panel, in which S. maritima was grouped with Chelicerata species. (2) Topology arrangement corresponding to Figure S4 central panel, in which S. maritima branches off before the split of Pancrustacea and Chelicerata. (3) Topology arrangement corresponding to Figure S4 right-hand panel, in which S. maritima was grouped with Pancrustacea species.
(DOCX)
Enriched functional GO Terms for the ten largest clusters of duplicated S. maritima protein-coding genes specifically expanded in the centipede lineage, as compared with the whole genome.
(DOCX)
Statistics regarding the duplications of centipede genes relative to seven specific ages detected using all available trees on the phylome.
(DOCX)
Enriched functional GO terms for proteins duplicated at the different relative ages shown in Table S9. Columns show relative age, gene ontology namespace, the GO term id, and its name, respectively.
(DOCX)
Overview of S. maritima mitochondrial genome.
(DOCX)
Species used in the synteny analyses and the sources of their sequence data.
(DOCX)
Block-synteny summary statistics for pairs of species. Hs, Homo sapiens; Bf, B. floridae; Sm, S. maritima; Lg, Lottia gigantea; Ct, Capitella teleta; Nv, N. vectensis; Ta, Trichoplax adhaerens; Ag, Anopheles gambiae; Bm, B. mori.
(DOCX)
Summary of numbers of homeobox genes per class of Strigamia , Branchiostoma , and Tribolium .
(DOCX)
Names and identification numbers of all S. maritima homeobox genes along with their orthologues from the beetle, T. castaneum , and amphioxus, B. floridae .
(XLS)
One-to-one S. maritima to human orthologues starting from genes on S. maritima scaffold 48457, which contains SmaHox3a . The third column is the chromosomal location of the human orthologue. Human Hox chromosomes are 2, 7, 12, and 17 and the ParaHox chromosomes are 4, 5, 13, and X.
(DOCX)
Evolutionary conservation of RNA processing modes in the S. maritima and D. melanogaster Hox clusters. Type of RNA processing event concerning each one of the S. maritima (left) and D. melanogaster (right) Hox genes. We note that orthologous genes in both species undergo similar types of RNA processing: the three posterior-most Hox genes: Ubx, abd-a, and Abd-b display a specific type of APA (tandem APA) in both S. maritima and D. melanogaster (conserved patterns highlighted by red asterisks) providing an example of what might be a feature present in the ancestral Hox cluster to insects and myriapods. Nonetheless, for most other Hox genes, RNA processing patterns differ markedly between S. maritima and D. melanogaster, indicating that the conserved incidence of alternative RNA processing across arthropods can only be proposed for the posterior-most Hox genes.
(PDF)
Details of SmGr family genes and proteins. Columns are: Gene, the gene and protein name we are assigning (suffixes are PSE, pseudogene; FIX, assembly was repaired; JOI, gene model spans scaffolds); OGS, the official gene number in the 13,233 proteins (prefix is Smar_temp_); Scaffold, the genome assembly scaffold ID, prefix is scf718000 (amongst 14,739 scaffolds in assembly Smar05272011); Coordinates, the nucleotide range from the first position of the start codon to the last position of the stop codon in the scaffold; Strand – + is forward and − is reverse; introns, number of introns; ESTs, presence of an EST contig with appropriate splicing in one of the three transcriptome assemblies (F, female; M, male; E, eggs); AAs, number of encoded amino acids in the protein; comments, comments on the OGS gene model, repairs to the genome assembly, and pseudogene status (numbers in parentheses are the number of obvious pseudogenizing mutations).
(DOC)
Total numbers of biogenic amine receptors in different species.
(DOCX)
A comparison between the D. melanogaster and S. maritima biogenic amine receptors. The orthologues are given next to each other. When there is no orthologue, a dash (–) is written instead.
(XLSX)
Genes encoding neuropeptide precursors and neuropeptide receptors annotated in S. maritima . Abbreviations: ACP, adipokinetic hormone/corazonin-related neuropeptide; AKH, adipokinetic hormone; ADF, antidiuretic factor; AST, allatostatin; CCAP, crustacean cardio-active peptides; DH (Calc.-like), calcitonin-like diuretic hormone; DH (CRF-like), corticotropin releasing factor-like diuretic hormone; EH, eclosion hormone; ETH, ecdysis triggering hormone; GPA2, glycoprotein hormone A2; GPB5, glycoprotein hormone B5; ILP, insulin-like peptide; ITP, ion transport peptide; NPF, neuropeptide F; NPLP, neuropeptide-like precursor; PDF, pigment dispersing factor; PTTH, prothoracicotropic hormone; sNPF, short neuropeptide F.
(EPS)
Presence or absence of neuropeptide signaling systems in arthropods. The centipede S. maritima contains two CCHamide-1, two eclosion hormone and two FMRFamide genes (2 p). In some cases neuropeptide precursors could not be identified, but the corresponding receptor genes are present (R). We assume that this is due to sequencing gaps. For abbreviations see Table S21.
(DOC)
Genes commonly implicated in arthropod juvenoids biosynthesis (green) and degradation (blue), and their potential regulators (purple) [98]–[101]. Common abbreviations, and presence in the centipede S. maritima.
(DOCX)
List of genes commonly implicated as potential regulators of arthropod juvenoids biosynthesis (purple) [98]–[101]. Common abbreviations, and presence in the centipede S. maritima.
(DOCX)
Wnt genes in the genome of S. maritima . SMAR, the gene identification number, and scaffold, the scaffold identification number. Wnt 1, 6, and 10 are clustered together on the same scaffold (yellow highlighting), which is likely a remnant of the ancestral wnt gene cluster (see text for details).
(PDF)
Selenoproteins in the S. maritima genome.
(DOCX)
Histone encoding loci of S. maritima .
(DOCX)
Number of loci within the genomes of arthropod species encoding the five classes of histones. Orthologues for A. aegypti, D. pulex, T. urticae, and I. scapularis were obtained by BLAST analysis. Orthologues for A. mellifera and A. pisum were obtained from published literature [108],[109].
(DOCX)
Germ line and RNAi genes annotated in the S. maritima genome. The name of the Drosophila orthologue is shown unless indicated with “(Mo),” for mouse.
(DOCX)
Details of the manually annotated genes of S. maritima .
(XLSX)
One2One_GOTerms_GenomeIDs for Orthology-based functional annotation.
(XLSX)
Gustatory receptor sequences.
(XLSX)
Supporting Methods Text.
(DOCX)
Acknowledgments
We thank Paul Kersey, Monica Munoz-Torres, and Jamie Walters for sharing their experience of community annotation projects; Rolf Sommer and Werner Mayer for assistance with the identification of S. maritima associated nematode sequences; Nipam Patel and all authors of the NHGRI Ecdysozoan Sequencing Proposal who initiated this project; P. Woznicki and F. Marec for sharing data on the karyotype of S. maritime; Geordie and Irene at BlarMhor for shelter and sustenance during the field collection of centipedes.
Abbreviations
- FGF
fibroblast growth factor
- GR
gustatory receptor
- GPCR
G protein-coupled receptor
- JH
juvenile hormone
- OR
odorant receptor
- RR
Rebers and Riddiford
- TGF
transforming growth factor
Funding Statement
This work was supported by the following grants: NHGRI U54 HG003273 to R.A.G.; EU Marie Curie ITN #215781 “Evonet” to M.A.; a Wellcome Trust Value in People (VIP) award to C.B., a Wellcome Trust graduate studentship WT089615MA to J.E.G., and a Wellcome Trust Investigator Award (098410/Z/12/Z) to C.R.A.; “Marine Rhythms of Life” of the University of Vienna, an FWF (http://www.fwf.ac.at/) START award (#AY0041321) and HFSP (http://www.hfsp.org/) research grant (#RGY0082/2010) to K.T-R; MFPL Vienna International PostDoctoral Program for Molecular Life Sciences (funded by Austrian Ministry of Science and Research and City of Vienna, Cultural Department - Science and Research) to T.K.; Direct Grant (4053034) of the Chinese University of Hong Kong to J.H.L.H.; NHGRI HG004164 to G.M.; Danish Research Agency (FNU), Carlsberg Foundation, and Lundbeck Foundation to C.J.P.G.; U.S. National Institutes of Health R01AI55624 to J.H.W.; Royal Society University Research fellowship to F.M.J.; P.D.E. was supported by the BBSRC via the Babraham Institute. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Arthropod Genomes Consortium (2014) List of sequenced arthropod genomes. Available: http://arthropodgenomes.org/wiki/Sequenced_genomes.
- 2. Bracken-Grissom H, Collins AG, Collins T, Crandall K, Distel D, et al. (2014) The Global Invertebrate Genomics Alliance (GIGA): developing community resources to study diverse invertebrate genomes. J Hered 105: 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Edgecombe GD (2011) Phylogenetic relationships of Myriapoda. Minelli A, editor. The Myriapoda. Leiden: Brill. pp. 1–20. [Google Scholar]
- 4. Giribet G, Edgecombe GD, Wheeler WC (2001) Arthropod phylogeny based on eight molecular loci and morphology. Nature 157–160. [DOI] [PubMed] [Google Scholar]
- 5. Rota-Stabelli O, Telford MJ (2008) A multi criterion approach for the selection of optimal outgroups in phylogeny: recovering some support for Mandibulata over Myriochelata using mitogenomics. Mol Phylogenet Evol 48: 103–111. [DOI] [PubMed] [Google Scholar]
- 6. Edgecombe GD, Giribet G (2007) Evolutionary biology of centipedes (Myriapoda; Chilopoda). Ann Rev Entomol 52: 151–170. [DOI] [PubMed] [Google Scholar]
- 7. Simakov O, Marletaz F, Cho SJ, Edsinger-Gonzales E, Havlak P, et al. (2013) Insights into bilaterian evolution from three spiralian genomes. Nature 493: 526–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Edgecombe GD (2004) Morphological data, extant Myriapoda, and the myriapod stem-group. Contrib Zool 73: 207–252. [Google Scholar]
- 9. Bitsch C, Bitsch J (2004) Phylogenetic relationships of basal hexapods among the mandibulate arthropods: a cladistic analysis based on comparative morphological characters. Zool Scr 33: 511–550. [Google Scholar]
- 10. Rota-Stabelli O, Daley AC, Pisani D (2013) Molecular timetrees reveal a Cambrian colonization of land and a new scenario for ecdysozoan evolution. Curr Biol 23: 392–398. [DOI] [PubMed] [Google Scholar]
- 11. Scholtz G, Edgecombe GD (2006) The evolution of arthropod heads: reconciling morphological, developmental and palaeontological evidence. Dev Genes Evol 216: 395–415. [DOI] [PubMed] [Google Scholar]
- 12. Mallatt JM, Garey JR, Shultz JW (2004) Ecdysozoan phylogeny and Bayesian inference: first use of nearly complete 28S and 18S rRNA gene sequences to classify the arthropods and their kin. Mol Phylogenet Evol 31: 178–191. [DOI] [PubMed] [Google Scholar]
- 13. Pisani D, Poling LL, Lyons-Weiler M, Hedges SB (2004) The colonization of land by animals: molecular phylogeny and divergence times among arthropods. BMC Biol 2: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bourlat SJ, Nielsen C, Economou AD, Telford MJ (2008) Testing the new animal phylogeny: a phylum level molecular analysis of the animal kingdom. Mol Phylogenet Evol 49: 23–31. [DOI] [PubMed] [Google Scholar]
- 15. Rota-Stabelli O, Campbell L, Brinkmann H, Edgecombe GD, Longhorn SJ, et al. (2011) A congruent solution to arthropod phylogeny: phylogenomics, microRNAs and morphology support monophyletic Mandibulata. Proc Roy Soc B 278: 298–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Regier JC, Shultz JW, Zwick A, Hussey A, Ball B, et al. (2010) Arthropod relationships revealed by phylogenomic analysis of nuclear protein-coding sequences. Nature 463: 1079–1083. [DOI] [PubMed] [Google Scholar]
- 17. Rehm P, Meusemann K, Borner J, Misof B, Burmester T (2014) Phylogenetic position of Myriapoda revealed by 454 transcriptome sequencing. Mol Phylogenet Evol [DOI] [PubMed] [Google Scholar]
- 18. Kraus O, Kraus M (1994) Phylogenetic system of the Tracheata (Mandibulata): on “Myriapoda”:Insecta interrelationships, phylogenetic age and primary ecological niches. Verh Naturwiss Ver Hambg 34: 5–31. [Google Scholar]
- 19. Cook CE, Smith ML, Telford MJ, Bastianello A, Akam M (2001) Hox genes and the phylogeny of the arthropods. Curr Biol 11: 759–763. [DOI] [PubMed] [Google Scholar]
- 20. Cook CE, Yue Q, Akam M (2005) Mitochondrial genomes suggest that hexapods and crustaceans are mutually paraphyletic. Proc Biol Sci 272: 1295–1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Regier JC, Shultz JW, Kambic RE (2005) Pancrustacean phylogeny: hexapods are terrestrial crustaceans and maxillopods are not monophyletic. Proc Biol Sci 272: 395–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gregory TR (2014) Animal Genome Size Database. Available: http://www.genomesize.com.
- 23. Arthur W, Chipman AD (2005) The centipede Strigamia maritima: what it can tell us about the development and evolution of segmentation. Bioessays 27: 653–660. [DOI] [PubMed] [Google Scholar]
- 24. Brena C, Akam M (2012) The embryonic development of the centipede Strigamia maritima . Dev Biol 363: 290–307. [DOI] [PubMed] [Google Scholar]
- 25. Lewis JGE (1961) The life history and ecology of the littoral centipede Strigamia ( = Scolioplanes) maritima (Leach). Proc Zool Soc Lond 137: 221–248. [Google Scholar]
- 26. Chipman AD, Akam M (2008) The segmentation cascade in the centipede Strigamia maritima: involvement of the Notch pathway and pair-rule gene homologues. Dev Biol 319: 160–169. [DOI] [PubMed] [Google Scholar]
- 27. Chipman AD, Arthur W, Akam M (2004) Early development and segment formation in the centipede Strigamia maritima (Geophilomorpha). Evol Dev 6: 78–89. [DOI] [PubMed] [Google Scholar]
- 28. Chipman AD, Arthur W, Akam M (2004) A double segment periodicity underlies segment generation in centipede development. Curr Biol 14: 1250–1255. [DOI] [PubMed] [Google Scholar]
- 29. Green J, Akam M (2013) Evolution of the pair rule gene network: Insights from a centipede. Dev Biol 382: 235–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kettle C, Johnstone J, Jowett T, Arthur H, Arthur W (2003) The pattern of segment formation, as revealed by engrailed expression, in a centipede with a variable number of segments. Evol Dev 5: 198–207. [DOI] [PubMed] [Google Scholar]
- 31. Brena C, Green J, Akam M (2013) Early embryonic determination of the sexual dimorphism in segment number in geophilomorph centipedes. Evodevo 4: 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Brena C, Akam M (2013) An analysis of segmentation dynamics throughout embryogenesis in the centipede Strigamia maritima . BMC Biology 11: 112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Vedel V, Apostolou Z, Arthur W, Akam M, Brena C (2010) An early temperature-sensitive period for the plasticity of segment number in the centipede Strigamia maritima . Evol Dev 12: 347–352. [DOI] [PubMed] [Google Scholar]
- 34. Giribet G, Carranza S, Riutort M, Baguña J, Ribera C (1999) Internal phylogeny of the Chilopoda (Myriapoda, Arthropoda) using complete 18S rDNA and partial 28S rDNA sequences. Phil Trans Roy Soc Lond B 354: 215–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mundel P (1979) The centipedes (Chilopoda) of the Mazon Creek. Nitecki MH, editor. Mazon Creek fossils. New York: Academic Press. pp. 361–378. [Google Scholar]
- 36.Minelli A (2011) Chilopoda – general morphology. Minelli A, editor. The Myriapoda. Leiden: Brill. pp. 43–66. [Google Scholar]
- 37.Müller CHG, Sombke A, Hilken G, Rosenberg J (2011) Chilopoda – sense organs. Minelli A, editor. The Myriapoda. Leiden: Brill. pp. 235–278. [Google Scholar]
- 38. Plateau F (1886) Recherches sur la perception de la lumière par les Myriapodes aveugles. J Anat Physiol 22: 431–457. [Google Scholar]
- 39. Mackay TFC, Richards S, Stone EA, Barbadilla A, Ayroles JF, et al. (2012) The Drosophila melanogaster genetic reference panel. Nature 482: 173–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Huerta-Cepas J, Capella-Gutierrez S, Pryszcz LP, Denisov I, Kormes D, et al. (2011) PhylomeDB v3.0: an expanding repository of genome-wide collections of trees, alignments and phylogeny-based orthology and paralogy predictions. Nuc Acid Res 39: D556–D560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Gabaldón T (2008) Large-scale assignment of orthology: back to phylogenetics? Genome Biol 9: 235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Huerta-Cepas J, Gabaldón T (2011) Assigning duplication events to relative temporal scales in genome-wide studies. Bioinformatics 27: 38–45. [DOI] [PubMed] [Google Scholar]
- 43. Negrisolo E, Minelli A, Valle G (2004) The mitochondrial genome of the house centipede Scutigera and the monophyly versus paraphyly of myriapods. Mol Biol Evol 21: 770–780. [DOI] [PubMed] [Google Scholar]
- 44. Putnam NH, Butts T, Ferrier DEK, Furlong RF, Hellsten U, et al. (2008) The amphioxus genome and the evolution of the chordate karyotype. Nature 453: 1064–1071. [DOI] [PubMed] [Google Scholar]
- 45. Putnam NH, Srivastava M, Hellsten U, Dirks B, Chapman J, et al. (2007) Sea anemone genome reveals ancestral eumetazoan gene repertoire and genomic organization. Science 317: 86–94. [DOI] [PubMed] [Google Scholar]
- 46. Zdobnov EM, von Mering C, Letunic I, Bork P (2005) Consistency of genome-based methods in measuring metazoan evolution. FEBS lett 579: 3355–3361. [DOI] [PubMed] [Google Scholar]
- 47. Denoeud F, Henriet S, Mungpakdee S, Aury J-M, Da Silva C, et al. (2010) Plasticity of animal genome architecture unmasked by rapid evolution of a pelagic tunicate. Science 330: 1381–1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Panfilio KA, Akam M (2007) A comparison of Hox3 and Zen protein coding sequences in taxa that span the Hox3/zen divergence. Dev Genes Evol 217: 323–329. [DOI] [PubMed] [Google Scholar]
- 49. Garcia-Fernandez J (2005) The genesis and evolution of homeobox gene clusters. Nat Rev Genet 6: 881–892. [DOI] [PubMed] [Google Scholar]
- 50. Hui JHL, McDougall C, Monteiro AS, Holland PWH, Arendt D, et al. (2012) Extensive chordate and annelid macrosynteny reveals ancestral homeobox gene oganization. Mol Biol Evol 29: 157–165. [DOI] [PubMed] [Google Scholar]
- 51. Pollard SL, Holland PWH (2000) Evidence for 14 homeobox gene clusters in human genome ancestry. Curr Biol 10: 1059–1062. [DOI] [PubMed] [Google Scholar]
- 52. Butts T, Holland PWH, Ferrier DE (2008) The Urbilaterian Super-Hox cluster. Trends Genet 24: 259–262. [DOI] [PubMed] [Google Scholar]
- 53. Penalva-Arana DC, Lynch M, Robertson HM (2009) The chemoreceptor genes of the waterflea Daphnia pulex: many Grs but no Ors. BMC Evol Biol 9: 79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Robertson HM, Warr CG, Carlson JR (2003) Molecular evolution of the insect chemoreceptor gene superfamily in Drosophila melanogaster . P Natl Acad Sci U S A 100: 14537–14542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Vieira FG, Rozas J (2011) Comparative genomics of the odorant-binding and chemosensory protein gene families across the Arthropoda: Origin and rvolutionary history of the chemosensory system. Genome Biol Evol 3: 476–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Pelosi P (1994) Odorant-binding proteins. Crit Rev Biochem Mol 29: 199–228. [DOI] [PubMed] [Google Scholar]
- 57. Vogt RG, Riddiford LM (1981) Pheromone binding and inactivation by moth antennae. Nature 293: 161–163. [DOI] [PubMed] [Google Scholar]
- 58. Angeli S, Ceron F, Scaloni A, Monti M, Monteforti G, et al. (1999) Purification, structural characterization, cloning and immunocytochemical localization of chemoreception proteins from Schistocerca gregaria . Eur J Biochem 262: 745–754. [DOI] [PubMed] [Google Scholar]
- 59. Pelosi P, Zhou JJ, Ban LP, Calvello M (2006) Soluble proteins in insect chemical communication. Cell Mol Life Sci 63: 1658–1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Starostina E, Xu AG, Lin HP, Pikielny CW (2009) A Drosophila protein family implicated in pheromone perception is related to Tay-Sachs GM2-activator protein. J Biol Chem 284: 585–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Xu A, Park SK, D'Mello S, Kim E, Wang Q, et al. (2002) Novel genes expressed in subsets of chemosensory sensilla on the front legs of male Drosophila melanogaster . Cell Tissue Res 307: 381–392. [DOI] [PubMed] [Google Scholar]
- 62. Clyne PJ, Warr CG, Carlson JR (2000) Candidate taste receptors in Drosophila . Science 287: 1830–1834. [DOI] [PubMed] [Google Scholar]
- 63. Scott K, Brady R, Cravchik A, Morozov P, Rzhetsky A, et al. (2001) A chemosensory gene family encoding candidate gustatory and olfactory receptors in Drosophila . Cell 104: 661–673. [DOI] [PubMed] [Google Scholar]
- 64. Clyne PJ, Warr CG, Freeman MR, Lessing D, Kim JH, et al. (1999) A novel family of divergent seven-transmembrane proteins: candidate odorant receptors in Drosophila . Neuron 22: 327–338. [DOI] [PubMed] [Google Scholar]
- 65. Gao Q, Chess A (1999) Identification of candidate Drosophila olfactory receptors from genomic DNA sequence. Genomics 60: 31–39. [DOI] [PubMed] [Google Scholar]
- 66. Benton R, Vannice KS, Gomez-Diaz C, Vosshall LB (2009) Variant ionotropic glutamate receptors as chemosensory receptors in Drosophila . Cell 136: 149–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Croset V, Rytz R, Cummins SF, Budd A, Brawand D, et al. (2010) Ancient protostome origin of chemosensory ionotropic glutamate receptors and the evolution of insect taste and olfaction. PLoS Genet 6: e1001064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Weil E (1958) Zur Biologie der einheimischen Geophiliden. Z Angew Entomol 42: 173–209. [Google Scholar]
- 69. Xiang Y, Yuan QA, Vogt N, Looger LL, Jan LY, et al. (2010) Light-avoidance-mediating photoreceptors tile the Drosophila larval body wall. Nature 468: 921–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Zhan S, Merlin C, Boore JL, Reppert SM (2011) The monarch butterfly genome yields insights into long-distance migration. Cell 147: 1171–1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Benna C, Bonaccorsi S, Wulbeck C, Helfrich-Forster C, Gatti M, et al. (2010) Drosophila timeless2 Is required for chromosome stability and circadian photoreception. Curr Biol 20: 346–352. [DOI] [PubMed] [Google Scholar]
- 72. George H, Terracol R (1997) The vrille gene of Drosophila is a maternal enhancer of decapentaplegic and encodes a new member of the bZIP family of transcription factors. Genetics 146: 1345–1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Reddy KL, Rovani MK, Wohlwill A, Katzen A, Storti RV (2006) The Drosophila Par domain protein I gene, Pdp1, is a regulator of larval growth, mitosis and endoreplication. Dev Biol 289: 100–114. [DOI] [PubMed] [Google Scholar]
- 74. Avivi A, Albrecht U, Oster H, Joel A, Beiles A, et al. (2001) Biological clock in total darkness: The Clock/MOP3 circadian system of the blind subterranean mole rat. Proc Natl Acad Sci U S A 98: 13751–13756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Avivi A, Oster H, Joel A, Beiles A, Albrecht U, et al. (2004) Circadian genes in a blind subterranean mammal III: molecular cloning and circadian regulation of cryptochrome genes in the blind subterranean mole rat, Spalax ehrenbergi superspecies. J Biol Rhyth 19: 22–34. [DOI] [PubMed] [Google Scholar]
- 76. Goldman BD, Goldman SL, Riccio AP, Terkel J (1997) Circadian patterns of locomotor activity and body temperature in blind mole-rats, Spalax ehrenbergi . J Biol Rhyth 12: 348–361. [DOI] [PubMed] [Google Scholar]
- 77. Crandall KA, Hillis DM (1997) Rhodopsin evolution in the dark. Nature 387: 667–668. [DOI] [PubMed] [Google Scholar]
- 78. Willis JH (2010) Structural cuticular proteins from arthropods: annotation, nomenclature, and sequence characteristics in the genomics era. Insect Biochem Molec Biol 40: 189–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Rebers JE, Riddiford LM (1988) Structure and expression of a Manduca sexta larval cuticle gene homologous to Drosophila cuticle genes. J Mol Biol 203: 411–423. [DOI] [PubMed] [Google Scholar]
- 80. Rebers JE, Willis JH (2001) A conserved domain in arthropod cuticular proteins binds chitin. Insect Biochem Molec Biol 31: 1083–1093. [DOI] [PubMed] [Google Scholar]
- 81. Fredriksson R, Schioth HB (2005) The repertoire of G-protein-coupled receptors in fully sequenced genomes. Mol Pharmacol 67: 1414–1425. [DOI] [PubMed] [Google Scholar]
- 82. Ritter SL, Hall RA (2009) Fine-tuning of GPCR activity by receptor-interacting proteins. Nat Rev Mol Cell Bio 10: 819–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Hill RJ, Billas IML, Bonneton F, Graham LD, Lawrence MC (2013) Ecdysone Receptors: from the Ashburner model to structural biology. Annu Rev Entomol 58: 251–271. [DOI] [PubMed] [Google Scholar]
- 84. Jindra M, Palli SR, Riddiford LM (2013) The juvenile hormone signaling pathway in insect development. Annu Rev Entomol 58: 181–204. [DOI] [PubMed] [Google Scholar]
- 85. Srivastava DP, Yu EJ, Kennedy K, Chatwin H, Reale V, et al. (2005) Rapid, nongenomic responses to ecdysteroids and catecholamines mediated by a novel Drosophila G-protein-coupled receptor. J Neurosci 25: 6145–6155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Evans PD, Maqueira B (2005) Insect octopamine receptors: a new classification scheme based on studies of cloned Drosophila G-protein coupled receptors. Invert Neurosci 5: 111–118. [DOI] [PubMed] [Google Scholar]
- 87. Hauser F, Neupert S, Williamson M, Predel R, Tanaka Y, et al. (2010) Genomics and peptidomics of neuropeptides and protein hormones present in the parasitic wasp Nasonia vitripennis . J Proteome Res 9: 5296–5310. [DOI] [PubMed] [Google Scholar]
- 88. Hauser F, Cazzamali G, Williamson M, Park Y, Li B, et al. (2008) A genome-wide inventory of neurohormone GPCRs in the red flour beetle Tribolium castaneum . Front Neuroendocrin 29: 142–165. [DOI] [PubMed] [Google Scholar]
- 89. Stay B, Tobe SS (2007) The role of allatostatins in juvenile hormone synthesis in insects and crustaceans. Annu Rev Entomol 52: 277–299. [DOI] [PubMed] [Google Scholar]
- 90. Weaver RJ, Audsley N (2009) Neuropeptide regulators of juvenile hormone synthesis: structures, functions, distribution, and unanswered questions. Trends Comp Endocrinol Neuro 1163: 316–329. [DOI] [PubMed] [Google Scholar]
- 91. Grbic M, Van Leeuwen T, Clark RM, Rombauts S, Rouze P, et al. (2011) The genome of Tetranychus urticae reveals herbivorous pest adaptations. Nature 479: 487–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Hui JHL, Hayward A, Bendena WG, Takahashi T, Tobe SS (2010) Evolution and functional divergence of enzymes involved in sesquiterpenoid hormone biosynthesis in crustaceans and insects. Peptides 31: 451–455. [DOI] [PubMed] [Google Scholar]
- 93. Van der Zee M, da Fonseca RN, Roth S (2008) TGF beta signaling in Tribolium: vertebrate-like components in a beetle. Dev Genes Evol 218: 203–213. [DOI] [PubMed] [Google Scholar]
- 94. Lowery JW, LaVigne AW, Kokabu S, Rosen V (2013) Comparative genomics identifies the mouse Bmp3 promoter and an upstream evolutionary conserved region (ECR) in mammals. PLoS ONE 8: e57840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Cho SJ, Valles Y, Giani VC, Seaver EC, Weisblat DA (2010) Evolutionary dynamics of the wnt gene family: a lophotrochozoan perspective. Mol Biol Evol 27: 1645–1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Prud'homme B, Lartillot N, Balavoine G, Adoutte A, Vervoort M (2002) Phylogenetic analysis of the Wnt gene family: insights from lophotrochozoan members. Curr Biol 12: 1395–1400. [DOI] [PubMed] [Google Scholar]
- 97. Janssen R, Le Gouar M, Pechmann M, Poulin F, Bolognesi R, et al. (2010) Conservation, loss, and redeployment of Wnt ligands in protostomes: implications for understanding the evolution of segment formation. Bmc Evolutionary Biology 10: 374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Murat S, Hopfen C, McGregor AP (2010) The function and evolution of Wnt genes in arthropods. Arthropod Struct Dev 39: 446–452. [DOI] [PubMed] [Google Scholar]
- 99. Nusse R (2001) An ancient cluster of Wnt paralogues. Trends Genet 17: 443–443. [DOI] [PubMed] [Google Scholar]
- 100. McGinnis N, Ragnhildstveit E, Veraksa A, McGinnis W (1998) A cap ‘n’ collar protein isoform contains a selective Hox repressor function. Development 125: 4553–4564. [DOI] [PubMed] [Google Scholar]
- 101. Iwanaga S, Lee BL (2005) Recent advances in the innate immunity of invertebrate animals. J Biochem Mol Biol 38: 128–150. [DOI] [PubMed] [Google Scholar]
- 102. Hoffmann JA, Kafatos FC, Janeway CA, Ezekowitz RAB (1999) Phylogenetic perspectives in innate immunity. Science 284: 1313–1318. [DOI] [PubMed] [Google Scholar]
- 103. Lemaitre B, Hoffmann J (2007) The host defense of Drosophila melanogaster . Annu Rev Immunol 25: 697–743. [DOI] [PubMed] [Google Scholar]
- 104. Dong YM, Dimopoulos G (2009) Anopheles fibrinogen-related proteins provide expanded pattern recognition capacity against bacteria and malaria parasites. J Biol Chem 284: 9835–9844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Waterhouse RM, Kriventseva EV, Meister S, Xi ZY, Alvarez KS, et al. (2007) Evolutionary dynamics of immune-related genes and pathways in disease-vector mosquitoes. Science 316: 1738–1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Watson FL, Puttmann-Holgado R, Thomas F, Lamar DL, Hughes M, et al. (2005) Extensive diversity of Ig-superfamily proteins in the immune system of insects. Science 309: 1874–1878. [DOI] [PubMed] [Google Scholar]
- 107. Brites D, Brena C, Ebert D, Du Pasquier L (2013) More than one way to produce protein diversity: duplication and limited alternative splicing of an adhesion molecule gene in basal arthropods. Evolution 67: 2999–3011. [DOI] [PubMed] [Google Scholar]
- 108. Obbard DJ, Gordon KHJ, Buck AH, Jiggins FM (2009) The evolution of RNAi as a defence against viruses and transposable elements. Philos Trans Roy Soc B 364: 99–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Squires JE, Berry MJ (2008) Eukaryotic selenoprotein synthesis: mechanistic insight incorporating new factors and new functions for old factors. IUBMB Life 60: 232–235. [DOI] [PubMed] [Google Scholar]
- 110. Mariotti M, Ridge PG, Zhang Y, Lobanov AV, Pringle TH, et al. (2012) Composition and evolution of the vertebrate and mammalian selenoproteomes. PLoS ONE 7: e33066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Chapple CE, Guigo R (2008) Relaxation of selective constraints causes independent selenoprotein etinction in insect genomes. PLoS ONE 3: e2968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Kim HY, Fomenko DE, Yoon YE, Gladyshev VN (2006) Catalytic advantages provided by selenocysteine in methionine-S-sulfoxide reductases. Biochemistry 45: 13697–13704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Corona M, Robinson GE (2006) Genes of the antioxidant system of the honey bee: annotation and phylogeny. Insect Mol Biol 15: 687–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Feng S, Cokus SJ, Zhang X, Chen PY, Bostick M, et al. (2010) Conservation and divergence of methylation patterning in plants and animals. Proc Natl Acad Sci U S A 107: 8689–8694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Suzuki MM, Kerr AR, De Sousa D, Bird A (2007) CpG methylation is targeted to transcription units in an invertebrate genome. Genome Res 17: 625–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Zemach A, McDaniel IE, Silva P, Zilberman D (2010) Genome-wide evolutionary analysis of eukaryotic DNA methylation. Science 328: 916–919. [DOI] [PubMed] [Google Scholar]
- 117. Foret S, Kucharski R, Pellegrini M, Feng S, Jacobsen SE, et al. (2012) DNA methylation dynamics, metabolic fluxes, gene splicing, and alternative phenotypes in honey bees. Proc Natl Acad Sci U S A 109: 4968–4973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Laurent L, Wong E, Li G, Huynh T, Tsirigos A, et al. (2010) Dynamic changes in the human methylome during differentiation. Genome Res 20: 320–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Elango N, Hunt BG, Goodisman MA, Yi SV (2009) DNA methylation is widespread and associated with differential gene expression in castes of the honeybee, Apis mellifera. Proc Natl Acad Sci U S A 106: 11206–11211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Hunt BG, Brisson JA, Yi SV, Goodisman MAD (2010) Functional conservation of DNA methylation in the pea aphid and the honeybee. Genome Biol Evol 2: 719–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Park J, Peng ZG, Zeng J, Elango N, Park T, et al. (2011) Comparative analyses of DNA methylation and sequence evolution using Nasonia genomes. Mol Biol Evol 28: 3345–3354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Richards S, Gibbs RA, Weinstock GM, Brown SJ, Denell R, et al. (2008) The genome of the model beetle and pest Tribolium castaneum . Nature 452: 949–955. [DOI] [PubMed] [Google Scholar]
- 123. Kriaucionis S, Heintz N (2009) The nuclear DNA base 5-hydroxymethylcytosine is present in Purkinje neurons and the brain. Science 324: 929–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Tahiliani M, Koh KP, Shen Y, Pastor WA, Bandukwala H, et al. (2009) Conversion of 5-methylcytosine to 5-hydroxymethylcytosine in mammalian DNA by MLL partner TET1. Science 324: 930–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Kozomara A, Griffiths-Jones S (2011) miRBase: integrating microRNA annotation and deep-sequencing data. Nuc Acid Res 39: D152–D157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Wheeler BM, Heimberg AM, Moy VN, Sperling EA, Holstein TW, et al. (2009) The deep evolution of metazoan microRNAs. Evol Dev 11: 50–68. [DOI] [PubMed] [Google Scholar]
- 127. Reinhart BJ, Slack FJ, Basson M, Pasquinelli AE, Bettinger JC, et al. (2000) The 21-nucleotide let-7 RNA regulates developmental timing in Caenorhabditis elegans . Nature 403: 901–906. [DOI] [PubMed] [Google Scholar]
- 128. Christodoulou F, Raible F, Tomer R, Simakov O, Trachana K, et al. (2010) Ancient animal microRNAs and the evolution of tissue identity. Nature 463: 1084–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Caygill EE, Johnston LA (2008) Temporal regulation of metamorphic processes in Drosophila by the let-7 and miR-125 heterochronic microRNAs. Curr Biol 18: 943–950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Marco A, Hui JHL, Ronshaugen M, Griffiths-Jones S (2010) Functional shifts in insect microRNA evolution. Genome Biol Evol 2: 686–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. McTaggart SJ, Conlon C, Colbourne JK, Blaxter ML, Little TJ (2009) The components of the Daphnia pulex immune system as revealed by complete genome sequencing. BMC Genomics 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Dasmahapatra KK, Walters JR, Briscoe AD, Davey JW, Whibley A, et al. (2012) Butterfly genome reveals promiscuous exchange of mimicry adaptations among species. Nature 487: 94–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Frequency histogram showing the distribution of gene lengths in the S. maritima genome. Gene length data used in this plot are available in File S4.
(PDF)
Multi-gene phylogeny for the 18 species included in the phylogenomics analysis. 1,491 widespread single-copy sets of orthologue sequences in at least 15 out of the 18 species were concatenated into a single alignment of 842,150 columns. Then, a maximum-likelihood tree was inferred using LG as evolutionary model by using PhyML.
(PDF)
Multi-gene phylogeny for 12 species included in the phylogenomics analysis plus five additional Chelicerata species. 1,491 widespread single-copy sets of orthologue sequences were concatenated into a single alignment of 829,729 positions. Then, a maximum-likelihood tree was inferred using LG as the evolutionary model by using PhyML.
(PDF)
Alternative topological placements of S. maritima relative to the main arthropod groups considered in the study: Chelicerata and Pancrustacea. Internal organization of each group was initially collapsed and, therefore, optimized during maximum-likelihood reconstruction.
(PDF)
Clusters of genes specifically expanded in the centipede lineage. On the plot, only clusters grouping five or more protein-coding genes were considered. The data underlying this plot are available in File S4.
(PDF)
Mitochondrial gene organisation. Shaded regions represent differences from the ground pattern. Gene translocations in Myriapoda have been noted in Scutigerella causeyae (Myriapoda: Symphyla) [49]. The previous example of the small conserved region trnaF-nad5-H-nad4-nad4L on the minus strand between Limulus, Lithobius, and Strigamia is not a conserved feature in all Chilopoda, because Scutigera colepotrata have an interruption between nad5 and H-nad4 with elements on the minus and plus strands accompanied by a translocation of nad4L to a position immediately preceding nad5.
(PDF)
Classification of all S. maritima (Sma) homeodomains (excluding Pax2/5/8/sv) via phylogenetic analysis using T. castaneum (Tca) and B. floridae (Bfl) homeodomains. This phylogenetic analysis was constructed using neighbour-joining with a JTT distance matrix and 1,000 bootstrap replicates. Gene classes are indicated by colours. The genes coloured in grey are those genes that cannot be assigned to known classes. Further classification was performed using additional domains outside the homeodomain and by performing additional phylogenetic analysis for particular gene classes using maximum-likelihood and bayesian approaches. Pax2/5/8/sv is excluded due to the gene possessing only a partial homeobox.
(PDF)
Phylogenetic analysis of ANTP class homeodomains of S. maritima (Sma) using T. castaneum (Tca) and B. floridae (Bfl) for comparison. These phylogenetic analyses were constructed using neighbour-joining with a JTT distance matrix, 1,000 bootstrap replicates (support values in black). Nodes with support equal to or above 500 in the maximum-likelihood (LG+G) analysis are in blue and nodes with posterior probabilities equal to or above 0.5 (LG+G) in the Bayesian analysis are in red.
(PDF)
Phylogenetic analysis of PRD class homeodomains of S. maritima (Sma) using T. castaneum (Tca) and B. floridae (Bfl) for comparison. These phylogenetic analyses were constructed using neighbour-joining with a JTT distance matrix, 1,000 bootstrap replicates (support values in black). Nodes with support equal to or above 500 in the maximum-likelihood (LG+G) analysis are in blue and nodes with posterior probabilities equal to or above 0.5 (LG+G) in the Bayesian analysis are in red.
(PDF)
Phylogenetic analysis of HNF class homeodomains of S. maritima (Sma) using B. floridae (Bfl), human ( Homo sapiens , Hsa), and sea anemone ( N. vectensis , Nve) for comparison. These phylogenetic analyses were constructed using neighbour-joining with a JTT distance matrix, 1,000 bootstrap replicates (support values in black). Nodes with support equal to or above 500 in the maximum-likelihood (LG+G) analysis are in blue and nodes with posterior probabilities equal to or above 0.5 (LG+G) in the Bayesian analysis are in red.
(PDF)
Phylogenetic analysis of Xlox/Hox3 genes of S. maritima (Sma) using a selection of Hox1, Hox2, Hox3, Hox4, and Xlox sequences. This analysis was based upon the whole coding sequence of the genes, and was constructed using neighbour-joining with a JTT distance matrix and 1,000 bootstrap replicates. The blue support value (of 333) is the node that reveals the affinity between the Xlox/Hox3 genes of S. maritima and Xlox sequences. Ame, A. mellifera; Bfl, B. floridae; Cte, Capitella teleta; Dme, D. melanogaster; Lgi, Lottia gigantea; and Tca, T. castaneum.
(PDF)
Multiple alignment of relevant residues of the Hox1, Hox2, Hox3, Hox4, and Xlox sequences of different lineages compared to S. maritima Hox3a and Hox3b sequences. Three paired class genes are included as an outgroup. The grading of purple colouring of the amino acids shows the identity level of these sequences. The red rectangles in the multiple alignment delimit the core of the hexapeptide motif and the homeodomain. This is the alignment used to construct the phylogenetic tree in Figure S13. Ame, A. mellifera; Bfl, B. floridae; Cte, Capitella teleta; Dme, D. melanogaster; Lgi, Lottia gigantea; and Tca, T. castaneum.
(PDF)
Phylogenetic analysis of S. maritima Xlox/Hox3 homeodomain and hexapeptide motifs using a selection of Hox1, Hox2, Hox3, Hox4, and Xlox sequences. This analysis used a section of the coding sequence including the hexapeptide and some flanking residues plus the homeodomain (alignment in Figure S12). Three paired class genes are included as an outgroup. This phylogeny was constructed using neighbour-joining with the JTT distance matrix and 1,000 bootstrap replicates. Maximum likelihood support values are shown in blue and Bayesian posterior probabilities in red. Ame, A. mellifera; Bfl, B. floridae; Cte, Capitella teleta; Dme, D. melanogaster; Lgi, Lottia gigantean; Tca, T. castaneum.
(PDF)
Fisher's exact test to distinguish whether S. maritima scaffold 48457 has significant synteny conservation with ParaHox or Hox chromosomes of humans. No significant Hox or ParaHox association is found.
(PDF)
Phylogenetic analysis of TALE class homeodomains of S. maritima (Sma) using T. castaneum (Tca) and B. floridae (Bfl), including the Iroquois/Irx genes. These phylogenetic analyses were constructed using neighbour-joining with a JTT distance matrix, 1,000 bootstrap replicates (support values in black). Nodes with support equal to or above 500 in the maximum-likelihood (LG+G) analysis are in blue and nodes with posterior probabilities equal to or above 0.5 (LG+G) in the Bayesian analysis are in red.
(PDF)
RNA processing in the Hox cluster of S. maritima . The transcriptome of S. maritima (Sm) eggs (blue), females (green), and males (red) was mapped to the Hox gene cluster (top panel; see Figure 4 in the main text) and transcript models were inferred for each gene within the cluster (shaded area) taking into account the presence of ORF and polyadenylation signals (PAS) to support the existence of RNA processing events. We note the occurrence of more than one mRNA isoform of six S. maritima Hox genes (i.e., Antp, Ubx, abd-A, lab, Dfd, pb). In all these six cases alternative polyadenylation (APA) generates mRNAs bearing distinct 3′ UTRs (alternative UTR sizes at the bottom). Alternative splicing (AS) with concomitant alternative promoter use (APU) events concern two S. maritima Hox genes Dfd and ftz (see alternative ORF sizes at the bottom). We also see that some genes such as S. maritima Ubx display high heterogeneity in 3′UTR sequences within the embryonic transcriptome (“eggs” data) suggesting the possibility that S. maritima Ubx APA might be developmentally controlled and/or display a tissue-specific pattern (see inset for further details on symbols).
(PDF)
RNA processing in the S. maritima and D. melanogaster Hox clusters. (A) The incidence of alternatively processed mRNAs is comparable between S. maritima and D. melanogaster, in that over 75% of the S. maritima Hox genes undergo RNA processing of one type or another. Similarly, seven out of the eight Drosophila Hox genes produce different mRNA isoforms (FlyBase, http://flybase.org/). (B) Three D. melanogaster Hox genes undergo AS (blue) and five produce different transcripts via APA (red, FlyBase http://flybase.org/). In addition five fruit fly Hox genes form different RNA species by APU (green). (C) Classification of all alternatively processed mRNA events in the S. maritima Hox cluster based on the same categorisation as in (B). Note that patterns of AS and APA affecting S. maritima and D. melanogaster Hox genes are relatively comparable; in contrast, APU seems more prevalent in the Drosophila (five out of eight genes) than in the centipede (two out of nine genes) Hox genes.
(PDF)
Phylogenetic tree of the S. maritima , D. pulex , I. scapularis , and representative insect GRs, part one. This is a corrected distance tree and was rooted at the midpoint in the absence of a clear outgroup, an approach that clearly indicates the distinctiveness of the centipede GRs. It is a more detailed version of Figure 5A. The S. maritima, D. pulex, I. scapularis, and representative insect gene/protein names are highlighted in red, blue, brown, and green, respectively, as are the branches leading to them to emphasize gene lineages. Bootstrap support levels in percentage of 10,000 replications of neighbour-joining with uncorrected distance is shown above major branches. Comments on major gene lineages are on the right. Suffixes after the gene/protein names are: PSE, pseudogene; FIX, sequence fixed with raw reads; JOI, gene model joined across scaffolds. Note than in Figure 5A for space reasons the IsGr47 and 59 proteins are included in the carbon dioxide and sugar receptor groupings, respectively; however, there is no bootstrap support for these branches, and no such functional assignment is claimed. Similarly, it is unlikely that the DpGr57/58 proteins are fructose receptors.
(PDF)
Phylogenetic tree of the S. maritima , D. pulex , I. scapularis , and representative insect GRs, part two. This is a corrected distance tree and was rooted at the midpoint in the absence of a clear outgroup, an approach that clearly indicates the distinctiveness of the centipede GRs. It is a more detailed version of Figure 5A. The S. maritima, D. pulex, I. scapularis, and representative insect gene/protein names are highlighted in red, blue, brown, and green, respectively, as are the branches leading to them to emphasize gene lineages. Bootstrap support levels in percentage of 10,000 replications of neighbour-joining with uncorrected distance is shown above major branches. Comments on major gene lineages are on the right. Suffixes after the gene/protein names are: PSE, pseudogene; FIX, sequence fixed with raw reads; JOI, gene model joined across scaffolds. Note than in Figure 5A for space reasons the IsGr47 and 59 proteins are included in the carbon dioxide and sugar receptor groupings, respectively; however, there is no bootstrap support for these branches, and no such functional assignment is claimed. Similarly, it is unlikely that the DpGr57/58 proteins are fructose receptors.
(PDF)
Neuropeptide precursor sequences identified in the S. maritima genome. The putative signal peptides (predicted by SignalP) are underlined, the putative active neuropeptides or protein hormones (based on similarity to neuropeptides or protein hormones identified in other invertebrates) are marked in yellow. Green indicates putative basic cleavage sites flanking the putative neuropeptides. Glycines used for amidation are shown in blue, cysteines proposed to form cysteine bridges are shown in red. Dots indicate missing N- or C-termini.
(DOCX)
Examples of tandem duplications of neuropeptide receptor genes. Structure of the two inotocin receptor genes found head-to-head on opposite strands of scaffold JH431865 (A). Structure of the two SIFamide receptor genes found tail-to-head on the same strand of scaffold JH432116 (B).
(PDF)
Schematic diagram showing sesquiterpenoids/juvenoids synthesis (upper) and degradation (lower) pathways in arthropods. Molecules/hormones in synthesis are shown in bold, enzymes are shown in italics, and species/clades are shown in bold italics.
(PDF)
Phylogenetic analysis of the TGFβ ligands in arthropods. See Text S1 for details. Abbreviations: Ag, Anopheles gambiae; Am, A. mellifera; Ap, Acyrthosiphon pisum; Ca, Clogmia albipunctata; Dm, Drosophila melanogaster; Dp, D. pulex; Is, I. scapularis; Lg, Lottia gigantea Ma, Megaselia abdita; Nv, Nasonia vitripennis; Ph, Pediculus humanus; Tc, T. castaneum;.
(EPS)
Range of Wnt genes present in S. maritima . Wnt genes present and number of Wnt subfamilies absent in S. maritima in comparison with other arthropods and three non-arthropod outgroups.
(TIF)
Phylogeny of FGFR genes indicating that FGFR genes duplicated independently in S. maritima and D. melanogaster . See text for details. Alignment was performed using Clustal-Omega (http://www.ebi.ac.uk/Tools/services/web). The evolutionary history was inferred using the neighbour-joining method with bootstrapping to determine node support values (10,000 replicates). The evolutionary distances were computed using the Poisson correction method. Evolutionary analyses were conducted in MEGA5.
(EPS)
Phylogeny including the three FGF genes of S. maritima . See text for details. Alignment was performed using Clustal-Omega (http://www.ebi.ac.uk/Tools/services/web). The evolutionary history was inferred using the neighbour-joining method with bootstrapping to determine node support values (10,000 replicates). The evolutionary distances were computed using the Poisson correction method. Evolutionary analyses were conducted in MEGA5.
(EPS)
Cap ‘n’ collar (cnc) genes. (A) The two genes are located on different scaffolds. Cnc1 is a long transcript consisting of 11 exons. Cnc2 is shorter (eight exons), the three exons at the 3′ end of the gene that encode the C-terminal region of the protein including the conserved domain (B) show a similar structure. (B) S. maritima Cnc protein structure. Both proteins contain the bZip domain in a similar position at the C-terminus. Cnc1 encodes a long protein (925 amino acids). Bits of the N-terminal region (blue lines) align with D. melanogaster Cnc isoform C and T. castaneum Cnc variant A. (C) Cnc protein sequence alignment, only showing the aligning bits in the N-terminal region. Blue lines show short stretches of sequence that form a consensus motif. These motifs are not present in the proteins encoded by Sm-cnc2, Dm-cnc isoforms A and B, and T. castaneum cnc variant B.
(JPG)
Frequency histograms of observed versus expected dinucleotide content in S. maritima gene bodies. (A–P) The y-axis depicts the number of genes with the specific dinucleotide[o/e] values given on the x-axis. The distribution of all dinucleotide pairs, with the exception of CpG, is best described as a unimodal distribution. The distribution of CpG dinucleotides is best described as a trimodal distribution, with “high” and “low” CpG[o/e] classes. The data underlying this figure are available in File S5.
(TIF)
Frequency histogram of CpG[o/e] observed in 1,000 bp windows of the S. maritima genome. The y-axis depicts the number of genes with the specific CpG[o/e] values given on the x-axis. The distribution of CpG[o/e] in S. maritima genome is a bimodal distribution, with a high CpG[o/e] peak observed similar to that observed in the gene bodies (Figure 9). The data underlying this figure are available in File S6.
(TIF)
Contrasting patterns of DNA methylation, as measured by over- and underrepresentation of CpG dinucleotides in coding regions (CpG(o/e)), within arthropod species. In all graphs the y-axis depicts the number of genes with the specific CpG(o/e) values given on the x-axis. (A) D. melanogaster coding regions show a unimodal peak reflective of the lack of DNA methylation in this species. (B) A. mellifera shows a bimodal peak consisting of genes with a lower than expected CpG(o/e) (green distribution) and a higher than expected CpG(o/e) (blue distribution). The presence of a bimodal distribution in this species is consistent with depletion of CpG dinculeotides in the coding regions of genes over evolutionary time as a result of DNA methylation. (C) A single unimodal peak is also observed for Tetranychus urticae, a species that has very low levels of DNA methylation. (D) The S. maritima distribution is best explained as a mixture of three distinct distributions that we have deemed “low” (green distribution), “medium” (blue distribution), and “high” (grey distribution). The genes within the low distribution likely contain genes that are historically methylated, whilst the “high” distribution can be explained by regions of the genome that are comparatively CpG-rich (as determined by the analysis of the S. maritima genome, Figure S29). The data underlying this figure are available in File S7.
(PDF)
Chromosomal organisation of histone gene clusters in S. maritima . In insects such as Drosophila [115] and the pea aphid [109] histone encoding genes are present in quintet clusters, each cluster containing one gene from each of the five classes of histone. Only one such cluster could be identified in S. maritima (A). The other four clusters identified in the S. maritima genome (B–D) all consist of two to three copies of a histone encoding gene of a single class. It is possible that these have arisen as a result of recent gene duplication.
(EPS)
S. maritima vasa DEAD-box helicase germline gene phylogeny. Maximum likelihood tree of vasa/PL10 family genes. One gene is a likely vasa orthologue (SMAR015390), one groups with the PL10 family (SMAR005518), and the majority group in an apparently distinct DEAD-box-containing clade. Bootstrap values for 2,000 replicates are shown at each node. Accession numbers for protein sequences are as follows: Apis Belle (XP_391829.3), Apis Vasa (NP_001035345.1), Danio PL10 (NP_571016.2), Danio Vasa (AAI29276.1), Drosophila Belle (NP_536783.1), Drosophila Vasa (NP_723899.1), Gryllus Vasa (BAG65665.1), Mus Mvh (NP_001139357.1), Mus PL10 (NP_149068.1), Nasonia Belle (XP_001605842.1), Nasonia Vasa (XP_001603956.2), Nematostella PL10 (XP_001627306.1), Nematostella Vasa 1 (XP_001628238.1), Nematostella Vasa 2 (XP_001639051.1), Oncopeltus Vasa (AGJ83330.1), Parhyale Vasa (ABX76969.1), Tribolium Belle (NP_001153721.1), Tribolium Vasa (NP_001034520.2), Xenopus PL10 (NP_001080283.1), Xenopus VLG1 (NP_001081728.1).
(EPS)
Phylogenomic inventory of meiotic genes in arthropods. Red genes are specific to meiosis in model species in which functional data are available. “+” and “−” indicate the presence and absence of orthologues, respectively. Numbers indicate copy number of duplicated genes.
(PDF)
Patterns of microRNA gain and loss across the animal kingdom with the inclusion of S. maritima . The number of microRNAs that were gained or lost at each node are shown in green and red, respectively, and names are listed below each taxon. MicroRNAs that are found in the S.maritima genome are in bold, and families for which more than one homologue is found are marked with an asterisk. The tree depicts the Mandibulata hypothesis rather than the Myriochelata, as in [124].
(EPS)
Detailed overview for the repetitive elements in S. maritima . For each group the number of elements (putative families), the number of their fragments or copies in the genome, the cumulative length, the proportion of the assembly, and some features are shown. This includes elements containing nested inserts of other elements (n), elements that appear to be complete (i.e., all typical structural and coding parts present, even if containing stop codons or frameshifts), elements with a RT or Tase domain detected (n), elements that potentially could be active as they contain an intact ORF with all the typical domains even though they could lack other structural features like terminal repeats, and elements that contain an intact ORF for the RT domain or parts of the Tase domain and could thus be partly active. The elements that could not be categorized or contained features of protein coding regions are shown at the bottom, whereby they probably do not belong to the transposable elements.
(XLSX)
Set of species used in the comparative genomics analyses related to the S. maritima genome. Columns include, in this order, scientific names, the species code according to UNIPROT, the number of the longest unique transcript used in the analyses, the data source, and the date in which data were retrieved.
(DOCX)
Orthologues detected between a given species and S. maritima . First column indicates how many trees have been used to detect such orthologues. Columns “uniq” refers to the number of orthologues detected for each pair of species after removing redundancy. In one-to-many and many-to-many orthology relationships it is possible to count a given protein more than once. Regarding the ratios values, “all” column refers to the orthology ratio computed using all orthologue pairs meanwhile “uniq” refers to the ratio computed using “uniq” columns.
(DOCX)
Orthology ratios for a given species related to S. maritima . This table is similar to Table S3, but in this case orthology relationships with ten or more proteins for any of the species are discarded in order to avoid biases introduced by species-specific gene family expansions.
(DOCX)
Newly added Chelicerata species used to increase the taxon sampling for the species phylogeny. First column indicates the scientific species name, the second one indicates which strategy has been used to identify single copy protein-coding genes. Third column shows how many single-copy genes have been identified in each species from the initial set of 1,491 used to reconstruct the species phylogeny. Last two columns show the data source and the date on which data were retrieved.
(DOCX)
Results after applying the different statistical tests implemented in CONSEL for the alternative placement of S. maritima relative to Pancrustacea and Chelicerata groups of species (as shown in Figure S4) in the context of the 18 species used for the phylogenomics analyses. The “item” column relates to Figure S4 as follows: (1) topology arrangement corresponding to Figure S4 left-hand panel, in which S. maritima was grouped with Chelicerata species. (2) Topology arrangement corresponding to Figure S4 central panel, in which S. maritima branches off before the split of Pancrustacea and Chelicerata. (3) Topology arrangement corresponding to Figure S4 right-hand panel, in which S. maritima was grouped with Pancrustacea species.
(DOCX)
Results after applying the different statistical tests implemented in CONSEL for the alternative placement of S. maritima relative to the two arthropod groups, Pancrustacea and Chelicerata (as shown in Figure S4), with the inclusion of extra chelicerates. Taxon sampling for the Chelicerata was increased after including sequences from five additional species. In order to reduce any potential bias introduced by distant and/or fast-evolving out-groups, six out-group species from the initial set were removed. The “item” column relates to Figure S4 as follows: (1) topology arrangement corresponding to Figure S4 left-hand panel, in which S. maritima was grouped with Chelicerata species. (2) Topology arrangement corresponding to Figure S4 central panel, in which S. maritima branches off before the split of Pancrustacea and Chelicerata. (3) Topology arrangement corresponding to Figure S4 right-hand panel, in which S. maritima was grouped with Pancrustacea species.
(DOCX)
Enriched functional GO Terms for the ten largest clusters of duplicated S. maritima protein-coding genes specifically expanded in the centipede lineage, as compared with the whole genome.
(DOCX)
Statistics regarding the duplications of centipede genes relative to seven specific ages detected using all available trees on the phylome.
(DOCX)
Enriched functional GO terms for proteins duplicated at the different relative ages shown in Table S9. Columns show relative age, gene ontology namespace, the GO term id, and its name, respectively.
(DOCX)
Overview of S. maritima mitochondrial genome.
(DOCX)
Species used in the synteny analyses and the sources of their sequence data.
(DOCX)
Block-synteny summary statistics for pairs of species. Hs, Homo sapiens; Bf, B. floridae; Sm, S. maritima; Lg, Lottia gigantea; Ct, Capitella teleta; Nv, N. vectensis; Ta, Trichoplax adhaerens; Ag, Anopheles gambiae; Bm, B. mori.
(DOCX)
Summary of numbers of homeobox genes per class of Strigamia , Branchiostoma , and Tribolium .
(DOCX)
Names and identification numbers of all S. maritima homeobox genes along with their orthologues from the beetle, T. castaneum , and amphioxus, B. floridae .
(XLS)
One-to-one S. maritima to human orthologues starting from genes on S. maritima scaffold 48457, which contains SmaHox3a . The third column is the chromosomal location of the human orthologue. Human Hox chromosomes are 2, 7, 12, and 17 and the ParaHox chromosomes are 4, 5, 13, and X.
(DOCX)
Evolutionary conservation of RNA processing modes in the S. maritima and D. melanogaster Hox clusters. Type of RNA processing event concerning each one of the S. maritima (left) and D. melanogaster (right) Hox genes. We note that orthologous genes in both species undergo similar types of RNA processing: the three posterior-most Hox genes: Ubx, abd-a, and Abd-b display a specific type of APA (tandem APA) in both S. maritima and D. melanogaster (conserved patterns highlighted by red asterisks) providing an example of what might be a feature present in the ancestral Hox cluster to insects and myriapods. Nonetheless, for most other Hox genes, RNA processing patterns differ markedly between S. maritima and D. melanogaster, indicating that the conserved incidence of alternative RNA processing across arthropods can only be proposed for the posterior-most Hox genes.
(PDF)
Details of SmGr family genes and proteins. Columns are: Gene, the gene and protein name we are assigning (suffixes are PSE, pseudogene; FIX, assembly was repaired; JOI, gene model spans scaffolds); OGS, the official gene number in the 13,233 proteins (prefix is Smar_temp_); Scaffold, the genome assembly scaffold ID, prefix is scf718000 (amongst 14,739 scaffolds in assembly Smar05272011); Coordinates, the nucleotide range from the first position of the start codon to the last position of the stop codon in the scaffold; Strand – + is forward and − is reverse; introns, number of introns; ESTs, presence of an EST contig with appropriate splicing in one of the three transcriptome assemblies (F, female; M, male; E, eggs); AAs, number of encoded amino acids in the protein; comments, comments on the OGS gene model, repairs to the genome assembly, and pseudogene status (numbers in parentheses are the number of obvious pseudogenizing mutations).
(DOC)
Total numbers of biogenic amine receptors in different species.
(DOCX)
A comparison between the D. melanogaster and S. maritima biogenic amine receptors. The orthologues are given next to each other. When there is no orthologue, a dash (–) is written instead.
(XLSX)
Genes encoding neuropeptide precursors and neuropeptide receptors annotated in S. maritima . Abbreviations: ACP, adipokinetic hormone/corazonin-related neuropeptide; AKH, adipokinetic hormone; ADF, antidiuretic factor; AST, allatostatin; CCAP, crustacean cardio-active peptides; DH (Calc.-like), calcitonin-like diuretic hormone; DH (CRF-like), corticotropin releasing factor-like diuretic hormone; EH, eclosion hormone; ETH, ecdysis triggering hormone; GPA2, glycoprotein hormone A2; GPB5, glycoprotein hormone B5; ILP, insulin-like peptide; ITP, ion transport peptide; NPF, neuropeptide F; NPLP, neuropeptide-like precursor; PDF, pigment dispersing factor; PTTH, prothoracicotropic hormone; sNPF, short neuropeptide F.
(EPS)
Presence or absence of neuropeptide signaling systems in arthropods. The centipede S. maritima contains two CCHamide-1, two eclosion hormone and two FMRFamide genes (2 p). In some cases neuropeptide precursors could not be identified, but the corresponding receptor genes are present (R). We assume that this is due to sequencing gaps. For abbreviations see Table S21.
(DOC)
Genes commonly implicated in arthropod juvenoids biosynthesis (green) and degradation (blue), and their potential regulators (purple) [98]–[101]. Common abbreviations, and presence in the centipede S. maritima.
(DOCX)
List of genes commonly implicated as potential regulators of arthropod juvenoids biosynthesis (purple) [98]–[101]. Common abbreviations, and presence in the centipede S. maritima.
(DOCX)
Wnt genes in the genome of S. maritima . SMAR, the gene identification number, and scaffold, the scaffold identification number. Wnt 1, 6, and 10 are clustered together on the same scaffold (yellow highlighting), which is likely a remnant of the ancestral wnt gene cluster (see text for details).
(PDF)
Selenoproteins in the S. maritima genome.
(DOCX)
Histone encoding loci of S. maritima .
(DOCX)
Number of loci within the genomes of arthropod species encoding the five classes of histones. Orthologues for A. aegypti, D. pulex, T. urticae, and I. scapularis were obtained by BLAST analysis. Orthologues for A. mellifera and A. pisum were obtained from published literature [108],[109].
(DOCX)
Germ line and RNAi genes annotated in the S. maritima genome. The name of the Drosophila orthologue is shown unless indicated with “(Mo),” for mouse.
(DOCX)
Details of the manually annotated genes of S. maritima .
(XLSX)
One2One_GOTerms_GenomeIDs for Orthology-based functional annotation.
(XLSX)
Gustatory receptor sequences.
(XLSX)
Supporting Methods Text.
(DOCX)