SYNOPSIS
The discipline that investigates the biological effects of ultraviolet radiation on the immune system is called photoimmunology. Photoimmunology evolved from an interest in understanding the role of the immune system in skin cancer development, and why immunosuppressed organ transplant recipients are at greatly increased risk for cutaneous neoplasms. Ultraviolet radiation-induced damage DNA modifies the antigen presenting function of cutaneous dendritic cells, biases the immune response towards the generation of regulatory T-cells and stimulates epidermal keratinocyte production of immunosuppressive cytokines. In addition to contributing to an understanding of the pathogenesis of non-melanoma skin cancer, the knowledge acquired about the immunological effects of ultraviolet radiation exposure has provided an understanding of its role in the pathogenesis of other photodermatologic diseases such as polymorphous light eruption, chronic actinic dermatitis and cutaneous lupus erythematosus. This information has also been helpful in developing more effective and safer phototherapeutic devices for the treatment of a variety of cutaneous diseases.
Keywords: Photoimmunology, non-melanoma skin cancer, ultraviolet radiation, polymorphous light eruption, chronic actinic dermatitis, cutaneous lupus erythematosus, phototherapy, photosensitivity
The area of photodermatology that investigates the complex inter-relationship between ultraviolet (UV) radiation and the body’s immune system is referred to as photoimmunology. Its foundation derives from clinical observations in patients with UV-induced skin cancers, but the acquisition of knowledge about the interactions of ultraviolet radiation with the immune system has implications beyond skin cancer. It has helped us to understand the mechanisms by which phototherapy is effective in psoriasis and other dermatological diseases, and, in so doing, has expanded the spectrum of illnesses amenable to ultraviolet radiation treatments. Furthermore, photoimmunology has enhanced our understanding of the role of the immune system in several different photodermatologic disorders.
EVIDENCE OF PHOTOIMMUNOLOGICAL EFFECTS OF UV RADIATION IN HUMANS
The possibility that ultraviolet radiation, especially within the UVB range (290–320 nm), might modulate immunological function was suspected long before there was supportive experimental evidence, and was based on careful observations in individuals who developed solar UV-radiation-induced skin cancers. In contrast to many of the cancers that arise in other organs, cutaneous squamous cell carcinomas (SCC) develop gradually, rarely metastasize, and are associated with a chronic inflammatory infiltrate. SCC develop from actinic keratoses, over 25% of which undergo spontaneous regression, presumably by activation of cell-mediated immune defenses directed at antigens expressed by the pre-neoplastic cells1.
It has also been observed that cutaneous squamous cell carcinomas are more frequent in immunosuppressed individuals. Over 50% of renal transplant recipients on long term immunosuppressive therapy have developed at least one non-melanoma skin cancer.2–5 Although there is a 10-fold increased risk of basal cell carcinomas (BCCs) in organ transplant recipients, the likelihood of developing SCCs is even greater by 65–250 times6, resulting in an inverted SCC:BCC ratio. The predisposition to skin cancer is not restricted to transplant patients. Lymphoma and chronic lymphocytic leukemia patients, who also have subtle defects in cellular immune function, have an increased incidence of basal cell and squamous cell carcinomas as well.7, 8
Immunologic abnormalities have been detected in skin cancer patients who are otherwise immune-competent. These patients demonstrate suppressed reactions to skin test antigens and decreased sensitization rates to the contact allergen dinitrochlorobenzene (DNCB), which are immune responses mediated by T lymphocytes.9, 10 In addition, when BCCs are examined histologically, a disproportionate number of regulatory T-cells are present in the inflammatory infiltrate.11 Finally, people who have received large numbers of psoralen plus UVA photochemotherapy (PUVA) treatments are known to have an increased incidence of skin cancer.12, 13 These patients have been reported to have decreased immunization rates to contact allergens,14, 15 and reduced numbers of circulating peripheral blood CD4+ T-cells.16, 17
EXPERIMENTAL EVIDENCE FOR THE PHOTOIMMUNOLOGICAL EFFECTS OF UV RADIATION
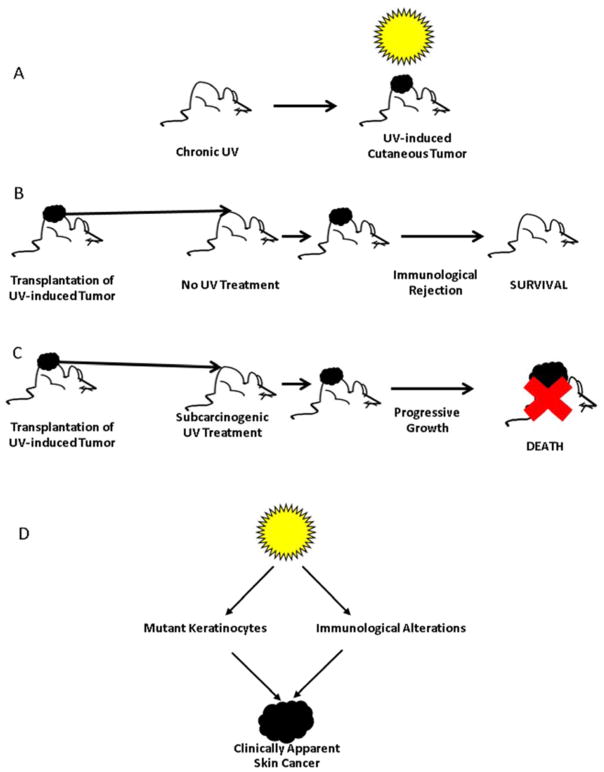
More direct evidence of the ability of ultraviolet radiation to impair immune responses is derived from a classic series of experiments in animal models.18 Mice that are chronically exposed to UV radiation, like humans, develop UV-induced tumors (Figure 1A). When these tumors are removed and are transplanted to the skin of genetically identical recipient mice, the tumors initially engraft but do not enlarge, and ultimately regress because a host immune response develops against the tumors (Figure 1B). On the other hand, when the same tumors are transplanted to genetically identical recipients that have received subcarcinogenic doses of UV radiation, the tumors grow progressively, are not immunologically eradicated, and ultimately kill their host (Figure 1C). Conclusions that can be derived from these experiments are: 1) ultraviolet radiation, in addition to causing mutations in keratinocytes, also prevents activation of host immune responses that have evolved to destroy mutant keratinocytes before they can develop into clinically apparent tumors; and 2) only when there are both mutant keratinocytes and UV-induced immunological alterations is it possible for skin tumors to occur (Figure 1D).
Figure 1.
UV-induced immune suppression and photocarcinogenesis. (A) Chronic exposure of mice, as in humans, results in the development of UV-induced non-melanoma skin cancers. (B) UV-induced tumors that are transplanted to genetically identical recipients that have not been exposed to UV radiation results in rejection of the tumor by the host immune response. (C) UV-induced tumors that are transplanted to genetically identical recipients that have received subcarcinogenic doses of UV radiation grow progressively and ultimately result in death of the recipient. (D) UV-induced tumors only develop when mutations in keratinocytes and UV-induced immunosuppression occurs.
MECHANISMS OF UV-INDUCED IMMUNE SUPPRESSION
There has been great interest in determining the mechanisms by which ultraviolet radiation mediates its effects on the immune system. Those studies have focused on five features: 1) the role of regulatory T-cells; 2) the contribution to alterations in antigen presenting cell function; 3) the effect of UV-induced cytokines and soluble mediators; 4) the molecular target that initiates UV-induced immune suppression; and 5) the participation of toll-like receptors and innate immunity.
Regulatory T-cells
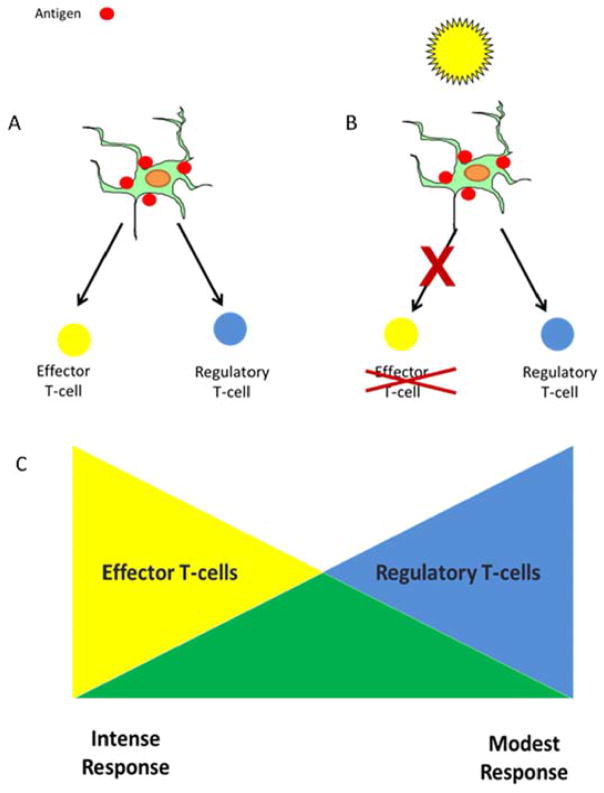
Studies have shown that UV radiation alters T-cell mediated immunity and, in so doing, causes immune suppression. Under normal circumstances, cutaneous exposure to antigens, such as contact allergens or tumor antigens expressed on skin cancers, results in the generation of both effector and regulatory T-lymphocytes which are specific for the exposed antigen (Figure 2A). Effector cells promote an immune response directed against the inciting antigen, whereas regulatory T-cells dampen the reaction. The overall magnitude of the response depends on the ratio of effector to regulatory T-cells that develop (Figure 2C). When large numbers of effector T-cells develop and small numbers of regulatory T-cells are present, a vigorous immune response occurs, whereas in situations in which smaller numbers of effector T-cells and proportionally larger numbers of regulatory T-cells are generated, there is a modest immune response. Following UV exposure, the generation of regulatory T-cells is unaffected, whereas the number of effector T-cells is diminished (Figure 2B).19 The disproportionate number of regulatory T-cells relative to effector T-cells leads to a suppressed immune response (Figure 2C). The regulatory T-cells that occur following UV exposure carry the phenotypic markers CD4+, CD25+, CTLA4+, and FoxP3+, and secrete the immunosuppressive cytokine interleukin-10 (IL-10).20, 21 In addition, a second population of cells called NKT cells have characteristics of both natural killer cells and T-cells, and express the CD4+ and DX5+ (CD49b+) proteins. NKT cells suppress immune responses following UV radiation exposure, and produce the Th2 cytokine IL-4, which suppresses anti-tumor immunity.22
Figure 2.
Cell-mediated immune responses reflect the balance between regulatory and effector T-cells. (A) When antigens, including tumor antigens, are present in the skin, they are taken up by cutaneous dendritic cells, which is a necessary precondition for the generation of effector and regulatory Tlymphocytes. (B) When the skin has been UV-irradiated and then encounters an antigen, the generation of regulatory T-cells proceeds unimpeded, but the generation of effector T-cells is diminished. (C) The overall magnitude of the cutaneous cell-mediated immune response represents the balance between the effector and regulatory T-cells that are generated. Following UV exposure, there are relatively more regulatory T-cells, resulting in a more modest response.
Antigen Presenting Cells
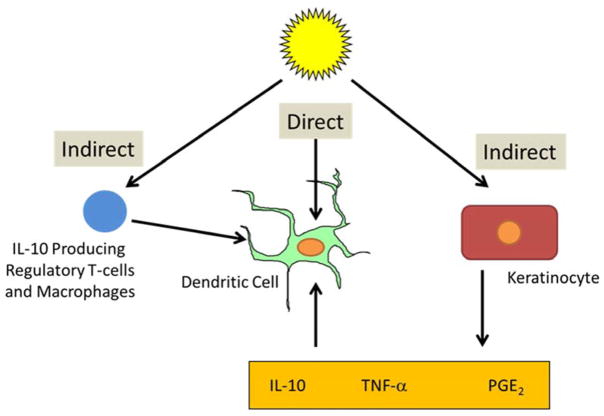
The recognition that regulatory T-cells contribute to the suppressed immune response following UV exposure coincided with the discovery that T-cells are only activated when antigen is presented to them by antigen presenting cells (APCs). The skin contains several populations of antigen presenting cells, including epidermal Langerhans cells, different types of dermal dendritic cells, and macrophages/monocytes, some of which migrate into the skin after UVB exposure.23 Depending on the type and status of the antigen presenting cell, different subpopulations of T-cells are activated. UV radiation has been shown to have a deleterious influence on cutaneous dendritic cell function for effector T-cell activation and less of an effect on antigen presenting cell function for regulatory T-cells (Figure 2).24, 25 Recent evidence from animal models indicates that epidermal Langerhans cells are required for the generation of regulatory T-cells following UV exposure.26 Ultraviolet radiation produces its effects on cutaneous dendritic cells both through indirect and direct effects on the cells (Figure 3). Indirect effects include stimulation of keratinocytes to produce immunosuppressive soluble mediators, such as IL-4, IL-10, tumor necrosis factor (TNF)-α and prostaglandin (PGE2),27–30 and stimulation of the migration of immunosuppressive macrophages into sites of UV-injury.31, 32 Also, the regulatory T-cells that are generated following UV exposure have a negative influence on the presentation of antigen to effector T-cells, thereby serving as a positive feedback loop for immunosuppression.
Figure 3.
Direct and indirect effects of UV radiation on the antigen presenting function of cutaneous dendritic cells. Following UV exposure, the antigen presenting function of cutaneous dendritic cells is altered by direct effects on the antigen presenting cell itself and by indirect effects. The indirect effects are mediated by UV-irradiated keratinocyte production of soluble mediators such as IL-10, prostaglandin E2, and TNF-α, which then act on cutaneous dendritic cells. In addition, macrophages, which migrate into the skin following UV exposure, and regulatory T-cells both secrete the immunosuppressive molecule IL-10, which diminishes the capacity of cutaneous antigen presenting cells to activate effector T-cells.
Initial Molecular Events
DNA is now generally considered to be the molecular structure within cells that initiates the immunosuppressive effects of UV radiation.33–35 Those wavelengths within the solar spectrum that are most effective at causing immunosuppression lie primarily within the UVB range and correspond closely with those that are most damaging to DNA.36 In fact, patients with xeroderma pigmentosum (XP), a disease in which there is an inherited defect in DNA repair, have an increased propensity to develop actinic keratoses, BCCs, SCCs and melanomas at an unusually early age. XP patients also have impaired delayed type hypersensitivity (DTH) responses, reduced circulating CD4/CD8 T-cell ratios, defective natural killer (NK) cell function and impaired production of interferon-γ, further supporting the concept of DNA as the molecular target of UVR-induced immunosuppression.37–41 In animal models, UV-induced immune suppression can be reversed by topical application of enzymes that repair DNA damage.33, 34, 42 When used in XP patients, topical application of DNA repair enzymes prevents the development of actinic keratoses and basal cell carcinomas.43
Trans-urocanic acid is present in large amounts in the stratum corneum of the skin and undergoes photoisomerization to its cis-conformation following UV exposure. Cis-urocanic acid has been shown to be a mediator of UV-induced immunosuppression.44 Recent studies have shown that cis-urocanic acid mediates its immunosuppressive effects by interfering with the repair of UV-induced DNA damage.35
Cytokines and Other Soluble Mediators
UV radiation is known to stimulate epidermal production of a variety of soluble mediators. These include TNF-α,45 prostaglandin E2,46, 47 serotonin48, platelet activating factor,49, 50 and neuropeptides, such as calcitonin gene related peptide (CGRP) and α-melanocyte-stimulating hormone (α-MSH).51 UVR is also proficient at generating reactive oxygen intermediates.52 These molecules have been shown to be important mediators of UV-induced immunosuppression, and interfering with their activity reverses their immunosuppressive effects in experimental animal models (Figure 4).28, 29, 47, 48, 50, 53–55 Many of these agents, including cis-urocanic acid, platelet activating factor and serotonin, produce their immunosuppressive effects by interfering with repair of DNA damage.35 CGRP inhibits Langerhans cell antigen presenting function,56 and this may be the mechanism by which it causes UV-induced immune suppression. α-MSH is a stimulus for IL-10 production by keratinocytes and monocytes.57, 58 Two other cytokines that play a prominent role in UV-induced immunosuppression are IL-10 and IL-12. IL-10 is an immunosuppressive cytokine produced by UV-irradiated keratinocytes, macrophages that migrate into UV-irradiated skin, and regulatory T-cells that are generated following UV exposure.59–61 IL-10 acts on dendritic cells in such a manner as to enhance the generation of UV-induced regulatory T-cells. IL-12, on the other hand, promotes T-cell mediated immunity by supporting the production of effector T-cells which produce the pro-inflammatory cytokine interferon-γ. Studies in animal models have shown that administration of IL-12 will reverse the immunosuppressive effects of UV radiation.62, 63 IL-12 stimulates the production of enzymes that repair UV-damaged DNA,64 which may also contribute to its ability to abrogate the immunosuppressive effects of UV. Interestingly, polyphenols present in green tea and other natural and dietary products have been shown to prevent UV-induced immunosuppression and carcinogenesis, at least in part, by stimulating the production of IL-12.65–68
Figure 4.
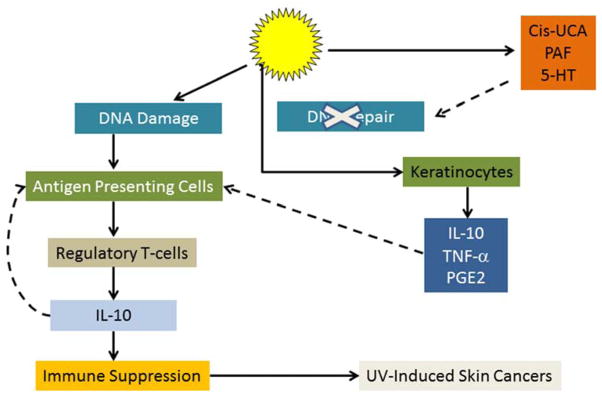
Mechanism of by which UV radiation suppresses cutaneous cell-mediated immune responses. Following UV exposure, DNA damage occurs, which, in addition to its other effects, initiates photoimmunosuppression. Furthermore, UV exposure of the skin results in the generation of soluble mediators including serotonin (5-HT), cis-urocanic acid (Cis-UCA) and platelet activating factor (PAF), all of which antagonize activation of DNA repair enzymes. In addition, UV exposure prompts keratinocytes to produce immunosuppressive mediators which alter the antigen presenting function of cutaneous dendritic cells. Regulatory T-cells are generated that produce IL-10, which suppresses host T-cell mediated defense mechanisms and facilitates the growth and development of UV-induced tumors. Solid line: stimulatory. Dashed line: inhibitory.
Toll-like Receptors and Innate Immunity
Toll-like receptors (TLRs) are highly conserved molecules that are present on the surface of immune cells and epithelial cells including epidermal keratinocytes.69 They are important in the activation of several pathways of the innate immune response. TLRs recognize patterns within antigens that are foreign to the immune system, which include foreign pathogens, pathogen associated molecular patterns (PAMPs) as well as endogenous antigens that are altered from their normal state, damage associated molecular patterns (DAMPs). Of the 13 TLRs that have been identified thus far, two, TLR3 and TLR4 have been shown to be involved in the recognition of ultraviolet radiation-induced damage of RNA and DNA, respectively. These receptors initiate pathways that ultimately enhance UV-induced immunosuppression.
TLR3 is a cell surface receptor, present on keratinocytes. Following UV exposure, damage to keratinocytes occurs, which results in the release of double stranded, small nuclear RNA (snRNA).70 Once released, these snRNAs induce the expression of TLR3 on non-irradiated cells, and then bind to the same TLR3 receptors, the expression of which they have provoked. TLR3 activation causes keratinocytes to produce pro-inflammatory cytokines such as IL-6 and TNF-α. TNF-α is a known mediator of UV-induced immune suppression. Direct evidence of TLR3 in UV-induced immune suppression has come from studies in experimental animal models. UV-irradiated mice expressing TLR3 develop a suppressed T-cell mediated allergic contact hypersensitivity response, while the immune response mice that are genetically deficient in TLR3 is normal.
A receptor involved in recognition of UV-induced DNA damage is TLR4.71 As was mentioned, UV-damaged DNA can be repaired by DNA repair enzymes. In the skin, TLR4 is found primarily on dendritic cells. Following UV-exposure, dendritic cells in the skin that express the TLR4 molecule have a diminished capacity to synthesize IL-12. As was mentioned (vide supra), IL-12 stimulates the synthesis of DNA repair enzymes. The decrease in DNA repair in cutaneous dendritic cells renders them unable to effectively activate effector T cells and leads to suppression of the cell-mediated immune response. In contrast, in experimental systems, TLR4-deficient animals repair UV-damaged DNA normally and do not exhibit UV-induced immune suppression.
PHOTOIMMUNOLOGICAL DISEASES
The information derived from animal models about the photoimmunological effects of ultraviolet radiation, coupled with observations made in patients, have been the basis for theories about the immunopathogenesis of a number of photodermatolog disorders, including polymorphous light eruption, chronic actinic dermatitis and cutaneous lupus erythematosus.
Polymorphous Light Eruption
Polymorphous light eruption (PMLE) is the most common photodermatosis with prevalence rates ranging from 1–21%, depending on the geographic location surveyed.72–74 Wavelengths within the UVA are most commonly reported to prompt the inflammatory reaction, although in some patients, UVB and even UVC75 have been associated with flares of the disease. PMLE has many features in common with delayed-type hypersensitivity (DTH) reactions in the skin. Within a few hours of sun exposure, CD4+ T-cells can be detected infiltrating the UV-exposed site.76 This is followed over the next few days by an influx of CD8+ T-cells. In addition, the adhesion molecules E-selectin (CD62), vascular cell adhesion molecule 1 (VCAM-1, CD106) and intercellular adhesion molecule-1 (ICAM-1CD54), all of which facilitate the migration and retention of T-cells into inflamed skin, can easily be detected in PMLE skin, whereas they are not found in normal skin.77 Although they are not first-line therapies for the disease, the fact that PMLE can be controlled with immunosuppressive agents, such as azathioprine and cyclosporine, also is consistent with the concept that it is an immunologically mediated disease.
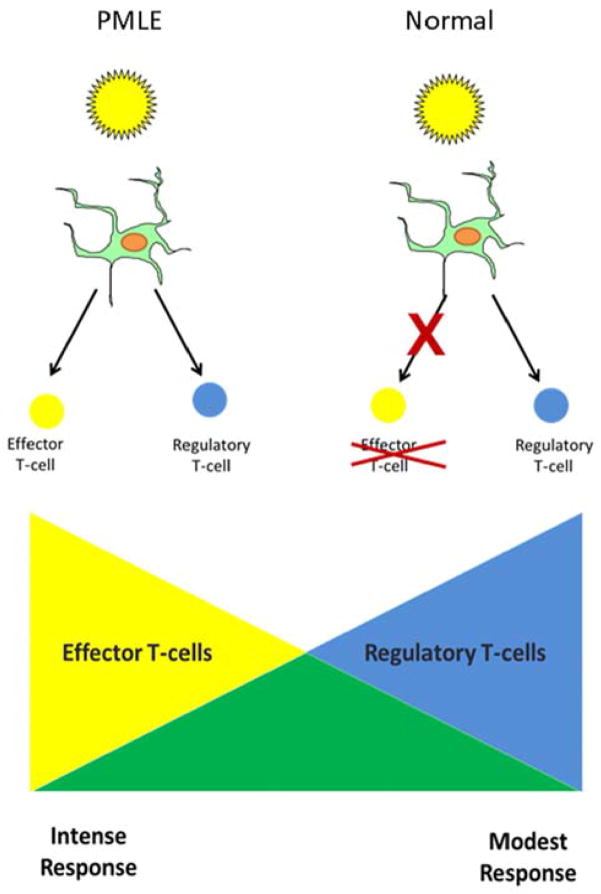
It has been proposed that UV is less effective at suppressing cell-mediated immune responses in PMLE patients (Figure 5). There is decreased migration of CD11b+ macrophages, which are known to secrete IL-10, into UV-irradiated skin in PMLE patients.78 Moreover, a series of studies in which attempts were made to sensitize PMLE and normal control subjects to the contact allergen DNCB through UV-irradiated skin, demonstrated that UV-induced suppression of the contact allergic reaction was more effective in control subjects than in PMLE patients.79 In other words, patients with PMLE are resistant to the immunosuppressive effects of UV radiation.
Figure 5.
Proposed photopathogenesis of polymorphous light eruption (PMLE). In contrast to normal individuals who have a suppressed immune response following UV exposure, the immune response in patients with polymorphous light eruption is not suppressed.
Chronic Actinic Dermatitis
Patients with chronic actinic dermatitis (CAD) have an exquisite photosensitivity that results in a subacute to chronic inflammatory process which begins in sun exposed skin and can generalize to non-sun exposed cutaneous surfaces.80–82 Chronic actinic dermatitis patients may or may not have a positive photoallergic response, but even in those who do, the dermatitis persists despite removal of the substance causing the problem. Many of these patients initially have a photosensitivity to UVB, but this frequently extends to the UVA and even visible light.
The photopathogenesis of chronic actinic dermatitis is incompletely understood, but is thought to be immunologic in nature.82 Biopsies of involved skin demonstrate leukocyte epidermotropism, dermal infiltrates comprised primarily of T-cells, and keratinocytes that express major histocompatibility class II molecules, features which are also present in DTH reactions in the skin.82–84 Another characteristic suggesting that chronic actinic dermatitis is immunologically-mediated is that the disease responds to immunosuppressive agents.80, 82
There are two theories about the immunopathogenesis of chronic actinic dermatitis.82 The first postulates that these patients have an exaggerated immune reaction to a photoantigen. This type of reaction is suppressed in normal individuals, but for some reason, is activated in those with chronic actinic dermatitis. The second theory proposes that there is cross-reactivity between a photoallergen and endogenous antigens. In this scenario, a photoallergen initiates the immune response. Because of cross-reactivity, the endogenous antigen allows the response to persist even though the photoallergen is no longer present.
Cutaneous Lupus Erythematosus
Patients with cutaneous lupus erythematosus are frequently photosensitive, as sun sensitivity is one of the defining characteristics of the disease.85, 86 Rates vary depending on the type of lupus. 87,{Kim, 2013 #9253}88 Wavelengths within the UVA and/or UVB are responsible for production of cutaneous lesions.89, 90
UV exposure can prompt the development of lupus, aggravate the activity in existing skin lesions, and provoke systemic manifestations of the disease. With the exception of tumid lupus, cutaneous lupus is associated with vacuolar destructive changes in the basal cell layer of the epidermis. All forms of cutaneous lupus are associated with a dermal mononuclear infiltrate.
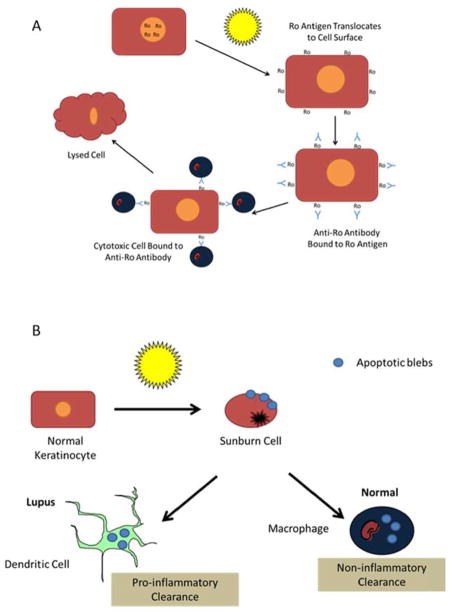
Antibodies to the Ro antigen are commonly present in subacute and neonatal lupus erythematosus (Figure 6A). The Ro antigen is normally present within the nucleus of cells, but when keratinocytes are exposed to UV radiation, it translocates to the cell surface membrane.91 In that location, anti-Ro antibodies can bind to the molecule. It is hypothesized that cells which express Fc receptors for IgG are able to attach to the Fc portion of the anti-Ro antibody and exert a cytotoxic effect, resulting in keratinocyte lysis.
Figure 6.
Two proposed models of the photopathogenesis of cutaneous lupus. (A) In subjects with Ro positive lupus, UV exposure of the skin results in translocation of the Ro protein to the surface of the keratinocyte, which enables anti-Ro antibodies to bind. Cytotoxic cells that express Fc receptors for the IgG molecule bind to the Fc portion of the anti-Ro antibody, which is attached to UV-irradiated keratinocytes expressing the Ro antigen on their surface. Thus, cytotoxicity occurs, leading to keratinocyte lysis. (B) Following UV exposure, apoptotic keratinocytes (sunburn cells) result. Apoptotic blebs are released from the dying keratinocytes. In unaffected individuals, these apoptotic blebs are removed by macrophages via non-inflammatory mechanisms. In lupus patients, the apoptotic bodies are taken up by dendritic cells and enter a pro-inflammatory pathway.
Cell destruction in cutaneous lupus may also occur via apoptotic keratinocytes and activation of a pro-inflammatory pathway (Figure 6B).92, 93 Following UV exposure, keratinocytes undergo apoptosis, which can be observed histologically as a sunburn cell. Under normal conditions, the apoptotic blebs that are released from degenerating keratinocytes are taken up by macrophages and are cleared by non-inflammatory pathways. In lupus patients, however, these apoptotic blebs may be taken up by dendritic cells, which then can activate cell-mediated immune processes that eventuate in formation of cutaneous lupus lesions.
PHOTOIMMUNOLOGICAL EFFECTS OF PHOTOTHERAPY
Our ability to develop new phototherapeutic modalities is due to the recognition that different types of ultraviolet radiation have unique molecular and biologic effects.94, 95 Most of the radiation within the UVB (290–320 nm) range penetrates only to the superficial dermis. Its primary effects on DNA are to cause cyclobutane pyrimidine dimers, although it is known to cause photochemical changes as well. In the stratum corneum, UV exposure converts trans-urocanic acid to its cis- isomer. Direct cellular targets of UVB are primarily Langerhans cells and keratinocytes in the epidermis; UVB depletes the epidermis of Langerhans cells and stimulates keratinocytes both to produce immunosuppressive cytokines and to alter the expression of adhesion molecules that are required for adherence of T-cells to keratinocytes. These immunological effects help to explain why UVB radiation, and more recently narrowband UVB, have been effective in treating psoriasis,96, 97 atopic dermatitis98–100 and early forms of cutaneous T-cell lymphoma.101, 102
In contrast, longer wavelength UVA1 radiation (340–400 nm) penetrates more deeply into the skin and has its major effects in the mid- to lower dermis.95 The major molecular consequence of UVA1 exposure is the production of reactive oxygen intermediates. UVA1 depletes the dermis of mast cells and dendritic cells, causes apoptosis of dermal CD4+ T-cells, and stimulates the production of matrix metalloproteinases by fibroblasts. These distinguishing characteristics of UVA1 have been exploited for therapeutic benefit for acute flares of atopic dermatitis and dyshidrosis, the pruritus associated with mastocytosis, as well as morphea,103 scleroderma103 and other sclerodermoid inflammatory syndromes.104 In atopic dermatitis, UVA1 has been shown to be more successful than combined UVA/UVB phototherapy.105, 106 It has also been shown to be effective in cutaneous T-cell lymphoma (CTCL),107 in which UVA1 acts directly on CD4+ T-cells and causes apoptosis.108 Its efficacy in morphea and other sclerodermoid conditions is due to its ability to augment matrix metalloproteinase-1, which degrades collagen.109, 110 In mastocytosis, UVA1 has been effective because of its ability to deplete the skin of mast cells.103
The primary molecular defect in psoralen plus UVA (PUVA) photochemotherapy differs from that of both UVB and UVA1.95 The addition of psoralen results in the production of bifunctional adducts with DNA. Like UVA1, UVA used in PUVA photochemotherapy penetrates into the mid- to lower dermis, thereby altering dermal dendritic cells, stimulating fibroblast production of IL-1 and damaging dermal CD4+ T-cells. These effects help to explain its efficacy in psoriasis111 and cutaneous T-cell lymphoma.112, 113
CONCLUSION
Ultraviolet radiation has been shown to alter immunological function, both in the skin and systemically. In so doing, it contributes to the pathogenesis of non-melanoma skin cancer and participates in the development of lupus and other photosensitivity diseases in which immune mechanisms play a role. On the other hand, the immunological effects of ultraviolet radiation have been exploited as a therapeutic modality to treat diseases such as psoriasis, atopic dermatitis, cutaneous T-cell lymphoma, morphea and mastocytosis.
KEY POINTS.
Photoimmunology investigates the immunological effects of ultraviolet radiation exposure.
Photoimmunology originated from observations about the biologic behavior of non-melanoma skin cancers and the recognition that immunosuppressed organ transplant recipients were at increased risk for sunlight-induced skin cancers.
Experiments in animal models showed that alterations in the host cell-mediated immune response are necessary for skin cancers to develop.
UV mediates its effects by causing a disproportionate increase in regulatory T-cells, altering the function of antigen presenting cutaneous dendritic cells, and stimulating the production of soluble immunosuppressive mediators.
Knowledge generated about UV effects on the immune system has contributed to a broader understanding of the pathogenesis of a number of photosensitivity diseases and to the generation of more effective and safer forms of phototherapy.
Acknowledgments
Funded by NIH Grants and Contracts: P30 AR050948, P30 CA013148, N01 CN05014-69 and by VA Merit Review 18-103-02.
Footnotes
DISCLOSURE STATEMENT
Conflicts of Interest:
Craig A. Elmets, M.D. None
Cather Cala None
Hui Xu, Ph.D. None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Marks R, Rennie G, Selwood TS. Spontaneous remission of solar keratoses: the case for conservative management. Br J Dermatol. 1986;115:649–655. doi: 10.1111/j.1365-2133.1986.tb06644.x. [DOI] [PubMed] [Google Scholar]
- 2.Hartevelt MM, Bouwes Bavinck JN, Kootte AM, et al. Incidence of skin cancer after renal transplantation in the Netherlands. Transplantation. 1990;49:506–509. doi: 10.1097/00007890-199003000-00006. [DOI] [PubMed] [Google Scholar]
- 3.Bouwes-Bavinck JN, Vermeer BJ, van-der-Woude FJ, et al. Relation between skin cancer and HLA antigens in renal-transplant patients. N Engl J Med. 1991;325:843–848. doi: 10.1056/NEJM199109193251203. [DOI] [PubMed] [Google Scholar]
- 4.Webb MC, Compton F, Andrews PA, et al. Skin tumours posttransplantation: a retrospective analysis of 28 years’ experience at a single centre. Transplant Proc. 1997;29:828–830. doi: 10.1016/s0041-1345(96)00152-2. [DOI] [PubMed] [Google Scholar]
- 5.Jensen P, Moller B, Hansen S. Skin cancer in kidney and heart transplant recipients and different long-term immunosuppressive therapy regimens. J Am Acad Dermatol. 2000;42:307. doi: 10.1016/s0190-9622(00)90154-3. [DOI] [PubMed] [Google Scholar]
- 6.Euvrard S, Kanitakis J, Claudy A. Skin cancers after organ transplantation. N Engl J Med. 2003;348:1681–1691. doi: 10.1056/NEJMra022137. [DOI] [PubMed] [Google Scholar]
- 7.Mellemgaard A, Geisler CH, Storm HH. Risk of kidney cancer and other second solid malignancies in patients with chronic lymphocytic leukemia. Eur J Haematol. 1994;53:218–222. doi: 10.1111/j.1600-0609.1994.tb00192.x. [DOI] [PubMed] [Google Scholar]
- 8.Maule M, Scelo G, Pastore G, et al. Risk of second malignant neoplasms after childhood leukemia and lymphoma: an international study. J Natl Cancer Inst. 2007;99:790–800. doi: 10.1093/jnci/djk180. [DOI] [PubMed] [Google Scholar]
- 9.Weimar VM, Ceilley RI, Goeken JA. Cell-mediated immunity in patients with basal and squamous cell skin cancer. J Am Acad Dermatol. 1980;2:143–147. doi: 10.1016/s0190-9622(80)80393-8. [DOI] [PubMed] [Google Scholar]
- 10.Yoshikawa T, Rae V, Bruins-Slot W, et al. Susceptibility to effects of UVB radiation on induction of contact hypersensitivity as a risk factor for skin cancer in man. J Invest Dermatol. 1990;95:530–536. doi: 10.1111/1523-1747.ep12504877. [DOI] [PubMed] [Google Scholar]
- 11.Kaporis HG, Guttman-Yassky E, Lowes MA, et al. Human basal cell carcinoma is associated with Foxp3+ T cells in a Th2 dominant microenvironment. J Invest Dermatol. 2007;127:2391–2398. doi: 10.1038/sj.jid.5700884. [DOI] [PubMed] [Google Scholar]
- 12.Stern RS, Liebman EJ, Vakeva L. Oral psoralen and ultraviolet-A light (PUVA) treatment of psoriasis and persistent risk of nonmelanoma skin cancer. PUVA Follow-up Study. J Natl Cancer Inst. 1998;90:1278–1284. doi: 10.1093/jnci/90.17.1278. [DOI] [PubMed] [Google Scholar]
- 13.Stern RS. Carcinogenic risk of psoralen plus ultraviolet radiation therapy: evidence in humans. Natl Cancer Inst Monogr. 1984;66:211–216. [PubMed] [Google Scholar]
- 14.Volden G, Molin L, Thomsen K. PUVA-induced suppression contact sensitivity to mustine hydrochloride in mycosis fungoides. Br Med J. 1978;2:865–866. doi: 10.1136/bmj.2.6141.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Volden G, Molin L, Thomsen K. PUVA-induced suppression of contact sensitivity to mustine hydrochloride in mycosis fungoides. Br Med J. 1978;2:865–866. doi: 10.1136/bmj.2.6141.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moscicki RA, Morison WL, Parrish JA, et al. Reduction of the fraction of circulating helper-inducer T cells identified by monoclonal antibodies in psoriatic patients treated with long-term psoralen/ultraviolet-A radiation (PUVA) J Invest Dermatol. 1982;79:205–208. doi: 10.1111/1523-1747.ep12500058. [DOI] [PubMed] [Google Scholar]
- 17.Morison WL, Wimberly J, Parrish JA, et al. Abnormal lymphocyte function following long-term PUVA therapy for psoriasis. Br J Dermatol. 1983;108:445–450. doi: 10.1111/j.1365-2133.1983.tb04597.x. [DOI] [PubMed] [Google Scholar]
- 18.Kripke ML. Antigenicity of murine skin tumors induced by ultraviolet light. J Natl Cancer Inst. 1974;53:1333–1336. doi: 10.1093/jnci/53.5.1333. [DOI] [PubMed] [Google Scholar]
- 19.Elmets CA, Bergstresser PR, Tigelaar RE, et al. Analysis of mechanism of unresponsiveness produced by haptens painted on skin exposed to low dose ultraviolet radiation. J Exp Med. 1983;158:781–794. doi: 10.1084/jem.158.3.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schwarz A, Maeda A, Wild MK, et al. Ultraviolet radiation-induced regulatory T cells not only inhibit the induction but can suppress the effector phase of contact hypersensitivity. J Immunol. 2004;172:1036–1043. doi: 10.4049/jimmunol.172.2.1036. [DOI] [PubMed] [Google Scholar]
- 21.Schwarz A, Navid F, Sparwasser T, et al. In vivo reprogramming of UV radiation-induced regulatory T-cell migration to inhibit the elicitation of contact hypersensitivity. J Allergy Clin Immunol. 2011;128:826–833. doi: 10.1016/j.jaci.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 22.Moodycliffe AM, Nghiem D, Clydesdale G, et al. Immune suppression and skin cancer development: regulation by NKT cells. Nat Immunol. 2000;1:521–525. doi: 10.1038/82782. [DOI] [PubMed] [Google Scholar]
- 23.Xu H, Timares L, Elmets CA. Host defenses in the skin. In: Rich RR, Fleisher TA, Shearer WT, et al., editors. Clinical immunology: principles and practice. 4. Elsevier Saunders; 2013. pp. 228–238. [Google Scholar]
- 24.Ullrich SE, Byrne SN. The immunologic revolution: photoimmunology. J Invest Dermatol. 2012;132:896–905. doi: 10.1038/jid.2011.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schwarz T. 25 years of UV-induced immunosuppression mediated by T cells-from disregarded T suppressor cells to highly respected regulatory T cells. Photochem Photobiol. 2008;84:10–18. doi: 10.1111/j.1751-1097.2007.00223.x. [DOI] [PubMed] [Google Scholar]
- 26.Schwarz A, Noordegraaf M, Maeda A, et al. Langerhans cells are required for UVR-induced immunosuppression. J Invest Dermatol. 2010;130:1419–1427. doi: 10.1038/jid.2009.429. [DOI] [PubMed] [Google Scholar]
- 27.Shreedhar V, Giese T, Sung VW, et al. A cytokine cascade including prostaglandin E2, IL-4, and IL-10 is responsible for UV-induced systemic immune suppression. J Immunol. 1998;160:3783–3789. [PubMed] [Google Scholar]
- 28.Kurimoto I, Streilein JW. Tumor necrosis factor-alpha impairs contact hypersensitivity induction after ultraviolet B radiation via TNF-receptor 2 (p75) Exp Dermatol. 1999;8:495–500. doi: 10.1111/j.1600-0625.1999.tb00308.x. [DOI] [PubMed] [Google Scholar]
- 29.Streilein JW. Sunlight and skin-associated lymphoid tissues (SALT): if UVB is the trigger and TNF alpha is its mediator, what is the message? J Invest Dermatol. 1993;100:47S–52S. doi: 10.1111/1523-1747.ep12355578. [DOI] [PubMed] [Google Scholar]
- 30.Yoshikawa T, Streilein JW. Genetic basis of the effects of ultraviolet light B on cutaneous immunity. Evidence that polymorphism at the TNFa and Lps loci governs susceptibility. Immunogenetics. 1990;32:398–405. doi: 10.1007/BF00241633. [DOI] [PubMed] [Google Scholar]
- 31.Meunier L, Bata-Csorgo Z, Cooper KD. In human dermis, ultraviolet radiation induces expansion of a CD36+ CD11b+ CD1− macrophage subset by infiltration and proliferation; CD1+ Langerhans-like dendritic antigen-presenting cells are concomitantly depleted. J Invest Dermatol. 1995;105:782–788. doi: 10.1111/1523-1747.ep12326032. [DOI] [PubMed] [Google Scholar]
- 32.Cooper KD, Oberhelman L, Hamilton TA, et al. UV exposure reduces immunization rates and promotes tolerance to epicutaneous antigens in humans: relationship to dose, CD1a-DR+ epidermal macrophage induction, and Langerhans cell depletion. Proc Natl Acad Sci U S A. 1992;89:8497–8501. doi: 10.1073/pnas.89.18.8497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Applegate LA, Ley RD, Alcalay J, et al. Identification of the molecular target for the suppression of contact hypersensitivity by ultraviolet radiation. J Exp Med. 1989;170:1117–1131. doi: 10.1084/jem.170.4.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kripke ML, Cox PA, Alas LG, et al. Pyrimidine dimers in DNA initiate systemic immunosuppression in UV-irradiated mice. Proc Natl Acad Sci USA. 1992;89:7516–7520. doi: 10.1073/pnas.89.16.7516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sreevidya CS, Fukunaga A, Khaskhely NM, et al. Agents that reverse UV-Induced immune suppression and photocarcinogenesis affect DNA repair. The Journal of investigative dermatology. 2010;130:1428–1437. doi: 10.1038/jid.2009.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Elmets CA, LeVine MJ, Bickers DR. Action spectrum studies for induction of immunologic unresponsiveness to dinitrofluorobenzene following in vivo low dose ultraviolet radiation. Photochem Photobiol. 1985;42:391–397. doi: 10.1111/j.1751-1097.1985.tb01586.x. [DOI] [PubMed] [Google Scholar]
- 37.Norris PG, Limb GA, Hamblin AS, et al. Immune function, mutant frequency and cancer risk in the DNA repair defective genodermatoses xeroderma pigmentosum, Cockayne’s syndrome and trichothiodystrophy. J Invest Dermatol. 1990;94:94–100. doi: 10.1111/1523-1747.ep12873952. [DOI] [PubMed] [Google Scholar]
- 38.Gaspari AA, Fleisher TA, Kraemer KH. Impaired interferon production and natural killer cell activation in patients with the skin cancer-prone disorder, xeroderma pigmentosum. J Clin Invest. 1993;92:1135–1142. doi: 10.1172/JCI116682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wysenbeek AJ, Weiss H, Duczyminer-Kahana M, et al. Immunologic alterations in xeroderma pigmentosum patients. Cancer. 1986;58:219–221. doi: 10.1002/1097-0142(19860715)58:2<219::aid-cncr2820580203>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 40.Morison WL, Bucana C, Hashem N, et al. Impaired immune function in patients with xeroderma pigmentosum. Cancer Res. 1985;45:3929–3931. [PubMed] [Google Scholar]
- 41.Dupuy JM, Lafforet D. A defect of cellular immunity in Xeroderma pigmentosum. Clinical immunology and immunopathology. 1974;3:52–58. doi: 10.1016/0090-1229(74)90022-1. [DOI] [PubMed] [Google Scholar]
- 42.Cafardi JA, Elmets CA. T4 endonuclease V: review and application to dermatology. Expert Opin Biol Ther. 2008;8:829–838. doi: 10.1517/14712598.8.6.829. [DOI] [PubMed] [Google Scholar]
- 43.Yarosh D, Klein J, O’Connor A, et al. Effect of topically applied T4 endonuclease V in liposomes on skin cancer in xeroderma pigmentosum: a randomised study. Lancet. 2001;357:926–929. doi: 10.1016/s0140-6736(00)04214-8. [DOI] [PubMed] [Google Scholar]
- 44.Noonan FP, DeFabo EC, Morrison H. Cis-urocanic acid, a product formed by ultraviolet B irradiation of the skin, initiates an antigen presentation defect in splenic dendritic cells in vivo. J Invest Dermatol. 1988;90:92–99. doi: 10.1111/1523-1747.ep12462045. [DOI] [PubMed] [Google Scholar]
- 45.Kock A, Schwarz T, Kirnbauer R, et al. Human keratinocytes are a source for tumor necrosis factor a: evidence for synthesis and release upon stimulation with endotoxin or ultraviolet light. J Exp Med. 1990;172:1609–1614. doi: 10.1084/jem.172.6.1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pentland AP, Mahoney M, Jacobs SC, et al. Enhanced prostaglandin synthesis after ultraviolet injury is mediated by endogenous histamine stimulation. J Clin Invest. 1990;86:566–574. doi: 10.1172/JCI114746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chung HT, Burnham DK, Robertson B, et al. Involvement of prostaglandins in the immune alterations caused by the exposure of mice to ultraviolet radiation. J Immunol. 1986;137:2478–2484. [PubMed] [Google Scholar]
- 48.Walterscheid JP, Nghiem DX, Kazimi N, et al. Cis-urocanic acid, a sunlight-induced immunosuppressive factor, activates immune suppression via the 5-HT2A receptor. Proc Natl Acad Sci U S A. 2006;103:17420–17425. doi: 10.1073/pnas.0603119103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dy LC, Pei Y, Travers JB. Augmentation of ultraviolet B radiation-induced tumor necrosis factor production by the epidermal platelet-activating factor receptor. J Biol Chem. 1999;274:26917–26921. doi: 10.1074/jbc.274.38.26917. [DOI] [PubMed] [Google Scholar]
- 50.Zhang Q, Yao Y, Konger RL, et al. UVB radiation-mediated inhibition of contact hypersensitivity reactions is dependent on the platelet-activating factor system. J Invest Dermatol. 2008;128:1780–1787. doi: 10.1038/sj.jid.5701251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Seiffert K, Granstein RD. Neuropeptides and neuroendocrine hormones in ultraviolet radiation-induced immunosuppression. Methods. 2002;28:97–103. doi: 10.1016/s1046-2023(02)00214-1. [DOI] [PubMed] [Google Scholar]
- 52.Black HS. Potential involvement of free radical reactions in ultraviolet light-mediated cutaneous damage. Photochem Photobiol. 1987;46:213–221. doi: 10.1111/j.1751-1097.1987.tb04759.x. [DOI] [PubMed] [Google Scholar]
- 53.Garssen J, Buckley TL, Van Loveren H. A role for neuropeptides in UVB-induced systemic immunosuppression. Photochem Photobiol. 1998;68:205–210. [PubMed] [Google Scholar]
- 54.Legat FJ, Jaiani LT, Wolf P, et al. The role of calcitonin gene-related peptide in cutaneous immunosuppression induced by repeated subinflammatory ultraviolet irradiation exposure. Exp Dermatol. 2004;13:242–250. doi: 10.1111/j.0906-6705.2004.00185.x. [DOI] [PubMed] [Google Scholar]
- 55.Grabbe S, Bhardwaj RS, Mahnke K, et al. alpha-Melanocyte-stimulating hormone induces hapten-specific tolerance in mice. J Immunol. 1996;156:473–478. [PubMed] [Google Scholar]
- 56.Hosoi J, Murphy GF, Egan CL, et al. Regulation of Langerhans cell function by nerves containing calcitonin gene-related peptide. Nature. 1993;363:159–163. doi: 10.1038/363159a0. [DOI] [PubMed] [Google Scholar]
- 57.Bhardwaj RS, Schwarz A, Becher E, et al. Pro-opiomelanocortin-derived peptides induce IL-10 production in human monocytes. J Immunol. 1996;156:2517–2521. [PubMed] [Google Scholar]
- 58.Redondo P, Garcia-Foncillas J, Okroujnov I, et al. Alpha-MSH regulates interleukin-10 expression by human keratinocytes. Arch Dermatol Res. 1998;290:425–428. doi: 10.1007/s004030050330. [DOI] [PubMed] [Google Scholar]
- 59.Rivas JM, Ullrich SE. The role of IL-4, IL-10, and TNF-alpha in the immune suppression induced by ultraviolet radiation. J Leukoc Biol. 1994;56:769–775. doi: 10.1002/jlb.56.6.769. [DOI] [PubMed] [Google Scholar]
- 60.Rivas JM, Ullrich SE. Systemic suppression of delayed-type hypersensitivity by supernatants from UV-irradiated keratinocytes. An essential role for keratinocyte-derived IL-10. J Immunol. 1992;149:3865–3871. [PubMed] [Google Scholar]
- 61.Toichi E, Lu KQ, Swick AR, et al. Skin-infiltrating monocytes/macrophages migrate to draining lymph nodes and produce IL-10 after contact sensitizer exposure to UV-irradiated skin. J Invest Dermatol. 2008;128:2705–2715. doi: 10.1038/jid.2008.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schwarz A, Maeda A, Kernebeck K, et al. Prevention of UV radiation-induced immunosuppression by IL-12 is dependent on DNA repair. J Exp Med. 2005;201:173–179. doi: 10.1084/jem.20041212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Schmitt DA, Walterscheid JP, Ullrich SE. Reversal of ultraviolet radiation-induced immune suppression by recombinant interleukin-12: suppression of cytokine production. Immunology. 2000;101:90–96. doi: 10.1046/j.1365-2567.2000.00084.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Schwarz A, Stander S, Berneburg M, et al. Interleukin-12 suppresses ultraviolet radiation-induced apoptosis by inducing DNA repair. Nature cell biology. 2002;4:26–31. doi: 10.1038/ncb717. [DOI] [PubMed] [Google Scholar]
- 65.Sharma SD, Katiyar SK. Dietary grape-seed proanthocyanidin inhibition of ultraviolet B-induced immune suppression is associated with induction of IL-12. Carcinogenesis. 2006;27:95–102. doi: 10.1093/carcin/bgi169. [DOI] [PubMed] [Google Scholar]
- 66.Vaid M, Singh T, Li A, et al. Proanthocyanidins inhibit UV-induced immunosuppression through IL-12-dependent stimulation of CD8+ effector T cells and inactivation of CD4+ T cells. Cancer Prevention Research. 2011;4:238–247. doi: 10.1158/1940-6207.CAPR-10-0224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Katiyar S, Elmets CA, Katiyar SK. Green tea and skin cancer: photoimmunology, angiogenesis and DNA repair. J Nutr Biochem. 2007;18:287–296. doi: 10.1016/j.jnutbio.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 68.Meeran SM, Katiyar S, Elmets CA, et al. Silymarin inhibits UV radiation-induced immunosuppression through augmentation of interleukin-12 in mice. Mol Cancer Ther. 2006;5:1660–1668. doi: 10.1158/1535-7163.MCT-06-0095. [DOI] [PubMed] [Google Scholar]
- 69.Takeda K, Kaisho T, Akira S. Toll-like receptors. Annu Rev Immunol. 2003;21:335–376. doi: 10.1146/annurev.immunol.21.120601.141126. [DOI] [PubMed] [Google Scholar]
- 70.Bernard JJ, Cowing-Zitron C, Nakatsuji T, et al. Ultraviolet radiation damages self noncoding RNA and is detected by TLR3. Nat Med. 2012;18:1286–1290. doi: 10.1038/nm.2861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ahmad I, Simanyi E, Guroji P, et al. Toll-Like Receptor-4 Deficiency Enhances Repair of Ultraviolet Radiation Induced Cutaneous DNA Damage by Nucleotide Excision Repair Mechanism. J Invest Dermatol. 2013 doi: 10.1038/jid.2013.530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ros AM, Wennersten G. Current aspects of polymorphous light eruptions in Sweden. Photodermatol. 1986;3:298–302. [PubMed] [Google Scholar]
- 73.Honigsmann H. Polymorphous light eruption. Photodermatol Photoimmunol Photomed. 2008;24:155–161. doi: 10.1111/j.1600-0781.2008.00343.x. [DOI] [PubMed] [Google Scholar]
- 74.Khoo SW, Tay YK, Tham SN. Photodermatoses in a Singapore skin referral centre. Clin Exp Dermatol. 1996;21:263–268. doi: 10.1111/j.1365-2230.1996.tb00090.x. [DOI] [PubMed] [Google Scholar]
- 75.Majoie IM, van Weelden H, Sybesma IM, et al. Polymorphous light eruption-like skin lesions in welders caused by ultraviolet C light. J Am Acad Dermatol. 2010;62:150–151. doi: 10.1016/j.jaad.2009.01.041. [DOI] [PubMed] [Google Scholar]
- 76.Norris PG, Morris J, McGibbon DM, et al. Polymorphic light eruption: an immunopathological study of evolving lesions. Br J Dermatol. 1989;120:173–183. doi: 10.1111/j.1365-2133.1989.tb07781.x. [DOI] [PubMed] [Google Scholar]
- 77.Norris PG, Barker JN, Allen MH, et al. Adhesion molecule expression in polymorphic light eruption. J Invest Dermatol. 1992;99:504–508. doi: 10.1111/1523-1747.ep12616175. [DOI] [PubMed] [Google Scholar]
- 78.Kolgen W, Van Weelden H, Den Hengst S, et al. CD11b+ cells and ultraviolet-B-resistant CD1a+ cells in skin of patients with polymorphous light eruption. J Invest Dermatol. 1999;113:4–10. doi: 10.1046/j.1523-1747.1999.00625.x. [DOI] [PubMed] [Google Scholar]
- 79.Palmer RA, Friedmann PS. Ultraviolet radiation causes less immunosuppression in patients with polymorphic light eruption than in controls. J Invest Dermatol. 2004;122:291–294. doi: 10.1046/j.0022-202X.2004.22213.x. [DOI] [PubMed] [Google Scholar]
- 80.Roelandts R. Chronic actinic dermatitis. J Am Acad Dermatol. 1993;28:240–249. doi: 10.1016/0190-9622(93)70034-q. [DOI] [PubMed] [Google Scholar]
- 81.Que SK, Brauer JA, Soter NA, et al. Chronic actinic dermatitis: an analysis at a single institution over 25 years. Dermatitis: contact, atopic, occupational, drug. 2011;22:147–154. [PubMed] [Google Scholar]
- 82.Hawk JLM, Lim HW. Chronic actinic dermatitis. In: Lim HW, Honigsmann H, Hawk JLM, editors. Photodermatology. New York: Informa Health; 2007. pp. 169–183. [Google Scholar]
- 83.du Menage HP, Sattar NK, Haskard DO, et al. A study of the kinetics and pattern of E-selectin, VCAM-1 and ICAM-1 expression in chronic actinic dermatitis. Br J Dermatol. 1996;134:262–268. [PubMed] [Google Scholar]
- 84.Fujita M, Miyachi Y, Horio T, et al. Immunohistochemical comparison of actinic reticuloid with allergic contact dermatitis. Journal of Dermatological Science. 1990;1:289–296. doi: 10.1016/0923-1811(90)90122-t. [DOI] [PubMed] [Google Scholar]
- 85.Foering K, Chang AY, Piette EW, et al. Characterization of clinical photosensitivity in cutaneous lupus erythematosus. J Am Acad Dermatol. 2013;69:205–213. doi: 10.1016/j.jaad.2013.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Foering K, Goreshi R, Klein R, et al. Prevalence of self-report photosensitivity in cutaneous lupus erythematosus. J Am Acad Dermatol. 2012;66:220–228. doi: 10.1016/j.jaad.2010.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kim A, Chong BF. Photosensitivity in cutaneous lupus erythematosus. Photodermatol Photoimmunol Photomed. 2013;29:4–11. doi: 10.1111/phpp.12018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Scheinfeld N, Deleo VA. Photosensitivity in lupus erythematosus. Photodermatol Photoimmunol Photomed. 2004;20:272–279. doi: 10.1111/j.1600-0781.2004.00094.x. [DOI] [PubMed] [Google Scholar]
- 89.Kuhn A, Wozniacka A, Szepietowski JC, et al. Photoprovocation in cutaneous lupus erythematosus: a multicenter study evaluating a standardized protocol. J Invest Dermatol. 2011;131:1622–1630. doi: 10.1038/jid.2011.101. [DOI] [PubMed] [Google Scholar]
- 90.Lehmann P, Holzle E, Kind P, et al. Experimental reproduction of skin lesions in lupus erythematosus by UVA and UVB radiation. J Am Acad Dermatol. 1990;22:181–187. doi: 10.1016/0190-9622(90)70020-i. [DOI] [PubMed] [Google Scholar]
- 91.LeFeber WP, Norris DA, Ryan SR, et al. Ultraviolet light induces binding of antibodies to selected nuclear antigens on cultured human keratinocytes. J Clin Invest. 1984;74:1545–1551. doi: 10.1172/JCI111569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Orteu CH, Sontheimer RD, Dutz JP. The pathophysiology of photosensitivity in lupus erythematosus. Photodermatol Photoimmunol Photomed. 2001;17:95–113. doi: 10.1034/j.1600-0781.2001.170301.x. [DOI] [PubMed] [Google Scholar]
- 93.Lin JH, Dutz JP, Sontheimer RD, et al. Pathophysiology of cutaneous lupus erythematosus. Clinical reviews in allergy & immunology. 2007;33:85–106. doi: 10.1007/s12016-007-0031-x. [DOI] [PubMed] [Google Scholar]
- 94.Cafardi JA, Pollack BP, Elmets CA. Phototherapy. In: Goldsmith LA, et al., editors. Fitzpatrick’s Dermatology in General Medicine. 8. New York: McGraw Hill; 2012. pp. 2841–2850. [Google Scholar]
- 95.Krutmann J, Morita A, Elmets CA. Mechanisms of Photo(chemo)therapy. In: Krutmann J, Hönigsmann H, Elmets CA, editors. Dermatological Phototherapy and Photodiagnostic Methods. 2. Berlin: Springer-Verlag; 2009. pp. 63–77. [Google Scholar]
- 96.van Weelden H, De La Faille HB, Young E, et al. A new development in UVB phototherapy of psoriasis. Br J Dermatol. 1988;119:11–19. doi: 10.1111/j.1365-2133.1988.tb07096.x. [DOI] [PubMed] [Google Scholar]
- 97.Parrish JA, Jaenicke KF. Action spectrum for phototherapy of psoriasis. J Invest Dermatol. 1981;76:359–362. doi: 10.1111/1523-1747.ep12520022. [DOI] [PubMed] [Google Scholar]
- 98.Clayton TH, Clark SM, Turner D, et al. The treatment of severe atopic dermatitis in childhood with narrowband ultraviolet B phototherapy. Clin Exp Dermatol. 2007;32:28–33. doi: 10.1111/j.1365-2230.2006.02292.x. [DOI] [PubMed] [Google Scholar]
- 99.Ersoy-Evans S, Altaykan A, Sahin S, et al. Phototherapy in childhood. Pediatr Dermatol. 2008;25:599–605. doi: 10.1111/j.1525-1470.2008.00773.x. [DOI] [PubMed] [Google Scholar]
- 100.Jury CS, McHenry P, Burden AD, et al. Narrowband ultraviolet B (UVB) phototherapy in children. Clin Exp Dermatol. 2006;31:196–199. doi: 10.1111/j.1365-2230.2006.02061.x. [DOI] [PubMed] [Google Scholar]
- 101.Gathers RC, Scherschun L, Malick F, et al. Narrowband UVB phototherapy for early-stage mycosis fungoides. J Am Acad Dermatol. 2002;47:191–197. doi: 10.1067/mjd.2002.120911. [DOI] [PubMed] [Google Scholar]
- 102.Boztepe G, Sahin S, Ayhan M, et al. Narrowband ultraviolet B phototherapy to clear and maintain clearance in patients with mycosis fungoides. J Am Acad Dermatol. 2005;53:242–246. doi: 10.1016/j.jaad.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 103.Stege H, Schopf E, Ruzicka T, et al. High-dose UVA1 for urticaria pigmentosa. Lancet. 1996;347:64. doi: 10.1016/s0140-6736(96)91600-1. [DOI] [PubMed] [Google Scholar]
- 104.Tuchinda C, Kerr HA, Taylor CR, et al. UVA1 phototherapy for cutaneous diseases: an experience of 92 cases in the United States. Photodermatol Photoimmunol Photomed. 2006;22:247–253. doi: 10.1111/j.1600-0781.2006.00245.x. [DOI] [PubMed] [Google Scholar]
- 105.Krutmann J, Czech W, Diepgen T, et al. High-dose UVA1 therapy in the treatment of patients with atopic dermatitis. J Am Acad Dermatol. 1992;26:225–230. doi: 10.1016/0190-9622(92)70031-a. [DOI] [PubMed] [Google Scholar]
- 106.Krutmann J, Diepgen TL, Luger TA, et al. High-dose UVA1 therapy for atopic dermatitis: results of a multicenter trial. J Am Acad Dermatol. 1998;38:589–593. doi: 10.1016/s0190-9622(98)70123-9. [DOI] [PubMed] [Google Scholar]
- 107.Plettenberg H, Stege H, Megahed M, et al. Ultraviolet A1 (340–400 nm) phototherapy for cutaneous T-cell lymphoma. J Am Acad Dermatol. 1999;41:47–50. doi: 10.1016/s0190-9622(99)70405-6. [DOI] [PubMed] [Google Scholar]
- 108.Morita A, Werfel T, Stege H, et al. Evidence that singlet oxygen-induced human T helper cell apoptosis is the basic mechanism of ultraviolet-A radiation phototherapy. J Exp Med. 1997;186:1763–1768. doi: 10.1084/jem.186.10.1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Yin L, Yamauchi R, Tsuji T, et al. The expression of matrix metalloproteinase-1 mRNA induced by ultraviolet A1 (340–400 nm) is phototherapy relevant to the glutathione (GSH) content in skin fibroblasts of systemic sclerosis. J Dermatol. 2003;30:173–180. doi: 10.1111/j.1346-8138.2003.tb00368.x. [DOI] [PubMed] [Google Scholar]
- 110.Wlaschek M, Briviba K, Stricklin GP, et al. Singlet oxygen may mediate the ultraviolet A-induced synthesis of interstitial collagenase. J Invest Dermatol. 1995;104:194–198. doi: 10.1111/1523-1747.ep12612751. [DOI] [PubMed] [Google Scholar]
- 111.Parrish JA, Fitzpatrick TB, Tanenbaum L, et al. Photochemotherapy of psoriasis with oral methoxsalen and longwave ultraviolet light. N Engl J Med. 1974;291:1207–1211. doi: 10.1056/NEJM197412052912301. [DOI] [PubMed] [Google Scholar]
- 112.Rupoli S, Goteri G, Pulini S, et al. Long-term experience with low-dose interferon-alpha and PUVA in the management of early mycosis fungoides. Eur J Haematol. 2005;75:136–145. doi: 10.1111/j.1600-0609.2005.00497.x. [DOI] [PubMed] [Google Scholar]
- 113.Herrmann JJ, Roenigk HH, Jr, Honigsmann H. Ultraviolet radiation for treatment of cutaneous T-cell lymphoma. Hematol Oncol Clin North Am. 1995;9:1077–1088. [PubMed] [Google Scholar]