Abstract
Despite recent advances in behavioral interventions for cannabis use disorders, effect sizes remain modest, and few individuals achieve long-term abstinence. One strategy to enhance outcomes is the addition of pharmacotherapy to complement behavioral treatment, but to date no efficacious medications targeting cannabis use disorders in adults through large, randomized controlled trials have been identified. The National Institute on Drug Abuse Clinical Trials Network (NIDA CTN) is currently conducting a study to test the efficacy of N-acetylcysteine (NAC) versus placebo (PBO), added to contingency management, for cannabis cessation in adults (ages 18–50). This study was designed to replicate positive findings from a study in cannabis-dependent adolescents that found greater odds of abstinence with NAC compared to PBO. This paper describes the design and implementation of an ongoing 12-week, intent-to-treat, double-blind, randomized, placebo-controlled study with one follow-up visit four weeks post-treatment. Approximately 300 treatment-seeking cannabis-dependent adults will be randomized to NAC or PBO across six study sites in the United States. The primary objective of this 12-week study is to evaluate the efficacy of twice-daily orally-administered NAC (1200 mg) versus matched PBO, added to contingency management, on cannabis abstinence. NAC is among the first medications to demonstrate increased odds of abstinence in a randomized controlled study among cannabis users in any age group. The current study will assess the cannabis cessation efficacy of NAC combined with a behavioral intervention in adults, providing a novel and timely contribution to the evidence base for the treatment of cannabis use disorders.
Keywords: N-acetylcysteine, cannabis, marijuana, pharmacotherapy, randomized trials, clinical study design
1.0 Introduction
Cannabis is the most commonly used illicit substance in the United States (US), and rates of use continue to rise [1]. While the public perception of risks is diminishing, cannabis use is associated with substantial health-related effects and impairments [2, 3]. Cannabis use disorders (CUDs) are increasingly prevalent and frequently lead individuals to seek treatment, with approximately 305,000 individuals entering inpatient and outpatient substance use disorder treatment for CUDs in the US in 2012 (17.5% of all admissions) [4]. Daily cannabis use among adolescents is rapidly rising, which is expected to lead to increased rates of CUDs, especially among young adults [5]. A recent meta-analysis showed modest benefit with behavioral treatments targeting CUDs (i.e., contingency management, relapse prevention, motivational interviewing, cognitive behavioral therapy, and combinations) compared to control conditions [6], yet there remains substantial room for improvement, as the majority of patients fail to achieve sustained periods of abstinence [7–9]. The development of pharmacological interventions to complement behavioral treatments represents a potential avenue to improve outcomes [10–14], and contingency management (CM) has specifically been identified as a preferred evidence-based behavioral platform to conduct pharmacotherapeutic efficacy trials [15]. Several promising agents to treat CUDs exist [16, 17], but to date, an effective medication targeting cannabis dependence in adults has not been established [12, 14, 18, 19], leaving clinicians and patients with limited options.
Preclinical research has demonstrated dysregulation in glutamatergic signaling within a corticostriatal circuit (prefrontal cortex-nucleus accumbens) following periods of self-administration and withdrawal across multiple substances of abuse [20–24]. These data indicate that glutamate plays a key role in drug-seeking and reinstatement models, suggesting that glutamate is a promising neurochemical target for medication development to treat substance use disorders (SUDs) [25, 26]. Further, evidence suggests that cannabinoid administration disrupts normal glutamate functioning [27–33] and indirectly disinhibits dopamine transmission [34, 35]. Among potential glutamate-targeted pharmacotherapies for SUDs, N-acetylcysteine (NAC) has emerged as a particularly strong candidate [21, 25, 26]. NAC is an N-acetyl pro-drug of the naturally occurring amino acid cysteine, and stimulates cystine-glutamate exchange, thus increasing non-synaptic glial release of glutamate [36]. NAC has also been shown to reduce the reinstatement of drug-seeking in animal models across multiple substances [36–43].
In addition to promising preclinical findings, NAC also has a favorable safety profile. NAC is approved by the US Food and Drug Administration (FDA) as a mucolytic agent for bronchopulmonary disorders [44] and as an oral or intravenous antidote to treat acetaminophen poisoning [45]. NAC is also available as an inexpensive over-the-counter product commonly sold as a nutritional supplement. Preliminary clinical studies have suggested efficacy for NAC as a pharmacotherapuetic agent for several psychiatric disorders, including compulsive disorders and SUDs [46, 47]. Specific to CUDs, a single-site, randomized controlled study showed that NAC compared to PBO, when paired with CM to promote abstinence, doubled the odds of negative urine cannabinoid tests among cannabis-dependent adolescents [16]. NAC is among the first medications to improve measures of abstinence in a randomized placebo-controlled study among cannabis users in any age group, but has yet to be tested among adult cannabis users.
With rates of cannabis use continuing to rise [1] and largely ineffective treatment options for those wishing to quit, it is vital and timely to identify efficacious treatment options and extend promising treatments to new and diverse populations of cannabis users. In response to the study conducted by Gray and colleagues [16], a study has been developed through the National Institute on Drug Abuse Clinical Trials Network (NIDA CTN) to evaluate the efficacy of NAC compared to PBO, in combination with CM, to promote abstinence from cannabis in adults. This paper details the design and implementation of CTN-0053, Achieving Cannabis Cessation - Evaluating N-Acetylcysteine Treatment (ACCENT).
2.0 Study Design and Procedures
2.1 Study Overview and Design
The ACCENT study is a 12-week, double-blind, randomized, placebo-controlled, multisite study that will enroll approximately 300 cannabis-dependent adults. The primary objective of this 12-week study is to evaluate the efficacy of NAC versus matched PBO, when added to contingency management, on cannabis abstinence throughout the treatment phase. Secondary objectives include evaluating the efficacy of NAC, compared to PBO, each paired with CM, on several measures of abstinence (e.g., 2- and 4-week continuous abstinence at the end of treatment, etc.) and other cannabis-related measures, such as craving, withdrawal, compulsive use, and cannabis-associated problems.
The ACCENT study protocol was developed by a multidisciplinary group of researchers and community treatment program providers with expertise in clinical trials methodology, pharmacology, statistics, cannabis use disorders, and substance use disorder treatment, which make up the ACCENT protocol development team. The Southern Consortium node (based at the Medical University of South Carolina [MUSC] in Charleston, SC) of the CTN is responsible for scientific oversight of the study and serves as the Lead Investigators. The ACCENT study is registered under an investigational new drug (IND) application from the US FDA (IND 78,927; Gray).
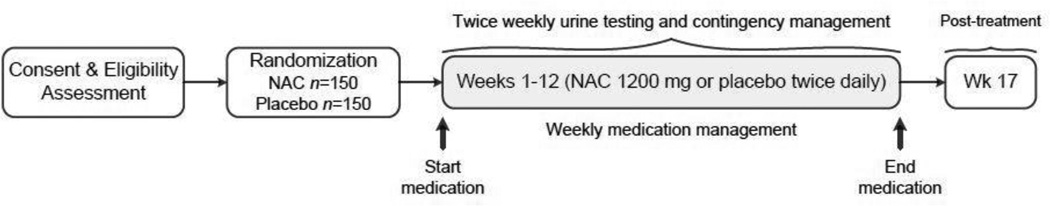
An overview of the ACCENT study design is shown in Figure 1. The screening/baseline assessment visit occurs after interested individuals have been pre-screened to ascertain preliminary eligibility status and have provided informed consent to participate in the study. Following consent and a thorough eligibility screening assessment, participants are randomized to receive NAC or matched PBO twice daily for 12 weeks. Participants then attend two weekly clinic visits during the 12-week treatment period to provide urine samples, complete assessments, and receive contingent reinforcement. One weekly study visit is a long visit (“a” visit), in which participants complete weekly assessments and meet with the medical clinician to review any medication side effects, adverse events, and to address medication adherence. The second weekly visit is a brief “drop-in” visit (“b” visit) that lasts approximately 10–15 minutes in which participants provide a urine sample and receive contingent reinforcement. Along with medication, all participants concurrently receive CM twice weekly during treatment. The CM schedule employs escalating cash reinforcement with resets, targeting both attendance at scheduled visits, and cannabis abstinence as determined by a negative qualitative urine cannabinoid dipstick test (Quicktox® Drug Screen Dipcard). Participants then return for one post-treatment follow-up visit 16 weeks after randomization (week 17).
Figure 1.
Overview of study design.
2.2 Study Design Decisions
2.2.1 Replication of the Adolescent Study
The ACCENT study was designed to replicate and extend the positive efficacy study of NAC in the treatment of CUDs conducted by Gray and colleagues with cannabis-dependent adolescents [16], with the inclusion of some key modifications. First, the length of the treatment phase in the adolescent study was 8 weeks of NAC or matched PBO. This was extended to 12 weeks of treatment in the current adult study. The Lead Investigators and the NIDA-appointed external protocol review board felt that 12 weeks of active treatment was consistent with the recommendations of the NIDA guidelines, which state that three months is the minimum amount of time that an individual should be engaged in treatment for SUDs [48].
Another important modification to the ACCENT study from the adolescent trial was the magnitude of cash reinforcement being used. CM is being used in the ACCENT study to reinforce abstinence from cannabis, as well as attendance at scheduled study visits. This “two-tiered” CM schedule was used in the adolescent study, but was set at a higher magnitude of reinforcement for the ACCENT study. The starting attendance and abstinence compensation for the adolescent study was set at $5 with escalations of $2 per visit. Participants could earn a total of $640 in that study. It was reasoned that adults may require increased compensation for engagement in the ACCENT study since they may have competing employment and family obligations, making attendance more difficult compared to an adolescent population. Therefore, to maximally reinforce completion of study visits, the starting attendance compensation in the ACCENT study is set at $10. In total, participants may earn a maximum of $1100 for uninterrupted study attendance and abstinence (see Section 2.3.3. for further details on the CM schedule).
Finally, while the ACCENT study was intended to replicate the results of the adolescent trial [16] in adult cannabis users, it is also intended to extend these results and contribute novel and innovative findings to researchers and clinicians. The ACCENT study is enrolling a large number of participants across several sites within the US. This will contribute greatly to the diversity of the study sample and add to the generalizability of study results.
2.2.2 Randomization
Eligible participants who return for the randomization visit are assigned to one of the two treatment groups (NAC or PBO) for 12 weeks of treatment. Assignment is on a 1:1 ratio to one of the two groups, and is stratified by two important criteria: 1) study site; and 2) self-reported tobacco smoking status, which emerged as an important randomization stratum because the literature from both laboratory and outpatient studies have shown that tobacco users have greater odds of relapse to cannabis compared to non-tobacco users [49, 50] and tend to have poorer cannabis treatment outcomes [51]. The randomization procedure is conducted centrally through the CTN Data and Statistics Center (DSC).
2.2.3 Efficacy Outcomes
To assess for an effect of treatment on abstinence from cannabis, it was decided by the Lead Investigators and the protocol development team that the ACCENT study would consider the entire 12-week treatment period as the primary outcome. This decision was made in order to closely replicate findings from the Gray and colleagues adolescent study [16], which also considered the entire treatment period. This design decision is somewhat inconsistent with recommendations from the CTN Treatment Effect and Assessment Measures (TEAM) Task Force, which advocates for the assessment of a treatment effect only in the last four weeks of the active treatment phase. The feasibility of using the CTN TEAM Task Force recommendation within the ACCENT study was assessed and found that, with the proposed sample of 300 participants, the study is reasonably powered for four-week end-of-treatment abstinence as a secondary outcome (details of the power calculation can be found in Section 8.0). Therefore, results from the ACCENT study will be powered to detect efficacy for both the entire treatment period (12 weeks) and the last four weeks of active treatment (weeks 10–13). Recommendations from the CTN TEAM Task Force can be found at: http://ctndisseminationlibrary.org/PDF/522.pdf.
2.3 Clinical Interventions
2.3.1 Pharmacological Intervention/Adherence
United States Pharmacopeia (USP) grade NAC powder was encapsulated in 600 mg quantities (two 600 mg capsules per dose plus 25 mg of riboflavin to each capsule). Matched PBO capsules (containing 25 mg of riboflavin and corn starch) were also prepared. All capsules are packaged in 7-day blister packs, with individual labels for day/date of each dose. Participants are given a two-week supply of medication to take home, with instruction to take two capsules twice daily, in approximately twelve-hour intervals. This dose was chosen due to its demonstrated tolerability and evidence of effect on cannabis use in cannabis-dependent adolescents [16].
During weekly visits, study personnel review medication logs and perform pill counts to monitor medication adherence. By assessing adherence via pill count, the extent of adherence is obtained, and the use of blister packs with labeled day/date of individual doses provides added benefit in the assessment and enhancement of adherence [52–54]. Weekly riboflavin measurement is also being used for biological confirmation of medication adherence, with 25 mg of riboflavin in each NAC or PBO capsule. Urine aliquots from weekly study visits are sent to a central laboratory (Clinical Neurobiology Laboratory at MUSC) to be tested for riboflavin. Urine riboflavin levels are determined via a fluorescence plate reader [55]. Levels of >1500 ng/mL are considered consistent with adherence as defined by taking 80–100% of prescribed study medication per week [56]. Additional methods to assess adherence were considered, such as the Medication Event Monitoring System (MEMS), but were not included due to added cost with unclear added benefit [57]. Studies of pill count combined with patient self-report have demonstrated good concordance with MEMS cap data in primary care populations [58].
2.3.2 Medication Management
To ensure that each site is providing consistent but minimal support and encouragement to study participants, non-manualized medication management is performed by the medical clinician weekly throughout treatment. This is a low-intensity intervention that emphasizes medication adherence, retention, and abstinence, but does not incorporate more intensive modalities, such as cognitive behavioral therapy or 12-step facilitation. Medical clinicians received training on medication management at the national ACCENT protocol training by the study principal investigator (Kevin M. Gray), and additionally were urged to use clinical judgment and skills when meeting with participants to discuss adherence to study medication.
2.3.3 Contingency Management (CM)
All participants receive the behavioral intervention in the form of twice-weekly CM. Cash rewards are provided to study participants for (a) cannabis abstinence, and (b) visit attendance (i.e., attending scheduled study visits). This CM intervention is based on prior use of CM in the treatment of cannabis use disorders previously reported in the literature [9, 15, 59–62]. This “two-tiered” CM approach was used with significant effect on both study retention and cannabis abstinence in a prior young adult CM study [59]. An additional potential benefit of the “two-tiered” approach is that it increases early exposure to contingent rewards. Among frequent cannabis users, urine cannabinoid metabolites may take two to four weeks to test negative after initiation of abstinence. In the context of a CM procedure that only rewards negative urine testing, several participants, even if abstaining from use, would potentially not be eligible for rewards for two to four weeks after achieving abstinence. Rewarding attendance may help to sustain motivation among participants initiating a quit attempt until urine tests yield negative results. Study staff are also sensitive to the possibility of delayed negative urine tests, and were trained to encourage continued abstinence among study participants. The Lead Investigators are particularly interested in this two-tiered method of reinforcement given the lack of single-tiered CM (reward only for substance abstinence) effect on study retention in a prior adolescent smoking cessation study [63].
CM feedback and rewards are delivered twice weekly during treatment, starting at the week 1b visit (i.e., “drop-in” short visit), which includes escalating schedules of cash reinforcement with resets. An escalating reinforcement schedule, in which participants are able to earn rewards of increasing monetary value over successive displays of desired behavior, is being used. The reinforcement schedule is shown in Table 2. For attendance, the reward for attending the first scheduled visit is $10. For each successive visit at which participants attend scheduled study visit, the reward increases by $2, up to a maximum of $30. If participants attend all scheduled visits, they receive a total of $610 during the 12-week treatment period. If participants subsequently fail to attend a study visit, they do not receive an attendance-contingent reward at that visit, and the attendance-contingent reward value for the next session is re-set to $10. For abstinence, the initial reward is $5. For each successive visit at which participants are abstinent, the reward increases by $2, up to a maximum of $25. If participants have a negative urine cannabinoid dipstick test at each visit during the 12-week treatment period, they receive a total of $490. If participants test positive for cannabinoids at a subsequent visit, they do not receive an abstinence-contingent reward at that visit, and the abstinent-contingent reward value for the next negative urine cannabinoid test is re-set to $5. If, at a given visit, the participants test positive but adhere with study procedures, they may still collect the attendance reward as scheduled, but is not eligible for abstinence reward. The rate of contingent reward escalation and the total potential contingent reward are comparable to those in previous cannabis cessation studies [9, 59–62]. The escalating reinforcement schedule with reset contingency is used in the current study as it has been shown to be more effective than fixed schedule or escalating schedule without reset contingency [64].
Table 2.
Contingency management (CM) reward schedule. Maximum possible compensation if a participant attends and is abstinent at each visit. EOT = End of treatment.
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | EOT |
CM Total Possible |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| b | a | b | a | b | a | b | a | b | a | b | a | b | a | b | a | b | a | b | a | b | a | b | |||
| Attendance | $10 | $12 | $14 | $16 | $18 | $20 | $22 | $24 | $26 | $28 | $30 | $30 | $30 | $30 | $30 | $30 | $30 | $30 | $30 | $30 | $30 | $30 | $30 | $30 | $610 |
| Abstinence | $5 | $7 | $9 | $11 | $13 | $15 | $17 | $19 | $21 | $23 | $25 | $25 | $25 | $25 | $25 | $25 | $25 | $25 | $25 | $25 | $25 | $25 | $25 | $25 | $490 |
| CM Total Possible | $15 | $19 | $23 | $27 | $31 | $35 | $39 | $43 | $47 | $51 | $55 | $55 | $55 | $55 | $55 | $55 | $55 | $55 | $55 | $55 | $55 | $55 | $55 | $55 | $1100 |
Contingent rewards are delivered in the form of cash payment. Prior research indicates that this form of contingent compensation, when compared with the use of gift cards or vouchers, is associated with improved research follow-up and retention rates and does not increase drug use or perception of coercion [65–67]. Alternative CM designs were considered, such as prize-based reinforcement, which has demonstrated improved cost-effectiveness in some settings [68]. However, no controlled trials supporting the efficacy of prize-based CM targeting cannabis cessation were found to support this approach. Additionally, given the positive primary outcome noted in the adolescent study, which used an escalating cash-based CM schedule with resets [16], the Lead Investigators and protocol development team determined that it would be prudent to closely mirror that study’s CM design.
3.0 Study Sites
3.1 Site Selection
Potential study sites that were considered for inclusion consisted of both academic research settings and community treatment programs that were part of the NIDA CTN. The CTN is comprised of centers across the US that vary widely in terms of patient demographics, services provided, level of care, etc. [69]. Sites interested in participating in the ACCENT study initially completed surveys that described their previous performance in conducting research, potential participant pools, recruitment strategies, and any regulatory issues. Sites were first selected based on answers to the survey. Sites then completed extensive telephone interviews and had in-person site visits to judge their appropriateness for recruitment and implementation of the ACCENT study. Sites were required to: 1) have access to a medical clinician (e.g., physician, physician’s assistant, nurse practitioner, etc.) to perform medical assessments, determine participant eligibility, regulate the medication dose appropriately, evaluate severity and relatedness of adverse events (AEs), and provide the medication management intervention; 2) have access to, or the ability to contract with, a pharmacy/pharmacist to store/dispense study medications if required by state and/or local regulations; 3) be able to provide after-hours clinical backup for study-related emergencies; 4) provide adequate space to accommodate research staff and study protocol procedures including on-site urine collection/testing and space to conduct study assessments; 5) be willing to provide cash incentives for CM purposes; and 6) be able to recruit enough individuals with cannabis dependence to meet recruitment goals.
Sample size estimations concluded that 300 participants would be adequate to power the study for efficacy. Six sites will enroll 4–6 subjects per month in order to complete recruitment over a 12-month period. Recruitment is being reviewed periodically to ensure adequate enrollment of women and minorities in the trial based on demographics of the study sites (38% women, 20% Hispanic). In addition, efforts are being made to recruit a study sample that reflects, or exceeds, the proportion of minorities in the communities where the sites are located. The six sites participating in the ACCENT are Behavioral Health Services of Pickens County (Pickens, SC), The APT Foundation (New Haven, CT), University of Kentucky Medical Center (Lexington, KY), University of California, Los Angeles Integrated Substance Abuse Programs (Los Angeles, CA), The University of Texas Health Science Center at San Antonio (San Antonio, TX), and CODA, Inc. (Portland, OR).
3.2 Training of Study Sites
Staffing for the protocol include the following: a site principal investigator, a full-time study coordinator, a full-time research assistant, a part-time research assistant/recruiter, a part-time medical clinician and a part-time phlebotomist. Research staff members were trained in the specifics of the protocol during a two-and-a-half-day national protocol training, hosted by the Lead Investigators in Charleston, South Carolina. The principal investigator, study coordinator, research assistant and medical clinician for each site attended. The training was also streamed live for additional staff and video-recorded using Adobe® Connect for staff hired after the training. Training modules (16 in total) each had a quiz that required 80% accuracy to be considered proficient. Separate practicum training exercises were completed for the informed consent document, the online data entry system, and administration of the Mini International Neuropsychiatric Interview Plus (M.I.N.I. 6.0) [70].
4.0 Participant Eligibility
A detailed list of study inclusion and exclusion criteria and the rationale for each criterion, are shown in Table 1. The study sample will consist of men and women aged 18–50. This age range was chosen since it encompasses the vast majority of adults over the age of 18 who enter treatment for CUDs [4]. The Lead Investigators and the protocol development team felt that participants over the age of 50 would not be representative of those patients who typically present for CUD treatment. The study will include those who are interested, but not currently enrolled in treatment for CUDs and meet criteria for CUDs in the past 30 days [71]. The Lead Investigators chose to use the Diagnostic and Statistical Manual of Mental Disorders-IV (DSM-IV) checklist [72] for SUD criteria determination as well as the Mini International Neuropsychiatric Interview Plus (M.I.N.I. 6.0) [70], while adding an additional question related to craving in order to have the ability to make a DSM-5 diagnosis as well. The substance withdrawal item from DSM-IV was also modified to capture cannabis withdrawal, which is consistent with DSM-5. Notably, although DSM-5 criteria were published just prior to the initiation of the ACCENT study, the Lead Investigators chose to use DSM-IV criteria for identifying SUDs and co-occurring psychiatric disorders because there was not yet a DSM-5 version of the MINI available at the time of study initiation. Also of note, DSM criteria capture past year SUDs, but in addition to past year problematic use, the ACCENT study required current (past 30 day) problematic use to be eligible for the study. This was done to ensure that participants were actively using cannabis at the time of study enrollment.
Table 1.
Study eligibility criteria and rationale.
| Criteria | Description | Rationale | |
|---|---|---|---|
| Inclusion | 1 | Age 18 – 50 years | Definition of study sample; encompasses large majority of adults seeking treatment for cannabis use disorders [4] |
| 2 | Able to understand study and give consent | Good Clinical Practice requirement | |
| 3 | DSM-IV diagnosis of cannabis dependence | Definition of study sample | |
| 4 | Interested in treatment | To help ensure that participant will provide useful data | |
| 5 | Positive urine cannabinoid test during screening | To ensure enrollment of individuals who might benefit | |
| 6 | Agree to use birth control | Safety of NAC during pregnancy not established | |
| Exclusion | 1 | Allergy or intolerance to NAC | Safety |
| 2 | Pregnancy or lactation | Safety of NAC during pregnancy/lactation not established | |
| 3 | Use of NAC or NAC-containing supplements | Safety; integrity of randomization | |
| 4 | Use of hazardous concurrent medications | Safety—potential high-risk drug-drug interactions | |
| 5 | Current treatment enrollment | Concurrent treatments may confound results | |
| 6 | Use of synthetic cannabinoids | Safety; Possible confound | |
| 7 | Other substance dependence | Definition of study sample | |
| 8 | UDS positive aside from cannabinoids | To help ensure that cannabis is primary substance | |
| 9 | UDS positive for amphetamines without valid prescription | To allow individuals receiving pharmacotherapy for co-occurring ADHD to participate | |
| 10 | Buprenorphine or methadone maintenance | Definition of study population | |
| 11 | Recent history of asthma | Safety | |
| 12 | Uncontrolled medical or psychiatric illness that could put participant at risk | Safety | |
| 13 | Risk of homicide or suicide | Safety | |
In an effort to be more inclusive, it was decided by the Lead Investigators and the protocol development team to enroll participants who were interested in achieving abstinence from cannabis, but also in reducing their cannabis use. Since the ACCENT study provides contingent reinforcement based on abstinence, it was felt that even those only motivated to reduce at the beginning of the study may be sufficiently motivated by incentives to abstain completely during the study. This design decision was also consistent with the adolescent trial [16], which included cannabis-dependent adolescents motivated to quit or reduce their cannabis use. Additionally, this may be more representative of clinical presentations of CUDs, in which individuals may wish to reduce their use to recreational levels without stopping entirely.
Participants are required to have a positive urine cannabinoid test during the screening visit as an entry criterion to help avoid enrollment of individuals who have already successfully achieved cannabis abstinence. The ACCENT study was not designed to test the effects of NAC on relapse prevention, which is a different empirical question from the one being asked. The adolescent study conducted by Gray and colleagues [16] found that 91% of participants had positive urine cannabinoid tests at screening, suggesting that this criteria would not unnecessarily exclude the majority of participants. Self-reported use of synthetic cannabinoids during the 30 days prior to screening is exclusionary to avoid enrollment of individuals who may be at increased risk of switching to use of synthetic cannabinoid products as a means for continuing substance use, but still receiving incentives for cannabis abstinence. It should be noted that biological tests for synthetic cannabinoids are not being conducted routinely in the ACCENT trial, but only after the first negative urinary cannabinoid test during the 12-week treatment period.
Study participants must not meet criteria for other SUDs (except for nicotine) and must not be receiving agonist treatment for opioid dependence. Use of other drugs that qualifies an individual for a SUD has the potential to interfere and may confound results from the ACCENT study. The impact of other SUD treatment and/or opioid replacement medications on the treatment intervention in the ACCENT study are unknown and could bias clinical response. Co-occurring nicotine dependence was deemed acceptable for inclusion for feasibility purposes, given the high frequency of co-use of tobacco and cannabis, though tobacco use status is being considered for randomization purposes (see Section 2.2.2 Randomization). Participants must not have a positive urine drug screen (UDS) at randomization for any substance other than cannabis. However, participants with a diagnosis of attention deficit hyperactivity disorder (ADHD) with a valid stimulant prescription could participate and a positive UDS for amphetamines would be excused. This was considered appropriate for feasibility purposes, given the high frequency of co-occurrence of ADHD and SUDs among young adults [73, 74].
Several inclusion and exclusion criteria were chosen to ensure the safety of study participants. Female participants who are pregnant or breastfeeding are not eligible to participate due to the unknown safety risks of NAC during pregnancy and lactation. Additionally, women of childbearing potential must agree to use some form of birth control during the study due to the unknown risks associated with NAC use during pregnancy. Any known allergy or intolerance to NAC is exclusionary, as is the use of carbamazepine or nitroglycerin. Participants with a recent history of asthma (last 3 years) are excluded since allergic reactions to intravenous NAC have been shown to be more likely in those with asthma [75]. Finally, those with a history of seizure disorder, bipolar disorder, schizophrenia, or other significant or unstable medical or psychiatric illness that may place the participant at increased risk are excluded from the study.
5.0 Measures/Assessments
5.1 Cannabis Use/Abstinence
Cannabis use and abstinence are assessed through several qualitative, quantitative, and self-reported measures and assessments. A qualitative urine drug dipstick test (Quicktox® Drug Screen Dipcard) is performed at each visit to test for 10 substances (i.e., benzodiazepines, amphetamines, cannabis, methamphetamines, opiates, cocaine, ecstasy, oxycodone, methadone, and barbiturates). A separate dipstick test for buprenorphine (CLIAwavied™, Inc. Single Drug Dipstick Test) is used only at screening and randomization visits. The cannabis results from the dipstick test are being used for twice-weekly CM purposes. All collected urine samples are being tested for adulteration with the UrineCheck 7 Adulterant Test Strips (Lifeloc Technologies, Inc.), as well as temperature strips within the urine collection cups. The observation of providing urine samples is not routine or required in the ACCENT study. The Lead Investigators felt that the temperature and adulteration testing being conducted was adequate to ensure the authenticity of samples. Since sites included academic research settings as well as community treatment programs, not all sites had appropriate facilities to observe urines easily. Therefore, it was felt that observed urines would not substantially add benefit beyond the adulterant and temperature testing. However, individual study sites are not prohibited from observing urine samples (given proper approval from their Institutional Review Board) if this is routine practice in their clinic. One of the two weekly urine samples is additionally being sent to a central laboratory (Clinical Neurobiology Laboratory at MUSC) for quantitative testing of Creatinine-corrected metabolites of Δ9-tetrahydrocannabinol (THC COOH) [76, 77], which is being run by the central laboratory on the ARCHITECT c4000 system for immunoassay testing. Qualitative urine cannabinoid testing (dichotomized using standard cutoff of 50 ng/mL) conducted at study sites (at baseline, at randomization, weekly throughout treatment, and at post-treatment follow-up) is the primary outcome of the efficacy assessments. Upon the first negative urine cannabinoid dipstick test that occurs after randomization, a urine Synthetic Cannabinoid Test [78] is conducted by a second laboratory (Soft Landings Laboratory) to ensure that cannabis cessation is not achieved via substitution of synthetic cannabinoids (e.g., K2, Spice). Currently, the assay tests for the presence of six synthetic cannabinoids (JWH 018, JWH 018N, JWH 0815, JWH 073, JWH 0734, and JWH 073N). The number of synthetic cannabinoids being tested may change throughout the ACCENT study to reflect new formulations that may emerge during the enrollment period. The Lead Investigators and protocol development team felt that it was important to test for synthetic cannabinoids initially during cannabis abstinence, but felt it would be too costly to maintain throughout the active treatment period. By testing for synthetic cannabinoids at the first negative cannabinoid result, there is greater certainty that participants are not substituting synthetics during the initial period of abstinence, arguably the most likely time they would substitute to deal with withdrawal and craving. Synthetic cannabinoid test results will not be immediately available to research staff, and so they will not be able to address the use of synthetic cannabinoids with participants during their study enrollment. These results will, however, be accounted for in the statistical analyses of cannabis abstinence should they be positive.
Self-reported measures of cannabis use are also collected throughout study participation. The Timeline Follow-Back (TLFB) procedure [79] is being used to elicit the participant’s self-reported use of substances. At screening, cannabis and other substance use are assessed for the 30-day period prior to screening. Since standard TLFB procedures do not account for precise quantity and potency measures of cannabis, an additional cannabis quantification procedure is being conducted at screening to supplement the TLFB. Participants are asked to quantify cannabis use by weighing out amounts of an inert cannabis surrogate (motherwort) and reporting on that amount’s potency through dollar value estimates. Methods of use in the past 30 days are quantified using this system (e.g., bowls, bongs, blunts, oral ingestion, etc.). These procedures have been used previously [80] and provide superior estimates of cannabis use. Methods of cannabis use that are not easily quantifiable with this measurement technique are being collected via the TLFB and grouped based on preparation (e.g., resin, hash, hash oil, edible formulations, and assorted high potency extracts). The TLFB is administered weekly throughout the active treatment phase and through the end of the follow-up period to document the participant’s self-reported use of cannabis and other substances. Capturing quantitative, qualitative, and detailed self-reported measures of cannabis use will allow for comprehensive comparisons between methods at the end of the study.
Several other cannabis-specific assessments will be collected, including: the Cannabis Withdrawal Scale (CWS) [81], Marijuana Craving Questionnaire (MCQ) [82, 83], and the Marijuana Problem Scale [84]. Additionally, the Obsessive Compulsive Drug Use Scale [85] has been adapted to specify cannabis as the primary substance, and will be used to assess obsessive and compulsive cannabis use-related symptoms.
5.2 Medical/Psychological Assessments
At the screening assessment, a medical history and physical exam is performed by appropriately credentialed medical personnel (e.g., physician, physician’s assistant, nurse practitioner) to assess whether individuals are medically stable for study inclusion. For safety purposes, all medications taken by participants for the 30 days prior to screening and during the study are documented. Vital signs including height (screening visit only), weight, and blood pressure are recorded at screening, every fourth week during active treatment, and at the follow-up visit. Appropriately qualified and trained study personnel assess for any medical or psychiatric side effects. AEs are recorded at each visit.
A battery of psychological assessments are also conducted at screening and some throughout the study, including: the Hospital Anxiety and Depression Scale (HADS) [86, 87], and The Pittsburgh Sleep Quality Index (PSQI) [88]. The Concise Health Risk Tracking—Self Report (CHRT-SR) [89] is a participant self-reported assessment of suicidality and related thoughts and behaviors that is administered at each weekly study visit. This scale is designed to quickly and easily track suicidality in a manner consistent with the Columbia Classification Algorithm of Suicide Assessment (C-CASA) [90]. The CHRT-SR assesses high risk suicide ideation by a positive response (Agree or Strongly Agree) on any of the last three questions (thoughts of, thoughts of how, and/or a specific plan to commit suicide) and prompt a clinician assessment for suicide risk before leaving the clinic. Additionally, tobacco smoking status is assessed and nicotine dependence is determined via the Fagerström Test for Nicotine Dependence (FTND) [91].
For safety purposes, a urine pregnancy test is performed at screening, randomization, weeks 5 and 9, and at End of Treatment for all women (Quicktox® Pregnancy Urine Test Cassette). If a woman is found to be pregnant at any point during the study, she will be allowed to continue in the study but be withdrawn from study medications and followed until resolution of the pregnancy.
5.3 Genetic Testing
The ACCENT study involves an optional blood draw, which may occur any time after randomization. Although not specifically tied to this study, participants are asked if he or she is willing to provide a 10 mL blood sample that will be sent to the Rutgers University Cell and DNA repository. Refusal to provide a blood sample has no influence on study participation. Efforts are being made across studies conducted through the NIDA CTN research platform to collect and bank blood samples for genetic testing. The ACCENT study is able to contribute samples from cannabis-dependent adults who are racially and ethnically diverse for better understanding of cannabis dependence and pharmacotherapeutic response. Further information regarding policies for access and distribution of DNA and clinical data from NIDA-funded studies on the genetics of addiction vulnerability may be found at: http://www.drugabuse.gov/about-nida/organization/workgroups-interest-groups-consortia/genetics-workgroup-gwg/nida-genetics-consortium-ngc.
6.0 Safety Monitoring
Unlike many other potential candidate medications for cannabis dependence [10], NAC has a long-established safety record, with FDA approval since 1963. NAC has been used safely for several decades, often at doses greatly exceeding those proposed for the present study [92, 93]. A meta-analysis of studies evaluating long-term oral treatment with NAC for prevention of chronic bronchitis found that NAC was well-tolerated, with generally mild, most commonly gastrointestinal adverse effects that did not require treatment interruption [44]. Systemic allergic reactions to NAC have been observed, but only with intravenous administration [94].
Despite NAC’s favorable safety profile, every effort is being made in the current study to ensure the medical and psychiatric safety of study participants. Medical clinicians meet with study participants on a weekly basis to assess AEs, and determine the severity, causality and seriousness of each event. For this study, mild, unrelated events are not entered into the data system. All other AEs are considered reportable, and must be entered into the data system within 7 days. Serious AEs are defined per federal regulations [95], and must be reported within 24 hours of the sites’ knowledge of the event. All reported AEs and SAEs, are reviewed centrally by the CTN appointed study Medical Monitor at the Clinical Coordinating Center (CCC) and summarized by system organ class and preferred term using MedDRA™ (The Medical Dictionary for Regulatory Activities). Additional information collected includes any corrective action taken by the medical clinician (e.g. dosage reduction). If a participant experiences intolerable adverse effects with study medication that are not remedied by a dose reduction, the medication may be discontinued while the participant continues to participate in all non-medication study interventions and procedures. Given the relative safety of NAC, routine blood work is not being conducted throughout the ACCENT study. Also due to the relative safety of this medication, it is highly unlikely that there would be a need to break the blind. However, in the case of an unintended pregnancy or medical emergency, or when knowledge of the assigned treatment group would be necessary for medical management, a process is in place to break the blind. This decision will be made by the CCC Medical Monitor in consultation with the Lead PI and site medical clinician.
Patient safety, study validity, and data integrity matters are monitored by the Data and Safety Monitoring Board (DSMB). The NIDA Center for Clinical Trials Network (CCTN) has appointed a CTN DSMB in accordance with the National Institutes of Health requirements to provide independent oversight of CTN trials. The DSMB reviews the research protocol and plans and make recommendations to assure that participant safety, study validity and data integrity are addressed appropriately. Throughout this study, the DSMB assesses study progress, factors that may affect study outcome, safety and outcome data, critical efficacy endpoints and factors or scientific discoveries external to the study that may have ethical considerations or may affect the risk benefit analysis of this study.
6.1 Participant Withdrawal
Every effort is being made to retain participants in the study. If a participant experiences intolerable AEs due to study medication that are not remedied by a dose reduction, the medication may be discontinued while the participant continues to participate in all non-medication study procedures. Given the intent-to-treat design of this study, participants are not withdrawn if they are non-adherent to study medication or the visit schedule.
6.2 Clinical Deterioration Plan
A clinical deterioration “rescue” plan is in place for participants who experience psychiatric deterioration or marked increases in substance use during the study. Symptoms are monitored closely throughout the study to assess for deterioration. Appropriate intervention will be arranged for any participant demonstrating gross clinical deterioration. The rescue measures include immediate assessment by the site medical clinician for a comprehensive psychiatric and substance use evaluation and referral for appropriate clinical intervention.
7.0 Outcomes and Data Analysis
7.1 Primary and Secondary Outcomes
The primary outcome measure for the ACCENT study is the odds of negative quantitative urine cannabinoid tests submitted during 12 weeks of active treatment, compared between the treatment groups (NAC versus PBO). Abstinence is based on the quantitative weekly urine samples analyzed by the central laboratory at MUSC. The first urine sample contributing to the primary outcome is collected at week 2, since the week 1a (long) visit urine sample is collected prior to randomization. The last urine sample contributing to the primary outcome is collected at the End of Treatment (week 13) visit. Thus, each participant contributes 12 indicators of abstinence, one for each week of treatment, and the primary outcome measure for each participant is then a vector of binary variables of length 12. Primary analysis is based on an intent-to-treat evaluation of all participants randomized into the study, with missing urine specimens imputed as positive in the analysis (i.e., missing data are assumed to be missing not at random). A sensitivity analysis will be performed to determine how the missing data affects study outcomes. Several strategies will be evaluated to determine the optimal method for dealing with missing data, including sophisticated imputation methods that have been recommended for alcohol clinical trials [96].
Secondary outcomes include the effect of NAC versus PBO, each added to CM, on the following: 1) end-of-treatment cannabis abstinence, measured by negative urine cannabinoid testing throughout the last two weeks (urine samples collected during weeks 12–13) of treatment as well as the last four weeks (urine samples collected during weeks 10–13); 2) odds of negative weekly urine cannabinoid tests during the first 8 weeks (urine samples collected during weeks 2–9) of active treatment; 3) two- and four-week modified end-of-treatment cannabis abstinence, anchored at week 8, based on urine samples collected during weeks 8–9 and weeks 6–9 respectively; 4) two- and four-week end-of-treatment self-reported cannabis abstinence confirmed by negative urine cannabinoid tests; 5) other cannabis-related measures (e.g., craving, withdrawal, compulsive use, cannabis-associated problems); as well as several other comparisons (i.e., other substance use, quality of life), and sub-analyses based on medication adherence criteria. It is hypothesized that NAC added to CM will result in different rates of cannabis abstinence compared with PBO + CM, regardless of the measure of abstinence (e.g., two-week end-of-treatment abstinence based on urine testing, or four-week end-of-treatment abstinence based on self-report). While there are several outcomes specified a priori, there will not be formal multiple comparison adjustment for these tests since they are secondary and seek to replicate prior results or generate new hypotheses. However, results from secondary analyses will be interpreted with caution.
7.2 Analysis of Outcomes
7.2.1 Primary Analysis
For the primary outcome measure, a longitudinal logistic model will be used to analyze the odds of a negative urine cannabinoid test as an indicator of abstinence across all 12 weeks of treatment. At each week, the primary outcome will be an indicator of whether the urine sample at that visit was negative for cannabinoids. Since each participant will contribute up to 12 outcomes to the model, generalized estimating equations (GEEs) [97] will be used to adjust for this correlation, with missing urine specimens imputed as positive in the analysis as noted previously. This is a commonly used imputation strategy among SUD treatment studies [98–103]. The primary analysis will assess various correlation structures between observations and select the best fitting structure using the Quasi-likelihood under the Independence Model Criterion (QIC) [104]. GEEs are a method of estimating parameters from a generalized linear model where there possibly is unknown correlation between outcomes. In this case, the correlation being accounted for is the lack of independence of weekly cannabis abstinence indicators for a participant over time. An advantage of utilizing GEEs with the robust variance estimator is that the results are fairly robust to misspecification of the underlying correlation between outcomes. The longitudinal model will include the main effect of treatment, the main effect of time, site effects, the effect of baseline tobacco smoking status and a time-by-treatment interaction. Testing of the treatment difference will evaluate whether the coefficient of the main effect of treatment assignment is significantly different from zero. The primary outcome will be evaluated using a two-sided test with a type I error rate of 5%. All analyses will follow the intent-to-treat principle, where all randomized participants are analyzed and the covariate of interest is the treatment assignment, not the treatment received. The primary outcome analyses will be conducted by the DSC of the NIDA CTN coordinated by The EMMES Corporation to ensure independent oversight of study results.
7.2.2 Secondary Analyses
For the end-of-treatment measure, a logistic model will be used to analyze the odds of abstinence during the last two weeks and last four weeks evaluation period. For the end-of-treatment logistic models, the binary outcome measure for each participant is defined as whether they contributed all cannabinoid-negative urine samples during the specified period. For example, during the four week end-of-treatment analysis, participants are abstinent only if they contribute four cannabinoid-negative urine samples. Thus, missing urine samples are imputed as positive and thus an individual with any missing urine samples during the four week period will be counted as non-abstinent. Additional analyses will be performed that mimic those conducted for the adolescent study [16]. The first analysis will be the comparison of the odds of a weekly cannabinoid-negative urine drug screen during the first 8 weeks of treatment across treatment assignments. This analysis will be conducted similar to primary outcome analysis, but for the first 8 weeks of the evaluation period. A modified end-of-treatment cannabis abstinence analysis anchored at week 8 will also be performed using a logistic model similar to end-of-treatment measure. Conducting these analyses will allow direct comparison of results with the adolescent study which utilized only 8 weeks of active treatment. All of the end-of-treatment analyses will be repeated using a different definition of abstinence that requires both a negative UDS as well as self-reported abstinence on TLFB.
8.0 Sample Size Calculation
A vital component of the proposed study was to closely mimic a similarly designed adolescent study [16]. The sample size calculation for the ACCENT study was conducted using the simulation described below. The general approach was to simulate the data based on parameters from the adolescent study, then analyze each simulated data set using a GEE model with the following covariates: main effect of time, main effect of treatment assignment, baseline smoking status and a time-by-treatment interaction. The test of the main effect of treatment was calculated for each simulated data set. The power for each particular parameter combination was the proportion of simulated data sets that yielded a statistically significant test of the main effect of treatment at the 0.05 level.
The generation of simulated data requires the following parameters to generate the simulated data: (1) the proportion of cannabinoid-negative UDS in each week in the PBO arm, (2) the missing data patterns, (3) the correlation between weekly abstinence outcomes within a participant, (4) the proportion of participants that self-report as smokers at baseline, (5) site effects that allow for variation in the odds of cannabinoid-abstinence to differ across sites, and (6) a clinically meaningful measure of treatment effect. The first four quantities were obtained from analyzing the adolescent study. That study was only eight weeks long, so for the simulation it was assumed that the odds of a cannabinoid-negative urine were the same for weeks 9–13 as for weeks 4–8 of the adolescent study. Since the adolescent study included participants regardless of their cannabinoid-positivity at baseline, the estimated proportion of cannabinoid-abstinence urines samples during the active treatment phase were estimated using only the 52 placebo participants that had a cannabinoid-positive urine at baseline. The resulting estimated proportions of cannabinoid-negative UDS during active treatment: 20%, 33%, 40%, 41%, 40%, 37%, 36%, and 50%. Of the possible 256 (=28) missing data patterns in the adolescent study, there were 46 (44%) participants who were fully observed, 26 (25%) who contributed no UDS post-randomization, 12 (11%) dropped out during active treatment, and 21 (20%) had intermittent missing UDS. The correlation between weekly cannabinoid-abstinence indicators estimated from the adolescent study was 0.75, and 57% of randomized participants self-reported being a cigarette smoker. Note that the model used assumed an auto-regressive lag-1 correlation structure since it specifies that urine samples collected farther apart in time are less correlated compared to those closer together in time, however, GEEs are generally robust to misspecification of the correlation structure. The estimated weekly cannabinoid-abstinence proportions were perturbed to capture site variability, such that the deviations for the six sites from the log-odds of abstinence at any given week were: −0.05, +0.05, −0.075, +0.075, −0.1, and +0.1. To generate data in the NAC plus CM arm, an odds ratio (OR) of 2 was used since it had been determined a priori to be clinically meaningful. Using the above simulation setup, for each participant a string of 12 binary indicators for weekly abstinent/non-abstinent status was simulated using the abstinent proportion at each week incorporating the week effect, missing data pattern, correlation and site effect, as described above. In addition 57% of the participants were randomly assigned to be cigarette smokers at baseline. Simulated data were then analyzed using a GEE model incorporating the overall main treatment effect, a week effect, site effects, a week-by-treatment interaction effect and an effect of baseline cigarette smoking status. While a sample size of 252 would be sufficient to detect an OR of 2 for the primary outcome analysis, it does not allow for sufficient power in replicating the adolescent study (8-week study). Since replication of the adolescent study was vital to the ACCENT study, we designed this study to be powered to detect treatment effects for both the 12- and 8-week anchored outcome measures, though the primary outcome only included treatment effect across the 12-week treatment period. A sample size of 300 participants will provide sufficient power (~80%) to detect the treatment difference corresponding to an OR of 2 with 6 sites, for analyses anchored at both the 12-week (ACCENT) and 8-week (adolescent study). In addition, the CTN TEAM Task Force recommends testing for a treatment effect only in the last four weeks of the active treatment phase. The feasibility of using the CTN TEAM Task Force recommendation within this study was assessed and the proposed sample of 300 participants would provide reasonable power (~80%, OR = 2.25 with 6 sites) for a four-week end-of-treatment abstinence measure as a secondary outcome. A unique feature of the sample size calculation for the ACCENT study is that a design decision was based on being powered to detect a treatment effect for the primary outcome measure as well as a key secondary outcome measure.
9.0 Discussion
9.1 Current Status of the Study
Recruitment of study participants commenced in January 2014, with all six study sites having Institutional Review Board (IRB) approvals and open to enrollment as of February 2014. Recruitment is expected to be completed by March 2015, with the final follow-up visits occurring in July 2015. At the time of writing this manuscript, recruitment is ahead of schedule and indicators of treatment exposure (doses of medication taken) and retention in study procedures are all excellent. Primary and secondary outcomes will be reported in future manuscripts.
9.2 Value of Findings from ACCENT in Treating CUDs
Cannabis use disorders are increasingly prevalent, accounting for 17.5% of all admissions to SUD treatment in 2012, which encompasses approximately 305,000 individuals in the US alone [4]. Treatment admissions due to CUDs are greater than those for cocaine (121,000), methamphetamine (116,000), and heroin (285,000) [4]. It is expected that rates of CUDs will continue to rise [5], therefore increasing the number of individuals seeking treatment for problematic use. The public perception of risks associated with cannabis use continues to diminish [5], which may increase the pool of individuals using cannabis regularly, and progressing to dependence. Unfortunately, established treatments convey limited efficacy for all age groups. The development of a safe and efficacious medication to complement behavioral treatment is a critical step in addressing a significant and growing public health problem. Based on the positive effect in the initial clinical study in adolescent cannabis users [16] and a favorable safety profile, NAC is an excellent candidate for clinical evaluation for the treatment of cannabis dependence among all age groups.
The results from the ACCENT study have the potential to improve treatment for CUDs among adults and could have a marked impact on clinical practice. With the recent implementation of the 2010 Patient Protection and Affordable Care Act (ACA), previously uninsured individuals will have increased access to primary care and treatment for SUDs. This is expected to create added pressure for evidence-based treatment options for those seeking treatments for SUDs. Therefore, it is of great importance, now more than ever, to have efficacious treatment options available for those wishing to reduce their use of cannabis or abstain completely. NAC is well-tolerated and readily available as an over-the-counter product sold as a nutritional supplement (should be noted that some, but not all over-the-counter NAC, is USP-grade NAC, which is the formulation used in research studies). This safety profile and availability may help clinicians to be more comfortable in recommending NAC as a pharmacotherapy for patients interested in reducing their cannabis use or quitting entirely.
By conducting this study within the NIDA CTN, the generalizability of study findings is enhanced. The multi-site study design being used by ACCENT promotes diversity in age, race, and ethnicity of study participants. Study sites include both academic medical centers and community treatment programs across the US, all with varying regulations and cultures surrounding cannabis. This may yield diversity with regards to CUD severity, and type and quantity of cannabis use and abstinence achieved.
In addition to the primary outcome of NAC’s efficacy for cannabis cessation, several secondary outcomes will be of value to researchers and clinicians investigating and treating cannabis dependence. This study is utilizing several measures of cannabis use and abstinence, which will provide direct comparisons of different quantification methods.
9.3. NAC for SUDs
Preclinical literature has provided support for targeting glutamatergic dysregulation as a treatment for SUDs with the use of glutamatergic agents that restore normal functioning. This demonstrated dysregulation appears to be consistent across several different substances of abuse [20, 21, 25], which lends support for employing glutamatergic agents across substances of abuse or in the case of poly-substance dependence. NAC may have the potential to be used in the treatment of several psychiatric disorders [46] and SUDs [47], though not all randomized trials have yielded positive results with NAC [105, 106]. The focus on CUDs in the ACCENT study may provide a template for similar work with other substances, among those who are dependent on several substances, or who have co-occurring psychiatric disorders, though the current study will not be able to answer questions regarding those populations. Future studies should work to address the benefit of NAC as a pharmacotherapy for CUDs in populations with co-occurring psychiatry disorders, should the results of the NAC trial be positive.
9.4 Study Limitations
There are some notable limitations of the ACCENT protocol that deserve discussion. First, the use of CM to reinforce abstinence from cannabis in this study may be seen as unrealistic for large-scale dissemination. Indeed, there are difficulties in the wide-spread implementation of CM as a behavioral treatment; however, it has been suggested that CM provides an ideal evidence-based platform for pharmacotherapy trials and treatments [15]. Continued work on the dissemination of CM, in conjunction with pharmacotherapy, will be important for future research and implementation studies. Second, biological verification of abstinence from cannabis may take 2–4 weeks with the tests that are currently available. This is not ideal for cannabis treatment trials, as false positives may emerge and discourage participants from remaining abstinent. It is expect that some false positives will occur early in abstinence in the ACCENT study, but it is also expected that the generous attendance CM schedule will assist in keeping participants engaged in treatment until the tests yield negative results. Third, the ACCENT study is excluding participants over the age of 50, which could be seen as limiting the generalizability of study results within and outside the US. Finally, this study has one follow-up visit four weeks after the treatment phase. The follow-up visit is largely intended to assess safety, and not necessarily continued efficacy, but we recognize that long-term and sustained efficacy would need to be established and assessed via future study.
9.5 Concluding Remarks
NAC is one of the first medications to demonstrate cessation benefit in a randomized controlled study among cannabis users in any age group, but has not yet been demonstrated in adults. While the results from the adolescent study by Gray and colleagues found that NAC was effective in promoting abstinence from cannabis [16], those findings require replication and extension among adult cannabis users. The ACCENT study was designed to mirror the methods from the adolescent study, with appropriate modifications for an adult population. If results from the ACCENT study show efficacy of NAC, plus CM, in promoting abstinence from cannabis among adults, this will be the first, multi-site, randomized controlled, intent-to-treat demonstration of an effective and safe pharmacotherapy for CUDs among a diverse population of adult cannabis users. Future studies should then work to address the clinical utility of NAC in the absence of a behavioral intervention or work to incorporate behavioral platforms that are easily adopted and delivered to patients. These results have the potential for application to clinical care and to improve outcomes for those seeking treatment for CUDs.
Acknowledgements
This work was supported by the National Institute on Drug Abuse National Drug Clinical Trials Network Grants U10DA013727 (Kathleen T. Brady), U10DA15831 (Kathleen M. Carroll and Roger D. Weiss), NIDA K24DA022288 (Roger D. Weiss), U10DA013045 (Walter Ling), U10DA013034 (Maxine L. Stitzer and Robert P. Schwartz), U10DA013732 (Theresa Winhusen), U10DA013714 (James L. Sorensen and Dennis McCarty); and NIDA Contracts N01DA92217 and N01DA102221 (The EMMES Corporation). The authors would like to thank the staff at the study sites and the regional research and training programs of the NIDA CTN who participated in the development and implementation of this study. Special thanks to Ashley Morrill, Ricardo Cantu, Sarah Brewer, and Christine Horne for their hard work and dedication to the implementation of the ACCENT study. The opinions in this manuscript are those of the authors and do not represent the official position of the U.S. government.
Disclosure Statement: Dr. Gray has received research funding from Supernus Pharmaceuticals and Merck, Inc., for unrelated projects. Dr. Winhusen has received research funding from Pfizer Inc. for an unrelated project. Dr. Levin has received medications for NIDA-funded studies from US World Meds and has been a consultant to GW Pharmaceuticals.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Substance Abuse and Mental Health Services Administration. Results from the 2012 National Survey on Drug Use and Health: Summary of National Findings. Rockville, MD: U.S. Department of Health and Human Services, Substance Abuse and Mental Health Services Administration; 2013. [Google Scholar]
- 2.Budney AJ, Moore BA. Development and consequences of cannabis dependence. J Clin Pharmacol. 2002;42:28S–33S. doi: 10.1002/j.1552-4604.2002.tb06000.x. [DOI] [PubMed] [Google Scholar]
- 3.Hall W, Degenhardt L. Adverse health effects of non-medical cannabis use. Lancet. 2009;374:1383–1391. doi: 10.1016/S0140-6736(09)61037-0. [DOI] [PubMed] [Google Scholar]
- 4.Substance Abuse and Mental Health Data Archive. Substance Abuse and Mental Health Services Administration. Ann Arbor, MI: United States Department of Health and Human Services; 2013. Treatment Episode Data Set: Admissions (TEDS-A) 2012. [Google Scholar]
- 5.Johnston LD, O'Malley PM, Bachman JG, Schulenberg JE. Monitoring the future national results on drug use 2012 Overview, Key Findings on Adolescent Drug Use. Ann Arbor, MI: Institute for Social Research, University of Michigan; 2013. [Google Scholar]
- 6.Davis ML, Powers MB, Handelsman P, Medina JL, Zvolensky M, Smits JA. Behavioral Therapies for Treatment-Seeking Cannabis Users: A Meta-Analysis of Randomized Controlled Trials. Eval Health Prof. 2014 doi: 10.1177/0163278714529970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Budney AJ, Roffman R, Stephens RS, Walker D. Marijuana dependence and its treatment. Addiction science & clinical practice. 2007;4:4–16. doi: 10.1151/ascp07414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McRae AL, Budney AJ, Brady KT. Treatment of marijuana dependence: a review of the literature. Journal of substance abuse treatment. 2003;24:369–376. doi: 10.1016/s0740-5472(03)00041-2. [DOI] [PubMed] [Google Scholar]
- 9.Kadden RM, Litt MD, Kabela-Cormier E, Petry NM. Abstinence rates following behavioral treatments for marijuana dependence. Addictive behaviors. 2007;32:1220–1236. doi: 10.1016/j.addbeh.2006.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hart CL. Increasing treatment options for cannabis dependence: a review of potential pharmacotherapies. Drug Alcohol Depend. 2005;80:147–159. doi: 10.1016/j.drugalcdep.2005.03.027. [DOI] [PubMed] [Google Scholar]
- 11.Vandrey R, Haney M. Pharmacotherapy for cannabis dependence: how close are we? CNS drugs. 2009;23:543–553. doi: 10.2165/00023210-200923070-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weinstein AM, Gorelick DA. Pharmacological treatment of cannabis dependence. Current pharmaceutical design. 2011;17:1351–1358. doi: 10.2174/138161211796150846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Benyamina A, Lecacheux M, Blecha L, Reynaud M, Lukasiewcz M. Pharmacotherapy and psychotherapy in cannabis withdrawal and dependence. Expert review of neurotherapeutics. 2008;8:479–491. doi: 10.1586/14737175.8.3.479. [DOI] [PubMed] [Google Scholar]
- 14.Danovitch I, Gorelick DA. State of the art treatments for cannabis dependence. Psychiatr Clin North Am. 2012;35:309–326. doi: 10.1016/j.psc.2012.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carroll KM, Rounsaville BJ. A perfect platform: combining contingency management with medications for drug abuse. Am J Drug Alcohol Abuse. 2007;33:343–365. doi: 10.1080/00952990701301319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gray KM, Carpenter MJ, Baker NL, DeSantis SM, Kryway E, Hartwell KJ, et al. A double-blind randomized controlled trial of N-acetylcysteine in cannabis-dependent adolescents. The American Journal of Psychiatry. 2012;169:805–812. doi: 10.1176/appi.ajp.2012.12010055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mason BJ, Crean R, Goodell V, Light JM, Quello S, Shadan F, et al. A proof-of-concept randomized controlled study of gabapentin: effects on cannabis use withdrawal and executive function deficits in cannabis-dependent adults. Neuropsychopharmacology. 2012;37:1689–1698. doi: 10.1038/npp.2012.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Levin FR, Mariani JJ, Brooks DJ, Pavlicova M, Cheng W, Nunes EV. Dronabinol for the treatment of cannabis dependence: a randomized, double-blind, placebo-controlled trial. Drug Alcohol Depend. 2011;116:142–150. doi: 10.1016/j.drugalcdep.2010.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Allsop DJ, Copeland J, Lintzeris N, Dunlop AJ, Montebello M, Sadler C, et al. Nabiximols as an agonist replacement therapy during cannabis withdrawal: a randomized clinical trial. JAMA psychiatry. 2014;71:281–291. doi: 10.1001/jamapsychiatry.2013.3947. [DOI] [PubMed] [Google Scholar]
- 20.Gass JT, Olive MF. Glutamatergic substrates of drug addiction and alcoholism. Biochemical pharmacology. 2008;75:218–265. doi: 10.1016/j.bcp.2007.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kalivas PW, Lalumiere RT, Knackstedt L, Shen H. Glutamate transmission in addiction. Neuropharmacology. 2009;56(Suppl 1):169–173. doi: 10.1016/j.neuropharm.2008.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gipson CD, Reissner KJ, Kupchik YM, Smith AC, Stankeviciute N, Hensley-Simon ME, et al. Reinstatement of nicotine seeking is mediated by glutamatergic plasticity. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:9124–9129. doi: 10.1073/pnas.1220591110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.LaLumiere RT, Kalivas PW. Glutamate release in the nucleus accumbens core is necessary for heroin seeking. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2008;28:3170–3177. doi: 10.1523/JNEUROSCI.5129-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McFarland K, Lapish CC, Kalivas PW. Prefrontal glutamate release into the core of the nucleus accumbens mediates cocaine-induced reinstatement of drug-seeking behavior. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2003;23:3531–3537. doi: 10.1523/JNEUROSCI.23-08-03531.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kalivas PW, Volkow ND. New medications for drug addiction hiding in glutamatergic neuroplasticity. Molecular psychiatry. 2011;16:974–986. doi: 10.1038/mp.2011.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Olive MF, Cleva RM, Kalivas PW, Malcolm RJ. Glutamatergic medications for the treatment of drug and behavioral addictions. Pharmacology, biochemistry, and behavior. 2012;100:801–810. doi: 10.1016/j.pbb.2011.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hoffman AF, Lupica CR. Direct actions of cannabinoids on synaptic transmission in the nucleus accumbens: a comparison with opioids. Journal of neurophysiology. 2001;85:72–83. doi: 10.1152/jn.2001.85.1.72. [DOI] [PubMed] [Google Scholar]
- 28.Pistis M, Muntoni AL, Pillolla G, Gessa GL. Cannabinoids inhibit excitatory inputs to neurons in the shell of the nucleus accumbens: an in vivo electrophysiological study. The European journal of neuroscience. 2002;15:1795–1802. doi: 10.1046/j.1460-9568.2002.02019.x. [DOI] [PubMed] [Google Scholar]
- 29.Doherty J, Dingledine R. Functional interactions between cannabinoid and metabotropic glutamate receptors in the central nervous system. Current opinion in pharmacology. 2003;3:46–53. doi: 10.1016/s1471-4892(02)00014-0. [DOI] [PubMed] [Google Scholar]
- 30.Robbe D, Alonso G, Manzoni OJ. Exogenous and endogenous cannabinoids control synaptic transmission in mice nucleus accumbens. Ann N Y Acad Sci. 2003;1003:212–225. doi: 10.1196/annals.1300.013. [DOI] [PubMed] [Google Scholar]
- 31.Schoffelmeer AN, Hogenboom F, Wardeh G, De Vries TJ. Interactions between CB1 cannabinoid and mu opioid receptors mediating inhibition of neurotransmitter release in rat nucleus accumbens core. Neuropharmacology. 2006;51:773–781. doi: 10.1016/j.neuropharm.2006.05.019. [DOI] [PubMed] [Google Scholar]
- 32.Hoffman AF, Laaris N, Kawamura M, Masino SA, Lupica CR. Control of cannabinoid CB1 receptor function on glutamate axon terminals by endogenous adenosine acting at A1 receptors. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2010;30:545–555. doi: 10.1523/JNEUROSCI.4920-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pistis M, Ferraro L, Pira L, Flore G, Tanganelli S, Gessa GL, et al. Delta(9)-tetrahydrocannabinol decreases extracellular GABA and increases extracellular glutamate and dopamine levels in the rat prefrontal cortex: an in vivo microdialysis study. Brain research. 2002;948:155–158. doi: 10.1016/s0006-8993(02)03055-x. [DOI] [PubMed] [Google Scholar]
- 34.Robbe D, Alonso G, Duchamp F, Bockaert J, Manzoni OJ. Localization and mechanisms of action of cannabinoid receptors at the glutamatergic synapses of the mouse nucleus accumbens. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2001;21:109–116. doi: 10.1523/JNEUROSCI.21-01-00109.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Parolaro D, Vigano D, Rubino T. Endocannabinoids and drug dependence. Current drug targets CNS and neurological disorders. 2005;4:643–655. doi: 10.2174/156800705774933014. [DOI] [PubMed] [Google Scholar]
- 36.Baker DA, McFarland K, Lake RW, Shen H, Tang XC, Toda S, et al. Neuroadaptations in cystine-glutamate exchange underlie cocaine relapse. Nature neuroscience. 2003;6:743–749. doi: 10.1038/nn1069. [DOI] [PubMed] [Google Scholar]
- 37.Moran MM, McFarland K, Melendez RI, Kalivas PW, Seamans JK. Cystine/glutamate exchange regulates metabotropic glutamate receptor presynaptic inhibition of excitatory transmission and vulnerability to cocaine seeking. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2005;25:6389–6393. doi: 10.1523/JNEUROSCI.1007-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Madayag A, Lobner D, Kau KS, Mantsch JR, Abdulhameed O, Hearing M, et al. Repeated N-acetylcysteine administration alters plasticity-dependent effects of cocaine. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2007;27:13968–13976. doi: 10.1523/JNEUROSCI.2808-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moussawi K, Zhou W, Shen H, Reichel CM, See RE, Carr DB, et al. Reversing cocaine-induced synaptic potentiation provides enduring protection from relapse. Proc Natl Acad Sci U S A. 2011;108:385–390. doi: 10.1073/pnas.1011265108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Reichel CM, Moussawi K, Do PH, Kalivas PW, See RE. Chronic N-acetylcysteine during abstinence or extinction after cocaine self-administration produces enduring reductions in drug seeking. The Journal of pharmacology and experimental therapeutics. 2011;337:487–493. doi: 10.1124/jpet.111.179317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Amen SL, Piacentine LB, Ahmad ME, Li SJ, Mantsch JR, Risinger RC, et al. Repeated N-acetyl cysteine reduces cocaine seeking in rodents and craving in cocaine-dependent humans. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2011;36:871–878. doi: 10.1038/npp.2010.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhou W, Kalivas PW. N-acetylcysteine reduces extinction responding and induces enduring reductions in cue- and heroin-induced drug-seeking. Biol Psychiatry. 2008;63:338–340. doi: 10.1016/j.biopsych.2007.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Knackstedt LA, LaRowe S, Mardikian P, Malcolm R, Upadhyaya H, Hedden S, et al. The role of cystine-glutamate exchange in nicotine dependence in rats and humans. Biological psychiatry. 2009;65:841–845. doi: 10.1016/j.biopsych.2008.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Grandjean EM, Berthet P, Ruffmann R, Leuenberger P. Efficacy of oral long-term N-acetylcysteine in chronic bronchopulmonary disease: a meta-analysis of published double-blind, placebo-controlled clinical trials. Clinical therapeutics. 2000;22:209–221. doi: 10.1016/S0149-2918(00)88479-9. [DOI] [PubMed] [Google Scholar]
- 45.Smilkstein MJ, Knapp GL, Kulig KW, Rumack BH. Efficacy of oral N-acetylcysteine in the treatment of acetaminophen overdose. Analysis of the national multicenter study (1976 to 1985) The New England journal of medicine. 1988;319:1557–1562. doi: 10.1056/NEJM198812153192401. [DOI] [PubMed] [Google Scholar]
- 46.Berk M, Malhi GS, Gray LJ, Dean OM. The promise of N-acetylcysteine in neuropsychiatry. Trends in pharmacological sciences. 2013;34:167–177. doi: 10.1016/j.tips.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 47.McClure EA, Gipson CD, Malcolm RJ, Kalivas PW, Gray KM. Potential role of N-acetylcysteine in the management of substance use disorders. CNS drugs. 2014;28:95–106. doi: 10.1007/s40263-014-0142-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.National Institutes of Health. Principles of Drug Addiction Treatment: A Reserach-based Guide. 3rd ed. Bethesda, MD: National Institute on Drug Abuse; 2012. [Google Scholar]
- 49.Haney M, Bedi G, Cooper ZD, Glass A, Vosburg SK, Comer SD, et al. Predictors of marijuana relapse in the human laboratory: robust impact of tobacco cigarette smoking status. Biological psychiatry. 2013;73:242–248. doi: 10.1016/j.biopsych.2012.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.de Dios MA, Vaughan EL, Stanton CA, Niaura R. Adolescent tobacco use and substance abuse treatment outcomes. Journal of substance abuse treatment. 2009;37:17–24. doi: 10.1016/j.jsat.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Peters EN, Budney AJ, Carroll KM. Clinical correlates of co-occurring cannabis and tobacco use: a systematic review. Addiction (Abingdon, England) 2012;107:1404–1417. doi: 10.1111/j.1360-0443.2012.03843.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wright JM, Htun Y, Leong MG, Forman P, Ballard RC. Evaluation of the use of calendar blister packaging on patient compliance with STD syndromic treatment regimens. Sexually transmitted diseases. 1999;26:556–563. doi: 10.1097/00007435-199911000-00004. [DOI] [PubMed] [Google Scholar]
- 53.Huang HY, Maguire MG, Miller ER, 3rd, Appel LJ. Impact of pill organizers and blister packs on adherence to pill taking in two vitamin supplementation trials. Am J Epidemiol. 2000;152:780–787. doi: 10.1093/aje/152.8.780. [DOI] [PubMed] [Google Scholar]
- 54.Simmons D, Upjohn M, Gamble GD. Can medication packaging improve glycemic control and blood pressure in type 2 diabetes? Results from a randomized controlled trial. Diabetes care. 2000;23:153–156. doi: 10.2337/diacare.23.2.153. [DOI] [PubMed] [Google Scholar]
- 55.Anton RF. New methodologies for pharmacological treatment trials for alcohol dependence. Alcohol Clin Exp Res. 1996;20:3A–9A. doi: 10.1111/j.1530-0277.1996.tb01183.x. [DOI] [PubMed] [Google Scholar]
- 56.Malcolm R, Kajdasz DK, Herron J, Anton RF, Brady KT. A double-blind, placebo-controlled outpatient trial of pergolide for cocaine dependence. Drug Alcohol Depend. 2000;60:161–168. doi: 10.1016/s0376-8716(99)00151-9. [DOI] [PubMed] [Google Scholar]
- 57.Farmer KC. Methods for measuring and monitoring medication regimen adherence in clinical trials and clinical practice. Clin Ther. 1999;21:1074–1090. doi: 10.1016/S0149-2918(99)80026-5. discussion 3. [DOI] [PubMed] [Google Scholar]
- 58.Matsuyama JR, Mason BJ, Jue SG. Pharmacists' interventions using an electronic medication-event monitoring device's adherence data versus pill counts. Ann Pharmacother. 1993;27:851–855. doi: 10.1177/106002809302700705. [DOI] [PubMed] [Google Scholar]
- 59.Carroll KM, Easton CJ, Nich C, Hunkele KA, Neavins TM, Sinha R, et al. The use of contingency management and motivational/skills-building therapy to treat young adults with marijuana dependence. J Consult Clin Psychol. 2006;74:955–966. doi: 10.1037/0022-006X.74.5.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kamon J, Budney A, Stanger C. A contingency management intervention for adolescent marijuana abuse and conduct problems. J Am Acad Child Adolesc Psychiatry. 2005;44:513–521. doi: 10.1097/01.chi.0000159949.82759.64. [DOI] [PubMed] [Google Scholar]
- 61.Stanger C, Budney AJ, Kamon JL, Thostensen J. A randomized trial of contingency management for adolescent marijuana abuse and dependence. Drug Alcohol Depend. 2009;105:240–247. doi: 10.1016/j.drugalcdep.2009.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Budney AJ, Moore BA, Rocha HL, Higgins ST. Clinical trial of abstinence-based vouchers and cognitive-behavioral therapy for cannabis dependence. J Consult Clin Psychol. 2006;74:307–316. doi: 10.1037/0022-006X.4.2.307. [DOI] [PubMed] [Google Scholar]
- 63.Gray KM, Carpenter MJ, Baker NL, Hartwell KJ, Lewis AL, Hiott DW, et al. Bupropion SR and contingency management for adolescent smoking cessation. Journal of substance abuse treatment. 2011;40:77–86. doi: 10.1016/j.jsat.2010.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Roll JM, Higgins ST. A within-subject comparison of three different schedules of reinforcement of drug abstinence using cigarette smoking as an exemplar. Drug Alcohol Depend. 2000;58:103–109. doi: 10.1016/s0376-8716(99)00073-3. [DOI] [PubMed] [Google Scholar]
- 65.Croft JR, Festinger DS, Dugosh KL, Marlowe DB, Rosenwasser BJ. Does size matter? Salience of follow-up payments in drug abuse research. Irb. 2007;29:15–19. [PubMed] [Google Scholar]
- 66.Festinger DS, Marlowe DB, Dugosh KL, Croft JR, Arabia PL. Higher magnitude cash payments improve research follow-up rates without increasing drug use or perceived coercion. Drug Alcohol Depend. 2008;96:128–135. doi: 10.1016/j.drugalcdep.2008.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Vandrey R, Bigelow GE, Stitzer ML. Contingency management in cocaine abusers: a dose-effect comparison of goods-based versus cash-based incentives. Experimental and clinical psychopharmacology. 2007;15:338–343. doi: 10.1037/1064-1297.15.4.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Olmstead TA, Petry NM. The cost-effectiveness of prize-based and voucher-based contingency management in a population of cocaine- or opioid-dependent outpatients. Drug Alcohol Depend. 2009;102:108–115. doi: 10.1016/j.drugalcdep.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.McCarty D, Fuller B, Kaskutas LA, Wendt WW, Nunes EV, Miller M, et al. Treatment programs in the National Drug Abuse Treatment Clinical Trials Network. Drug Alcohol Depend. 2008;92:200–207. doi: 10.1016/j.drugalcdep.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(Suppl 20):22–33. quiz 4–57. [PubMed] [Google Scholar]
- 71.Association AP. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Arlington, VA: American Psychiatric Publishing; 2013. [Google Scholar]
- 72.First MSR, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition. (SCID-I/P) New York: Biometrics Research, New York State Psychiatric Institute; 1994. [Google Scholar]
- 73.Wilens TE, Spencer TJ. Understanding attention-deficit/hyperactivity disorder from childhood to adulthood. Postgraduate medicine. 2010;122:97–109. doi: 10.3810/pgm.2010.09.2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zulauf CA, Sprich SE, Safren SA, Wilens TE. The complicated relationship between attention deficit/hyperactivity disorder and substance use disorders. Curr Psychiatry Rep. 2014;16:436. doi: 10.1007/s11920-013-0436-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Schmidt LE, Dalhoff K. Risk factors in the development of adverse reactions to N-acetylcysteine in patients with paracetamol poisoning. British journal of clinical pharmacology. 2001;51:87–91. doi: 10.1046/j.1365-2125.2001.01305.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Huestis MA, Cone EJ. Differentiating new marijuana use from residual drug excretion in occasional marijuana users. Journal of analytical toxicology. 1998;22:445–454. doi: 10.1093/jat/22.6.445. [DOI] [PubMed] [Google Scholar]
- 77.Schwilke EW, Gullberg RG, Darwin WD, Chiang CN, Cadet JL, Gorelick DA, et al. Differentiating new cannabis use from residual urinary cannabinoid excretion in chronic, daily cannabis users. Addiction. 2011;106:499–506. doi: 10.1111/j.1360-0443.2010.03228.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Moran CL, Le VH, Chimalakonda KC, Smedley AL, Lackey FD, Owen SN, et al. Quantitative measurement of JWH-018 and JWH-073 metabolites excreted in human urine. Analytical chemistry. 2011;83:4228–4236. doi: 10.1021/ac2005636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sobell LC, Sobell MB, Leo GI, Cancilla A. Reliability of a timeline method: assessing normal drinkers' reports of recent drinking and a comparative evaluation across several populations. British journal of addiction. 1988;83:393–402. doi: 10.1111/j.1360-0443.1988.tb00485.x. [DOI] [PubMed] [Google Scholar]
- 80.Mariani JJ, Brooks D, Haney M, Levin FR. Quantification and comparison of marijuana smoking practices: blunts, joints, and pipes. Drug Alcohol Depend. 2011;113:249–251. doi: 10.1016/j.drugalcdep.2010.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Allsop DJ, Norberg MM, Copeland J, Fu S, Budney AJ. The Cannabis Withdrawal Scale development: patterns and predictors of cannabis withdrawal and distress. Drug Alcohol Depend. 2011;119:123–129. doi: 10.1016/j.drugalcdep.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 82.Heishman SJ, Evans RJ, Singleton EG, Levin KH, Copersino ML, Gorelick DA. Reliability and validity of a short form of the Marijuana Craving Questionnaire. Drug and alcohol dependence. 2009;102:35–40. doi: 10.1016/j.drugalcdep.2008.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Heishman SJ, Singleton EG, Liguori A. Marijuana Craving Questionnaire: development and initial validation of a self-report instrument. Addiction. 2001;96:1023–1034. doi: 10.1046/j.1360-0443.2001.967102312.x. [DOI] [PubMed] [Google Scholar]
- 84.Stephens RS, Roffman RA, Curtin L. Comparison of extended versus brief treatments for marijuana use. Journal of consulting and clinical psychology. 2000;68:898–908. [PubMed] [Google Scholar]
- 85.Franken IH, Hendriksa VM, van den Brink W. Initial validation of two opiate craving questionnaires the obsessive compulsive drug use scale and the desires for drug questionnaire. Addictive behaviors. 2002;27:675–685. doi: 10.1016/s0306-4603(01)00201-5. [DOI] [PubMed] [Google Scholar]
- 86.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta psychiatrica Scandinavica. 1983;67:361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 87.Bjelland I, Dahl AA, Haug TT, Neckelmann D. The validity of the Hospital Anxiety and Depression Scale. An updated literature review. Journal of psychosomatic research. 2002;52:69–77. doi: 10.1016/s0022-3999(01)00296-3. [DOI] [PubMed] [Google Scholar]
- 88.Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry research. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 89.Trivedi MH, Wisniewski SR, Morris DW, Fava M, Gollan JK, Warden D, et al. Concise Health Risk Tracking scale: a brief self-report and clinician rating of suicidal risk. J Clin Psychiatry. 2011;72:757–764. doi: 10.4088/JCP.11m06837. [DOI] [PubMed] [Google Scholar]
- 90.Posner KBD, Lucas C, Gould M, Stanley B, Brown G, Fisher P, Zelazny J, Burke A, Oquendo M, Mann J. Columbia-Suicide Severity Rating Scale (C-SSRS) 2007 [Google Scholar]
- 91.Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. British journal of addiction. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- 92.Marzullo L. An update of N-acetylcysteine treatment for acute acetaminophen toxicity in children. Current opinion in pediatrics. 2005;17:239–245. doi: 10.1097/01.mop.0000152622.05168.9e. [DOI] [PubMed] [Google Scholar]
- 93.Mucomyst [package insert] New York City, NY: Bristol-Myers Squibb Company; 2007. [Google Scholar]
- 94.Bailey B, McGuigan MA. Management of anaphylactoid reactions to intravenous N-acetylcysteine. Annals of emergency medicine. 1998;31:710–715. doi: 10.1016/s0196-0644(98)70229-x. [DOI] [PubMed] [Google Scholar]
- 95.Food and Drug Administration. Investigational New Drug Application (IND) 2013 Apr [Google Scholar]
- 96.Hallgren KA, Witkiewitz K. Missing data in alcohol clinical trials: a comparison of methods. Alcohol Clin Exp Res. 2013;37:2152–2160. doi: 10.1111/acer.12205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Liang KY, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73:13–22. [Google Scholar]
- 98.Campbell AN, Nunes EV, Matthews AG, Stitzer M, Miele GM, Polsky D, et al. Internet-Delivered Treatment for Substance Abuse: A Multisite Randomized Controlled Trial. Am J Psychiatry. 2014 doi: 10.1176/appi.ajp.2014.13081055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Subramaniam GA, Warden D, Minhajuddin A, Fishman MJ, Stitzer ML, Adinoff B, et al. Predictors of abstinence: National Institute of Drug Abuse multisite buprenorphine/naloxone treatment trial in opioid-dependent youth. J Am Acad Child Adolesc Psychiatry. 2011;50:1120–1128. doi: 10.1016/j.jaac.2011.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Cropsey KL, Lane PS, Hale GJ, Jackson DO, Clark CB, Ingersoll KS, et al. Results of a pilot randomized controlled trial of buprenorphine for opioid dependent women in the criminal justice system. Drug Alcohol Depend. 2011;119:172–178. doi: 10.1016/j.drugalcdep.2011.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Dackis CA, Kampman KM, Lynch KG, Pettinati HM, O'Brien CP. A double-blind, placebo-controlled trial of modafinil for cocaine dependence. Neuropsychopharmacology. 2005;30:205–211. doi: 10.1038/sj.npp.1300600. [DOI] [PubMed] [Google Scholar]
- 102.Shoptaw S, Kintaudi PC, Charuvastra C, Ling W. A screening trial of amantadine as a medication for cocaine dependence. Drug Alcohol Depend. 2002;66:217–224. doi: 10.1016/s0376-8716(01)00205-8. [DOI] [PubMed] [Google Scholar]
- 103.Najavits LM, Gastfriend DR, Barber JP, Reif S, Muenz LR, Blaine J, et al. Cocaine dependence with and without PTSD among subjects in the National Institute on Drug Abuse Collaborative Cocaine Treatment Study. Am J Psychiatry. 1998;155:214–219. doi: 10.1176/ajp.155.2.214. [DOI] [PubMed] [Google Scholar]
- 104.Pan W. Akaike's information criterion in generalized estimating equations. Biometrics. 2001;57:120–125. doi: 10.1111/j.0006-341x.2001.00120.x. [DOI] [PubMed] [Google Scholar]
- 105.LaRowe SD, Kalivas PW, Nicholas JS, Randall PK, Mardikian PN, Malcolm RJ. A double-blind placebo-controlled trial of N-acetylcysteine in the treatment of cocaine dependence. Am J Addict. 2013;22:443–452. doi: 10.1111/j.1521-0391.2013.12034.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Grant JE, Odlaug BL, Kim SW. A double-blind, placebo-controlled study of N-acetyl cysteine plus naltrexone for methamphetamine dependence. European neuropsychopharmacology : the journal of the European College of Neuropsychopharmacology. 2010;20:823–828. doi: 10.1016/j.euroneuro.2010.06.018. [DOI] [PubMed] [Google Scholar]