SUMMARY
Presynaptic resting Ca2+ influences synaptic vesicle (SV) release probability. Here, we report that a TRPV channel, Inactive (Iav), maintains presynaptic resting [Ca2+] by promoting Ca2+ release from the endoplasmic reticulum in Drosophila motor neurons, and is required for both synapse development and neurotransmission. We find that Iav activates the Ca2+/calmodulin-dependent protein phosphatase, calcineurin, which is essential for presynaptic microtubule stabilization at the neuromuscular junction. Thus, loss of Iav induces destabilization of presynaptic microtubules resulting in diminished synaptic growth. Interestingly, expression of human TRPV1 in Iav-deficient motor neurons rescues these defects. We also show that the absence of Iav causes lower SV release probability and diminished synaptic transmission, whereas Iav overexpression elevates these synaptic parameters. Together, our findings indicate that Iav acts as a key regulator of synaptic development and function by influencing presynaptic resting [Ca2+].
INTRODUCTION
At any time, the Ca2+ concentration ([Ca2+]) within presynaptic terminals is a function of the complex interplay between events driving Ca2+ elevation and Ca2+ sequestration. Presynaptic Ca2+ elevation, which may occur due to depolarization-induced opening of voltage gated Ca2+ channels (VGCCs) triggers SV exocytosis (Catterall, 2000). While VGCCs are closed, the resting [Ca2+] is insufficient to trigger SV release, but influences SV release probability and sculpts the spatiotemporal dynamics of synaptic transmission (Awatramani et al., 2005; Zucker and Regehr, 2002). However, whether resting [Ca2+] is involved in other aspects of presynaptic function and whether specific non-excitatory channels set the resting [Ca2+] remain unknown.
We sought to evaluate the synaptic function of Ca2+ channels belonging to the Transient Receptor Potential (TRP) superfamily, which are voltage-independent channels that regulate diverse neuronal pathways (Venkatachalam and Montell, 2007). However, the biggest hurdle to evaluating the role of TRP channels in synapse development and function is the extent of functional redundancy between the different vertebrate TRP genes (Venkatachalam and Montell, 2007). This problem can be overcome by using Drosophila because flies express only 13 TRP genes compared to the 27 in vertebrates (Venkatachalam and Montell, 2007). Moreover, loss of function mutations in all 13 Drosophila TRP genes are available (Fowler and Montell, 2012). We found that loss of function mutations in a Drosophila TRPV channel gene, inactive (iav) (Gong et al., 2004), result in decreased synaptic growth and diminished neurotransmission. Our results indicate that Iav functions in motor neurons to regulate ER Ca2+ release and is required for maintaining presynaptic resting [Ca2+], which is essential for microtubule stability, synaptic growth, and SV release probability.
RESULTS
Inactive is required for synapse growth and morphology
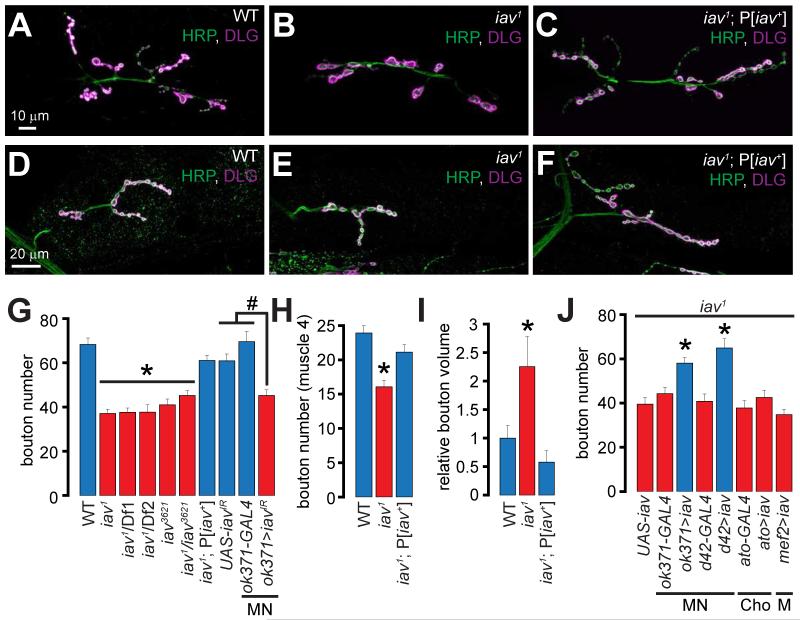
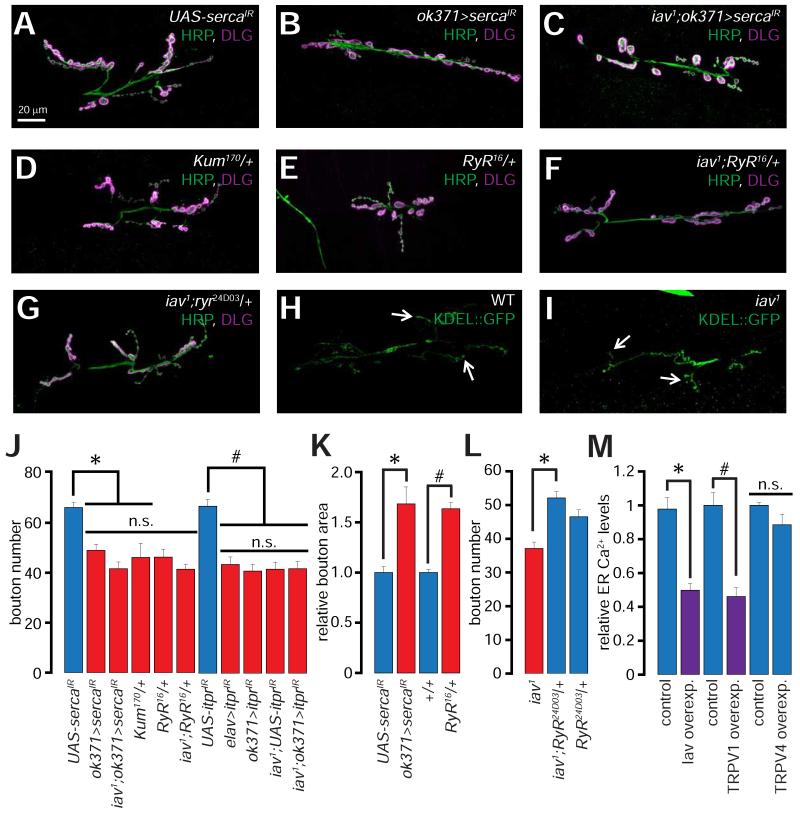
We determined the number of synaptic boutons at the Drosophila 3rd instar larval NMJs in wild-type and loss-of-function alleles of the TRP channel genes indicated in Supplemental table 1 using antibodies against HRP (Horseradish peroxidase: detects a carbohydrate moiety present on numerous neuronal glycoproteins (Snow et al., 1987) and DLG (Discs large: Drosophila ortholog of PSD-95 (Cho et al., 1992)). Only the larvae lacking iav (Gong et al., 2004) exhibit fewer synaptic boutons (muscles 6/7), which are 2-fold larger than control boutons (Supplemental table 1, Figures 1A-1B, 1G, and 1I). The iav mutants (iav1) also exhibit diminished bouton numbers at the NMJs on muscle 4 (Figures 1D-1E and 1H). The alterations in bouton numbers and size are rescued by a genomic wild-type iav transgene (P[iav+]) (Figures 1C, 1F and 1G-1I). The iav1/Df1, iav1/Df2, iav3621, and iav1 /iav3621 larvae (Gong et al., 2004) also show similar alterations in the bouton number and morphology (Figure 1G).
Figure 1. Alterations in synaptic growth and morphology in iav1.
(A-C) Confocal images of NMJs on muscles 6/7 from larvae of the indicated genotypes stained with antibodies against the presynaptic marker, HRP (green) and the postsynaptic marker, DLG (magenta). Scale bar shown in (A) also applies to (B-C).
(D-F) Confocal images of NMJs on muscle 4 from larvae of the indicated genotypes stained with antibodies against HRP (green) and DLG (magenta). Scale bar shown in (D) also applies to (E-F).
(G) Quantification of the number of boutons at NMJs on muscles 6/7 in larvae of the indicated genotypes. *, p < 0.0001, one-way ANOVA (comparing all the iav1 alleles with WT and iav1;P[iav+]; #, p = 2.5×10−5, one-way ANOVA (comparing ok371>iavIR with GAL4 and UAS controls); n=8-30 NMJs per genotype.
(H) Quantification of the number of boutons at NMJs on muscle 4 in larvae of the indicated genotypes. *, p = 6.3×10−6, one-way ANOVA (comparing all the data sets shown), n=8-14 NMJs per genotype.
(I) Quantification of the volume/bouton in larvae of the indicated genotypes. *, p = 0.007, one-way ANOVA (comparing all the data sets shown), n≥7 NMJs per genotype.
(J) Quantification of the bouton number in larvae of the indicated genotypes. *, p <10−6, one-way ANOVA (comparing the data sets shown in the blue bars with those in the red bars), n=11-20 NMJs per genotype.
All values represent mean ±SEM. Please consult Supplementary Files for values. Abbreviations: MN, motor neuron; Cho, chordotonal organ; M, muscle.
Iav functions in motor neurons to regulate NMJ synapse growth
To assess whether synaptic growth defects in iav1 are due to a requirement for Iav in motor neurons (MNs), we first expressed an RNAi against iav (UAS-iavIR) in MNs using the VGLUTok371-GAL4 (ok371-GAL4) driver. Expression of iavIR in wild-type MNs induces a significant decrease in NMJ bouton number—a phenotype not observed in the UAS/GAL4 controls (Figure 1G). Furthermore, expression of UAS-iav in the iav1 MNs using two separate drivers, ok371-GAL4 and d42-GAL4, rescues the synaptic growth defects (Figure 1J).
Iav is expressed in chordotonal neurons where it is required for hearing (Gong et al., 2004). However, we found that expression of UAS-iav in the iav1 chordotonal organs using ato-Gal4 (iav1; ato>iav) does not suppress the NMJ phenotype (Figure 1J). Expression of iav in mutant muscles (iav1; mef2>iav) also does not restore the bouton number in iav1 (Figure 1J). These data indicate that Iav functions cell-autonomously in MNs to drive synaptic growth.
Human TRPV1, but neither human TRPV4 nor Drosophila Nanchung, rescue the iav1 synaptic growth phenotype
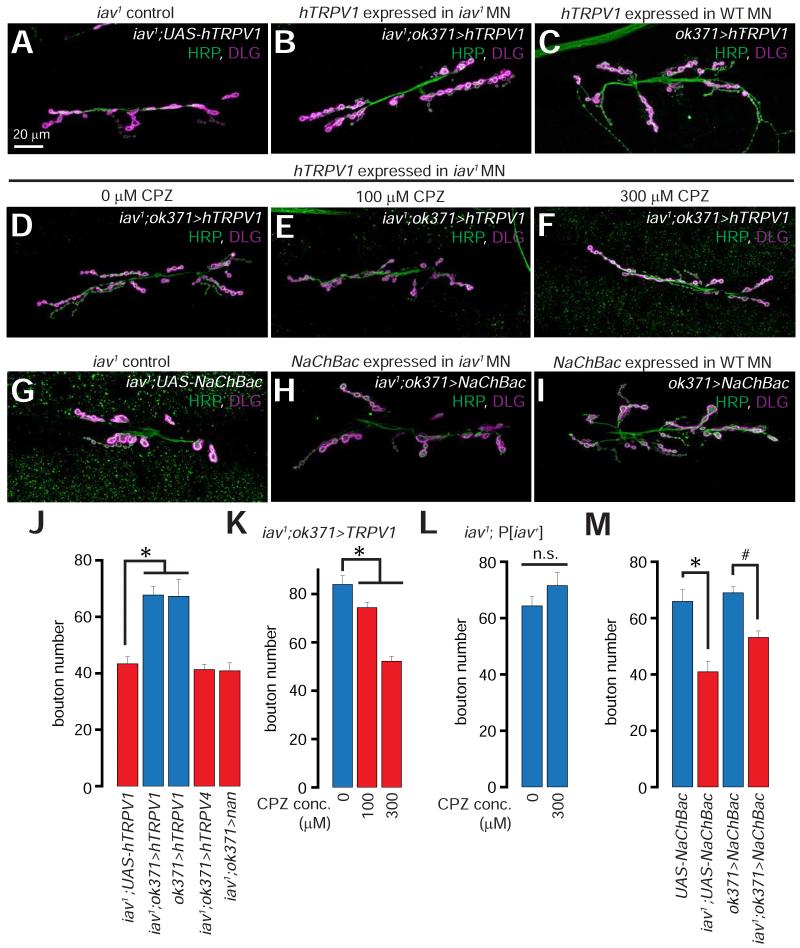
Within the hTRPV channel subfamily (hTRPV1-6, 25-27% identity and 39-46% similarity with Iav), hTRPV1 and hTRPV4 are the most extensively studied channels (Caterina et al., 2000; Caterina et al., 1999; Güler et al., 2002; Watanabe et al., 2002), whose activity can be manipulated by a host of pharmacological agents (Watanabe et al., 2002; Xia et al., 2011a; Xia et al., 2011b). Here, we asked whether expression of either hTRPV1 of hTRPV4 in iav1 MNs would suppress the synaptic growth defects (see Figure S1 for a comparison of the amino acid sequences of Iav, hTRPV1, and hTRPV4). Remarkably, expression of hTRPV1 in the iav1 MNs (iav1; ok371>hTRPV1) suppresses the diminished synaptic growth (Figures 2A-2B, and 2J). However, expression of hTRPV1 in wild-type MNs (ok371>hTRPV1) does not promote the formation of additional boutons (Figures 2C and 2J). We also pharmacologically inhibited the hTRPV1 channels expressed in the iav1 MNs by feeding the iav1; ok371>hTRPV1 larvae the TRPV1 antagonist, capsazepine (CPZ) (Zygmunt et al., 1999). CPZ reverses the hTRPV1-mediated suppression of the iav1 synaptic growth phenotype in a dose-dependent manner (Figures 2D-2F and 2K), whereas even 300 μM of CPZ does not decrease synaptic growth in control iav1; P[iav+] neurons that lack hTRPV1 (Figure 2L). In contrast, expression of neither hTRPV4 nor Drosophila Nanchung (Nan—the second TRPV gene in Drosophila (Kim et al., 2003)) in the iav1 MNs suppresses the synaptic growth defects (Figure 2J).
Figure 2. Suppression of iav1 synaptic growth defects by presynaptic expression of human TRPV1, but not human TRPV4.
(A-C) Confocal images of larval NMJs from larvae of the indicated genotypes stained with antibodies against HRP (green) and DLG (magenta).
(D-F) Confocal images of larval NMJs from larvae of the indicated genotypes that were fed the indicated concentrations of capsazepine (CPZ) stained with antibodies against HRP (green) and DLG (magenta).
(G-I) Confocal images of NMJs from larvae of the indicated genotypes stained with antibodies against HRP (green) and DLG (magenta).
Scale bar shown in (A) also applies to (B-I).
(J) Quantification of the NMJ bouton number in larvae of the indicated genotypes. *, p = 5.2×10−4, one-way ANOVA, n = 9-12 NMJs per genotype.
(K-L) Quantification of the NMJ bouton numbers in larvae of the indicated genotypes that were fed the indicated concentrations of CPZ. The overall increased baseline of the bouton number in all the genotypes here was a result of raising the flies on instant food. In (K), *, p = 10−9, one-way ANOVA, n = 14-28 NMJs per genotype. In (L), n.s. represents “not significant”, p = 0.22, unpaired Student’s t-test, n = 15 NMJs per genotype.
(M) Quantification of the NMJ bouton number in larvae of the indicated genotypes. *, p = 4.4×10−4, unpaired Student’s t-test, n = 8-9 NMJs per genotype; #, p = 1.3×10−5, unpaired Student’s t-test, n = 18-24 NMJs per genotype.
All values represent mean ±SEM. Abbreviations: WT, wild-type; MN, motor neuron; n.s., not significant.
To test whether elevating MN neuronal activity is sufficient to suppress the iav1 synaptic growth phenotype, we expressed a constitutively active bacterial Na+ channel, NaChBac (Kuzmenkin et al., 2004; Luan et al., 2006), in the iav1 MNs. NaChBac depolarizes the host neurons and decreases their threshold for firing action potentials (Kuzmenkin et al., 2004). In contrast to the rescue we observed with expression of hTRPV1, the iav1 NMJ synaptic growth defects persist following the expression of NaChBac (Figures 2G-2I, and 2M). These data indicate that the synaptic growth phenotype at the iav1 NMJs depends on the selective loss of TRPV channel activity rather than diminished MN excitability.
Decreased microtubule stability in iav1 motor neurons
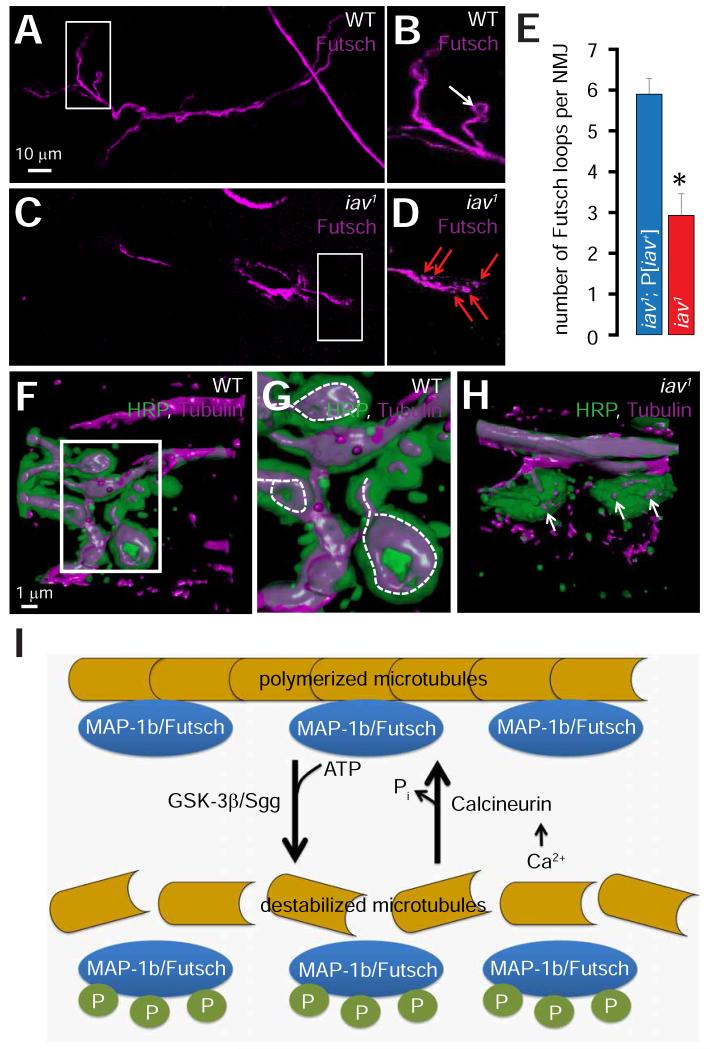
The decrease in synaptic bouton number with a concomitant increase in bouton volume in iav1 is reminiscent of the phenotype observed in larvae lacking genes such as wingless (Miech et al., 2008; Packard et al., 2002), vapb (encoding Vesicle Associated Membrane Protein-B) (Nishimura et al., 2004; Pennetta et al., 2002), futsch (the fly ortholog of the gene encoding mammalian microtubule associated protein-1b (MAP-1b)) (Roos et al., 2000; Zhang et al., 2001), and pp2A-B’ (a subunit of the PP2A protein phosphatase) (Viquez et al., 2006). Because diminished stability of presynaptic microtubules underlies the synaptic defects in these mutants, we assessed the structure of the presynaptic microtubules in iav1. First, we found that the number of the characteristic Futsch loops is reduced by ~50% within iav1 synapses compared to controls (Figures 3A-3E). Moreover, in contrast to the Futsch loops within control boutons (Figure 3B, arrow points to a Futsch loop), Futsch appears punctate within some iav1 boutons (Figure 3D, red arrows point to Futsch punctae). Next, 3D reconstructions of synaptic boutons stained with an anti-Tubulin antibody revealed the characteristic loop-like structures that microtubules form within wild-type boutons (Figures 3F and dashed lines in 3G). However, microtubules appear fragmented in iav1 boutons (Figure 3H, arrows) further indicating that the presynaptic microtubules exhibit diminished stability at the iav1 NMJs.
Figure 3. Disruption of the presynaptic microtubule cytoskeleton in iav1.
(A) Confocal image of an NMJ from wild-type (WT) larvae stained with antibodies against Futsch (magenta).
(B) Magnification of the boxed region in (A). White arrow points to a synaptic Futsch loop.
(C) Same as (A) but in iav1 larvae. Scale bar shown in (A) also applies to (C).
(D) Same as (B) but in iav1 larvae. Red arrows point to synaptic Futsch punctae.
(E) Quantification of the number of Futsch loops per NMJ in the indicated genotypes. *, p = 3.6×10−4, Student’s t-test, n = 10-14 NMJs per genotype.
(F) 3-D reconstruction of a wild-type (WT) synaptic bouton stained with antibodies against HRP (green) and tubulin (magenta).
(G) Magnification of the boxed region in (F). Dashed-lines represent synaptic microtubule loops.
(H) Same as (F) but in iav1 larvae. Arrows point to fragmented microtubules. Scale bar shown in (F) also applies to (H).
(I) Schematic depicting the role of Futsch phosphorylation status on the regulation of presynaptic microtubule stability.
All values represent mean ±SEM. Abbreviations: MAP-1b, microtubule associated protein-1b; P, phosphorylation.
Diminished calcineurin activity underlies the synaptic growth defects in iav1
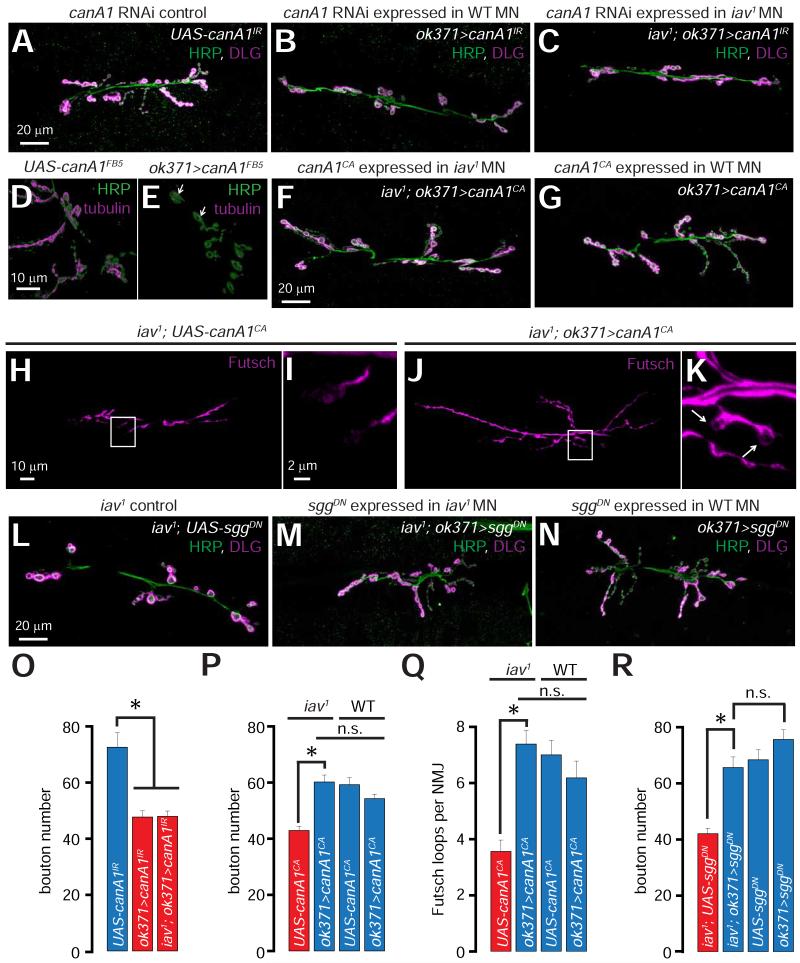
The stability of presynaptic microtubules depends on the level and/or phosphorylation status of Futsch (Franco et al., 2004; Miech et al., 2008; Packard et al., 2002; Roos et al., 2000) (Figure 3I). Thus, futsch-deficient larvae exhibit diminished bouton numbers and increased bouton size (Roos et al., 2000)—a phenotype similar to that observed in iav1. The Drosophila homolog of glycogen synthase kinase-3β (GSK-3β), Shaggy (Sgg), phosphorylates Futsch, causing the latter to dissociate from microtubules (Franco et al., 2004; Gogel et al., 2006; Miech et al., 2008) (Figure 3I). Following the dissociation of Futsch, microtubules become destabilized and fragmented (Figure 3I) resulting in the formation of fewer boutons, which are increased in size (Franco et al., 2004; Miech et al., 2008). The protein phosphatase, calcineurin, counteracts the kinase activity of GSK-3β by dephosphorylating microtubule-associated proteins resulting in microtubule stabilization (Figure 3I) (Gong et al., 2000a; Gong et al., 2000b). Because calcineurin is a Ca2+/calmodulin-activated protein phosphatase (Lynch and Michalak, 2003; Rinne et al., 2009), we hypothesized that loss of Iav-dependent cytosolic Ca2+ elevations may result in diminished calcineurin activity, which in-turn may affect microtubule stability (Figure 3I). Indeed, knocking-down Drosophila calcineurin (canA1) in wild-type MNs using two RNAi lines (UAS-canA1IR and UAS-canA1FB5) (Dijkers and O’Farrell, 2007) results in decreased number of synaptic boutons compared to controls (Figures 4A-4B and 4O and S2A-S2B and S2G). Furthermore, larvae that carried the canA1 loss of function allele (Nakai et al., 2011) in trans with a deficiency uncovering canA1 locus (canA1−/Df-canA1) exhibit decreased bouton numbers compared to the canA1−/+ heterozygotes (Figures S2C-S2D and S2G). However, MN specific knockdown of canA1 in iav1 does not further decrease synaptic growth (Figures 4C and 4O), suggesting that canA1 and iav may function in a common pathway. Loss of CanA1 also results in an increase in bouton area (Figure S2H) and destabilization of presynaptic microtubules (Figures 4D-4E, white arrows in Figure 4E point to fragmented tubulin within boutons). These data indicate that presynaptic loss of calcineurin decreases synaptic growth, increases the size of the boutons, and causes presynaptic microtubule destabilization at the larval NMJ—phenotypes that bear striking resemblance to those displayed by iav1.
Figure 4. Role of calcineurin in the iav1 synaptic growth phenotype.
(A-C) Confocal images of NMJs from larvae of the indicated genotypes stained with antibodies against HRP (green) and DLG (magenta). Scale bar shown in (A) also applies to (B-C).
(D-E) 3-D reconstruction of synaptic boutons from larvae of the indicated genotypes stained with antibodies against HRP (green) and tubulin (magenta). Please note that only the microtubules within the NMJ boutons are shown by applying an “HRP mask” (see Experimental Procedures). Scale bar shown in (D) also applies to (E).
(F-G) Confocal images of NMJs from larvae of the indicated genotypes stained with antibodies against HRP (green) and DLG (magenta). Scale bar shown in (F) also applies to (G).
(H and J) Confocal images of NMJs from larvae of the indicated genotypes stained with antibodies against Futsch (magenta). Scale bar shown in (H) also applies to (J).
(I and K) Magnification of the boxed regions in (H) and (J) respectively. Scale bar shown in (I) also applies to (K). Arrows in (K) point to Futsch loops.
(L-N) Confocal images of NMJs from larvae of the indicated genotypes stained with antibodies against HRP (green) and DLG (magenta). Scale bar shown in (L) also applies to (M-N).
(O) Quantification of the NMJ bouton number in larvae of the indicated genotypes. *, p = 4.2×10−6, one-way ANOVA, n = 12-16 NMJs per genotype.
(P) Quantification of the NMJ bouton number in larvae of the indicated genotypes. Horizontal bars above the graph indicate data from WT and iav1. *, p = 2.9×10−6, unpaired Student’s t-test, n.s. represents p>0.05, n = 16-18 NMJs per genotype
(Q) Quantification of the number of Futsch loops per NMJ in larvae of the indicated genotypes. Horizontal bars above the graph indicate data from WT and iav1. *, p = 1.8×10−6, Student’s t-test, n.s. represents p>0.05, n = 13-16 NMJs per genotype.
(R) Quantification of the NMJ bouton number in larvae of the indicated genotypes. *, p = 1.4×10−6, Student’s t-test, n.s. represents p>0.05, n = 10-18 NMJs per genotype.
All values represent mean ±SEM. Abbreviations: MN, motor neuron; WT, wild-type; n.s., not significant.
We also found that expression of a constitutively active CanA1, which does not require elevations in cytosolic Ca2+ to be fully active (UAS-canA1CA (Dijkers and O’Farrell, 2007)), in the iav1 MNs suppresses the synaptic growth defects (Figures 4F and 4P), whereas expression of CanA1CA in wild-type MNs does not affect the overall bouton numbers (Figures 4G and 4P). Expression of CanA1CA in the iav1 MNs also restores the bouton size (Figure S2H) and the number of Futsch loops within the NMJ boutons (Figures 4H-4K and 4Q), but does not affect the number of Futsch loops in wild-type animals (Figure 4Q). However, overexpression of CanA1CA in the MNs of the futsch hypomorphs, futschN94 (Roos et al., 2000), does not suppress the observed synaptic growth deficits (Figure S2I). These epistatic analyses indicate that futsch functions downstream of canA1, which in turn functions downstream of iav, in the regulation of synaptic growth.
Finally, if calcineurin function is decreased in iav1, lowering the activity of the counteracting kinase, Sgg, may suppress the iav1 synaptic growth phenotype. Indeed, expression of dominant negative sgg (sggDN) in the iav1 MNs also suppresses the synaptic growth defects (Figures 4L-4N and 4R). Together, these findings indicate that diminished calcineurin activity underlies the synaptic growth defects observed in iav1.
ER Ca2+ release regulates NMJ synapse morphology and development
Because calcineurin function is promoted by ER Ca2+ release (Lynch and Michalak, 2003; Rinne et al., 2009)), we first assessed whether ER Ca2+ release plays a role in NMJ synapse development. If so, depleting the MN ER Ca2+ stores may alter the number and size of the NMJ boutons. Indeed, RNAi-mediated knockdown of the ER Ca2+ pump, SERCA (ok371> sercaIR), which is required for maintaining Ca2+ levels within the ER lumen (Dormer et al., 1993; Sanyal et al., 2005)), leads to decreased synaptic growth (Figures 5A-5B and 5J). Similar results were obtained with Kum170, a dominant-negative SERCA allele (Sanyal et al., 2005) (Figures 5D and 5J). Knockdown of SERCA in MNs also results in an increase in the size of NMJ boutons (Figure 5K)—morphological alterations reminiscent of the iav1 synapses.
Figure 5. Role of ER Ca2+ release in NMJ synapse development.
(A-G) Confocal images of NMJs from larvae of the indicated genotypes stained with antibodies against HRP (green) and DLG (magenta).
(H-I) Confocal images of NMJs from larvae of the indicated genotypes expressing Lysozyme-KDEL::GFP (KDEL-GFP) stained with antibodies against GFP (green). Arrows point to distal boutons.
Scale bar shown in (A) also applies to (B-G).
(J) Quantification of the NMJ bouton numbers in larvae of the indicated genotypes. *, p = 5.5×10−9, one-way ANOVA (comparing the indicated data sets), n = 9-27 NMJs per genotype; #, p = 1.3×10−8, one-way ANOVA (comparing the indicated data sets), n = 13-14 NMJs per genotype.
(K) Quantification of the relative bouton area in larvae of the indicated genotypes. *, p = 6.4×10−4, unpaired Student’s t-test, n = 9-11 NMJs per genotype. #, p = 6.2×10−10, unpaired Student’s t-test, n = 14-16 NMJs per genotype.
(L) Quantification of the NMJ bouton numbers in larvae of the indicated genotypes. *, p = 3×10−6, unpaired Student’s t-test, n = 17-21 NMJs per genotype.
(M) Quantification of the relative ER Ca2+ levels as assessed by fura-2 imaging in control N2A cells and in N2A cells overexpressing (overexp.) the indicated TRPV channel. *, p = 3.4×10−10, unpaired Student’s t-test, n = 8-9 independent coverslips containing control or iav transfected cells (number of cells per field ≥8). #, p = 4×10−5, unpaired Student’s t-test, n = 8 independent coverslips containing control or TRPV1 transfected cells (number of cells per field ≥10).
All values represent mean ±SEM. Abbreviations: WT, wild-type; n.s., not significant.
Next, we examined the effects of lowering ER Ca2+ release by decreasing the levels of the genes encoding the ER Ca2+ release channels, ryanodine receptor and inositol trisphosphate receptor (RyR and itpr respectively) (Hasan and Rosbash, 1992). Loss of a single copy of RyR (RyR16/+), which results in diminished ER Ca2+ release (Sullivan et al., 2000), leads to a decrease in the number and an increase in the size of the NMJ boutons (Figures 5E and 5J-5K). Similarly, presynaptic expression of an RNAi against itpr (UAS-itprIR) using pan-neuronal or MN specific drivers (elav>itprIR and ok371>itprIR respectively) leads to reduced NMJ bouton numbers (Figure 5J). Importantly, none of these manipulations enhance the iav1 synaptic phenotypes (Figures 5C, 5F, and 5J; n.s., p > 0.05, one-way ANOVA). We also asked whether promoting ER Ca2+ release in iav1 would suppress the synaptic growth defects. To promote ER Ca2+ release we used the RyR24D03/+ flies that carry one extra copy of the RyR gene and exhibit elevated ER Ca2+ release (Gao et al., 2013). Remarkably, introduction of RyR24D03/+ leads to a partial suppression of the iav1-associated alterations in bouton numbers (Figures 5G and 5L). These data indicate that the iav1 synaptic growth defects arise as a result of diminished Ca2+ release from the ER.
Because the MN ER traverses the axons and is found at the NMJs, defects in extension of ER into the axon terminus may also result in decreased Ca2+ release. Thus, we evaluated ER distribution in the iav1 NMJs by expressing the ER marker, Lysozyme-KDEL::GFP (using UAS-Lyso::GFP-KDEL; herein referred to as KDEL-GFP). Consistent with previous observations (Chouhan et al., 2010), we found that ER is distributed throughout the MN axons, and is also located within some, but not all, of the synaptic boutons (Figure 5H; arrows indicate ER in distal boutons). Despite an obvious decrease in the total number of boutons, presynaptic KDEL-GFP distribution is largely unchanged in iav1 compared to controls (Figure 5I; arrows indicate ER in distal boutons). Therefore, ER trafficking to the axon terminal is not significantly altered in iav1.
Expression of channels that promote ER Ca2+ release results in a partial depletion of the ER Ca2+ stores in cultured cells (Wegierski et al., 2009). We evaluated whether Iav, TRPV1, and TRPV4 expression in Neuro2A (N2A) cells results in lower ER Ca2+ levels. Expression of either Iav or TRPV1 in N2A cells results in a ~50% decrease in ER Ca2+ levels (Figure 5M). However, expression of TRPV4 does not affect the ER Ca2+ content (Figure 5M). Together, these data are consistent with a role for Iav and TRPV1 in regulating ER Ca2+ release, which is essential for synaptic development.
Iav and hTRPV1, but not hTRPV4, localize to the ER
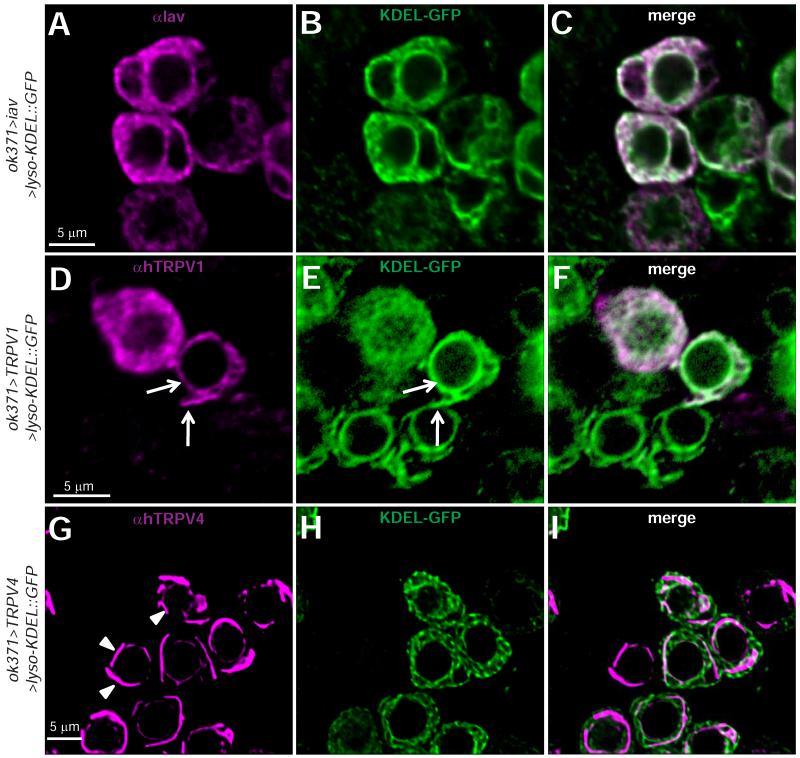
Next, we sought to identify the subcellular distribution of Iav and TRPV1 in larval MNs. Although we could detect Iav in larval chordotonal organs using established antibodies (Gong et al., 2004) (Figure S3A), we could not detect Iav in wild-type larval MNs using these antibodies. These findings are consistent with the difficulties in observing the native expression patterns of some Ca2+ channels owing to their low expression levels (Ly et al., 2008; Venkatachalam et al., 2008). Therefore, we evaluated whether Iav overexpression, which suppresses the iav1 phenotypes, would reveal the protein’s subcellular distribution. We found that overexpressed Iav colocalized with KDEL::GFP in MN cell bodies (Figures 6A-6C) and muscle (Figure S3B). Using anti-TRPV1 antibodies (Tominaga et al., 1998), we found that hTRPV1 also colocalizes with KDEL-GFP in the MN cell bodies (Figures 6D-6F). In contrast, hTRPV4 overexpressed in MNs does not show overlap with KDEL-GFP (Figures 6G-6I, arrowheads point to hTRPV4 expression). Hence, both Iav and hTRPV1, which suppress the NMJ defects observed in iav1, are expressed in the ER, whereas hTRPV4, which does not suppress the iav1 NMJ growth defects, is not localized to the ER.
Figure 6. Subcellular distribution of Iav, hTRPV1, and hTRPV4 in Drosophila larval motor neurons.
(A-C) Confocal images of MN cell bodies in ventral nerve cord dissected from larvae expressing Iav and KDEL-GFP in motor neurons stained with αIAV (A, magenta), αGFP (B, green), and merge (C). Scale bar shown in (A) also applies to (B-C).
(D-F) Same as (A-C) but with larvae overexpressing hTRPV1 instead of Iav. An antibody against hTRPV1 was used. Scale bar shown in (D) also applies to (E-F). Arrows indicate colocalization between hTRPV1 and KDEL-GFP in the nuclear envelope and other regions of the ER.
(G-I) Same as (A-C) but with larvae overexpressing hTRPV4 instead of Iav. An antibody against hTRPV4 was used. Scale bar shown in (G) also applies to (H-I). The arrowheads point to “tubular” structures that are decorated by hTRPV4 in motor neuron cell bodies.
Loss of Iav results in decreases in presynaptic resting [Ca2+]
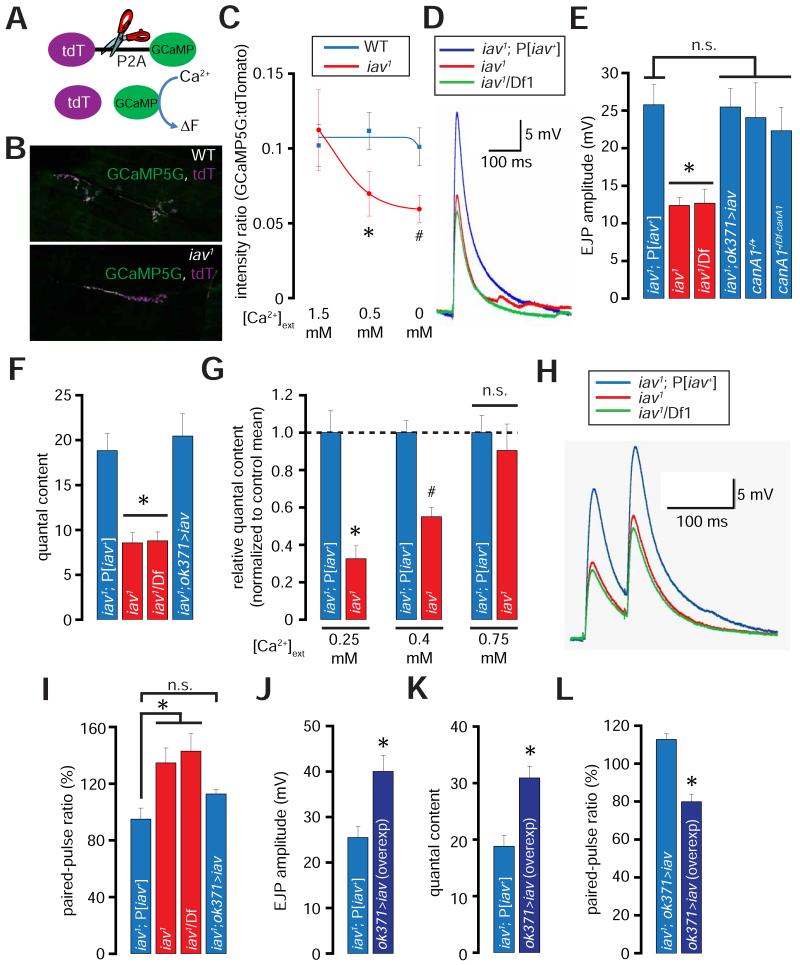
Next, we asked whether loss of Iav results in diminished cytosolic [Ca2+] at NMJ termini. To evaluate resting [Ca2+] within presynaptic boutons, we expressed GCaMP5G (GCaMP) (Akerboom et al., 2012) linked via a 2A peptide (P2A) to tdTomato (tdT)—a Ca2+ insensitive fluorescent protein (Figure 7A) (Daniels et al., 2014). The 2A peptide is cleaved by native endoproteases in Drosophila neurons (Inagaki et al., 2012), leading to the separation of GCaMP5G from tdTomato—both of which are expressed at the NMJ (Figure 7B). Because the two fluorophores are translated as a single polypeptide, the levels of tdTomato can be used to normalize the levels of GCaMP5G. This ratiometric normalization is important for determining resting [Ca2+], which does not involve the measurement of robust alterations from baseline. Our analysis revealed that the relative ratio of the GCaMP5G:tdTomato fluorescence intensities in WT and iav1 are comparable when the recordings are performed in extracellular bath solution containing 1.5 mM [Ca2+] (Figure 7C). However, when the bath [Ca2+] over the same NMJs is dropped to 0.5 mM and 0 mM, the intensity ratios show a significant decline in iav1 boutons (Figure 7C). Remarkably, the intensity ratio at WT NMJs does not change even after 9 minutes in a solution containing no Ca2+. These data indicate that the resting cytosolic [Ca2+] at NMJ bouton terminals is sustained by release of Ca2+ from intracellular stores and the ability of these stores to maintain presynaptic resting [Ca2+] is compromised in iav1.
Figure 7. Diminished presynaptic resting Ca2+ levels and neurotransmission at the iav1 synapses.
(A) Structure of tdTomato-P2A-GCaMP5 (tdT-P2A-GCaMP). Cleavage at the P2A site (indicated by scissors) disengages the two fluorophores.
(B) Confocal images showing the expression of GCaMP5 and tdTomato (tdT) at the NMJ in larvae of the indicated genotypes.
(C) Quantification of the GCaMP5:tdTomato intensity ratios in wild-type (blue line) and iav1 (red line) at the indicated [Ca2+]ext. The blue and red curves were obtained by fitting the respective mean values to sigmoidal functions using Origin6 (OriginLab corporation). *, p = 0.04, unpaired Student’s t-test, n = 7-8 NMJs per genotype; #, p = 0.02, unpaired Student’s t-test, n = 7-8 NMJs per genotype.
(D) EJP traces obtained from recordings performed on larval NMJs of the indicated genotypes ([Ca2+]ext=0.5 mM).
(E) Quantification of the amplitude of the EJPs obtained from recordings performed on larvae of the indicated genotypes. *, p = 5.1×10−4, one-way ANOVA (comparing all the data sets shown), n = 6-8 NMJs per genotype.
(F) Quantification of the quantal contents obtained from recordings performed on larvae of the indicated genotypes. *, p = 1.1×10−5, one-way ANOVA (comparing all the data sets shown), n = 6-8 animals per genotype.
(G) Quantification of the relative quantal contents in iav1 normalized to the iav1; P[iav+] means at the indicated [Ca2+]ext. *, p = 3.5×10−4, unpaired Student’s t-test, n = 5-7 NMJs per genotype; #, p = 5.3×10−5, unpaired Student’s t-test, n = 7-10 NMJs per genotype.
(H) EJP trace obtained from paired-pulse recordings performed on larval NMJs of the indicated genotypes ([Ca2+]ext=0.5 mM).
(I) Quantification of the paired-pulse ratio (% change in the amplitude of the second EJP to that of the first EJP when the two were separated by duration of 50 ms) in the larvae of the indicated genotypes. *, p = 0.01, one-way ANOVA, n = 5-7 animals per genotype.
(J) Quantification of the EJP amplitudes in larvae of the indicated genotypes. *, p = 0.01, Student’s t-test, n = 6 NMJs per genotype.
(K) Quantification of the quantal content in larvae of the indicated genotypes. *, p = 0.002, unpaired Student’s t-test, n = 6 NMJs per genotype.
(L) Quantification of the paired-pulse ratio in larvae of the indicated genotypes. *, p = 1.5×10−4, unpaired Student’s t-test, n = 5-6 NMJs per genotype.
All values represent mean ±SEM. Abbreviations: WT, wild-type; n.s., not significant.
Loss of Iav results in diminished synaptic transmission and SV release probability
We hypothesized that owing to the critical roles of presynaptic Ca2+ in SV release and synaptic transmission (Jahn and Fasshauer, 2012), evoked excitatory junctional potentials (EJPs) may be diminished at iav1 NMJs. Indeed, at 0.5 mM extracellular [Ca2+] ([Ca2+]ext), the EJP amplitudes are ~50% lower at the iav1 NMJs compared to controls (Figures 7D-7E). However, canA1 deficient NMJs, which also show a decrease in the number of synaptic boutons, do not exhibit a corresponding decrease in EJP amplitude (Figure 7E). Thus, although Iav regulates NMJ growth and morphology via calcineurin, Iav regulates synaptic transmission independent of calcineurin.
We also found that the amplitude and frequency of spontaneous mini-EJPs (mEJPs) are unchanged in iav1 (Figures S4A-S4B). Hence, the quantal content (EJP amplitude/mEJP amplitude), indicative of the number of SVs released per evoked event, is ~50% decreased in iav1 (Figure 7F). The diminished EJP and quantal content in iav1 are rescued by expression of UAS-iav in MNs (Figures 7E-7F).
If the decreased quantal content at iav1 NMJs is a consequence of a decrease in presynaptic resting [Ca2+], raising [Ca2+]ext during neurotransmission may restore the quantal content. To test this hypothesis, we recorded quantal contents at [Ca2+]ext concentrations of 0.25 mM, 0.4 mM, and 0.75 mM. We found that the relative decrease in quantal content in iav1 is most severe at 0.25 mM [Ca2+]ext (~70% lower than in controls), and becomes progressively less pronounced at higher [Ca2+]ext (Figures S4C and 7G). At 0.75 mM [Ca2+]ext, the quantal content in iav1 is not significantly different than in controls (Figures S4C and 7G, p = 0.6, unpaired Student’s t-test, n = 4-5 NMJs per genotype). These data indicate that the synaptic transmission defects in iav1 are primarily a consequence of diminished presynaptic resting Ca2+ levels, which can be suppressed by increasing the amounts of Ca2+ entering the boutons through the VGCC.
Examination of the presynaptic ultrastructure by electron microscopy revealed that the iav1 NMJs do not display significant alterations in other parameters involved in regulating SV release (Figures S5A-S5C). Previous studies have found a 3rd-4th order dependence of neurotransmitter release upon [Ca2+]ext, and this cooperativity is decreased in mutations affecting the levels of the SV Ca2+ sensor, Synaptotagmin (Jan and Jan, 1976; Littleton et al., 1994). The Ca2+ cooperativity also reflects the number of VGCCs participating in the release of a single SV (Matveev et al., 2011). We found that Ca2+ cooperativity of neurotransmitter release in both controls and iav1 larvae, as determined by the slopes of the double-logarithmic plots of quantal content and [Ca2+]ext (Jan and Jan, 1976; Littleton et al., 1994) remains unchanged (Figure S4C, slopes in control and iav1 are 3.4 and 3.5 respectively). Taken with the lack of alterations in SV number or distribution in iav1, these data argue against a role for an exocytic block in the iav1 neurotransmission defects.
Cytosolic Ca2+ concentration at axon terminals influences SV release probability, which can be evaluated using the paired-pulse ratio of evoked potentials (Zhang et al., 2009; Zucker and Regehr, 2002). When the resting [Ca2+] at a synapse is low, stimulus-induced elevation in presynaptic [Ca2+] will induce the exocytosis of relatively fewer synaptic vesicles resulting in a smaller evoked EJP. However, if a second stimulus, i.e. the paired-pulse, is provided before the resting Ca2+ returns to baseline, the presynaptic Ca2+ will elevate sufficiently leading to the exocytosis of the remaining SVs. Therefore, the higher the ratio of amplitudes of evoked responses following the first and second pulses respectively (known as paired pulse facilitation), the lower is the probability of SV release, and vice versa. Consistent with the observation that the iav1 NMJ synapses have reduced SV release probability, the paired-pulse ratio is increased by ~50% at iav1 NMJs (Figures 7H-7I). Expression of UAS-iav in the iav1 MNs restores the SV release probability (Figure 7I).
Overexpression of Iav in wild-type MNs results in increased SV release probability and neurotransmission
If Iav is a determinant of presynaptic resting Ca2+ levels, raising its expression level may sensitize the synapse to releasing more SVs. Indeed, overexpression of iav in wild-type MNs leads to a ~50% increase in EJP amplitude and quantal content (Figures 7J-7K). We also found that the elevation in EJP amplitudes following iav overexpression is a consequence of increased SV release probability as determined by a decrease in the paired-pulse ratio (Figure 7L). Interestingly, overexpression of iav in MNs also results in the formation of fewer synaptic boutons indicating that the number of synaptic boutons at the larval NMJ exhibits a bell-shaped dependence on the expression levels of Iav (Figure S5H). We also found that overexpression of RyR or Itpr also leads to the formation of fewer synaptic boutons at the Drosophila NMJ (data not shown). Thus, the consequence of iav overexpression on the number of synaptic boutons is likely due to an increase in presynaptic Ca2+ rather than a specific effect of Iav per se. However, overexpression of iav in the MNs does not lead to an increase in the total number of punctae formed by the active zone (AZ) specific structural protein, Bruchpilot (Brp) (Kittel et al., 2006) (Figure S5I, p = 0.4, unpaired Student’s t-test). Therefore, SV release increases following iav overexpression are a consequence of elevated resting Ca2+ levels rather than due to alterations in the number of SV release sites.
DISCUSSION
Iav-mediated calcineurin activation regulates presynaptic microtubule stability, bouton morphology, and bouton numbers
Here we show that Iav functions in larval MNs to regulate the development and function of the NMJ. Although expression UAS-iav in the iav1 MNs rescues the defects in NMJ growth and synaptic transmission, this does not alter the iav1 locomotion defects (data not shown). In contrast, expression of UAS-iav in the iav1 chordotonal neurons suppresses the locomotion defects (data not shown) but does not impact the NMJ phenotypes. Thus, the iav1 NMJ and proprioceptive phenotypes arise separately in the MNs and chordotonal neurons respectively.
The phenotype of diminished synaptic bouton numbers with an increase in bouton size observed in iav1 occurs due to destabilization of presynaptic microtubules. Furthermore, our investigation revealed that in the absence of Iav, presynaptic microtubule stability and bouton numbers are diminished due to decreased calcineurin activity. Thus, constitutively active calcineurin suppresses these iav1 phenotypes. However, expression of the constitutively active calcineurin does not suppress the synaptic growth defects in the futsch hypomorphs, indicating that futsch functions downstream of calcineurin. Previous studies have also implicated the other Ser/Thr protein phosphatase, PP2A, in regulating microtubule stability and NMJ development by antagonizing Sgg (Viquez et al., 2009; Viquez et al., 2006). Because PP2A is Ca2+-independent, these observations suggest that distinct signals can lead to similar alterations in NMJ synapse morphology via Futsch.
The transcription factor, nuclear factor of activated T-cells (NFAT) is activated by calcineurin (Clipstone and Crabtree, 1992; Jain et al., 1993). However, our findings suggest the iav1 and calcineurin mutant phenotypes studied here are unlikely to be NFAT dependent because NFAT knockouts display an increase in bouton number (Freeman et al., 2011) rather than the decrease observed in the iav1 and calcineurin mutants. Thus, in the context of NMJ synapse development, calcineurin appears to function via Futsch rather than NFAT.
Iav regulates ER Ca2+ release and presynaptic resting [Ca2+] in Drosophila larval MNs
Several lines of evidence indicate that Iav and TRPV1 regulate ER Ca2+ release. First, decreasing ER Ca2+ release independent of Iav recapitulates the iav1 synaptic growth and morphological phenotypes. Moreover, loss of SERCA results in decreased EJP amplitude but normal mini frequency and amplitudes (Sanyal et al., 2005)—defects similar to those observed in iav1. Second, elevated ER Ca2+ release via RyR suppresses the iav1 synaptic growth and morphological phenotypes. Third, expression of Iav or TRPV1 in N2A cells results in the partial depletion of ER stores. Fourth, overexpressed Iav and TRPV1 are localized to the ER in the MN cell bodies. Interestingly, native TRPV1 in mammalian DRG neurons have been shown to localize to the ER and also permit ER Ca2+ release (Castro et al., 2009; Gallego-Sandin et al., 2009), although the biological significance of TRPV1 in the ER has so far remained unknown. The ER localization of Iav and TRPV1 is not simply an artifact of overexpression of a membrane protein because overexpressed hTRPV4 is not localized to the ER in MN cell bodies. Most importantly, Iav is required for maintaining synaptic resting [Ca2+] in the absence of extracellular Ca2+. These data strongly support the notion that Iav regulates Ca2+ release from an intracellular store to maintain the presynaptic resting [Ca2+]. Several studies have found that the ionic environment in the synaptic cleft could be tightly insulated such that [Ca2+] in synaptic clefts can drop dramatically during bursts of synaptic transmission thereby severely limiting Ca2+ entry into the presynaptic termini (Borst and Sakmann, 1999; Egelman and Montague, 1999; Rabl and Thoreson, 2002; Rusakov and Fine, 2003; Stanley, 2000). Although the synaptic cleft in a Drosophila NMJ is likely permeable to extracellular ions because these synapses are not tightly insulated by glial cells (Fuentes-Medel et al., 2009), more tightly insulated synapses such as those in vertebrate CNS might be more dependent on ER Ca2+ release to maintain the strength of synaptic transmission during intense stimulation.
The role of Iav in maintaining presynaptic resting [Ca2+] is not uncovered till the extracellular [Ca2+] is dropped to 0.5 mM. We speculate that endogenous decreases in synaptic [Ca2+] to the lower end of the 0.5-1.5 mM range in iav1 might result in subthreshold activation of calcineurin. However, since the extracellular [Ca2+] at a Drosophila larval NMJ is not known, we cannot rule out the possibility that Iav regulates the activity of calcineurin via a mechanism independent of the resting [Ca2+].
Our findings also allow us to speculate that Iav could function in homeostatic control of presynaptic [Ca2+]. The activity of Iav may be suppressed at resting [Ca2+] via proteins such as calmodulin such that a drop in resting [Ca2+] could result in Iav disinhibition and channel opening. Indeed, ER Ca2+ release via TRPV1 has been shown to be strongly suppressed by calmodulin binding to a C-terminal calmodulin binding domain on TRPV1 (Gallego-Sandin et al., 2009). Interestingly, Iav also contains a C-terminal calmodulin binding domain, which may underlie the homeostatic control of Iav activity.
Evoked neurotransmission at the Drosophila larval NMJ depends on Iav dosage and presynaptic [Ca2+] rather than the number of release sites
Owing to its function in the regulation of presynaptic resting [Ca2+], Iav influences SV release probability and the amplitude of evoked neurotransmission without affecting the Ca2+ cooperativity of SV release. Furthermore, Iav regulates neurotransmission in a dose dependent manner consistent with a critical function of the protein in evoked neurotransmission. Although calcineurin inhibits SV cycling at the Drosophila NMJ (Kuromi et al., 1997) and promotes synaptic growth, calcineurin does not play a role in Iav-dependent regulation of synaptic transmission. Moreover, our findings indicate that presynaptic Ca2+, rather than the number of SV release sites, is the major factor in regulating the evoked SV release. Thus, at higher extracellular [Ca2+], the Ca2+ entering via the VGCC compensates for the lower resting [Ca2+] in iav1, thereby resulting in normal synaptic transmission despite the reduction in the number of NMJ boutons and active zones (number of Brp punctae per NMJ, iav1;P[iav+] = 413.1, iav1 = 339.4, p = 0.01, Student’s t-test, n = 11 NMJs per genotype). Thus, synaptic transmission does not change proportionally with the number of synaptic boutons or even the number of active zones per synapse. Indeed, only ~50% of active zones at a Drosophila larval NMJ participate in SV release (Peled and Isacoff, 2011) and although both vapb and futsch mutants have fewer synaptic boutons, they exhibit elevated evoked synaptic transmission (Chai et al., 2008; Zhang et al., 2001).
EXPERIMENTAL PROCEDURES
Immunohistochemistry and confocal imaging
Wandering 3rd instar larvae were filleted in ice-cold PBS by cutting the body wall open along the dorsal midline and removing the visceral organs except the brain and nerves. The fillet was fixed in 4% PFA in PBS for 30 minutes. The fixed fillets were washed with 0.1% Triton X-100 in PBS before incubation with primary antibodies overnight at 4°C. Antibody dilutions: 1:200 rabbit anti-HRP (Jackson ImmunoResearch), 1:100 mouse anti-DLG (Parnas et al., 2001), 1:50 mouse anti-NC82 (Wagh et al., 2006), 1:50 mouse anti-Futsch (Fujita et al., 1982), 1:100 mouse anti-alpha-tubulin (Thazhath et al., 2002), 1:200 mouse anti-GFP (Invitrogen), 1:200 rabbit anti-TRPV1 and anti-TRPV4 (Alomone Labs), and 1:200 GFP-Booster (Chromotek). The rat anti-Iav antibody (anti-serum GNIEb (Gong et al., 2004)) was precleared by incubating the antibody with fixed iav1 fillets at a concentration of 1:100. Subsequently, the precleared antibody was used at dilution of 1:5. The monoclonal antibodies against DLG, NC82, Futsch, and alpha-tubulin were obtained from the Developmental Studies Hybridoma Bank developed under the auspices of the NICHD and maintained by The University of Iowa, Department of Biology, Iowa City, IA 52242. The samples were then washed and probed with fluorophore-conjugated secondary antibodies (1:400, Alexa Fluor 488/568/647) (Invitrogen) at room temperature for 1.5 hours and then mounted on glass slides with Vectashield (Vector Labs). Confocal images were obtained using a Nikon A1 Confocal Laser Microscope System (Nikon). For NMJ bouton counting, a 60× oil objective was used to focus on the NMJs on abdominal segment 3.
Evaluation of NMJ presynaptic [Ca2+] using tdTomato-P2A-GCaMP5G
3rd instar larval fillets were prepared in ice-cold HL-3.1, which contained (in mM): 70 NaCl, 5 KCl, 1.5 CaCl2, 4 MgCl2, 10 NaHCO3, 5 trehelose, 115 sucrose, and 5 HEPES. 7 mM L-glutamic acid was added to HL-3.1 to desensitize glutamate receptors and prevent muscle contraction during the course of experiment. The VNCs was severed from the dorsal brain lobes to prevent peristalsis. Dissected fillets were allowed to equilibrate to room temperature in HL3.1 containing 1.5 mM Ca2+ for at least 15 minutes before imaging. Type 1b boutons on muscle M13 of abdominal segment A4 were brought to focus with a 100× water-immersion objective on an Olympus BX51Wl microscope. Images were captured with an Andor Technology EMCCD camera (DU860) under the control of Ando IQ software. Subsequently, fluorescence signals were captured for tdTomato and for GCaMP5G. The bath solution was then exchanged to HL3.1 containing 0.5 mM CaCl2. Two minutes after bath exchange, fluorescence signals were captured at the same settings. Bath solution was then further exchanged to nominally Ca2+ free HL3.1. Fluorescence signals were then captured again 2 minutes and 9 minutes after bath exchange. Recorded images were analyzed using Andor IQ software. Regions not containing an axon terminal and close to a region of interest (ROI) were selected as background. Average pixel intensity from the background regions was subtracted from that of ROI for each fluorescence channel.
Ca2+ imaging in N2A cells
N2A cells were cultured in DMEM (Invitrogen) supplemented with 5% fetal bovine serum. Cells were transfected with TRPV cDNA or control vector using X-tremeGENE 9 DNA transfection reagent (Roche) at 1:4 DNA-to-reagent ratio according to the manufacturer’s instructions. One day later, transfected cells were trypsinized and seeded onto poly-D-lysine coated glass coverslips. Another day alter, cells were loaded with 10 μM fura2-AM (Invitrogen) in culture medium for 30 minutes. The glass coverslips were mounted onto a chamber containing 500 μL of bath solution (125 mM NaCl, 5 mM KCl, 10 mM MgSO4, 10 mM KH2PO4, 1 mM CaCl2, 5.5 mM glucose, and 5 mM HEPES; pH 7.4). Fura-2 signals, which represent cytosolic free [Ca2+] were recorded by 340/380 nm excitation and 510 nm emission, using a Nikon TiE Wide-Field Fluorescence Imaging System (Nikon). The background subtracted emission ratio (R340/380) was measured and calculated by NIS Elements imaging software (Nikon).
To evaluate the total ER Ca2+ content, we completely released the ER Ca2+ stores using thapsigargin (TG) while simultaneously evaluating the resulting elevation in cytosolic Ca2+, which is indicative of the total ER Ca2+ levels (Wegierski et al., 2009). Baseline fura-2 fluorescence was first acquired for 1 minute, before replacing the bath solution with Ca2+-free bath solution. Three minutes after removing bath Ca2+, 5 μM thapsigargin was added to the bath, and the fluorescence images were recorded for another 6 minutes. The amplitude of the R340/380 was taken to be the total ER Ca2+ content of those cells.
Supplementary Material
ACKNOWLEDGEMENTS
We thank the Bloomington Drosophila Stock Center for fly stocks, Dr. C. Montell for the trpgG4 and UAS-nan flies, Dr. T. Aigaki for the canA1 deficient flies, Dr. S. Sanyal for the kum170 flies, Dr. P. O’Farrell for the UAS-canA1FB5 and UAS-canA1CA lines, Drs. D. Sandstrom and B. White for the RyR24D03 flies, Dr. C. Kim for the anti-Iav antibodies, and Dr. R. Daniels for the UAS-tdTomato-P2A-GCaMP5G flies. We also thank Dr. C-K Yao and H. Hu for technical help. We are grateful to N. Haelterman, Dr. N. Giagtzoglou, Dr. S. Yamamoto, Dr. H. Sandoval, and Dr. M. Jaiswal for helpful discussions. H.J.B. is an Investigator of the HHMI. This work was supported by the NINDS grant, R01NS081301 (K.V.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no conflicts of interest.
REFERENCES
- Akerboom J, Chen TW, Wardill TJ, Tian L, Marvin JS, Mutlu S, Calderon NC, Esposti F, Borghuis BG, Sun XR, et al. Optimization of a GCaMP calcium indicator for neural activity imaging. J Neurosci. 2012;32:13819–13840. doi: 10.1523/JNEUROSCI.2601-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awatramani GB, Price GD, Trussell LO. Modulation of transmitter release by presynaptic resting potential and background calcium levels. Neuron. 2005;48:109–121. doi: 10.1016/j.neuron.2005.08.038. [DOI] [PubMed] [Google Scholar]
- Borst JG, Sakmann B. Depletion of calcium in the synaptic cleft of a calyx-type synapse in the rat brainstem. J Physiol. 1999;521(Pt 1):123–133. doi: 10.1111/j.1469-7793.1999.00123.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro J, Aromataris EC, Rychkov GY, Barritt GJ. A small component of the endoplasmic reticulum is required for store-operated Ca2+ channel activation in liver cells: evidence from studies using TRPV1 and taurodeoxycholic acid. Biochem J. 2009;418:553–566. doi: 10.1042/BJ20081052. [DOI] [PubMed] [Google Scholar]
- Caterina MJ, Leffler A, Malmberg AB, Martin WJ, Trafton J, Petersen-Zeitz KR, Koltzenburg M, Basbaum AI, Julius D. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science. 2000;288:306–313. doi: 10.1126/science.288.5464.306. [DOI] [PubMed] [Google Scholar]
- Caterina MJ, Rosen TA, Tominaga M, Brake AJ, Julius D. A capsaicin-receptor homologue with a high threshold for noxious heat. Nature. 1999;398:436–441. doi: 10.1038/18906. [DOI] [PubMed] [Google Scholar]
- Catterall WA. Structure and regulation of voltage-gated Ca2+ channels. Annu Rev Cell Dev Biol. 2000;16:521–555. doi: 10.1146/annurev.cellbio.16.1.521. [DOI] [PubMed] [Google Scholar]
- Chai A, Withers J, Koh YH, Parry K, Bao H, Zhang B, Budnik V, Pennetta G. hVAPB, the causative gene of a heterogeneous group of motor neuron diseases in humans, is functionally interchangeable with its Drosophila homologue DVAP-33A at the neuromuscular junction. Hum Mol Genet. 2008;17:266–280. doi: 10.1093/hmg/ddm303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho KO, Hunt CA, Kennedy MB. The rat brain postsynaptic density fraction contains a homolog of the Drosophila discs-large tumor suppressor protein. Neuron. 1992;9:929–942. doi: 10.1016/0896-6273(92)90245-9. [DOI] [PubMed] [Google Scholar]
- Chouhan AK, Zhang J, Zinsmaier KE, Macleod GT. Presynaptic mitochondria in functionally different motor neurons exhibit similar affinities for Ca2+ but exert little influence as Ca2+ buffers at nerve firing rates in situ. J Neurosci. 2010;30:1869–1881. doi: 10.1523/JNEUROSCI.4701-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clipstone NA, Crabtree GR. Identification of calcineurin as a key signalling enzyme in T-lymphocyte activation. Nature. 1992;357:695–697. doi: 10.1038/357695a0. [DOI] [PubMed] [Google Scholar]
- Daniels RW, Rossano AJ, Macleod GT, Ganetzky B. Expression of multiple transgenes from a single construct using viral 2A peptides in Drosophila. PLoS One. 2014;9:e100637. doi: 10.1371/journal.pone.0100637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dijkers PF, O’Farrell PH. Drosophila calcineurin promotes induction of innate immune responses. Curr Biol. 2007;17:2087–2093. doi: 10.1016/j.cub.2007.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dormer RL, Capurro DE, Morris R, Webb R. Demonstration of two isoforms of the SERCA-2b type Ca2+,Mg(2+)-ATPase in pancreatic endoplasmic reticulum. Biochim Biophys Acta. 1993;1152:225–230. doi: 10.1016/0005-2736(93)90253-v. [DOI] [PubMed] [Google Scholar]
- Egelman DM, Montague PR. Calcium dynamics in the extracellular space of mammalian neural tissue. Biophys J. 1999;76:1856–1867. doi: 10.1016/s0006-3495(99)77345-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler MA, Montell C. Drosophila TRP channels and animal behavior. Life Sci. 2012 doi: 10.1016/j.lfs.2012.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco B, Bogdanik L, Bobinnec Y, Debec A, Bockaert J, Parmentier ML, Grau Y. Shaggy, the homolog of glycogen synthase kinase 3, controls neuromuscular junction growth in Drosophila. J Neurosci. 2004;24:6573–6577. doi: 10.1523/JNEUROSCI.1580-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman A, Franciscovich A, Bowers M, Sandstrom DJ, Sanyal S. NFAT regulates pre-synaptic development and activity-dependent plasticity in Drosophila. Mol Cell Neurosci. 2011;46:535–547. doi: 10.1016/j.mcn.2010.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuentes-Medel Y, Logan MA, Ashley J, Ataman B, Budnik V, Freeman MR. Glia and muscle sculpt neuromuscular arbors by engulfing destabilized synaptic boutons and shed presynaptic debris. PLoS Biol. 2009;7:e1000184. doi: 10.1371/journal.pbio.1000184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita SC, Zipursky SL, Benzer S, Ferrus A, Shotwell SL. Monoclonal antibodies against the Drosophila nervous system. Proc Natl Acad Sci U S A. 1982;79:7929–7933. doi: 10.1073/pnas.79.24.7929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallego-Sandin S, Rodriguez-Garcia A, Alonso MT, Garcia-Sancho J. The endoplasmic reticulum of dorsal root ganglion neurons contains functional TRPV1 channels. J Biol Chem. 2009;284:32591–32601. doi: 10.1074/jbc.M109.019687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao S, Sandstrom DJ, Smith HE, High B, Marsh JW, Nash HA. Drosophila ryanodine receptors mediate general anesthesia by halothane. Anesthesiology. 2013;118:587–601. doi: 10.1097/ALN.0b013e31827e52c6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogel S, Wakefield S, Tear G, Klambt C, Gordon-Weeks PR. The Drosophila microtubule associated protein Futsch is phosphorylated by Shaggy/Zeste-white 3 at an homologous GSK3beta phosphorylation site in MAP1B. Mol Cell Neurosci. 2006;33:188–199. doi: 10.1016/j.mcn.2006.07.004. [DOI] [PubMed] [Google Scholar]
- Gong CX, Lidsky T, Wegiel J, Zuck L, Grundke-Iqbal I, Iqbal K. Phosphorylation of microtubule-associated protein tau is regulated by protein phosphatase 2A in mammalian brain. Implications for neurofibrillary degeneration in Alzheimer’s disease. J Biol Chem. 2000a;275:5535–5544. doi: 10.1074/jbc.275.8.5535. [DOI] [PubMed] [Google Scholar]
- Gong CX, Wegiel J, Lidsky T, Zuck L, Avila J, Wisniewski HM, Grundke-Iqbal I, Iqbal K. Regulation of phosphorylation of neuronal microtubule-associated proteins MAP1b and MAP2 by protein phosphatase-2A and -2B in rat brain. Brain Res. 2000b;853:299–309. doi: 10.1016/s0006-8993(99)02294-5. [DOI] [PubMed] [Google Scholar]
- Gong Z, Son W, Chung YD, Kim J, Shin DW, McClung CA, Lee Y, Lee HW, Chang DJ, Kaang BK, et al. Two interdependent TRPV channel subunits, inactive and Nanchung, mediate hearing in Drosophila. J Neurosci. 2004;24:9059–9066. doi: 10.1523/JNEUROSCI.1645-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Güler AD, Lee H, Iida T, Shimizu I, Tominaga M, Caterina M. Heat-evoked activation of the ion channel, TRPV4. J Neurosci. 2002;22:6408–6414. doi: 10.1523/JNEUROSCI.22-15-06408.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasan G, Rosbash M. Drosophila homologs of two mammalian intracellular Ca(2+)-release channels: identification and expression patterns of the inositol 1,4,5-triphosphate and the ryanodine receptor genes. Development. 1992;116:967–975. doi: 10.1242/dev.116.4.967. [DOI] [PubMed] [Google Scholar]
- Inagaki HK, Ben-Tabou de-Leon S, Wong AM, Jagadish S, Ishimoto H, Barnea G, Kitamoto T, Axel R, Anderson DJ. Visualizing neuromodulation in vivo: TANGO-mapping of dopamine signaling reveals appetite control of sugar sensing. Cell. 2012;148:583–595. doi: 10.1016/j.cell.2011.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahn R, Fasshauer D. Molecular machines governing exocytosis of synaptic vesicles. Nature. 2012;490:201–207. doi: 10.1038/nature11320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain J, McCaffrey PG, Miner Z, Kerppola TK, Lambert JN, Verdine GL, Curran T, Rao A. The T-cell transcription factor NFATp is a substrate for calcineurin and interacts with Fos and Jun. Nature. 1993;365:352–355. doi: 10.1038/365352a0. [DOI] [PubMed] [Google Scholar]
- Jan LY, Jan YN. Properties of the larval neuromuscular junction in Drosophila melanogaster. J Physiol. 1976;262:189–214. doi: 10.1113/jphysiol.1976.sp011592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Chung YD, Park DY, Choi S, Shin DW, Soh H, Lee HW, Son W, Yim J, Park CS, et al. A TRPV family ion channel required for hearing in Drosophila. Nature. 2003;424:81–84. doi: 10.1038/nature01733. [DOI] [PubMed] [Google Scholar]
- Kuromi H, Yoshihara M, Kidokoro Y. An inhibitory role of calcineurin in endocytosis of synaptic vesicles at nerve terminals of Drosophila larvae. Neurosci Res. 1997;27:101–113. doi: 10.1016/s0168-0102(96)01132-7. [DOI] [PubMed] [Google Scholar]
- Kuzmenkin A, Bezanilla F, Correa AM. Gating of the bacterial sodium channel, NaChBac: voltage-dependent charge movement and gating currents. J Gen Physiol. 2004;124:349–356. doi: 10.1085/jgp.200409139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Littleton JT, Stern M, Perin M, Bellen HJ. Calcium dependence of neurotransmitter release and rate of spontaneous vesicle fusions are altered in Drosophila synaptotagmin mutants. Proc Natl Acad Sci U S A. 1994;91:10888–10892. doi: 10.1073/pnas.91.23.10888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luan H, Lemon WC, Peabody NC, Pohl JB, Zelensky PK, Wang D, Nitabach MN, Holmes TC, White BH. Functional dissection of a neuronal network required for cuticle tanning and wing expansion in Drosophila. J Neurosci. 2006;26:573–584. doi: 10.1523/JNEUROSCI.3916-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ly CV, Yao CK, Verstreken P, Ohyama T, Bellen HJ. straightjacket is required for the synaptic stabilization of cacophony, a voltage-gated calcium channel alpha1 subunit. J Cell Biol. 2008;181:157–170. doi: 10.1083/jcb.200712152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch J, Michalak M. Calreticulin is an upstream regulator of calcineurin. Biochem Biophys Res Commun. 2003;311:1173–1179. doi: 10.1016/j.bbrc.2003.08.040. [DOI] [PubMed] [Google Scholar]
- Matveev V, Bertram R, Sherman A. Calcium cooperativity of exocytosis as a measure of Ca(2)+ channel domain overlap. Brain Res. 2011;1398:126–138. doi: 10.1016/j.brainres.2011.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miech C, Pauer HU, He X, Schwarz TL. Presynaptic local signaling by a canonical wingless pathway regulates development of the Drosophila neuromuscular junction. J Neurosci. 2008;28:10875–10884. doi: 10.1523/JNEUROSCI.0164-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakai Y, Horiuchi J, Tsuda M, Takeo S, Akahori S, Matsuo T, Kume K, Aigaki T. Calcineurin and its regulator sra/DSCR1 are essential for sleep in Drosophila. J Neurosci. 2011;31:12759–12766. doi: 10.1523/JNEUROSCI.1337-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura AL, Mitne-Neto M, Silva HC, Richieri-Costa A, Middleton S, Cascio D, Kok F, Oliveira JR, Gillingwater T, Webb J, et al. A mutation in the vesicle-trafficking protein VAPB causes late-onset spinal muscular atrophy and amyotrophic lateral sclerosis. Am J Hum Genet. 2004;75:822–831. doi: 10.1086/425287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packard M, Koo ES, Gorczyca M, Sharpe J, Cumberledge S, Budnik V. The Drosophila Wnt, wingless, provides an essential signal for pre- and postsynaptic differentiation. Cell. 2002;111:319–330. doi: 10.1016/s0092-8674(02)01047-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parnas D, Haghighi AP, Fetter RD, Kim SW, Goodman CS. Regulation of postsynaptic structure and protein localization by the Rho-type guanine nucleotide exchange factor dPix. Neuron. 2001;32:415–424. doi: 10.1016/s0896-6273(01)00485-8. [DOI] [PubMed] [Google Scholar]
- Peled ES, Isacoff EY. Optical quantal analysis of synaptic transmission in wild-type and rab3-mutant Drosophila motor axons. Nat Neurosci. 2011;14:519–526. doi: 10.1038/nn.2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennetta G, Hiesinger PR, Fabian-Fine R, Meinertzhagen IA, Bellen HJ. Drosophila VAP-33A directs bouton formation at neuromuscular junctions in a dosage-dependent manner. Neuron. 2002;35:291–306. doi: 10.1016/s0896-6273(02)00769-9. [DOI] [PubMed] [Google Scholar]
- Rabl K, Thoreson WB. Calcium-dependent inactivation and depletion of synaptic cleft calcium ions combine to regulate rod calcium currents under physiological conditions. Eur J Neurosci. 2002;16:2070–2077. doi: 10.1046/j.1460-9568.2002.02277.x. [DOI] [PubMed] [Google Scholar]
- Rinne A, Banach K, Blatter LA. Regulation of nuclear factor of activated T cells (NFAT) in vascular endothelial cells. J Mol Cell Cardiol. 2009;47:400–410. doi: 10.1016/j.yjmcc.2009.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roos J, Hummel T, Ng N, Klambt C, Davis GW. Drosophila Futsch regulates synaptic microtubule organization and is necessary for synaptic growth. Neuron. 2000;26:371–382. doi: 10.1016/s0896-6273(00)81170-8. [DOI] [PubMed] [Google Scholar]
- Rusakov DA, Fine A. Extracellular Ca2+ depletion contributes to fast activity-dependent modulation of synaptic transmission in the brain. Neuron. 2003;37:287–297. doi: 10.1016/s0896-6273(03)00025-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanyal S, Consoulas C, Kuromi H, Basole A, Mukai L, Kidokoro Y, Krishnan KS, Ramaswami M. Analysis of conditional paralytic mutants in Drosophila sarco-endoplasmic reticulum calcium ATPase reveals novel mechanisms for regulating membrane excitability. Genetics. 2005;169:737–750. doi: 10.1534/genetics.104.031930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snow PM, Patel NH, Harrelson AL, Goodman CS. Neural-specific carbohydrate moiety shared by many surface glycoproteins in Drosophila and grasshopper embryos. J Neurosci. 1987;7:4137–4144. doi: 10.1523/JNEUROSCI.07-12-04137.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley EF. Presynaptic calcium channels and the depletion of synaptic cleft calcium ions. J Neurophysiol. 2000;83:477–482. doi: 10.1152/jn.2000.83.1.477. [DOI] [PubMed] [Google Scholar]
- Sullivan KM, Scott K, Zuker CS, Rubin GM. The ryanodine receptor is essential for larval development in Drosophila melanogaster. Proc Natl Acad Sci U S A. 2000;97:5942–5947. doi: 10.1073/pnas.110145997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thazhath R, Liu C, Gaertig J. Polyglycylation domain of beta-tubulin maintains axonemal architecture and affects cytokinesis in Tetrahymena. Nat Cell Biol. 2002;4:256–259. doi: 10.1038/ncb764. [DOI] [PubMed] [Google Scholar]
- Tominaga M, Caterina MJ, Malmberg AB, Rosen TA, Gilbert H, Skinner K, Raumann BE, Basbaum AI, Julius D. The cloned capsaicin receptor integrates multiple pain-producing stimuli. Neuron. 1998;21:531–543. doi: 10.1016/s0896-6273(00)80564-4. [DOI] [PubMed] [Google Scholar]
- Venkatachalam K, Long AA, Elsaesser R, Nikolaeva D, Broadie K, Montell C. Motor deficit in a Drosophila model of mucolipidosis type IV due to defective clearance of apoptotic cells. Cell. 2008;135:838–851. doi: 10.1016/j.cell.2008.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatachalam K, Montell C. TRP channels. Annu Rev Biochem. 2007;76:387–417. doi: 10.1146/annurev.biochem.75.103004.142819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viquez NM, Fuger P, Valakh V, Daniels RW, Rasse TM, DiAntonio A. PP2A and GSK-3beta act antagonistically to regulate active zone development. J Neurosci. 2009;29:11484–11494. doi: 10.1523/JNEUROSCI.5584-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viquez NM, Li CR, Wairkar YP, DiAntonio A. The B’ protein phosphatase 2A regulatory subunit well-rounded regulates synaptic growth and cytoskeletal stability at the Drosophila neuromuscular junction. J Neurosci. 2006;26:9293–9303. doi: 10.1523/JNEUROSCI.1740-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagh DA, Rasse TM, Asan E, Hofbauer A, Schwenkert I, Durrbeck H, Buchner S, Dabauvalle MC, Schmidt M, Qin G, et al. Bruchpilot, a protein with homology to ELKS/CAST, is required for structural integrity and function of synaptic active zones in Drosophila. Neuron. 2006;49:833–844. doi: 10.1016/j.neuron.2006.02.008. [DOI] [PubMed] [Google Scholar]
- Watanabe H, Davis JB, Smart D, Jerman JC, Smith GD, Hayes P, Vriens J, Cairns W, Wissenbach U, Prenen J, et al. Activation of TRPV4 channels (hVRL-2/mTRP12) by phorbol derivatives. J Biol Chem. 2002;277:13569–13577. doi: 10.1074/jbc.M200062200. [DOI] [PubMed] [Google Scholar]
- Wegierski T, Steffl D, Kopp C, Tauber R, Buchholz B, Nitschke R, Kuehn EW, Walz G, Kottgen M. TRPP2 channels regulate apoptosis through the Ca2+ concentration in the endoplasmic reticulum. EMBO J. 2009;28:490–499. doi: 10.1038/emboj.2008.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia R, Dekermendjian K, Lullau E, Dekker N. TRPV1: a therapy target that attracts the pharmaceutical interests. Adv Exp Med Biol. 2011a;704:637–665. doi: 10.1007/978-94-007-0265-3_34. [DOI] [PubMed] [Google Scholar]
- Xia R, Samad TA, Btesh J, Jiang LH, Kays I, Stjernborg L, Dekker N. TRPV1 signaling: mechanistic understanding and therapeutic potential. Curr Top Med Chem. 2011b;11:2180–2191. doi: 10.2174/156802611796904843. [DOI] [PubMed] [Google Scholar]
- Zhang C, Wu B, Beglopoulos V, Wines-Samuelson M, Zhang D, Dragatsis I, Sudhof TC, Shen J. Presenilins are essential for regulating neurotransmitter release. Nature. 2009;460:632–636. doi: 10.1038/nature08177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YQ, Bailey AM, Matthies HJ, Renden RB, Smith MA, Speese SD, Rubin GM, Broadie K. Drosophila fragile X-related gene regulates the MAP1B homolog Futsch to control synaptic structure and function. Cell. 2001;107:591–603. doi: 10.1016/s0092-8674(01)00589-x. [DOI] [PubMed] [Google Scholar]
- Zucker RS, Regehr WG. Short-term synaptic plasticity. Annu Rev Physiol. 2002;64:355–405. doi: 10.1146/annurev.physiol.64.092501.114547. [DOI] [PubMed] [Google Scholar]
- Zygmunt PM, Petersson J, Andersson DA, Chuang H, Sorgard M, Di Marzo V, Julius D, Hogestatt ED. Vanilloid receptors on sensory nerves mediate the vasodilator action of anandamide. Nature. 1999;400:452–457. doi: 10.1038/22761. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.