Abstract
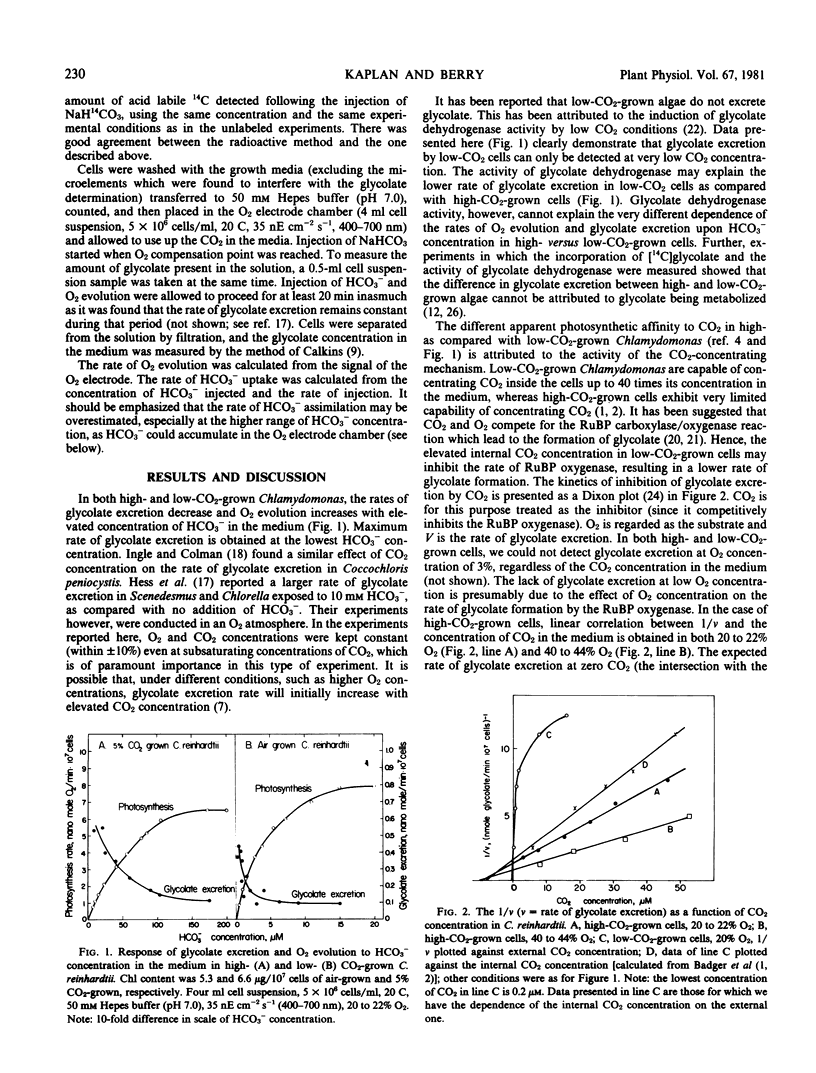
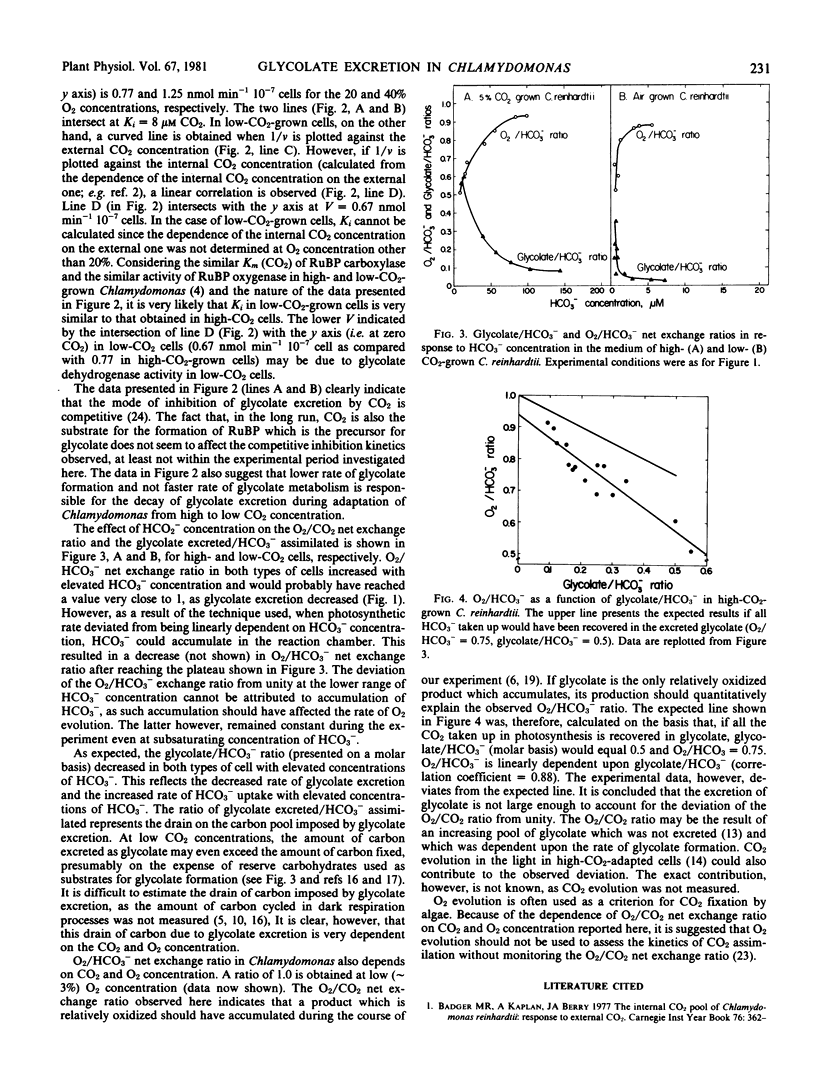
Chlamydomonas reinhardtii cells were grown in high (5% v/v) or low (0.03% v/v) CO2 concentration in air. O2 evolution, HCO3− assimilation, and glycolate excretion were measured in response to O2 and CO2 concentration. Both low- and high-CO2-grown cells excrete glycolate. In low-CO2-grown cells, however, glycolate excretion is observed only at much lower CO2 concentrations in the medium, as compared with high-CO2-adapted cells. It is postulated that the activity of the CO2-concentrating mechanism in low-CO2-grown cells is responsible for the different dependence of glycolate excretion on external CO2 concentration in low- versus high-CO2-adapted cells.
The O2/CO2 net exchange ratio is dependent on the CO2 concentration in the medium and is linearly dependent on the fraction of glycolate excreted per CO2 taken up. Glycolate excretion, however, is too low to account for the deviation of the O2/CO2 net exchange ratio from unity.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Björkman O. Comparative studies on photosynthesis in higher plants. Photophysiology. 1973;8:1–63. doi: 10.1016/b978-0-12-282608-5.50007-2. [DOI] [PubMed] [Google Scholar]
- Bruin W. J., Nelson E. B., Tolbert N. E. Glycolate pathway in green algae. Plant Physiol. 1970 Sep;46(3):386–391. doi: 10.1104/pp.46.3.386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Codd G. A., Lord J. M., Merrett M. J. The glycollate oxidising enzyme of algae. FEBS Lett. 1969 Dec 30;5(5):341–342. doi: 10.1016/0014-5793(69)80352-2. [DOI] [PubMed] [Google Scholar]
- Colman B., Miller A. G., Grodzinski B. A study of the control of glycolate excretion in chlorella. Plant Physiol. 1974 Mar;53(3):395–397. doi: 10.1104/pp.53.3.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause G. H., Thorne S. W., Lorimer G. H. Glycolate synthesis by intact chloroplasts. Studies with inhibitors of photophosphorylation. Arch Biochem Biophys. 1977 Oct;183(2):471–479. doi: 10.1016/0003-9861(77)90382-4. [DOI] [PubMed] [Google Scholar]
- Lorimer G. H., Krause G. H., Berry J. A. The incorporation of (18O)oxygen into glycolate by intact isolated chloroplasts. FEBS Lett. 1977 Jun 15;78(2):199–202. doi: 10.1016/0014-5793(77)80305-0. [DOI] [PubMed] [Google Scholar]
- Nelson E. B., Tolbert N. E. The regulation of glycolate metabolism in Chlamydomonas reinhardtii. Biochim Biophys Acta. 1969 Jul 30;184(2):263–270. doi: 10.1016/0304-4165(69)90028-2. [DOI] [PubMed] [Google Scholar]
- Radmer R., Kok B., Ollinger O. Kinetics and Apparent K(m) of Oxygen Cycle under Conditions of Limiting Carbon Dioxide Fixation. Plant Physiol. 1978 Jun;61(6):915–917. doi: 10.1104/pp.61.6.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sueoka N. MITOTIC REPLICATION OF DEOXYRIBONUCLEIC ACID IN CHLAMYDOMONAS REINHARDI. Proc Natl Acad Sci U S A. 1960 Jan;46(1):83–91. doi: 10.1073/pnas.46.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WARBURG O., KRIPPAHL G. [Glycolic acid formation in Chlorella]. Z Naturforsch B. 1960 Mar;15B:197–199. [PubMed] [Google Scholar]


