Abstract
Accurate transmission of genetic material relies on the coupling of chromosomes to spindle microtubules by kinetochores. These linkages are regulated by the conserved Aurora B/Ipl1 kinase to ensure that sister chromatids are properly attached to spindle microtubules. Kinetochore–microtubule attachments require the essential Ndc80 complex, which contains two globular ends linked by large coiled-coil domains. In this study, we isolated a novel ndc80 mutant in Saccharomyces cerevisiae that contains mutations in the coiled-coil domain. This ndc80 mutant accumulates erroneous kinetochore–microtubule attachments, resulting in misalignment of kinetochores on the mitotic spindle. Genetic analyses with suppressors of the ndc80 mutant and in vitro cross-linking experiments suggest that the kinetochore misalignment in vivo stems from a defect in the ability of the Ndc80 complex to stably fold at a hinge in the coiled coil. Previous studies proposed that the Ndc80 complex can exist in multiple conformations: elongated during metaphase and bent during anaphase. However, the distinct functions of individual conformations in vivo are unknown. Here, our analysis revealed a tightly folded conformation of the Ndc80 complex that is likely required early in mitosis. This conformation is mediated by a direct, intracomplex interaction and involves a greater degree of folding than the bent form of the complex at anaphase. Furthermore, our results suggest that this conformation is functionally important in vivo for efficient error correction by Aurora B/Ipl1 and, consequently, to ensure proper kinetochore alignment early in mitosis.
Keywords: Hec1, linker scanning, mass spectrometry, optical trap, syntelic
KINETOCHORES mediate the linkage between chromosomes and spindle microtubules during mitosis. This attachment is highly regulated to promote the fidelity of chromosome segregation. At metaphase, replicated chromosomes are attached to microtubules emanating from opposite poles, in a “bioriented” alignment. This ensures that each daughter cell receives a full complement of genetic material after chromosome segregation. Errors can occur in the form of “syntelic” attachments, when both sister kinetochores attach to microtubules emanating from the same pole. These erroneous kinetochore–microtubule attachments are detected and detached by the conserved Aurora B/Ipl1 kinase (Biggins et al. 2001; Biggins and Murray 2001; Tanaka et al. 2002; Pinsky et al. 2006). In the current prevailing model, Aurora B/Ipl1 corrects syntelic attachments by destabilizing linkages that are under low levels of tension (Nicklas and Koch 1969; Biggins and Murray 2001; Tanaka et al. 2002; Liu et al. 2009; Cane et al. 2013). The Aurora B/Ipl1 error detection system is coupled to the spindle checkpoint, a separate surveillance system that delays anaphase until all kinetochores are attached to spindle microtubules (reviewed in Hauf 2013). Together, these systems ensure that the physical separation of replicated chromatids does not occur until all kinetochore–microtubule attachments are bioriented.
The attachment of spindle microtubules to kinetochores requires the conserved Ndc80 complex (Figure 1A), a flexible rod-shaped heterotetramer composed of Ndc80, Nuf2, Spc24, and Spc25 (Osborne et al. 1994; Janke et al. 2001; Wigge and Kilmartin 2001; Wei et al. 2005; Ciferri et al. 2008; Wang et al. 2008). The Ndc80 complex has a well-characterized role in microtubule binding, mediated by N-terminal calponin homology domains (Wei et al. 2007; Powers et al. 2009; Alushin et al. 2010). At the other end of the complex, the globular domains of Spc24 and Spc25 link the Ndc80 complex to other kinetochore components (De Wulf et al. 2003; Malvezzi et al. 2013; Nishino et al. 2013). Between the two structurally defined ends, the Ndc80 complex is composed of predicted coiled-coil domains from all four components (Wei et al. 2005; Wang et al. 2008). The coiled-coil domains are interrupted by a disordered “loop” region that is thought to enable flexion of the complex, as observed for recombinant Ndc80 complexes on negative-stain electron micrographs (Wang et al. 2008). The Ndc80 complex adopts elongated and bent conformations in vivo during metaphase and anaphase, respectively (Joglekar et al. 2009; Aravamudhan et al. 2014). However, it remains to be determined if these conformations arise from the flexibility of the complex at the loop observed in vitro. Furthermore, no previous study has examined whether the bending flexibility of the Ndc80 complex is important in vivo.
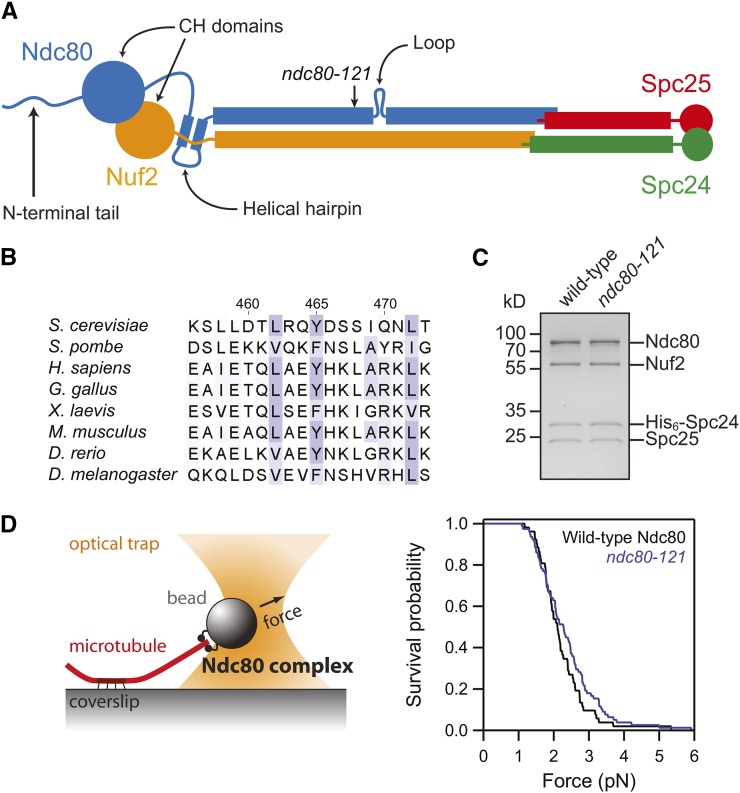
Figure 1.
The ndc80-121 mutations affect conserved residues in Ndc80 and do not disrupt assembly of an intact Ndc80 complex. (A) Schematic of the Ndc80 complex, with the site of the ndc80-121 mutations noted. (B) Sequence alignment of the region of Ndc80 mutated in the ndc80-121 allele (Y465C I469Q). Alignments were performed with ClustalW2 (Larkin et al. 2007) and residues are colored based on Blosum62 scores in Jalview (Waterhouse et al. 2009). (C) Coomassie-stained gel of recombinant Ndc80 complex containing the ndc80-121 mutations. The mutant complex migrated similarly to the wild-type complex by gel filtration and was collected at the same elution volume. (D) Left: schematic of rupture force assay. Right: survival vs. force curves for beads coated with wild-type (black trace, n = 52) or mutant (blue trace, n = 78) Ndc80 complexes. The two distributions are not significantly different, as determined by the Kolmogorov–Smirnov test (P = 0.4).
Our recent results from a mutagenesis screen suggest that several small regions of the coiled coil are essential for cell viability, including one region near the predicted loop (Tien et al. 2013). Here, biochemical characterization of the Ndc80 complex was combined with genetic analysis of a novel ndc80 mutant to reveal that the loop region acts as a hinge in vivo, enabling the complex to adopt a tightly folded conformation. This conformation is mediated by a direct, intracomplex interaction between two regions of the complex on either side of the loop. Mutations in one of these regions impede the ability of Aurora B/Ipl1 to correct aberrant kinetochore–microtubule attachments prior to metaphase. Therefore, the tightly folded conformation of the Ndc80 complex is likely required to promote kinetochore biorientation early in mitosis.
Materials and Methods
Strains
All strains used in this study (Supporting Information, Table S1) were derived from W303.
Protein expression and purification
Recombinant Saccharomyces cerevisiae Ndc80 complex was expressed from two dicistronic plasmids (encoding Ndc80/Nuf2 and His6-Spc24/Spc25) and purified as previously described (Wei et al. 2005; Powers et al. 2009).
Immunoprecipitation
For immunoprecipitation of Nuf2-TAP from ndc80-121 cultures, 2 liters of JTY30-1A (ndc80-121 NUF2-TAP) cells were grown to ∼100 Klett units in YPD at 25°. JTY30-4A (NDC80NUF2-TAP) cells served as a wild-type control. Cultures were shifted to 37° for 100 min and harvested by centrifugation. Pellets were cryogenically ground into cell dust using a PM100 (Retsch) and stored at −80°, as per the protocol from the Rout laboratory (http://lab.rockefeller.edu/rout/assets/file/protocols). For each condition, 4 g of cell dust were resuspended in lysis buffer (20 mM HEPES, pH 7.4, 300 mM NaCl, 100 μM GTP, 1 mM MgCl2, 1 mM dithiothreitol, 4 μg·ml−1 pepstatin, 4 μg·ml−1 leupeptin, 4 μg·ml−1 aprotinin, 4 μg·ml−1 chymostatin, 1 mM phenylmethanesulfonyl fluoride, 1 mM sodium pyrophosphate, 1 mM sodium fluoride, 1 mM β-glycerophosphate, 5% glycerol, and 0.5% Triton X-100), homogenized, and cleared by centrifugation at 2000 × g for 10 min at 4°. An aliquot (250 µl) of 60 mg·ml−1 Dynabeads (Invitrogen) conjugated with rabbit IgG (MP Biomedicals) was added to the clarified lysate and incubated for 30 min at 4°. Beads were then washed three times with 150 µl of wash buffer (20 mM HEPES, pH 7.4, 200 mM NaCl, 100 μM GTP, 1 mM MgCl2, 1 mM dithiothreitol, 4 μg·ml−1 pepstatin, 4 μg·ml−1 leupeptin, 4 μg·ml−1 aprotinin, 4 μg·ml−1 chymostatin, 1 mM phenylmethanesulfonyl fluoride, 1 mM sodium pyrophosphate, 1 mM sodium fluoride, 1 mM β-glycerophosphate, and 5% glycerol), washed once with 150 µl of Tobacco Etch Virus (TEV) buffer (40 mM HEPES, pH 7.4, 200 mM NaCl, 2 mM MgCl2, 1 mM EDTA, 1 mM dithiothreitol, 1 mM GTP, and 5% glycerol), and resuspended in 100 µl TEV buffer. TEV was added to 67 nM and the reaction was incubated for 2 hr at 4°. Trichloroacetic acid protein precipitation was performed on 60 µl of eluate after TEV cleavage. Immunoprecipitated proteins were identified by mass spectrometry and MudPIT analysis.
Fluorescence microscopy
The CellAsic ONIX microfluidics system (Millipore) was used for time-lapse imaging of synchronized cells. For G1 synchronization, MATa cells (Table S1) were grown to ∼60 Klett units at 25° and arrested for a total of 1.5 generations with α-factor. One generation into the arrest, cells were briefly sonicated and 50 µl were loaded onto an Y04C CellAsic ONIX plate. The arrest was completed on the plate before releasing into media lacking α-factor. For metaphase arrests, cells with an auxin-inducible Cdc20 degron (Table S1) were grown to ∼50 Klett units at 25°, arrested with 500 μM 3-indoleacetic acid (Sigma-Aldrich) for 3 hr, and loaded onto a Y04C plate. All flow rates were at ∼12 µl·hr−1. After completing the arrests, plate and objective heaters were raised to 37° (t = 0) and cells were imaged. Time-lapse images of cells were taken at 7.5-min intervals, with three z-sections spanning 2.4 μm, binned 1 × 1, using the DeltaVision system (Applied Precision) equipped with an IX70 inverted microscope (Olympus), a Plan Apo ×60 objective (1.40 NA), and a CoolSnap HQ digital camera (Photometrics). Exposures were 0.1 s for GFP and 0.15 s for mCherry.
To assay for chromosome biorientation, CEN3 was visualized using LacI-GFP bound to a LacO-array adjacent to the centromere (Table S1). LacI-GFP is under control of the pCUP1 promoter and imaged using uninduced conditions. For G1 synchronization, MATa cells were grown to ∼60 Klett units at 25° and arrested for 1.5 generations with α-factor. To release from the arrest, cells were collected by filtration, washed with three volumes of YPD, sonicated, and shifted to 37° medium. At 100 min after the release, ∼500 µl of cells were harvested by centrifugation and resuspended in media made with yeast nitrogen base without copper (ForMedium). For metaphase arrests, cells with an auxin-inducible Cdc20 degron (Table S1) were first synchronized in G1 with α-factor, then released into media containing 500 μM 3-indoleacetic acid (Sigma-Aldrich) for 1 hr at 25°. The culture was then shifted to 37° for 100 min before harvesting. For imaging, cells were mounted for microscopy as previously described (Muller et al. 2005) (instructional video at http://youtu.be/ZrZVbFg9NE8), except that agarose pads were made with yeast nitrogen base without copper (ForMedium). Images were taken with seven z-sections spanning 4.2 μm, binned 1 × 1, using the DeltaVision system (as above) equipped with a U Plan Apo ×100 objective (1.35 NA). Exposures were 0.4 s for GFP and 0.3 s for mCherry.
To image kinetochores, MATa cells containing Nuf2-GFP and Spc110-mCherry (Table S1) were synchronized in G1 with α-factor (as above) and released into 37° medium for 100 min. After harvesting, cells were mounted on agarose pads (Muller et al. 2005) and imaged using the DeltaVision system (as above) equipped with a U Plan Apo ×100 objective (1.35 NA). Images were taken with seven z-sections spanning 4.2 μm, binned 1 × 1. Exposures were 0.4 s for GFP and 0.3 s for mCherry. To determine the kinetochore intensity ratio, the intensities of Nuf2-GFP and Spc110-mCherry were measured (ImageJ). For each spindle, custom programs (available upon request) written in Igor Pro (Wavemetrics) identified the positions of spindle pole bodies based on Gaussian fits to the Spc110-mCherry signal, and calculated the integrated GFP fluorescence intensity on each half of the spindle. The kinetochore intensity ratio is defined as the integrated intensity of the brighter half of the spindle divided by the intensity of the dimmer half (and thus, always ≥1). Box plots of the data were constructed in Igor Pro and statistical analyses were performed using the Kolmogorov–Smirnov test.
In vitro rupture force assay
Recombinant Ndc80 complex was loaded onto 0.44-µm polystyrene beads at a ratio of ∼2000 complexes per bead. Bead functionalization was carried out in incubation buffer (BRB80 containing 8 mg·ml−1 BSA and 1 mM DTT) for 1 hr at 4° in a total volume of 60 µl. Beads were then pelleted (16,000 × g for 10 min at 4°) and washed with ∼200 µl incubation buffer to remove any unbound Ndc80 complex. Flow chambers were prepared as previously described (Franck et al. 2010). Beads were introduced into the flow chamber in assay buffer (BRB80 containing 8 mg·ml−1 BSA, 1 mg·ml−1 κ-casein, 1 mM DTT, 1 mM GTP, and 1.4 mg·ml−1 tubulin) supplemented with an oxygen scavenging system (250 μg·ml−1 glucose oxidase, 30 μg·ml−1 catalase, and 30 mM glucose). Free tubulin in the assay buffer assembled to form dynamic microtubule extensions from coverslip-anchored Guanosine-5′-[(α,β)-methyleno]triphosphate (GMPCPP)-stabilized microtubule seeds.
A custom optical trap instrument was used to manipulate individual beads in the flow chamber. Rupture force assays were performed as previously described (Franck et al. 2010; Tien et al. 2010). Each bead was pulled to the end of a dynamic microtubule under a test force of 0.5–1 pN exerted by the trap. Applied force was then increased at a constant rate (0.25 pN·s−1) until the attachment ruptured. The rupture force was indicated by the maximum force attained during each event prior to detachment and determined after the experiment during data analysis from records of force vs. time (using custom software, available upon request, written in Igor Pro, Wavemetrics).
Cross-linking of recombinant Ndc80 complex and mass spectrometry analysis
Ndc80 complex (44 µg in 143 µL reaction volume) was cross-linked for 2 min at room temperature with disuccinimidyl suberate (Pierce, 0.3 mM final). The reaction mix was quenched with 10 µl of 500 mM NH4HCO3 and the buffer was exchanged to HB500 (40 mM HEPES, 500 mM NaCl, pH 7.5) using protein desalting spin columns (Pierce) according to the manufacturer’s protocol. Cross-linked proteins were subsequently reduced with 10 mM dithiothreitol for 30 min at 37°, alkylated with 15 mM iodoacetamide for 30 min at room temperature, and digested with trypsin (at a substrate-to-enzyme ratio of 60:1) overnight at room temperature with shaking. Samples were acidified with 5 M HCl and stored at −80°.
Samples (1.5 µg) were loaded onto a fused-silica capillary tip column (75 µm i.d.) packed with 40 cm of Reprosil-Pur C18-AQ (3-µm bead diameter, Dr. Maisch). Peptides were eluted from the column at 250 nL·min−1 using a gradient of 2–35% acetonitrile (in 0.1% formic acid) over 120 min, followed by 35–60% acetonitrile over 10 min. Mass spectrometry was performed on a Q-Exactive (Thermo Scientific), operated using data-dependent acquisition where a maximum of six MS/MS spectra were acquired per MS spectrum (scan range of m/z 400–1600). At m/z 200, the resolution for MS and MS/MS was 70,000 and 35,000, respectively.
Cross-linked peptides were identified using the Kojak cross-link identification software (available at https://code.google.com/p/kojak-ms/). The results of Kojak were exported directly to Percolator (Kall et al. 2007) to produce a statistically validated set of cross-linked peptide identifications at a false discovery rate threshold of 5%.
Results
Isolation of the ndc80-121 temperature-sensitive allele
The Ndc80 complex features two globular ends linked by a long, rod-shaped segment composed of predicted coiled-coil domains from all four components (Figure 1A). We recently performed an insertional mutagenesis screen to uncover essential regions of NDC80 in S. cerevisiae (Tien et al. 2013). The functions of four of these essential regions, identified as clusters of lethal insertions, have not yet been identified. Insertions from one such cluster located near the disordered loop were found to confer a temperature-sensitive phenotype (Table S2), allowing us to study the function of this loop-proximal region of NDC80 in vivo. These temperature-sensitive insertions in NDC80 mapped to consecutive residues in the protein, disrupting the region from a highly conserved aromatic, Y465, through I469 (Table S2, Figure 1B). We isolated a minimal mutation that was sufficient to recapitulate the temperature-sensitive phenotype: Y465C and I469Q (Figure 1A, Table S2). This allele was named ndc80-121.
The ndc80-121 mutations do not affect assembly of the complex or its ability to bind microtubules
We first examined the effects of the ndc80-121 mutations on the integrity of the mutant Ndc80 complex. When expressed and purified recombinantly, the ndc80-121 mutations do not disrupt assembly of the heterotetrameric Ndc80 complex (Figure 1C). All four components of the Ndc80 complex were coimmunoprecipitated with Nuf2-TAP from ndc80-121 yeast cells shifted to the restrictive temperature of 37° (Table S3). These results demonstrate that the temperature-sensitive phenotype of ndc80-121 cells is not a result of degradation of the Ndc80 complex. Furthermore, the temperature shift to 37° does not lead to disassembly of mutant Ndc80 complexes. We then asked if these mutations disrupt the microtubule attachment strength of the Ndc80 complex, as reported for other temperature-sensitive ndc80 mutants (e.g., ndc80-1; Wigge et al. 1998; Pinsky et al. 2006; Akiyoshi et al. 2010). Using an in vitro rupture force assay (Franck et al. 2010), we determined that microtubule attachments mediated by wild-type Ndc80 complex ruptured at 2.2 ± 0.1 pN on average (Figure 1D), as previously observed (Tien et al. 2010). The attachment strength of the mutant complex was indistinguishable, yielding a mean rupture force of 2.4 ± 0.1 pN (Figure 1D). Therefore, the ndc80-121 mutations do not affect composition of the Ndc80 complex or its ability to bind microtubules (also see below). Instead, the mutations likely disrupt a specific function of the coiled-coil domain near the loop region.
The ndc80-121 mutations cause a mitotic arrest
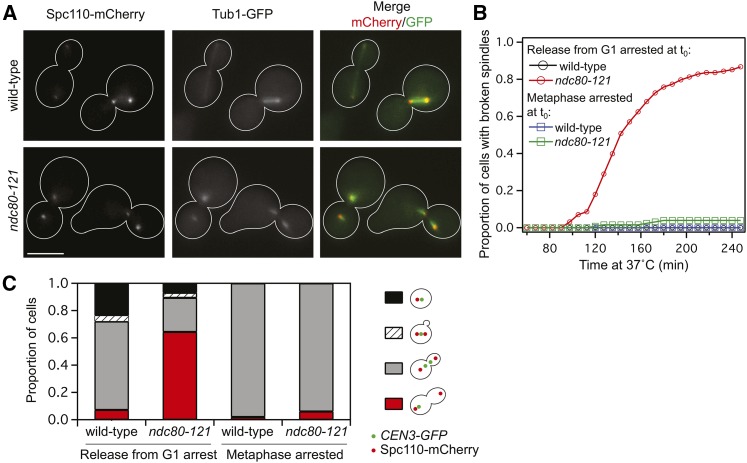
The ndc80-121 allele allows us to interrogate the functions of this uncharacterized region of Ndc80 in vivo. To track budding and progression through the cell cycle, we synchronized wild-type and ndc80-121 cells in G1 and released to the restrictive temperature. While wild-type cells progressed through mitosis normally (Figure S1A), ndc80-121 cells accumulated large buds, indicative of a mitotic arrest (Figure S1B). As ndc80-121 cells entered mitosis, spindle pole bodies (marked by Spc110-mCherry) were able to separate. However, microtubules (visualized by Tub1-GFP) frequently failed to orient along the spindle axis after pole separation (Figure 2A), indicating that ndc80-121 cells arrest with broken spindles at the restrictive temperature (Figure 2B). This suggests that ndc80-121 cells might also have a defect in chromosome segregation. To track chromosome biorientation, we utilized a LacO/LacI system to visualize individual centromeres by fluorescence (see Materials and Methods). Consistent with an inability to biorient chromosomes, 64% of ndc80-121 cells did not have separated CEN3-GFP spots after 100 min at 37° (compared to 7% of wild-type cells) (Figure 2C).
Figure 2.
ndc80-121 cells arrest early in mitosis with broken spindles. (A) Representative images of intact spindles in wild-type cells and broken spindles in ndc80-121 cells at 37°. Spindle pole bodies were labeled with Spc110-mCherry and microtubules were marked by Tub1-GFP. Bar, 5 µm. (B) Quantification of broken spindles in wild-type and ndc80-121 cells, which were scored based on microtubule morphology as judged by Tub1-GFP or Stu2-GFP fluorescence. Accumulation of broken spindles was determined after synchronization in G1 and release into 37° medium (n = 119 wild-type cells and n = 128 ndc80-121 cells), or during a continuous metaphase arrest at 37° (n = 81 wild-type cells and n = 43 ndc80-121 cells). (C) Biorientation of CEN3 (visualized using a LacO/LacI-GFP system) in wild-type and ndc80-121 cells after G1 synchronization and release into 37° medium for 100 min (n = 182 wild-type cells and n = 216 ndc80-121 cells), or during a continuous metaphase arrest at 37° for 100 min (n = 142 wild-type cells and n = 166 ndc80-121 cells).
Using the broken spindle phenotype as a readout, we found that the spindle integrity defect observed in ndc80-121 cells does not occur if biorientation is first established. We arrested ndc80-121 cells in metaphase at the permissive temperature using a Cdc20-degron system (Nishimura et al. 2009) and subsequently shifted to the restrictive temperature while continuing the metaphase arrest. Similar to wild-type cells, ndc80-121 cells remained in metaphase; spindles did not break, and chromosome biorientation (visualized by CEN3-GFP) was maintained (Figure 2, B and C). Therefore, metaphase kinetochores in ndc80-121 cells can support microtubule attachments against the tensile forces exerted across bioriented chromosomes. The ndc80-121 mutations preclude cells from establishing, but not maintaining, a bioriented spindle at the restrictive temperature.
The mitotic defects in ndc80-121 cells are detected by Ipl1
During mitosis, aberrant kinetochore–microtubule attachments are thought to be detected and detached by the conserved Ipl1/Aurora B kinase (Biggins and Murray 2001; Tanaka et al. 2002; Pinsky et al. 2006). The detachment of kinetochores by Ipl1 causes a “wait” signal to be generated, and anaphase is delayed until proper kinetochore–microtubule attachments are made (Pinsky et al. 2006). To determine the basis for mitotic arrest in ndc80-121 cells, we deleted the checkpoint component MAD1 and monitored cell cycle progression. These ndc80-121 mad1∆ cells bypassed the mitotic arrest seen at the restrictive temperature in ndc801-121 cells (Figure S1C). Similarly, ndc80-121 ipl1-321 cells did not arrest in mitosis at the restrictive temperature (Figure S1D). Therefore, the spindle checkpoint is functional in ndc80-121 cells, and their spindle checkpoint-dependent arrest requires Ipl1.
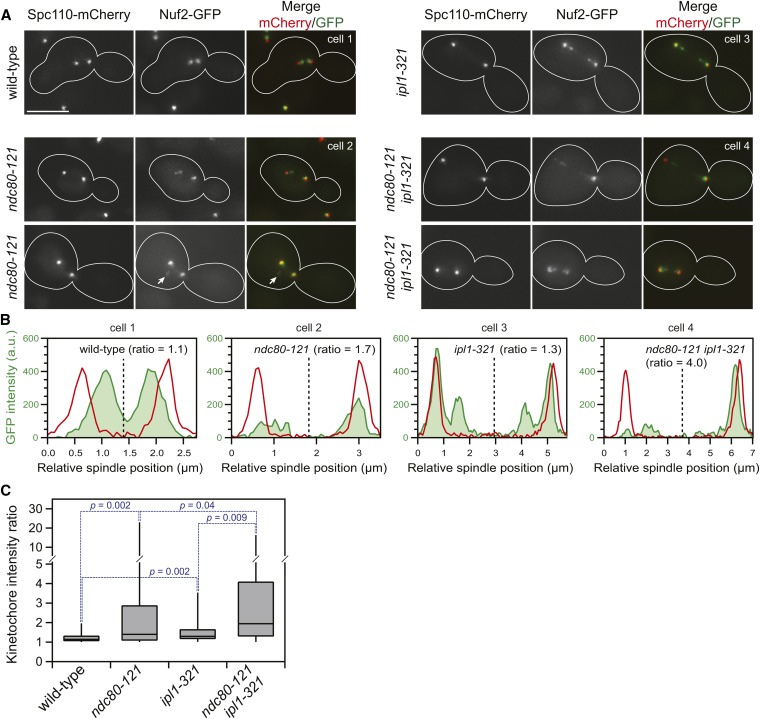
The dependence of the arrest on Mad1 indicates that ndc80-121 cells fail to silence the spindle checkpoint wait signal, which is likely generated by unattached kinetochores. To determine if unattached kinetochores persist in ndc80-121 cells, we visualized kinetochores directly by fluorescence microscopy (with Nuf2-GFP; Figure 3A). During mitosis, kinetochores in wild-type cells are clustered into two distinct spots. By contrast, ndc80-121 cells have multiple Nuf2-GFP foci located both on and off the spindle axis. Kinetochore declustering off the spindle axis (Figure 3A, arrow) indicates the presence of unattached kinetochores (Anderson et al. 2009). At the restrictive temperature, the checkpoint arrest of ndc80-121 cells also requires Ipl1 (Figure S1D), suggesting that unattached kinetochores are generated in an Ipl1-dependent manner (Pinsky et al. 2006). Indeed, kinetochore declustering off the spindle axis was observed 10-fold more frequently in ndc80-121 cells (18%, n = 187 cells) relative to ndc80-121 ipl1-321 cells (2%, n = 95 cells). These results indicate that the ndc80-121 mutations do not directly lead to detachment of kinetochores from microtubules, but rather cause kinetochores to make erroneous attachments that are subsequently recognized and detached by Ipl1.
Figure 3.
Kinetochores are declustered in ndc80-121 cells and are targeted by Ipl1. (A) Representative images of kinetochores (Nuf2-GFP) in wild-type, ndc80-121, ipl1-321, and ndc80-121 ipl1-321 cells after synchronization in G1 and release into 37° medium for 100 min. Arrow denotes declustered kinetochores off the spindle axis. Bar, 5 µm. (B) From the representative images (cells 1–4), the integrated kinetochore fluorescence (green area) was measured for each half of the spindle, and the kinetochore intensity ratio was calculated. The spindle midline was determined based on the positions of the spindle pole bodies (Spc110-mCherry, red lines). (C) Summary of kinetochore intensity ratios of wild-type and mutant cells (n = 40 cells for each condition). The box and whisker plots denote the 0th, 25th, 50th, 75th, and 100th percentiles of the dataset. Statistical comparisons between the distributions were performed using the Kolmogorov–Smirnov test.
To directly assess whether ndc80-121 cells are defective in kinetochore alignment, we measured their ability to distribute kinetochores equally between the two halves of the spindle. This metric, termed the kinetochore intensity ratio, is obtained by dividing the kinetochore fluorescence intensity of the brighter half of the spindle by the dimmer half (and is thus, by definition, always ≥1) (Figure 3, B and C). In wild-type cells, kinetochore distribution was almost perfectly symmetrical, with a median kinetochore intensity ratio of 1.1. Consistent with the requirement of Ipl1 in mitotic error correction, ip1l-321 cells exhibited a slightly asymmetric kinetochore distribution, with a median ratio of 1.3. ndc80-121 cells showed a similar kinetochore alignment defect, with a median kinetochore intensity ratio of 1.4. These mutations had a combinatorial effect on kinetochore asymmetry (ndc80-121 ipl1-321 cells exhibited a median ratio of 1.9), indicating that the activity of Ipl1 mitigates the kinetochore alignment defect in ndc80-121 cells. Taken together, our results demonstrate that the ndc80-121 mutations cause the formation of aberrant kinetochore–microtubule attachments that are detached by Ipl1.
Folding of the Ndc80 complex is required for its function in vivo
One possible function of the region in Ndc80 affected by the ndc80-121 mutations could be the recruitment of an essential binding partner. To identify this putative binding partner, we first performed a screen to isolate dosage-dependent suppressors of the ndc80-121 mutations. ndc80-121 cells were transformed with a library containing fragments of the yeast genome cloned into a high-copy vector (Nasmyth and Tatchell 1980) and screened for growth at the restrictive temperature. We screened ∼2 × 105 plasmids (with 5- to 20-kb fragments, resulting in >70 times coverage of the genome) and isolated 41 suppressor fragments, all of which contain the wild-type NDC80 gene. Results from this screen indicate that overexpression of wild-type NDC80 can suppress the ndc80-121 mutations. However, no extragenic suppressors were found.
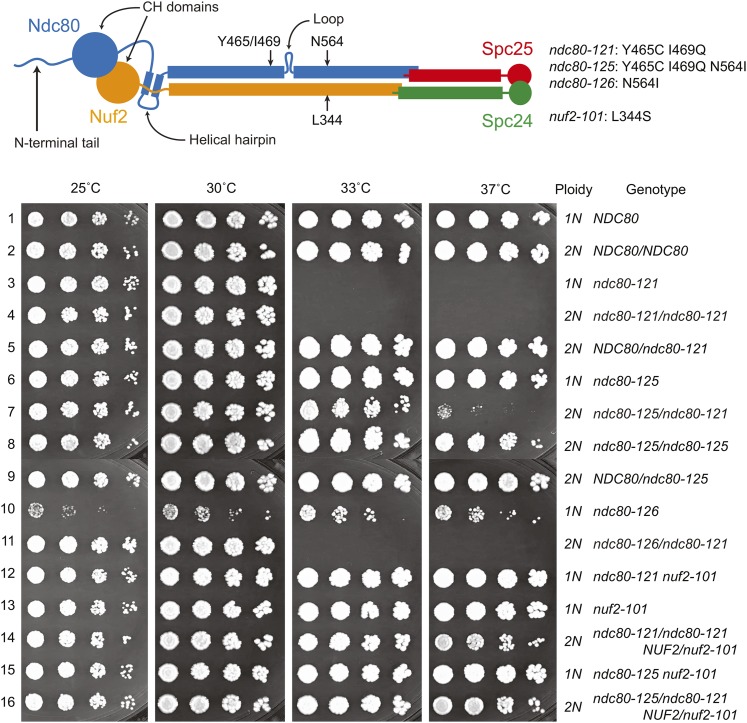
In an independent attempt to identify putative interaction partners, we performed a spontaneous suppressor screen on ndc80-121 cells to isolate mutations that permit growth at the restrictive temperature. In total, we identified four unique suppressor mutations from screening ∼5 × 108 ndc80-121 cells. In NDC80, we found one suppressor mutation that restores the wild-type sequence encoding residue 465 (C465Y) (a mutation restoring the wild-type sequence encoding residue 469 (Q469I) was not isolated likely because it requires two base pair substitutions). Additionally, we found two intragenic suppressor mutations in NDC80. The first intragenic suppressor mutation, C465F reintroduces an aromatic residue at position 465. The second intragenic suppressor mutation, encoding N564I, is 95 residues downstream of the ndc80-121 mutations. We named this allele (coding for Ndc80Y465C I469Q N564I) ndc80-125 (Figure 4). We also generated an allele consisting of the suppressor mutation alone (coding for Ndc80N564I), named ndc80-126. The ndc80-126 mutation confers a slow-growth phenotype, which is completely suppressed in the ndc80-125 allele (the combination of ndc80-126 and ndc80-121 mutations, encoding Ndc80Y465C I469Q N564I). Thus, the ndc80-121 and ndc80-126 mutations show reciprocal suppression (Figure 4, rows 3, 6, and 10), suggesting that the mutations disrupt the same physical interaction (Honts et al. 1994). Lastly, we isolated one extragenic suppressor mutation in NUF2, encoding L344S, and named this allele nuf2-101 (Figure 4). This mutation in Nuf2 is located toward the C terminus of the protein and is predicted to be positioned close to the suppressor mutation Ndc80N564I in the assembled tetrameric complex (see below). The findings from the two suppressor screens suggest that the ndc80-121 mutations do not directly affect recruitment of another kinetochore component, but rather disrupt an interaction between two parts of the Ndc80 complex.
Figure 4.
Mutational analyses provide genetic evidence for a folded conformation of the Ndc80 complex in vivo. A fivefold dilution series of cells (Table S1) was plated and grown at the indicated temperatures. Two suppressor mutations (encoding N564I in Ndc80 and L344S in Nuf2) can individually rescue growth of ndc80-121 cells at the restrictive temperature (rows 6 and 12). Mutant alleles encode the following amino acid changes: ndc80-121 (Y465C I469Q), ndc80-125 (Y465C I469Q N564I), ndc80-126 (N564I), and nuf2-101 (L344S). In a heterozygous diploid, ndc80-121/ndc80-121 is temperature sensitive (row 4). The N564I intragenic suppressor mutation in Ndc80 can rescue the ndc80-121 phenotype in cis, but not in trans (compare rows 7 and 11). The ndc80-126 allele confers a slow-growth phenotype, which is rescued by the mutations in ndc80-121 (compare rows 6 and 10).
It was previously proposed from electron microscopy studies that the Ndc80 complex exhibits flexibility in vitro by bending about the loop region (Wang et al. 2008). The ndc80-121 mutations and their suppressors lie on opposite sides of the loop. If this genetic interaction reflects a physical interaction, it can be explained by tight folding of the complex at the loop. Therefore, we reasoned that in diploid cells, the intragenic suppressor mutation should only rescue ndc80-121 in cis (i.e., when introduced into the same molecule of Ndc80). Similar to ndc80-121 haploid cells, ndc80-121/ndc80-121 homozygous diploid cells are temperature sensitive (Figure 4, row 4). A single copy of the intragenic suppressor mutation introduced in cis partially rescues the temperature sensitivity in diploid cells (Figure 4, row 7, ndc80-125/ndc80-121; Ndc80Y465C I469Q N564I/Ndc80Y465C I469Q). However, diploid cells containing the mutant and suppressor mutations on separate alleles are temperature sensitive (Figure 4, row 11, ndc80-126/ndc80-121; Ndc80N564I/Ndc80Y465C I469Q). Failure of Ndc80N564I to rescue function of Ndc80Y465C I469Q in trans suggests that a physical interaction occurs within a single Ndc80 protein (rather than between two or more copies of Ndc80 at the kinetochore). Our characterization of the ndc80-121 allele and its suppressors suggest that hinging of the Ndc80 complex about its loop is essential for its function in vivo.
The S. cerevisiae Ndc80 complex can adopt a tightly folded conformation
Additional evidence that the Ndc80 complex adopts a folded conformation came by assessing the structure of recombinant complex in vitro by protein cross-linking paired with mass spectrometry analysis. This approach allows the identification of interacting regions within the complex (Figure S2, Table S4, and Table S5). Previously, 26 cross-links were identified within the human Ndc80 complex (Maiolica et al. 2007). In addition to providing a higher resolution map of how the Ndc80 complex is organized, cross-linking can also capture transient conformations of the complex. Recombinant S. cerevisiae Ndc80 complex was cross-linked with disuccinimidyl suberate, which targets primary amine groups in lysines and free N termini of proteins. After digestion of the complex with trypsin and mass spectrometric analysis, four different types of peptides (Figure S2A) were identified using Kojak (an open-source application for efficient identification of cross-linked peptides; Hoopmann et al. 2014): (i) unlinked peptides, (ii) mono-linked peptides, (iii) loop-linked peptides (cross-link between two amino acids within a single peptide), and (iv) cross-linked peptides (cross-link between two different peptides). Of the 138 lysines in the Ndc80 complex, 128 were identified in mono-links (93%; Figure S2B), suggesting that we have nearly saturated available reactive sites with the cross-linking reagent. Structural information can be determined from the cross-linked peptides, which represent pairs of primary amine groups whose backbone α-carbons are within ∼30 Å of one another in the three-dimensional structure of the complex (Herzog et al. 2012). In total, our approach revealed 277 unique cross-links and 85 unique loop-links within the Ndc80 complex with ≥95% confidence (Figure S2, C and D, Table S4, and Table S5). The cross-links observed are in excellent agreement with the current structural model of the Ndc80 complex and confirm the location of the tetramerization domain as previously proposed (Wei et al. 2005; Maiolica et al. 2007; Ciferri et al. 2008; Tien et al. 2013). The N-terminal tail of Ndc80 cross-linked to multiple regions of the complex (Figure S2, C and D). This is consistent with the extended length of the tail (at least 15 nm, Aravamudhan et al. 2014) and its predicted disordered nature (Wei et al. 2005, 2007; Ciferri et al. 2008; Alushin et al. 2010), allowing it to reach multiple parts of the complex in a cross-linking reaction.
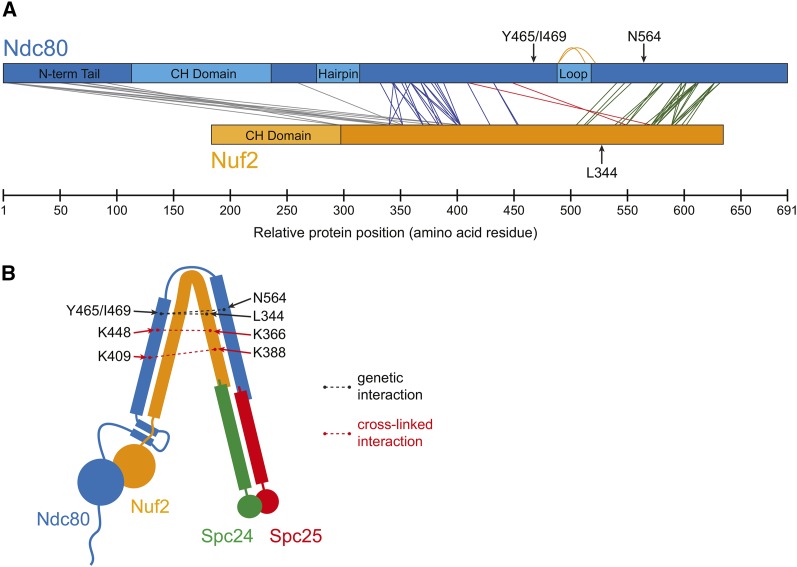
Our cross-links establish the register in the coiled coil formed by Ndc80 and Nuf2, revealing two tightly paired segments (Figure 5A, blue and green lines). In each of these regions, the sequences of Ndc80 and Nuf2 maintain a near-constant register, as expected for parallel helices in coiled-coil domains. The region of Ndc80 from K332 to K432 pairs with the Nuf2 region between K169 and K271. Here, the sequences of Ndc80 and Nuf2 are offset by 162 ± 13 residues (n = 23 cross-links; Figure 5A, blue lines). In the second tightly paired segment (Ndc80 from K541 to K632 pairs with Nuf2 from K322 to K409), the offset increases to 216 ± 9 residues (n = 27 cross-links; Figure 5A, green lines). The offset of the two spontaneous suppressor mutations of ndc80-121 (Nuf2L344S and Ndc80N564I) is 220 residues, suggesting they map to directly interacting heptad repeats in the second tightly paired coiled-coil segment. Between the two tightly paired segments, we identified two cross-links that are consistent with a predicted ∼50-residue interruption caused by the loop: Ndc80K489-Ndc80K513 and Ndc80K489-Ndc80K522 (Figure 5A, orange lines). These cross-links are separated by 24 and 33 residues, respectively. Our cross-linker is too short to span a continuous α-helical segment of even 24 residues in a coiled coil (∼1.47-Å rise per residue; discussed in Lupas and Gruber 2005). Instead, these cross-links indicate that the two predicted ends of the loop lie close together. Our cross-links are consistent with and extend the previous results obtained with the human Ndc80 complex and suggest that the coiled coils and interrupting loop are conserved structural features (Maiolica et al. 2007).
Figure 5.
Cross-linking analysis reveals a tightly folded conformation of the Ndc80 complex in vitro. (A) From cross-linking analysis of the Ndc80 complex, cross-links between Ndc80 and Nuf2 are shown. Cross-links showing the register between Ndc80 and Nuf2 coiled coils are presented as blue and green lines. Orange lines denote loop-links that support the presence of a loop predicted in the coiled-coil region of Ndc80. Cross-links consistent with a tightly folded conformation of the Ndc80 complex are shown as red lines. Full cross-linking results are diagrammed in Figure S2 and listed in Table S4 and Table S5. (B) A tightly folded conformation of the Ndc80 complex can explain the observed genetic and cross-linked interactions.
We found two cross-links that contradict the clear coiled-coil registrations between Ndc80 and Nuf2 (Figure 5A, red lines). These cross-links (Ndc80K409-Nuf2K388 and Ndc80K448-Nuf2K366), which were identified with high confidence (q = 0.01) and manually validated, connect regions of the complex separated by >130 residues in Ndc80 (Figure 5A). They can be explained if the Ndc80 complex forms a tightly folded conformation by hinging about its loop to bring the two coiled-coil segments in close proximity (Figure 5B). Folding of the complex, as predicted by the cross-links, also brings the suppressor mutations in close proximity to the temperature-sensitive ndc80-121 mutations. Our characterization of the ndc80-121 mutant suggests that this conformational change in the complex is required for kinetochore alignment and biorientation during mitosis.
Discussion
The Ndc80 complex folds in half at a flexible loop in Ndc80 early in mitosis
The Ndc80 protein is hypothesized to contain a flexible loop, based on a break in the predicted coiled-coil character (Figure S3). It was previously proposed that the loop acts as a hinge, conferring the flexibility observed for recombinant Ndc80 complexes on negative-stain electron micrographs (Wang et al. 2008). Super-resolution microscopy experiments further suggested that different conformations of the Ndc80 complex exist in vivo; the distance between the two ends of the Ndc80 complex decreases from ∼40 nm to ∼20 nm during the metaphase-to-anaphase transition in S. cerevisiae (Aravamudhan et al. 2014). Altogether, these results predict that the Ndc80 complex undergoes a conformational change through bending at the loop region. Here, we provide two independent lines of evidence that support and extend this model and further show that folding of the complex is of physiological importance. First, we identified cross-links that are consistent with a tightly folded conformation of the Ndc80 complex, bending at the loop region (Figure 5B, dotted red lines). A previous study with the human Ndc80 complex also observed one cross-link that bridges two distant regions of the complex (Maiolica et al. 2007). Second, the ndc80-121 mutant allele and its suppressor mutations demonstrate a genetic interaction between two parts of Ndc80 that can be explained by a folded conformation of the complex (Figure 5B, dotted black lines). We favor a model in which the ndc80-121 temperature-sensitive phenotype results from a weaker interaction between Ndc80Y465/I469 (ndc80-121 mutation sites) and Ndc80N564/Nuf2L344 (spontaneous suppressor mutation sites). Furthermore, our observation that the intragenic Ndc80N564I suppressor mutation can only rescue the ndc80-121 phenotype in cis suggests that this physical interaction occurs within a single complex.
Several observations support our hypothesis that the ndc80-121 mutations destabilize a physical interaction that underlies the tightly folded conformation of the Ndc80 complex. First, the reciprocal suppression between the ndc80-121 and ndc80-126 alleles suggests that the corresponding mutations affect a physical interaction between the two halves of a folded Ndc80 complex (Honts et al. 1994). Second, the ndc80-121 mutant is temperature sensitive, suggesting that both higher temperature and the ndc80-121 mutations decrease the stability of the folded conformation. By contrast, the suppressor mutation ndc80-126 confers slow growth at all temperatures, suggesting that this mutation hyperstabilizes the folded conformation, which functions poorly at all growth temperatures. When the ndc80-121 and ndc80-126 mutations are combined in cis (i.e., the ndc80-125 allele), their opposing effects on the stability of the folded conformation balance out and restore normal Ndc80 function. Finally, the ndc80-121 mutant displays no defects when shifted to the restrictive temperature in metaphase, when the Ndc80 complex has been shown to be in an extended conformation (Joglekar et al. 2009; Aravamudhan et al. 2014). We propose that the mutant complex prematurely adopts a “metaphase-like” extended conformation prior to biorientation, and that the phenotype of ndc80-121 cells results from an inability to stabilize a closed conformation early in mitosis.
ndc80-121 cells are defective in the resolution of aberrant kinetochore–microtubule attachments
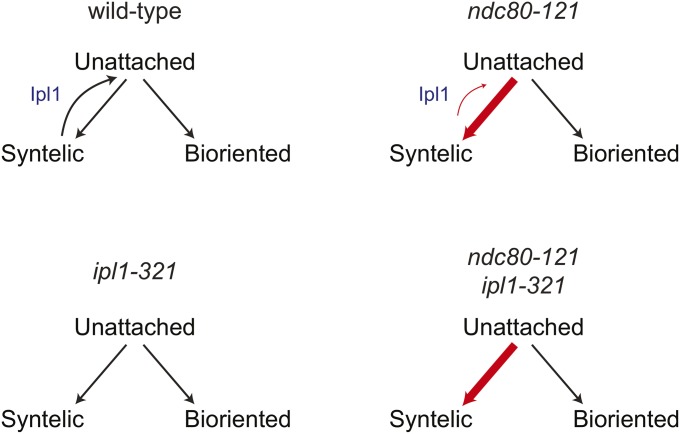
When shifted to the restrictive temperature prior to entry into mitosis, the ndc80-121 mutant arrests in mitosis with defects in chromosome biorientation. We show that ndc80-121 cells contain aberrant kinetochore–microtubule attachments that are detached in an Ipl1-dependent manner. It is generally accepted that Ipl1 selectively detaches kinetochores that experience low levels of tension, which can include kinetochores that form either syntelic or weak microtubule attachments (Nicklas and Koch 1969; Biggins and Murray 2001; Tanaka et al. 2002; Liu et al. 2009; Cane et al. 2013). We find that the ndc80-121 mutations do not weaken the microtubule attachment strength of the Ndc80 complex in vitro and that metaphase kinetochores in ndc80-121 cells can support tension across bioriented chromosomes at the restrictive temperature in vivo. Furthermore, ndc80-121 ipl1-321 cells are not arrested by the spindle checkpoint, indicating that the ndc80-121 mutations do not by themselves produce a major attachment defect without the detachment-promoting activity of Ipl1. Based on these observations, we favor a model where the Ipl1-dependent arrest of ndc80-121 cells is due to the presence of syntelic attachments (that are targeted for detachment by Ipl1), which are not fully resolved before spindle breakage. Consistent with a higher prevalence of syntelic attachments, kinetochores in ndc80-121 cells are asymmetrically distributed on the spindle, similar to those in ipl1-321 cells. This kinetochore alignment defect is further exacerbated in the ndc80-121 ipl1-321 double mutant. This observation rules out the possibility that kinetochore–microtubule attachments in ndc80-121 cells are correct, but anomalously targeted by Ipl1. The more severe kinetochore alignment defect in ndc80-121 ipl1-321 as compared to ipl1-321 cells further suggests that ndc80-121 kinetochores have a greater tendency to form incorrect attachments than wild-type kinetochores (Figure 6). This could result from changes in the conformation of the Ndc80 complex that favor syntelic attachment geometry. In an alternative, but not mutually exclusive model, aberrant attachments in ndc80-121 cells are not efficiently detached in response to Ipl1 activity (Figure 6). In both scenarios, the persistence of erroneous attachments in ndc80-121 cells overwhelms the Ipl1-dependent error correction machinery, resulting in spindle breakage.
Figure 6.
A model for how ndc80-121 cells generate kinetochore alignment defects. The ndc80-121 allele causes more stable syntelic attachments, which are not effectively resolved by Ipl1 kinase.
The structural architecture of the Ndc80 complex, including its inherent flexibility, is highly conserved (Maiolica et al. 2007; Wang et al. 2008). Furthermore, the ndc80-121 mutations affect conserved residues in Ndc80 (Figure 1B), suggesting that a physical interaction stabilizes a folded conformation of the Ndc80 complex in higher eukaryotes. Together, our results suggest that folding of Ndc80 complex and its role in the resolution of aberrant kinetochore–microtubule attachments are common features in eukaryotic cells.
Supplementary Material
Acknowledgments
We thank Rachel Klevit, Michelle Giarmarco, Justin Decarreau, and Linda Wordeman for advice and technical assistance. We also thank members of the Davis lab and the Seattle Mitosis group for helpful discussions and Matthew Miller for critical reading of this manuscript. This work was supported by a National Sciences and Engineering Research Council of Canada scholarship to J.F.T.; National Institutes of Health grant no. T32 GM008268 to N.T.U.; National Center for Research Resources grant no. S10 RR26406 to C.L.A.; and National Institute of General Medical Sciences grant nos. R01 GM040506 to T.N.D. and R01 GM079373 to C.L.A. This work was also supported in part by the National Science Foundation MRI grant no. 0923536, and the National Institute of General Medical Sciences under grant nos. 2P50 GM076547/Center for Systems Biology, and GM087221, and grant no. S10RR027584 to R.L.M. M.R., M.J.M., B.R.F. and J.R.Y. were supported by grant no. P41 GM103533 to T.N.D.
Footnotes
Supporting information is available online at http://www.genetics.org/lookup/suppl/doi:10.1534/genetics.114.167775/-/DC1.
Communicating editor: O. Cohen-Fix
Literature Cited
- Akiyoshi B., Sarangapani K. K., Powers A. F., Nelson C. R., Reichow S. L., et al. , 2010. Tension directly stabilizes reconstituted kinetochore-microtubule attachments. Nature 468: 576–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alushin G. M., Ramey V. H., Pasqualato S., Ball D. A., Grigorieff N., et al. , 2010. The Ndc80 kinetochore complex forms oligomeric arrays along microtubules. Nature 467: 805–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson M., Haase J., Yeh E., Bloom K., 2009. Function and assembly of DNA looping, clustering, and microtubule attachment complexes within a eukaryotic kinetochore. Mol. Biol. Cell 20: 4131–4139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aravamudhan P., Felzer-Kim I., Gurunathan K., Joglekar A. P., 2014. Assembling the protein architecture of the budding yeast kinetochore-microtubule attachment using FRET. Curr. Biol. 24: 1437–1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biggins S., Murray A. W., 2001. The budding yeast protein kinase Ipl1/Aurora allows the absence of tension to activate the spindle checkpoint. Genes Dev. 15: 3118–3129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biggins S., Bhalla N., Chang A., Smith D. L., Murray A. W., 2001. Genes involved in sister chromatid separation and segregation in the budding yeast Saccharomyces cerevisiae. Genetics 159: 453–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cane S., Ye A. A., Luks-Morgan S. J., Maresca T. J., 2013. Elevated polar ejection forces stabilize kinetochore-microtubule attachments. J. Cell Biol. 200: 203–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciferri C., Pasqualato S., Screpanti E., Varetti G., Santaguida S., et al. , 2008. Implications for kinetochore-microtubule attachment from the structure of an engineered Ndc80 complex. Cell 133: 427–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Wulf P., McAinsh A. D., Sorger P. K., 2003. Hierarchical assembly of the budding yeast kinetochore from multiple subcomplexes. Genes Dev. 17: 2902–2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franck A. D., Powers A. F., Gestaut D. R., Davis T. N., Asbury C. L., 2010. Direct physical study of kinetochore-microtubule interactions by reconstitution and interrogation with an optical force clamp. Methods 51: 242–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauf S., 2013. The spindle assembly checkpoint: progress and persistent puzzles. Biochem. Soc. Trans. 41: 1755–1760. [DOI] [PubMed] [Google Scholar]
- Herzog F., Kahraman A., Boehringer D., Mak R., Bracher A., et al. , 2012. Structural probing of a protein phosphatase 2A network by chemical cross-linking and mass spectrometry. Science 337: 1348–1352. [DOI] [PubMed] [Google Scholar]
- Honts J. E., Sandrock T. S., Brower S. M., O’Dell J. L., Adams A. E., 1994. Actin mutations that show suppression with fimbrin mutations identify a likely fimbrin-binding site on actin. J. Cell Biol. 126: 413–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janke C., Ortiz J., Lechner J., Shevchenko A., Magiera M. M., et al. , 2001. The budding yeast proteins Spc24p and Spc25p interact with Ndc80p and Nuf2p at the kinetochore and are important for kinetochore clustering and checkpoint control. EMBO J. 20: 777–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joglekar A. P., Bloom K., Salmon E. D., 2009. In vivo protein architecture of the eukaryotic kinetochore with nanometer scale accuracy. Curr. Biol. 19: 694–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kall L., Canterbury J. D., Weston J., Noble W. S., MacCoss M. J., 2007. Semi-supervised learning for peptide identification from shotgun proteomics datasets. Nat. Methods 4: 923–925. [DOI] [PubMed] [Google Scholar]
- Larkin M. A., Blackshields G., Brown N. P., Chenna R., McGettigan P. A., et al. , 2007. Clustal W and Clustal X version 2.0. Bioinformatics 23: 2947–2948. [DOI] [PubMed] [Google Scholar]
- Liu D., Vader G., Vromans M. J., Lampson M. A., Lens S. M., 2009. Sensing chromosome bi-orientation by spatial separation of aurora B kinase from kinetochore substrates. Science 323: 1350–1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupas A. N., Gruber M., 2005. The structure of alpha-helical coiled coils. Adv. Protein Chem. 70: 37–78. [DOI] [PubMed] [Google Scholar]
- Maiolica A., Cittaro D., Borsotti D., Sennels L., Ciferri C., et al. , 2007. Structural analysis of multiprotein complexes by cross-linking, mass spectrometry, and database searching. Mol. Cell. Proteomics 6: 2200–2211. [DOI] [PubMed] [Google Scholar]
- Malvezzi F., Litos G., Schleiffer A., Heuck A., Mechtler K., et al. , 2013. A structural basis for kinetochore recruitment of the Ndc80 complex via two distinct centromere receptors. EMBO J. 32: 409–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller E. G. D., Snydsman B. E., Novik I., Hailey D. W., Gestaut D. R., et al. , 2005. The organization of the core proteins of the yeast spindle pole body. Mol. Biol. Cell 16: 3341–3352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasmyth K. A., Tatchell K., 1980. The structure of transposable yeast mating type loci. Cell 19: 753–764. [DOI] [PubMed] [Google Scholar]
- Nicklas R. B., Koch C. A., 1969. Chromosome micromanipulation. 3. Spindle fiber tension and the reorientation of mal-oriented chromosomes. J. Cell Biol. 43: 40–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura K., Fukagawa T., Takisawa H., Kakimoto T., Kanemaki M., 2009. An auxin-based degron system for the rapid depletion of proteins in nonplant cells. Nat. Methods 6: 917–922. [DOI] [PubMed] [Google Scholar]
- Nishino T., Rago F., Hori T., Tomii K., Cheeseman I. M., et al. , 2013. CENP-T provides a structural platform for outer kinetochore assembly. EMBO J. 32: 424–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborne M. A., Schlenstedt G., Jinks T., Silver P. A., 1994. Nuf2, a spindle pole body-associated protein required for nuclear division in yeast. J. Cell Biol. 125: 853–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinsky B. A., Kung C., Shokat K. M., Biggins S., 2006. The Ipl1-Aurora protein kinase activates the spindle checkpoint by creating unattached kinetochores. Nat. Cell Biol. 8: 78–83. [DOI] [PubMed] [Google Scholar]
- Powers A. F., Franck A. D., Gestaut D. R., Cooper J., Gracyzk B., et al. , 2009. The Ndc80 kinetochore complex forms load-bearing attachments to dynamic microtubule tips via biased diffusion. Cell 136: 865–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka T. U., Rachidi N., Janke C., Pereira G., Galova M., et al. , 2002. Evidence that the Ipl1-Sli15 (Aurora kinase-INCENP) complex promotes chromosome bi-orientation by altering kinetochore-spindle pole connections. Cell 108: 317–329. [DOI] [PubMed] [Google Scholar]
- Tien J. F., Umbreit N. T., Gestaut D. R., Franck A. D., Cooper J., et al. , 2010. Cooperation of the Dam1 and Ndc80 kinetochore complexes enhances microtubule coupling and is regulated by aurora B. J. Cell Biol. 189: 713–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tien J. F., Fong K. K., Umbreit N. T., Payen C., Zelter A., et al. , 2013. Coupling unbiased mutagenesis to high-throughput DNA sequencing uncovers functional domains in the Ndc80 kinetochore protein of Saccharomyces cerevisiae. Genetics 195: 159–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H. W., Long S., Ciferri C., Westermann S., Drubin D., et al. , 2008. Architecture and flexibility of the yeast Ndc80 kinetochore complex. J. Mol. Biol. 383: 894–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterhouse A. M., Procter J. B., Martin D. M., Clamp M., Barton G. J., 2009. Jalview Version 2–a multiple sequence alignment editor and analysis workbench. Bioinformatics 25: 1189–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei R. R., Sorger P. K., Harrison S. C., 2005. Molecular organization of the Ndc80 complex, an essential kinetochore component. Proc. Natl. Acad. Sci. USA 102: 5363–5367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei R. R., Al-Bassam J., Harrison S. C., 2007. The Ndc80/HEC1 complex is a contact point for kinetochore-microtubule attachment. Nat. Struct. Mol. Biol. 14: 54–59. [DOI] [PubMed] [Google Scholar]
- Wigge P. A., Kilmartin J. V., 2001. The Ndc80p complex from Saccharomyces cerevisiae contains conserved centromere components and has a function in chromosome segregation. J. Cell Biol. 152: 349–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigge P. A., Jensen O. N., Holmes S., Soues S., Mann M., et al. , 1998. Analysis of the Saccharomyces spindle pole by matrix-assisted laser desorption/ionization (MALDI) mass spectrometry. J. Cell Biol. 141: 967–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.