Abstract
Partially purified ribulose-1,5-bisphosphate carboxylase (EC 4.1.1.39) was isolated from diploid and tetraploid cultivars of ryegrass (Lolium perenne L.) using two separate methods. The apparent Km (CO2) values for the enzymes prepared by either method did not differ significantly between diploid and tetraploid when assayed by two separate techniques. The unpurified enzymes from freshly lysed (and fully functional) protoplasts of both diploid and tetraploid cultivars gave virtually identical apparent Km (CO2) values. There was no indication of large differences in affinity for CO2 of illuminated intact protoplasts from the two cultivars.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bahr J. T., Jensen R. G. Activation of ribulose bisphosphate carboxylase in intact chloroplasts by CO2 and light. Arch Biochem Biophys. 1978 Jan 15;185(1):39–48. doi: 10.1016/0003-9861(78)90141-8. [DOI] [PubMed] [Google Scholar]
- Cooper T. G., Filmer D. The active species of "CO2" utilized by ribulose diphosphate carboxylase. J Biol Chem. 1969 Feb 10;244(3):1081–1083. [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Edwards G. E., Robinson S. P., Tyler N. J., Walker D. A. Photosynthesis by isolated protoplasts, protoplast extracts, and chloroplasts of wheat: influence of orthophosphate, pyrophosphate, and adenylates. Plant Physiol. 1978 Aug;62(2):313–319. doi: 10.1104/pp.62.2.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lilley R. M., Walker D. A. Carbon dioxide assimilation by leaves, isolated chloroplasts, and ribulose bisphosphate carboxylase from spinach. Plant Physiol. 1975 Jun;55(6):1087–1092. doi: 10.1104/pp.55.6.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorimer G. H., Badger M. R., Andrews T. J. D-Ribulose-1,5-bisphosphate carboxylase-oxygenase. Improved methods for the activation and assay of catalytic activities. Anal Biochem. 1977 Mar;78(1):66–75. doi: 10.1016/0003-2697(77)90009-4. [DOI] [PubMed] [Google Scholar]
- Lorimer G. H., Badger M. R., Andrews T. J. The activation of ribulose-1,5-bisphosphate carboxylase by carbon dioxide and magnesium ions. Equilibria, kinetics, a suggested mechanism, and physiological implications. Biochemistry. 1976 Feb 10;15(3):529–536. doi: 10.1021/bi00648a012. [DOI] [PubMed] [Google Scholar]
- Markwell M. A., Haas S. M., Bieber L. L., Tolbert N. E. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal Biochem. 1978 Jun 15;87(1):206–210. doi: 10.1016/0003-2697(78)90586-9. [DOI] [PubMed] [Google Scholar]
- ORNSTEIN L. DISC ELECTROPHORESIS. I. BACKGROUND AND THEORY. Ann N Y Acad Sci. 1964 Dec 28;121:321–349. doi: 10.1111/j.1749-6632.1964.tb14207.x. [DOI] [PubMed] [Google Scholar]
- PATTERSON M. S., GREENE R. C. MEASUREMENT OF LOW ENERGY BETA-EMITTERS IN AQUEOUS SOLUTION BY LIQUID SCINTILLATION COUNTING OF EMULSIONS. Anal Chem. 1965 Jun;37:854–857. doi: 10.1021/ac60226a017. [DOI] [PubMed] [Google Scholar]
- Paech C., Pierce J., McCurry S. D., Tolbert N. E. Inhibition of ribulose-1,5-biphosphate carboxylase/oxygenase by ribulose-1,5-bisphosphate epimerization and degradation products. Biochem Biophys Res Commun. 1978 Aug 14;83(3):1084–1092. doi: 10.1016/0006-291x(78)91506-1. [DOI] [PubMed] [Google Scholar]
- Peterson G. L. A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal Biochem. 1977 Dec;83(2):346–356. doi: 10.1016/0003-2697(77)90043-4. [DOI] [PubMed] [Google Scholar]
- RICKLI E. E., GHAZANFAR S. A., GIBBONS B. H., EDSALL J. T. CARBONIC ANHYDRASES FROM HUMAN ERYTHROCYTES. PREPARATION AND PROPERTIES OF TWO ENZYMES. J Biol Chem. 1964 Apr;239:1065–1078. [PubMed] [Google Scholar]
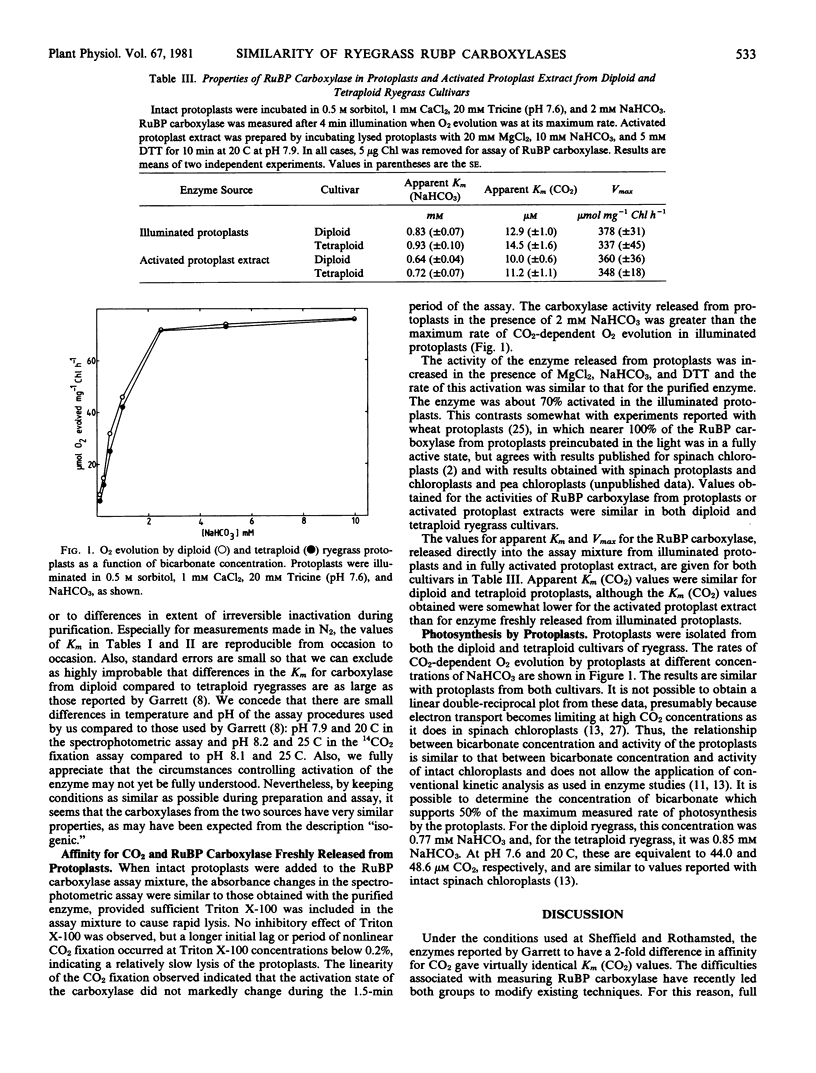
- Rathnam C. K., Chollet R. Photosynthetic and Photorespiratory Carbon Metabolism in Mesophyll Protoplasts and Chloroplasts Isolated from Isogenic Diploid and Tetraploid Cultivars of Ryegrass (Lolium perenne L.). Plant Physiol. 1980 Mar;65(3):489–494. doi: 10.1104/pp.65.3.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson S. P., McNeil P. H., Walker D. A. Ribulose bisphosphate carboxylase--lack of dark inactivation of the enzyme in experiments with protoplasts. FEBS Lett. 1979 Jan 15;97(2):296–300. doi: 10.1016/0014-5793(79)80106-4. [DOI] [PubMed] [Google Scholar]
- Shapiro A. L., Viñuela E., Maizel J. V., Jr Molecular weight estimation of polypeptide chains by electrophoresis in SDS-polyacrylamide gels. Biochem Biophys Res Commun. 1967 Sep 7;28(5):815–820. doi: 10.1016/0006-291x(67)90391-9. [DOI] [PubMed] [Google Scholar]
- WILKINSON G. N. Statistical estimations in enzyme kinetics. Biochem J. 1961 Aug;80:324–332. doi: 10.1042/bj0800324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]


